Introduction
Endometrial cancer (EC) is the most common
malignancy of the female genital tract, with an estimated incidence
of 63,230 new cases in 2018 in the United States (1). With the increasing prevalence of major
EC risk factors, such as obesity, diabetes and hypertension, a
rising trend has also been recognized in younger women (2). EC is generally classified into two
subtypes, defined as type I or endometrioid endometrial cancer
(EEC) and type II or non-endometrioid endometrial cancer (NEEC)
(3,4). This dualistic classification underlies
different patterns of molecular alterations, pathogenesis and
clinical outcome (4).
Type I EC, which is the most frequent uterine
malignancy (80% of all cases), is an estrogen-dependent lesion,
often seen in conjunction with endometrial hyperplasia, usually
developed in peri- and early postmenopausal women (4). Type II EC affects older patients, is
not preceded by hyperplasia and comprises more aggressive
histologic subtypes, such as papillary serous, clear cell
carcinomas and carcinosarcomas (3,5). In
general, type I EC has a good prognosis, with a 10-year overall
survival rate exceeding 80% (6,7).
Surprisingly, despite optimal risk-adapted treatment, a small but
substantial number of patients exhibits recurrence and poor
survival. In such cases available risk factors or biological
markers are not able to reliably predict the poor clinical course.
Indeed, it is currently established that a classification only
based on morphologic features is inconsistent and that
molecular-based classification is desirable for optimal treatment
and prognosis of such cancers. Nevertheless, although several
genetic alterations have been associated with increased risk of EC
(8–10) and mutations in genes such as ATR
have been established to be associated with poor clinical outcomes
in EEC (11), new molecular markers
need to be identified for early diagnosis and treatment
purposes.
In 2013, researchers at The Cancer Genome Atlas
(TCGA) performed an integrated genomic, transcriptomic and
proteomic characterization of 373 endometrial carcinomas by
applying array- and sequencing-based technologies, thus drawing a
new classification of endometrial cancers according to four main
molecular categories according to their genomic features (12). This may guide post-surgical therapy
in patients affected with aggressive tumors. Yet, since taking
these results into clinical practice is currently cost-prohibitive,
molecular surrogates corresponding to each of the subgroups
(13), able to successfully
classify all patients in the TCGA cohort and to minimize false
negatives in the CN-high poor prognosticator group, were
identified.
The Encyclopedia of DNA Elements (ENCODE) project
estimates that almost 62–75% of the DNA is transcribed into RNA,
but only 2% of the transcriptome is finally translated into
proteins (14). However, this
non-coding part of the genome plays many key roles in several
biological processes and diseases such as in cancers (15). Thus, the landscape of non-coding
RNAs (ncRNAs) is increasing day by day and has emerged as a major
source of biomarkers (16,17), being considered as priority
transcripts to be monitored for functional significance in both
pathogenesis and progression of ECs. Based on their size, ncRNAs
are divided in two main categories: Small ncRNAs [sncRNAs (<200
nt)] and long ncRNAs [lncRNAs (>200 nt)]. Moreover, they are
connected to each other, with some lncRNAs acting on
post-transcriptional regulation through the modulation of certain
sncRNA subgroups (18).
Among sncRNAs, four major functional groups have
been identified in mammals: microRNAs (miRNAs),
PIWI-interacting-RNAs (piRNAs), small nucleolar-RNAs (snoRNAs) and
endogenous small interfering-RNAs (endo-siRNAs) (19). sncRNAs can exert large-scale and
diverse effects on cellular processes by regulating gene
expression, protein translation and genomic organization. In
particular, lncRNAs may contain also miRNA-binding sites and can
compete with miRNAs for interaction with their target mRNAs and
thereby block their effects on these mRNAs.
The classification of lncRNAs is more complex than
that of sncRNAs, as they can be divided on the basis of their size,
association with annotated protein-coding genes, or with other DNA
elements of known function (18,20).
Thus, lncRNAs represent a heterogeneous group of RNAs, which have
been identified as important molecules in several biological
processes, performing tumor suppression and oncogenic functions in
various types of cancer (18).
Using small RNA sequencing and microarrays, we
previously determined significant differences in sncRNA expression
patterns between normal, hyperplastic and neoplastic endometrium of
patients affected with EEC (21).
This led to the definition of a sncRNA signature (129 miRNAs, 2 of
which were not previously described, 10 piRNAs and 3 snoRNAs)
recapitulating neoplastic transformation.
In the present study, we aimed to analyze the role
of lncRNAs in EEC and to examine a possible connection with the
sncRNAs previously observed. To this aim, we first performed lncRNA
profiling in a set of EEC samples and paired normal tissues, to
identify lncRNA molecules more likely to be associated with the
cancerous state. These were also confirmed in a set of 20 paired
cancer-normal profiles deposited in the TCGA database. The 80 more
highly differentially expressed were found to be significantly
associated with different tumor grade among 405 EEC RNA profiles in
TCGA. Finally, we identified a small/long RNA functional core whose
deregulation was predicted to be strongly associated with
endometrial neoplastic transformation. Indeed, computational
analysis identified, among the most affected functional pathways,
focal adhesion, MAPK and Wnt signaling, confirming previously
published data (21).
Materials and methods
Patients and tissue collection
Six patients, who underwent type A radical
hysterectomy with or without pelvic lymphadenectomy for EC, were
enrolled in the present study at the University of Salerno. Samples
were collected from December 2013 to November 2014 in the Division
of Gynecology and Obstetrics, ‘SS. Giovanni di Dio e Ruggi
d'Aragona’ University Hospital of Salerno (Italy). Average age of
patients was 64 (range, 51–80 years). The study protocol received
institutional review board (IRB) approval before the beginning of
the study, in accordance with the code of ethics of the Declaration
of Helsinki, and informed consent was obtained from all
patients.
From each patient a sample of neoplastic tissue and
corresponding normal endometrium were collected, washed and stored
at −80°C until analysis. The histology of each EC was classified
according to World Health Organization criteria, whereas surgical
staging followed FIGO (International Federation of Gynaecology and
Obstetrics) standards.
RNA isolation and quality
controls
Tissue specimen were disrupted and homogenized using
TissueLyser LT (Qiagen, Hilden, Germany). Total RNA was extracted
with miRVana RNA Isolation kit (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer's protocol. Before
use, the RNA concentration in each sample was assayed with a
NanoDrop ND-2000c (Thermo Fisher Scientific, Inc.) and its quality
was assessed with the Agilent 2100 Bioanalyzer using Agilent RNA
6000 Nano kit (Agilent Technologies, Inc., Santa Clara, CA,
USA).
RNA sequencing and data analysis
RNA-Seq libraries were prepared as previously
described (22). Briefly, 1 µg of
total RNA was used in a library preparation according to the
Illumina TruSeq Stranded Total RNA Sample Preparation protocol
(Illumina, Inc., San Diego, CA, USA). Each library was sequenced on
HiSeq 2500 (Illumina) at a concentration of 8 pM for 200 cycles
plus 7 additional cycles for index sequencing in the paired-mode
(2×100 base pair).
RNA-Seq data analysis was performed as described in
the study by Tarallo et al (23). In details, fastQ underwent to
quality control using FastQC tool [http://www.bioinformatics.babraham.ac.uk/projects/fastqc/].
The mapping of paired-end reads was performed on reference genome
assembly hg19 using STAR (version 2.5.2b) (24). The quantification of lncRNAs
expressed for each sample was performed using HTSeq-Count algorithm
(25), while differential
expression analysis was performed with DESeq2 (26). Only lncRNAs showing a cut-off of
|fold change| ≥1.5 and adjusted P-value (P-adj) ≤0.05 were
considered for further analysis.
TCGA raw data, corresponding to either 20 tumor and
paired normal or 405 EEC tissues, were analyzed as described above
and compared to the in-house investigated samples.
Bioinformatic analyses
Pearson correlation between in-house differentially
expressed lncRNAs and paired tumor-normal TCGA data was computed
using R software (https://www.r-project.org/).
Non-negative matrix factorization (NMF) was computed
considering the normalized expression values in endometrial cancer
data taken from TCGA, considering the list of in-house
differentially expressed lncRNAs showing |fold change| ≥3 and P-adj
≤0.05 using the R package ‘nmf’ available in CRAN (27).
Kaplan-Meier survival analysis and Cox
proportional-hazard regression was generated using MedCalc 18.5
software (https://www.medcalc.org/). lncRNA and
mRNA targets of the selected miRNAs were computed using miRWalk 2.0
(28). The miRNA, lncRNA and mRNA
associated competing endogenous RNA (ceRNA) network was designed as
described by the study of Wang et al (29). Gene Ontology analysis was performed
using Co-LncRNA tool (30).
Results
Long non-coding RNA profiling in EEC
tissues
lncRNA expression profiling was performed by
next-generation sequencing in samples of type I endometrial cancer
(EEC), to evaluate their possible deregulation during
carcinogenesis.
To this aim, six patients were selected out of a
larger cohort following initial characterization according to
defined clinicopathological parameters (Table I). For each patient, two endometrial
biopsies were obtained from pathological and adjacent normal tissue
(normal tissues indicated as: 3N, 4N, 14N, 15N, 18N and 19N; tumor
tissue samples indicated as: 3T, 4T, 14T, 15T, 18T and 19T). Normal
tissues were collected as far as possible from the area presenting
a cancerous lesion, to reduce the possibility of
cross-contamination.
 | Table I.Clinicopathological features of the
tissue samples analyzed from 6 patients affected with endometrioid
endometrial cancer. |
Table I.
Clinicopathological features of the
tissue samples analyzed from 6 patients affected with endometrioid
endometrial cancer.
|
Characteristics | N | Patient nos. |
|---|
| Age (years) |
|
>55 | 5 | 3, 14, 15, 18,
19 |
|
≤55 | 1 | 4 |
| Tissue
categories |
|
Normal | 6 | 3N, 4N, 14N, 15N,
18N, 19N |
|
Endometrial cancer | 6 | 3T, 4T, 14T, 15T,
18T, 19T |
| Stage (FIGO) |
|
I–II | 5 | 3T, 4T, 14T, 15T,
18T |
|
III–IV | 1 | 19T |
| Histological
grade |
| G1 | 3 | 4T, 14T, 15T |
| G2 | 1 | 3T |
| G3 | 2 | 18T, 19T |
| Lymph node
metastasis |
|
Positive | 0 |
|
|
Negative | 5 | 3T, 4T, 15T, 18T,
19T, |
| Nx | 1 | 14T |
| Invasion |
| T1 and
T2 | 5 | 3T, 4T, 14T, 15T,
18T |
| T3 and
T4 | 1 | 19T |
More than 200 million sequences were obtained by
RNA-Seq analysis for the 12 samples sequenced, that after filtering
out low quality reads and trimming the adaptors led to
approximately 20 million reads/sample.
The obtained reads were aligned against the human
genome reference (hg19), paying particular care in lncRNA
identification. lncRNA molecules included in the human genome are
classified in seven categories [long intergenic non-coding RNA
(lincRNA), antisense, sense_overlapping, processed_transcript,
sense_intronic, bidirectional lncRNA and miRNA] according to
GENCODE gene annotation, the largest manually curated catalog of
human lncRNAs (31). More than
24,000 RNA molecules, considering both coding and non-coding ones,
were identified in total in the investigated samples and the
percentage of lncRNAs belonging to the aforementioned categories is
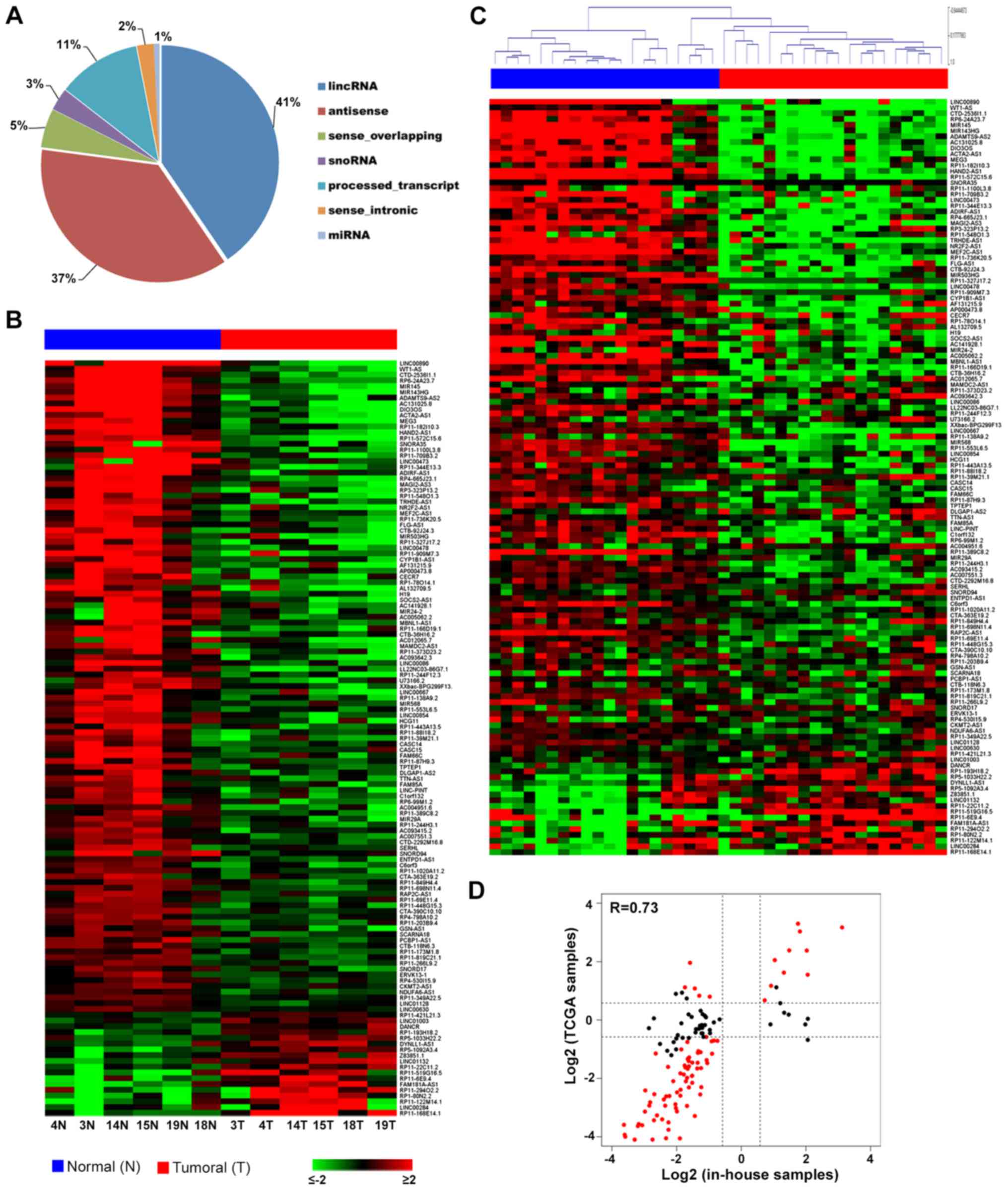
reported in Fig. 1A. In particular,
most of the lncRNAs expressed within the investigated samples
belonged to two main categories: lincRNAs (41%) and antisense
transcripts (37%).
Then, since deregulation of lncRNAs has been
associated with a broad range of physiological defects in multiple
diseases, including cancer, to investigate whether lncRNA
expression patterns may change during endometrial carcinogenesis,
differential expression analysis was performed (tumor vs. normal)
and a pool of lncRNAs specifically deregulated in cancerous tissues
was identified.
In particular, 131 lncRNAs (Fig. 1B) differentially expressed between
EEC samples and the corresponding normal endometrium were found
considering |fold change (FC)| ≥1.5 and P-adj ≤0.05, suggesting
their involvement in endometrial carcinogenesis. The full list of
statistically significant differentially expressed RNAs is
available at ArrayExpress (E-MTAB-7041).
To identify the most relevant lncRNAs in the
development of EEC, we performed genome-wide analysis by comparing
our data with TCGA molecular RNA-Seq profiles of 20 patients for
which data from primary EEC tumors and the corresponding normal
endometrial tissues were available. By analyzing the expression of
the 131 lncRNAs identified in our patients to be deregulated (|FC|
≥1.5 and P-adj ≤0.05), it emerged that most of them had the same
trend in the TCGA dataset, discriminating between cancer and normal
tissues (Fig. 1C). In particular,
comparing their expression levels, we observed a 0.73 correlation
between the two datasets (Fig. 1D).
It is also interesting to observe that the lncRNAs having an
opposite trend between the two sets of samples were mostly those
showing little changes among our samples and being not
statistically significant in the TCGA group.
To confirm the consistency of our results, we
investigated in particular the behavior of 80 lncRNAs whose
expression levels were strongly modulated in tumor samples (|FC| ≥3
and P-adj ≤0.05) among the molecular RNA-Seq profiles of 405
primary EEC tumors deposited in TCGA database.
Thus, we performed integrative analysis in order to
identify molecularly distinct tumor EEC subgroups associated with
specific clinico-biological features. Unsupervised hierarchical
clustering revealed five clusters of lncRNAs that correlated with
specific clinical parameters. In particular, the most statistically
relevant and discriminating factor resulted in histological grading
(P<0.01 by Chi-square test) (Fig.
2A).
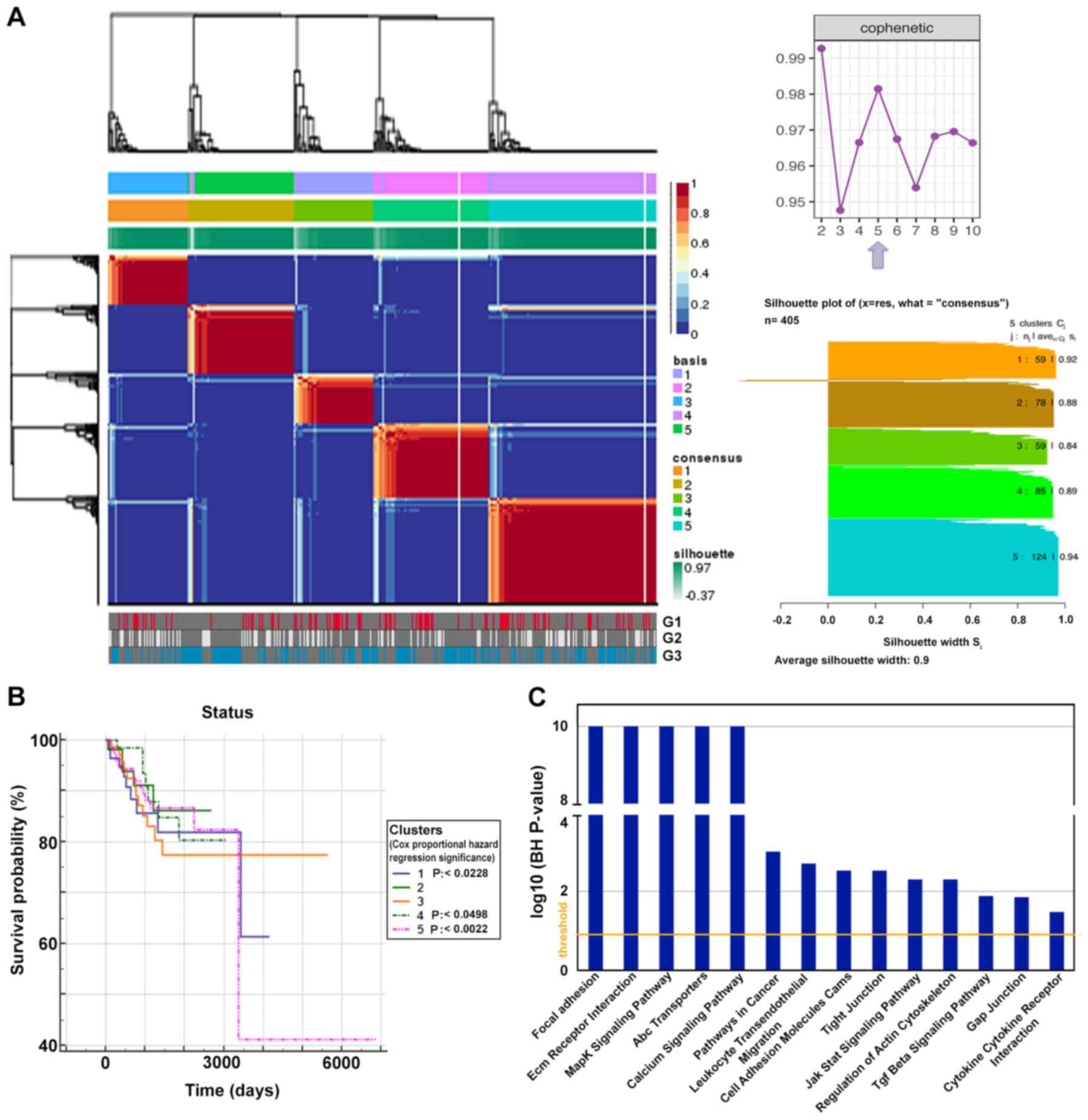 | Figure 2.lncRNAs as markers of endometrial
carcinogenesis. (A) Unsupervised clustering (non-negative matrix
factorization), using 80 differentially expressed lncRNAs in own
dataset compared to TCGA sample (|FC| ≥3 and P-adj ≤0.05),
depicting five clusters. In the lower panel, sample stratification
according to tumor grade is shown; in the upper right panel a plot
shows the change of cophenetic coefficient at rank 2 to 10, the
arrow displays the optimal number of subgroups, In the lower right
panel, silhouette plots of the 5 consensus groups, the number of
members and average silhouette width are shown. (B) Kaplan-Meier
curves showing the overall survival trends among the five clusters
according to transcriptional subtypes (log-rank test for trend
P-value=0.5890). (C) Functional pathways mainly affected after
lncRNA-mRNA co-expression analysis performed with the Co-LncRNA
tool (BH P-value ≤0.05). lncRNAs, long non-coding RNAs; TCGA, The
Cancer Genome Atlas; P-adj, adjusted P-value; FC, fold change. |
Moreover, these five clusters hold statistically
different weight on survival (P=0.5890), identifying five subgroups
of EECs with slightly different trends in overall survival, as
shown in the Kaplan-Meier survival curve of Fig. 2B. Additionally, we analyzed the
survival of patients separately in each of the five groups using
clinical stage and grade as covariates by applying Cox
proportional-hazards regression model. In this way, we found that
clusters 1 and 4 were significantly associated with survival in
patients at stage 3 (P<0.0228 and P<0.0498, respectively),
while survival was strongly associated in patients belonging to
cluster 5 classified in stage 4 (P<0.0022). All these patients
showed a significantly worse survival.
mRNA-lncRNA co-expression and ceRNA
network construction
To better investigate the functional significance of
our observations, we correlated the 80 most deregulated lncRNAs
with mRNA profiles obtained in the tumor tissues analyzed here.
This led to the identification of a set of pathways specifically
affected by mRNA-lncRNA co-expression, including MAPK, JAK/Stat and
TGF-β signaling (Fig. 2C) that was
found also to be affected by sncRNAs in our previous study
(21).
Indeed, by integrating these data with previous
results from studies of sncRNAs in EEC and considering the possible
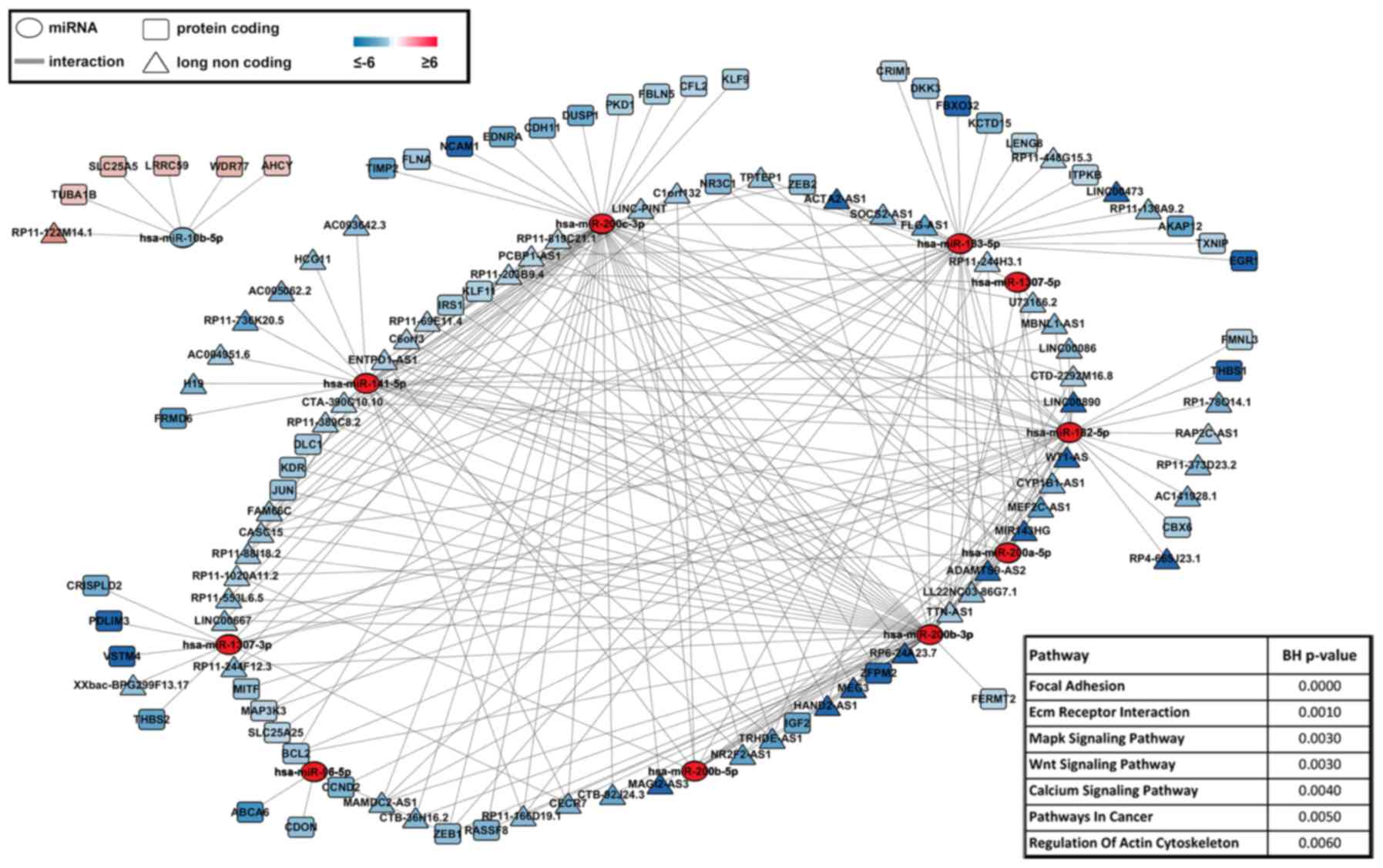
interplay between miRNAs-mRNAs-lncRNAs, a ceRNA network was
obtained, revealing miRNAs specifically targeting either mRNAs or
lncRNAs differentially expressed in EEC. To this aim, the list of
129 miRNAs representing a signature of endometrial neoplastic
transformation was filtered applying P-value correction (Bonferroni
correction), leading to 11 more significant miRNAs. An integrative
network was then drawn to show miRNA-mRNA-lncRNA correlations
(Fig. 3). In details, the analysis
was focused on miRNAs, thus, showing mRNA and lncRNA targets having
a negative correlation with respect to miRNA expression. In this
context, the most significantly affected functional pathways were
found, among others, focal adhesion, ECM receptor interaction, MAPK
and Wnt signaling (Fig. 3),
suggesting that specific small/long transcripts combination may be
directly involved in EEC onset and progression and thus being key
markers to be monitored in diagnosis and follow-up of the
disease.
Discussion
Molecular classification of ECs represents an
objective that clinicians are trying to achieve in order to provide
independent prognostic information beyond established risk
factors.
In the past few years, pragmatic molecular
classifiers have been developed, able to identify four
prognostically distinct molecular subgroups that may change the
current risk stratification systems (12,13,32).
Indeed, the evolution of genomic classification in ECs has the
potential to be used routinely in therapeutic protocol selection
and in stratifying cases in future clinical trials (13).
Nevertheless, mounting evidence has emphasized the
roles and clinical significance of non-coding RNAs and, in
particular lncRNAs, among others in endometrial cancer (33,34).
Several studies have shown the potential of lncRNAs
as therapeutic targets and investigated their involvement in cancer
pathogenesis (35,36). Indeed, limited information is
available on EC (37–44). Clarification of their role in the
onset, progression and follow-up of this tumor is warranted to help
molecular classification and targeted therapy of this cancer
histotype.
In the present study, we first evaluated the
differential expression of lncRNAs between endometrial tumor
samples and paired normal tissues of six EEC patients and then we
compared and validated our results with different EEC datasets
present in TCGA database. In this way, we identified a set of
lncRNAs discriminating between normal and cancerous endometrium,
common between those described here and publicly available data.
Moreover, deep investigation of this lncRNAs dataset among a group
of 405 EEC RNA-Seq profiles in TGCA revealed that 80 lncRNAs,
differentially expressed with a higher fold, were statistically
distributed in five clusters, reflecting tumor grade stratification
and associated with different survival trends.
A number of studies have demonstrated that a large
number of miRNA-binding sites are present on lncRNAs, suggesting
how these transcripts can serve also as competing endogenous RNAs
controlling ‘free’ miRNA levels and functions (45,46).
Importantly, lncRNAs could compete with mRNAs for miRNAs, and
thereby regulate miRNA-mediated transcript repression (45,46).
Therefore, we built a ceRNA network, depicting key interactions
occurring among differentially expressed mRNAs, lncRNAs and miRNAs
in our EEC samples and determining a functional impact within
crucial pathways in EC, which may represent potential core
regulators to be monitored for early diagnosis and therapeutic
purposes. Indeed, understanding molecular interactions has becoming
a common tool especially in cancer research, given the limited
information provided to date by studies focusing on a single
molecule (47–49).
In this regard, our results strengthen and integrate
the current knowledge concerning EEC molecular markers, thus
providing novel details about their functional correlations. In
this way, a functional multi-molecule core has been identified,
showing miRNA members mostly deregulated in EEC and the
corresponding mRNA and lncRNA targets inversely correlated with
miRNA expression. From this snapshot it emerges, for example, the
coexistence between downregulation of miR-10b that has been also
proved to be downregulated in other types of cancer, such as
gastric and cervical ones (50,51)
and upregulation of its long-RNA targets. Moreover, we confirmed
upregulation of almost the entire miR-200 family (miR-200a/b/c and
miR-141), an event already well documented in EEC, where these RNAs
target mainly genes involved in tissue transformation responsible
for epithelial-to-mesenchymal transition (EMT) (52,53).
In this context, we observed an inverse correlation, among others,
with the well known miR-200 targets ZEB1 and ZEB2, transcriptional
repressors of E-cadherin whose expression is restored in EMT
(53) and KLF9, whose
downregulation in EEC has been previously demonstrated (54,55).
Downregulation was also retrieved for TIMP2 representing, together
with the above mentioned mRNAs, a key gene involved in the
differentiation of EEC and proposed as a potential therapeutic
target (56–58). More interestingly, an inverse
correlation was also found with several downregulated lncRNAs,
including ADAMTS9-AS2, MEG3 and HAND2-AS1, already demonstrated to
act as tumor suppressors. Indeed, ADAMTS9-AS2 has been associated
with the inhibition of cancer progression and migration in lung and
glioma cells; its expression has been correlated with poor
prognosis through a mechanism involving the interaction with DNMT1
(59,60). In addition, a role of MEG3 in EEC
tumorigenesis and progression through the modulation of Notch and
PI3K pathways has been proved (40,61).
In several cancer types, MEG3 has been shown to sequester various
microRNAs from protein and/or target mRNAs resulting in altered
protein activity, translation and degradation; in particular
involvement of this lncRNA in the regulation of the miR-200 family
has been demonstrated, confirming what was revealed by the ceRNA
model constructed here (Fig. 3)
(62). Finally, HAND2-AS1 has been
shown to be involved in inhibition of EEC invasion and metastasis,
through the downregulation of neuromedin U (63), and it has been proposed to play a
role in the tumorigenesis of muscle-invasive bladder cancer through
the interaction with many several miRNAs (including, of note, also
miR-183) (64).
In conclusion, in the present study we identified a
functional core, constituted by a specific combination of
miRNAs-mRNAs-lncRNAs expression, which represents a molecular
signature of potential usefulness to monitor EEC progression, for
follow-up and prognosis of this disease.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Italian
Ministry of Education University and Research (Flagship Project
InterOmics), the Italian Association for Cancer Research (AIRC,
Grant IG-17426), the University of Salerno (FARB) and the
Genomix4Life Srl.
Availability of data and materials
The results shown here are in part based upon data
generated by the TCGA Research Network: http://cancergenome.nih.gov/. The datasets generated
and analyzed in this study are available in the EBI ArrayExpress
database (http://www.ebi.ac.uk/arrayexpress) with Accession
Number E-MTAB-7039 (raw data) and E-MTAB-7041.
Authors' contributions
AW, FR, FZ and MG designed and coordinated the
study; MAC, AC and MR selected the patients and MAC collected the
clinical samples; AC, FR, GN, MR, PS and RT carried out the
experimental work; AR and GG performed the data analyses; AC, MR
and RT wrote the manuscript; MR, AC, PS, AR, MAC, GN, GG, FZ, AW,
RT, FR and MG contributed to data interpretation and discussion and
to manuscript revision. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The study protocol received approval by the Ethics
Committee of the ‘SS. Giovanni di Dio e Ruggi d'Aragona’ University
of Salerno Hospital (n.er 91/13.12.2013) before the beginning of
the study, in accordance with The Code of Ethics of the Declaration
of Helsinki, and informed consent was obtained from all patients
included.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Pasquale Saggese is a PhD student of the Research
Doctorate in ‘Biomedical Science and Technology’ (XXXI cycle),
‘Roma TRE’ University.
Glossary
Abbreviations
Abbreviations:
|
EC
|
endometrial cancer
|
|
EEC
|
endometrioid endometrial cancer
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
FC
|
fold change
|
|
lincRNA
|
long intergenic non-coding RNA
|
|
lncRNA
|
long non-coding RNA
|
|
miRNA
|
microRNA
|
|
ncRNA
|
non-coding RNA
|
|
ceRNA
|
competing endogenous RNA
|
|
NEEC
|
non-endometrioid endometrial
cancer
|
|
P-adj
|
adjusted P-value
|
|
piRNA
|
PIWI-interacting RNA
|
|
sncRNA
|
small non-coding RNA
|
|
TCGA
|
The Cancer Genome Atlas
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Koh WJ, Greer BE, Abu-Rustum NR, Apte SM,
Campos SM, Chan J, Cho KR, Cohn D, Crispens MA, Dupont N, et al:
Uterine neoplasms, version 1.2014. J Natl Compr Canc Netw.
12:248–280. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zannoni GF, Scambia G and Gallo D: The
dualistic model of endometrial cancer: The challenge of classifying
grade 3 endometrioid carcinoma. Gynecol Oncol. 127:262–263. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amant F, Cadron I, Fuso L, Berteloot P, de
Jonge E, Jacomen G, Van Robaeys J, Neven P, Moerman P and Vergote
I: Endometrial carcinosarcomas have a different prognosis and
pattern of spread compared to high-risk epithelial endometrial
cancer. Gynecol Oncol. 98:274–280. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lajer H, Elnegaard S, Christensen RD,
Ortoft G, Schledermann DE and Mogensen O: Survival after stage IA
endometrial cancer; can follow-up be altered? A prospective
nationwide Danish survey. Acta Obstet Gynecol Scand. 91:976–982.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kitchener HC and Trimble EL: Endometrial
Cancer Working Group of the Gynecologic Cancer Intergroup:
Endometrial cancer state of the science meeting. Int J Gynecol
Cancer. 19:134–140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Torricelli F, Nicoli D, Bellazzi R,
Ciarrocchi A, Farnetti E, Mastrofilippo V, Zamponi R, La Sala GB,
Casali B and Mandato VD: Computational development of a
molecular-based approach to improve risk stratification of
endometrial cancer patients. Oncotarget. 9:25517–25528. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee PJ, McNulty S, Duncavage EJ, Heusel JW
and Hagemann IS: Clinical targeted next-generation sequencing shows
increased mutational load in endometrioid-type endometrial
adenocarcinoma with deficient DNA mismatch repair. Int J Gynecol
Pathol. 37:581–589. 2017.
|
|
10
|
DeLair DF, Burke KA, Selenica P, Lim RS,
Scott SN, Middha S, Mohanty AS, Cheng DT, Berger MF, Soslow RA and
Weigelt B: The genetic landscape of endometrial clear cell
carcinomas. J Pathol. 243:230–241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zighelboim I, Schmidt AP, Gao F, Thaker
PH, Powell MA, Rader JS, Gibb RK, Mutch DG and Goodfellow PJ: ATR
mutation in endometrioid endometrial cancer is associated with poor
clinical outcomes. J Clin Oncol. 27:3091–3096. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cancer Genome Atlas Research Network, ;
Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H,
Robertson AG, Pashtan I, Shen R, et al: Integrated genomic
characterization of endometrial carcinoma. Nature. 497:67–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Talhouk A, McConechy MK, Leung S, Li-Chang
HH, Kwon JS, Melnyk N, Yang W, Senz J, Boyd N, Karnezis AN, et al:
A clinically applicable molecular-based classification for
endometrial cancers. Br J Cancer. 113:299–310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
ENCODE Project Consortium. An integrated
encyclopedia of DNA elements in the human genome. Nature.
489:57–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wilusz JE, Sunwoo H and Spector DL: Long
noncoding RNAs: Functional surprises from the RNA world. Genes Dev.
23:1494–1504. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gibb EA, Vucic EA, Enfield KS, Stewart GL,
Lonergan KM, Kennett JY, Becker-Santos DD, MacAulay CE, Lam S,
Brown CJ, et al: Human cancer long non-coding RNA transcriptomes.
PLoS One. 6:e259152011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gibb EA, Brown CJ and Lam WL: The
functional role of long non-coding RNA in human carcinomas. Mol
Cancer. 10:382011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Angrand PO, Vennin C, Le Bourhis X and
Adriaenssens E: The role of long non-coding RNAs in genome
formatting and expression. Front Genet. 6:1652015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
St Laurent G, Wahlestedt C and Kapranov P:
The landscape of long noncoding RNA classification. Trends Genet.
31:239–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ravo M, Cordella A, Rinaldi A, Bruno G,
Alexandrova E, Saggese P, Nassa G, Giurato G, Tarallo R, Marchese
G, et al: Small non-coding RNA deregulation in endometrial
carcinogenesis. Oncotarget. 6:4677–4691. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nassa G, Giurato G, Cimmino G, Rizzo F,
Ravo M, Salvati A, Nyman TA, Zhu Y, Vesterlund M, Lehtiö J, et al:
Splicing of platelet resident pre-mRNAs upon activation by
physiological stimuli results in functionally relevant proteome
modifications. Sci Rep. 8:4982018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tarallo R, Giurato G, Bruno G, Ravo M,
Rizzo F, Salvati A, Ricciardi L, Marchese G, Cordella A, Rocco T,
et al: The nuclear receptor ERbeta engages AGO2 in regulation of
gene transcription, RNA splicing and RISC loading. Genome Biol.
18:1892017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dobin A, Davis CA, Schlesinger F, Drenkow
J, Zaleski C, Jha S, Batut P, Chaisson M and Gingeras TR: STAR:
Ultrafast universal RNA-seq aligner. Bioinformatics. 29:15–21.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Anders S, Pyl PT and Huber W: HTSeq-a
Python framework to work with high-throughput sequencing data.
Bioinformatics. 31:166–169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15:5502014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gaujoux R and Seoighe C: A flexible R
package for nonnegative matrix factorization. BMC Bioinformatics.
11:3672010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dweep H and Gretz N: miRWalk2.0: A
comprehensive atlas of microRNA-target interactions. Nat Methods.
12:6972015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang W, Zhuang Q, Ji K, Wen B, Lin P, Zhao
Y, Li W and Yan C: Identification of miRNA, lncRNA and
mRNA-associated ceRNA networks and potential biomarker for MELAS
with mitochondrial DNA A3243G mutation. Sci Rep. 7:416392017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao Z, Bai J, Wu A, Wang Y, Zhang J, Wang
Z, Li Y, Xu J and Li X: Co-LncRNA: Investigating the lncRNA
combinatorial effects in GO annotations and KEGG pathways based on
human RNA-Seq data. Database. 2015:bav0822015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Derrien T, Johnson R, Bussotti G, Tanzer
A, Djebali S, Tilgner H, Guernec G, Martin D, Merkel A, Knowles DG,
et al: The GENCODE v7 catalog of human long noncoding RNAs:
Analysis of their gene structure, evolution, and expression. Genome
Res. 22:1775–1789. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Talhouk A and McAlpine JN: New
classification of endometrial cancers: The development and
potential applications of genomic-based classification in research
and clinical care. Gynecol Oncol Res Pract. 3:142016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vallone C, Rigon G, Gulia C, Baffa A,
Votino R, Morosetti G, Zaami S, Briganti V, Catania F, Gaffi M, et
al: Non-coding RNAs and endometrial cancer. Genes. 9:E1872018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu J, Qian Y, Ye M, Fu Z, Jia X, Li W, Xu
P, Lv M, Huang L, Wang L, et al: Distinct expression profile of
lncRNA in endometrial carcinoma. Oncol Rep. 36:3405–3412. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fatima R, Akhade VS, Pal D and Rao SM:
Long noncoding RNAs in development and cancer: Potential biomarkers
and therapeutic targets. Mol Cell Ther. 3:52015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen BJ, Byrne FL, Takenaka K, Modesitt
SC, Olzomer EM, Mills JD, Farrell R, Hoehn KL and Janitz M:
Transcriptome landscape of long intergenic non-coding RNAs in
endometrial cancer. Gynecol Oncol. 147:654–662. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang J, Ke P, Guo L, Wang W, Tan H, Liang
Y and Yao S: Lentivirus-mediated RNA interference targeting the
long noncoding RNA HOTAIR inhibits proliferation and invasion of
endometrial carcinoma cells in vitro and in vivo. Int J Gynecol
Cancer. 24:635–642. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shang C, Lang B, Ao CN and Meng L: Long
non-coding RNA tumor suppressor candidate 7 advances chemotherapy
sensitivity of endometrial carcinoma through targeted silencing of
miR-23b. Tumour Biol. 39:10104283177078832017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun KX, Wu DD, Chen S, Zhao Y and Zong ZH:
LncRNA MEG3 inhibit endometrial carcinoma tumorigenesis and
progression through PI3K pathway. Apoptosis. 22:1543–1552. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang D, Wang D, Wang N, Long Z and Ren X:
Long non-coding RNA BANCR promotes endometrial cancer cell
proliferation and invasion by regulating MMP2 and MMP1 via ERK/MAPK
signaling pathway. Cell Physiol Biochem. 40:644–656. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang L, Zhang J, Jiang A, Liu Q, Li C,
Yang C and Xiu J: Expression profile of long non-coding RNAs is
altered in endometrial cancer. Int J Clin Exp Med. 8:5010–5021.
2015.PubMed/NCBI
|
|
43
|
Xin W, Liu X, Ding J, Zhao J, Zhou Y, Wu Q
and Hua K: Long non-coding RNA derived miR-205-5p modulates human
endometrial cancer by targeting PTEN. Am J Transl Res. 7:2433–2441.
2015.PubMed/NCBI
|
|
44
|
Zhai W, Li X, Wu S, Zhang Y, Pang H and
Chen W: Microarray expression profile of lncRNAs and the
upregulated ASLNC04080 lncRNA in human endometrial carcinoma. Int J
Oncol. 46:2125–2137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The Rosetta Stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cheng DL, Xiang YY, Ji LJ and Lu XJ:
Competing endogenous RNA interplay in cancer: Mechanism,
methodology, and perspectives. Tumour Biol. 36:479–488. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lu M, Xu X, Xi B, Dai Q, Li C, Su L, Zhou
X, Tang M, Yao Y and Yang J: Molecular network-based identification
of competing endogenous RNAs in thyroid carcinoma. Genes.
9:E442018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu C, Zhang YH, Deng Q, Li Y, Huang T,
Zhou S and Cai YD: Cancer-related triplets of mRNA-lncRNA-miRNA
revealed by integrative network in uterine corpus endometrial
carcinoma. Biomed Res Int. 2017:38595822017.PubMed/NCBI
|
|
50
|
Jia H, Zhang Z, Zou D, Wang B, Yan Y, Luo
M, Dong L, Yin H, Gong B, Li Z, et al: MicroRNA-10a is
down-regulated by DNA methylation and functions as a tumor
suppressor in gastric cancer cells. PLoS One. 9:e880572014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zou D, Zhou Q, Wang D, Guan L, Yuan L and
Li S: The downregulation of MicroRNA-10b and its role in cervical
cancer. Oncol Res. 24:99–108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Snowdon J, Zhang X, Childs T, Tron VA and
Feilotter H: The microRNA-200 family is upregulated in endometrial
carcinoma. PLoS One. 6:e228282011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Panda H, Pelakh L, Chuang TD, Luo X,
Bukulmez O and Chegini N: Endometrial miR-200c is altered during
transformation into cancerous states and targets the expression of
ZEBsVEGFAFLT1IKKbetaKLF9, and FBLN5. Reprod Sci.
19:786–796. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Korani M, Fallah S, Tehranian A,
Nourbakhsh M, Samadikuchaksaraei A, Pour MS and Maleki J: The
evaluation of the FOXO1, KLF9 and YT521 genes expression in human
endometrial cancer. Clin Lab. 59:483–489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Simmons CD, Pabona JM, Heard ME, Friedman
TM, Spataro MT, Godley AL, Simmen FA, Burnett AF and Simmen RC:
Krüppel-like factor 9 loss-of-expression in human endometrial
carcinoma links altered expression of growth-regulatory genes with
aberrant proliferative response to estrogen. Biol Reprod.
85:378–385. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu Y, Nan F, Lu K and Wang Y, Liu Y, Wei
S, Wu R and Wang Y: Identification of key genes in endometrioid
endometrial adenocarcinoma via TCGA database. Cancer Biomark.
21:11–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Honkavuori-Toivola M, Santala M, Soini Y,
Turpeenniemi- Hujanen T and Talvensaari-Mattila A: Combination of
strong MMP-2 and weak TIMP-2 immunostainings is a significant
prognostic factor in endometrial carcinoma. Dis Markers.
35:261–266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dai Y, Xia W, Song T, Su X, Li J, Li S,
Chen Y, Wang W, Ding H, Liu X, et al: MicroRNA-200b is
overexpressed in endometrial adenocarcinomas and enhances
MMP2 activity by downregulating TIMP2 in human
endometrial cancer cell line HEC-1A cells. Nucleic Acid Ther.
23:29–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liu C, Yang Z, Deng Z, Zhou Y, Gong Q,
Zhao R and Chen T: Upregulated lncRNA ADAMTS9-AS2 suppresses
progression of lung cancer through inhibition of miR-223-3p and
promotion of TGFBR3. IUBMB Life. 70:536–546. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yao J, Zhou B, Zhang J, Geng P, Liu K, Zhu
Y and Zhu W: A new tumor suppressor LncRNA ADAMTS9-AS2 is regulated
by DNMT1 and inhibits migration of glioma cells. Tumour Biol.
35:7935–7944. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Guo Q, Qian Z, Yan D, Li L and Huang L:
LncRNA-MEG3 inhibits cell proliferation of endometrial carcinoma by
repressing Notch signaling. Biomed Pharmacother. 82:589–594. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Terashima M, Tange S, Ishimura A and
Suzuki T: MEG3 long noncoding RNA contributes to the epigenetic
regulation of epithelial-mesenchymal transition in lung cancer cell
lines. J Biol Chem. 292:82–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yang X, Wang CC, Lee WYW, Trovik J, Chung
TKH and Kwong J: Long non-coding RNA HAND2-AS1 inhibits invasion
and metastasis in endometrioid endometrial carcinoma through
inactivating neuromedin U. Cancer Lett. 413:23–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wang H, Niu L, Jiang S, Zhai J, Wang P,
Kong F and Jin X: Comprehensive analysis of aberrantly expressed
profiles of lncRNAs and miRNAs with associated ceRNA network in
muscle-invasive bladder cancer. Oncotarget. 7:86174–86185. 2016.
View Article : Google Scholar : PubMed/NCBI
|