Introduction
Prostate cancer (PCa) is one of the most common
cancers and the second leading cause of cancer-related deaths in
men worldwide (1). Although there
has been substantial progress in the treatment of primary PCa,
distant bone metastasis remains incurable, due to the lack of
effective therapeutic avenues, contributing to the mortality of PCa
(2). Therefore, exploring a novel
marker to predict bone metastasis of PCa will facilitate the
development of antimetastatic therapeutic strategies against
PCa.
miRNAs are a class of small non-coding regulatory
RNAs that mechanistically function by binding to the 3′
untranslated region (3′UTR) of downstream mRNAs, giving rise to
mRNA degradation or repression of translation (3,4), and
play crucial roles in many biological processes, including cell
apoptosis, proliferation and differentiation (3). Numerous studies have demonstrated that
aberrant expression of miRNAs is implicated in the progression and
metastasis of several human cancers (5–9).
Furthermore, several miRNAs have been identified as critical
mediators of bone metastasis in human cancer (10,11),
holding promise as a potential predictive factor for bone
metastasis. Our previous studies demonstrated that downregulation
of miR-145 by loss of wild-type p53 in PC-3 cells promoted bone
metastasis of PCa by regulating several positive regulators of EMT,
including ZEB2 and HEF1 (12–14).
Therefore, it is imperative to discern a novel miRNA to predict
bone metastasis of PCa.
In the present study, we found no evident difference
in miR-505-3p expression in PCa tissues compared with that in
adjacent normal tissues. Notably, miR-505-3p was significantly
reduced in bone metastatic PCa tissues, and miR-505-3p expression
was inversely associated with poor clinicopathological
characteristics in PCa patients. More importantly, low expression
of miR-505-3p was positively associated with shorter bone
metastasis-free survival in PCa patients, but was not associated
with overall survival of PCa patients. Our results further revealed
that upregulation of miR-505-3p inhibited TGF-β signaling activity
by targeting SMAD2 and SMAD3, which further inhibited the invasion
and migration abilities of PCa cells. Therefore, our results
demonstrated that downregulation of miR-505-3p was associated with
poor bone metastasis-free survival in PCa, suggesting that
miR-505-3p may be used as a novel marker to predict bone metastasis
of PCa.
Materials and methods
Cell lines and cell culture
Human RWPE-1, DU145, LNCaP, 22RV1, PC-3 and VCaP
cells were obtained from the American Type Culture Collection
(ATCC; American Type Culture Collection, Manassas, VA, USA) and
cultured according to the manufacturer's instructions. The C4-2B
cell line was purchased from MD Anderson Cancer Center (Houston,
TX, USA). RWPE-1 cells were grown in defined keratinocyte-SFM (1X)
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
LNCaP, 22RV1, C4-2B and PC-3 cells were grown in RPMI-1640 medium
supplemented with 10% FBS, while DU145 and VCaP cells were grown in
Dulbecco's modified Eagle's medium (all from Invitrogen; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS. All cells were
incubated at 37°C in a humidified atmosphere with 5%
CO2.
Patients and tumor tissues
The 127 archived PCa tissues, including 81 non-bone
metastatic PCa tissues and 46 bone metastatic PCa tissues were
obtained during surgery or needle biopsy at Jiangmen Central
Hospital (Guangzhou, China). Patients were diagnosed based on
clinical and pathological evidence, and the specimens were
immediately snap-frozen and stored in liquid nitrogen tanks. For
the use of these clinical materials for research purposes, prior
informed consents from patients and approval from the Institutional
Research Ethics Committee were obtained from Jiangmen Central
Hospital (Jiangmen, China). The clinicopathological features of the
patients are presented in Table
I.
 | Table I.The relationship between the
expression level of miR-505-3p and the clinicopathological
characteristics in 127 PCa patients. |
Table I.
The relationship between the
expression level of miR-505-3p and the clinicopathological
characteristics in 127 PCa patients.
|
|
| miR-505-3p
expression |
|
|---|
|
|
|
|
|
|---|
| Parameters | Number of
cases | Low | High | P-values |
|---|
| Age (years) |
|
≤72 | 58 | 29 | 29 | 0.935 |
|
>72 | 69 | 35 | 34 |
|
| T
classification |
|
T1-T2 | 50 | 28 | 22 | 0.309 |
|
T3-T4 | 77 | 36 | 41 |
|
| N
classification |
| N0 | 93 | 38 | 55 | <0.001 |
| N1 | 34 | 26 | 8 |
|
| M
classification |
| M0 | 78 | 18 | 60 | <0.001 |
| M1 | 49 | 46 | 3 |
|
| Gleason score |
| ≤7 | 48 | 15 | 33 | <0.01 |
|
>7 | 79 | 49 | 30 |
|
| Serum PSA at
diagnosis, µg/ml |
|
<77.6 | 52 | 20 | 32 | <0.05 |
|
>77.6 | 75 | 44 | 31 |
|
| BM status |
|
nBM | 81 | 24 | 57 | <0.001 |
| BM | 46 | 40 | 6 |
|
RNA extraction, reverse transcription,
and real-time PCR
Total RNA from tissues or cells was extracted using
TRIzol (Life Technologies; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Messenger RNA (mRNA)
and miRNA were reverse-transcribed from total mRNA using the Revert
Aid First Strand cDNA Synthesis kit (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Real-time PCR was
performed according to a standard method, as previously described
(15). The list of primers used is
presented in Table II. Primers for
U6 and miR-505-3p were synthesized and purified by Guangzhou
RiboBio Co., Ltd. U6 or glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) was used as an endogenous control for miRNA or mRNA,
respectively. Relative fold expression was calculated using the
comparative threshold cycle (2−∆∆Cq) method (16).
 | Table II.List of primers used for real-time
RT-PCR. |
Table II.
List of primers used for real-time
RT-PCR.
| Primers |
|
|---|
| CTGF | F:
GCTACCACATTTCCTACCTAGAAATCA |
|
| R:
GACAGTCCGTCAAAACAGATTGTT |
| PTHRP | F:
ACTCGCTCTGCCTGGTTAGA |
|
| R:
GGAGGTGTCAGACAGGTGGT |
| IL11 | F:
TGAAGACTCGGCTGTGACC |
|
| R:
CCTCACGGAAGGACTGTCTC |
| SMAD2 | F:
CACGCTAGGAAAACAGCCTC |
|
| R:
TCGGAAGAGGAAGGAACAAA |
| SMAD3 | F:
CGGCAGTAGATGACATGAGG |
|
| R:
TCAACACCAAGTGCATCACC |
| GAPDH | F:
ATTCCACCCATGGCAAATTC |
|
| R:
TGGGATTTCCATTGATGACAAG |
Plasmid, small interfering RNA and
transfection
The (CAGAC) 12/pGL3 TGF-β/Smad-responsive luciferase
reporter plasmid and control plasmids (cat. no. 32177; Clontech;
Takara Bio, Inc., Tokyo, Japan) were used to quantitatively assess
the transcriptional activity of TGF-β signaling components. The
3′UTR regions of SMAD2 and SMAD3 were PCR-amplified from genomic
DNA and cloned into the pmirGLO luciferase reporter vector (Promega
Corporation, Madison, WI, USA). The miR-505-3p mimics were obtained
from Guangzhou RiboBio Co., Ltd. Transfection of siRNAs and
plasmids was performed using Lipofectamine 3000 (Life Technologies;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions.
Invasion and migration assays
Migration and invasion were performed using
Transwell chambers consisting of 8-mm membrane filter inserts
(Corning Incorporated, Corning, NY, USA) coated with or without
Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) as previously
described (17).
Luciferase assay
Cells (4×104) were seeded in triplicate
in 24-well plates and cultured for 24 h as previously described
(18). Cells were transfected with
250 ng pTGF-β/Smad reporter luciferase plasmid, or
pmirGLO-SMAD2-3′UTR and -SMAD3-3′UTR luciferase plasmid, plus 5 ng
pRL-TK Renilla plasmid (Promega Corporation) using
Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Luciferase and
Renilla signals were assessed 36 h after transfection using
a Dual-Luciferase Reporter Assay kit (Promega Corporation)
according to the manufacturer's protocol.
RNA immunoprecipitation
Cells were co-transfected with HA-Ago2 (cat. no.
10822; Addgene, Inc., Cambridge, MA, USA), followed by HA-Ago2
immunoprecipitation using an HA-antibody (1:1,000; cat. no. H3663;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), as previously
described (19). Real-time PCR
analysis of the IP material was used to assess the association of
SMAD2 and SMAD3 mRNA with the RISC complex.
Western blotting
Western blotting was performed according to a
standard method, as previously described (20). Proteins were visualized using ECL
reagents (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Antibodies against SMAD2 (cat. no. 5339), SMAD3 (cat. no.
9523), pSMAD2/3 (cat. no. 9510) and SMAD2/3 (cat. no. 8685) were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA),
and p84 (cat. no. PA5-27816) was obtained from Invitrogen; Thermo
Fisher Scientific, Inc. All antibodies aforementioned were diluted
1:1,000. The membranes were stripped and reprobed with an
anti-α-tubulin antibody (1:5,000; cat. no. ab7291, Abcam,
Cambridge, UK) as the loading control.
Generation and analysis of TCGA
datasets and Gene Set Enrichment Analysis (GSEA)
The miRNA expression levels and clinical profile
datasets from PCa patients were downloaded from The Cancer Genome
Atlas (TCGA; http://tcga-data.nci.nih.gov/tcga/). The log2 values
of miRNAs in each sample were analyzed using Excel 2010 and
GraphPad 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA).
The miRNA expression levels of all PCa tissues were statistically
analyzed using paired t-tests or unpaired t-tests. The miRNA
expression levels in each sample were analyzed as previously
described (21). Briefly the high
and low expression levels of miR-505-3p were stratified by the
medium expression level of miR-505-3p in PCa tissues. Gene set
enrichment analysis was performed using Molecular Signatures
Database (MSigDB) v5.2.
TargetScan and miRanda analysis
TargetScan (http://www.targetscan.org/vert_71/) and miRanda
(http://34.236.212.39/microrna/home.do) datasets were
analyzed as previously described respectively (22,23).
Statistical analysis
All values are presented as the means ± standard
deviation (SD). Significant differences were determined using
GraphPad 5.0 software. Student's t-test was used to determine
significant differences between two groups. The chi-square test was
used to analyze the relationship between miR-505-3p expression and
clinicopathological characteristics. Survival curves were plotted
using the Kaplan Meier method and compared by log-rank test.
P<0.05 was considered to indicate a statistically significant
difference. All experiments were repeated three times.
Results
miR-505-3p is downregulated in bone
metastatic PCa tissues
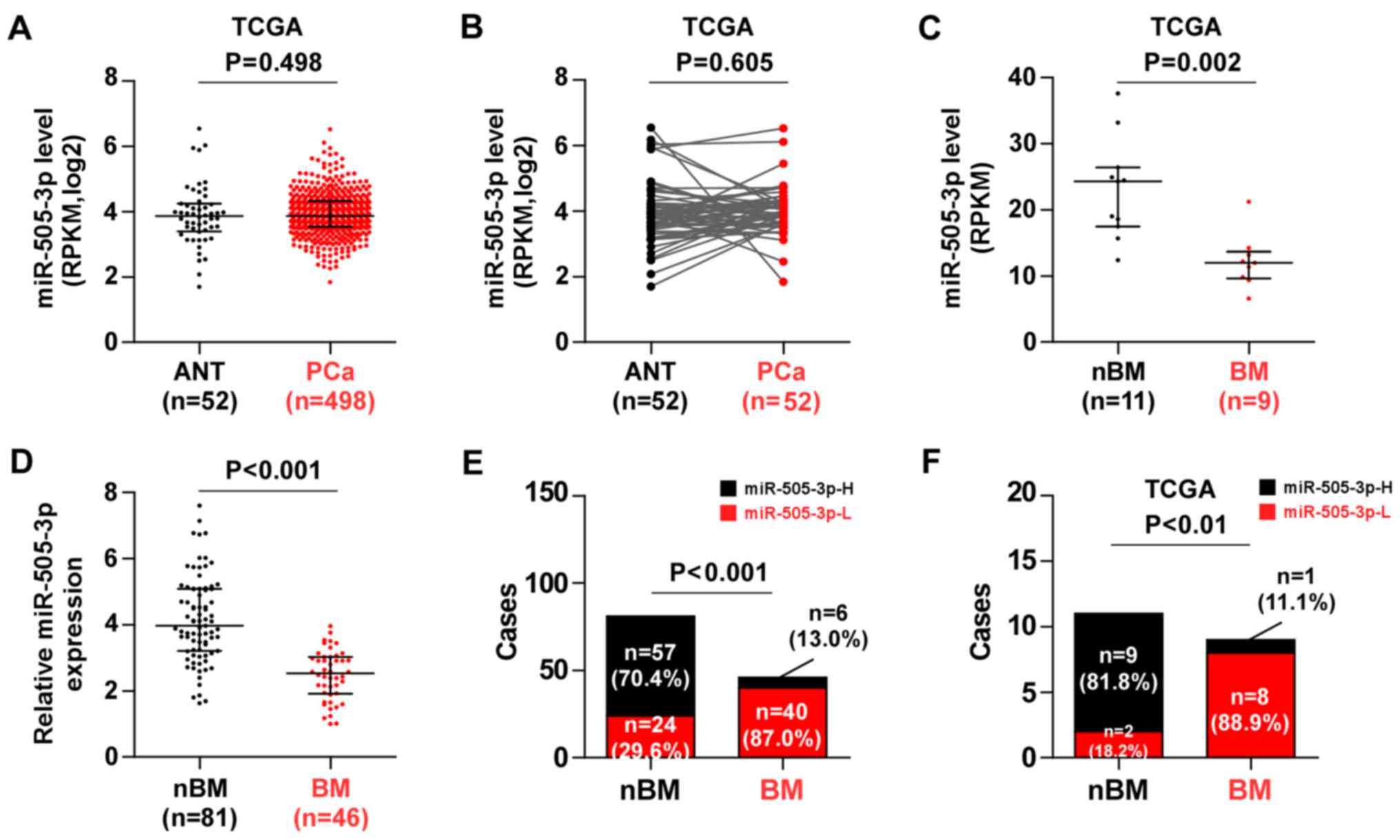
To investigate the aberrant miRNA expression between
bone metastatic PCa tissues and non-bone metastatic PCa tissues, we
analyzed the miRNA sequencing datasets of PCa tissues from TCGA. We
found that that there was no significant difference in miR-505-3p
expression between 498 PCa tissues and 52 adjacent normal tissues
(ANT) (Fig. 1A), or between 52
paired PCa tissues compared with the expression in the matched ANT
(Fig. 1B). Notably, miR-505-3p
expression was significantly downregulated in bone metastatic PCa
tissues compared with the expression in non-bone metastatic PCa
tissues (Fig. 1C). The miR-505-3p
expression in our 81 non-bone metastatic PCa tissues and 46 bone
metastatic PCa tissues was further validated via real-time PCR and
the results revealed that miR-505-3p expression was significantly
downregulated in bone metastatic PCa tissues compared with the
expression in non-bone metastatic PCa tissues (Fig. 1D). The percentage of low miR-505-3p
expression was much higher in bone metastatic PCa tissues compared
to that in non-bone metastatic PCa tissues (Fig. 1E), which was consistent with the
results of TCGA (Fig. 1F).
Therefore, these results indicated that downregulation of
miR-505-3p was involved in bone metastasis of PCa.
Low expression of miR-505-3p is
ssociated with advanced clinicopathological features and poor bone
metastasis-free survival in PCa patients
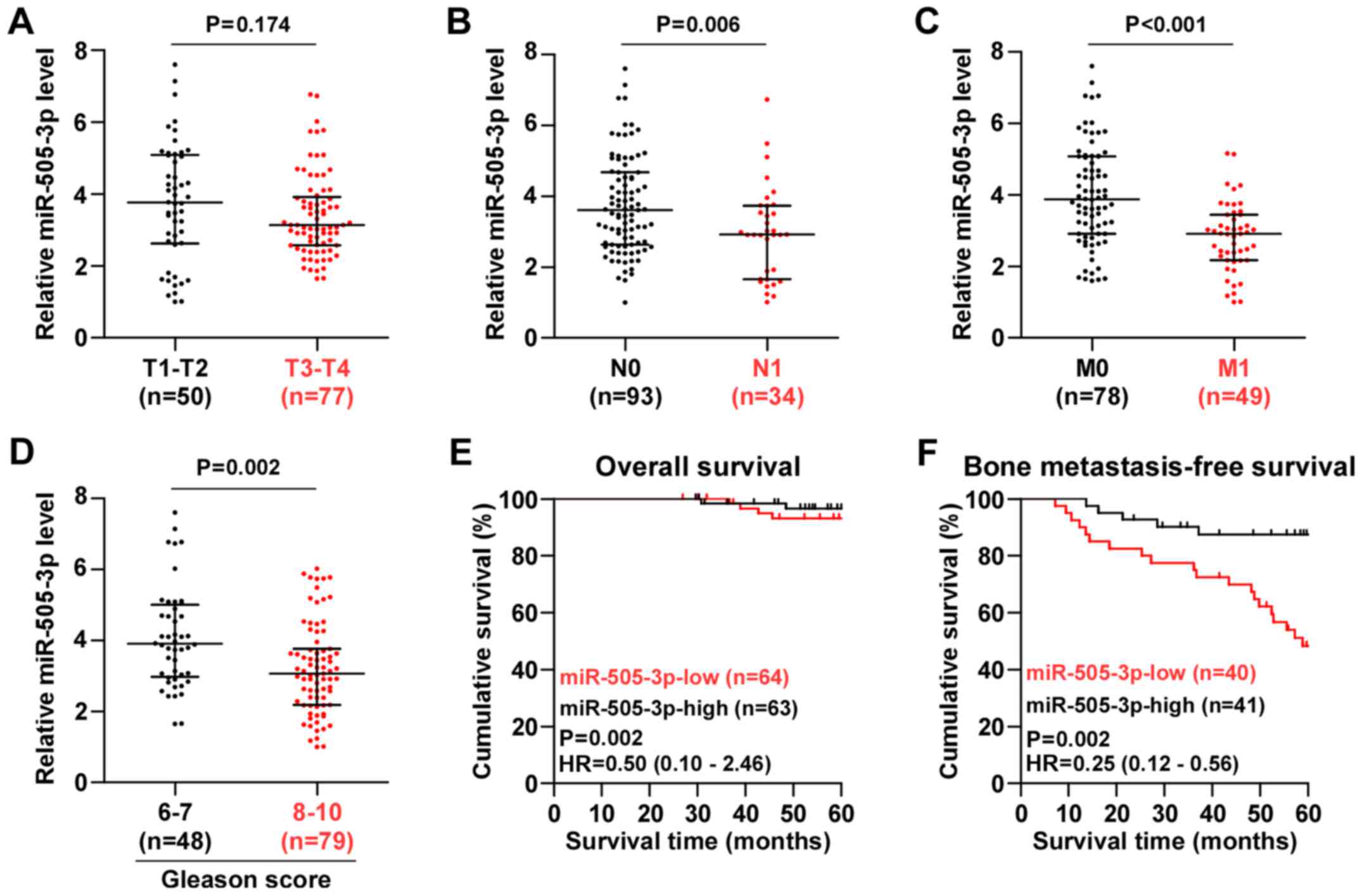
We further investigated the clinical association of
miR-505-3p expression with clinicopathological characteristics in
PCa patients and found that miR-505-3p expression was downregulated
in N1, M1 or Gleason grade (>7) PCa tissues, but not in T3-T4
PCa tissues (Fig. 2A-D).
Statistical analysis of PCa tissue samples revealed that miR-505-3p
expression was inversely associated with serum PSA levels, Gleason
grade, N classification, M classification and bone metastasis
status in PCa patients (Table I).
Kaplan Meier analysis revealed that miR-505-3p expression levels
had no effect on the overall 5-year survival in PCa patients
(Fig. 2E); whereas low expression
of miR-505-3p was strongly and positively associated with shorter
bone metastasis-free survival in PCa patients (Fig. 2F). Thus, our results demonstrated
that downregulation of miR-505-3p predicted poor bone
metastasis-free survival in PCa patients.
Upregulation of miR-505-3p inhibits
invasion and migration in PCa cells
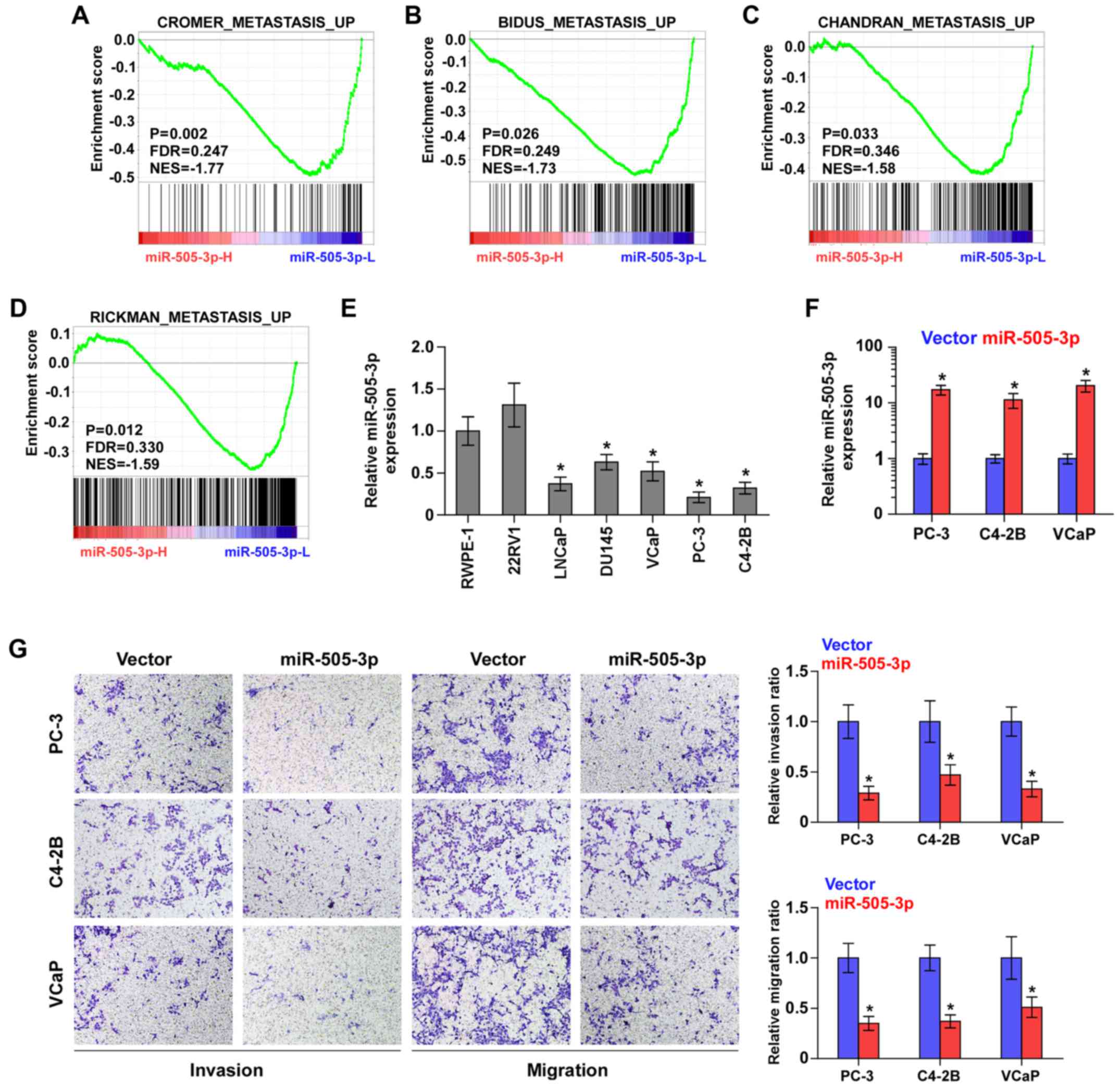
To determine the biological role of miR-505-3p in
bone metastasis of PCa, we performed gene set enrichment (GSEA)
analysis of miR-505-3p expression against the oncogenic signatures
collection of MSigDB, and the results revealed that downregulation
of miR-505-3p was significantly and positively associated with
metastatic propensity (Fig. 3A-D).
Therefore, we further examined whether miR-505-3p has an effect on
the invasion and migration of PCa cells. We first examined
miR-505-3p expression in normal prostate cells (RWPE-1) and 6 PCa
cell lines and found that miR-505-3p expression was differentially
downregulated in metastatic cell lines, including lymph node
metastatic cell line LNCaP, brain metastatic PCa cells DU145 and
bone metastatic PCa cell lines (PC-3, C4-2B and VCaP), particularly
in PC-3 and C4-2B, but slightly elevated in primary PCa 22RV1 cells
(Fig. 3E). This finding further
elucidated the pro-metastatic roles of miR-505-3p downregulation in
PCa. According to the expression levels of miR-505-3p shown in
Fig. 3E, we upregulated miR-505-3p
in bone metastatic PC-3, C4-2B and VCaP cells via transfection with
miR-505-3p mimics to further evaluate the effects of miR-505-3p on
the invasion and migration of PCa cells (Fig. 3F). As shown in Fig. 3G, miR-505-3p overexpression
significantly decreased the invasive and migratory abilities of PCa
cells. Therefore, these results indicated that low expression of
miR-505-3p was implicated in bone metastasis of PCa cells via
promotion of the invasion and migration abilities of PCa cells.
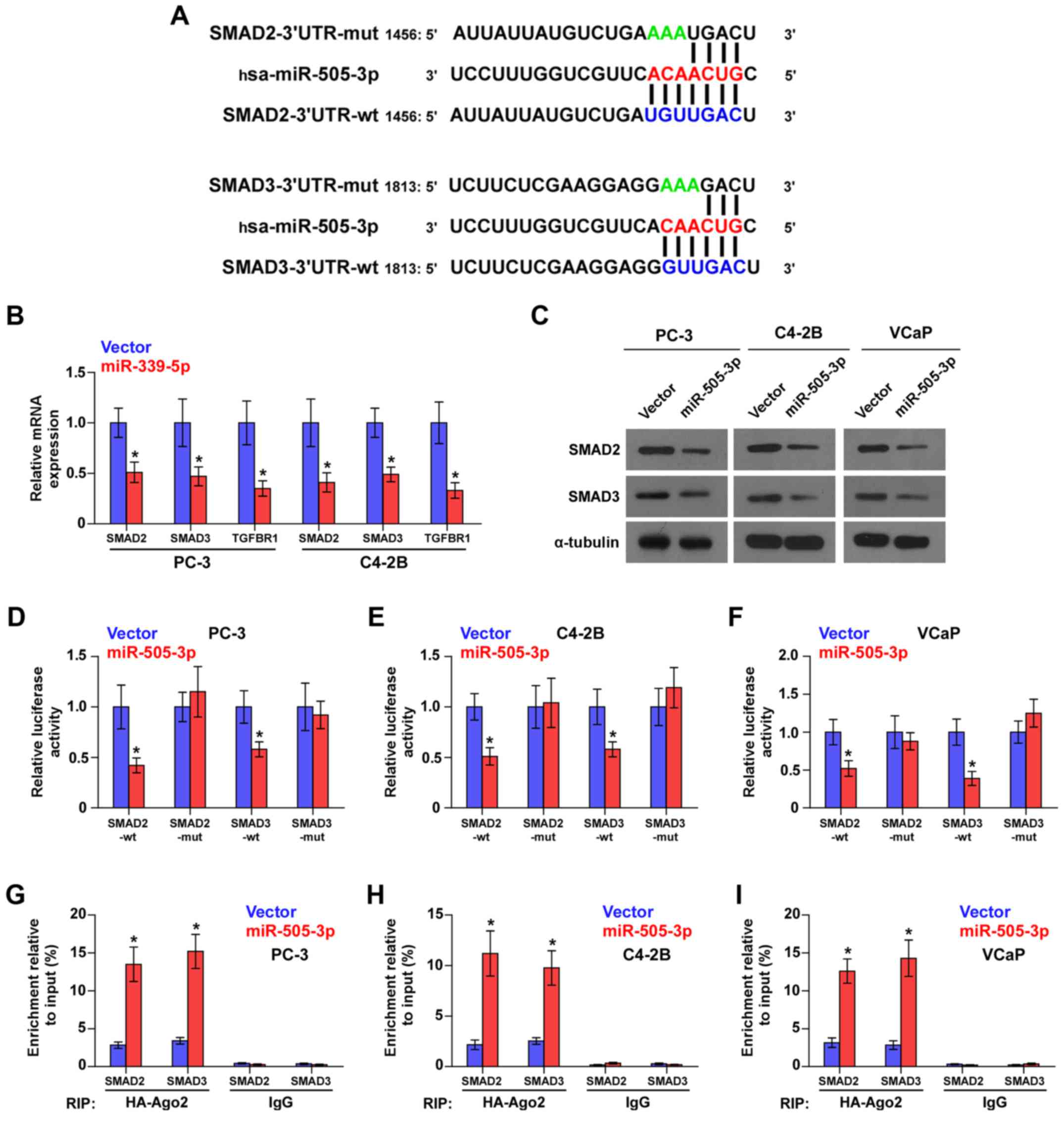
miR-505-3p inhibits TGF-β signaling
activity by targeting SMAD2 and SMAD3
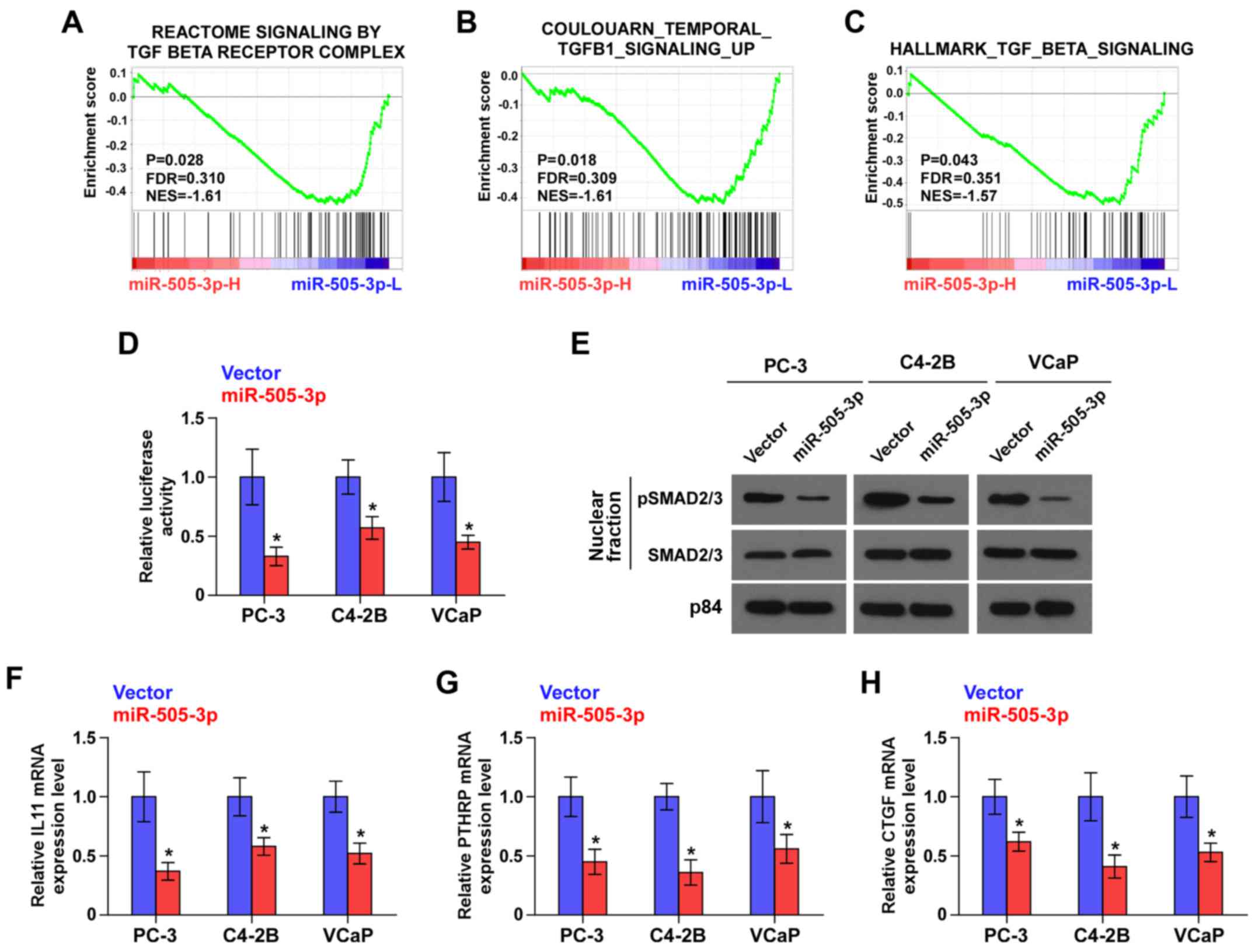
GSEA analysis of miR-505-3p expression revealed that
downregulation of miR-505-3p was positively associated with TGF-β
signaling activity (Fig. 4A-C).
These results indicated that miR-505-3p may negatively regulate the
TGF-β signaling pathway, which has been demonstrated to play an
important role in bone metastasis of PCa (24). As shown in Fig. 4D, miR-505-3p overexpression reduced
the transcriptional activity of the TGF-β/Smad-responsive
luciferase reporter in PCa cells. Cellular fractionation and
western blot analysis revealed that overexpression of miR-505-3p
decreased nuclear accumulation of pSMAD2/3 (Fig. 4E). Real-time PCR analysis revealed
that upregulation of miR-505-3p differentially reduced downstream
target genes of TGF-β signaling in PCa cells, including IL11, PTHRP
and CTGF (Fig. 4F-H). Thus, these
results indicated that miR-505-3p inhibited the activity of TGF-β
signaling in PCa cells.
miR-505-3p directly targets SMAD2 and
SMAD3 in PCa cells
Using the publicly available algorithms TargetScan
and miRanda, we found that the critical downstream effectors of
TGF-β signaling SMAD2 and SMAD3 were potential targets of
miR-505-3p (Fig. 5A). Real-time-PCR
and western blot analysis revealed that miR-505-3p overexpression
reduced the mRNA and protein expression levels of SMAD2 and SMAD3
(Fig. 5B and C). Luciferase assays
revealed that miR-505-3p overexpression suppressed the luciferase
reporter activity of SMAD2 and SMAD3, but not the expression of
SMAD2 and SMAD3 with the 3′UTR mutation within the
miR-505-3p-binding seed regions in PCa cells (Fig. 5D-F). Microribonucleoprotein (miRNP)
immunoprecipitation (IP) assays revealed a direct association of
miR-505-3p with SMAD2 and SMAD3 transcripts (Fig. 5G-I), further elucidating the direct
suppressive effects of miR-505-3p on SMAD2 and SMAD3. Collectively,
these results demonstrated that miR-505-3p inhibited the activity
of TGF-β signaling by targeting SMAD2 and SMAD3in PCa cells.
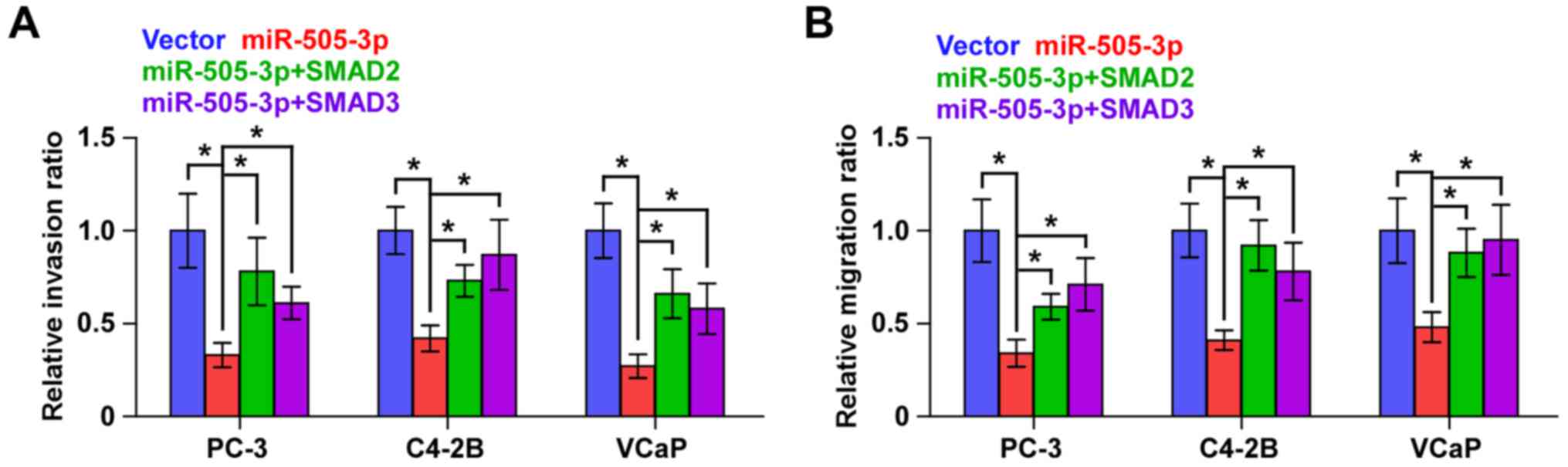
SMAD2 and SMAD3 reverse the effects of
miR-505-3p on the invasion and migration in PCa cells
We further investigated whether SMAD2 and SMAD3 had
an effect on the invasion and migration abilities in
miR-505-3p-overexpressing PCa cells and found that individual
upregulation of SMDA2 and SMAD3 markedly reversed the inhibitory
effects of miR-505-3p on the invasion and migration abilities in
PCa cells (Fig. 6). These results
indicated that miR-505-3p suppresses the invasion and migration
abilities by inhibiting SMAD2 and SMAD3 activity in PCa cells.
Discussion
The primary findings of the present study provide
novel insights into the important predictive role of miR-505-3p in
bone metastasis of PCa. In the present study, we reported that
miR-505-3p was significantly downregulated in bone metastatic PCa
tissues, and that miR-505-3p expression was inversely associated
with poor clinicopathological characteristics and bone
metastasis-free survival in PCa patients. Our results further
revealed that upregulation of miR-505-3p inhibited TGF-β signaling
activity by targeting SMAD2 and SMAD3, which further inhibited the
invasion and migration abilities of PCa cells. Therefore, our
results provide evidence that miR-505-3p may serve as a novel
marker to predict bone metastasis of PCa.
As one of the originally identified miRNAs,
miR-505-3p has been reported to be downregulated in several types
of cancers, which predicted poor prognosis in cancer patients
(25,26). However, miR-505-3p was found to be
upregulated in hepatocellular carcinoma, which facilitated the
identification of small-size, early-stage, α-fetoprotein-negative
hepatocellular carcinoma in patients at risk (27). These findings indicated that the
anticancer or pro-cancer role of miR-505-3p in cancer is tumor
type-dependent. However, the expression level and biological role
of miR-505-3p in bone metastasis of PCa remains largely unknown. In
the present study, we found that miR-505-3p expression was
downregulated in bone metastatic PCa tissues, which was positively
associated with advanced clinicopathological characteristics and
poor bone metastasis-free survival in PCa patients. Upregulation of
miR-505-3p inhibited the invasion and migration abilities of PCa
cells by targeting SMAD2 and SMAD3, leading to the inactivation of
TGF-β signaling activity. Therefore, our findings demonstrated that
miR-505-3p plays a tumor-suppressive role in bone metastasis of PCa
by suppressing the activity of the TGF-β signaling pathway.
Constitutive activation of TGF-β signaling has been
reported in bone metastasis of PCa. Fournier et al have
reported that TGF-β upregulated the negative regulator of the TGF-β
pathway PMEPA1, which inhibited bone metastasis of PCa. Notably,
disruption of this negative feedback loop using PMEPA1 siRNA
promoted bone metastases in vivo (28). Furthermore, downregulation of the
negative regulator of the TGF-β signaling PICK1 caused by oncogenic
miR-210-3p contributed to bone metastasis in PCa (24). In the present study, we found that
the critical downstream effectors of TGF-β signaling SMAD2 and
SMAD3 were potential targets of miR-505-3p. Through a series of
mechanistic experiments, including real-time-PCR, western blotting,
luciferase assay and microribonucleoprotein immunoprecipitation,
our results demonstrated that miR-505-3p directly targeted SMAD2
and SMAD3 in PCa cells, resulting in the suppression of TGF-β
signaling activity. Since the crucial roles of TGF-β signaling in
bone metastasis in a variety of cancers (29–33),
including PCa (24,28), have been well established, our
results uncovered a novel mechanism responsible for the
constitutive activation of TGF-β signaling in PCa, providing
theoretical evidence that miR-505-3p plays a tumor suppressive role
in bone metastasis of PCa.
miRNAs alone or in clusters have been widely
documented to serve as potential markers for the diagnosis and
prognosis of cancer. Several studies have shown that high levels of
miR-96 were significantly correlated with poor overall and
recurrence-free survival in PCa patients (34,35).
Moreover, a miRNA classifier, including miR-505-3p, served as a
potential biomarker for hepatocellular carcinoma, which was
valuable in the detection of preclinical hepatocellular carcinoma
(27). However, the clinical
significance of miR-505-3p in PCa remains largely unknown. In this
study, our results demonstratd that low expression of miR-505-3p
was positively associated with poor clinicopathological
characteristics and shorter bone metastasis-free survival in PCa
patients, but had no significant effect on the overall survival in
PCa patients. These findings indicated that miR-505-3p holds
promise as a novel prognostic marker for bone metastasis of
PCa.
miRNAs can not only serve as potential markers for
the diagnosis and prognosis of cancer, but also provide data for
the optimization and personalization of therapy in the treatment of
cancer. miR-505-3p has been reported as a tumor inhibitor in
several human cancer types, suggesting the therapeutic application
of miR-505-3p in cancer treatment. For example, lentivirus-mediated
miR-505-3p upregulation suppressed cervical cancer cell
proliferation and invasion in vitro. Notably, upregulation
of miR-505-3p markedly reduced tumorigenicity of cervical cancer
cells in vivo (26). In
endometrial carcinoma, overexpression of miR-505 reduced
tumorigenicity and inhibited the growth of xenograft tumors in a
mouse model of endometrial carcinoma (36). Furthermore, several lines of
evidence have reported that miR-505-3p expression levels can
predict complete chemotherapeutic response in cancer (37–39).
Therefore, it is tempting to investigate, although it remains to be
studied, the therapeutic application of miR-505-3p in bone
metastasis of PCa via an intra-prostatic injection mouse model or
clinical trial.
In summary, our results demonstrated that miR-505-3p
negatively regulated TGF-β signaling and was inversely associated
with bone metastasis-free survival in PCa. An improved
understanding of the underlying molecular mechanisms by which
miR-505-3p inhibits bone metastasis of PCa and the prognostic
values of miR-505-3p expression for bone metastasis of PCa will
increase our knowledge of the biological basis of the development
of PCa bone metastasis and facilitate the development of
antimetastatic therapeutic strategies against PCa.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by grants
from the National Natural Science Foundation of China (nos.
81402227 and 81503281), and the Guangdong Natural Science
Foundation (no. 2017A020215162).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
PX and PH conceived, designed, and wrote the study.
YT, BW and XL performed the experiments. XH and WZ analyzed the
miR-505-3p expression and clinical correlation with
clinicopathological characteristics. SH and XP performed the
clinical analysis and drafted the manuscript. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
For the use of the clinical samples for research
purposes, prior informed consents from patients and approval from
the Institutional Research Ethics Committee were obtained from
Jiangmen Central Hospital (Jiangmen, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nelson WG, De Marzo AM and Isaacs WB:
Prostate cancer. N Engl J Med. 349:366–381. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sabbatini P, Larson SM, Kremer A, Zhang
ZF, Sun M, Yeung H, Imbriaco M, Horak I, Conolly M, Ding C, et al:
Prognostic significance of extent of disease in bone in patients
with androgen-independent prostate cancer. J Clin Oncol.
17:948–957. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ventura A and Jacks T: MicroRNAs and
cancer: Short RNAs go a long way. Cell. 136:586–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khew-Goodall Y and Goodall GJ:
Myc-modulated miR-9 makes more metastases. Nat cell Biol.
12:209–211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hao GJ, Ding YH, Wen H, Li XF, Zhang W, Su
HY, Liu DM and Xie NL: Attenuation of deregulated miR-369-3p
expression sensitizes non-small cell lung cancer cells to cisplatin
via modulation of the nucleotide sugar transporter SLC35F5. Biochem
Biophys Res Commun. 488:501–508. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ren D, Lin B, Zhang X, Peng Y, Ye Z, Ma Y,
Liang Y, Cao L, Li X, Li R, et al: Maintenance of cancer stemness
by miR-196b-5p contributes to chemoresistance of colorectal cancer
cells via activating STAT3 signaling pathway. Oncotarget.
8:49807–49823. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao G, Li Y and Wang T: Potentiation of
docetaxel sensitivity by miR-638 via regulation of STARD10 pathway
in human breast cancer cells. Biochem Biophys Res Commun.
487:255–261. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang X, Liu J, Zang D, Wu S, Liu A, Zhu
J, Wu G, Li J and Jiang L: Upregulation of miR-572
transcriptionally suppresses SOCS1 and p21 and contributes to human
ovarian cancer progression. Oncotarget. 6:15180–15193.
2015.PubMed/NCBI
|
|
9
|
Ren D, Yang Q, Dai Y, Guo W, Du H, Song L
and Peng X: Oncogenic miR-210-3p promotes prostate cancer cell EMT
and bone metastasis via NF-kappaB signaling pathway. Mol Cancer.
16:1172017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Z, Liu Z, Zhang Y, Li Y, Liu B and
Zhang K: miR-24 represses metastasis of human osteosarcoma cells by
targeting Ack1 via AKT/MMPs pathway. Biochem Biophys Res Commun.
486:211–217. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siu MK, Tsai YC, Chang YS, Yin JJ, Suau F,
Chen WY and Liu YN: Transforming growth factor-beta promotes
prostate bone metastasis through induction of microRNA-96 and
activation of the mTOR pathway. Oncogene. 34:4767–4776. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ren D, Wang M, Guo W, Zhao X, Tu X, Huang
S, Zou X and Peng X: Wild-type p53 suppresses the
epithelial-mesenchymal transition and stemness in PC-3 prostate
cancer cells by modulating miR145. Int J Oncol. 42:1473–1481. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren D, Wang M, Guo W, Huang S, Wang Z,
Zhao X, Du H, Song L and Peng X: Double-negative feedback loop
between ZEB2 and miR-145 regulates epithelial-mesenchymal
transition and stem cell properties in prostate cancer cells. Cell
Tissue Res. 358:763–778. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo W, Ren D, Chen X, Tu X, Huang S, Wang
M, Song L, Zou X and Peng X: HEF1 promotes epithelial mesenchymal
transition and bone invasion in prostate cancer under the
regulation of microRNA-145. J Cell Biochem. 114:1606–1615. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang M, Ren D, Guo W, Huang S, Wang Z, Li
Q, Du H, Song L and Peng X: N-cadherin promotes
epithelial-mesenchymal transition and cancer stem cell-like traits
via ErbB signaling in prostate cancer cells. Int J Oncol.
48:595–606. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang X, Ren D, Guo L, Wang L, Wu S, Lin
C, Ye L, Zhu J, Li J, Song L, et al: Thymosin beta 10 is a key
regulator of tumorigenesis and metastasis and a novel serum marker
in breast cancer. Breast cancer Res. 19:152017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang X, Zhang L, Lin B, Chai X, Li R,
Liao Y, Deng X, Liu Q, Yang W, Cai Y, et al: Phospholipid
phosphatase 4 promotes proliferation and tumorigenesis, and
activates Ca2+-permeable cationic channel in lung
carcinoma cells. Mol Cancer. 16:1472017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li X, Liu F, Lin B, Luo H, Liu M, Wu J, Li
C, Li R, Zhang X, Zhou K, et al: miR150 inhibits proliferation and
tumorigenicity via retarding G1/S phase transition in
nasopharyngeal carcinoma. Int J Oncol. 2017.
|
|
20
|
Wang M, Ren D, Guo W, Wang Z, Huang S, Du
H, Song L and Peng X: Loss of miR-100 enhances migration, invasion,
epithelial-mesenchymal transition and stemness properties in
prostate cancer cells through targeting Argonaute 2. Int J Oncol.
45:362–372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheadle C, Vawter MP, Freed WJ and Becker
KG: Analysis of microarray data using Z score transformation. J Mol
Diagn. 5:73–81. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:2015. View Article : Google Scholar
|
|
23
|
Betel D, Wilson M, Gabow A, Marks DS and
Sander C: The microRNA.org resource: Targets and expression.
Nucleic Acids Res. 36:D149–D153. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dai Y, Ren D, Yang Q, Cui Y, Guo W, Lai Y,
Du H, Lin C, Li J, Song L, et al: The TGF-β signalling negative
regulator PICK1 represses prostate cancer metastasis to bone. Br J
Cancer. 117:685–694. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu M, Kuang Y, Wang M, Han X and Yang Q: A
microRNA expression signature as a predictor of survival for colon
adenocarcinoma. Neoplasma. 64:56–64. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma C, Xu B, Husaiyin S, Wang L,
Wusainahong K, Ma J, Zhu K and Niyazi M: MicroRNA-505 predicts
prognosis and acts as tumor inhibitor in cervical carcinoma with
inverse association with FZD4. Biosmed Pharmacother. 92:586–594.
2017. View Article : Google Scholar
|
|
27
|
Lin XJ, Chong Y, Guo ZW, Xie C, Yang XJ,
Zhang Q, Li SP, Xiong Y, Yuan Y, Min J, et al: A serum microRNA
classifier for early detection of hepatocellular carcinoma: A
multicentre, retrospective, longitudinal biomarker identification
study with a nested case-control study. Lancet Oncol. 16:804–815.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fournier PG, Juarez P, Jiang G, Clines GA,
Niewolna M, Kim HS, Walton HW, Peng XH, Liu Y, Mohammad KS, et al:
The TGF-beta signaling regulator PMEPA1 suppresses prostate cancer
metastases to bone. Cancer Cell. 27:809–821. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
No authors listed. TGF β induces a
pro-bone metastasis program in prostate cancer. Cancer Discov.
5(OF23)2015.
|
|
30
|
Yin JJ, Selander K, Chirgwin JM, Dallas M,
Grubbs BG, Wieser R, Massague J, Mundy GR and Guise TA: TGF-beta
signaling blockade inhibits PTHrP secretion by breast cancer cells
and bone metastases development. J Clin Invest. 103:197–206. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kang Y, Siegel PM, Shu W, Drobnjak M,
Kakonen SM, Cordon-Cardo C, Guise TA and Massague J: A multigenic
program mediating breast cancer metastasis to bone. Cancer Cell.
3:537–549. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sethi N, Dai X, Winter CG and Kang Y:
Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast
cancer by engaging Notch signaling in bone cells. Cancer Cell.
19:192–205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Javelaud D, Mohammad KS, McKenna CR,
Fournier P, Luciani F, Niewolna M, Andre J, Delmas V, Larue L,
Guise TA, et al: Stable overexpression of Smad7 in human melanoma
cells impairs bone metastasis. Cancer Res. 67:2317–2324. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schaefer A, Jung M, Mollenkopf HJ, Wagner
I, Stephan C, Jentzmik F, Miller K, Lein M, Kristiansen G and Jung
K: Diagnostic and prognostic implications of microRNA profiling in
prostate carcinoma. Int J Cancer. 126:1166–1176. 2010.PubMed/NCBI
|
|
35
|
Haflidadottir BS, Larne O, Martin M,
Persson M, Edsjo A, Bjartell A and Ceder Y: Upregulation of miR-96
enhances cellular proliferation of prostate cancer cells through
FOXO1. PLoS One. 8:e724002013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen S, Sun KX, Liu BL, Zong ZH and Zhao
Y: MicroRNA-505 functions as a tumor suppressor in endometrial
cancer by targeting TGF-alpha. Mol Cancer. 15:112016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Skinner HD, Lee JH, Bhutani MS, Weston B,
Hofstetter W, Komaki R, Shiozaki H, Wadhwa R, Sudo K, Elimova E, et
al: A validated miRNA profile predicts response to therapy in
esophageal adenocarcinoma. Cancer. 120:3635–3641. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ramachandran SS, Muiwo P, Ahmad HM, Pandey
RM, Singh S, Bakhshi S, Kumar L, Bhattacharya A and Gupta YK:
miR-505-5p and miR-193b-3p: Potential biomarkers of imatinib
response in patients with chronic myeloid leukemia. Leuk Lymphoma.
58:1981–1984. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Molina-Pinelo S, Carnero A, Rivera F,
Estevez-Garcia P, Bozada JM, Limon ML, Benavent M, Gomez J, Pastor
MD, Chaves M, et al: MiR-107 and miR-99a-3p predict chemotherapy
response in patients with advanced colorectal cancer. BMC Cancer.
14:6562014. View Article : Google Scholar : PubMed/NCBI
|