Introduction
Pituitary adenomas (PAs) are clinically common
intracranial neoplasms (1).
Non-functioning pituitary adenomas (NFPAs) account for nearly half
of all PAs (2). The majority of
NFPAs are detected due to neurological manifestations, including
headaches, vision and visual field disorders or incidental imaging
examinations (3). The first-line
treatment is surgical resection, but the clinical efficacy depends
on the tumour size in addition to the surgeon's ability (4,5) as
numerous macroadenomas invade important surrounding structures,
including the cavernous sinus, internal carotid artery and optic
chiasm (6). This invasion makes it
difficult to achieve total resection. Following partial tumour
resection, any residual tumour are a potential source of
regeneration (7). Even certain
tumour types that are completely resected during the initial
surgery still have a risk of regrowth (8). For non-functioning pituitary
macroadenoma tumour types, the recurrence rate may range from 12 to
58% (9). A previous study
demonstrated that NFPA recurrence and regrowth was likely
associated with the biomarkers high-mobility group protein
HMG-1/HMG-Y and mouse double minute 2 homolog (10). In a separate study, fascin
actin-bundling protein 1 was suggested to increase the risk of PA
recurrence (11). However, the
molecular mechanisms of tumour regrowth and recurrence are unclear
and require further research.
Postoperative radiation is considered an effective
adjunctive therapy that is able to reduce recurrence. However, it
is often difficult to determine whether radiation therapy is a
reasonable therapeutic method for a patient, as it may result in
progressive hypopituitarism and other long-term complications
(12). Therefore, radiotherapy
exclusively benefits patients with a high risk of tumour recurrence
following surgery, and its use is controversial in patients without
definite evidence of tumour regrowth (13). A method for predicting recurrence
during the initial surgery will help to determine if adjunctive
radiation therapy is required.
Circular RNAs (circRNAs) are covalently closed RNAs
that have been identified to regulate mammalian gene expression and
may be novel markers for cancer diagnosis (14–18).
The levels of hsa_circ_100269 are decreased in gastric cancer (GC)
tissues, and a signalling pathway involving hsa_circ_100269 and
hsa_miR_630 serves a critical role in GC cell growth (19). hsa_circ_001988 is downregulated in
colorectal cancer, and its expression is associated with
differentiation and perineural invasion (20). The expression levels of
hsa_circ_0001649 are downregulated in hepatocellular carcinoma and
colorectal cancer, indicating that hsa_circ_0001649 may be a novel
biomarker (21,22). Additionally, circRNAs serve a strong
regulatory function in carcinoma; for example, they are able to
function as efficient microRNA (miRNA/miR) sponges (15,23,24).
circRNAs sponge miRNAs through complementary sequences to reduce
the function of the microRNAs (25). For instance, the circRNA cerebellar
degeneration-related protein 1, which contains miR-7 binding sites,
is able to reduce the growth of human cells by inhibiting miR-7
binding (26,27); and circRNA homeodomain interacting
protein kinase 3 binds directly to miR-124 (28,29),
thus regulating cancer development. In hepatocellular carcinoma,
hsa_circ_0067934 accelerates tumour growth and metastasis by
modulating the miR-1324/Frizzled class receptor 5/Wnt/β-catenin
axis (30). In addition,
hsa_circ_100290 sponges the miR-29 family in oral cancer (31). Accordingly, circRNAs may be novel
potential cancer biomarkers and therapeutic targets. Thus, the
identification of pituitary-associated circRNAs, in addition to
their clinical roles and molecular mechanisms, is crucial for
understanding the development and progression of NFPA.
In the present study, tumour regrowth from residual
cells and tumour reappearance following total resection were
collectively referred to as tumour recurrence. The aim was to
develop a circRNA signature that was associated with recurrence and
could be used for treatment guidance in patients with NFPA.
Materials and methods
Patients and samples
A total of 73 specimens were obtained from patients
who underwent surgery at Beijing Tiantan Hospital (Beijing, China)
between October 2007 and July 2014. The patients included 34 males
and 39 females, with a median age of 55 years (range 25–73 years)
and a median follow-up of 78 months (range 36–121 months). Tumors
with Hardy-Wilson classification grade IV and/or Knosp
classification grades III and IV (32,33)
were defined as invasive. Table I
includes the clinical and pathological features of the adenomas.
All tissue samples were immediately snap-frozen and stored in
liquid nitrogen or at −80°C. The tumour type was determined based
on pathological diagnosis and the preoperative clinical and
biochemical examination results. This study was ethically approved
by the Tiantan Hospital Ethics Committee of Beijing Tiantan
Hospital, and written informed consent was obtained from each
patient prior to the study.
 | Table I.Summary of patient demographics and
clinical characteristics. |
Table I.
Summary of patient demographics and
clinical characteristics.
| Characteristic | Training set
(n) | Testing set
(n) | Total (n) |
|---|
| Sex |
|
Female | 19 | 20 | 39 |
|
Male | 18 | 16 | 34 |
| Age, years |
|
≤55 | 19 | 20 | 39 |
|
>55 | 18 | 16 | 34 |
| Vision and visual
field disorders |
|
| 0 |
|
Yes | 18 | 28 | 46 |
| No | 19 | 8 | 27 |
| Headache |
|
Yes | 18 | 18 | 36 |
| No | 19 | 18 | 37 |
| Hypogonadism |
|
Yes | 2 | 8 | 10 |
| No | 35 | 28 | 63 |
| Knosp
classification |
| I | 6 | 5 | 11 |
| II | 9 | 9 | 18 |
|
III | 8 | 8 | 16 |
| IV | 14 | 14 | 28 |
| Hardy
classification |
| I | 0 | 2 | 2 |
| II | 15 | 2 | 17 |
|
III | 7 | 10 | 17 |
| IV | 15 | 0 | 15 |
| Pathological
type |
|
Gonadotrophin | 12 | 9 | 21 |
|
Mult | 4 | 1 | 5 |
| Null
cell | 21 | 25 | 46 |
| Silent
GH | 0 | 1 | 1 |
| Invasion |
|
Yes | 25 | 23 | 48 |
| No | 12 | 13 | 25 |
Total RNA isolation and circRNA
microarray
Total RNA from a tumour was isolated and purified
using a phenol-free mirVana™ miRNA Isolation kit (cat. no. AM1561;
Ambion; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
according to the manufacturer's protocol, and the purity and
quantity were measured using a Nanodrop spectrophotometer.
Subsequently, these 73 total RNA samples (1.8 < Optical Density
260 < 2.1) were used to produce fluorescence-labelled cRNA
targets for the SBC human ceRNA array V1.0 (4×180 K). An Agilent
Microarray scanner (Agilent Technologies, Inc., Santa Clara, CA,
US) was used to scan the slides and hybridize the labelled cRNA
targets. Feature Extraction software 10.7 (Agilent Technologies,
Inc.) was used to extract the data. The R 3.2.3 software (algorithm
‘mean’ and ‘var’; www.r-project.org/) was used to normalize the raw data
and for data processing.
Selection of circRNA subsets
associated with progression-free survival (PFS)
The present study used the R 3.2.3 (www.r-project.org/) program algorithm ‘sample’ to
randomly divide the dataset into training (n=37) and test groups
(n=36). The association between the continuous expression level of
each circRNA and patient PFS in the training set was determined
using univariable Cox regression analysis: A gene with a univariate
Cox coefficient >0 was considered to be a risk factor, <0 was
considered to be a protective factor and -log10
P>1.30 (-log100.05) was regarded as significant.
Selection of circRNA prognostic
signature based on a risk score model
Next, a model for selecting circRNA signatures with
prognostic indicators was developed as follows:
Risk score = ∑Ni = 1 (Explg*Coef)
(30,34).
Where N is the number of predictive circRNAs, Explg
indicates the expression of circRNAs and Coef is the evaluated
regression coefficient of the circRNAs determined from the
univariable Cox regression analysis.
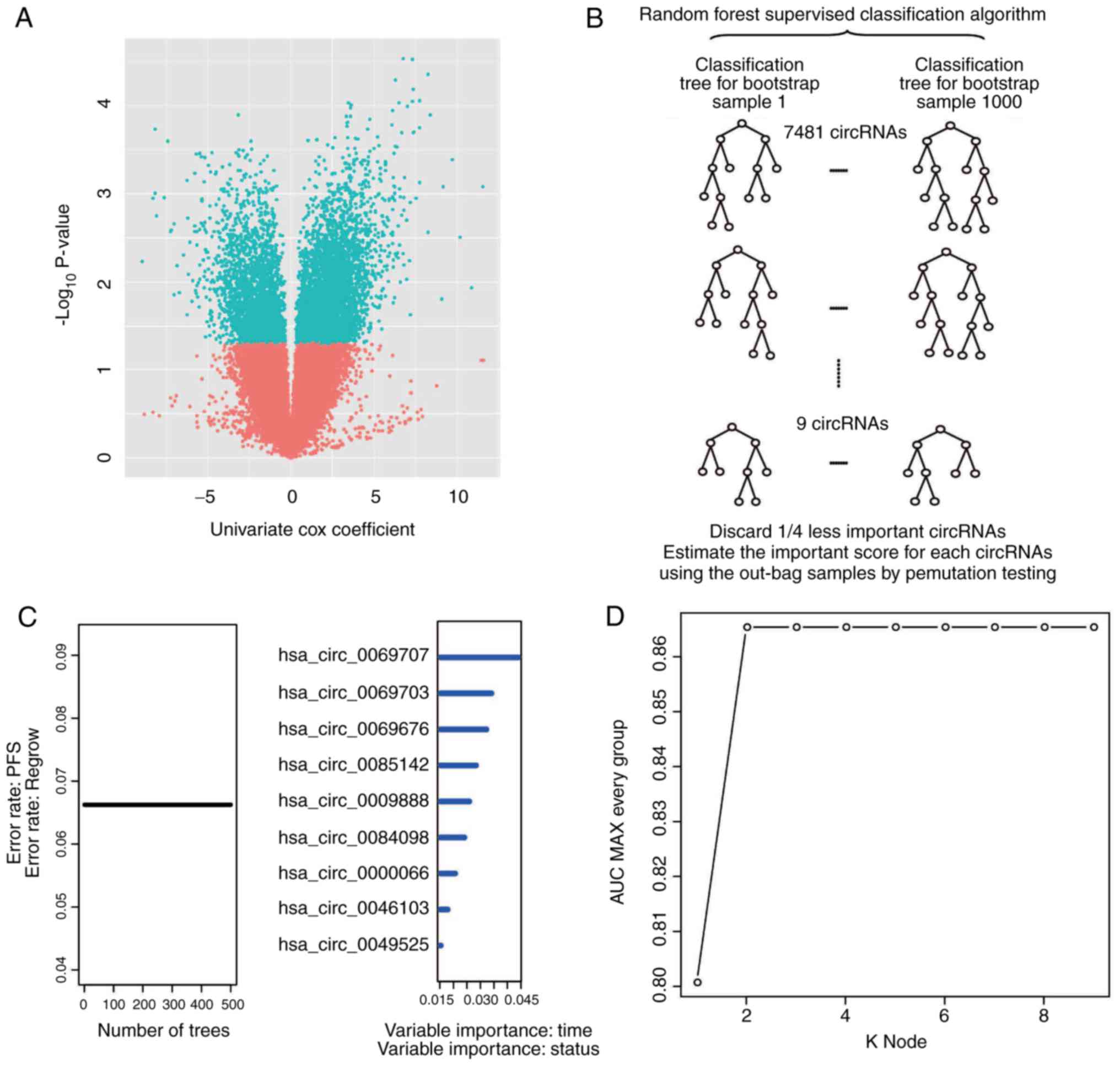
circRNAs were screened using a random survival
forest-variable hunting (RSFVH) algorithm, and then a total of 9
circRNAs were selected (30) as
fewer circRNAs provide a greater value. All 9 of the circRNAs were
analysed. The total of all 9 node permutations and combinations was
29−1=511 and the sensitivity and specificity of the
survival prediction of the risk score of the 29−1=511
signatures in the training dataset were compared with
time-dependent receiver operating characteristic (ROC) curves, and
the area under the curve (AUROC) values were obtained. In the
training test, the marginal value was obtained from the median risk
score. According to the median risk score, the patients with NFPA
were separated into a high-risk group and a low-risk group in each
dataset.
Statistical analysis
The equality of survival distributions in different
groups for the training and test NFPA cohorts was assessed using
Kaplan-Meier survival analyses, and two-sided log-rank tests were
used to evaluate the statistical significance. Furthermore, the
association with clinical features was analysed via χ2
tests. The risk grade and other clinical features in the available
data when independent were tested using multivariate Cox regression
analysis and data stratification analysis. P<0.05 was considered
to indicate a statistically significant difference. The R 3.2.3
program was used for the analysis of all data and to generate
plots; and the pROC, survival, ggplot2 and random forest SRC
packages were downloaded from Bioconductor (www.r-project.org) (35,36).
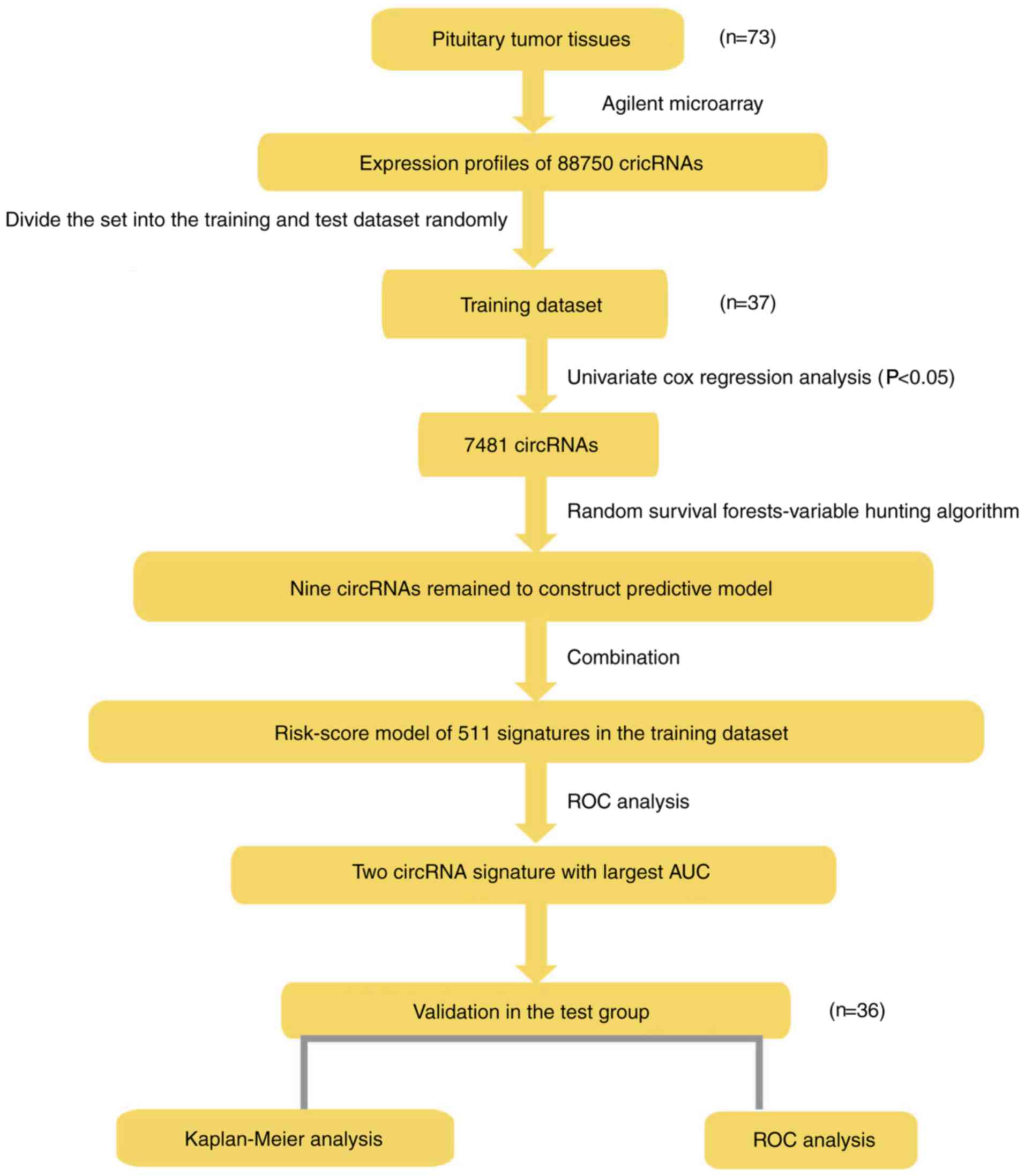
The analysis sequence used for development of a risk scoring model
and to verify the validity of signatures is presented in Fig. 1.
Functional analysis of circRNAs with
prognostic value
Pearson's correlation coefficients were used to
determine the co-expression association between prognostic circRNAs
and protein-coding genes. Gene ontology (GO) (37,38)
and Kyoto Encyclopaedia Gene and Genome (KEGG) (39–41)
enrichment analyses using the Cytoscape plugin ClueGo (version
3.2.3) were performed to determine the co-expressed protein-coding
genes and prognostic circRNAs for predicting the biological
function of prognostic circRNAs (42). Cytoscape is a functional annotation
tool used for assessing the over-representation of a category of
interest gene concentration. Enrichment analyses were performed
using functional annotation maps and functional annotation
clustering options and were restricted to the KEGG pathway and GO
terminology in the ‘biological process’ category. Functional
annotations with P<0.05 were considered to indicate a
statistically significant difference.
Results
Selection of a prognostic circRNA
signature with a high predictive power for recurrence
progression
The present study examined the pituitary tumour
tissues of 73 patients using an Agilent microarray. From this
analysis, a total of 88,750 circRNAs were identified. Next, the
patients were randomly divided into two groups: A training set
(n=37) and a test set (n=36). The training set was used to select
the circRNA prognostic signature. The test set was used to evaluate
the discriminative power of the circRNA signature, which was
obtained independently. In this model selection section, all
analyses were based on the training set.
First, univariate Cox proportional hazards
regression analysis of circRNA expression using recurrence as the
dependent variable was performed. A total of 7,481 circRNAs (data
not shown) were determined to be correlated (P<0.05) with
patient recurrence/progression and a volcano plot was used to
present the univariate Cox results (Fig. 2A). Next, a RSFVH algorithm was used
to select 9 circRNAs that were most closely associated with
progression/relapse in the set of 7,481 circRNAs, according to the
replacement vital score of the RSFVH algorithm (Fig. 2B).
The application of time-dependent ROC curves and the
comparison of the sensitivity and specificity of the risk score
survival prediction may result in better predictive
characteristics. The risk score is based on the risk score model of
29−1=511 combinations of the circRNA training set (data
not shown) for each circRNA of each patient in the training
dataset. All risk scores for the circRNA signatures were calculated
using this method. Then, the circRNA combination of
hsa_circ_0000066 and hsa_circ_0069707, which had the largest AUROC,
was selected (Fig. 2C and D;
Table II). A risk score for the
combination of hsa_circ_0000066 and hsa_circ_0069707 was obtained
as follows: Risk score=(2.38 × expression value of RP11-702B10.1) +
(8.27 × expression value of hsa_circ_0069707). The AUROC for the
circRNA signature in the model was 0.87 in the training set. Thus,
the optimal survival prediction value was obtained based on the
expression-based risk score.
 | Table II.Identities of circRNAs in the
prognostic expression signature and their univariable cox
association with prognosis. |
Table II.
Identities of circRNAs in the
prognostic expression signature and their univariable cox
association with prognosis.
|
|
|
|
|
|
|
| Chromosome
location |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Database ID | Best
transcript | Gene symbol | Genomic length |
Coefficienta |
P-valuea | Gene expression
level association with poor prognosis | (GRCh38/hg38) |
|---|
|
hsa_circ_0000066b | NM_018150 | RNF220 | 132 | 2.38 | <0.001 | High |
chr1:44890310-44890442:-1 |
|
hsa_circ_0069707b | NM_015030 | FRYL | 402 | 8.27 | <0.001 | High |
chr4:48712535-48782316:-1 |
Evaluation of the circRNA signature
for predicting the recurrence and PFS of patients with NFPA
Subsequently, the predictive power of the circRNA
signature was assessed. The test set (n=36) was utilized for an
independent evaluation, and the training set (n=37) was evaluated
in parallel for comparison.
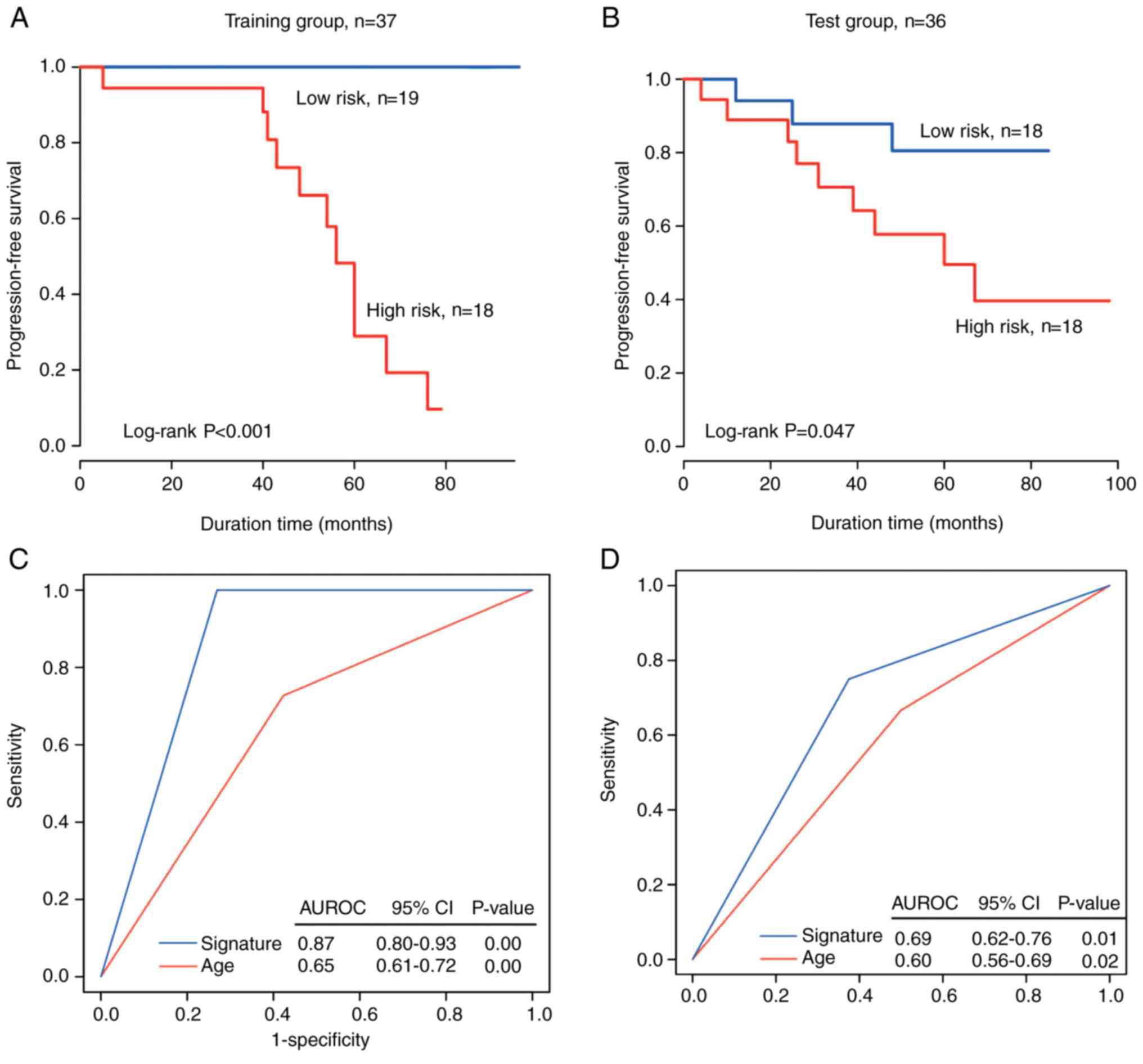
In the training set, based on the median value of
the risk score of the circRNA signature being used as the cutoff
value, the patients were separated into a high-risk group (n=18)
and a low-risk group (n=19). The PFS time was significantly shorter
in the high-risk patients compared with the low-risk patients
(median survival: 56 months vs. >80.2 months; P<0.001;
Fig. 3A). The rate of no recurrence
or progression in high-risk patients was <1% and that in
low-risk patients was >90%.
In the independent test set, the patients were
separated into two groups (n=18). Verification of the recurrence
prediction ability of the circRNA signature was performed using
Kaplan-Meier curves for the high-risk and low-risk groups in the
test dataset; the data were plotted and are presented in Fig. 3B, revealing a significant difference
in PFS between the two groups (median PFS time: 70.2 months vs.
>60 months; log-rank sum test, P=0.047). The rate of no
recurrence/progression was ~16.6% in the high-risk group and 50% in
the low-risk group.
CircRNA signature is independent of
clinicopathological features
The clinical significance of the circRNA signature
in NFPAs was obtained by associating the signature with a range of
clinical pathological parameters from the dataset (n=73). As
presented in Table III, except
for age, vision and visual field disorders, and headache, there was
no significant association between the circRNA markers and clinical
pathological variables, including sex and histological grade
(Table III).
 | Table III.Association of the circRNA signature
with clinicopathological characteristics in patients with pituitary
adenoma. |
Table III.
Association of the circRNA signature
with clinicopathological characteristics in patients with pituitary
adenoma.
|
| Training group |
| Test group |
| Entire group |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Variables | Low risk | High risk | P-value | Low risk | High risk | P-value | Low risk | High risk | P-value |
|---|
| Sex |
|
| 0.62 |
|
| >0.99 |
|
| 0.73 |
|
Female | 11 | 8 |
| 10 | 10 |
| 21 | 18 |
|
|
Male | 8 | 10 |
| 8 | 8 |
| 16 | 18 |
|
| Age, years |
|
| 0.41 |
|
| 0.09 |
|
| 0.05 |
|
≤55 | 8 | 11 |
| 7 | 13 |
| 15 | 24 |
|
|
>55 | 11 | 7 |
| 11 | 5 |
| 22 | 12 |
|
| Vision and visual
field disorders |
|
| >0.99 |
|
| 0.05 |
|
| 0.13 |
|
Yes | 13 | 13 |
| 11 | 17 |
| 24 | 30 |
|
| No | 6 | 5 |
| 7 | 1 |
| 13 | 6 |
|
| Headache |
|
| 0.03 |
|
| 0.74 |
|
| 0.29 |
|
Yes | 13 | 5 |
| 8 | 10 |
| 21 | 15 |
|
| No | 6 | 13 |
| 10 | 8 |
| 16 | 21 |
|
| Hypogonadism |
|
| >0.99 |
|
| 0.30 |
|
| 0.34 |
|
Yes | 1 | 1 |
| 2 | 5 |
| 3 | 6 |
|
| No | 18 | 17 |
| 15 | 13 |
| 33 | 30 |
|
| Knosp
classification |
|
| 0.49 |
|
| 0.10 |
|
| 0.93 |
| I | 2 | 4 |
| 4 | 1 |
| 6 | 5 |
|
| II | 6 | 3 |
| 2 | 7 |
| 8 | 10 |
|
|
III | 5 | 3 |
| 3 | 5 |
| 8 | 8 |
|
| IV | 6 | 8 |
| 9 | 5 |
| 15 | 13 |
|
| Hardy
classification |
|
| 0.52 |
|
| 0.43 |
|
| 0.27 |
| II | 6 | 9 |
| 10 | 13 |
| 16 | 22 |
|
|
III | 4 | 3 |
| 3 | 3 |
| 7 | 6 |
|
| IV | 9 | 6 |
| 5 | 2 |
| 14 | 8 |
|
| Pathological
type |
|
| 0.48 |
|
| 0.47 |
|
| 0.49 |
|
Gonadotroph | 4 | 8 |
| 4 | 5 |
| 8 | 13 |
|
|
Mult | 2 | 2 |
| 1 | 0 |
| 3 | 2 |
|
| Null
cell | 13 | 8 |
| 13 | 12 |
| 26 | 20 |
|
| Silent
GH |
|
|
| 0 | 1 |
| 0 | 1 |
|
| Invasion |
|
| >0.99 |
|
| >0.99 |
|
| 0.93 |
|
Yes | 13 | 12 |
| 12 | 11 |
| 25 | 23 |
|
| No | 6 | 6 |
| 6 | 7 |
| 12 | 13 |
|
To assess whether the ability of the circRNA markers
in predicting the progression of recurrence was independent of
other clinical features, multivariable Cox regression analysis was
performed using the NFPA dataset and a signature-based risk score
based on two circRNAs and other clinical features. The analysis
indicated that the circRNA marker risk score was independent of the
clinical characteristics in predicting survival, but that the
signature was significantly associated with survival (high-risk
group vs. low-risk group; hazard ratio=10.07, 95% confidence
interval, 2.92–34.77; P<0.001, n=73; Table IV).
 | Table IV.Univariable and multivariable Cox
regression analysis of the circRNA signature and survival of
patients with non-functioning pituitary adenoma in the training,
test group and entire group. |
Table IV.
Univariable and multivariable Cox
regression analysis of the circRNA signature and survival of
patients with non-functioning pituitary adenoma in the training,
test group and entire group.
|
|
| Training set
(n=37) | Test set
(n=36) | Entire dataset
(n=73) |
|---|
|
|
|
|
|
|
|---|
|
|
|
| 95% CI of HR |
|
| 95% CI of HR |
|
| 95% CI of HR |
|
|---|
| Variables | Comparison | HR | Lower | Upper | P-value | HR | Lower | Upper | P-value | HR | Lower | Upper | P-value |
|---|
| Univariable
analysis |
|
Signature | High-risk vs. low
risk | 2.36 | 1.59 | 3.51 | <0.001 | 1.04 | 0.88 | 1.22 | 0.67 | 9.89 | 2.92 | 33.54 | <0.001 |
|
Sex | Male vs.
female | 1.45 | 0.42 | 4.99 | 0.55 | 0.58 | 0.17 | 1.93 | 0.38 | 0.96 | 0.42 | 2.18 | 0.93 |
| Age,
years | >55 vs. ≤55 | 0.42 | 0.11 | 1.59 | 0.20 | 0.78 | 0.23 | 2.60 | 0.68 | 0.58 | 0.24 | 1.40 | 0.23 |
| Knosp
classification | I–II vs.
III–IV | 0.97 | 0.56 | 1.67 | 0.91 | 1.20 | 0.69 | 2.09 | 0.52 | 1.08 | 0.74 | 1.58 | 0.68 |
| Multivariable
analysis |
|
Signature | High-risk vs. low
risk | 3.42 | 1.82 | 6.41 | <0.001 | 1.08 | 0.88 | 1.33 | 0.45 | 10.07 | 2.92 | 34.77 | <0.001 |
|
Sex | Male vs.
female | 0.40 | 0.03 | 4.69 | 0.47 | 0.50 | 0.13 | 1.84 | 0.29 | 0.80 | 0.34 | 1.87 | 0.61 |
| Age,
years | >55 vs. ≤55 | 3.79 | 0.52 | 27.75 | 0.19 | 0.94 | 0.27 | 3.30 | 0.92 | 0.80 | 0.32 | 2.01 | 0.64 |
| Knosp
classification | I–II vs.
III–IV | 1.77 | 0.66 | 4.79 | 0.26 | 1.29 | 0.71 | 2.37 | 0.40 | 1.14 | 0.75 | 1.73 | 0.54 |
Comparing the predictive power of the
circRNA signature with age
In order to compare the sensitivity and specificity
of age and the circRNA-specific survival predictions, ROC analysis
was performed as a larger AUROC usually indicates a better
predictive model. In the dataset (n=37), the prognostic power of
the circRNA marker was significantly stronger compared with age for
the training and test groups (AUROC=0.87 and 0.69 for signature,
respectively, vs. AUROC=0.65 and 0.60 for age, respectively;
Fig. 3C and D). These data further
confirm that the circRNA marker is a novel predictive marker with a
high accuracy and thus has major clinical value.
Functional characterization of the
circRNAs in the prognostic signature
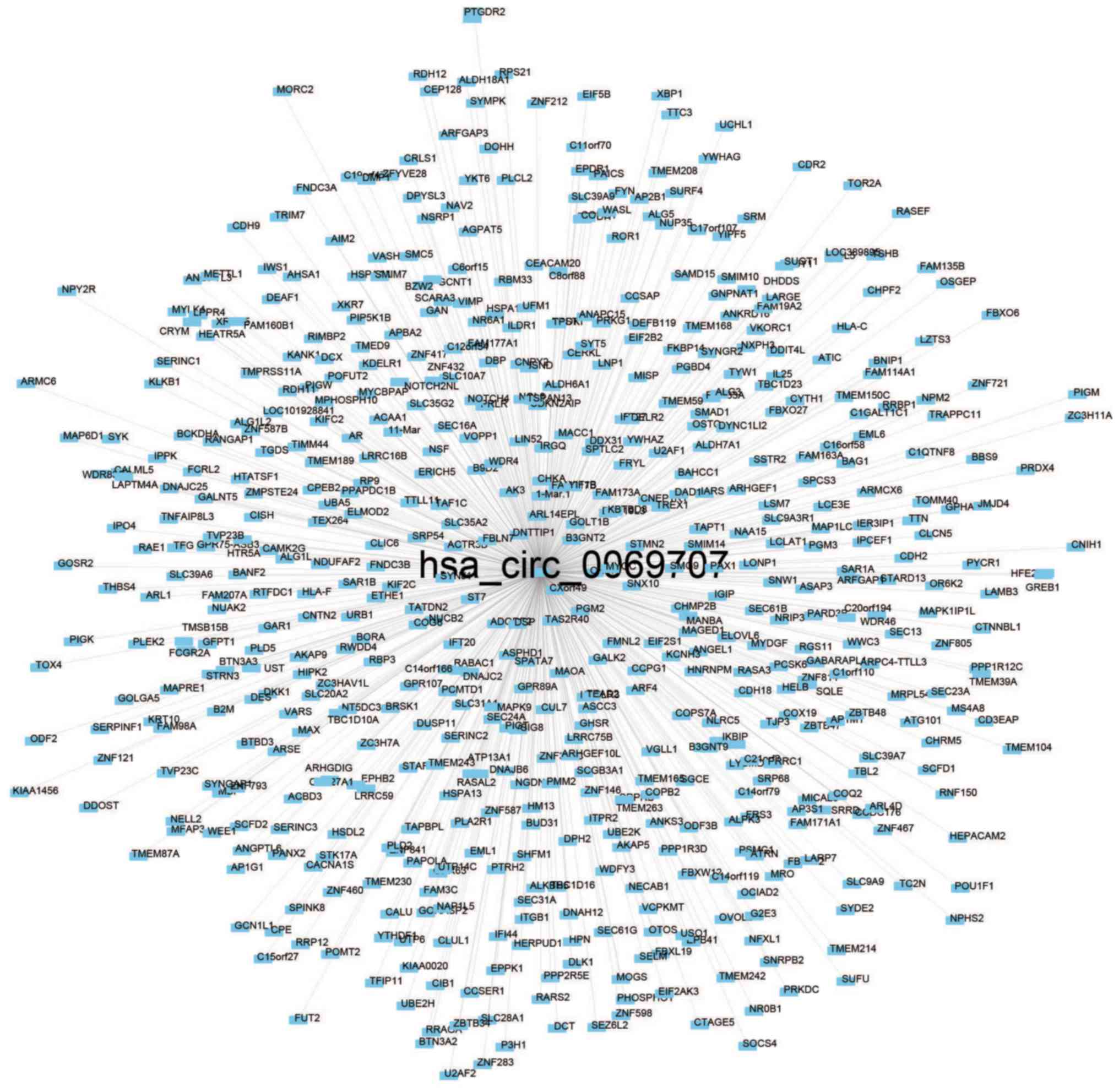
Pearson's correlation coefficient was used for 73
patients to calculate the co-expression association between the two
circRNAs (hsa_circ_0000066 and hsa_circ_0069707) and protein-coding
genes to determine the potential biological effects of these
molecular markers. Of the two circRNAs, at least one may be
associated with the expression levels of 623 protein-coding genes,
and the interaction network was determined with Cytoscape
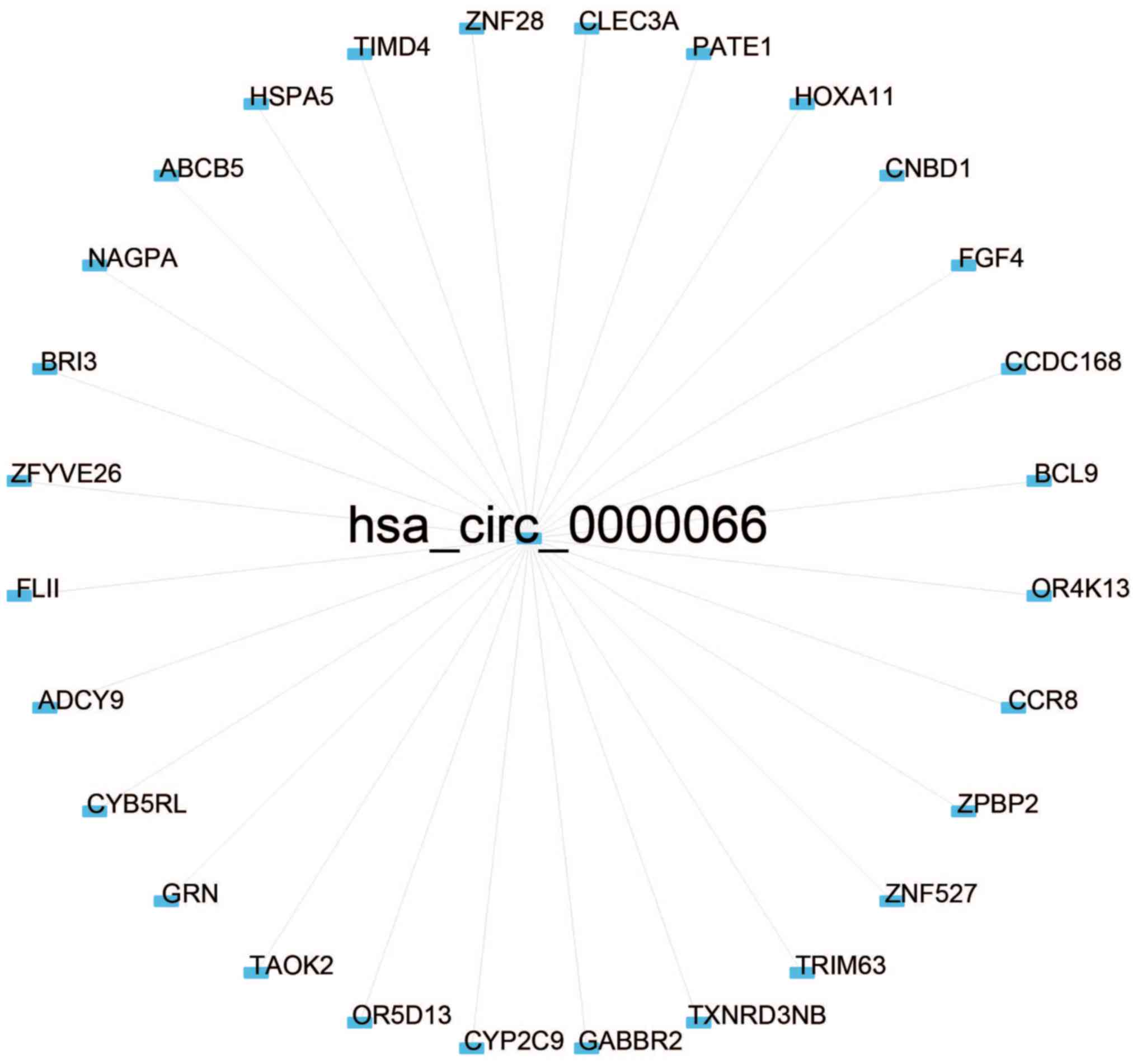
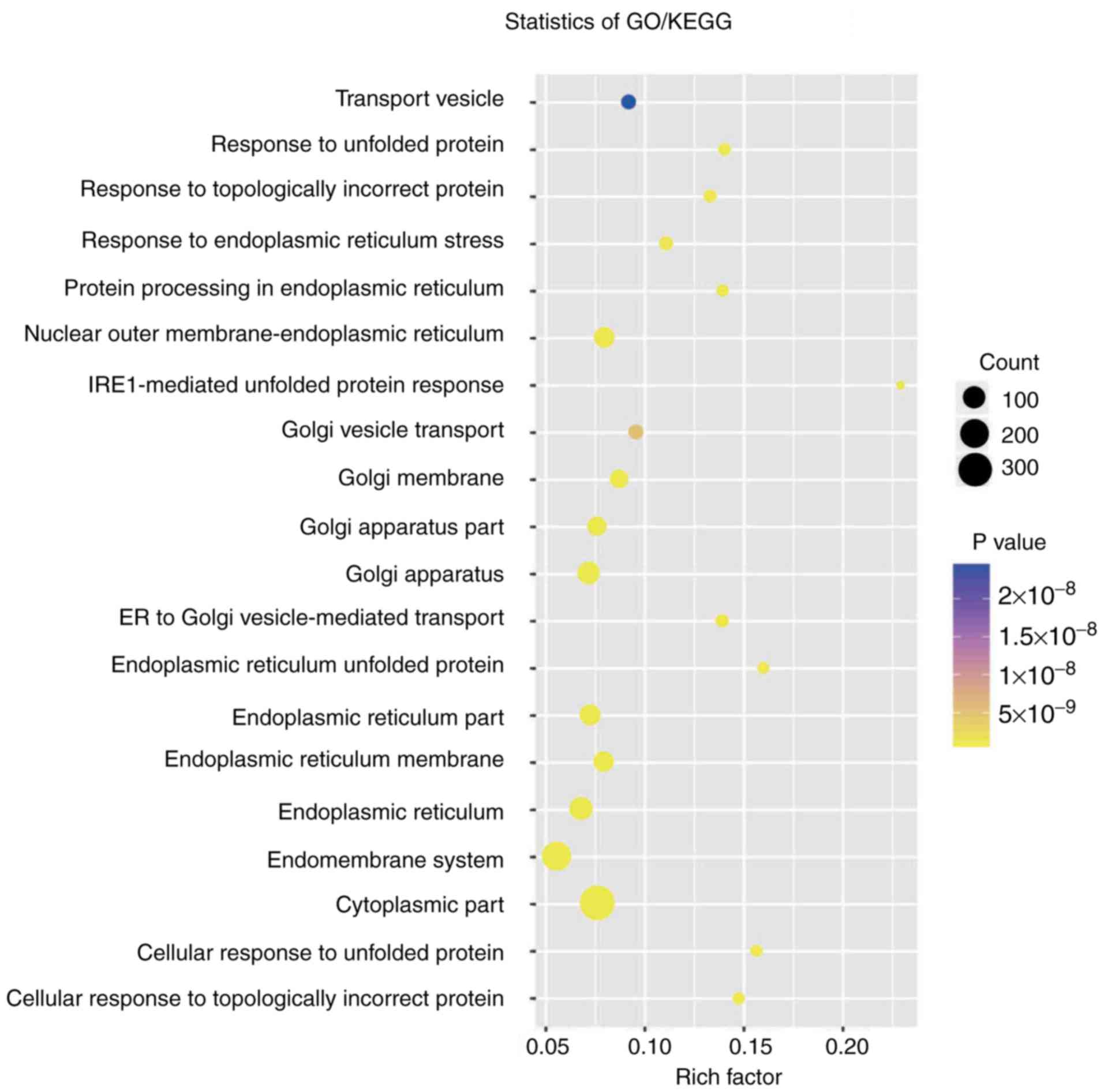
(Pearson's correlation coefficient >0.50; P<0.05; Figs. 4 and 5). Co-expressed protein-coding genes with
the whole human genome as the background were obtained via GO and
KEGG pathway function enrichment analysis. A total of 623
protein-coding genes were enriched in 57 GO/KEGG terms according to
GO functional annotation (data not shown). These important GO/KEGG
terms were used in the ClueGo plugin in Cytoscape to organize an
interaction network with similar functionality. Several groups of
functionally associated GO terms were analysed in the present study
and should be further investigated in the future. Based on the
present analysis, the two circRNAs likely to influence
protein-coding gene interactions and major biological processes,
including vesicle transport and the cellular response to unfolded
proteins, may be involved in tumorigenesis (Fig. 6).
Discussion
NFPA does not induce endocrine symptoms via hormone
hypersecretion, and thus differs from functioning PA. Surgical
resection is the preferred treatment (3–5).
Postoperative tumour recurrence is difficult to detect; regrowth is
discovered only when the tumour is large enough to be observed in
regular follow-up imaging examination or to cause notable symptoms
resulting from a tumour mass effect (43). This situation may exacerbate the
patient prognosis. Considering the severity of NFPA recurrence,
numerous studies are in progress to identify novel predictors that
may identify early recurrence (7).
The purpose of the present study was mainly to separate patients
into high-risk groups or low-risk group in order to identify the
most effective and least toxic treatment method.
CircRNAs are closed-loop RNAs that were originally
thought to be by-products of splicing or other mRNA processing
errors. However, current evidence demonstrates that circRNA may be
involved in disease development and even in the development of a
tumour (44). The level of
hsa_circRNA_100855 was revealed to be significantly (P<0.001)
higher in laryngeal squamous cell cancer tissues compared with in
the corresponding adjacent non-neoplastic tissues, and the levels
of hsa_circRNA_104912 were significantly (P<0.001) lower
(25). Filamin binding LIM protein
1 (FBLIM1) expression was regulated by circFBLIM1, which may serve
as a competing endogenous RNA through the sponging of miR-346 in
hepatocellular cancer (45). Zhang
et al (46) established a
four-circRNA-based classifier (hsa_circRNA_101308,
hsacircRNA_104423, hsa_circRNA_104916 and hsa_circRNA_100269) to
predict early recurrence for patients with stage III gastric cancer
following radical surgery. Therefore, these results suggest that
circRNAs may serve as novel diagnostic markers and treatment
targets.
To the best of our knowledge, the present study is
the first to identify a group of circRNAs expressed in NFPA. In the
present study, Cox's regression and RSFVH algorithm were used to
select 9 circRNAs that were most closely associated with
progression or relapse in the set of 7,481 circRNAs. Then, a risk
score survival prediction method that exhibited good predictive
characteristics was used to combine the circRNAs. Finally, a risk
score for the combination of hsa_circ_0000066 and hsa_circ_0069707
was obtained, which had the largest AUROC curve with the highest
predictive power. The two-circRNA-based classifier was able to
separate patients with NFPA into low-risk or high-risk early
recurrence groups. Disease recurrence may be predicted more
accurately by directly constructing an early recurrence model
rather than using traditional categorical indicators. According to
the present analysis, hsa_circ_0000066b is able to bind to
hsa_circ_0069707, and they work together to affect the PFS time of
patients by modulating the response of transport vesicles and cells
to unfolded proteins. Thus, hsa_circ_0000066b and hsa_circ_0069707
serve an important role in NFPA recurrence. Bioinformatics may
analyse and infer only the functions of these circRNAs; thus, it
remains necessary to confirm the biological effects of these two
circRNAs in tumourigenesis in experimental studies.
In addition to the limited availability of NFPA
sequencing data, the present study has a number of limitations that
need to be considered. First, the prognostic circRNAs confirmed
here are likely not the only circRNA candidates associated with
NFPA PFS as only a fraction of human circRNAs (88,750 out of
140,000+) were included in the present analysis.
Therefore, the present results should be further validated through
prospective and multi-centre studies. Secondly, the present study
lacks information on the mechanisms by which the two circRNAs
affect the prognosis of patients with NFPA. Further functional
experimental studies of primary cells or the 293 cell line should
be performed to determine whether these circRNA directly affect
NFPA progression. Lastly, although the present results were
outlined in the test dataset set as much as possible based on data
availability, this marker has not yet been prospectively tested in
clinical trials. However, despite these deficiencies, the
association between this circRNA signature and PFS in the present
dataset suggests that it is a potent prognostic marker for
NFPA.
In conclusion, to the best of our knowledge, the
present study is the first circRNA signature identified that
predicts tumour recurrence in patients with NFPA with a high
prediction accuracy and thus may be used for treatment guidance and
prognosis estimation.
Acknowledgements
The authors would like to thank Beijing Zhongke
Jingyun Technology Company (Beijing, China) for assisting with the
figures.
Funding
The present study was supported by the National High
Technology Research and Development Program of China (863 Program;
grant no. 2014AA020610), the National Natural Science Foundation of
China (grant no. 81771489), the Beijing Municipal Science &
Technology Commission (grant no. Z171100000117002) and the China
National Key Research and Development Program (grant no.
2017YFC0908300).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH, HG, QL, CL and YZ contributed to the conception
and design of the study. JG, ZW, YM and YS contributed to the
pathological identification and collection of the samples. JG, ZW
and YM contributed to the collection of the clinical and
pathological data of the patients. ML, LG and HW contributed to the
RNA extraction and array hybridization. JG and ZW contributed to
the interpretation of the data (statistical and computational
analysis) and the writing of the manuscript. YH, HG, QL, CL and YZ
contributed to the review and revision of the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committees of Beijing Tiantan Hospital (KY2013-015-02). Written
informed consent was obtained from all of the enrolled subjects,
and the study was performed in full compliance with all principles
of the Helsinki Declaration.
Patient consent for publication
Written informed consent was obtained from all
individual participants included in the study.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ROC
|
receiver operating characteristic
curve
|
|
AUROC
|
area under the ROC curve
|
|
CI
|
confidence interval
|
|
NFPA
|
non-functioning pituitary adenoma
|
|
circRNAs
|
circular RNAs
|
|
SD
|
standard deviation
|
References
|
1
|
Herman V, Fagin J, Gonsky R, Kovacs K and
Melmed S: Clonal origin of pituitary adenomas. J Clin Endocrinol
Metab. 71:1427–1433. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zatelli MC: Pathogenesis of
non-functioning pituitary adenomas. Pituitary. 21:130–137. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Greenman Y and Stern N: Non-functioning
pituitary adenomas. Best Pract Res Clin Endocrinol Metab.
23:625–638. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Donovan LE and Corenblum B: The natural
history of the pituitary incidentaloma. Arch Intern Med.
155:181–183. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shomali ME and Katznelson L: Medical
therapy of gonadotropin-producing and nonfunctioning pituitary
adenomas. Pituitary. 5:89–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scotti G, Yu CY, Dillon WP, Norman D,
Colombo N, Newton TH, De Groot J and Wilson CB: MR imaging of
cavernous sinus involvement by pituitary adenomas. AJR Am J
Roentgenol. 151:799–806. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brochier S, Galland F, Kujas M, Parker F,
Gaillard S, Raftopoulos C, Young J, Alexopoulou O, Maiter D and
Chanson P: Factors predicting relapse of nonfunctioning pituitary
macroadenomas after neurosurgery: A study of 142 patients. Eur J
Endocrinol. 163:193–200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Losa M, Mortini P, Barzaghi R, Ribotto P,
Terreni MR, Marzoli SB, Pieralli S and Giovanelli M: Early results
of surgery in patients with nonfunctioning pituitary adenoma and
analysis of the risk of tumor recurrence. J Neurosurg. 108:525–532.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li J, Chen Z, Tian L, Zhou C, He MY, Gao
Y, Wang S, Zhou F, Shi S, Feng X, et al: LncRNA profile study
reveals a three-lncRNA signature associated with the survival of
patients with oesophageal squamous cell carcinoma. Gut.
63:1700–1710. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yao X, Gao H, Li C, Wu L, Bai J, Wang J,
Li Y and Zhang Y: Analysis of Ki67, HMGA1, MDM2, and RB expression
in nonfunctioning pituitary adenomas. J Neurooncol. 132:199–206.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu C, Gao H, Cao L, Gui S, Liu Q, Li C,
Li D, Gong L and Zhang Y: The role of FSCN1 in migration and
invasion of pituitary adenomas. Mol Cell Endocrinol. 419:217–224.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Snead FE, Amdur RJ, Morris CG and
Mendenhall WM: Long-term outcomes of radiotherapy for pituitary
adenomas. Int J Radiat Oncol Biol Phys. 71:994–998. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sheehan JP, Starke RM, Mathieu D, Young B,
Sneed PK, Chiang VL, Lee JY, Kano H, Park KJ, Niranjan A, et al:
Gamma Knife radiosurgery for the management of nonfunctioning
pituitary adenomas: A multicenter study. J Neurosurg. 119:446–456.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen LL and Yang L: Regulation of circRNA
biogenesis. RNA Biol. 12:381–388. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hentze MW and Preiss T: Circular RNAs:
Splicing's enigma variations. EMBO J. 32:923–925. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Salzman J, Chen RE, Olsen MN, Wang PL and
Brown PO: Cell-type specific features of circular RNA expression.
PLoS Genet. 9:e10037772013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang HD, Jiang LH, Sun DW, Hou JC and Ji
ZL: CircRNA: A novel type of biomarker for cancer. Breast Cancer.
25:1–7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Liu H, Li W, Yu J, Li J, Shen Z,
Ye G, Qi X and Li G: CircRNA_100269 is downregulated in gastric
cancer and suppresses tumor cell growth by targeting miR-630.
Aging. 9:1585–1594. 2017.PubMed/NCBI
|
|
20
|
Wang X, Zhang Y, Huang L, Zhang J, Pan F,
Li B, Yan Y, Jia B, Liu H, Li S, et al: Decreased expression of
hsa_circ_001988 in colorectal cancer and its clinical
significances. Int J Clin Exp Pathol. 8:16020–16025.
2015.PubMed/NCBI
|
|
21
|
Ji W, Qiu C, Wang M, Mao N, Wu S and Dai
Y: Hsa_circ_0001649: A circular RNA and potential novel biomarker
for colorectal cancer. Biochem Biophys Res Commun. 497:122–126.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qin M, Liu G, Huo X, Tao X, Sun X, Ge Z,
Yang J, Fan J, Liu L and Qin W: Hsa_circ_0001649: A circular RNA
and potential novel biomarker for hepatocellular carcinoma. Cancer
Biomark. 16:161–169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guarnerio J, Bezzi M, Jeong JC, Paffenholz
SV, Berry K, Naldini MM, Lo-Coco F, Tay Y, Beck AH and Pandolfi PP:
Oncogenic role of fusion-circRNAs derived from cancer-associated
chromosomal translocations. Cell. 165:289–302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J,
Chen D, Gu J, He X and Huang S: Circular RNA is enriched and stable
in exosomes: A promising biomarker for cancer diagnosis. Cell Res.
25:981–984. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xuan L, Qu L, Zhou H, Wang P, Yu H, Wu T,
Wang X, Li Q, Tian L, Liu M, et al: Circular RNA: A novel biomarker
for progressive laryngeal cancer. Am J Transl Res. 8:932–939.
2016.PubMed/NCBI
|
|
26
|
Xu H, Guo S, Li W and Yu P: The circular
RNA Cdr1as, via miR-7 and its targets, regulates insulin
transcription and secretion in islet cells. Sci Rep. 5:124532015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao ZJ and Shen J: Circular RNA
participates in the carcinogenesis and the malignant behavior of
cancer. RNA Biol. 14:514–521. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qi X, Zhang L and Lu X: New insights into
the epithelial-to-mesenchymal transition in cancer. Trends
Pharmacol Sci. 37:246–248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zheng Q, Bao C, Guo W, Li S, Chen J, Chen
B, Luo Y, Lyu D, Li Y, Shi G, et al: Circular RNA profiling reveals
an abundant circHIPK3 that regulates cell growth by sponging
multiple miRNAs. Nat Commun. 7:112152016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo JC, Li CQ, Wang QY, Zhao JM, Ding JY,
Li EM and Xu LY: Protein-coding genes combined with long non-coding
RNAs predict prognosis in esophageal squamous cell carcinoma
patients as a novel clinical multi-dimensional signature. Mol
Biosyst. 12:3467–3477. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen L, Zhang S, Wu J, Cui J, Zhong L,
Zeng L and Ge S: circRNA_100290 plays a role in oral cancer by
functioning as a sponge of the miR-29 family. Oncogene.
36:4551–4561. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Knosp E, Steiner E, Kitz K and Matula C:
Pituitary adenomas with invasion of the cavernous sinus space: A
magnetic resonance imaging classification compared with surgical
findings. Neurosurgery. 33:610–617; discussion. 617–618. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Glebauskiene B, Liutkeviciene R,
Vilkeviciute A, Gudinaviciene I, Rocyte A, Simonaviciute D,
Mazetyte R, Kriauciuniene L and Zaliuniene D: Association of Ki-67
labelling index and IL-17A with pituitary adenoma. Biomed Res Int.
2018:74905852018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: Limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huber W, Carey VJ, Gentleman R, Anders S,
Carlson M, Carvalho BS, Bravo HC, Davis S, Gatto L, Girke T, et al:
Orchestrating high-throughput genomic analysis with Bioconductor.
Nat Methods. 12:115–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gentleman RC, Carey VJ, Bates DM, Bolstad
B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, et al:
Bioconductor: Open software development for computational biology
and bioinformatics. Genome Biol. 5:R802004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The gene
ontology consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
The Gene Ontology Consortium: Expansion of
the gene ontology knowledgebase and resources. Nucleic Acids Res.
45:D331–D338. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kanehisa M, Furumichi M, Tanabe M, Sato Y
and Morishima K: KEGG: New perspectives on genomes, pathways,
diseases and drugs. Nucleic Acids Res. 45:D353–D361. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kanehisa M, Sato Y, Kawashima M, Furumichi
M and Tanabe M: KEGG as a reference resource for gene and protein
annotation. Nucleic Acids Res. 44:D457–D462. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kanehisa M and Goto S: KE GG Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bindea G, Mlecnik B, Hackl H, Charoentong
P, Tosolini M, Kirilovsky A, Fridman WH, Pagès F, Trajanoski Z and
Galon J: ClueGO: A Cytoscape plug-in to decipher functionally
grouped gene ontology and pathway annotation networks.
Bioinformatics. 25:1091–1093. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lee MH, Lee JH, Seol HJ, Lee JI, Kim JH,
Kong DS and Nam DH: Clinical concerns about recurrence of
non-functioning pituitary adenoma. Brain Tumor Res Treat. 4:1–7.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Qu S, Yang X, Li X, Wang J, Gao Y, Shang
R, Sun W, Dou K and Li H: Circular RNA A new star of noncoding
RNAs. Cancer Lett. 365:141–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bai N, Peng E, Qiu X, Lyu N, Zhang Z, Tao
Y, Li X and Wang Z: circFBLIM1 act as a ceRNA to promote
hepatocellular cancer progression by sponging miR-346. J Exp Clin
Cancer Res. 37:1722018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang Y, Li J, Yu J, Liu H, Shen Z, Ye G,
Mou T, Qi X and Li G: Circular RNAs signature predicts the early
recurrence of stage III gastric cancer after radical surgery.
Oncotarget. 8:22936–22943. 2017.PubMed/NCBI
|