Introduction
In recent years cancer has become one of the most
serious threats to human health and life. Current cancer therapies,
such as radiotherapy and chemotherapy, are less effective and cause
various side effects, therefore, novel strategies for cancer
therapy are urgently needed. In the search for novel cancer
therapies, oncolytic virotherapy has recently appeared as an
appealing approach due to its ability to replicate in tumor cells
with consequent spread to other cells (1–5),
leading to significant oncolytic efficacy. In addition, oncolytic
virotherapy can specifically kill through additional mechanisms
such as arming therapeutic genes and causing tumor-specific
cytotoxic T lymphocytes (CTL). Therefore, oncolytic virotherapy
appears to be a promising approach to treat cancers that are
refractory to current treatments.
At present, various viruses are used as
replication-selective oncolytic viruses in the treatment of cancer,
such as the adenovirus, herpes virus, Newcastle disease virus, and
vaccinia virus (6–9). Among them, the vaccinia virus exhibits
notable benefits such as intravenous stability, efficient delivery,
large transgene-encoding capacity, verified ability to induce
efficient immune responses, and a safe, live vaccine administered
in humans. So far, a number of wild-type vaccinia strains have been
used as backbones in the design of oncolytic agents such as Wyeth
(10–16), Copenhagen (17) and Lister (18).
The vaccinia virus Tian Tan strain (VTT), the most
widely used vaccine in China, played a critical role during the
Chinese smallpox eradication campaign (19–21).
The biological characteristics of VTT have already been studied
systematically (22,23). Briefly, VTT has a wide host cell
range, and is less virulent than vaccinia virus Western Reserve
strain (WR) but still remains neurovirulent. Some attenuated
strains of VTT with lower toxicity were obtained by genetic
modification (24–26). Of these, vaccinia virus strain
Guang9 (VG9) displayed better attenuated properties as compared to
its parental strain by using a traditional single plaque
purification method (27–29). The neurovirulence and pathogenicity
of VG9 were also notably lower (30), while the immunogenicity of VG9 was
no less than that of VTT (31).
Thus far, the biological characteristics of VG9 have been well
studied and it is supposed to become an essential building block in
the construction of a recombinant vaccinia virus vector. However,
very few studies have evaluated the oncolytic efficacy of VG9, and
no clinical application has been performed. In this study, we
assessed the replication and cytotoxicity of VG9 in vitro,
and evaluated the antitumor effects in a murine melanoma tumor
model. Our findings will serve as a promising platform for further
cancer therapy.
Materials and methods
Cells and virus
Tumor cell lines including B16 (murine melanoma),
Hepa 1–6 (murine hepatoma), HeLa (human cervix carcinoma), SGC-7901
(human gastric carcinoma), A549 (human lung carcinoma), MDA-MB-231
(human breast carcinoma) and normal cell line L-02 cells (human
normal liver) were purchased from Shanghai Cell Collection
(Shanghai, China). Vero (African green monkey kidney epithelial),
BSC-40 (African green monkey kidney epithelial), and NIH3T3 (murine
embryo fibroblast) cell lines were purchased from the American Type
Culture Collection (ATCC; Manassas, VA, USA). All cells were
cultured under the conditions suggested by the ATCC.
The vaccinia virus of Tian Tan strain VG9 was a gift
from National Institutes for Food and Drug Control (NIFDC; Beijing,
China). The titer of VG9 was determined by a plaque-forming assay
on BSC-40 cells.
In vitro viral replication
The replication ability of VG9 was observed in
various cancer cell lines and normal cell lines at the multiplicity
of infection (MOI) of 0.1 PFU/cell. Cells pre-incubated in growth
medium containing 2% fetal bovine serum (FBS) for 2 h were then
washed and incubated in complete growth medium. Cells and
supernatant were harvested at different time points (24, 48 and 72
h), and viral titers were determined in BSC-40 cells after three
cycles of freezing and thawing.
In vitro cytotoxicity assay
Cells (104/well) seeded in 96 well plates were
infected with different MOIs of virus suspended in growth medium
containing 2% FBS. Following cell culture at different time points
(24, 48 and 72 h), 20 µl of 5 mg/ml
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
(MTT; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added to
each well. Cells were incubated at 37°C for 4 h, then the
supernatants were discarded, and 150 µl dimethylsulfoxide (DMSO)
was added to each well and mixed thoroughly. After 10 mins of
shaking, the color absorbance at 490 nm was measured by a
spectrophotometric system (SpectraMax M5e; Molecular Devices, LLC,
Sunnyvale, CA, USA).
Mice
The animal experiments were approved by the
Institutional Animal Care and Use Committees (IACUC) of Jiangsu
Institute of Nuclear Medicine (JSINM2010007). 20 female C57BL/6
immunocompetent mice (6 weeks old) were purchased from Shanghai
Laboratory Animals Center (SLAC; Shanghai, China). They were housed
under standard conditions (at 25°C, with 40–50% humidity and a
12-h/12-h light/dark cycle) and were given free access to diet and
water.
In vivo viral replication
To evaluate in vivo viral replication, mice
bearing subcutaneous B16 murine melanoma tumors were
intraperitoneally injected with VG9 (1×107 PFU). After 5
days, brain, lung, liver, spleen, kidney and tumor tissue were
harvested and homogenized. The viral yield was quantified by plaque
assay on BSC-40 cells.
Tumor models and antitumor
effects
To establish a murine melanoma tumor model,
approximately 5×105 B16 cells in 100 µl
phosphate-buffered saline (PBS) were injected subcutaneously into
the right flanks of C57BL/6 mice. PBS control), 107 PFU
of VG9 was injected intratumorally when tumors reached the size of
3–5 mm in diameter. Tumor growth was monitored every other day by
computed tomography CT) scan. The tumor volume was calculated as
the [(width)2 × length] × 0.52 (32). Mice were euthanized when tumors
reached their maximal permitted size according to the animal
regulations, and Kaplan-Meier survival curves were plotted.
Measurement of neutralizing antibody
to VG9
The titer of serum antibodies to virus was
determined by time-resolved fluoroimmunoassay (TRFIA) (33). After coating 96-well plates with VG9
(20 µg/ml) overnight, diluted serum samples were incubated with the
virus for 2 h. After the plates were washed 6 times,
Eu3+-labeled anti-mouse IgG secondary antibody (Cell
Signaling Technology, Danvers, MA, USA) incubation followed for 1
h. The fluorescent emission spectra of Eu3+ were
obtained on a PerkinElmer LS-55 fluorescence spectrometer
(PerkinElmer, Inc., Waltham, MA, USA) and time-resolved fluorescent
measurements were carried out with an AutoDELFIA-1235 automatic
analyzer (WALLAC; PerkinElmer, Inc.).
Cytotoxic T-lymphocyte study
After PBS or VG9-treatment for 13 days, spleens
harvested from the PBS or virus-treated or from normal control mice
were homogenized, filtered through a 40-µm nylon strainer (BD
Falcon; Becton Dickinson and Company, Franklin Lakes, NJ, USA) and
cultured for 24 h. B16 or Hepa 1–6 cells (1×104
cells/well) were cultured on 96-well plates and splenocytes were
added at ratios of 10:1. Cell viability was measured by MTT assay
after 48 h.
Thyroid samples
Three surgically removed thyroid samples from 3
patients (1 male, 2 females, median age 52 years) were collected at
the Department of Pathology of Jiangyuan Hospital Affiliated to
Jiangsu Institute of Nuclear Medicine (Wuxi, China) in December of
2016. All patients provided informed consent before enrollment in
the study, which was approved by the Ethics Committee of the
Jiangyuan Hospital Affiliated to Jiangsu Institute of Nuclear
Medicine.
Statistical analysis
Values are indicated as the mean ± standard
deviation (SD). Statistical analysis was calculated using the
Mann-Whitney test for non-parametric data or Student's t-test for 2
independent samples when appropriate. Survival was calculated by
Kaplan-Meier method, and differences between curves were assessed
by log-rank test. All statistics were generated by SPSS 19.0
software (IBM Corp., Armonk, NY, USA).
Results
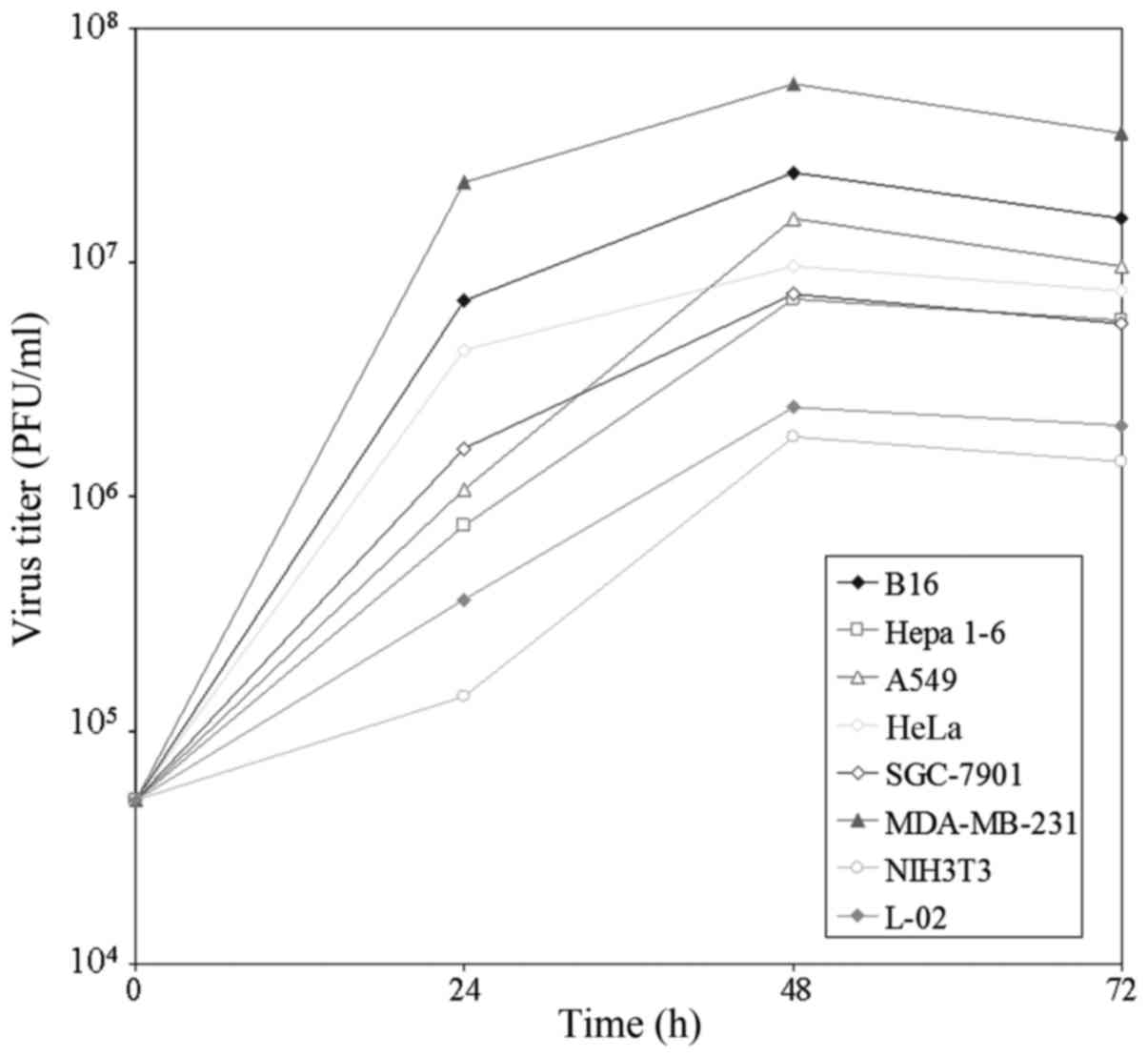
Replication of VG9 in vitro
The ability of VG9 to replicate and spread was
determined in various cancer cell lines and two normal cell lines.
The yield of infectious virus in cells at indicated time-points was
quantified by plaque assays in BSC-40 cells. As shown in Fig. 1, VG9 rapidly increased in all cell
types, reaching a maximum within 48 h. The value either remained
the same or changed slightly by 72 h. Maximum virus production
occurred in MDA-MB-231 cells, followed by B16 cells. VG9 titer in
the two normal cells was lower as compared to that of the cancer
cells. The results suggested natural tumor tropism of the vaccinia
virus.
Cytotoxic effect in vitro
The oncolytic potency of VG9 was evaluated in
various cell lines. Cells were cultured in 96-well plates and then
infected with increasing doses of viruses. After 3 days infection,
cell viability was assessed (Table
I). The sensitivity to virus-induced cell killing varied
between the cell lines. At an MOI of 1, >50% of all cancer cells
were killed. A viral MOI of 10 resulted in survival of <20% in
B16 cells or MDA-MB-231 cells; while ~20–40% in other cell lines.
Normal cells were poorly sensitive to virus-induced cell killing.
Even when infected with an MOI of 10, ~60–80% of normal cells
survived. The cell viability was also evaluated after infection at
different time points (Table II).
The results revealed that the cytotoxic effect of VG9 was
time-dependent.
 | Table I.Cell viability of various cell lines
infected with VG9 at different MOIsa. |
Table I.
Cell viability of various cell lines
infected with VG9 at different MOIsa.
|
| Cell viability
(%) |
|---|
|
|
|
|---|
| Cell lines | 0.01 MOI | 0.1 MOI | 1 MOI | 10 MOI |
|---|
| B16 | 77.15±2.15 | 37.50±2.47 | 26.77±1.77 | 12.30±1.18 |
| Hepa 1–6 | 59.73±1.41 | 37.93±0.08 | 33.47±1.05 | 27.14±1.21 |
| A549 | 62.55±3.73 | 53.32±1.69 | 46.91±0.93 | 40.40±1.90 |
| HeLa | 69.52±1.49 | 52.55±0.69 | 39.53±2.29 | 26.08±3.09 |
| SGC-7901 | 70.35±4.22 | 60.07±2.12 | 29.75±1.59 | 21.13±2.25 |
| MDA-MB-231 | 58.75±2.06 | 48.61±1.33 | 30.07±0.22 | 15.24±2.70 |
| NIH3T3 | 104.04±5.75 | 95.53±5.03 | 80.41±2.26 | 55.90±3.02 |
| L-02 | 96.60±1.96 | 99.50±1.17 | 95.25±2.93 | 79.85±4.04 |
 | Table II.Cell viability of various cell lines
infected with VG9 at different time-pointsa. |
Table II.
Cell viability of various cell lines
infected with VG9 at different time-pointsa.
|
| Cell viability
(%) |
|---|
|
|
|
|---|
| Cell lines | 24 h | 48 h | 72 h |
|---|
| B16 | 77.33±3.06 | 25.12±2.58 | 12.30±1.18 |
| Hepa 1–6 | 88.36±3.47 | 38.06±1.29 | 27.14±1.21 |
| A549 | 86.67±2.09 | 55.08±2.24 | 40.40±1.90 |
| HeLa | 80.36±2.11 | 40.06±1.38 | 26.08±3.09 |
| SGC-7901 | 83.02±2.58 | 38.13±3.26 | 21.13±2.25 |
| MDA-MB-231 | 72.06±3.48 | 34.61±1.33 | 15.24±2.70 |
| NIH3T3 | 102.88±3.26 | 80.41±2.66 | 55.90±3.02 |
| L-02 | 99.87±2.15 | 82.96±3.23 | 79.85±4.04 |
Replication of VG9 in vivo
The viral yield of VG9 in tumors and normal organ
tissues was evaluated 5 days after infection. Harvested viruses
were titered on BSC-40 cells and the yield was quantified per
milligram of tissue. The results presented in Table III indicated that the viral yields
of VG9 were significantly reduced in normal organs, while it was
recovered at higher amounts in tumor tissue.
 | Table III.Biodistribution of vaccinia viruses
in tumor and normal tissuesa. |
Table III.
Biodistribution of vaccinia viruses
in tumor and normal tissuesa.
| Tissue | VG9 |
|---|
| Tumor | 12.0 (7.2–16) ×
104 |
| Brain | 50 (0–160) |
| Lung | 0 (0–50) |
| Liver | 0 (0–20) |
| Spleen | 80 (16–240) |
| Kidney | 50 (30–90) |
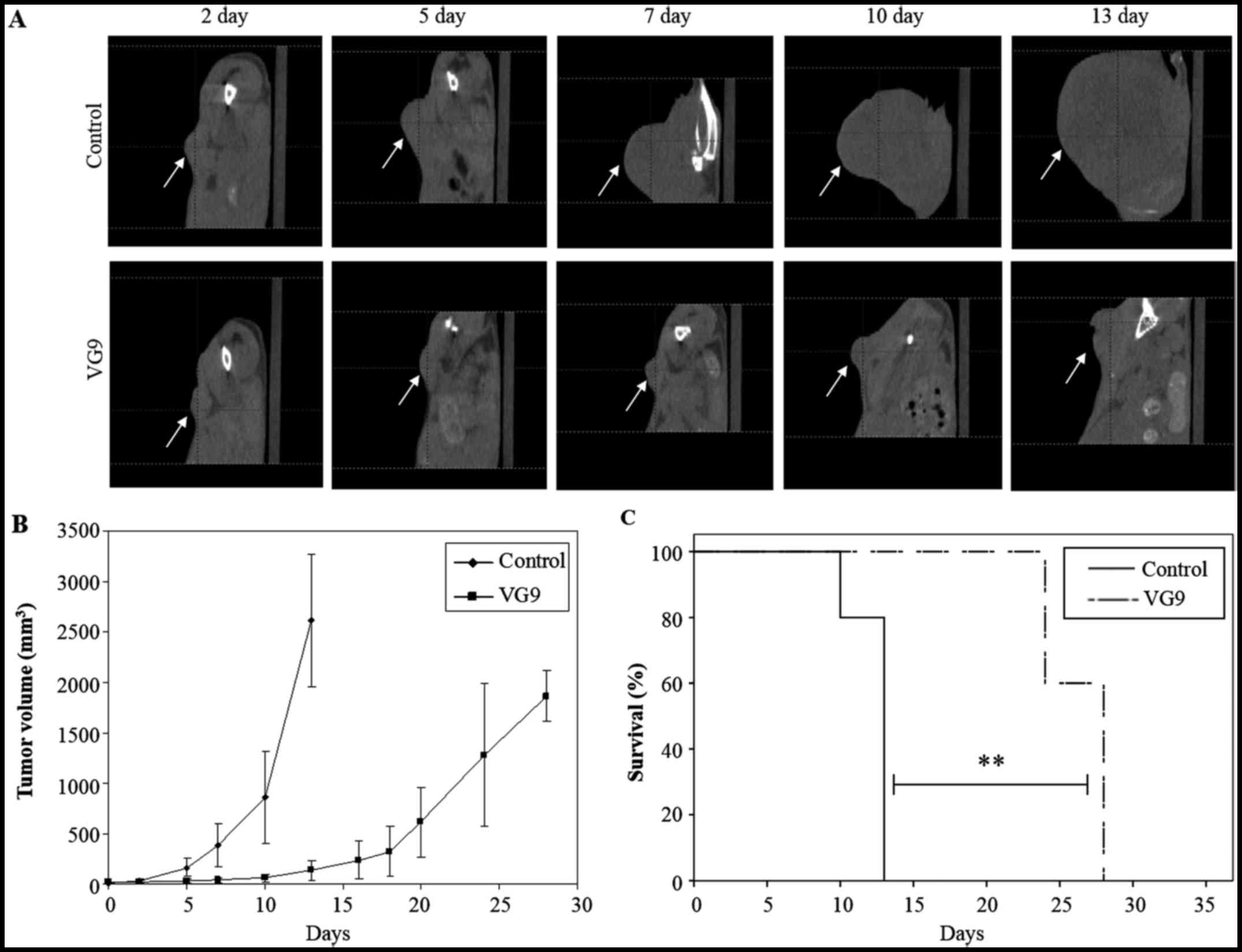
Antitumor effect of VG9 in vivo
The ability of VG9 to function as an oncolytic virus
was examined in a B16-murine melanoma tumor model. Immunocompetent
C57BL/6 mice bearing subcutaneous B16 murine melanoma tumors were
injected intratumorally with 1×107 PFU of VG9 or PBS
(control). Tumor development was monitored by CT (Fig. 2A). At 2 weeks from the initial
treatment, tumors in the control group had significantly increased
in size, while those in the VG9-treated groups had stabilized
(Fig. 2B). All control mice died
within 13 days, while VG9-treated mice lived longer with survival
extended up to 28 days (Fig.
2C).
Notably, the antitumor effect of VG9 was
attributable to the replication of the virus alone as no
therapeutic genes had been introduced into the virus. These results
strongly indicated that VG9 had a notable antitumor effect as an
oncolytic vaccinia virus.
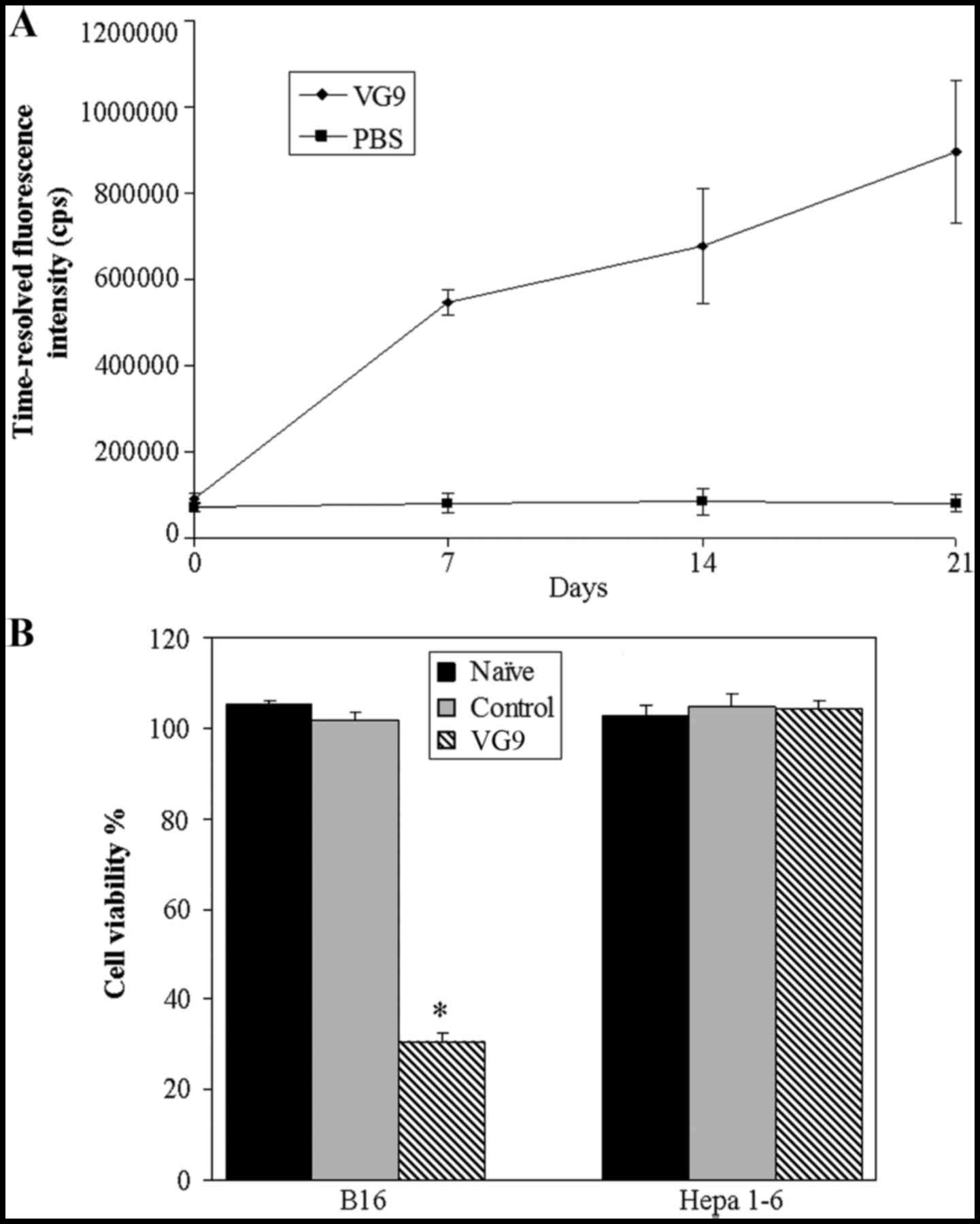
Immune response induced by VG9
To evaluate the immune response against the virus
itself, neutralizing antibody to virus was determined by
time-resolved fluoroimmunoassay (TRFIA). As shown in Fig. 3A, neutralizing antibodies to VG9
were detectable by day 7 after injection and elevated through day
21. To assess the immune response against the target tumor, we
evaluated tumor-specific CTL. Splenocytes harvested from
VG9-treated or PBS-treated mice harboring B16 tumors or normal
control mice were co-cultured with B16 or Hepa 1–6 cells. Cell
viability assays revealed that VG9 induced a notable increase in
B16-targeting CTL, while the effect was lost in Hepa 1–6 cells
(Fig. 3B), indicating that vaccinia
oncolysis induced tumor-specific immunity.
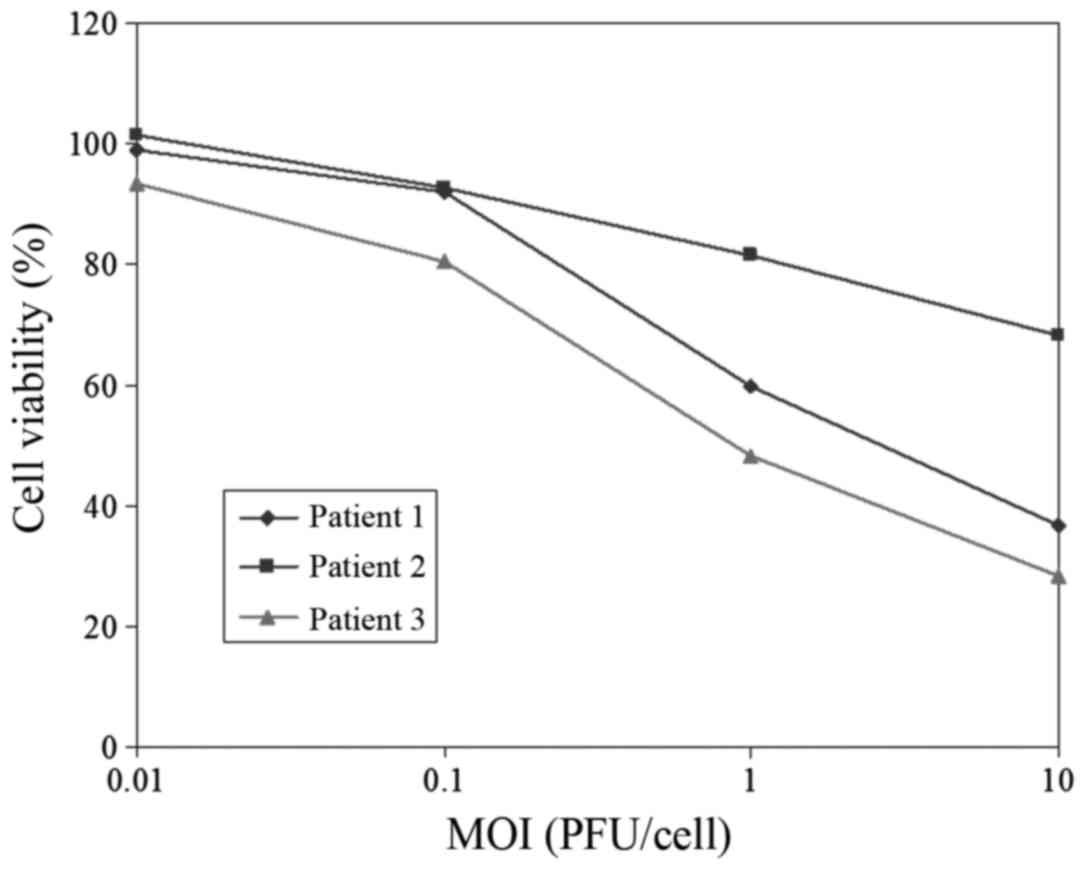
Oncolytic effect of VG9 on clinical
samples
To further investigate the oncolytic effect of VG9
on clinical human tumor samples, we obtained three surgically
resected human thyroid samples from Jiangyuan Hospital Affiliated
to Jiangsu Institute of Nuclear Medicine and the oncolytic potency
of VG9 was evaluated. Primary cells (104/well) from
fresh thyroid tissue were cultured in 96-well plates. Three days
after VG9 infection, cell viability was analyzed using the MTT
cytotoxicity assay (Fig. 4). The
results revealed that VG9 induced a cytotoxic effect in patient 1
and 3, while patient 2 was poorly sensitive to VG9-induced cell
killing. A pathological test indicated that the thyroid samples
from patients 1 and 3 were malignant while that of patient 2 was
benign.
Discussion
We are interested in the research of cancer therapy
using the vaccinia virus due to several favorable features. The
lifecycle of vaccinia virus is short with mature virions forming
within 6 h after infection (34),
resulting in a high titer produced within a short period of time.
The large transgene-encoding capacity of vaccinia virus facilitates
multiple therapeutic strategies. Its native promoters are strong
and efficient, leading to high levels of transgene expression using
its own enzyme systems. There is a long history of the use of the
vaccinia virus during the smallpox eradication and its biology is
clear, making it safe and easy to use in humans. Notably, many
laboratory studies and clinical trials have examined the
applicability of several vaccine strains including Wyeth,
Copenhagen and Lister. However, the potential of the Chinese
vaccine strain as an oncolytic agent was previously untested. In
this study, data characterizing the antitumor effect of Chinese
vaccine virus Guang9 strain (VG9) in vitro and in
vivo were presented. The results revealed that viral
replication and cytotoxicity of VG9 was potent in vitro, and
VG9 exhibited notable antitumor efficacy in inhibiting tumor
development in a murine melanoma tumor model.
VG9 was derived from the Chinese vaccine Tian Tan
strain (VTT) using consecutive plaque-cloning selection. According
to research, VG9 produced a smaller necrosis area and pock
diameter, less red swelling and lower incidences of fever and
hyperpyrexia (27–29). Although VG9 still had neurotoxicity
to a certain extent, the virulence was found to be lower than its
parental virus (VTT) in various animal models (30). In previous studies, the
neurovirulent vaccinia strain Western Reserve (WR), which has been
widely used in laboratories and extensively tested in clinical
trials, has an LD90 of 2.4 PFU, while VTT is about
5000-fold less virulent (23).
Collectively, we conclude that VG9 may become an ideal vaccinia
virus vector and a safer human vaccine. Some preliminary studies
have indicated that removing the thymidine kinase gene of the
vaccine virus may reduce the virulence as well as enhance tumor
targeting (35,36). Another approach to attenuate or
enhance tumor-selective replication is the introduction of selected
deletions in the viral genome (37–39).
These constructions based on VG9 hold promise and the detailed
oncolytic potency will be investigated in future studies. Our next
step to improve VG9 will be to insert various therapeutic genes
such as immune cytokine genes, suicide genes and enzyme-prodrug
genes, to elevate its potency as well as maintain its high tumor
selectivity.
Oncolytic viruses preferentially grow in tumor cells
due to their natural tropism for cell surface proteins that are
aberrantly expressed by tumor cells. In our in vitro study,
the cytotoxic effect on tumor cells was much stronger, while normal
cells were poorly sensitive to virally-induced cell killing. Our
in vitro study also revealed the differences between the
replication rates in different cancer cell lines. Vaccinia virus
replicates in cytoplasm and needs a nucleotide pool for replication
of the viral genome. Tumor cell lines have different pools of
functional nucleotides, which produce different replication rates
in various tumor cells. In addition, the growth rate of tumor cells
is another factor. The ability of viral replication was evidently
higher in fast-growing tumor cells, like highly malignant cells B16
and MDA-MB-231 cells. Another mechanism that may limit the overall
effectiveness of oncolytic viruses is the susceptibility of cancer
cells to apoptosis, which may be induced by viral infection or
other factors. If cells undergo apoptosis too rapidly, this will
reduce the time for viral replication and propagation.
The safety of the vaccinia virus is one of the most
essential considerations for clinical applications. Since being
used in smallpox vaccination programs globally, the safety of
oncolytic vaccine viruses in humans has been demonstrated and
specific antiviral agents are available (40,41).
Mild flu-like symptoms have been the primary side effects; no
treatment-related changes in the parameters of hematological,
hepatic, and renal function and no significant normal tissue
toxicity has been reported to date (10,12,42).
In this study, there was no significant toxicity and no mice died
even when 109 PFU of VG9 was injected (data not shown).
In some clinical studies, the dosage of the virus intravenous
injection was 108 PFU, while it was 107 PFU
for intratumoral injection. Upon 108 PFU of VG9
treatment, similar results were observed with an insignificant
change of the survival curve (data not shown). Furthermore, a
higher concentration of the virus is not easy to disperse in
tumors. Therefore, the dosage of 107 PFU was safe and
enough. Due to its excellent safety in humans, novel cancer
therapeutic strategies based on vaccinia backbones of the vaccinia
virus are feasible to design, owing to its fast replication cycle
and high selectivity for cancer tissue.
The rapid antiviral immune response and subsequent
virus clearance, which limit the use of repeated injections, are
potential limitations in the use of the vaccinia virus as an
antitumor agent (43). To address
this problem, one possible strategy is the administration of the
vaccinia virus concurrently with tumor-trafficking immune cells,
which would deliver viruses to their tumor targets (44). Another approach is using liposomes,
polyethylene glycol, neutralizing antibodies, or other biological
agents to disguise the vaccinia virus.
In this study, we revealed that the vaccinia strain
VG9 alone, without therapeutic genes, can induce an antitumor
effect by viral replication and consequent cell lysis. It has the
potential to be a novel platform for cancer treatment with the
ability to induce tumor destruction by multiple mechanisms and no
cross-resistance with traditional therapies. However, hurdles such
as the immune response, systemic distribution and intratumoral
spread are major potential limitations and must be addressed in
future studies.
Acknowledgements
We are grateful to the National Institutes for Food
and Drug Control (NIFDC) for providing the vaccinia virus of the
Tian Tan strain VG9.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81703061).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
LD and BH conceived the study. The manuscript was
written by LD and revised by ZH. LD contributed to the viral
replication. YZ carried out the cytotoxic assay. JF carried out the
animal study and contributed to the design of the in vivo
study. YD carried out the in vivo viral biodistribution. BZ
contributed to the time-resolved fluoroimmunoassay and data
acquisition. BH collected the clinical samples. ZH analyzed and
interpreted the data. JZ contributed to analysis of data for the
study. All authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The animal experiments was approved by the
Institutional Animal Care and Use Committees (IACUC) of Jiangsu
Institute of Nuclear Medicine (JSINM2010007). The study was
approved by the Ethics Committee of Jiangyuan Hospital Affiliated
to Jiangsu Institute of Nuclear Medicine (Wuxi, China). All
patients provided informed consent before enrollment in the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kirn D, Martuza RL and Zwiebel J:
Replication-selective virotherapy for cancer: Biological
principles, risk management and future directions. Nat Med.
7:781–787. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Everts B and van der Poel HG:
Replication-selective oncolytic viruses in the treatment of cancer.
Cancer Gene Ther. 12:141–161. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thorne SH, Hermiston T and Kirn D:
Oncolytic virotherapy: Approaches to tumor targeting and enhancing
antitumor effects. Semin Oncol. 32:537–548. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu TC, Galanis E and Kirn D: Clinical
trial results with oncolytic virotherapy: A century of promise, a
decade of progress. Nat Clin Pract Oncol. 4:101–117. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo ZS and Bartlett DL: Oncolytic viruses
as platform for multimodal cancer therapeutics: A promising land.
Cancer Gene Ther. 21:261–263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heise C, Sampson-Johannes A, Williams A,
McCormick F, Von Hoff DD and Kirn DH: ONY X-015, an E1B
gene-attenuated adenovirus, causes tumor-specific cytolysis and
antitumoral efficacy that can be augmented by standard
chemotherapeutic agents. Nat Med. 3:639–645. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Walker JR, McGeagh KG, Sundaresan P,
Jorgensen TJ, Rabkin SD and Martuza RL: Local and systemic therapy
of human prostate adenocarcinoma with the conditionally replicating
herpes simplex virus vector G207. Hum Gene Ther. 10:2237–2243.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Phuangsab A, Lorence RM, Reichard KW,
Peeples ME and Walter RJ: Newcastle disease virus therapy of human
tumor xenografts: Antitumor effects of local or systemic
administration. Cancer Lett. 172:27–36. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Puhlmann M, Gnant M, Brown CK, Alexander
HR and Bartlett DL: Thymidine kinase-deleted vaccinia virus
expressing purine nucleoside phosphorylase as a vector for
tumor-directed gene therapy. Hum Gene Ther. 10:649–657. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mastrangelo MJ, Maguire HC Jr, Eisenlohr
LC, Laughlin CE, Monken CE, McCue PA, Kovatich AJ and Lattime EC:
Intratumoral recombinant GM-CSF-encoding virus as gene therapy in
patients with cutaneous melanoma. Cancer Gene Ther. 6:409–422.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim JH, Oh JY, Park BH, Lee DE, Kim JS,
Park HE, Roh MS, Je JE, Yoon JH, Thorne SH, et al: Systemic armed
oncolytic and immunologic therapy for cancer with JX-594, a
targeted poxvirus expressing GM-CSF. Mol Ther. 14:361–370. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park BH, Hwang T, Liu TC, Sze DY, Kim JS,
Kwon HC, Oh SY, Han SY, Yoon JH, Hong SH, et al: Use of a targeted
oncolytic poxvirus, JX-594, in patients with refractory primary or
metastatic liver cancer: A phase I trial. Lancet Oncol. 9:533–542.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hwang TH, Moon A, Burke J, Ribas A,
Stephenson J, Breitbach CJ, Daneshmand M, De Silva N, Parato K,
Diallo JS, et al: A mechanistic proof-of-concept clinical trial
with JX-594, a targeted multi-mechanistic oncolytic poxvirus, in
patients with metastatic melanoma. Mol Ther. 19:1913–1922. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Heo J, Reid T, Ruo L, Breitbach CJ, Rose
S, Bloomston M, Cho M, Lim HY, Chung HC, Kim CW, et al: Randomized
dose-finding clinical trial of oncolytic immunotherapeutic vaccinia
JX-594 in liver cancer. Nat Med. 19:329–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thorne SH, Hwang TH, O'Gorman WE, Bartlett
DL, Sei S, Kanji F, Brown C, Werier J, Cho JH, Lee DE, et al:
Rational strain selection and engineering creates a broad-spectrum,
systemically effective oncolytic poxvirus, JX-963. J Clin Invest.
117:3350–3358. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kirn DH, Wang Y, Le Boeuf F, Bell J and
Thorne SH: Targeting of interferon-beta to produce a specific,
multi-mechanistic oncolytic vaccinia virus. PLoS Med. 4:e3532007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Foloppe J, Kintz J, Futin N, Findeli A,
Cordier P, Schlesinger Y, Hoffmann C, Tosch C, Balloul JM and Erbs
P: Targeted delivery of a suicide gene to human colorectal tumors
by a conditionally replicating vaccinia virus. Gene Ther.
15:1361–1371. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Q, Yu YA, Wang E, Chen N, Danner RL,
Munson PJ, Marincola FM and Szalay AA: Eradication of solid human
breast tumors in nude mice with an intravenously injected
light-emitting oncolytic vaccinia virus. Cancer Res.
67:10038–10046. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hui X and Yutu J: The eradication of
smallpox in Shanghai, China, October 1950-July 1951. Bull World
Health Organ. 59:913–917. 1981.PubMed/NCBI
|
|
20
|
Ma B: Variolation, pioneer of modern
immunology. Zhonghua Yi Shi Za Zhi. 25:139–144. 1995.(In Chinese).
PubMed/NCBI
|
|
21
|
Henderson DA: The eradication of smallpox
- an overview of the past, present, and future. Vaccine. 29((Suppl
4)): D7–D9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jin Q, Chen L, Chen S, Huang J, Feng Z,
Yuan J, Jin D, Bai H and Hou Y: Characterization of the complete
genomic sequence of the vaccinia virus Tian Tan strain. Sci China.
27:562–567. 1997.
|
|
23
|
Fang Q, Yang L, Zhu W, Liu L, Wang H, Yu
W, Xiao G, Tien P, Zhang L and Chen Z: Host range, growth property,
and virulence of the smallpox vaccine: Vaccinia virus Tian Tan
strain. Virology. 335:242–251. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang X, Lu B, Yu W, Fang Q, Liu L, Zhuang
K, Shen T, Wang H, Tian P, Zhang L, et al: A novel
replication-competent vaccinia vector MVTT is superior to MVA for
inducing high levels of neutralizing antibody via mucosal
vaccination. PLoS One. 4:e41802009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Z, Wang S, Zhang Q, Tian M, Hou J,
Wang R, Liu C, Ji X, Liu Y and Shao Y: Deletion of C7L and K1L
genes leads to significantly decreased virulence of recombinant
vaccinia virus TianTan. PLoS One. 8:e681152013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu W, Fang Q, Zhu W, Wang H, Tien P, Zhang
L and Chen Z: One time intranasal vaccination with a modified
vaccinia Tiantan strain MVTT(ZCI) protects animals against
pathogenic viral challenge. Vaccine. 28:2088–2096. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Products NiftCoPaB: A summary report on
the selection of VG9 vaccinia virus strain which acquired in the
collaborative work of the national smallpox vaccine. Compil Commun
Vac Virus Strains (Beijing). 45–49. 1974.
|
|
28
|
Products CIoB: Comparison of
reactogenicity in experimental animals of five vaccinia virus
strains of domestic and abroad. 7:70–73. 1978.
|
|
29
|
Products CIoB: Comparison of
reactogenicity and immunogenicity in experimental animals of five
vaccinia virus strains of domestic and abroad. 4:13–19. 1978.
|
|
30
|
Zhu R, Huang W, Wen Z, Wang W, Zhou Y and
YC W: Studies on the virulence of a novel attenuated vaccinia virus
VG9 strain in animals. Zhongguo Bingdubing Zazhi. 1:183–187.
2011.
|
|
31
|
Zhu R, Huang W, Wang Y and Yu Y:
Immunogenicity of an attenuated vaccinia virus VG9 strain. Zhongguo
Shengwuzhipinxue Zazhi. 9:347–350. 2011.
|
|
32
|
O'Reilly MS, Boehm T, Shing Y, Fukai N,
Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR and Folkman J:
Endostatin: An endogenous inhibitor of angiogenesis and tumor
growth. Cell. 88:277–285. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang B, Tao W, Shi J, Tang L and Jin J:
Determination of ochratoxin A by polyclonal antibodies based
sensitive time-resolved fluoroimmunoassay. Arch Toxicol.
80:481–485. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zeh HJ and Bartlett DL: Development of a
replication-selective, oncolytic poxvirus for the treatment of
human cancers. Cancer Gene Ther. 9:1001–1012. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Buller RM, Smith GL, Cremer K, Notkins AL
and Moss B: Decreased virulence of recombinant vaccinia virus
expression vectors is associated with a thymidine kinase-negative
phenotype. Nature. 317:813–815. 1985. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gnant MF, Puhlmann M, Bartlett DL and
Alexander HR Jr: Regional versus systemic delivery of recombinant
vaccinia virus as suicide gene therapy for murine liver metastases.
Ann Surg. 230:352–361. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ramsey-Ewing A and Moss B: Restriction of
vaccinia virus replication in CHO cells occurs at the stage of
viral intermediate protein synthesis. Virology. 206:pp. 984–993.
1995, View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kan S, Wang Y, Sun L, Jia P, Qi Y, Su J,
Liu L, Yang G, Liu L, Wang Z, et al: Attenuation of vaccinia Tian
Tan strain by removal of viral TC7L-TK2L and TA35R genes. PLoS One.
7:e319792012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guo ZS, Naik A, OMalley ME, Popovic P,
Demarco R, Hu Y, Yin X, Yang S, Zeh HJ, Moss B, et al: The enhanced
tumor selectivity of an oncolytic vaccinia lacking the host range
and antiapoptosis genes SPI-1 and SPI-2. Cancer Res. 65:9991–9998.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
De Clercq E: Cidofovir in the therapy and
short-term prophylaxis of poxvirus infections. Trends Pharmacol
Sci. 23:456–458. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wittek R: Vaccinia immune globulin:
Current policies, preparedness, and product safety and efficacy.
Int J Infect Dis. 10:193–201. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Breitbach CJ, Burke J, Jonker D,
Stephenson J, Haas AR, Chow LQ, Nieva J, Hwang TH, Moon A, Patt R,
et al: Intravenous delivery of a multi-mechanistic cancer-targeted
oncolytic poxvirus in humans. Nature. 477:99–102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Evgin L, Acuna SA, Tanese de Souza C,
Marguerie M, Lemay CG, Ilkow CS, Findlay CS, Falls T, Parato KA,
Hanwell D, et al: Complement inhibition prevents oncolytic vaccinia
virus neutralization in immune humans and cynomolgus macaques. Mol
Ther. 23:1066–1076. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Thorne SH, Liang W, Sampath P, Schmidt T,
Sikorski R, Beilhack A and Contag CH: Targeting localized immune
suppression within the tumor through repeat cycles of immune
cell-oncolytic virus combination therapy. Mol Ther. 18:1698–1705.
2010. View Article : Google Scholar : PubMed/NCBI
|