Introduction
Oncoprotein 18 (Op18)/stathmin is a highly
conserved, cytoplasmic, small molecular phosphoprotein that is
usually highly expressed in solid tumors (1). Op18 directly regulates the dynamic
equilibrium of microtubule polymerization and depolymerization by
inhibiting phosphorylation and activating dephosphorylation, which
is closely associated with cell cycle, transport of substances,
cell adhesion and migration, cell proliferation and
differentiation, and cell death (2–4).
Op18/stathmin has four phosphorylation sites, Ser16, Ser25, Ser38
and Ser63, which are regulated by a series of kinases, including
cell division cycle-2 (CDC2; also termed cyclin-dependent kinase
1), extracellular-regulated protein kinase (ERK) of the
mitogen-activated protein kinase family (5,6).
Op18/stathmin integrates and relays multiple intra- and
extracellular stimuli to mediate the maintenance of malignant
phenotypes and other biological behaviors of tumor cells.
Therefore, Op18 is a central target of a wide range of signaling
pathways (7,8).
Our previous studies demonstrated that NCI-H1299
cells, a non-small cell lung cancer (NSCLC) cell line, with a high
expression of Op18/stathmin has high resistance to Taxol (namely
paclitaxel, which has been widely applied clinically as a cytoxic
antiepithelioma chemotherapy drug derived from plants) compared
with other epithelial-derived tumor cell lines (CNE1, MGC gastric
cancer cells, MCF-7 breast cancer cells and Hep3b-2 hepatoma cells)
(1). Additionally, silencing
Op18/stathmin by RNA interference (RNAi) increased the sensitivity
of NCI-H1299 cells to Taxol (1).
Further in vitro experiments confirmed that autocrine
interleukin-10 (IL-10) was decreased in NCI-H1299 cells when
treated with a combination of Taxol and Op18/stathmin RNAi. In
athymic BALB/c mice that lack functional mature T cells and
have IL-10 production deficiency, autocrine IL-10 was identified to
be produced by NCI-H1299 cell xenografts in the sera (9). Furthermore, Op18/stathmin RNAi and
Taxol synergistically inhibited lung carcinogenesis and promoted
the differentiation of implanted tumors and sensitivity to Taxol.
The level of autocrine IL-10 was notably declined in the sera of
tumor-bearing mice (9).
In this study, the possibility of autocrine IL-10
feedback regulating Op18/stathmin signaling in NCI-H1299 cells and
its underlying molecular mechanisms were examined. The effects of
cross talk between autocrine IL-10 and Op18/stathmin signals on
lung cancer malignant phenotypes, drug sensitivity and tumor immune
escape were investigated.
Materials and methods
Cell culture
Human NSCLC NCI-H1299 cells (cat. no. CRL-5803;
American Type Culture Collection, Manassas, VA, USA) were cultured
in RPMI-1640 medium (cat. no. 01-100-1ACS; Biological Industries,
Kibbutz Beit Haemek, Israel) supplemented with 10% fetal bovine
serum (FBS; cat. no. 04-001-1ACS; Biological Industries), 100 IU/ml
penicillin, and 100 µg/ml streptomycin (cat. no. sv30010; Hyclone;
GE Healthcare Life Sciences, Logan, UT, USA) at 37°C in a
humidified atmosphere of 5% CO2.
Antibodies and reagents
The primary antibodies used are as follows:
Anti-Op18 (cat. no. 569391; Merck KGaA, Darmstadt, Germany);
anti-phospho-Op18-Ser25 (cat. no. ab194752),
anti-phospho-stathmin-Ser63 (cat. no. ab76583) and anti-IL-10 (cat.
no. ab134742) from Abcam (Cambridge, MA, USA); anti-CDC2 p34 (cat.
no. sc-954), anti-caspase-3 (cat. no. sc-7272), anti-ERK (cat. no.
sc-93), anti-phospho-ERK (cat. no. sc-7383), anti-β-actin (cat. no.
sc-47778) and anti-nuclear factor-κB (NF-κB; cat. no. sc-109) were
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA);
anti-phospho-Thr161-CDC2 (cat. no. 9114), anti-caspase-8 (cat. no.
9746), anti-caspase-9 (cat. no. 9502) and anti-phospho-Ser536-NF-κB
p65 (cat. no. 3031S) were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA). The secondary antibodies were horseradish
peroxidase (HRP)-conjugated goat anti-rabbit IgG (cat. no. sc-2004)
and rabbit anti-mouse IgG (cat. no. sc-358914; Santa Cruz). NF-κB
inhibitor pyrrolidine dithiocarbamate (PDTC; cat. no. 5108-96-3,
Sigma-Aldrich; Merck KGaA) (10,11)
and Taxol (cat. no. sc-201439; Santa Cruz Biotechnology, Inc.) were
separately dissolved in dimethyl sulfoxide (DMSO) and stored at
−20°C.
Plasmid construction
RNAi targeting Op18/stathmin was used to silence
Op18/stathmin. Small interfering RNA targeting the coding region of
Op18/stathmin at 5′-AGAGAAACTGACCCACAAA-3′ (GenBank no. 53305,
sequence 374–393) were inserted into the
BamH1/HindIII restriction sites of
pGCsilencer-U6/Neo/GFP (RNAi; Shanghai GeneChem Co., Ltd.,
Shanghai, China; sense gaAGAGAAACTGACCCACAAA, antisense
TTTGTGGGTCAGTTTCTCTtc). A nonsense sequence not targeting any
encoding gene was inserted into pGC-silencer-U6/Neo/GFP-Non (Non)
and pGC-silencer-U6/Neo/GFP (Blank) was an empty plasmid. The
plasmids (4 µg) were transiently transfected into cells at a
transfection efficiency of >80% in 250 µl incomplete RPMI-1640
medium containing 10 µl Lipofectamine™ 2000 (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA); 4 h later, the
medium replaced with complete medium for 24 h. All plasmids were
previously constructed by our laboratory (9,12).
MTT assays
Cells were seeded in 96-well plates at 5,000 cells
per well and supplemented with different concentrations of
anti-IL-10 antibody (0, 0.5 and 2.5 µg/ml; cat. no. ab134742;
Abcam). At 24, 48 and 72 h, 10 µl 0.5% MTT was added to the well
and incubated at 37°C for 4 h. The medium was then removed and 100
µl DMSO was added and incubated for 10 min. A total of six parallel
wells/sample was used. The optical density (OD) value was measured
using a microplate reader (BioTek Instruments, Inc., Winooski, VT,
USA) at 490 nm. The relative proliferation ratios were calculated
according to the formula: Relative proliferation ratio (%) =
(ODtreatment/ODcontrol) × 100. Cells treated
with DMSO were used as a control, and the OD value of the control
was set as 1.0.
Colony formation assays
A total of 2,000 cells per well were seeded in a
6-well plate in the presence of anti-IL-10 antibody (0, 0.5 and 2.5
µg/ml) and incubated for 1–2 weeks. Cell growth was suspended when
colonies were observed by the naked eye. The cells were fixed with
100% methanol for 10 min at room temperature and stained with 0.1%
crystal violet at room temperature for 30 min. The colonies with
>50 cells were counted with an inverted microscope. The relative
colony formation efficiency was calculated using the formula:
Relative colony formation efficiency (%) = (average colonies/2,000)
× 100.
Wound healing assays
When the confluence of the cells was ~80%, the cells
were washed three times with ice-cold phosphate-buffered saline
(PBS). A sterilized pipette tip (200 µl) was applied to wipe off
the cells along the longitudinal and parallel trails that were
marked in the back of the plate. The plate was washed with PBS, and
0, 0.5 and 2.5 µg/ml anti-IL-10 antibody was added. Cell migration
was observed with an inverted optical microscope at 0, 12, 24 and
36 h.
Transwell analysis
The upper Transwell chamber with 8.0 µm pore size of
polycarbonate membrane of polystyrene plates (Costar; Corning
Incorporated, NY, USA) was pretreated with heated serum-free
RPMI-1640 for 30 min, and 800 µl complete medium containing 10% FBS
was added to the lower chamber. A total of 10 µl cell suspension
(cell density, 2×105 cells/ml with 0.2% FBS of
RPMI-1640) was added to the upper chamber at 37°C for 24 h. The
cells in the upper Transwell chamber were wiped with a cotton swab,
and the cells were fixed with 100% methanol for 10 min and stained
with 0.1% crystal violet for 10 min at room temperature. The cells
that passed through the membrane were counted in five random visual
fields under an inverted microscope. The migrated cells were then
imaged and evaluated.
Flow cytometric analysis
A total of 2.5×105 cells per well were
seeded into 6-well plates in the presence of 2.5 µg/ml anti-IL-10
antibody combined with 100 nM Taxol. The cells were cultured for 24
h. The cells were harvested and fixed with 70% ethanol at 4°C
overnight for flow cytometric analysis. Cell apoptosis assay was
performed using a Fluorescence Activating Cell Sorter (Beijing
Dingguo Changsheng Biotechnology Co., Ltd., Beijing, China). In
brief, cells were resuspended and stained in 500 µl binding buffer
with 5 µl Annexin V-fluorescein isothiocyanate and 5 µl propidium
following the instruction of Annexin V-fluorescein isothiocyanate
Apoptosis Detection kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China). In addition, the cells were treated with different
concentrations (0, 5 and 10 µM) of PDTC for 24 h.
Western blot analysis
The cells were treated on ice with a cell lysis
buffer (50 mM Tris-HCl, 1 mM ethylenediaminetetraacetic acid, 2%
sodium dodecyl sulfate, 5 mM dithiothreitol and 10 mM
phenylmethylsulfonyl fluoride) for 30 min. The lysates were
collected and denatured in boiling water for 8 min and incubated on
ice for 5 min. The lysates were sonicated for 30 sec and
centrifuged at 4°C for 15 min. The concentration of the protein was
measured using BCA Protein Assay reagent (Pierce; Thermo Fisher
Scientific Inc.). Total protein (50 µg) was separated by 10–12%
SDS-polyacrylamide gel electrophoresis and electrotransferred onto
a nitrocellulose membrane. The membrane was blocked for 2 h with 5%
non-fat milk at room temperature in PBS with 0.05% Tween-20 and
then incubated with primary antibodies, anti-β-actin (1:2,000),
anti-Op18 (1:3,000), anti-phospho-Op18-Ser25,
anti-phospho-stathmin-Ser63, anti-IL-10, anti-phospho-Thr161-CDC2,
anti-CDC2 p34, anti-ERK, anti-phospho-ERK, anti-NF-κB,
anti-phospho-Ser536-NF-κB p65, anti-caspase-3, anti-caspase-8 and
anti-caspase-9 (all 1:1,000), overnight at 4°C. The membrane was
then incubated with HRP-conjugated secondary antibodies
(1:2,000-1:3,000) for 1.5 h at room temperature. The membrane was
visualized with an enhanced chemiluminescence detection kit (Thermo
Fisher Scientific, Inc.).
ELISA assays
Cells in the logarithmic growth phase were seeded in
24-well plates. When the cells reached 80% confluence, the media
was replaced with fresh RPMI-1640 medium. The cells were incubated
in phenol red-free media for 6, 12, 24 and 36 h, while other cells
were treated with 0, 5 and 10 µM PDTC in phenol red-free media for
24 h. The supernatant was collected for in vitro detection
of autocrine IL-10. The human IL-10 ELISA kit [cat. no. EK1102;
Multisciences (Lianke) Biotech Co., Ltd., Hangzhou, China] was
used.
Animal experiments
Male Kunming mice (4-week-old) were randomly divided
into four groups of 15, 30 and 45 day, and parallel controls (8
mice per group). NCI-H1299 cells at the logarithmic growth phase
were collected and resuspended in normal saline. A total of
4×106 cells in 0.5 ml were injected intraperitoneally
into each mouse. The control mice were injected with the same
volume of normal saline. Retro-orbital blood samples were collected
from anaesthetized mice at day 0, 15, 30 and 45; the mice were
respectively sacrificed at day 15, 30 and 45 at 15 days interval by
cervical dislocation for the collection of blood. Flow cytometry
(model no. BC-2800vet; Auto Hematology Analyzer; Shenzhen Mindray
Bio-Medical Electronics Co., Ltd., Shenzhen, China) was performed
to sort the leukocytes. Cell counting was performed by Servicebio,
Inc. (Woburn, MA, USA).
In addition, part of each blood sample was used to
detect IL-10 in the sera by ELISA. The mice were respectively
sacrificed at day 15, 30 and 45 at 15 days interval by cervical
dislocation for the collection of blood sample, and the tumors were
removed from 45-day mice. The 3–5 µm thickness of tissue sections
were fixed by 10% formalin overnight and stained with 10%
hematoxylin (×5, 10 min) and 1% eosin (1 min) at room temperature
and examined by inverted optical microscope. Histopathological
examination was performed by Professor Deyun Feng (Department of
Pathology, First Xiangya Hospital Affiliated to Central South
University, Changsha, China).
Statistical analysis
Statistical analysis was performed using the SPSS
17.0 statistical software (SPSS, Inc., Chicago, IL, USA). The data
are presented as the mean ± standard deviation. Analysis of
variance and least significant difference method was applied to
perform multiple comparisons between the groups, P<0.05 was
considered to indicate a statistically significant difference.
Results
Anti-IL-10 antibody inhibits cell
proliferation and colony formation
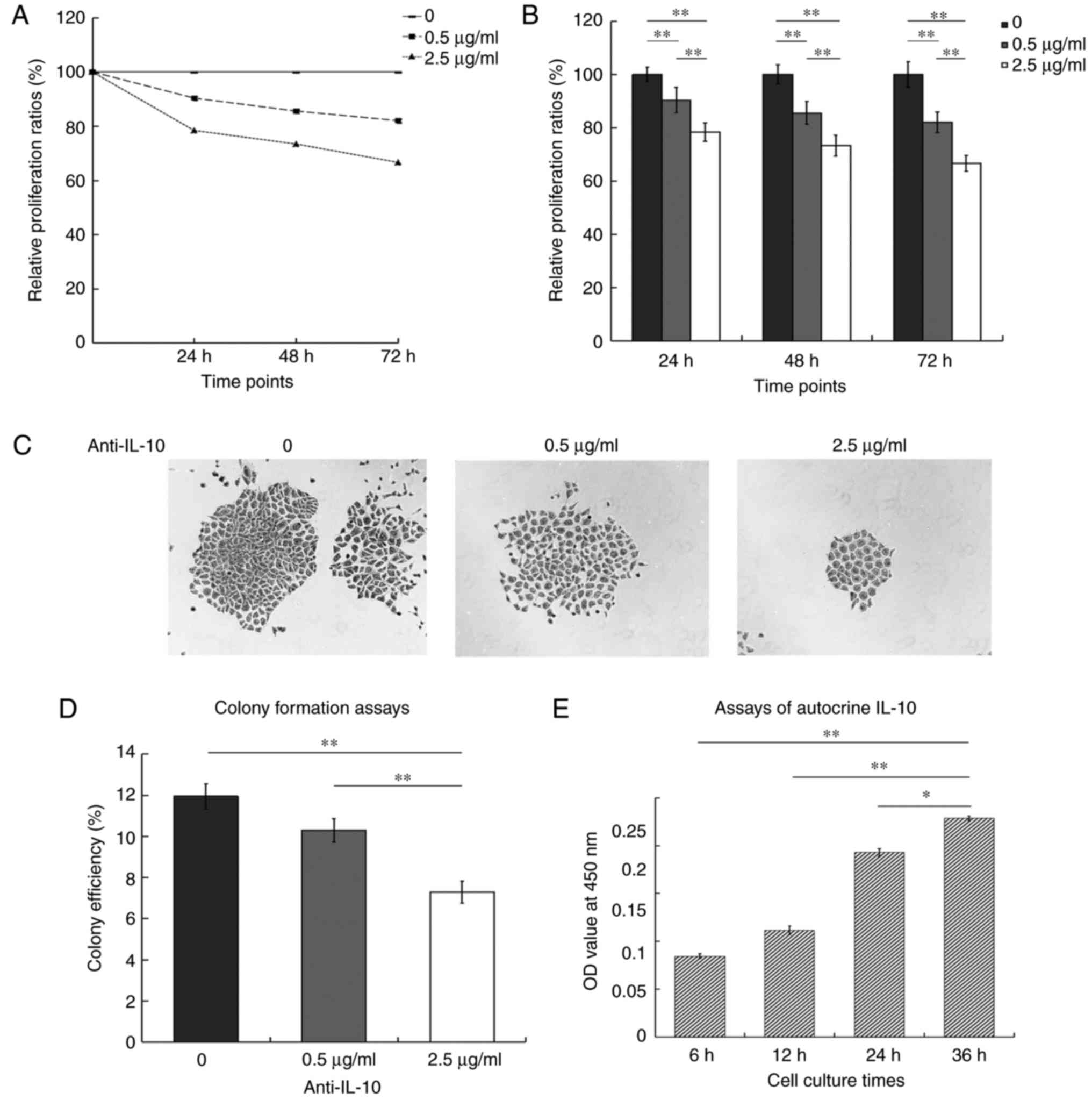
The relative proliferation ratios were 90.32, 85.54
and 82.02% at 24, 48 and 72 h in the presence of 0.5 µg/ml
anti-IL-10 antibody, and 78.38, 73.38 and 66.60% at 2.5 µg/ml
anti-IL-10 antibody. With the extension of time, the growth curve
was steeper for 2.5 µg/ml compared with 0.5 µg/ml. The treatment
with anti-IL-10 antibody inhibited cell proliferation in a
dose-dependent manner (Fig. 1A).
The histograms showed that the relative proliferation ratios of two
treated groups were markedly lower compared with the control. In
addition, the difference was also significant between two groups
that were treated with anti-IL-10 antibody at three time points
(P<0.01; Fig. 1B).
The colony formation analysis demonstrated that
there were many large colonies with closely adhered cells in the
control group. When the cells were exposed to the anti-IL-10
antibody, the number of colonies declined, and the cells became
sparse. Only a few of small colonies appeared when treated with 2.5
µg/ml anti-IL-10 (Fig. 1C). The
histograms indicated that the colony formation ratios were 11.95,
10.3 and 7.3% at 0, 0.5 and 2.5 µg/ml, respectively. There was no
significant difference between the control and the group treated
with 0.5 µg/ml anti-IL-10 (P>0.05). The number of colonies was
significantly decreased in the group treated with 2.5 µg/ml
anti-IL-10 antibody compared with the other two groups (P<0.01,
Fig. 1D). The ELISA assays for
autocrine IL-10 indicated that the OD values of the supernatants
were 0.0845, 0.1115, 0.193 and 0.2285 at 6, 12, 24 and 36 h,
respectively. The level of IL-10 reached a peak value at 36 h,
which confirmed that the production of autocrine IL-10 was markedly
increased with the increase in the duration of incubation in
culture. The differences were statistically significant between 36
h and the other time points (Fig.
1E).
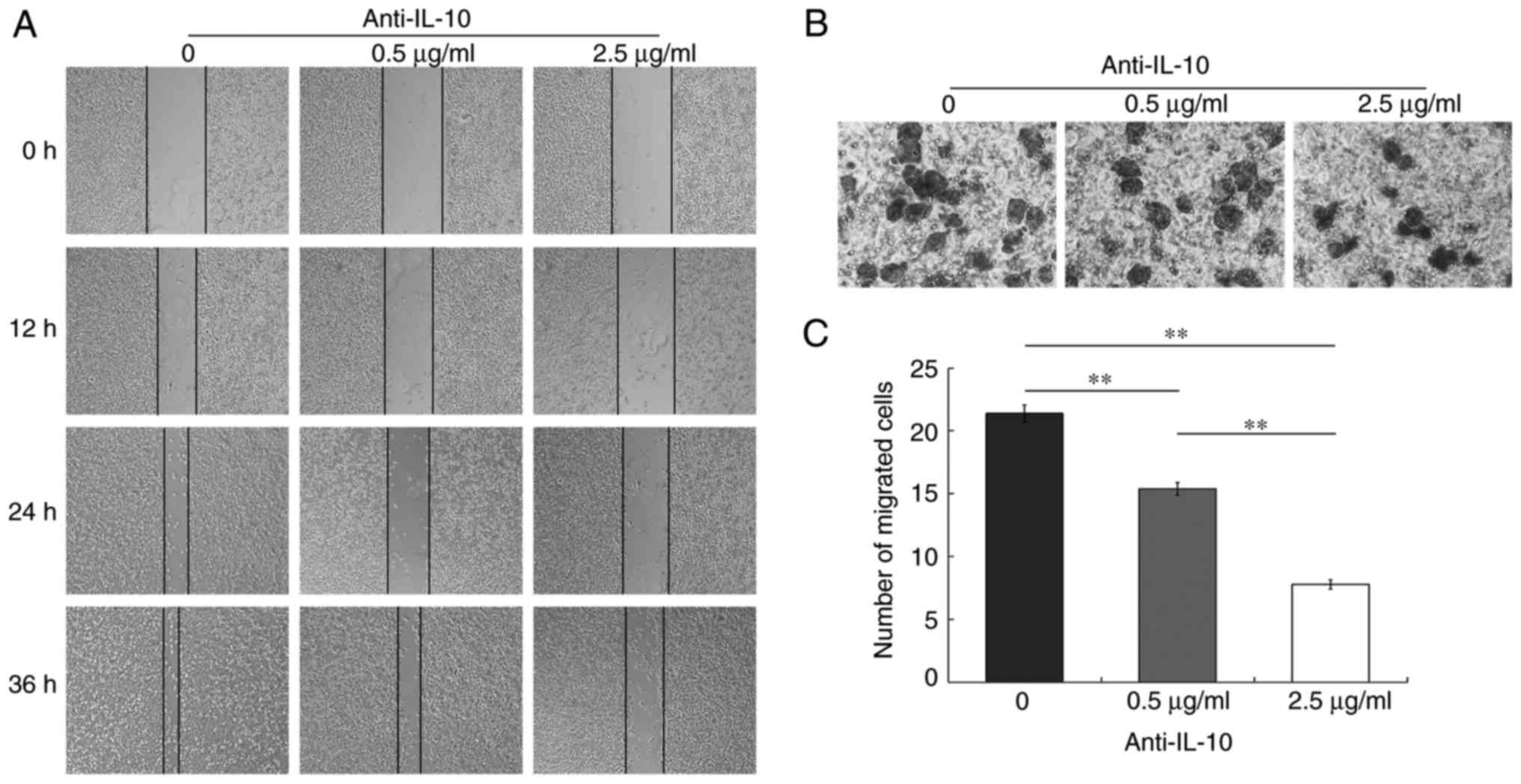
Cell migration is markedly inhibited
in the presence of anti-IL-10
The wound healing experiments indicated that there
were large blank areas at 12 h in all three groups. By 24 h, the
blank areas became narrower, and the blank area was the narrowest
in the 0 µg/ml control group. At 36 h, the wound nearly healed
fully in the control group and was partially recovered at 0.5 µg/ml
anti-IL-10 antibody; however, a large gap remained in the group
treated with 2.5 µg/ml anti-IL-10 antibody (Fig. 2A). The Transwell analysis confirmed
that the mean numbers of transmembrane cells per visual field were
21.4, 15.4 and 7.8 at 0, 0.5 and 2.5 µg/ml, respectively. Invasion
assay by Transwell demonstrated that the cells that invaded through
the matrix on Transwell membranes were irregular in shape. The
treatment with anti-IL-10 antibody led to the inhibition of cell
migration in a 3-D format (Fig.
2B). The histograms indicated that the number of transmembrane
cells was decreased in the two IL-10 antibody-treated groups
compared with the control. The differences were significant in the
group that was exposed to 2.5 µg/ml anti-IL-10 antibody compared
with other two other groups (P<0.01; Fig. 2C).
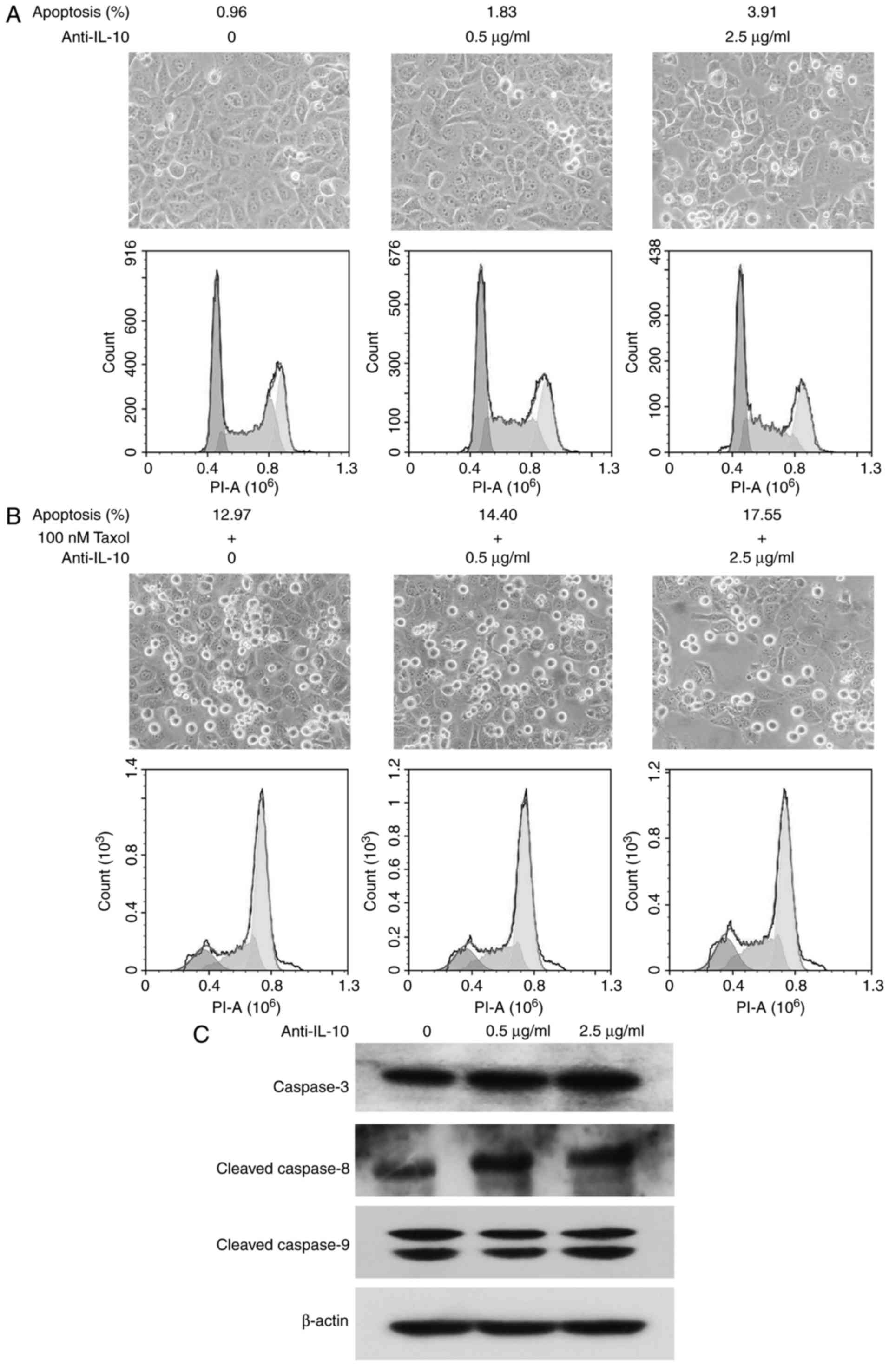
Anti-IL-10 antibody promotes cell
apoptosis and sensitivity to Taxol
Cell micrographs demonstrated that cells were
arranged closely in the three groups, but a small number of
floating cells appeared in the treated groups with the addition of
anti-IL-10 antibody (indicating that anti-IL-10 antibody promoted
cell apoptosis to some extent). Flow cytometric analysis
demonstrated that cellular apoptotic rates were 0.96, 1.83 and
3.91% in the 0, 0.5 and 2.5 µg/ml treatment groups, respectively,
which was slightly upregulated with the increase of anti-IL-10
antibody (Fig. 3A).
A large number of cells became detached in the three
groups treated with Taxol, cells became sparser with anti-IL-10
treatment, and the number of floating cells was highest in the
group that received 2.5 µg/ml anti-IL-10 and Taxol. Flow cytometric
assays demonstrated that cellular apoptotic ratios were 12.97,
14.40 and 17.55% at 0, 0.5 and 2.5 µg/ml anti-IL-10 antibody
treatment, respectively, combined with 100 nM Taxol, which implied
that neutralizing autocrine IL-10 promoted the sensitivity of
NCI-H1299 cells to Taxol (Fig.
3B).
Western blot analysis demonstrated that anti-IL-10
induced the expression of caspase-3, and cleavage of caspase-8 in
NCI-H1299 cells in a dose-dependent manner. However, there was no
effect on caspase-9 expression or cleavage (Fig. 3C).
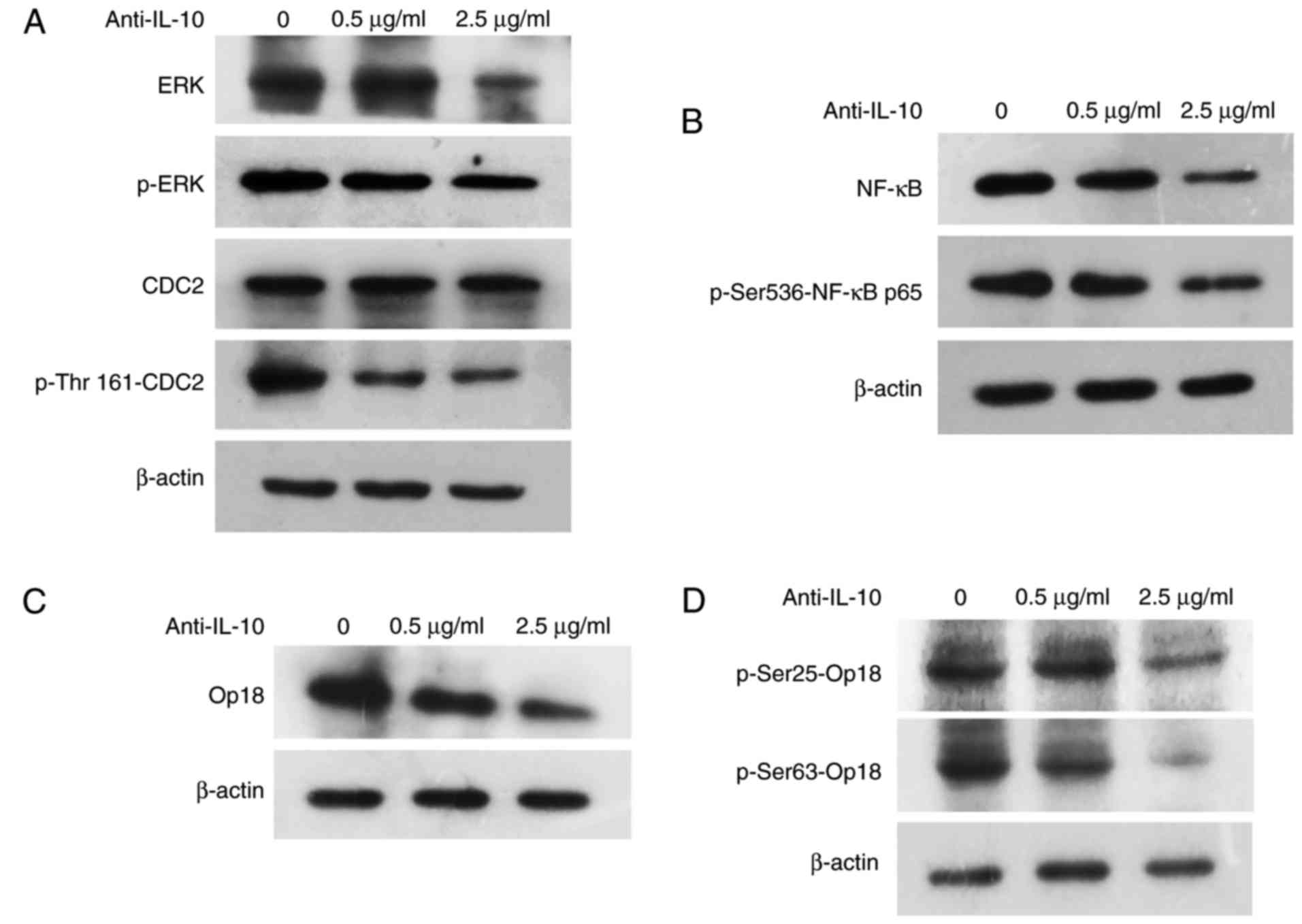
Neutralization of autocrine IL-10
decreases the activities of ERK, CDC2 and NF-κB, and reduces the
expression and phosphorylation of Op18/stathmin
Western blot analysis demonstrated that the addition
of anti-IL-10 decreased the expression and the phosphorylation of
ERK, and the phosphorylation of CDC2 at Thr161. However, anti-IL-10
did not affect CDC2 expression, which indicated that the activities
of ERK and CDC2, which are kinases upstream of Op18/stathmin, were
weakened by neutralization of autocrine IL-10 in vitro
(Fig. 4A). Similarly, anti-IL-10
antibody reduced the expression of NF-κB and its active subunit
p65, which is phosphorylated at Ser536, in a dose-dependent manner
(Fig. 4B). Further western blot
detection identified that the expression of Op18/stathmin and its
phosphorylated forms (Ser25 and Ser63) were gradually reduced with
the increase of anti-IL-10 antibody concentration (Fig. 4C and D).
Blocking NF-κB signaling promotes cell
apoptosis extent and decreases the activities of ERK and CDC2
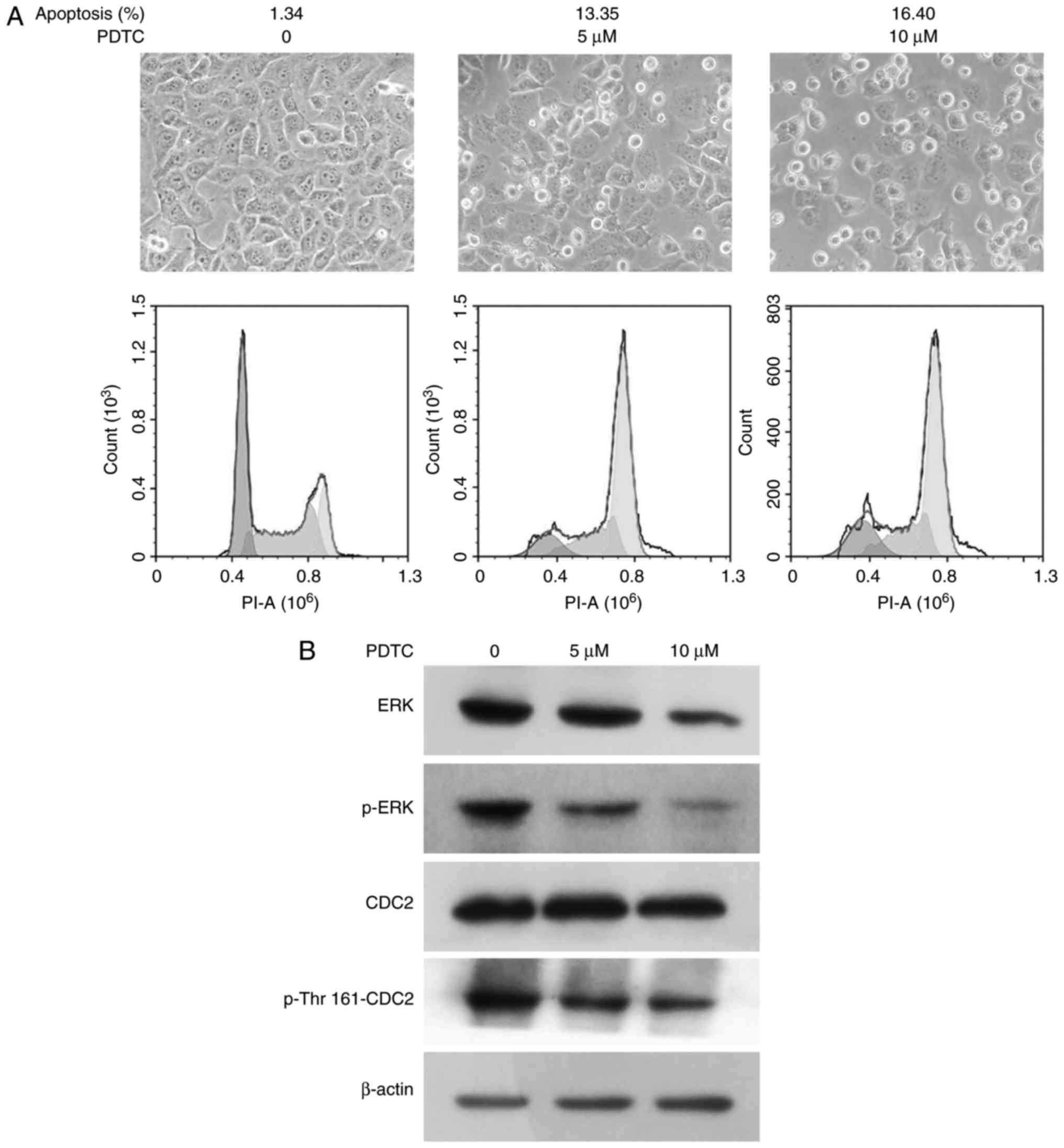
To examine the relevance of the transcription
factor, NF-κB and kinases (ERK and CDC2), PDTC, an inhibitor of
NF-κB was applied to block NF-κB signaling. The cells grew sparsely
in the presence of PDTC, with 10 µM PDTC as the highest
concentration. Flow cytometric analysis showed that the apoptotic
ratios were 1.34, 13.35 and 16.4% at 0, 5 and 10 µM PDTC,
respectively (Fig. 5A).
Western blot analysis demonstrated that PDTC also
decreased the expression and phosphorylation of ERK, and the
phosphorylation of CDC2 at Thr161 in a concentration-dependent
manner. However, PDTC did not exert any effects on CDC2 expression
(Fig. 5B).
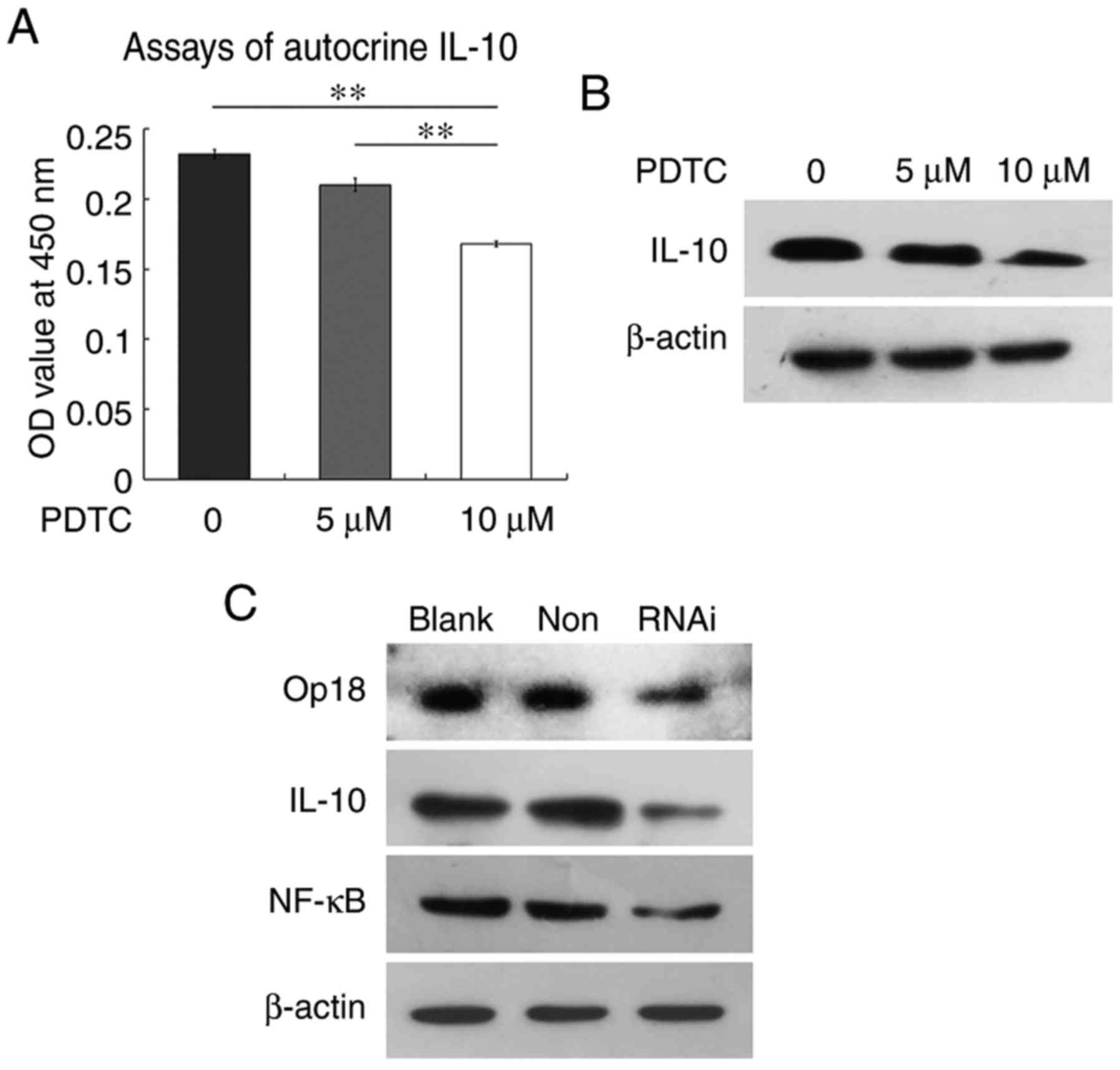
Blocking NF-κB or silencing
Op18/stathmin reduces the levels of autocrine IL-10 and protein
expression
ELISA assays for autocrine IL-10 demonstrated that
the OD values of supernatants were 0.232, 0.21 and 0.168 at 0, 5
and 10 µM PDTC treatment, respectively. The levels of autocrine
IL-10 were significantly downregulated at 10 µM PDTC compare with
the 0 and 5 µM treatment groups (P<0.01; Fig. 6A). Western blot analysis indicated
that PDTC also inhibited IL-10 protein expression in a
concentration-dependent manner (Fig.
6B). Similarly, silencing Op18/stathmin by RNAi effectively
reduced the expression of NF-κB and IL-10 (Fig. 6C). These findings suggested that
blocking NF-κB or silencing Op18/stathmin inhibited the levels of
IL-10 protein expression and autocrine release.
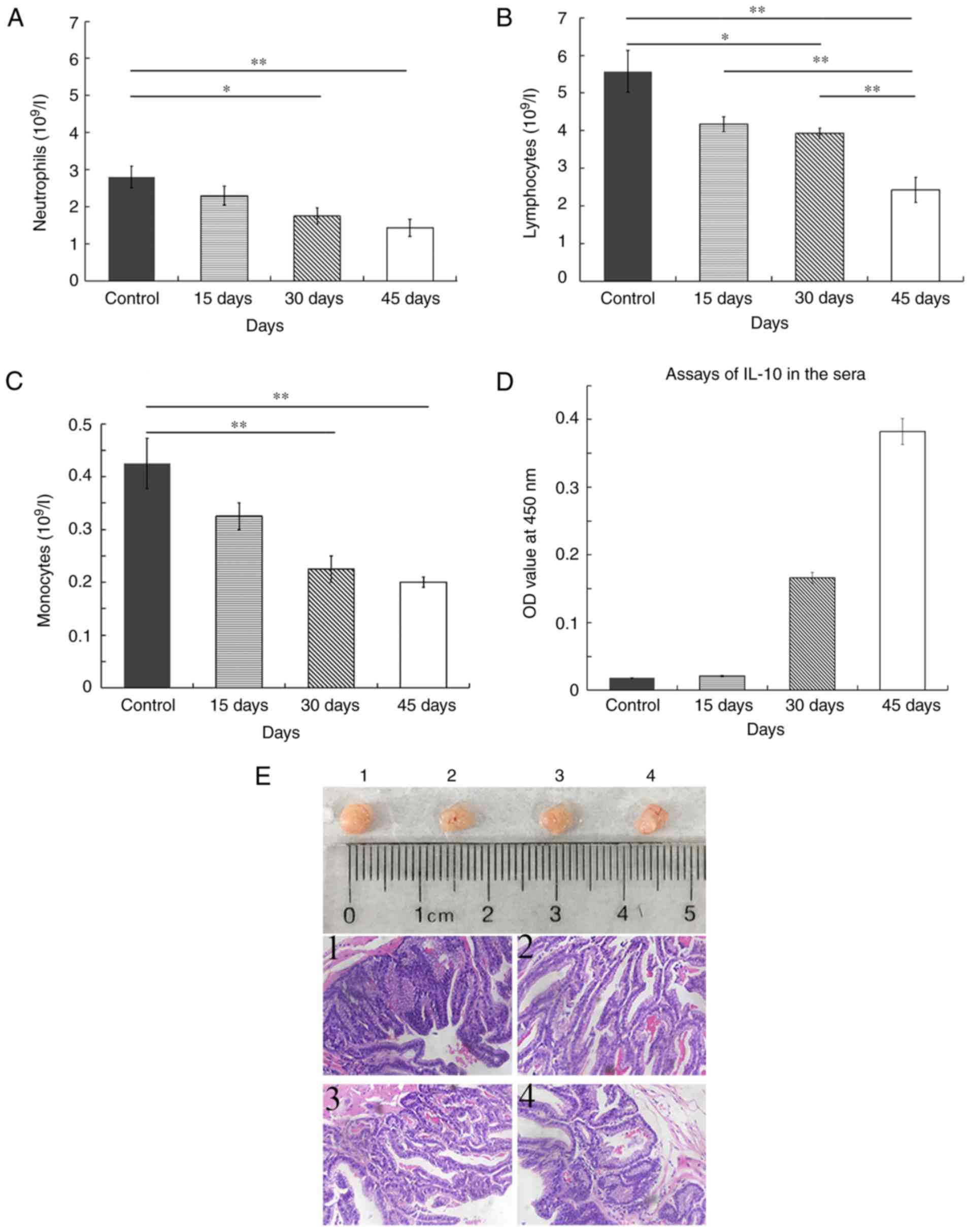
Release of autocrine IL-10 is involved
in the reduction in neutrophils, lymphocytes and monocytes in the
blood of xenografted mice
To examine whether autocrine IL-10 mediates tumor
immune evasion, we collected the blood of mice with xenograft of
NCI-H1299 cells on day 15, 30 and 45 for the detection of immune
cells by flow cytometry. The average number of neutrophils was
2.800×109/l in the control mice. The number of
neutrophils in mice that were inoculated was 2.300×109,
1.750×109 and 1.432×109 on day 15, 30 and 45,
respectively. The number of neutrophils was significantly reduced
on day 30 and 45 in tumor bearing mice compared with control mice
(P<0.01; Fig. 7A). The number of
lymphocytes in the four groups of mice was 5.575×109,
4.175×109, 3.925×109 and
2.425×109. The number of lymphocytes was significantly
decreased on day 45 compared with the other time points and the
control (P<0.01; Fig. 7B). The
mean number of monocytes was 0.425×109,
0.325×109, 0.225×109 and 0.200×109
in the control, 15, 30 and 40 days groups. A significant difference
in the number of monocytes was observed on day 30 and 45 compared
with the control (P<0.01; Fig.
7C). Therefore, the number of neutrophils, lymphocytes and
monocytes was all decreased in the blood of mice with xenografts.
The levels of IL-10 were low in the sera on day 15 and similar to
that of the control, which was gradually increased on day 30 and
reached to the peak on day 45 (Fig.
7D).
The images of tumors indicated that the dissected
tumors were identical in size in four random samples of mice with
NCI-H1299 ×enograft on day 45. Further histopathological
examination identified that tumor tissues presented typical lung
glandular cavity-like structure, and the cells were heterogeneous
in staining of the nuclei, which indicated that these tumors were
derived from the lung adenocarcinoma epithelial NCI-H1299 cells
that were intraperitoneally injected into the mice (Fig. 7E).
Discussion
It is well established that IL-10 is predominantly
secreted by the T-helper 2 cell subpopulation, and it has a crucial
role in humoral immune and inflammatory responses by stimulating
the activation and proliferation of B lymphocytes and antibody
production (13,14). Increasing evidence indicates that
tumor cells may also secrete IL-10 that is involved in
carcinogenesis and tumor immune evasion. Autocrine IL-10
predominantly functions to promote cell proliferation and
metastasis, and tolerance to chemotherapeutics. IL-10 also exerts
multiple immunosuppressive effects on host immune response
(15,16).
In the present study, the levels of autocrine IL-10
were significantly increased with increased incubation duration of
NCI-H1299 cells. In vitro neutralization of autocrine IL-10
resulted in the inhibition of cell proliferation, colony formation
and cell migration. Anti-IL-10 also increased the expression of
caspase-3 and cleavage of caspase-8, increased cell apoptosis and
the sensitivity of NCI-H1299 cells to Taxol. This indicated that
autocrine IL-10 is involved in tumor cell growth, cell metastasis
and invasion as well as the development of resistance to Taxol. A
previous study demonstrated that blocking autocrine IL-10 signaling
led to the activation of exogenous cell death pathways via
caspase-3 and −8 (17).
Other studies confirmed that the level of autocrine
IL-10 secreted by tumor cells is involved associated with patient
prognosis and curative effects of chemotherapeutics on patient
prognosis. By contrast to with normal and benign tumor cells, the
levels of IL-10 mRNA and protein expression are significantly
higher in laryngeal cancer or melanoma cells (18,19).
It was also previously demonstrated that overall survival was
lowest in NSCLC patients with high serum levels of IL-10 in the
compared with other cytokines (IL-1, IL-6, IL-8 and interferon-γ)
(20). Patients with diffuse large
B-cell lymphoma with elevated serum levels of IL-10 were likely to
have tolerance to chemotherapy and poor clinical outcome.
Inhibiting autocrine IL-10 using the non-toxic tellurium compound
ammonium trichloro(dioxoethylene-O,O')tellurate enhanced the
sensitivity of B and T-lymphoma cells to Taxol (21–23).
Similarly, the level of autocrine IL-10 was positively associated
with the malignancy of cervical cancer that is caused by human
papillomavirus (24).
Our previous studies indicated that Op18/stathmin
was a target of the kinases ERK and CDC2 in CNE1 cells. The
Epstein-Barr virus-encoded the latent membrane protein 1 was
demonstrated to regulate Op18/stathmin signaling by ERK and CDC2,
which was associated with acceleration of cell cycle progression
and cell proliferation. The levels of phospho-ERK and phospho-CDC2
Thr161 reflected the activities of ERK and CDC2 kinases (25,26).
Blocking CDC2 signaling with Purvalanol A or ERK with PD98059 in
NCI-H1299 cells decreased the phosphorylation of Op18/stathmin and
the increase of sensitivity to Taxol in NCI-H1299 cells (27,28).
This study identified that in vitro IL-10 neutralization
negatively regulated the activities of ERK and CDC2 by reducing the
phosphorylation of ERK and CDC2-Thr161, and decreased expression of
Op18/stathmin and its phosphorylation at Ser25 and Ser63, however
anti-IL-10 also induced downregulation of ERK expression. Whether
the reduced ERK phosphorylation was caused by decreased total
expression requires further investigation. In short, anti-IL-10
also increased the sensitivity of NCI-H1299 cells to Taxol via
regulation of ERK and CDC2 signaling.
Transcription factor NF-κB is closely associated
with cell proliferation, of which NF-κB p65 is the main active
subunit. The phosphorylation of p65 Ser536 is well established as a
marker of NF-κB activation, which has an important role in
tumorigenesis (29,30). In SW-1990 pancreatic cancer cells,
silencing Op18/stathmin downregulated NF-κB p65 and NF-κB
expression, and induced the arrest of cell proliferation and
metastasis (31). In cervical
cancer, serine protease inhibitor, kallikrein, markedly impeded
NF-κB activation by suppressing NF-κB inhibitor α degradation and
the phosphorylation of p65, which led to the inhibition of cell
growth (32). Our previous studies
indicated that silencing Op18/stathmin by targeting RNAi-inhibited
IL-10 autocrine in CNE1 and NCI-H1299 cells enhanced the
sensitivity of the cells to Taxol (9,12).
This study demonstrated that in vitro neutralizing autocrine
IL-10 attenuated NF-κB expression and activity. Blocking NF-κB
signaling using the inhibitor, PDTC, led to a decrease in the
activities of ERK and CDC2, and a decrease in the levels of
autocrine IL-10 and protein expression. Op18/stathmin RNAi also
decreased the expression of NF-κB and IL-10 in NCI-H1299 cells,
which suggested the presence of an autocrine
IL-10/NF-κB/ERK/CDC2/Op18/stathmin axis and a positive feedback
loop between Op18/stathmin and autocrine IL-10. The regulation of
Op18/stathmin signaling mediated by autocrine IL-10 may be
important for the maintenance of malignant tumor behaviors in
NCI-H1299 cells.
Autocrine IL-10 is usually regarded as an immune
inhibitory cytokine, which induces tumor immune evasion by reducing
the activation of antigen-presenting cells (33). In MC38 cell mouse colon cancer
models, the killing effects of cytotoxic T cells were markedly
reduced when the serum level of autocrine IL-10 was increased
(34). In chronic gastric mucosal
infections caused by Helicobacter pylori, the maturation of
dendritic cells (DCs) and antigen delivery was inhibited by
inducing the autocrine secretion of IL-10 from DCs, ultimately
leading to evasion of the host immune surveillance by
Helicobacter pylori and the development of gastric cancer
(35). In breast tumors, the high
level of IL-10 secreted by tumor-associated macrophages was
demonstrated to significantly prevent paclitaxel-induced apoptosis
of breast tumor cells. Neutralizing IL-10 using anti-IL-10 antibody
resulted in the attenuation of signal transducer and activator of
transcription 3 activation and decreased Bcl-2 mRNA expression.
This consequently enhanced the sensitivity of breast cancer cells
(36). The animal experiments in
the current study indicated that the number of neutrophils,
lymphocytes and monocytes were significantly decreased in the blood
of mice with NCI-H1299 cell xenografts at 45 days after
inoculation. The release of IL-10 was increased with the occurrence
and growth of xenografted tumors. This finding suggested that
autocrine IL-10 reduced the generation of neutrophils, lymphocytes
and monocytes, and thus affected neutrophil-mediated inflammation,
antigen processing, presentation of monocytes and the immune
effects of B- or T-lymphocytes, which ultimately resulted in tumor
immune evasion. The associated molecular mechanism required further
investigation.
Acknowledgements
We thank Professor Feng Deyun from the First
Affiliated Xiangya Hospital of Central South University (Changsha,
China) for histopathological examination.
Funding
This study was financed by the Key Scientific
Research Project of Colleges and Universities of Hunan Province
(grant no. 12A018), the National Natural Science Foundation of
China (grant no. 81272274) and the Natural Science Foundation of
Hunan Province (grant no 12JJ3104).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL was mainly responsible for the design of the
study and revision of the manuscript. The experiments, analysis and
interpretation of the data were mainly carried out by YZ. SC, FS,
DL, TY, MW, as members of the research team, participated in part
of the experiments, analysis and interpretation of the data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were reviewed and approved by
the Ethics Committee of Center for Scientific Research with Animal
Models of Changsha Medical University, Hunan, China (no.
2017-05-1). All applicable international, national and
institutional guidelines for the care and use of animals were
followed.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lin X, Liao Y, Xie J, Liu S, Su L and Zou
H: Op18/stathmin is involved in the resistance of taxol among
different epithelial carcinoma cell lines. Cancer Biother
Radiopharm. 29:376–386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yip YY, Yeap YY, Bogoyevitch MA and Ng DC:
Differences in c-Jun N-terminal kinase recognition and
phosphorylation of closely related stathmin-family members. Biochem
Biophys Res Commun. 446:248–254. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Machado-Neto JA, Lazarini M, Favaro P, de
Melo Campos P, Scopim-Ribeiro R, Franchi Junior GC, Nowill AE, Lima
PR, Costa FF, Benichou S, et al: ANKHD1 silencing inhibits Stathmin
1 activity, cell proliferation and migration of leukemia cells.
Biochim Biophys Acta. 1853:583–593. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Machado-Neto JA, de Melo Campos P, Favaro
P, Favaro P, Lazarini M, Lorand-Metze I, Costa FF, Olalla Saad ST
and Traina F: Stathmin 1 is involved in the highly proliferative
phenotype of high-risk myelodysplastic syndromes and acute leukemia
cells. Leuk Res. 38:251–257. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nemunaitis J: Stathmin 1: A protein with
many tasks. New biomarker and potential target in cancer. Expert
Opin Ther Targets. 16:631–634. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnsen JI, Aurelio ON, Kwaja Z, Jörgensen
GE, Pellegata NS, Plattner R, Stanbridge EJ and Cajot JF:
p53-mediated negative regulation of stathmin/Op18 expression is
associated with G2/M cell-cycle arrest. Int J Cancer.
88:685–691. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ke B, Wu LL, Liu N, Zhang RP, Wang CL and
Liang H: Overexpression of stathmin 1 is associated with poor
prognosis of patients with gastric cancer. Tumour Biol.
34:3137–3145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Watanabe A, Suzuki H, Yokobori T,
Tsukagoshi M, Altan B, Kubo N, Suzuki S, Araki K, Wada S,
Kashiwabara K, et al: Stathmin 1 regulates p27 expression,
proliferation and drug resistance, resulting in poor clinical
prognosis in cholangiocarcinoma. Cancer Sci. 105:690–696. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Long D, Yu T, Chen X, Liao Y and Lin X:
RNAi targeting STMN alleviates the resistance to taxol and
collectively contributes to down regulate the malignancy of NSCLC
cells in vitro and in vivo. Cell Biol Toxicol. 34:7–21. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee EH, Kim SS and Seo SR: Pyrrolidine
dithiocarbamate (PDTC) inhibits inflammatory signaling via
expression of regulator of calcineurin activity 1 (RCAN1):
Anti-inflammatory mechanism of PDTC through RCAN1 induction.
Biochem Pharmacol. 143:107–117. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ko EY, Cho SH, Kwon SH, Eom CY, Jeong MS,
Lee W, Kim SY, Heo SJ, Ahn G, Lee KP, et al: The roles of NF-kappaB
and ROS in regulation of pro-inflammatory mediators of inflammation
induction in LPS-stimulated zebrafish embryos. Fish Shellfish
Immunol. 68:525–529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin X, Yu T, Zhang L, Chen S, Chen X, Liao
Y, Long D and Shen F: Silencing Op18/stathmin by RNA interference
promotes the sensitivity of nasopharyngeal carcinoma cells to taxol
and high-grade differentiation of xenografted tumours in nude mice.
Basic Clin Pharmacol Toxicol. 119:611–620. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheng SB and Sharma S: Interleukin-10: A
pleiotropic regulator in pregnancy. Am J Reprod immunol.
73:487–500. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heine G, Drozdenko G, Grun JR, Chang HD,
Radbruch A and Worm M: Autocrine IL-10 promotes human B-cell
differentiation into IgM- or IgG-secreting plasmablasts. Eur J
Immunol. 44:1615–1621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ledeboer A, Breve JJ, Wierinckx A, van der
Jagt S, Bristow AF, Leysen JE, Tilders FJ and Van Dam AM:
Expression and regulation of interleukin-10 and interleukin-10
receptor in rat astroglial and microglial cells. Eur J Neurosci.
16:1175–1185. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rodriguez JA, Galeano L, Palacios DM,
Gómez C, Serrano ML, Bravo MM and Combita AL: Altered HLA class I
and HLA-G expression is associated with IL-10 expression in
patients with cervical cancer. Pathobiology. 79:72–83. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sakamaki K, Imai K, Tomii K and Miller DJ:
Evolutionary analyses of caspase-8 and its paralogs: Deep origins
of the apoptotic signaling pathways. Bioessays. 37:767–776. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Itakura E, Huang RR, Wen DR, Paul E,
Wunsch PH and Cochran AJ: IL-10 expression by primary tumor cells
correlates with melanoma progression from radial to vertical growth
phase and development of metastatic competence. Mod Pathol.
24:801–809. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mahipal A, Terai M, Berd D, Chervoneva I,
Patel K, Mastrangelo MJ and Sato T: Tumor-derived interleukin-10 as
a prognostic factor in stage III patients undergoing adjuvant
treatment with an autologous melanoma cell vaccine. Cancer Immunol
Immunother. 60:1039–1045. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barrera L, Montesservín E, Barrera A,
Ramírez-Tirado LA, Salinas-Parra F, Bañales-Méndez JL,
Sandoval-Ríos M and Arrieta Ó: Cytokine profile determined by
data-mining analysis set into clusters of non-small-cell lung
cancer patients according to prognosis. Ann Oncol. 26:428–435.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gupta M, Han JJ, Stenson M, Maurer M,
Wellik L, Hu G, Ziesmer S, Dogan A and Witzig TE: Elevated serum
IL-10 levels in diffuse large B-cell lymphoma: A mechanism of
aberrant JAK2 activation. Blood. 119:2844–2853. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lech-Maranda E, Baseggio L, Bienvenu J,
Charlot C, Berger F, Rigal D, Warzocha K, Coiffier B and Salles G:
Interleukin-10 gene promoter polymorphisms influence the clinical
outcome of diffuse large B-cell lymphoma. Blood. 103:3529–3534.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Danoch H, Kalechman Y, Albeck M, Longo DL
and Sredni B: Sensitizing B- and T-cell lymphoma cells to
paclitaxel/abraxane-induced death by AS101 via inhibition of the
VLA-4-IL10-survivin axis. Mol Cancer Res. 13:411–422. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bermudez-Morales VH, Peralta-Zaragoza O,
Alcocer-Gonzalez JM, Moreno J and Madrid-Marina V: IL-10 expression
is regulated by HPV E2 protein in cervical cancer cells. Mol Med
Rep. 4:369–375. 2011.PubMed/NCBI
|
|
25
|
Lin X, Tang M, Tao Y, Li L, Liu S, Guo L,
Li Z, Ma X, Xu J and Cao Y: Epstein-Barr virus-encoded LMP1
triggers regulation of the ERK-mediated Op18/stathmin signaling
pathway in association with cell cycle. Cancer Sci. 103:993–999.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin X, Liu S, Luo X, Ma X, Guo L, Li L, Li
Z, Tao Y and Cao Y: EBV-encoded LMP1 regulates Op18/stathmin
signaling pathway by cdc2 mediation in nasopharyngeal carcinoma
cells. Int J Cancer. 124:1020–1027. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin X, Liao Y, Chen X, Long D, Yu T and
Shen F: Regulation of oncoprotein 18/stathmin signaling by ERK
concerns the resistance to taxol in nonsmall cell lung cancer
cells. Cancer Biother Radiopharm. 31:37–43. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen X, Liao Y, Long D, Yu T, Shen F and
Lin X: The Cdc2/Cdk1 inhibitor, purvalanol A, enhances the
cytotoxic effects of taxol through Op18/stathmin in non-small cell
lung cancer cells in vitro. Int J Mol Med. 40:235–242. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hoesel B and Schmid JA: The complexity of
NF-kappaB signaling in inflammation and cancer. Mol Cancer.
12:862013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen JY, He XX, Ma C, Wu XM, Wan XL, Xing
ZK, Pei QQ, Dong XP, Liu DX, Xiong WC, et al: Netrin-1 promotes
glioma growth by activating NF-kappaB via UNC5A. Sci Rep.
7:54542017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu Y, Liu C, Cheng H, Xu Y, Jiang J, Xu J,
Long J, Liu L and Yu X: Stathmin, interacting with Nf-kappaB,
promotes tumor growth and predicts poor prognosis of pancreatic
cancer. Curr Mol Med. 14:328–339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang T, Shi F, Wang J, Liu Z and Su J:
Kallistatin Suppresses cell proliferation and invasion and promotes
apoptosis in cervical cancer through blocking NF-kappaB signaling.
Oncol Res. 25:809–817. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mittal SK and Roche PA: Suppression of
antigen presentation by IL-10. Curr Opin Immunol. 34:22–27. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tanikawa T, Wilke CM, Kryczek I, Chen GY,
Kao J, Núñez G and Zou W: Interleukin-10 ablation promotes tumor
development, growth, and metastasis. Cancer Res. 72:420–429. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rizzuti D, Ang M, Sokollik C, Wu T,
Abdullah M, Greenfield L, Fattouh R, Reardon C, Tang M, Diao J, et
al: Helicobacter pylori inhibits dendritic cell maturation via
interleukin-10-mediated activation of the signal transducer and
activator of transcription 3 pathway. J Innate Immun. 7:199–211.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang C, He L, He P, Liu Y, Wang W, He Y,
Du Y and Gao F: Increased drug resistance in breast cancer by
tumor-associated macrophages through IL-10/STAT3/bcl-2 signaling
pathway. Med Oncol. 32:3522015. View Article : Google Scholar : PubMed/NCBI
|