Introduction
Prostate cancer (PCa) is the second most frequently
diagnosed cancer in men worldwide (1), and remains a leading cause of
cancer-associated mortality (2).
The incidence rates for PCa increased between 2000 and 2011, with
an estimated 60,300 new cases in China in 2015 (3). Genetic variations affect the
initiation and progression of PCa, and men with a family history of
PCa have a two-fold increased risk of developing PCa, usually with
an earlier age of onset (4).
Genome-wide association studies (GWAS) have identified 100 risk
variants for PCa, which may explain ~33% of the familial risk of
the disease (5). However, the
biological function of numerous genetic variations remains to be
discovered.
Accumulating evidence suggests gene expression may
be altered by single nucleotide polymorphisms (SNPs) in the 3′
untranslated region (UTR), and one essential mechanism involves
regulation by microRNAs (miRNAs) (6,7).
miRNAs are small non-coding RNAs, composed of 18–24 nucleotides,
that are involved in the regulation of essential biological
processes, including cell proliferation, differentiation and
apoptosis (8,9). miRNAs modulate the expression of
protein-coding genes by binding to the full or part of their
complementary sequence in the 3′ or 5′UTR of the coding sequence
(10,11). Polymorphisms in miRNA binding sites
affect the binding efficacy of miRNAs and consequently alter the
expression of target genes (6,7,9,12,13).
Insulin-like growth factor 1 receptor (IGF1R) is a
transmembrane tyrosine kinase receptor, characterized by a
heterodimer of α- and β-chains, and is activated by its ligands
insulin-like growth factor 1 (IGF1) and insulin-like growth factor
2 (IGF2) (14). Activation of IGF1R
is associated with improved growth, proliferation, angiogenesis and
survival (15). Numerous studies
have demonstrated that IGF1R serves an important role in the risk
and progression of PCa (16,17).
In our previous study (18), an
association analysis for the reverse tyrosine kinase-extracellular
signal-regulated kinase pathway was performed based on the GWAS
data from the Consortium for Chinese Prostate Cancer Genetics
(ChinaPCa), which identified two SNPs (rs1815009 and rs2684788) in
the 3′UTR of IGF1R presented significant genotype distribution
between PCa and control samples.
Based on the important regulatory function of miRNAs
at the transcriptional level, two miRNA binding prediction tools
[TargetScan (19) and miRanda
(20)] were used to predict the
binding of miRNAs in the IGF1R 3′UTR. The predicted results
revealed that miR-133a and miR-133b may bind near rs1815009, and
miR-455 near rs2684788, within the IGF1R 3′UTR. In addition, a
thermodynamic model was applied to analyze the binding affinity of
miR-133a and miR-133b for rs1815009, and miR-455 for rs2684788
under different variants, which demonstrated that the binding of
these miRNAs would be affected. A dual-luciferase reporter assay
was performed on 293 cells to verify the binding affinity predicted
by the thermodynamic model. Finally, whether IGF1R expression was
affected by these miRNAs was investigated through overexpression of
miR-133a, miR-133b or mir-455 in PC3 and LNCaP cells.
Materials and methods
Thermodynamic model for the
miRNA-target interaction
The sequence of the 3′UTR of IGF1R was retrieved
from the University of California Santa Cruz database (http://genome.ucsc.edu/) in the human genome assembly
GRCh37/hg19. The targets for miRNA binding in the IGF1R 3′UTR
containing two polymorphisms, rs1815009 or rs2684788, were
predicted using the TargetScan7.1 (http://www.targetscan.org) (19) and miRanda databases (http://www.microrna.org) (20). Mature human sequences were
downloaded from the National Center for Biotechnology Information
(https://www.ncbi.nlm.nih.gov/). A
parameter-free thermodynamic model was used (21,22) to
investigate the binding affinity of miR-133a and miR-133b for the C
or T allele of rs1815009, and miR-455 for the C or T allele
ofrs2684788. In this model, ETarget represented the
energy of the dissociated mRNA target region with no miRNAs
interacting with it, EIntermediate indicated the energy
required to make the target region accessible for microRNA binding,
and EComplex is the energy of the microRNA-target
complex, the binding of which was consistent with the constraints
imposed by the seed region (6,7,23).
RNAfold software (Vienna RNA Package; http://rna.tbi.univie.ac.at/cgi-bin/RNAWebSuite/RNAfold.cgi)
was used to compute all ensemble free energies with the partition
function algorithm for folding and the default settings. The
RNAfold was modified to calculate the EIntermediate and
EComplex in order to construct the connectivity matrix
of the intermediate or complex product secondary structures, and
the corresponding structure-associated energies were calculated.
The target sequence size contained the relevant miRNA target seed
region, the relevant IGF1R SNP site and the nucleotides surrounding
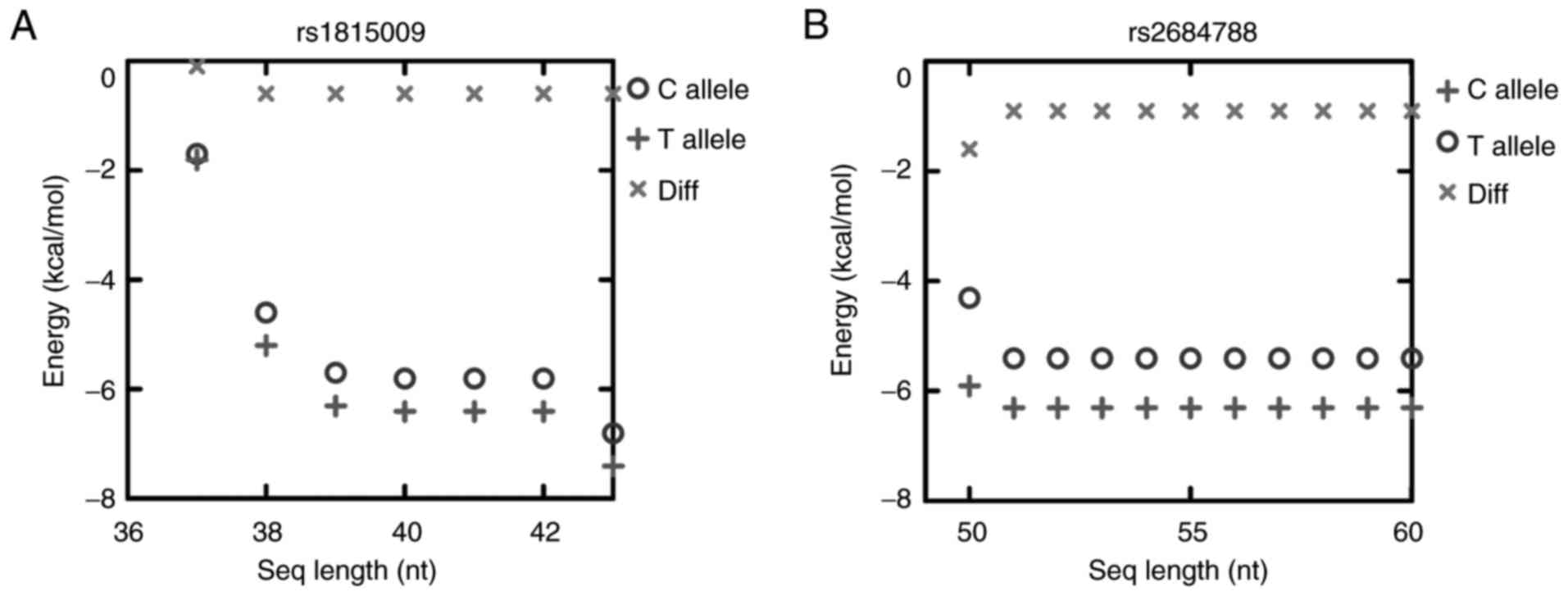
the SNP site. The site length provided for the RNAfold was 42 nt
for rs1815009 and 60 nt for rs2684788. The value of the selected
target size for rs1815009 and rs2684788 was chosen to reduce the
time complexity of the aforementioned computations, particularly as
the results were not altered when the length of the target was
extended (Fig. 1). The
ΔEa is the difference between ETarget and
EIntermediate, and ΔEb is the difference
between ETarget and EComplex (6,7).
Literature search
A thorough literature search was performed using
PubMed on miRNA expression profiling studies for PCa with the
following key words: Prostate and (cancer OR tumor OR tumor) and
(mirna OR microrna OR mir-). Additionally, miRNA expression
profiles for PCa were retrieved by searching the Gene Expression
Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo/) (24). Profiles whose data published between
June 14, 2007 and April 28, 2015 were selected. The inclusion
criteria were as follows: i) Experimental samples consisting of
prostate tumor and non-tumor tissues, for which the complete raw or
normalized microarray data was available; ii) data included
genome-wide miRNA expression; iii) Homo sapiens as the
studied organism; iv) samples that had not been subjected to
adjuvant therapy; and v) the number of PCa and normal controls was
>15. For the present study, the non-tumor tissues included
adjacent tissues of PCa or tissues from independent healthy donors,
but excluded benign prostatic hyperplasia. The data extracted from
each GEO dataset was as follows: Tumor sample type, country,
platform, experimental materials and number of samples (Table I). The list of miRNAs with
statistically significant expression in PCa was extracted from the
publications. All statistical analyses were performed using
GEO2R.
 | Table I.Brief overview of the GEO
datasets. |
Table I.
Brief overview of the GEO
datasets.
| Series ID | Tumor sample
type | Country | Platform | Experimental
materials | Number of
samples |
|---|
| GSE36803 | Primary | North American | Affymetrix GeneChip
array | Tissue (Homo
sapiens) | 21 pairs |
| GSE8126 | Primary | North American | OSU-CCC
hsa-miRNA-chip version 3 | Tissue (Homo
sapiens) | 60 T+16N |
| TCGA | Primary | North American | Illumina HiSeq 2000
miRNASeq | Tissue (Homo
sapiens) | 498T+52N |
Processing of data obtained from the
cancer genome atlas
The normalized miRNA-HiSeq expression values and PCa
clinical information was downloaded from the Cancer Genome Atlas
(TCGA) (http://cancergenome.nih.gov/). The
reads/million miRNA mapped (RPM) normalized values for miRNA
expression were further log2-transformed. The fold
change in miRNA expression between tumors and normal tissues was
calculated using the median-centered RPM values. The information
for TCGA dataset is presented in Table
I. The difference in expression between PCa and normal tissue
was analyzed using an independent sample t-test.
Plasmid construction and luciferase
reporter assay
Truncated UTR sequences containing the SNP region
were amplified by polymerase chain reaction (PCR) from genomic DNA,
then ScaI cloned into the 3′UTR of the pmirGLO-control
vector (Promega Corporation, Madison, WI, USA) following the
manufacturer's protocol. The 3′UTR reporter vector for IGF1R,
containing the C allele of rs1815009 and seed region of miR-133a or
miR-133b, was named pmirGLO-rs1815009-IGF1R-[C]. Plasmids carrying
the 3′UTR with the T allele were designated as
pmirGLO-rs1815009-IGF1R-[T]. Likewise, two 3′UTR constructs for
rs2684788 containing the C or T alleles were designated as
pmirGLO-rs2684788-IGF1R-[C] and pmirGLO-rs2684788-IGF1R-[T]. Each
plasmid was co-transfected with the miRNA precursor (pre-133a,
pre-133b, pre-455 or scrambled negative control; Shanghai
GenePharma Co., Ltd., Shanghai, China) in 293 cells cultured in
24-well plates. The following sequences were used: Pre-133a,
AATTCACAATGCTTTGCTAGAGCTGGTAAAATGGAACCAAATCGCCTCTTCAATGGATTTGGTCCCCTTCAACCAGCTGTAGCTATGCATTGAACCGG;
pre-133b,
AATTCCCTCAGAAGAAAGATGCCCCCTGCTCTGGCTGGTCAAACGGAACCAAGTCCGTCTTCCTGAGAGGTTTGGTCCCCTTCAACCAGCTACAGCAGGGCTGGCAATGCCCAGTCCTTGGAGAACCGG;
pre-455,
AATTCTCCCTGGCGTGAGGGTATGTGCCTTTGGACTACATCGTGGAAGCCAGCACCATGCAGTCCATGGGCATATACACTTGCCTCAAGGCCTATGTCATCACCGG;
and scrambled negative control, AAATGTACTGCGCGTGGAGAC. Following
transfection with Lipofectamine 2000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), 1.5×106
cells/ml cells were cultured for 24 h, then lysed in passive lysis
buffer, according to the Dual-Luciferase® Reporter Assay
system kit (Promega Corporation), and luciferase activity was
measured with a Tecan M1000 microplate reader (Tecan Group, Ltd.,
Mannedorf, Switzerland). A dual-luciferase system was utilized in
this assay, containing two luciferase enzymes, one containing the
luc2 reporter gene that reports miRNA activity, and
another containing the hRluc-neo reporter gene used as a control
reporter for normalization of gene expression. Normalization of the
empty pmirGLO vector was performed using the Renilla/firefly
luciferase ratios. Each luciferase assay was performed in
triplicate and the mean value was calculated.
Cell culture and transfection
The human PCa cell line PC3 was obtained from the
American Type Culture Collection (Manassas, VA, USA), and the human
PCa cell line LNCaP and the 293 cell line were purchased from the
Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). The PC3 cells were maintained in Dulbecco's
modified Eagle's medium (DMEM)/F12 (HyClone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 10% (v/v) fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.). LNCaP cells
were cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.)
with 10% (v/v) FBS, and 293 was cultured in DMEM supplemented with
10% (v/v) FBS, 1% (v/v) penicillin and streptomycin. All cultures
were cultured in an incubator set to 37°C with 5% CO2.
Then, 0.5 µg knockdown synthetic oligonucleotides (pre-133a,
pre-133b, pre-455, pre-455 inhibitor and pre-control) were
transfected when cells reached 80–90% confluence, using
Lipofectamine 3000 reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol.
DNA sequencing
PC3 and LNCaP cells at the log growth phase were
collected for DNA isolation. Genomic DNA was extracted using
MiniBEST Universal Genomic DNA Extraction kit (Takara Bio, Inc.,
Otsu, Japan), following the manufacturer's protocol. The
SNP-containing region was amplified with Premix Taq (Takara Bio,
Inc.). Sequencing of the PCR product was performed using the BigDye
Terminator Reaction Chemistry v3.1 sequencing kit (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol, and
sequence analyses were performed with DNASTAR 7.0 (DNASTAR,
Madison, WI, USA).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
After 48 h of transfection, the PC3 and LNCaP cells
were harvested for RNA isolation. Total RNA was extracted using the
mirVana™ miRNA Isolation kit (Ambion; Thermo Fisher
Scientific, Inc.), following the manufacturer's protocol. Total RNA
from PC3 and LNCaP cells was used to synthesize cDNA using the
PrimeScript™ RT Master Mix (Takara Bio, Inc.). The PCR
reaction conditions were as follows: 37°C for 15 min and 85°C for 5
sec. RT-qPCR was performed in triplicate using PowerUp™
SYBR®-Green Master mix with a 7500 Real-Time PCR System
(both form Applied Biosystems; Thermo Fisher Scientific, Inc.). The
RT-qPCR reaction conditions were as follows: 50°C for 5 min; 95°C
for 2 min; and 40 cycles of 95°C for 15 sec and 60°C for 1 min. The
primers used are listed in Table
II. The relative mRNA expression in the control and
experimental groups of PC3 and LNCaP was determined using the
2−ΔΔCq method (25), and
normalized to GAPDH or β-actin mRNA expression levels.
 | Table II.Primer sequences used in the present
study. |
Table II.
Primer sequences used in the present
study.
| Genes | Primers
(5′-3′) |
|---|
| IGF1R |
|
| F |
ATGCTGACCTCTGTTACCTCT |
| R |
GGCTTATTCCCCACAATGTAGTT |
| β-actin |
|
| F |
AACTCCATCATGAAGTGTGA |
| R |
ACTCCTGCTTGCTGATCCAC |
| GAPDH |
|
| F |
ATCTCTGCCCCCTCTGCTGA |
| R |
GATGACCTTGCCCACAGCCT |
| MMP2 |
|
| F |
AACTACGATGATGACCGCAAG |
| R |
GACAGACGGAAGTTCTTGGTG |
| CDH1 |
|
| F |
ACTCGTAACGACGTTGCACCA |
| R |
GGTCAGTATCAGCCGCTTTCAG |
| VEGFA |
|
| F |
CCTTGCTGCTCTACCTCCAC |
| R |
GAAGATGTCCACCAGGGTCTC |
| BCL2 |
|
| F |
TCGCCCTGTGGATGACTGA |
| R |
CAGAGACAGCCAGGAGAAATCA |
| SNP |
|
| F |
ACCCATCTCTCCCAGGACC |
| R |
CTGGCAGAAGGAGGTTGCAT |
| SNP2 |
|
| F |
CAGGCAGCACCATCTCTGTG |
| R |
CCAATGTGCACCGAGCATCT |
Western blot analysis
Post-transfected cells (PC3 and LNCaP) were
collected for protein isolation following 48 h of incubation. Total
protein was extracted with radioimmunoprecipitation assay buffer
(Beyotime Institute of Biotechnology, Beijing, China) with Halt
Protease Inhibitor Cocktail (100X; Thermo fisher Scientific, Inc.)
and quantified with the bicinchoninic acid assay, and 50 µg of
protein/lane was separated on a 12% SDS-PAGE gel and transferred to
a 0.2-µm polyvinylidene fluoride membrane (EMD Millipore,
Billerica, MA, USA). Blots were blocked with 5% milk, TBS and 0.1%
Tween-20 at room temperature for 2 h. Blots were incubated with the
primary anti-mouse IGF-IR antibody (cat. no., 3G5C1; 1:2,000; Novus
Biologicals, Ltd., Cambridge, UK) or tubulin (cat. no., 66240-1-lg;
1:2,000; ProteinTech Group, Inc., Chicago, IL, USA) at 4°C for 12
h. Subsequently, blots were incubated with the horseradish
peroxidase-conjugated Affinipure Goat Anti-Mouse IgG (H+L) second
antibody (cat. no., SA00001-1; 1:10,000; ProteinTech Group, Inc.)
at room temperature for 2 h. BeyoECL Plus (Beyotime Institute of
Biotechnology) was used as the visualization reagent. The intensity
of the protein bands was quantified using Odyssey 2.1 software
(LI-COR Biosciences, Lincoln, NE, USA).
Cell migration and invasion assay
Transfected LNCaP cells (pre-455, pre-455 inhibitor
and pre-control) growing in the log phase were harvested for use in
the migration and invasion assays. A total of 4×105
cells in serum-free RPMI-1640 (200 µl) were seeded into the upper
chamber of 8-µM pore Transwell plates (Costar; Corning
Incorporated; Corning, NY, USA), coated with or without Matrigel
(BD Biosciences, San Jose, CA, USA). RPMI-1640 (600 µl) filled with
10% (v/v) FBS was added to the lower chamber. The cells were
allowed to migrate towards RPMI-1640 containing 10% (v/v) FBS for
48 or 72 h at 37°C. Cells on the top surface of the insert were
removed by scraping, and the migrated and invasive cells were fixed
for 10 min using 100% methanol and stained for 20 min with 1%
crystal violet (both at room temperature). Subsequently, cells were
imaged at ×20 magnification using an inverted phase contrast
microscope (TS100-F; Nikon Corporation, Tokyo, Japan), removed the
crystal violet dye retained on the filters using 33% acetic acid
(600 µl) and then transferred the solution containing crystal
violet to 96-well plates (150 µl/well). Values for migration and
invasion were obtained by measuring absorbance of the solution at
570 nm using an ELISA reader (BioTek ELx-800; BioTek Instruments,
Inc., Winooski, VT, USA) (26), and
presented as the mean of at least three independent
experiments.
Statistical data analysis
The figures were generated with GraphPad Prism 5.0
(GraphPad Software, Inc., La Jolla, CA, USA). Data are presented as
the mean ± standard error the mean and were compared using a
Student's t-test (two-tailed). P<0.05 was considered to indicate
a statistically significant difference.
Results
rs1815009 (C/T) overlaps the predicted
binding sites of miR-133a and miR-133b, and rs2684788 (C/T)
overlaps miR-455 in the IGF1R 3′UTR
The alleles of rs1815009 and rs2684788 in the IGF1R
3′UTR in the present study were primarily based on GWAS data from
ChinaPCa. In our previous study, rs1815009 and rs2684788 were
identified to be significantly associated with PCa using the same
dataset (18). Among the PCa cases,
rs1815009 was identified to be a common variant, with a frequency
of ~52.12% for C the allele and 47.88% for the T allele, and
healthy individuals exhibited a common variant with a frequency of
~48.57% for the C allele and 51.43% for the T allele. In addition,
among the PCa cases, rs2684788 was demonstrated to have a frequency
of ~52.23% for the C allele and 47.77% for the T the allele, and
the healthy population exhibited a common variant with a frequency
of ~47.43% for the C allele and 52.57% for the T allele. The 3′UTR
was demonstrated to be an important region for miRNA binding to
genes. To clarify the function of these two polymorphisms on miRNA
binding, the target miRNA sequence was predicted using TargetScan
and miRanda. By combining the results obtained from TargetScan
(19) and miRanda (20), miR-133a, miR-133b and miR-455 were
predicted to bind to the IGF1R 3′UTR (Fig. 2A). Furthermore, rs1815009 was
located near the binding seed regions of miR-133a and miR-133b, and
rs2684788 was located near the binding seed region of miR-455
(Fig. 2B).
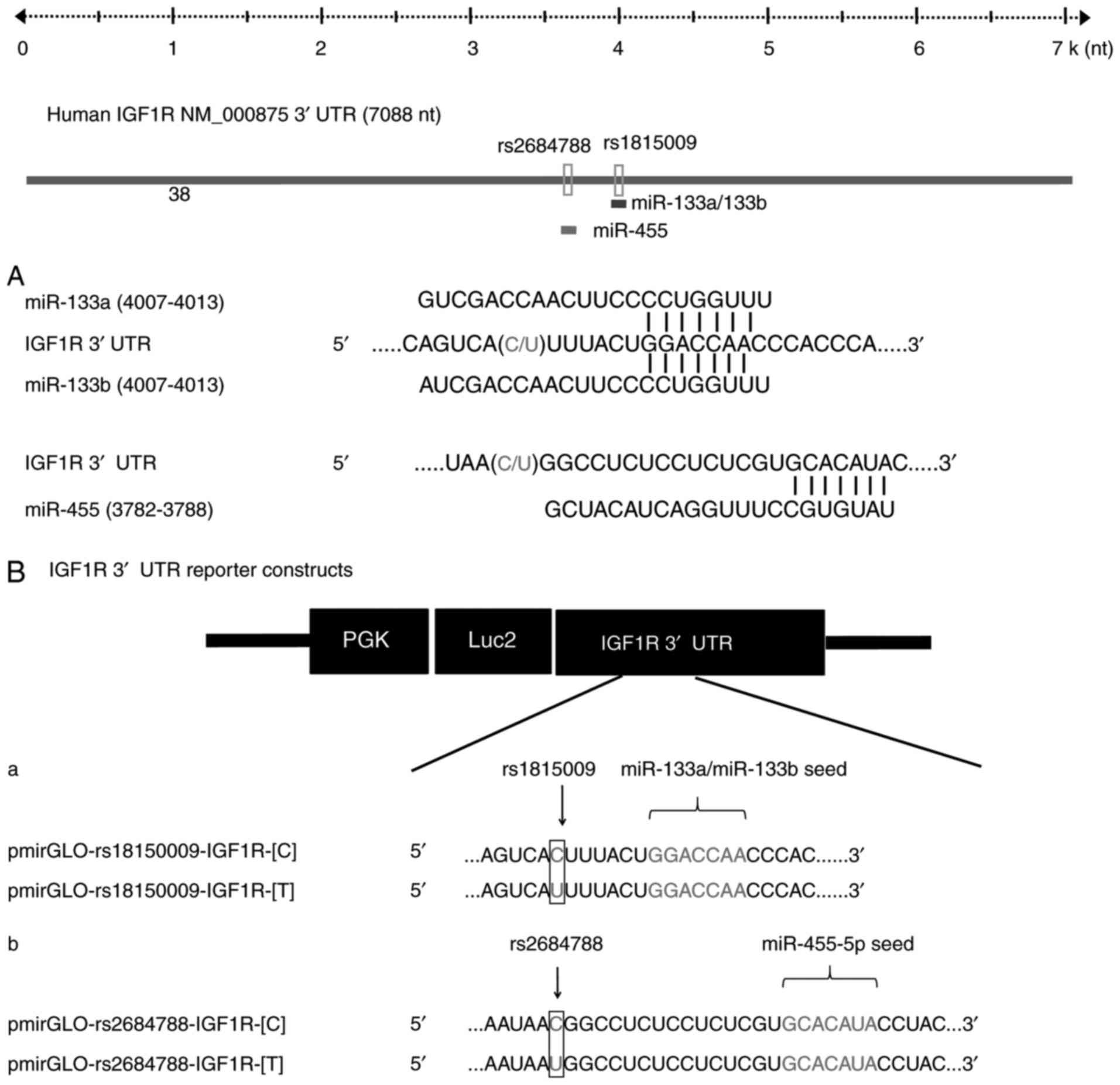 | Figure 2.Schematic depiction of miR-133a,
miR-133b and miR-455 binding regions within the IGF1R 3′UTR. (A)
Schematic representation of the IGF1R 3′UTR according to NCBI
reference sequence NM_000875.4 Numbering starts from the first
nucleotide of the 3′UTR, SNPs rs1815009 and rs2684788 are present
in positions 4000 and 3766, respectively. The seed sequences of
miR-133a and miR-133b are located in position 4007–4013, and the
seed region of miR-455 is located in the position 3782–3788. (B)
Schematic depiction of the constructs used for the luciferase
reporter assay. The sequences below demonstrated the nucleotides
that are mutated to generate the SNPs (a) rs1815009 and (b)
rs2684788 of the C allele (pmirGLO-rs1815009-IGF1R-[C] and
pmirGLO-rs2684788-IGF1R-[C]) and T allele
(pmirGLO-rs1815009-IGF1R-[T] and pmirGLO-rs2684788-IGF1R-[T]),
within the 3′UTR. IGF1R, insulin-like growth factor 1 receptor;
3′UTR, 3′ untranslated region; seq, sequence; miR, microRNA; SNP,
single nucleotide polymorphism. |
Minimum free energy (MFE) of miR-133a,
miR-133b and miR-455 binding to the IGF1R 3′UTR differs between
allelic variants of SNP rs1815009 (C/T) and rs2684788 (C/T)
In order to analyze the binding affinity of
miR-133a, miR-133b and miR-455 to the IGF1R 3′UTR of different
variants (rs1815009 and rs2684788), a parameter-free thermodynamic
model was generated (21). The free
energy of secondary structure-based molecules was calculated for
each stage of the binding process: ETarget represents
the energy of the dissociated mRNA target region;
EIntermediate is the energy required to make the target
region accessible for miRNA binding; and EComplex is the
energy of the miRNA-target complex (6,7,23). The
activation energy (ΔEa) was calculated as the difference
between EIntermediate and ETarget, whereas
the binding energy (ΔEb) was equivalent to the
interaction score, which is the difference between
EComplex and ETarget (6,7,23).
Compared with the C allele, the T allele of rs1815009 exhibited a
higher Etarget and lower ΔEa, indicating that
the binding affinity of miR-133a for the T allele was stronger
compared with that of the C allele. The same phenomenon was
observed for miR-133b. A higher Etarget and lower
ΔEa was identified for the rs2684788-IGF1R C allele,
suggesting that this allele was more accessible for miR-455
binding. Furthermore, the ΔEb of miR-133a or miR-133b to
the rs1815009-IGF1R C allele was increased compared with that of
the T allele, suggesting that miR-133a and miR-133b exhibited a
higher binding affinity for the C allele. Regarding the other SNP,
the ΔEb of the rs2684788-IGF1R C allele was reduced
compared with that for the T allele, suggesting that miR-455
exhibited a higher binding affinity for the T allele (Fig. 3).
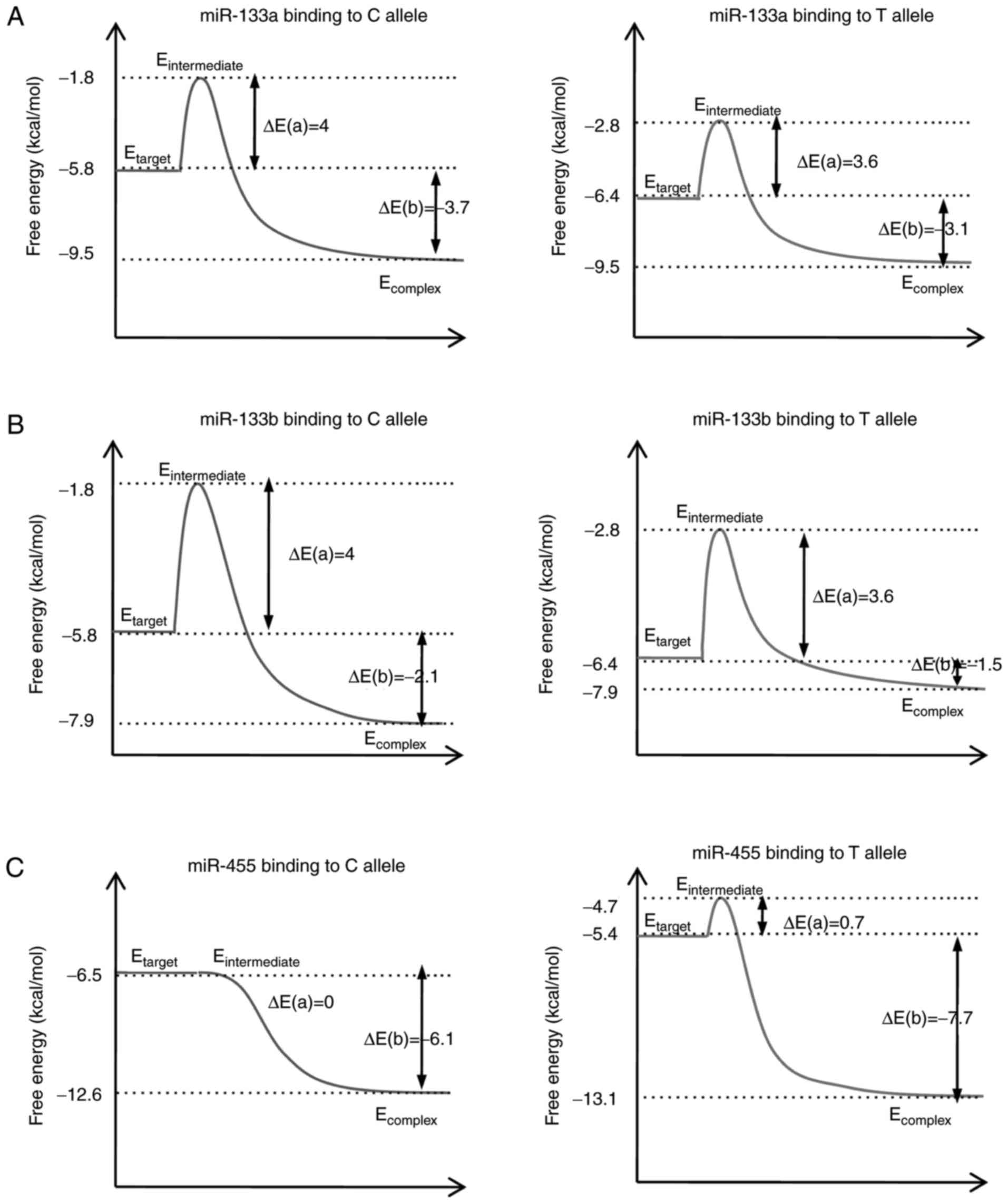 | Figure 3.Thermodynamic model of miR-133a,
miR-133b and miR-455 binding to the IGF1R 3′ untranslated region,
and the influence of single nucleotide polymorphisms. The secondary
structure-based energies for different miRNA-target binding
processes, where the ΔEa is the energy difference
between the transition state and the original target, and the
ΔEb is the energy difference between the complex and
target. Schematic for the binding energy diagram of (A) rs1815009
to miR-133a, (B) rs1815009 to miR-133b, and (C) rs2684788 to
miR-455. ΔEa, activation energy; ΔEb, binding
energy; IGF1R, insulin-like growth factor 1 receptor; seq,
sequence; miR, microRNA. |
Expression analysis of miR-133a,
miR-133b and miR-455 in GEO and TCGA
According to the inclusion criteria, four datasets
[GSE36803 (27), GSE8126 (28), GSE23022 (29) and TCGA datasets] were selected to
evaluate the miRNA expression in PCa. However, the GSE23022 dataset
is primarily derived from moderately differentiated PCa samples,
unlike the other three datasets which are from primary PCa samples,
therefore, this dataset was removed. In total, 579 carcinoma
samples and 89 normal samples were included in the present study.
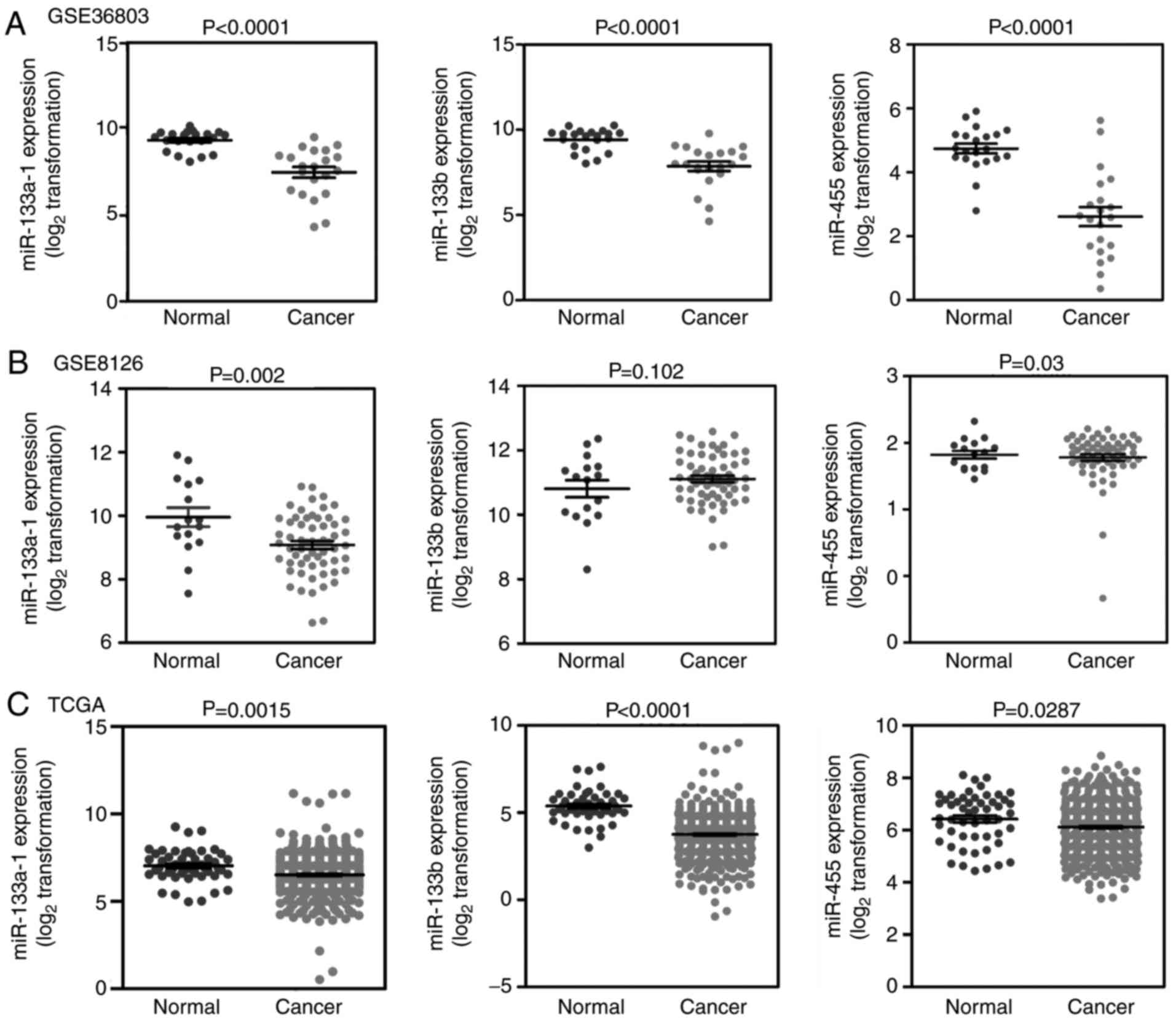
With the exception of miR-133b in the GSE8126 dataset, the
expression levels of all three miRNAs (miR-133a, miR-133b and
miR-455) in PCa tissues were significantly lower compared with that
of normal tissues in the three datasets (Fig. 4). This result indicated that
miR-133a, miR-133b and miR-455 are likely to be dysregulated in
PCa.
Effect of PCa susceptibility
polymorphisms rs1815009 and rs2684788 on miRNA binding
An in vitro luciferase reporter assay was
performed to verify whether the two SNPs, rs1815009 and rs2684788,
are able to affect miRNA binding. For each SNP, pmirGLO constructs
were generated carrying the C or T allele for rs1815009 and
rs2684788. These plasmids were designated as
pmirGLO-rs1815009-IGF1R-[C] and pmirGLO-rs1815009-IGF1R-[T] for
rs1815009, and pmirGLO-rs2684788-IGF1R-[C] and
pmirGLO-rs2684788-IGF1R-[C] for rs2684788 (Fig. 2). Each of the pmirGLO constructs was
analyzed for luciferase activity levels in 293 cells that
overexpressed the predicted interacting miRNAs or the scrambled
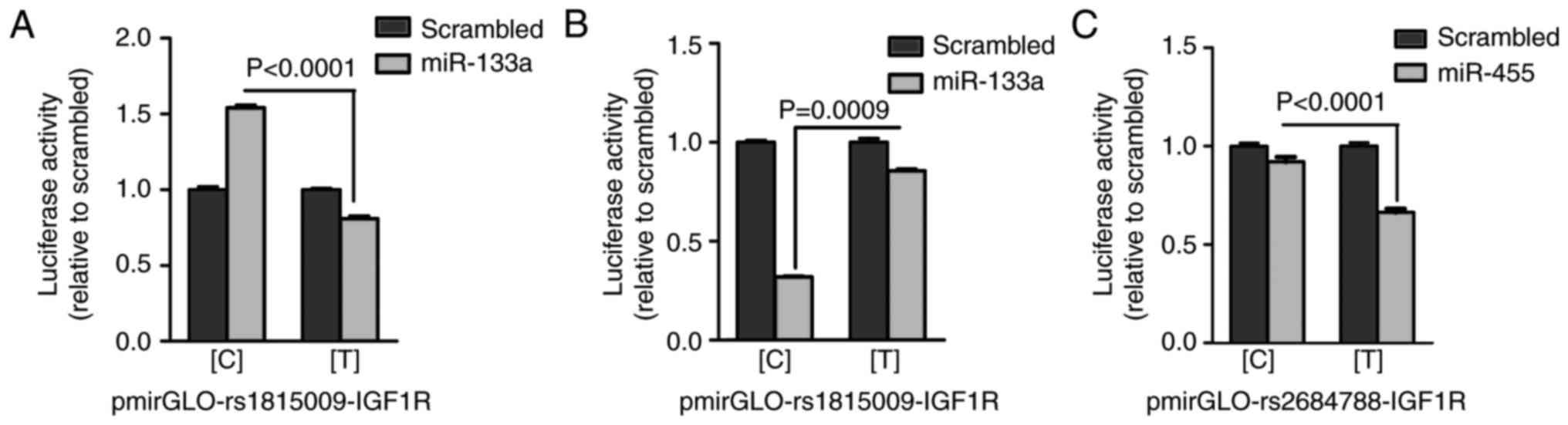
negative control. When miR-133a or the scrambled negative control
was co-transfected with pmirGLO-rs1815009-IGF1R constructs, the
luciferase activity for the C allele was significantly increased
compared with the T allele (Fig.
5A), suggesting a stabilizing rather than inhibitory role,
resulting in stronger binding of miR-133a to the rs1815009-IGF1R C
allele. Conversely, according to the parameter-free thermodynamic
model, it was predicted that the rs1815009-IGF1R C allele may
exhibit a higher binding affinity and decreased miR-133b binding
energy compared with the T allele, which was in agreement with the
results of the luciferase assay. These results suggest a
significantly higher suppressive effect of miR-133b when
interacting with the construct containing the C allele (Fig. 5B). Furthermore, the luciferase assay
demonstrated a statistically significant suppressive effect of
miR-455 on the construct carrying the T allele (Fig. 5C), indicating that miR-455 had a
primary binding efficacy for the rs2684788-IGF1R C allele. Overall,
the results of the in vitro luciferase reporter assays were
consistent with the MFE prediction.
Overexpression of miRNAs affect the
IGF1R mRNA and protein expression levels
To determine the effect of rs1815009 and rs2684788
on the mRNA and protein expression levels of endogenous IGF1R in
vitro, PCa cell lines (PC3 and LNCaP) were transfected with
miRNA precursors of the interacting miRNAs. RT-qPCR analysis
demonstrated that, with the exception of overexpression of miR-133a
in PC3 cells, forced expression of the interacting miRNAs
significantly suppressed IGF1R expression at the transcriptional
level in PCa cells (PC3 and LNCaP) compared with the miR-control
(Fig. 6). In addition, western blot
analysis demonstrated that miR-133b markedly downregulated the
expression of the IGF1R protein in LNCaP cells containing the
rs2684788-IGF1R C allele (Fig. 6D,
right). A similar suppressive effect was observed in PC3 cells that
overexpressed miR-455 (Fig. 6E,
right). Taken together, these results suggest that miR-133b
directly regulates IGF1R gene expression at the mRNA and protein
levels in LNCaP cells, whereas miR-133a regulates IGF1R gene
expression at the mRNA level in LNCaP cells, but not in PC3 cells.
Therefore, the rs2684788 SNP exhibited a functional effect on the
miR-455 binding efficacy in PC3 cells.
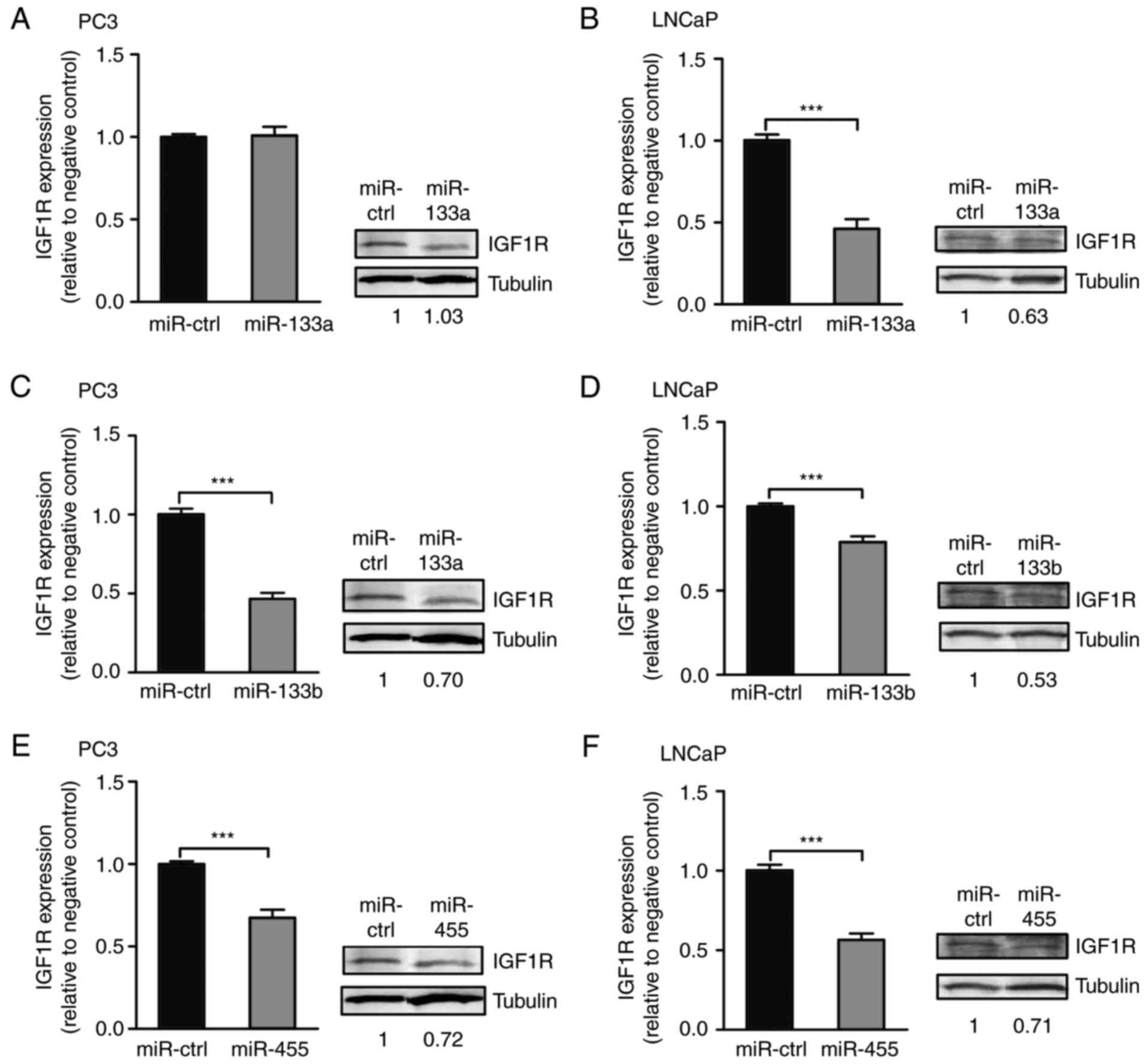 | Figure 6.Effect of miR-133a, miR-133b or
miR-455 overexpression on IGF1R at the mRNA and protein levels.
Results for the RT-qPCR and western blot analyses for miR-133a in
(A) PC3 and (B) LNCaP cells; for miR-133b in (C) PC3 and (D) LNCaP
cells; and miR-4 55 in (E) PC3 and (F) LNCaP cells. RT-qPCR
analysis of IGF1R is represented as relative expression in the
cells transfected with pre-133a, pre-133b, pre-455 or a scrambled
negative control, and the data were analyzed using β-actin as an
endogenous control for normalization. The western blots
demonstrated the IGF1R protein in the cells transfected with
pre-133a, pre-133b, pre-455 or a scrambled negative control. The
numbers represent the relative band intensities, measured by
densitometry analysis and normalized using tubulin as an endogenous
control. Statistical significance was calculated using a Student's
t-test. ***P<0.001. IGF1R, insulin-like growth factor 1
receptor; miR, microRNA; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; ctrl,
control. |
Overexpression of miRNAs affect genes
associated with invasion, migration and apoptosis at the mRNA
levels
In order to analyze the effect of miR-133a, miR-133b
and miR-455 on the expression of genes associated with the
biological activity of tumors, including cell invasion, migration
and apoptosis, miRNA precursors (pre-133a, pre-133b, pre-455 or
scrambled negative control) were transfected into PC3 and LNCaP
cell lines. Expression levels were determined 48 h after
transfection. Matrix metalloproteinase 2 (MMP-2) in transfected
LNCaP cells was demonstrated to be significantly suppressed by
miR-133a, miR-133b and miR-455, and was suppressed by miR-455 in
PC3 cells compared with the miR-control. E-cadherin (CDH1) was
significantly upregulated in LNCaP cells that overexpressed
miR-133a, and in PC3 cells that overexpressed miR-455, but
exhibited mild downregulation in LNCaP cells that overexpressed
miR-455 compared with the miR-control. Forced expression of
miR-133b and miR-455 in LNCaP cells resulted in mild suppression of
vascular endothelial growth factor A (VEGFA), but the
overexpression of miR-133a in LNCaP cells led to a more significant
downregulation at the transcriptional level. B-cell lymphoma-2
(BCL-2) was significant upregulated in LNCaP cells with forced
expression of miR-133a, but a suppressive effect was observed in
post-transfected LNCaP cells that overexpressed miR-133b compared
with the miR-control. These results are presented in Fig. 7.
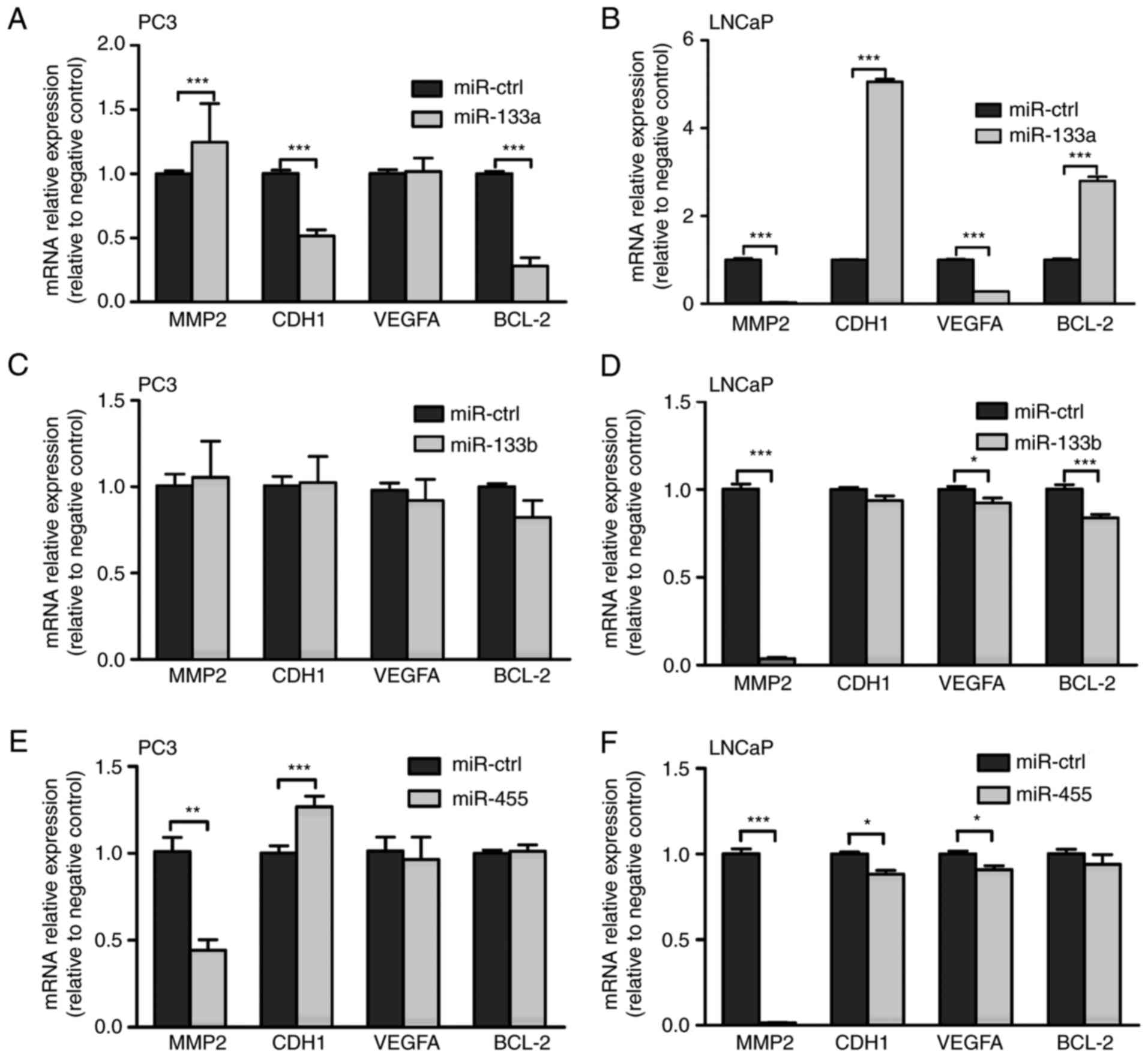 | Figure 7.Effect of miR-133a, miR-133b or
miR-455 overexpression on the transcription of genes associated
with the biological function of tumors. Results from reverse
transcription-quantitative polymerase chain reaction analysis of
MMP2, CDH1, VEGFA, BCL-2 in PC3 cells transfected with (A)
pre-133a, (C) pre-133b and (E) pre-455, and in LNCaP cells
transfected with (B) pre-133a, (D) pre-133b and (F) pre-455, using
GAPDH as an endogenous control for normalization. Statistical
significance was calculated using a Student's t-test. *P<0.05,
**P<0.01 and ***P<0.001. MMP2, matrix metalloproteinase-2;
CDH1, E-cadherin; VEGFA, vascular endothelial growth factor A;
BCL2, B-cell lymphoma-2; GAPDH, glyceraldehyde-phosphate
dehydrogenase; miR, micro-RNA; ctrl, control. |
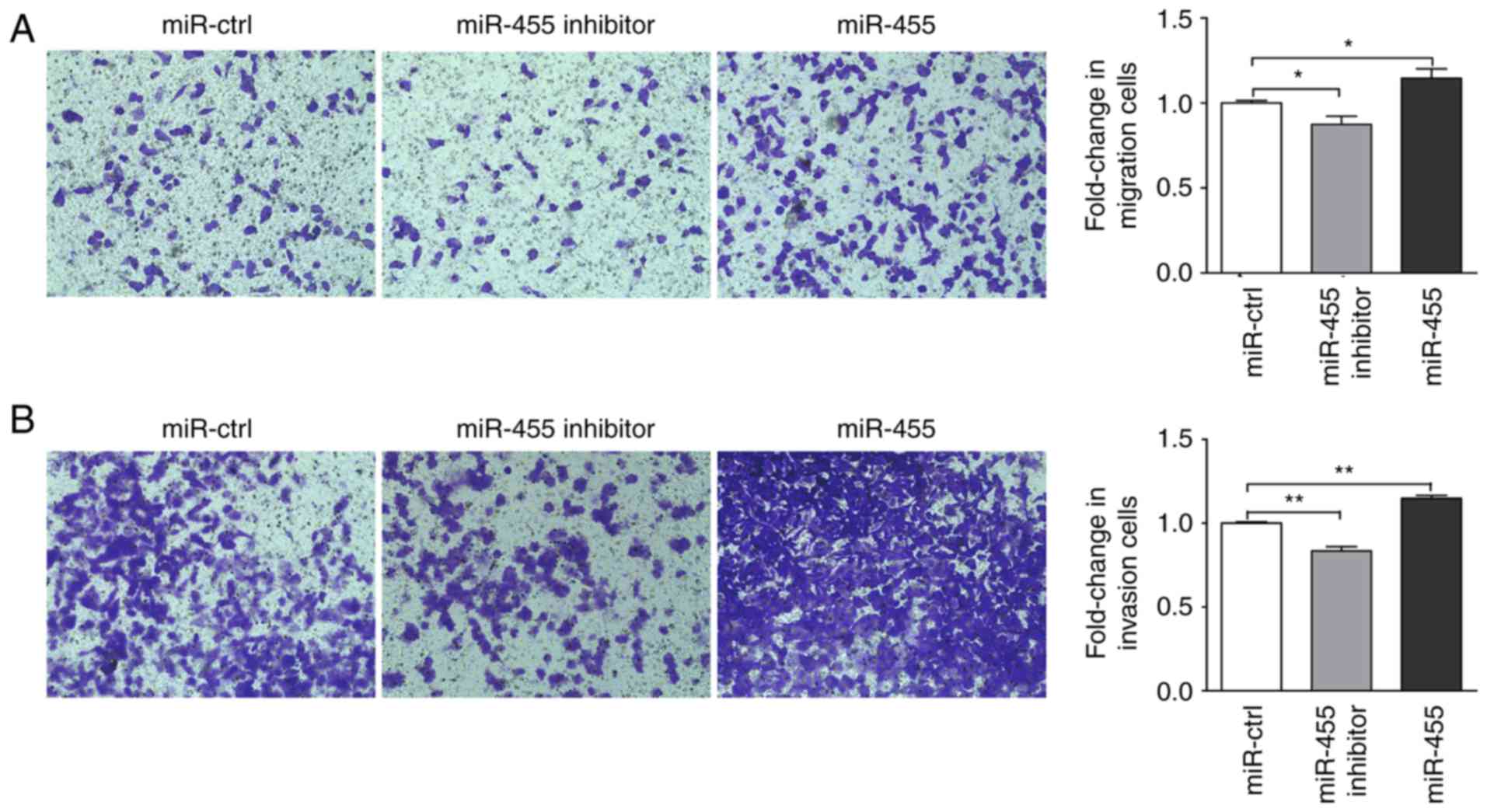
miR-455 enhances cell migration and
invasion in LNCaP cells
To analyze the effect of miR-455 on prostate cells
migration and invasion, post-transfected LNCaP cells were harvested
to a Transwell assay was performed. After 48 h incubation, the
migration rate was significantly increased in LNCaP cells with
miR-455 overexpression, and significantly suppressed in cells that
were treated with miR-455 inhibitor compared with the miR-control
(Fig. 8A). Similarly, after 72 h
incubation, the invasion rate in LNCaP cells with miR-455
overexpression was significantly increased, and significantly
decreased in LNCaP cells treated with miR-455 inhibitor compared
with the miR-control (Fig. 8B).
Discussion
Polymorphisms in miRNA target binding sites affect
the binding efficacy of miRNAs. Consequently, the miRNA binding
affinity for the target may be affected, which may potentially
alter the expression of the target gene, thereby contributing to
the susceptibility for developing certain diseases. Indeed, genetic
polymorphisms that alter miRNA binding have been associated with
disease risk in multiple studies (6,7,9,12,13).
From an association analysis based on the GWAS data of ChinaPCa, we
previously identified two SNPs (rs1815009 and 2684788) in the IGF1R
3′UTR with significant genotype distribution between PCa and
control samples (18). IGF1R serves
an important role in the risk and progression of PCa (16,17).
In the present study, bioinformatics analysis was used, which
revealed that rs1815009 is located near the binding seed regions of
miR-133a and miR-133b, and rs2684788 is located near the binding
seed region of miR-455. In addition, the binding affinity of these
three miRNAs was altered by different alleles, as predicted using a
parameter-free thermodynamic model (21). The expression of miR-133a, miR-133b
and miR-455 from three miRNA profiling datasets in GEO and TCGA
demonstrated a significant difference between PCa and normal
tissues. The in vitro luciferase reporter assays further
confirmed the binding affinity of these three miRNAs, which was
altered by different alleles. In PC3 cells, overexpression of the
three miRNAs resulted in suppression of VEGFA, with the
overexpression of miR-133a leading to the most significant level of
downregulation at the transcriptional level in LNCaP cells.
There are two copies of miR-133a, miR-133a-1 and
miR-133a-2, located on chromosomes 18q11.2 and 20q13.33,
respectively (28). miR-133 has
been demonstrated as a potent suppressor of non-muscle expression
of genes involved in cell fate determination during mouse and human
embryonic stem cell differentiation (30). miR-133a inhibits cell proliferation,
migration and invasion in PCa cells by targeting the epidermal
growth factor receptor (31). In
the present study, miR-133a in PCa tissues demonstrated lower
expression in a large number of samples from GEO and TCGA, and this
result was consistent with a previous report (32). Gong et al (33) reported that miR-133a was
downregulated in gastric cancer, and functions as a tumor
suppressor in vitro and in vivo, partly by repressing
IGF1R. Guo et al (34)
reported that miR-133a overexpression repressed IGF1R 3′UTR
reporter activity, and reduced the mRNA and protein levels of
endogenous IGF1R in ovarian cancer. Other studies have also
suggested that IGF1R is a potential target of miR-133a (35). In the present research, miR-133a was
predicted to bind to the IGF1R ' UTR. It was demonstrated that the
overexpression of miR-133a downregulated the mRNA expression of
IGF1R in LNCaP cells in the current study. The MFE indicated that
the C allele for rs1815009 had greater accessibility for miR-133a
binding compared with the T allele, which was consistent with the
results of luciferase reporter assays. In addition, a
population-based study on the ChinaPCa revealed that the C allele
for rs1815009 was a common variant with a higher frequency among
the cases with PCa, and a lower frequency among the healthy
individuals. This suggests that the rs1815009 C allele is a crucial
risk factor for PCa, via differential regulation of IGF1R with
miR-133a.
miR-133b, located on chromosome 6p12.2 (36), is generally considered to be a
muscle-specific molecule that enhances myoblast differentiation
(37). Although miR-133a and
miR-133b are located on different chromosomes in the human genome,
they have common target genes, including IGF1R, and are merely
distinguished by a single nucleotide at the 3′ end. Previous
studies have demonstrated that miR-133b serves a crucial role in
malignant tumors and non-muscle related diseases (38–40),
including PCa (40). miR-133b
expression was revealed to be significantly lower in colorectal
carcinoma tissues compared with healthy colon tissues and adjacent
non-tumor tissues, modulating cell apoptosis and invasion in
colorectal carcinoma (41). In the
present study, the expression of miR-133b in PCa tissue was lower
compared with that of normal controls in large samples obtained
from GEO and TCGA. miR-133b may serve an important role as a tumor
suppressor gene in osteosarcoma and overexpression of miR-133b has
been reported to decrease the expression of IGF1R (42). miR-133b was predicted to bind to the
IGF1R 3′UTR in the current study. Furthermore, miR-133b was
observed to bind more tightly to the C allele of rs1815009 within
the IGF1R 3′UTR using a thermodynamic model. Subsequently, the
miR-133b target region within the IGF1R 3′UTR and the binding
affinity were validated, in which miR-133b exhibited greater
suppression of the C allele compared with the T allele, as measured
using a dual-luciferase reporter assay. In addition, the
population-based study by ChinaPCa revealed the C allele for
rs1815009 was a common variant with a higher frequency among the
cases with PCa, and exhibited at a lower frequency among the
healthy population. Together with the results of IGF1R expression
at the mRNA and protein levels, these results suggest that the C
allele differentially regulates IGF1R with miR-133b. Therefore, the
C allele for rs1815009 is a key risk factor for PCa through binding
with miR-133a or miR-133b.
miR-455-5p is located on chromosome 6, and
accumulating evidence suggests that miR-455 serves essential roles
in human cancer. Sand et al (43) identified 16 significantly
upregulated miRNAs, including miR-455-5p, in basal cell carcinoma.
Lv et al (41) demonstrated
that miR-455-5p was significantly upregulated in thymic epithelial
tumor tissues. However, miR-455 was reported to be significantly
downregulated in a colon cancer sample, and overexpression of
miR-455 significantly inhibited the proliferation and invasion of
SW480 cells, but had no evident effect on apoptosis (44). Pantaleo et al (45) identified seven miRNAs, including
miR-455-5p that may alter IGF1R expression by targeting the IGF1R
3′UTR in gastrointestinal stromal tumor. The results for IGF1R
expression at the protein level suggested a significant suppressive
effect in PC3 cells containing the IGF1R-rs2684788 T allele, but
not in LNCaP cells carrying the IGF1R-rs2684788 C allele. In
addition, the population-based study of China PCa revealed that the
T allele for rs2684788 was a common variant with a higher frequency
among the cases with PCa, but exhibited at a lower frequency among
the healthy population. Along with the results for IGF1R expression
at the gene and protein levels, suggesting that the T allele of
rs2684788 is a key risk factor for PCa through altered regulation
of IGF1R by miR-455, as observed for prostate carcinoma.
MMP-2, one of the key enzymes involved in
extracellular matrix (ECM) degradation, serves an essential role in
the invasion and metastasis of various cancer types (46,47),
and is significantly associated with the malignancy and metastasis
of PCa (47). CDH1 gene, encoding
the E-cadherin protein, is associated with infiltrative tumor
growth patterns and lymph node metastasis in colorectal cancer
(48). Abnormal CDH1 expression has
been associated with numerous human diseases, including PCa
(49). VEGFA, a key angiogenic
factor, induces endothelial cell proliferation, differentiation and
migration, thus making VEGFA an important molecule in angiogenesis
(50). The oncogene BCL-2, a
repressor of apoptosis, has been reported to be upregulated in
numerous cancer types (51).
Elevated expression of BCL-2 is highly protective against apoptosis
in vitro, resulting in resistance to androgen depletion
in vivo (52). In the
present study, the expression levels of MMP-2 and VEGFA were
downregulated in LNCaP cells that overexpressed miR-133a, miR-133b
or miR-455. Furthermore, miR-133a and miR-133b have been identified
to be associated with the migration and invasion of
androgen-insensitive human PCa cells (31). In the present study, miR-455 was
also demonstrated to serve a role in the migration and invasion of
LNCaP cells that are androgen-sensitive. Notably, MMP2 expression
was regulated by mir-133b and mir-455. The expression level and
activity of MMP2/9 have been revealed to be regulated by ERK1/2 in
293 and monocytic cells (53). In
addition, aberrant miR-455-5p expression is partially regulated by
activated ERK signaling in non-small cell lung cancer (54). It is possible that ERK1/2 regulates
MMP2 by miR-455 in LNCaP cells. Future studies should focus on
identifying drugs, which are able to target this process.
In conclusion, two miRNA binding prediction tools
were used to predict the miRNAs that bind to the IGF1R 3′UTR, and
revealed that rs1815009 (C/T) overlaps miR-133a and miR-133b, and
rs2684788 (C/T) overlaps the predicted miR-455 binding site in the
IGF1R 3′UTR. Predictions from a thermodynamic model for
miRNA-target interaction and the results from the luciferase
reporter assay were integrated to demonstrate that miR-133a and
miR-133b may bind near rs1815009, and miR-455 near rs2684788, in
the IGF1R 3′UTR. In addition, public data analysis revealed that
miR-133a, miR-133b and miR-455 demonstrated significantly lower
expression in PCa tissue in the majority of public datasets.
Furthermore, overexpression of miR-455 significantly increased the
migration and invasion rates in LNCaP cells. The results of the
present study revealed that the association between rs1815009,
rs2684788 and PCa risk involves altered miRNA regulation. It may be
suggested that miR-133a, miR-133b and miR-455 are useful diagnostic
markers for PCa. However, future in vivo studies are
required to confirm the results of the current study.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81272853,
81472414, 81370857, 81360378 and 81460388), the Guangxi Scientific
Research and Technology Development Project (grant nos. 2013BC26299
and 1355005-3-17) and the Guangxi Natural Science Foundation (grant
nos. 2015GXNSFBB139008, 2014GXNSFBA118201 and 2013GXNSFFA019002),
and the Youth Science Foundation of Guangxi Medical University
(grant nos. GXMUYSF201201 and GXMUYSF201603).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HW, WH, YaH and QZ designed the study; HW, WH, XY
and YuH performed the experiments; HW, ZM, YaH, WL and YG analyzed
the data; and HW, XY, YaH and ZM wrote the manuscript. All authors
reviewed and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bratt O: What should a urologist know
about hereditary predisposition to prostate cancer? BJU Int.
99:743–748. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stegeman S, Amankwah E, Klein K, O'Mara
TA, Kim D, Lin HY, Permuth-Wey J, Sellers TA, Srinivasan S, Eeles
R, et al: A large-scale analysis of genetic variants within
putative miRNA binding sites in prostate cancer. Cancer Discov.
5:368–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang L, Liu Y, Song F, Zheng H, Hu L, Lu
H, Liu P, Hao X, Zhang W and Chen K: Functional SNP in the
microRNA-367 binding site in the 3′UTR of the calcium channel
ryanodine receptor gene 3 (RYR3) affects breast cancer risk and
calcification. Proc Natl Acad Sci USA. 108:13653–13658. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Minguzzi S, Selcuklu SD, Spillane C and
Parle-McDermott A: An NTD-associated polymorphism in the 3′UTR of
MTHFD1L can affect disease risk by altering miRNA binding. Hum
Mutat. 35:96–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng CJ, Bahal R, Babar IA, Pincus Z,
Barrera F, Liu C, Svoronos A, Braddock DT, Glazer PM, Engelman DM,
et al: MicroRNA silencing for cancer therapy targeted to the tumour
microenvironment. Nature. 518:107–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nicoloso MS, Sun H, Spizzo R, Kim H,
Wickramasinghe P, Shimizu M, Wojcik SE, Ferdin J, Kunej T, Xiao L,
et al: Single-nucleotide polymorphisms inside microRNA target sites
influence tumor susceptibility. Cancer Res. 70:2789–2798. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chandradoss SD, Schirle NT, Szczepaniak M,
MacRae IJ and Joo C: Dynamic search process underlies MicroRNA
targeting. Cell. 162:96–107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L, Zhang S, Yao J, Lowery FJ, Zhang
Q, Huang WC, Li P, Li M, Wang X, Zhang C, et al:
Microenvironment-induced PTEN loss by exosomal microRNA primes
brain metastasis outgrowth. Nature. 527:100–104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuosmanen SM, Viitala S, Laitinen T,
Peräkylä M, Pölönen P, Kansanen E, Leinonen H, Raju S,
Wienecke-Baldacchino A, Närvänen A, et al: The effects of sequence
variation on genome-wide NRF2 binding-new target genes and
regulatory SNPs. Nucleic Acids Res. 44:1760–1775. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Niu T, Liu N, Zhao M, Xie G, Zhang L, Li
J, Pei YF, Shen H, Fu X, He H, et al: Identification of a novel
FGFRL1 MicroRNA target site polymorphism for bone mineral density
in meta-analyses of genome-wide association studies. Hum Mol Genet.
24:4710–4727. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ullrich A, Gray A, Tam AW, Yang-Feng T,
Tsubokawa M, Collins C, Henzel W, Le Bon T, Kathuria S, Chen E, et
al: Insulin-like growth factor I receptor primary structure:
Comparison with insulin receptor suggests structural determinants
that define functional specificity. EMBO J. 5:2503–2512. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ofer P, Heidegger I, Eder IE, Schöpf B,
Neuwirt H, Geley S, Klocker H and Massoner P: Both IGF1R and INSR
knockdown exert antitumorigenic effects in prostate cancer in vitro
and in vivo. Mol Endocrinol. 29:1694–1707. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Huang Y, Christie A, Bowden M, Lee
GS, Kantoff PW and Sweeney CJ: Cabozantinib inhibits abiraterone's
upregulation of IGFIR phosphorylation and enhances its
anti-prostate cancer activity. Clin Cancer Res. 21:5578–5587. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Madan RA and Dahut WL: Prostate cancer:
Charting a course in metastatic castration-sensitive prostate
cancer. Nat Rev Urol. 12:368–369. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Y, Xin X, Li J, Xu J, Yu X, Li T, Mo
Z and Hu Y: RTK/ERK pathway under natural selection associated with
prostate cancer. PLoS One. 8:e782542013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:2015.doi: 10.7554/eLife.05005. View Article : Google Scholar
|
|
20
|
Betel D, Koppal A, Agius P, Sander C and
Leslie C: Comprehensive modeling of microRNA targets predicts
functional non-conserved and non-canonical sites. Genome Biol.
11:R902010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mathews DH, Disney MD, Childs JL,
Schroeder SJ, Zuker M and Turner DH: Incorporating chemical
modification constraints into a dynamic programming algorithm for
prediction of RNA secondary structure. Proc Natl Acad Sci USA.
101:7287–7292. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Turner DH and Mathews DH: NN DB The
nearest neighbor parameter database for predicting stability of
nucleic acid secondary structure. Nucleic Acids Res. 38:D280–D282.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kertesz M, Iovino N, Unnerstall U, Gaul U
and Segal E: The role of site accessibility in microRNA target
recognition. Nat Genet. 39:1278–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barrett T, Troup DB, Wilhite SE, Ledoux P,
Rudnev D, Evangelista C, Kim IF, Soboleva A, Tomashevsky M,
Marshall KA, et al: NCBI GEO: Archive for high-throughput
functional genomic data. Nucleic Acids Res. 37:D885–D890. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin HH, Liao CJ, Lee YC, Hu KH, Meng HW
and Chu ST: Lipocalin-2-induced cytokine production enhances
endometrial carcinoma cell survival and migration. Int J Biol Sci.
7:74–86. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin PC, Chiu YL, Banerjee S, Park K,
Mosquera JM, Giannopoulou E, Alves P, Tewari AK, Gerstein MB,
Beltran H, et al: Epigenetic repression of miR-31 disrupts androgen
receptor homeostasis and contributes to prostate cancer
progression. Cancer Res. 73:1232–1244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ambs S, Prueitt RL, Yi M, Hudson RS, Howe
TM, Petrocca F, Wallace TA, Liu CG, Volinia S, Calin GA, et al:
Genomic profiling of microRNA and messenger RNA reveals deregulated
microRNA expression in prostate cancer. Cancer Res. 68:6162–6170.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wach S, Nolte E, Szczyrba J, Stöhr R,
Hartmann A, Ørntoft T, Dyrskjøt L, Eltze E, Wieland W, Keck B, et
al: MicroRNA profiles of prostate carcinoma detected by
multiplatform microRNA screening. Int J Cancer. 130:611–621. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ivey KN, Muth A, Arnold J, King FW, Yeh
RF, Fish JE, Hsiao EC, Schwartz RJ, Conklin BR, Bernstein HS, et
al: MicroRNA regulation of cell lineages in mouse and human
embryonic stem cells. Cell Stem Cell. 2:219–229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tao J, Wu D, Xu B, Qian W, Li P, Lu Q, Yin
C and Zhang W: microRNA-133 inhibits cell proliferation, migration
and invasion in prostate cancer cells by targeting the epidermal
growth factor receptor. Oncol Rep. 27:1967–1975. 2012.PubMed/NCBI
|
|
32
|
Kojima S, Chiyomaru T, Kawakami K, Yoshino
H, Enokida H, Nohata N, Fuse M, Ichikawa T, Naya Y, Nakagawa M, et
al: Tumour suppressors miR-1 and miR-133a target the oncogenic
function of purine nucleoside phosphorylase (PNP) in prostate
cancer. Br J Cancer. 106:405–413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gong Y, Ren J, Liu K and Tang LM: Tumor
suppressor role of miR-133a in gastric cancer by repressing IGF1R.
World J Gastroenterol. 21:2949–2958. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Guo J, Xia B, Meng F and Lou G: miR-133a
suppresses ovarian cancer cell proliferation by directly targeting
insulin-like growth factor 1 receptor. Tumour Biol. 35:1557–1564.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang W, Liu K, Liu S, Ji B, Wang Y and
Liu Y: MicroRNA-133a functions as a tumor suppressor by targeting
IGF-1R in hepatocellular carcinoma. Tumour Biol. 36:9779–9788.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mitchelson KR and Qin WY: Roles of the
canonical myomiRs miR-1, −133 and −206 in cell development and
disease. World J Biol Chem. 6:162–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rao PK, Kumar RM, Farkhondeh M,
Baskerville S and Lodish HF: Myogenic factors that regulate
expression of muscle-specific microRNAs. Proc Natl Acad Sci USA.
103:8721–8726. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu L, Shao X, Gao W, Zhang Z, Liu P, Wang
R, Huang P, Yin Y and Shu Y: MicroRNA-133b inhibits the growth of
non-small-cell lung cancer by targeting the epidermal growth factor
receptor. FEBS J. 279:3800–3812. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Duan FT, Qian F, Fang K, Lin KY, Wang WT
and Chen YQ: miR-133b, a muscle-specific microRNA, is a novel
prognostic marker that participates in the progression of human
colorectal cancer via regulation of CXCR4 expression. Mol Cancer.
12:1642013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Karatas OF, Guzel E, Suer I, Ekici ID,
Caskurlu T, Creighton CJ, Ittmann M and Ozen M: miR-1 and miR-133b
are differentially expressed in patients with recurrent prostate
cancer. PLoS One. 9:e986752014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lv LV, Zhou J, Lin C, Hu G, Yi LU, DU J,
Gao K and Li X: DNA methylation is involved in the aberrant
expression of miR-133b in colorectal cancer cells. Oncol Lett.
10:907–912. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhao H, Li M, Li L, Yang X, Lan G and
Zhang Y: MiR-133b is down-regulated in human osteosarcoma and
inhibits osteosarcoma cells proliferation, migration and invasion,
and promotes apoptosis. PLoS One. 8:e835712013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sand M, Skrygan M, Sand D, Georgas D, Hahn
SA, Gambichler T, Altmeyer P and Bechara FG: Expression of
microRNAs in basal cell carcinoma. Br J Dermatol. 167:847–855.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hu J, Xu Y and Cai S: Specific microRNAs
as novel biomarkers for combination chemotherapy resistance
detection of colon adenocarcinoma. Eur J Med Res. 20:952015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pantaleo MA, Ravegnini G, Astolfi A,
Simeon V, Nannini M, Saponara M, Urbini M, Gatto L, Indio V,
Sammarini G, et al: Integrating miRNA and gene expression profiling
analysis revealed regulatory networks in gastrointestinal stromal
tumors. Epigenomics. 8:1347–1366. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Dufour A, Sampson NS, Zucker S and Cao J:
Role of the hemopexin domain of matrix metalloproteinases in cell
migration. J Cell Physiol. 217:643–651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kuniyasu H, Ukai R, Johnston D, Troncoso
P, Fidler IJ and Pettaway CA: The relative mRNA expression levels
of matrix metalloproteinase to E-cadherin in prostate biopsy
specimens distinguishes organ-confined from advanced prostate
cancer at radical prostatectomy. Clin Cancer Res. 9:2185–2194.
2003.PubMed/NCBI
|
|
48
|
Kim SA, Inamura K, Yamauchi M, Nishihara
R, Mima K, Sukawa Y, Li T, Yasunari M, Morikawa T, Fitzgerald KC,
et al: Loss of CDH1 (E-cadherin) expression is associated with
infiltrative tumour growth and lymph node metastasis. Br J Cancer.
114:199–206. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bonilla C, Mason T, Long L, Ahaghotu C,
Chen W, Zhao A, Coulibaly A, Bennett F, Aiken W, Tullock T, et al:
E-cadherin polymorphisms and haplotypes influence risk for prostate
cancer. Prostate. 66:546–556. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yip KW and Reed JC: Bcl-2 family proteins
and cancer. Oncogene. 27:6398–6406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Raffo AJ, Perlman H, Chen MW, Day ML,
Streitman JS and Buttyan R: Overexpression of bcl-2 protects
prostate cancer cells from apoptosis in vitro and confers
resistance to androgen depletion in vivo. Cancer Res.
55:44338–4445. 1995.
|
|
53
|
Huang WC, Chan SH, Jang TH, Chang JW, Ko
YC, Yen TC, Chiang SL, Chiang WF, Shieh TY, Liao CT, et al:
miRNA-491-5p and GIT1 serve as modulators and biomarkers for oral
squamous cell carcinoma invasion and metastasis. Cancer Res.
74:751–764. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang J, Wang Y, Sun D, Bu J, Ren F, Liu B,
Zhang S, Xu Z, Pang S and Xu S: miR-455-5p promotes cell growth and
invasion by targeting SOCO3 in non-small cell lung cancer.
Oncotarget. 8:114956–114965. 2017.PubMed/NCBI
|