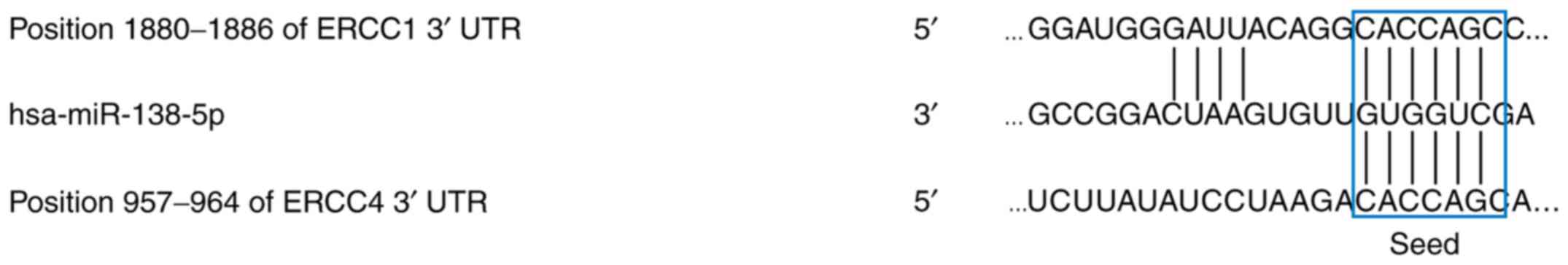Introduction
Gastric cancer is one of the most common and lethal
types of malignancy worldwide, ranking fourth in morbidity and
third in mortality (1). China has
the highest incidence of gastric cancer (2). Although there has been progress
regarding the diagnosis and treatment of gastric cancer over the
past few decades, the prognosis is generally poor, partially due to
the highly invasive and metastatic nature of this tumor. Treatment
of advanced gastric cancer primarily relies on palliative
chemotherapy, with platinum drugs remaining the cornerstone of
treatment. However, the majority of antineoplastic protocols that
include platinum-based drugs have a <50% efficacy rate, and the
development of primary and secondary resistance restrict the
applications of these drugs (3).
Therefore, the identification of novel biomarkers able to predict
the efficacy of platinum-based therapy and guide clinical treatment
is required.
Excision repair cross-complementing (ERCC) proteins
are key components of the nuclear excision repair (NER) signaling
pathway, which is a primary cause of cisplatin [also known as
diamminedichloroplatinum (II), DDP] resistance. Previous studies
have confirmed that increased expression of ERCC1 is associated
with cisplatin resistance in SGC7901/DDP cells (4). ERCC1 forms a heterodimer with ERCC4 to
excise the 5′ end of damaged DNA as part of the NER signaling
pathway. ERCC4- and ERCC1-deficient cells have been reported to be
40 times more sensitive to cisplatin compared with the parental
cells (5), supporting the role of
these enzymes in the NER signaling pathway and cisplatin
resistance. Increasing evidence suggests that microRNAs
(miRNAs/miRs) influence the efficacy of chemotherapeutic drugs by
regulating gene expression (6).
miR-138 is abnormally expressed in various tumors, and has been
associated with drug resistance in lung cancer (7–9),
cervical cancer (10) and
osteosarcoma (11) cell lines.
miR-138 promotes chemotherapeutic sensitivity by targeted
regulation of DNA repair, induction of apoptosis and inhibition of
the epithelial-mesenchymal transition (EMT) (8–12),
Nevertheless, little is known regarding the association between
miR-138-5p expression and chemotherapeutic sensitivity of gastric
cancer. In the present study, the expression of miR-138-5p was
examined in a gastric cancer cell line SGC7901 and its
cisplatin-resistant derivative SGC7901/DDP. The expression of two
putative miR-138-5p target genes and the effects of miR-138-5p
modulation on cisplatin sensitivity in the cell lines were also
determined.
Materials and methods
Cell culture
The human gastric adenocarcinoma cell lines
SGC7901/DDP and SGC7901 were obtained from Nanjing KeyGen Biotech
Co., Ltd. (Nanjing, China). The cisplatin-resistant SGC7901/DDP
cell line was developed from the parental SGC7901 line by exposure
to increasing concentrations of cisplatin over ~12 months. The
cells acquired resistance at 1 µg/ml cisplatin. We have also
previously confirmed that SGC7901/DDP cells are highly resistant to
cisplatin using cell viability assays (13). All cells were maintained in
RPMI-1640 containing 10% fetal calf serum, 100 U/ml penicillin, and
100 U/ml streptomycin (all from Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) in a humidified atmosphere with 5%
CO2 at 37°C. Cells were cultured in the absence
(SGC7901) or presence (SGC7901/DDP) of 800 ng/ml cisplatin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
RNA isolation
Total RNA was extracted from the gastric cancer
cells using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.)
following the manufacturer's protocol. RNA concentrations were
measured using a NanoDrop-2000 spectrophotometer with the
absorbance set at 260 nm. Aliquots of total RNA were used for the
microarray and reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) analyses.
Lentiviral infection
miR-138-5p-depleted or -overexpressing cell lines
were constructed using lentiviral-mediated short hairpin (sh)RNA
targeting or overexpression of miR-138-5p. Vectors encoding
miR-138-5p (pLV-miR-138-5p) or a negative control sequence
(pLV-miR-138-5p-NC) and the corresponding viruses (1×108
plaque-forming units) were all purchased from GeneCopoeia, Inc.
(Rockville, MD, USA). Lentiviral infection was performed according
to the manufacturer's protocol. Briefly, SGC7901/DDP cells were
cultured overnight in 12-well plates at a density of
1×105 cells/well, and then infected with the appropriate
lentivirus at a multiplicity of infection of 10 plaque-forming
units/cell. A total of 72 h after the transduction, SGC7901/DDP
cells were transferred to RMPI-1640 containing 2 µg/ml puromycin
and cultured for 3 days. Cells that survived were selected and used
for subsequent experiments.
A similar approach was used to knock down miR-138-5p
in SGC7901 cells, except that the cells were infected with
lentiviral vectors encoding a control sequence or short hairpin
(sh)RNA specific for miR-138-5p and then cultured for 5 days in
RPMI-1640 containing 150 µg/ml hygromycin. Cells that survived were
selected and used for subsequent experiments.
The lentiviral vectors co-expressed green
fluorescent protein (GFP) for overexpression or red fluorescent
protein (RFP) for silencing to allow verification of transduction
efficacy by fluorescence microscopy.
RT-qPCR analysis
RT-qPCR was performed on an Mx3000P qPCR system with
a SYBR Green single-step kit RT-qPCR kit (Biomics Biopharma,
Nantong, China) according to the manufacturer's protocol. Aliquots
of cDNA were amplified under the following conditions: 42°C for 30
min followed by 40 cycles of 20 sec at 95°C, 30 sec at 60°C and 30
sec at 72°C; and an extension step for 2 min at 72°C to obtain the
fluorescent signals. All miRNA levels were normalized to U6
expression. Primers used in this study were: miR-138-5p forward,
5′-AGCTGGTGTTGTGAATCAGGCCG-3′ and reverse, 5′-TGGTGTCGTGGAGTCG-3′;
U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′. The relative differences were
quantified using the 2−ΔΔCq method, where ΔΔCq is
calculated as the: Experimental
(CtmiR-138-5p-Ctu6)-control
(CtmiR-138-5p-Ctu6). The procedure was
performed in triplicate (14).
Microarray analysis
Total RNA was extracted from triplicate biological
samples of SGC7901/DDP and SGC7901 cells as aforementioned and sent
to Beijing CapitalBio Technology Co., Ltd. (Beijing, China) for
microarray analysis.
Expression profiling was performed using an
Affymetrix miRNA 4.0 Array, which contains 775 mature human miRNA
probe sets. The output data, which contained normalized miRNA
expression profiles, were analyzed in Excel spreadsheets. The two
groups of data were analyzed by t-tests to obtain P-values, and
false discovery rate adjustment was performed to generate corrected
P-values. The fold change in differentially expressed miRNAs
between SGC7901/DDP and SGC7901 cells was calculated, and Cluster
3.0 software (http://bonsai.hgc.jp/~mdehoon/software/cluster/software.htm)
was used to display the differential expression patterns. Genes
potentially targeted by the differentially expressed miRNAs were
identified using the online tools DIANAmT http://diana.imis.athena-innovation.gr/), miRanda
(http://www.microrna.org/microrna/home.do), miRDB
(http://www.mirdb.org/), miRWalk (http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/index.html),
RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/),
PICTAR4 (http://pictar.mdc-berlin.de), PICTAR5
(http://pictar.mdc-berlin.de), PITA
(http://genie.weizmann.ac.il/pubs/mir07/mir07_data.html),
RNA22 (https://cm.jefferson.edu/rna22/) and TargetScan
(http://www.targetscan.org/).
Western blotting
Total proteins were extracted from cultured SCG7901
cells and SGC7901/DDP cells with or without lentivirus infection by
cell lysis in radioimmunoprecipitation assay buffer containing
protease inhibitors (Beyotime Institute of Biotechnology, Shanghai,
China). Protein concentrations were quantitatively determined using
a BCA Protein Assay kit (Beyotime Institute of Biotechnology).
Lysate samples containing 60 µg total protein were electrophoresed
on a 10 or 8% SDS-PAGE and transferred to polyvinylidene difluoride
(PVDF) membranes (Beyotime Institute of Biotechnology). The PVDF
membrane was blocked with 5% non-fat milk for 1.5 h at room
temperature and probed overnight at 4°C with the following primary
antibodies: Rabbit anti-ERCC1 (1:200 dilution; cat. no. ab129267);
rabbit anti-ERCC4 (XPF) (1:300 dilution; cat. no. ab76948); rabbit
anti-β-actin (1:1,000 dilution; cat. no. ab8227); and rabbit
anti-GAPDH (1:1,000 dilution; cat. no. ab9485) (all from Abcam,
Cambridge, UK). Following washing with Tris-buffered saline
containing 0.1% Tween-20, the membranes were incubated with goat
anti-rabbit horseradish peroxidase-conjugated secondary antibody
(1:5,000 dilution; cat. no. 7074S; Shanghai Youningwei
Biotechnology Co. Ltd., Shanghai, China) for 4 h at 4°C. Protein
bands were visualized with an ECL Plus chemiluminescent kit (Thermo
Scientific Fisher, Inc.). Protein levels were quantified by
densitometry (Tanon2500 Fully Automatic Digital Gel Image Analysis
system; Tanon Science and Technology Co., Ltd., Shanghai, China)
and are presented as the fold change in expression after
normalization to GAPDH and β-actin.
Cisplatin sensitivity assay
Cisplatin sensitivity was determined using the
colorimetric MTT assay. SGC7901/DDP and SGC7901 cells, their
miR-138-5p-overexpressing or -silenced counterparts, and control
cells were resuspended at 1×105 cells/ml. Aliquots of
100 µl were distributed into 96-well plates, and the volumes were
made up to 200 µl/well with RPMI-1640. The cells were incubated for
24 h, and 180 µl of supernatant/well was then removed and replaced
with freshly prepared DDP at final concentrations of 40, 20, 2, 0.2
and 0.02 µg/ml for DDP-resistant groups; and at final
concentrations of 4, 2, 0.2, 0.02 and 0.002 µg/ml for DDP-sensitive
groups. These concentrations were selected because the peak
concentration of DDP in human plasma is 2.0 µg/ml (15). A total of 48 h later, 180 µl/well of
supernatant was removed and replaced with 180 µl fresh medium plus
20 µl of MTT/well (5 mg/ml, Sigma-Aldrich; Merck KgaA). The plate
was incubated for 4 h in a humidified atmosphere at 37°C, the
medium was removed, and 150 µl/well DMSO (Sigma-Aldrich; Merck
KGaA) was added. The plates were mixed for 10 min at 72°C to
dissolve the formazan crystals and the absorbance at 490 nm was
then measured using a spectrophotometer. The 50% inhibitory
concentration (IC50) of DDP was calculated based on the
relative viability of control wells lacking DDP. The assay was
performed in triplicate.
Statistical analysis
Statistical analysis was performed using SPSS 19.0
software (IBM Corp., Armonk, NY, USA). All data are presented as
the mean ± standard deviation. A two-tailed Student's t-test was
used to compare differences between two groups. One-way analysis of
variance and the Bonferroni post hoc test was used to compare
differences between multiple groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of miR-138-5p in
SGC7901/DDP and SGC7901 cells
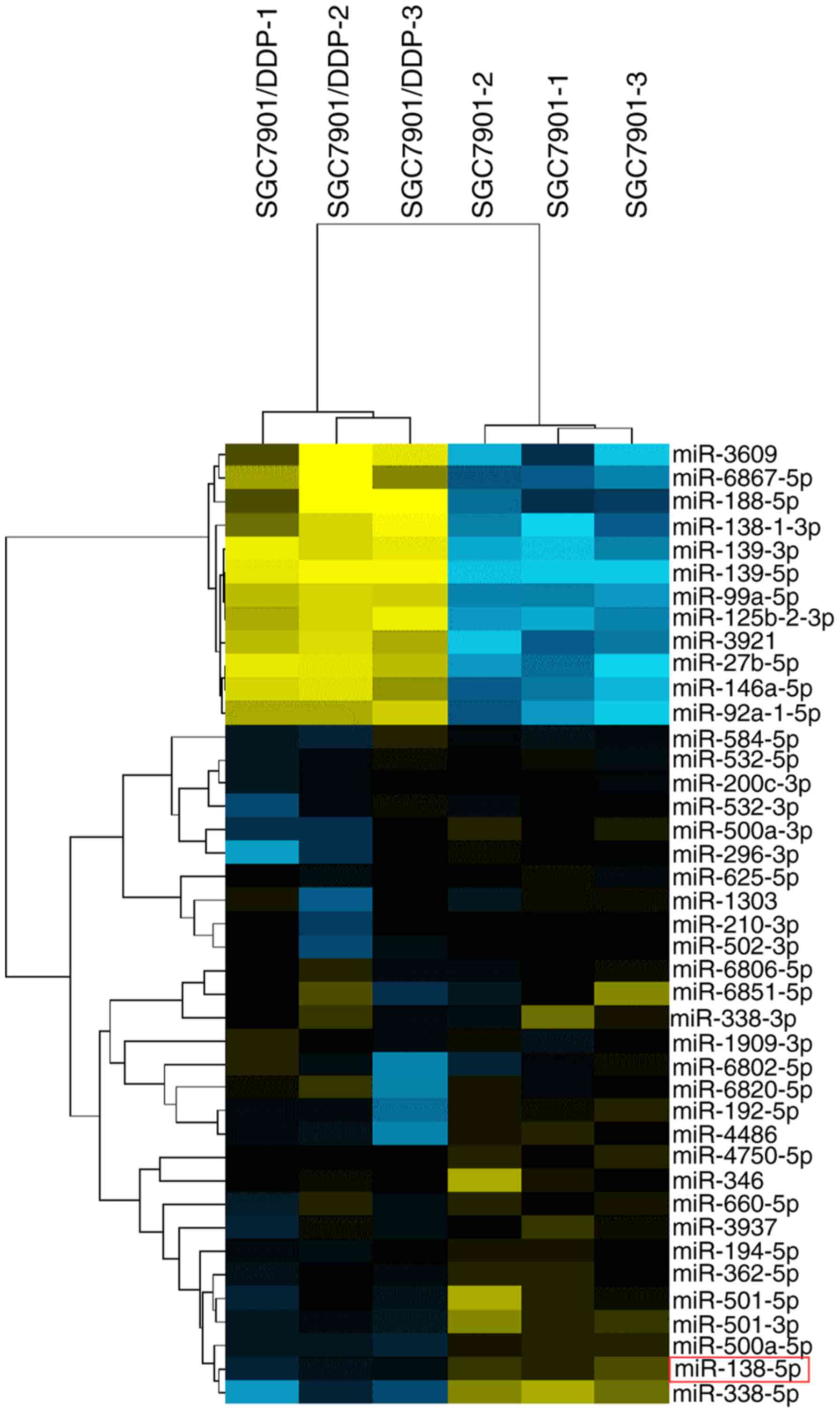
To identify miRNAs potentially involved in cisplatin
resistance, miRNA microarray analysis was performed on triplicate
samples of SGC7901 and SGC7901/DDP cells. As shown in Fig. 1 and Table I, 41 miRNAs were significantly
differentially expressed between SGC7901 and SGC7901/DDP cells,
using a threshold of ≥2-fold or ≤0.5-fold change and P<0.05. For
further analysis, miR-138-5p was selected, which exhibited the
largest difference in expression between the cisplatin-sensitive
and -resistant cell lines. This miRNA was of particular interest as
it has previously been reported to modulate drug resistance in lung
cancer by acting on the NER pathway (9,16). The
expression of miR-138-5p was ~3-fold lower in SGC7901/DDP cells
compared with SGC7901 cells (P<0.05; Fig. 1).
 | Table I.Fold change of differentially
expressed miRNAs in SGC7901/DDP and SGC7901 cells. |
Table I.
Fold change of differentially
expressed miRNAs in SGC7901/DDP and SGC7901 cells.
| miRNAs | Fold change | q-value
(%) |
|---|
| hsa-miR-139-5p | 3.9627 | 0 |
| hsa-miR-139-3p | 2.8456 | 0 |
| hsa-miR-3609 | 2.6297 | 1.0853785 |
| hsa-miR-27b-5p | 2.6106 | 0 |
|
hsa-miR-138-1-3p | 2.3803 | 1.0853785 |
|
hsa-miR-125b-2-3p | 2.3502 | 0 |
| hsa-miR-188-5p | 2.2048 | 3.1418851 |
| hsa-miR-99a-5p | 2.0877 | 0 |
|
hsa-miR-6867-5p | 2.0662 | 2.7032068 |
| hsa-miR-3921 | 2.0584 | 0 |
|
hsa-miR-146a-5p | 2.0484 | 0 |
|
hsa-miR-92a-1-5p | 2.0356 | 1.0853785 |
| hsa-miR-584-5p | 0.4914 | 2.7203157 |
|
hsa-miR-1909-3p | 0.4902 | 1.4046075 |
|
hsa-miR-6806-5p | 0.4859 | 0 |
| hsa-miR-532-5p | 0.4509 | 0 |
| hsa-miR-625-5p | 0.4481 | 0 |
|
hsa-miR-6851-5p | 0.4475 | 3.0810745 |
|
hsa-miR-200c-3p | 0.444 | 0 |
| hsa-miR-338-3p | 0.4315 | 0 |
| hsa-miR-532-3p | 0.4282 | 0 |
|
hsa-miR-6802-5p | 0.4274 | 2.7203157 |
|
hsa-miR-4750-5p | 0.4255 | 0 |
| hsa-miR-1303 | 0.4198 | 2.7203157 |
|
hsa-miR-6820-5p | 0.4108 | 1.5801834 |
| hsa-miR-210-3p | 0.3935 | 0 |
| hsa-miR-346 | 0.3795 | 0 |
| hsa-miR-660-5p | 0.3795 | 0 |
| hsa-miR-194-5p | 0.3763 | 0 |
| hsa-miR-502-3p | 0.3732 | 0 |
| hsa-miR-362-5p | 0.3497 | 0 |
| hsa-miR-3937 | 0.3483 | 0 |
|
hsa-miR-500a-3p | 0.3071 | 0 |
| hsa-miR-296-3p | 0.3033 | 0 |
| hsa-miR-192-5p | 0.2989 | 0 |
|
hsa-miR-500a-5p | 0.2901 | 0 |
| hsa-miR-4486 | 0.2887 | 0 |
| hsa-miR-501-5p | 0.2836 | 0 |
| hsa-miR-138-5p | 0.2686 | 0 |
| hsa-miR-501-3p | 0.2683 | 0 |
| hsa-miR-338-5p | 0.1619 | 0 |
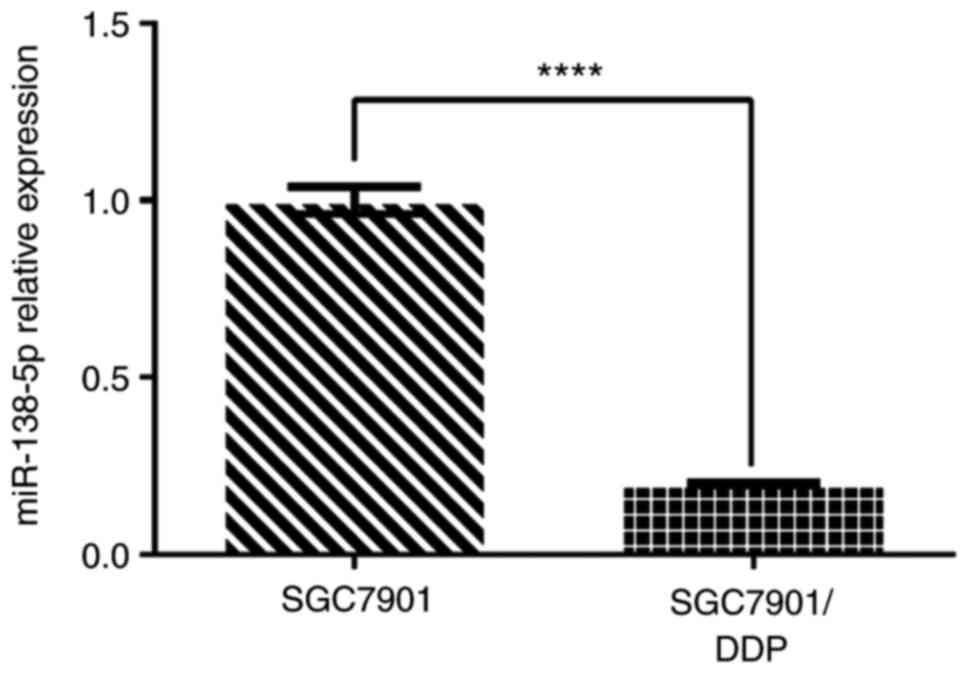
To confirm these findings, the expression of
miR-138-5p was examined by RT-qPCR. The analysis demonstrated that
miR-138-5p expression in SGC7901/DDP cells was ~5-fold lower
compared with that in SGC7901 cells (P<0.0001; Fig. 2). Thus, the PCR results were
consistent with the microarray analysis, confirming that miR-138-5p
was downregulated in cisplatin-resistant SGC7901/DDP cells compared
with the cisplatin-susceptible parental cells.
Predicted miR-138-5p target genes
To investigate potential target genes of miR-138-5p,
the predictive algorithms miRanda, miRWalk and PicTar, which screen
for complementary binding sequences between miRNAs and known gene
transcripts were used. These analysis identified the excision
repair proteins ERCC1 and ERCC4 as possible target genes of
miR-138-5p (Fig. 3).
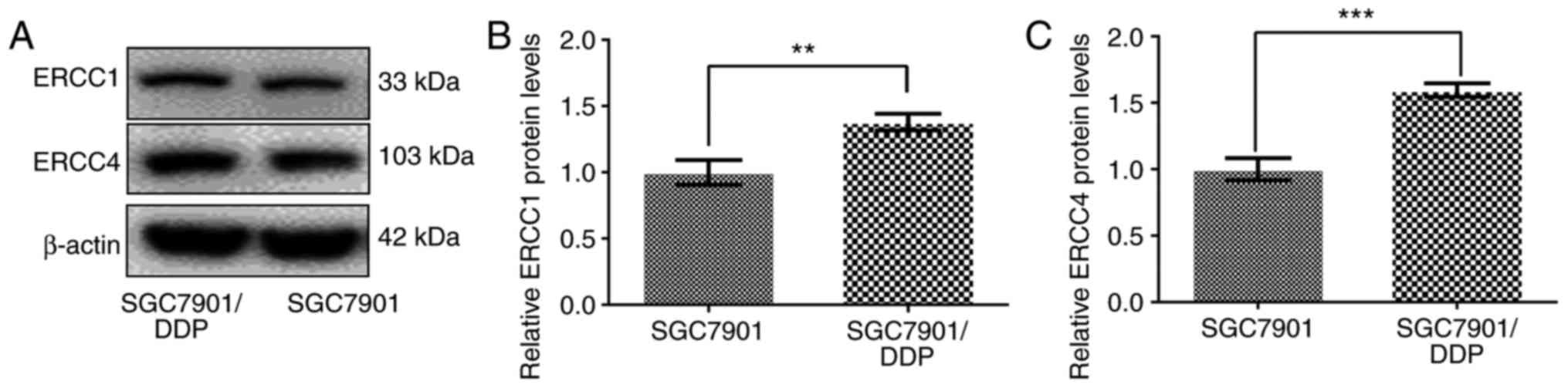
Expression of ERCC1 and ERCC4 in
SGC7901/DDP and SGC7901 cells
Western blot analysis was performed to
determine the expression levels of ERCC1 and ERCC4 in SGC7901/DDP
and SGC7901 cells. As shown in Fig.
4, ERCC1 and ERCC4 proteins were expressed at significantly
higher levels in SGC7901/DDP cells compared with that in SGC7901
cells (P<0.01).
Alterations in ERCC1 and ERCC4 expression
in miR-138-5p-overexpressing or miR-138-5p-depleted gastric cancer
cells
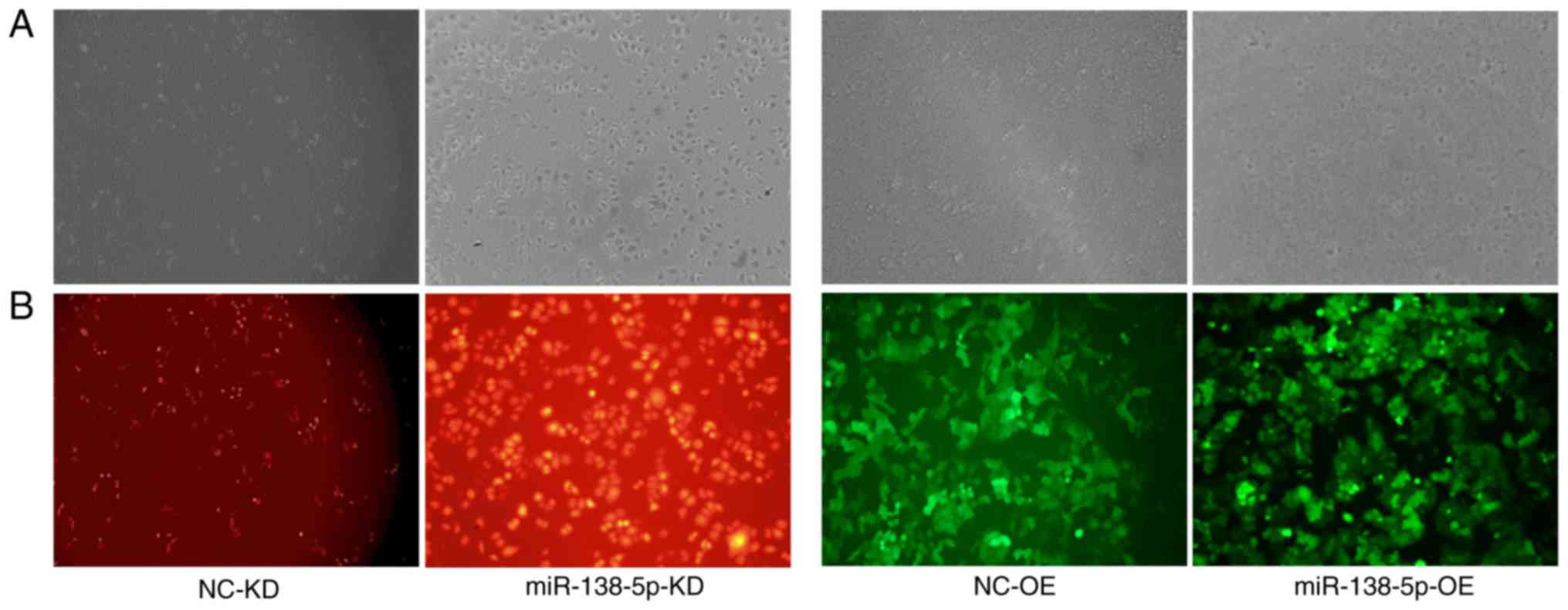
Fluorescence microscopy of infected
cells
To suppress miR-138-5p expression, SGC7901 cells
were infected with lentiviruses encoding RFP and a negative control
sequence or an shRNA targeting miR-138-5p, named
SGC7901-LV-NC-knockdown (KD) and SGC7901-LV-miR138-5p-KD cells,
respectively. Similarly, the effects of miR-138-5p overexpression
were investigated by SGC7901/DDP cell infection with lentiviruses
encoding GFP and a control sequence or miR-138-5p, named
SGC7901/DDP-LV-NC-overexpression (OE) and
SGC7901/DDP-LV-miR-138-5p-OE cells, respectively. Fluorescence
microscopy of the infected cells revealed successful transduction,
as demonstrated by abundant RFP and GFP expression in the
corresponding SGC7901 and SGC7901/DDP cells (Fig. 5).
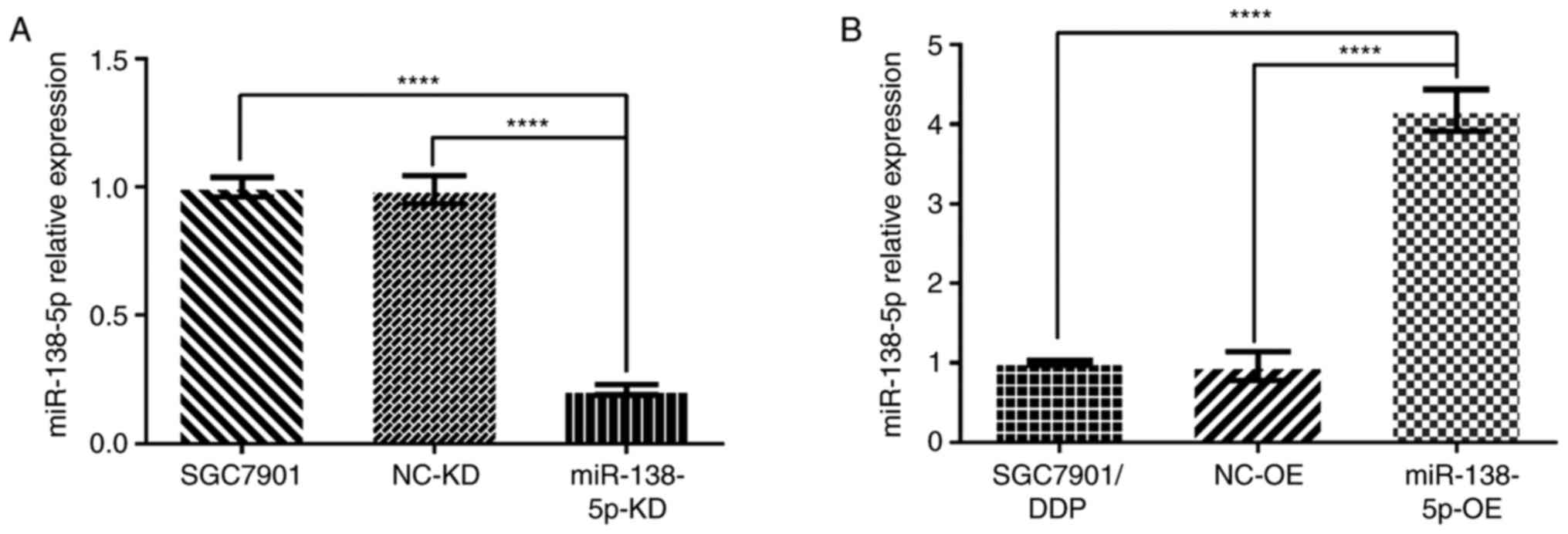
Verification of miR-138-5p expression
in transduced gastric cancer cells by RT-qPCR
RT-qPCR was used to verify the miR-138-5p expression
in SGC7901 and SGC7901/DDP cells. As shown in Fig. 6A, the levels of miR-138-5p in
uninfected SGC7901 cells and control SGC7901-LV-NC-KD cells were
similar. However, miR-138-5p was significantly reduced (~5-fold
lower) in the SGC7901-LV-miR-138-5p-KD cells compared with both
control cell groups (both P<0.0001; Fig. 6A). Similarly, miR-138-5p was
significantly upregulated (~4-fold increase) in
SGC7901/DDP-LV-miR-138-5p-OE cells compared with either SGC7901/DDP
and SGC7901/DDP-LV-NC-OE cells (both P<0.0001; Fig. 6B). Thus, miR-138-5p was successfully
knocked down or overexpressed in the transduced gastric cancer
cells.
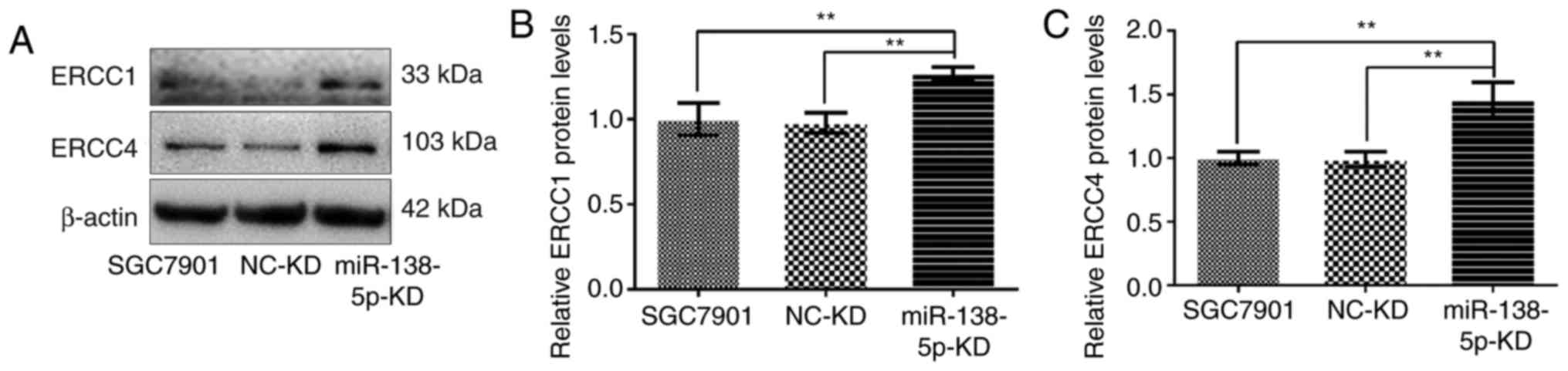
Expression of ERCC1 and ERCC4
following modulation of miR-138-5p expression in gastric cancer
cells
As ERCC1 and ERCC4 were identified as potential
target genes of miR-138-5p, their expression in SGC7901 or
SGC7901/DDP cells was evaluated by western blot analysis. Notably,
downregulation of miR-138-5p significantly increased the expression
of ERCC1 and ERCC4 compared with uninfected SGC7901 cells or
SGC7901-LV-NC-KD cells (P<0.01; Fig.
7).
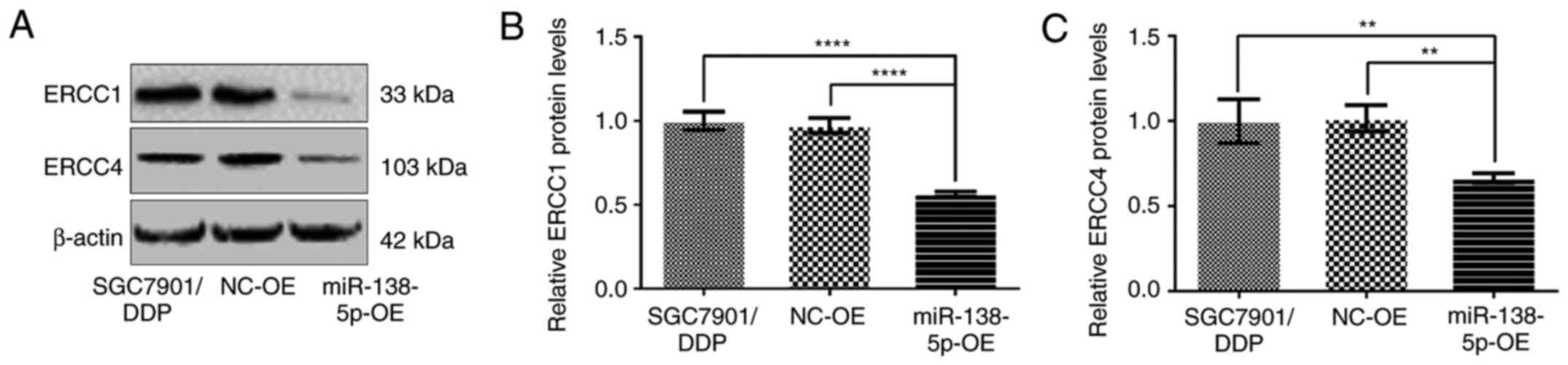
Conversely, the expression of ERCC1 and ERCC4 was
significantly diminished in SGC7901/DDP-LV-miR138-5p-OE cells
compared with control SGC7901/DDP and SGC7901/DDP-LV-NC-OE cells
(P<0.01; Fig. 8).
Cisplatin sensitivity of gastric
cancer cells following modulation of miR-138-5p expression
To evaluate the effects of miR-138-5p downregulation
or upregulation on the sensitivity of SGC7901/DDP or SGC7901 cells
to cisplatin, cells were treated with varying concentrations (40,
20, 2, 0.2 and 0.02 µg/ml in SGC7901/DDP,
SGC7901/DDP-LV-miR138-5p-OE and SGC7901/DDP-LV-NC-OE groups; and
with varying concentrations of 4, 2, 0.2, 0.02 and 0.002 µg/ml in
SGC7901, SGC7901-LV-NC-KD and SGC7901-LV-miR138-5p-KD groups) of
cisplatin for 48 h, and then cell viability was determined using an
MTT assay. Drug resistance was evaluated by calculating the
IC50 of DDP relative to untreated cells. It was
demonstrated that miR138-5p-overexpressing SGC7901/DDP cells were
significantly more sensitive to cisplatin compared with SGC7901/DDP
or SGC7901/DDP-LV-NC-OE cells, as evidenced by the IC50 values of
3.14±0.32, 6.81±0.12, and 6.89±0.14 µg/ml, respectively (P<0.01;
Fig. 9A). Conversely, the cisplatin
resistance of SGC7901-LV-miR138-5p-KD cells was significantly
increased following depletion of miR-138-5p compared with SGC7901
and SGC7901-LV-NC-KD cells (IC50 values 0.39±0.04, 0.20±0.01, and
0.25±0.05 µg/ml, P<0.01; Fig.
9B). Collectively, these data demonstrated that low miR-138-5p
levels and high ERCC1 and ERCC4 levels were associated with
cisplatin resistance in gastric cancer cells.
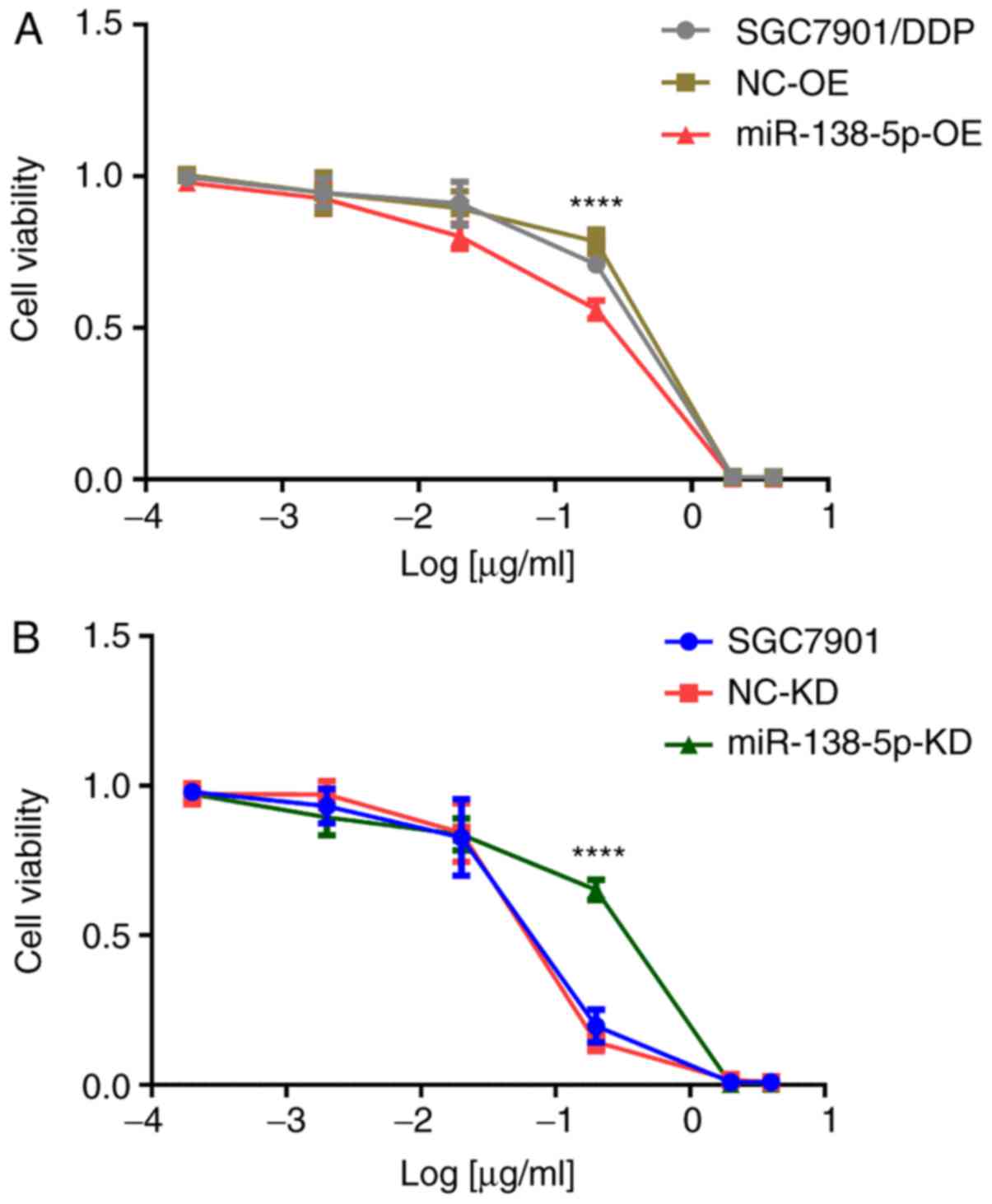 | Figure 9.Cell viability of cisplatin-treated
gastric cancer cells. (A) SGC7901/DDP cells treated for 48 h with
40, 20, 2, 0.2, and 0.02 µg/ml cisplatin and (B) SGC7901 cells
treated for 48 h with 4, 2, 0.2, 0.02 and 0.002 µg/ml cisplatin.
Data are presented as the mean ± standard deviation of experiments
performed in triplicate. ****P<0.01. NC, negative control; miR,
microRNA; KD, knockdown; OE, overexpression; DDP, cisplatin. |
Discussion
Cisplatin is a broad-spectrum antineoplastic drug
that is widely used in the treatment of cancer, including head and
neck, lung, breast, gastric, ovarian, and testicular cancer;
indeed, cisplatin is the cornerstone of numerous combination
chemotherapy regimens (17).
Primary and secondary resistance to cisplatin restricts its
clinical applications. Thus, the identification of novel biomarkers
that are able to predict the efficacy of platinum-based therapy is
required. The results of the present study demonstrated that
miR-138-5p expression modulates the sensitivity of human gastric
cancer cells to cisplatin, possibly by suppressing the expression
of the NER pathway components ERCC1 and ERCC4. Therefore, the
expression level of miR-138-5p and/or the ERCC proteins may be a
useful predictor of cisplatin efficacy, thereby guiding the
individualized treatment of patients with gastric cancer.
The majority of studies on miR-138-5p performed to
date have reported that it is an anti-oncogene (12,18).
For instance, Ma et al (19)
reported that miR-138-5p inhibited the proliferation of gallbladder
cancer cells by acting on the anti-apoptotic protein BAG family
molecular chaperone regulator 1. Yu et al (20) demonstrated that miR-138-5p inhibited
the proliferation of pancreatic cancer cells by reducing expression
of Forkhead box protein C1. Similarly, Chen et al (21) revealed that depletion of miR-138-5p
upregulated LIM domain kinase 1 in ovarian cancer, thereby
promoting metastasis. However, certain studies have reported
conflicting data. For example, in glioma, miR-138-5p is
overexpressed compared with normal cells, and promotes tumor
occurrence, development, metastasis and infiltration while
suppressing apoptosis (22–24). Therefore, miR-138-5p may be a ‘dual
function’ miRNA. A recent report suggested that the expression
level of miR-138-5p and its target gene PDK1 are prognostic factors
for non-small cell lung cancer (NSCLC) and are associated with
tumor-node-metastasis staging and lymph node metastasis (25). Regarding drug resistance, several
studies have suggested that miR-138-5p may influence chemotherapy
sensitivity via inhibition of DNA repair, induction of apoptosis,
and inhibition of EMT. For example, Yang et al (16) demonstrated that miR-138 exhibited a
direct effect on histone H2AX, thereby inhibiting DNA repair in
SCLC cells. Wang et al (9)
demonstrated that miR-138-5p promoted cisplatin resistance of a
NSCLC cell line (A549/DDP) by inhibiting ERCC1 expression and
impairing the DNA repair response. In addition, in osteosarcoma,
miR-138 increases caspase-3-mediated apoptosis via its target gene
histone-lysine N-methyltransferase EZH2, thereby increasing the
sensitivity to cisplatin (11).
miR-138 has also been reported to enhance the sensitivity of NSCLC
cells to adriamycin by targeting E-cadherin, Zinc finger
E-box-binding homeobox 2, and vimentin and suppressing EMT
(8). Han et al (7) revealed that the upregulation of
miR-138 and the subsequent effects on G1/S-specific cyclin-D3
enhanced the sensitivity of NSCLC to cisplatin. A similar study
have reported that miR-138 enhances the efficacy of 5-fluorouracil
and doxorubicin in the cervical cancer cell line HeLa via
downregulation of FAK (10).
However, the association between miR-138-5p and the resistance of
gastric cancer to chemotherapy remains unclear.
Expression of NER pathway constituents is associated
with resistance and sensitivity to platinum-based drugs (17,26).
ERCC1 is considered to serve a role in platinum-based drug
efficacy, since it is predictive of prognosis in lung (27), ovarian (28), cervical (29), bladder (30) and gastric (31) cancer. Indeed, ERCC4- and
ERCC1-deficient cells are 40 times more sensitive compared with
their parental cells to cisplatin (5). Stevens et al (32) demonstrated that the expression of
ERCC4 was associated with the sensitivity of ovarian and colon
cancer cells to cisplatin. Furthermore, in the hepatic cancer cell
line HepG2.2.15, miR-192-mediated regulation of ERCC3 and ERCC4
expression inhibited the NER pathway (33). Finally, depletion of ERCC4 in
Chinese hamster mutant cell lines increased cisplatin sensitivity
>3-fold compared with depletion of ERCC3, ERCC2, or ERCC5,
suggesting an important role for ERCC4 in NER-induced cisplatin
resistance (5). However, the
involvement of ERCC4 in this process has received little attention
to date.
The microarray analysis performed in the current
stud identified 41 differentially expressed miRNAs in the two
gastric cancer cell lines. miR-138 was chosen for subsequent
experiments as it has been previously reported to modulate drug
resistance in small cell lung cancer and NSCLC by acting on the NER
pathway (9,16), but it has not previously been
examined in gastric cancer. The current study demonstrated that
miR-138-5p was downregulated in cisplatin-resistant SGC7901/DDP
cells, and bioinformatics analysis identified ERCC1 and ERCC4 as
possible miR-138-5p target mRNAs. Indeed, the expression of the two
proteins was significantly higher in SGC7901/DDP compared with
SGC7901 cells. The ERCC proteins were downregulated by miR-138-5p
overexpression and, conversely, upregulated by miR-138-5p KD.
Furthermore, upregulation of miR-138-5p partially reversed the
cisplatin resistance of SGC7901/DDP cells, whereas miR-138-5p
downregulation in SGC7901 cells had the opposite effect, rendering
the cells more resistant to cisplatin. Collectively, these results
suggest that miR-138-5p may regulate DNA damage repair and
cisplatin resistance via its effects on ERCC1 and ERCC4. Thus,
miR-138-5p may be a novel target for drug-resistant gastric
cancer.
There are certain limitations to the present study.
First, the study lacks data from animal experiments or patients to
validate the in vitro findings. Second, the study is based
on a single gastric cancer cell line and its platinum-resistant
derivative. Third, miR-138-5p was not directly demonstrated to
modulate ERCC1/ERCC4 expression; for example, by performing
luciferase reporter assays with the wild-type and
miR-138-5p-binding mutant forms of the ERCC1 and ERCC4
3′-untranslated regions. Future studies plan to validate these
findings by investigating the association between miR-138-5p, ERCC
proteins and cisplatin resistance in gastric cancer patients.
In conclusion, microarray and RT-qPCR analysis
revealed that miR-138-5p was expressed at significantly lower
levels in cisplatin-resistant SGC7901/DDP compared with the
parental SGC7901 cells. The expression of ERCC1 and ERCC4 in the
cisplatin-resistant cell line was significantly decreased by
lentivirus-mediated overexpression of miR-138-5p, suggesting that
miR-138-5p may function as a chemosensitizer. In addition, the
expression of ERCC1 and ERCC4 in SGC7901 cells was significantly
increased by lentivirus-mediated downregulation of miR-138-5p,
leading to cisplatin resistance. The expression level of miR-138-5p
may be used to predict the chemosensitivity of patients with
gastric cancer treated with cisplatin, and may be a potential novel
target for reversing drug resistance.
Acknowledgements
The authors would like to thank Ms. Anne M. O'Rourke
for editing the English text of a draft of this manuscript.
Funding
The present study was supported by Foreign Science
and Technology Cooperation Project of Anhui Province (grant no.
1604b0602027).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HW, KG and XD conceived and designed the study. JN,
YJ, XX and YZ performed the experiments. CZ, YY and XX analyzed and
interpreted the data. JN and YY wrote the manuscript. JN, YJ and KG
reviewed and edited the manuscript. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Center MM, Jemal A, Lortet-Tieulent J,
Ward E, Ferlay J, Brawley O and Bray F: International variation in
prostate cancer incidence and mortality rates. Eur Urol.
61:1079–1092. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schlansky B and Sonnenberg A: Epidemiology
of noncardia gastric adenocarcinoma in the United States. Am J
Gastroenterol. 106:1978–1985. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li W, Jie Z, Li Z, Liu Y, Gan Q, Mao Y and
Wang X: ERCC1 siRNA ameliorates drug resistance to cisplatin in
gastric carcinoma cell lines. Mol Med Rep. 9:2423–2428. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Silva IU, McHugh PJ, Clingen PH and
Hartley JA: Defects in interstrand cross-link uncoupling do not
account for the extreme sensitivity of ERCC1 and XPF cells to
cisplatin. Nucleic Acids Res. 30:3848–56. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Buhagiar A and Ayers D: Chemoresistance,
cancer stem cells, and miRNA influences: The case for
neuroblastoma. Anal Cell Pathol. 2015:1506342015. View Article : Google Scholar
|
|
7
|
Han LP, Fu T, Lin Y, Miao JL and Jiang QF:
MicroRNA-138 negatively regulates non-small cell lung cancer cells
through the interaction with cyclin D3. Tumour Biol. 37:291–298.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jin Z, Guan L, Song Y, Xiang GM, Chen SX
and Gao B: MicroRNA-138 regulates chemoresistance in human
non-small cell lung cancer via epithelial mesenchymal transition.
Eur Rev Med Pharmacol. 20:1080–1086. 2016.
|
|
9
|
Wang Q, Zhong M, Liu W, Li J, Huang J and
Zheng L: Alterations of microRNAs in cisplatin-resistant human
non-small cell lung cancer cells (A549/DDP). Exp Lung Res.
37:427–434. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Golubovskaya VM, Sumbler B, Ho B, Yemma M
and Cance WG: miR-138 and miR-135 directly target focal adhesion
kinase, inhibit cell invasion, and increase sensitivity to
chemotherapy in cancer cells. Anticancer Agents Med Chem. 14:18–28.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu Z, Tang J, Wang J, Duan G, Zhou L and
Zhou X: miR-138 acts as a tumor suppressor by targeting EZH2 and
enhances cisplatin-induced apoptosis in osteosarcoma Cells. PLoS
One. 11:e01500262016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Wang Q, Wen R, Liang J, Zhong X,
Yang W, Su D and Tang J: miR-138 inhibits cell proliferation and
reverses epithelial-mesenchymal transition in non-small cell lung
cancer cells by targeting GIT1 and SEMA4C. J Cell Mol Med.
19:2793–2805. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie XQ, Zhao QH, Wang H and Gu KS:
Dysregulation of mRNA profile in cisplatin-resistant gastric cancer
cell line SGC7901. World J Gastroenterol. 23:1189–1202. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sileni VC, Fosser V, Maggian P, Padula E,
Beltrame M, Nicolini M and Arslan P: Pharmacokinetics and tumor
concentration of intraarterial and intravenous cisplatin in
patients with head and neck squamous cancer. Cancer Chemother
Pharmacol. 30:221–225. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang H, Luo J, Liu Z, Zhou R and Luo H:
MicroRNA-138 regulates DNA damage response in small cell lung
cancer cells by directly targeting H2AX. Cancer Invest. 33:126–236.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Y, Yang K, Sun X, Fang P, Shi H, Xu J,
Xie M and Li M: miR-138 suppresses airway smooth muscle cell
proliferation through the PI3K/AKT signaling pathway by targeting
PDK1. Exp Lung Res. 41:363–369. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma F, Zhang M, Gong W, Weng M and Quan Z:
miR-138 suppresses cell proliferation by targeting Bag-1 in
gallbladder carcinoma. PLoS One. 10:e01264992015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu C, Wang M, Li Z, Xiao J, Peng F, Guo X,
Deng Y, Jiang J and Sun C: MicroRNA-138-5p regulates pancreatic
cancer cell growth through targeting FOXC1. Cell Oncol (Dordr).
38:173–181. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen P, Zeng M, Zhao Y and Fang X:
Upregulation of Limk1 caused by microRNA-138 loss aggravates the
metastasis of ovarian cancer by activation of Limk1/cofilin
signaling. Oncol Rep. 32:2070–2076. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chan XH, Nama S, Gopal F, Rizk P, Ramasamy
S, Sundaram G, Ow GS, Ivshina AV, Tanavde V, Haybaeck J, et al:
Targeting glioma stem cells by functional inhibition of a
prosurvival oncomiR-138 in malignant gliomas. Cell Rep. 2:591–602.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stojcheva N, Schechtmann G, Sass S, Roth
P, Florea AM, Stefanski A, Stühler K, Wolter M, Müller NS, Theis
FJ, et al: MicroRNA-138 promotes acquired alkylator resistance in
glioblastoma by targeting the Bcl-2-interacting mediator BIM.
Oncotarget. 7:12937–12950. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Di Pascale F, Nama S, Muhuri M, Quah S,
Ismail HM, Chan XHD, Sundaram GM, Ramalingam R, Burke B and Sampath
P: C/EBPbeta mediates RNA polymerase III-driven transcription of
oncomiR-138 in malignant gliomas. Nucleic Acids Res. 46:336–349.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han L, Zhang G, Zhang N, Li H, Liu Y, Fu A
and Zheng Y: Prognostic potential of microRNA-138 and its target
mRNA PDK1 in sera for patients with non-small cell lung cancer. Med
Oncol. 31:1292014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Amable L: Cisplatin resistance and
opportunities for precision medicine. Pharmacol Res. 106:27–36.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Postel-Vinay S and Soria JC: ERCC1 as
predictor of platinum benefit in non-small-cell lung cancer. J Clin
Oncol. 35:384–386. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Du P, Wang Y, Chen L, Gan Y and Wu Q: High
ERCC1 expression is associated with platinum-resistance, but not
survival in patients with epithelial ovarian cancer. Oncol Lett.
12:857–862. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Muallem MZ, Marnitz S, Richter R, Kohler
C, Sehouli J and Arsenic R: ERCC1 expression as a predictive marker
of cervical cancer treated with cisplatin-based chemoradiation.
Anticancer Res. 34:401–406. 2014.PubMed/NCBI
|
|
30
|
Li S, Wu J, Chen Y, Tang W, Peng Q, Deng
Y, Xie L, Wang J, Huang S, Li R, et al: ERCC1 expression levels
predict the outcome of platinum-based chemotherapies in advanced
bladder cancer: A meta-analysis. Anticancer Drugs. 25:106–114.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wan J, Chao L, Lee AC and Chen Q: Higher
expression of ERCC1 may be associated with resistance to adjuvant
platinum-based chemotherapy in gastric cancer. Cancer Invest.
35:85–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stevens EV, Nishizuka S, Antony S, Reimers
M, Varma S, Young L, Munson PJ, Weinstein JN, Kohn EC and Pommier
Y: Predicting cisplatin and trabectedin drug sensitivity in ovarian
and colon cancers. Mol Cancer Ther. 7:10–18. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie QH, He XX, Chang Y, Sun SZ, Jiang X,
Li PY and Lin JS: miR-192 inhibits nucleotide excision repair by
targeting ERCC3 and ERCC4 in HepG2.2.15 cells. Biochem Biophys Res
Commun. 410:440–445. 2011. View Article : Google Scholar : PubMed/NCBI
|