Introduction
Hepatocellular carcinoma (HCC) is one of the most
prevalent malignancies worldwide, and most new HCC cases are found
in Asia, with about half in China alone (1). The long-term survival rate of patients
with HCC remains low, and HCC is the fifth most common cause of
cancer-related mortality worlwide (2). Differing from the Western hemisphere,
where alcohol abuse is the main factor in HCC development, the
major risk factor in China is the high prevalence of viral
hepatitis B infection (3). Given
the inconspicuous symptoms and lack of screening at the early
stages of HCC, a portion of patients with HCC present with
macrovascular invasion and intra/extrahepatic spread at the time of
diagnosis. Over past decades, understanding of the molecular
mechanisms of HCC has advanced significantly, and there has been a
robust increase in clinical trial activity, improving the long-term
survival outcomes of patients with advanced HCC. Compared with
yttrium-90 radiation therapy, transarterial bland
embolization/transarterial chemoembolization and ablation (or a
combination thereof), sorafenib is the only efficacious strategy
for prolonging life in patients with advanced HCC (4).
Sorafenib is an oral multi-targeting tyrosine kinase
inhibitor (TKI) that suppresses Fms-like tyrosine kinase 3,
vascular endothelial growth factor (VEGF) receptors,
platelet-derived growth factor (PDGF) receptors, and the RAF
serine/threonine kinases (5).
Several random clinical trials of sorafenib have reported
consistent improvement in overall survival for patients with
advanced HCC. Llovet et al first reported that the median
survival and time to radiologic progression for patients treated
with sorafenib were nearly 3 months longer than for those given
placebo (6). Vilgrain et al
demonstrated that patients with advanced HCC who received
continuous oral sorafenib (400 mg twice daily) had a median overall
survival of up to 9.9 months (7).
However, sorafenib can cause serious adverse effects, and drug
resistance develops frequently. The negative sorafenib responses
have been associated with the regulation of multiple intracellular
signaling pathways.
MicroRNAs (miRNAs), tiny non-coding RNA molecules,
play an important role in regulating multiple signaling pathways
and play differential roles in terms of sorafenib response,
including resistance (8). In
humans, >1,800 distinct miRNAs have been identified to date,
which account for ~5% of the transcribed genome and which modulate
30–80% of genes (9). The silencing
mechanism depends on the extent of complementarity between the
miRNA and mRNA target, resulting in either degradation or
inhibition of the mRNA target at the translational level (10,11).
The present study was designed to reveal the function of miR-223
and its mRNA target F-box and WD repeat domain-containing 7
(FBW7) on promoting HCC resistance to sorafenib.
Materials and methods
Cell lines, chemicals and
antibodies
Three human HCC cell lines (Huh7, SNU387 and SNU449)
were purchased from the Cell Bank of the Type Culture Collection of
Chinese Academy of Sciences, Shanghai Institute of Cell Biology,
Chinese Academy of Sciences (Shanghai, China). The Huh7 cells were
cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and the SNU449 and
SNU387 cells were cultured in RPMI-1640 complete medium (Gibco;
Thermo Fisher Scientific, Inc.) in a humidified incubator at 37°C
and 5% CO2. All media were supplemented with 10% fetal
bovine serum (FBS; Gibco™; Thermo Fisher Scientific, Inc.) and 100
U/ml mixture of streptomycin and penicillin. The chemicals and
antibodies used were sorafenib and diamidinophenylindole (DAPI)
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), anti-FBW7 antibody
(cat. no. ab105752; Abcam, Cambridge, MA, USA),
anti-glyceraldehyde-3-phosphate dehydrogenase antibody (GAPDH; cat.
no. 5174), goat anti-rabbit horseradish peroxidase (HRP) antibody
(cat. no. 7074) and goat anti-mouse HRP antibody (cat. no. 7056;
Cell Signaling Technology, Inc., Beverly, MA, USA).
Small interfering RNA (siRNA)
transfection
The gene-targeting siRNAs or a scramble control were
purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China).
Untreated Huh7, SNU387 and SNU449 cells were plated in 6-well
plates at 1×105 cells/well and supplemented with 2 ml
corresponding media. Then, 50 nm siRNA and 50 µl Invitrogen™
Lipofectamine 2000 transfection reagent (Thermo Fisher Scientific,
Inc.) were added to the plated cells when the cells were 20–30%
confluent, following the manufacturer's protocol. The cells were
collected for subsequent experiments after 48–72 h of
transfection.
Cell proliferation assay
All siRNA-transfected HCC cells were plated at
3×103 cells/well in 100 µl medium for 24 h. After
sorafenib or phosphate-buffered saline (PBS; control) treatment,
cell viability was detected using the Cell Counting Kit-8 (Dojindo
Laboratories, Kumamoto, Japan) in a microplate reader (ELx800;
BioTek Instruments, Inc., Winooski, VT, USA). The optical density
at 450 nm was recorded to calculate the median inhibitory
concentration (IC50).
Ethynyl deoxyuridine (EdU)
incorporation assay
HCC cell proliferation ability was detected using a
Click-iT EdU Imaging kit (Invitrogen; Thermo Fisher Scientific,
Inc.) following the manufacturer's protocol. Briefly, 50 µM
EdU/well was added to HCC cell monolayers that were 50–70%
confluent and incubated for 2 h. After washing three times with
PBS, the cells were fixed with 4% paraformaldehyde. Then, Apollo
fluorescent dye solution (Invitrogen; Thermo Fisher Scientific,
Inc.) was added and incubated for 30 min, and the cell
proliferation rate was visualized and calculated under a
fluorescence microscope (Olympus Corp., Tokyo, Japan).
Quantitative real-time PCR
(RT-PCR)
Total RNA was isolated from the HCC cells using
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. Real-time PCR was conducted using
a SYBR Premix Ex Taq kit (Takara Bio, Inc., Otsu, Japan) in a Roche
LightCycler system (Roche, Basel, Switzerland). All reactions were
performed in triplicate. The primers for the target genes were as
follows: miR-223 mimic forward primer, 5′-UGUCAGUUUGUCAAAUACCCCA-3′
and reverse primer, 5′-GGGUAUUUGACAAACUGACAUU-3′; miR-223
inhibitor, 5′-UGGGGUAUUUGACAAACUGACA-3′; FBW7 forward primer,
5′-CACTCAAAGTGTGGAATGCAGAGAC-3′ and reverse primer,
5′-GCATCTCGAGAACCGCTAACAA-3′; GAPDH forward primer,
5′-UGACCUCAACUACAUGGUUTT-3′ and reverse primer,
5′-AACCAUGUAGUUGAGGUCATT-3′.
Western blotting
Radioimmunoprecipitation assay (RIPA) buffer
supplemented with protease inhibitors was used to extract the total
proteins from the HCC cells. Then, a bicinchoninic acid (BCA) kit
(Thermo Fisher Scientific, Inc.) was used to quantify the protein
concentration. Samples (10 µl) containing 20–50 g protein were
separated using 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) at 120 V, and then electrophoretically
transferred to 0.45-µm polyvinylidene fluoride (PVDF) membranes
(EMD Millipore, Bedford, MA, USA) at 350 mA for 1 h. The membranes
were blocked with a 5% skim milk and 0.05% Tween-20 mixture. The
membranes were incubated with the corresponding primary antibody
(dilution 1:1,000) at 4°C for overnight and subsequently incubated
with the secondary HRP-conjugated antibody (dilution 1:2,000) at
room temperature for 1 h. The target protein expression levels were
visualized with enhanced chemiluminescence (GE Healthcare,
Piscataway, NJ, USA) in the western blotting detection system
Quantity One software (Bio-Rad Laboratories, Hercules, CA,
USA).
Statistical analysis
All experimental data are reported as the mean ± SD
(n=3). The two-tailed Student t-test and Fisher exact test were
used to analyze differences between groups. Statistical analysis
was conducted using SPSS 19.0 software (SPSS, Inc., Chicago, IL,
USA). All statistical results with a P-value <0.05 were
considered statistically significant.
Results
High miR-223 expression is correlated
with sorafenib resistance in HCC cells
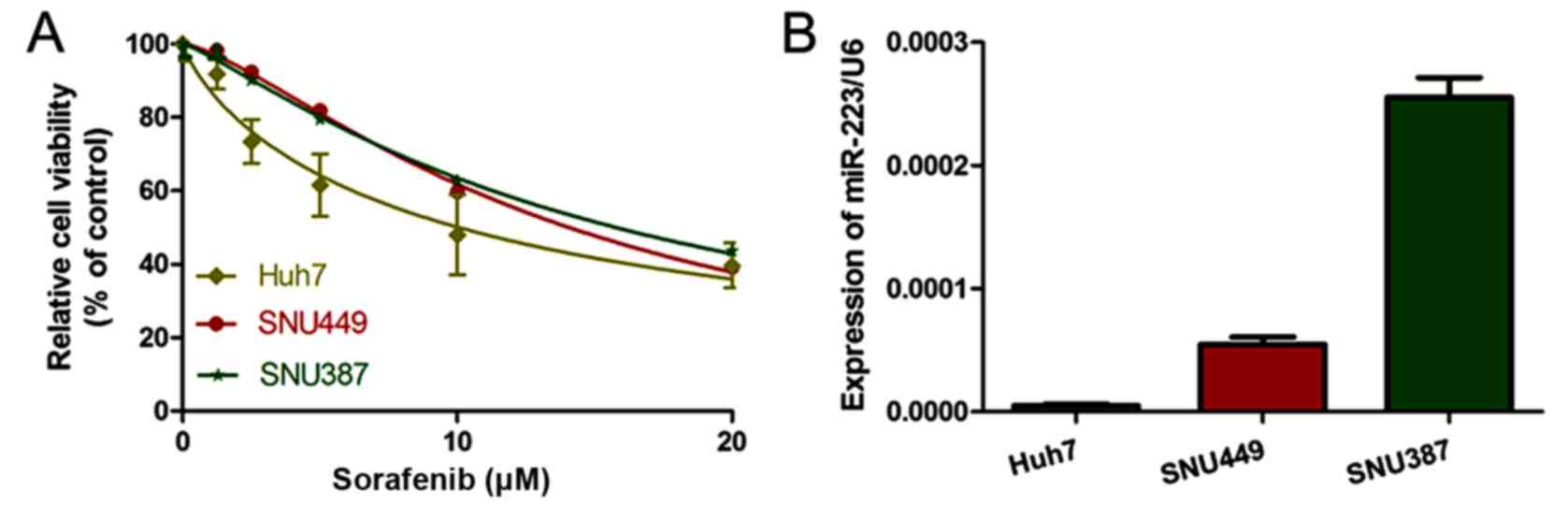
We detected altered cell viability of the three HCC
cell lines (SNU387, SNU449 and Huh7) in the presence of sorafenib
after 48 h. The sensitivity of the HCC cell lines to sorafenib,
from high to low, was Huh7, SNU449 and SNU387 (Fig. 1A). The IC50 of sorafenib
was 8.749±0.876, 13.4±1.05 and 15.72±1.58 µM in the Huh7, SNU449
and SNU387 cells, respectively (Table
I). Notably, miR-223 expression levels in the HCC cell lines
followed a similar trend to that of the sorafenib IC50
(Fig. 1B). This result suggests
that miR-223 potentially correlates with sorafenib resistance.
 | Table I.IC50 values of sorafenib
treatment in HCC cell lines. |
Table I.
IC50 values of sorafenib
treatment in HCC cell lines.
| Cell lines | Huh7 | SNU449 | SNU387 |
|---|
| IC50
(µM) | 8.749±0.876 | 13.4±1.05 | 15.72±1.58 |
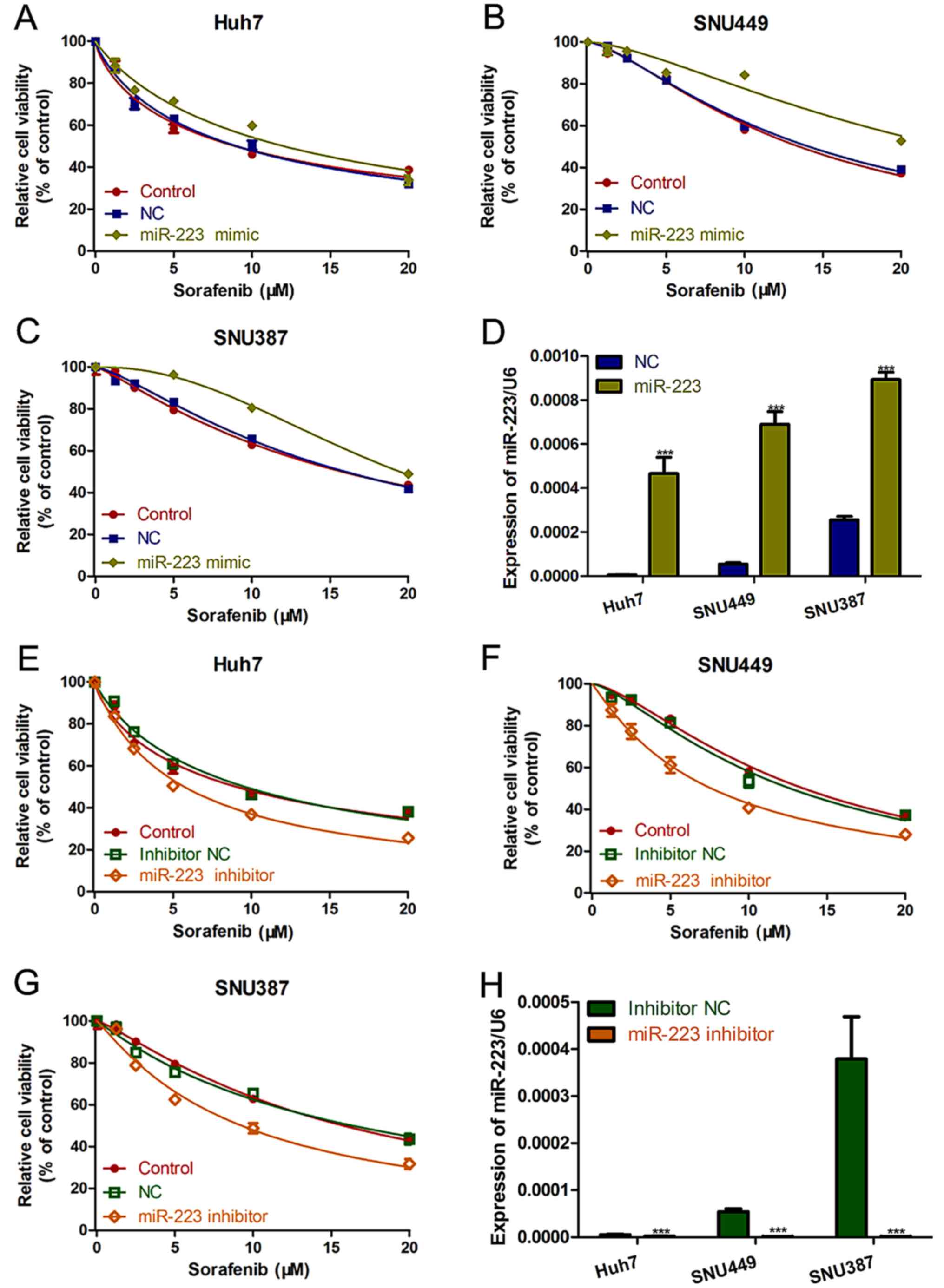
miR-223 knockdown increases HCC cell
sensitivity to sorafenib
To investigate the link between miR-223 and
sorafenib resistance, miR-223 mimic or miR-223 inhibitor were
packaged in lentivirus and transfected into the HCC cell lines to
induce miR-223 overexpression or knockdown, respectively. The cell
proliferation assay showed that, in all three HCC cell lines,
miR-223 upregulation increased cell viability in the presence of
sorafenib (Fig. 2A-C). On the
contrary, miR-223 knockdown significantly increased the therapeutic
effect of sorafenib on the HCC cells (Fig. 2E-G). qRT-PCR was used to determine
the expression of miR-223 with miR-223 mimic or miR-223 inhibitor
in HCC cells (Fig. 2D and H).
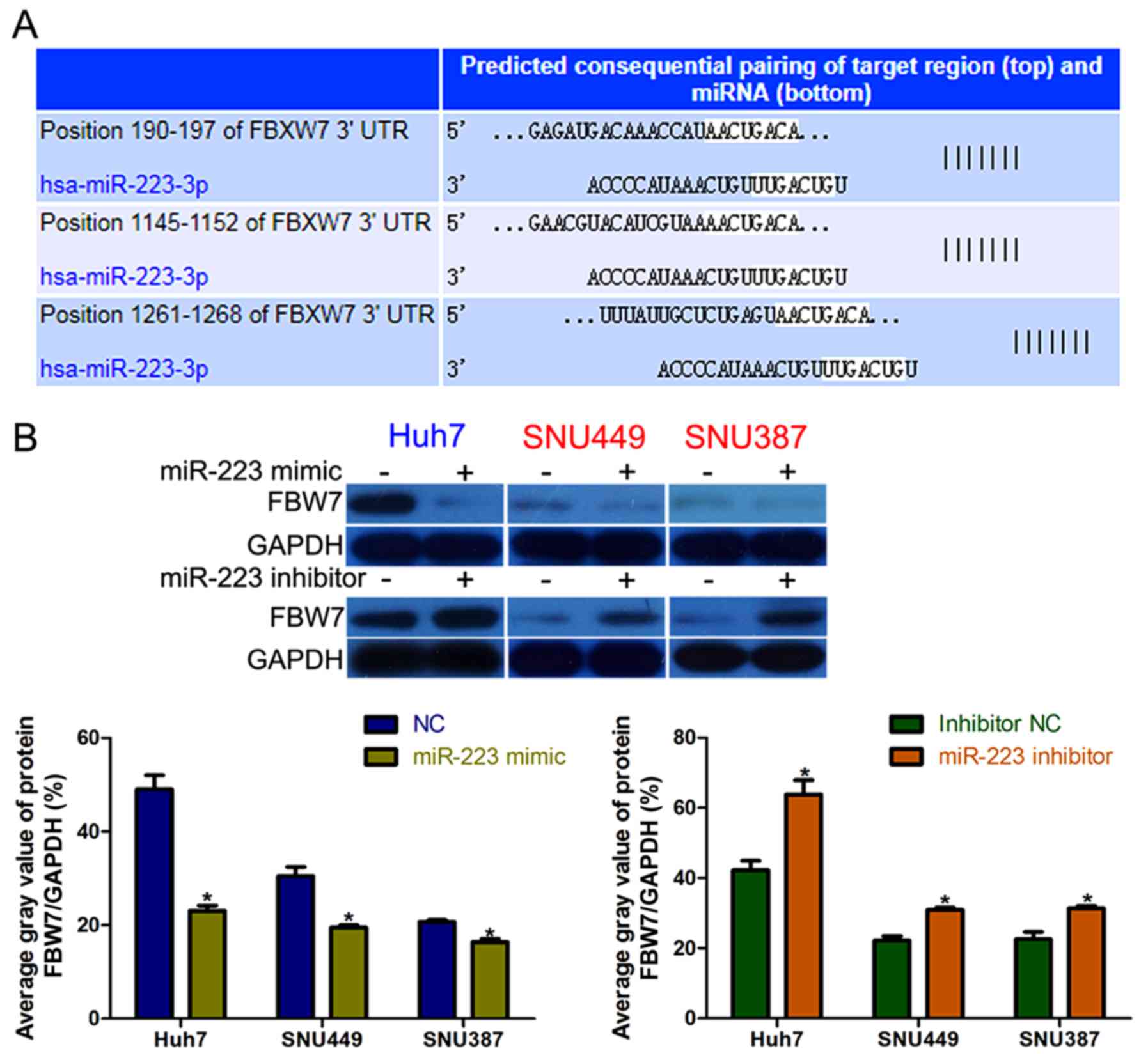
FBW7 is a direct and functional target
of miR-223 in HCC
The TargetScan web server was used to explore the
mechanism by which miR-223 exerts its function, and identified
FBW7 as a potential target of miR-223 in HCC cells (Fig. 3A). High miR-223 expression inhibited
FBW7 expression in an obvious manner, and the opposite
effect was observed in miR-223-knockdown HCC cells (Fig. 3B).
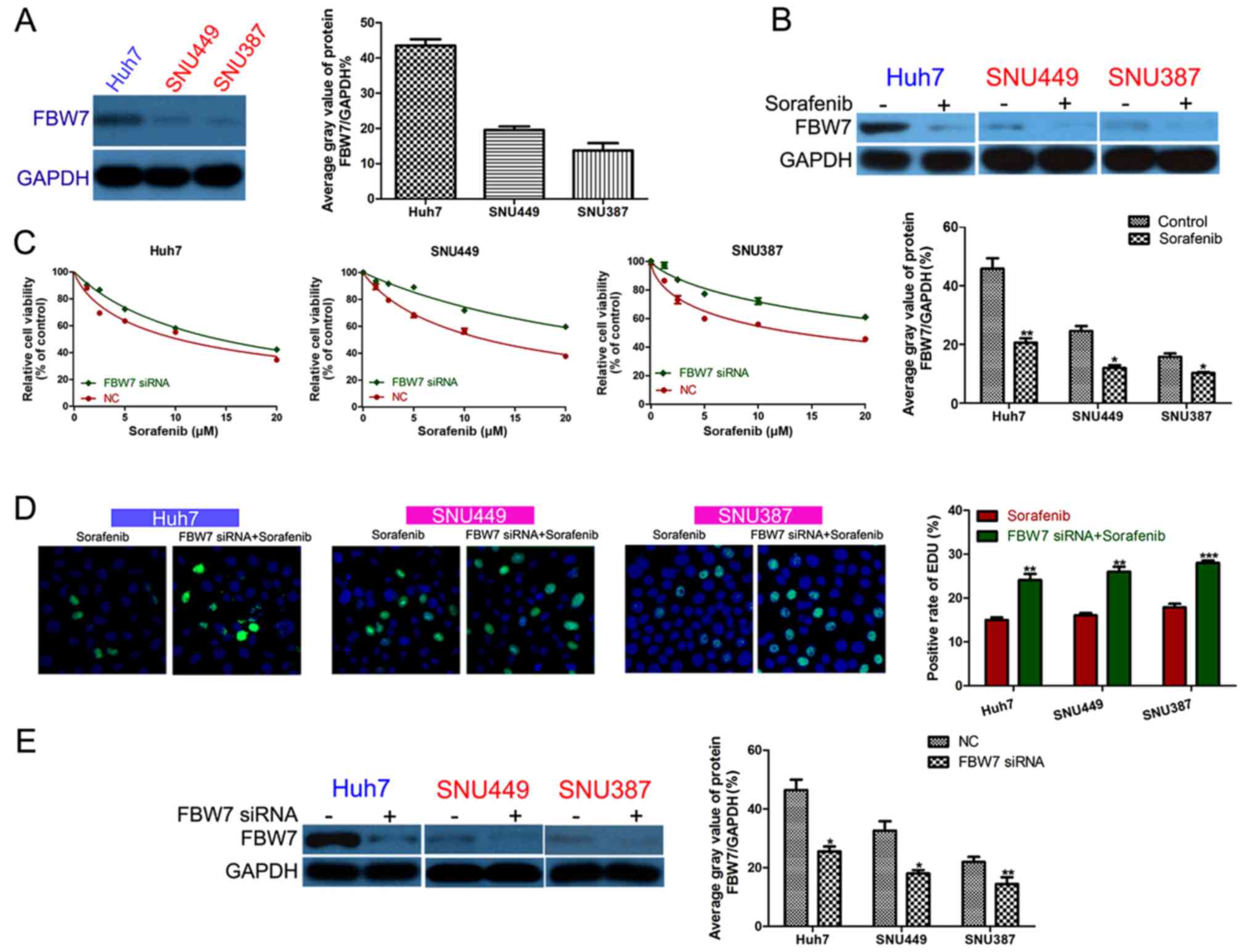
FBW7 increases HCC cell sensitivity to
sorafenib
We detected the FBW7 expression level in the
HCC cell lines and found that Huh7 cells had higher FBW7
expression than that noted in the SNU449 and SNU387 cells (Fig. 4A). FBW7 expression was
significantly inhibited in the surviving HCC cells after a 24-h
sorafenib treatment (Fig. 4B).
FBW7 siRNA was transfected into HCC cells to decrease
FBW7 expression (Fig. 4E).
After sorafenib treatment, HCC cells with FBW7 knockdown had
inhibited viability compared to the control group (Fig. 4C). Furthermore, FBW7
knockdown decreased HCC cell proliferation in the presence of
sorafenib (Fig. 4D).
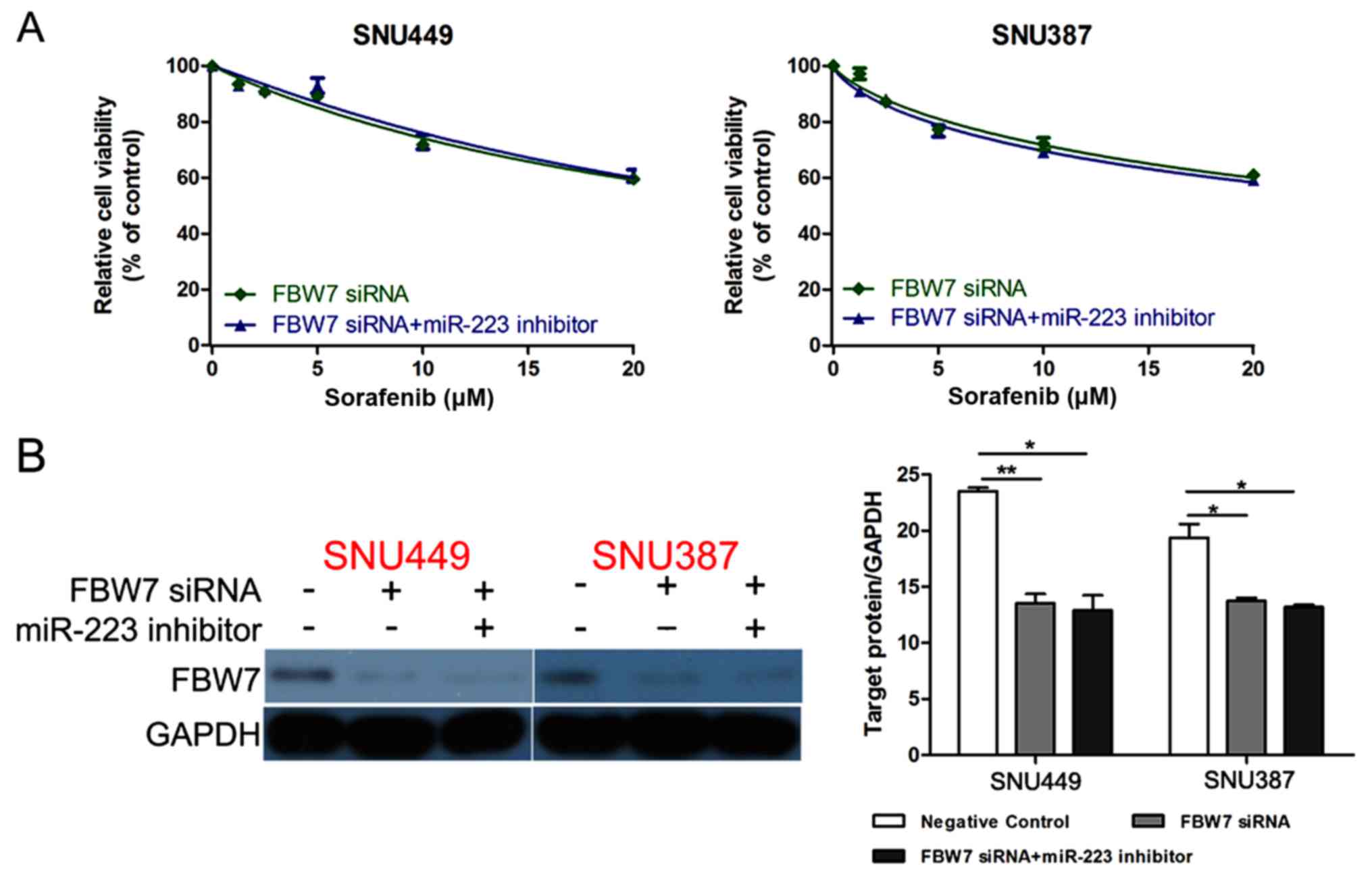
FBW7 reverses the effect of miR-223 in
promoting sorafenib resistance
FBW7 siRNA was transfected into SNU449 and
SNU387 cells together with miR-223 inhibitor to investigate whether
FBW7 knockdown could reverse the effect of miR-223 inhibitor
in promoting sorafenib sensitivity. As expected, the cell viability
was not different between the cells transfected with FBW7
siRNA and FBW7 siRNA+miR-223 inhibitor (Fig. 5A). Western blotting confirmed that
the FBW7 siRNA could eliminate the effect of miR-223
inhibitor on increasing FBW7 expression (Fig. 5B).
Discussion
Despite significant advances in the management and
treatment of patients with HCC over the last decades, the prognosis
remains poor. Unfortunately, HCC is very resistant to cytotoxic and
targeted therapies, even against the multikinase inhibitor,
sorafenib, the first and only approved systemic therapy that
improves the overall survival in patients with advanced HCC
(6); the gradually increasing rate
of sorafenib resistance has significantly limited its therapeutic
benefit. The reason for this limited effect and for the failure of
all targeted agents, including sorafenib, against HCC, varies, and
includes the molecular complexity of the tumor and the presence of
primary and acquired drug resistance mechanisms (12,13).
In most instances, the HCC cells that initially respond well to
anticancer drugs gradually display a loss of response and acquire
resistance during treatment, subsequently leading to HCC recurrence
(14). Recently, sorafenib
resistance has often been referred to as a ‘hot’ term used to
describe the impaired efficacy of sorafenib, especially for
patients with advanced HCC. A large body of mechanisms are involved
in the acquired resistance to sorafenib, such as the
phosphatidylinositol 3-kinase (PI3K)-AKT pathway,
epithelial-mesenchymal transition, epigenetic regulation, and
autophagy (12,15). There is an urgent need to understand
the underlying mechanism and identify new, promising
chemotherapeutic therapies.
A glance at the molecular mechanistic aspect reveals
the regulation of various signaling pathways potentially modulated
by miRNAs (16,17). Deregulation, i.e., either
downregulation or upregulation, of several miRNAs has been reported
in a series of in vivo, in vitro, and patient studies,
demonstrating that it may be responsible for the response to
sorafenib. Here, we explored the relationship between miR-223
expression and sorafenib resistance in HCC. Previously, miR-223 was
considered a potential diagnostic and prognostic biomarker of
various malignancies, including osteosarcoma (18), Barrett's esophagus (19), and esophageal squamous cell
carcinoma (20). Moreover, Han
et al revealed that miR-223 regulates the insulin-like
growth factor 1 receptor (IGF1R)/PI3K/AKT signaling pathway to
reverse epidermal growth factor receptor (EGFR) TKI resistance
(21). Our results revealed that
miR-223 expression levels correlate with HCC cell sensitivity to
sorafenib. Treating HCC cells with miR-223 inhibitor increased
their sensitivity to sorafenib in an obvious manner. These data
demonstrated that miR-223 is a suitable predictive biomarker of HCC
cell resistance to sorafenib. To further assess the function of
miR-223, we used TargetScan to predict the miR-223 target genes and
determined that FBW7 is a functional target of miR-223 in
HCC cells. miR-223 mimic markedly downregulated FBW7, and
miR-223 inhibitor had the opposite effect on FBW7
expression. Furthermore, FBW7 siRNA entirely eliminated the
effect of the miR-223 inhibitor on increasing HCC cell sensitivity
to sorafenib. These results strongly suggest that miR-223 regulates
HCC cell resistance to sorafenib by targeting FBW7.
Notably, a growing number of studies have observed
that FBW7 is also involved in regulating drug resistance
(22,23). Several groups have shown that the
loss of FBW7 led to elevated expression of the c-Jun, c-Myc,
and Notch-1 oncoproteins, all of which can promote cell growth,
although they can also provoke apoptosis as a side-effect. In the
present research, we transfected HCC cells with FBW7 siRNA,
consistent with previously published research (24). The results confirmed that
FBW7 knockdown significantly inhibited HCC cell sensitivity
to sorafenib. These results show an intimate relationship between
drug resistance and miR-223/FBW7 genetic status, and these
observations imply that the miRNA pathway can modulate FBW7
expression and activity directly, demonstrating that targeting
miR-223/FBW7 may open a new therapeutic window for drug
administration (25).
In conclusion, miR-223 expression is upregulated in
HCC cells with sorafenib resistance. miR-223 knockdown
significantly enhances HCC cell sensitivity to sorafenib by
increasing the expression of the target gene, FBW7,
suggesting that miR-223 may be a new therapeutic target for
overcoming sorafenib resistance.
Acknowledgements
Not applicable.
Funding
The present study was supported by Zhejiang Natural
Science Foundation (grant nos. LY16H160068 and LY15H160060).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
JY, SZ and XT conceived the research idea; WY, ZS
and XS performed the experiments; WZ, CC, LC and MZ analyzed the
data; SZ wrote the manuscript. All authors drafted, read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Poon D, Anderson BO, Chen LT, Tanaka K,
Lau WY, Van Cutsem E, Singh H, Chow WC, Ooi LL, Chow P, et al:
Management of hepatocellular carcinoma in Asia: Consensus statement
from the Asian Oncology Summit 2009. Lancet Oncol. 10:1111–1118.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forner A, Reig M and Bruix J:
Hepatocellular carcinoma. Lancet. 391:1301–1314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Njei B, Rotman Y, Ditah I and Lim JK:
Emerging trends in hepatocellular carcinoma incidence and
mortality. Hepatology. 61:191–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Finn RS, Zhu AX, Farah W, Almasri J, Zaiem
F, Prokop LJ, Murad MH and Mohammed K: Therapies for advanced stage
hepatocellular carcinoma with macrovascular invasion or metastatic
disease: A systematic review and meta-analysis. Hepatology.
67:422–435. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Escudier B, Eisen T, Stadler WM, Szczylik
C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA,
et al: Sorafenib in advanced clear-cell renal-cell carcinoma. N
Engl J Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vilgrain V, Pereira H, Assenat E, Guiu B,
Ilonca AD, Pageaux GP, Sibert A, Bouattour M, Lebtahi R, Allaham W,
et al: Efficacy and safety of selective internal radiotherapy with
yttrium-90 resin microspheres compared with sorafenib in locally
advanced and inoperable hepatocellular carcinoma (SARAH): An
open-label randomised controlled phase 3 trial. Lancet Oncol.
18:1624–1636. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanthaje S, Makol A and Chakraborti A:
Sorafenib response in hepatocellular carcinoma: MicroRNAs as tuning
forks. Hepatol Res. 48:5–14. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Axtell MJ, Westholm JO and Lai EC: Vive la
différence: Biogenesis and evolution of microRNAs in plants and
animals. Genome Biol. 12:2212011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu J and Clark AG: Impact of microRNA
regulation on variation in human gene expression. Genome Res.
22:1243–1254. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Berasain C: Hepatocellular carcinoma and
sorafenib: Too many resistance mechanisms? Gut. 62:1674–1675. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gauthier A and Ho M: Role of sorafenib in
the treatment of advanced hepatocellular carcinoma: An update.
Hepatol Res. 43:147–154. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Szakács G, Paterson JK, Ludwig JA,
Booth-Genthe C and Gottesman MM: Targeting multidrug resistance in
cancer. Nat Rev Drug Discov. 5:219–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu YJ, Zheng B, Wang HY and Chen L: New
knowledge of the mechanisms of sorafenib resistance in liver
cancer. Acta Pharmacol Sin. 38:614–622. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shenouda SK and Alahari SK: MicroRNA
function in cancer: Oncogene or a tumor suppressor? Cancer
Metastasis Rev. 28:369–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Davidson-Moncada J, Papavasiliou FN and
Tam W: MicroRNAs of the immune system: Roles in inflammation and
cancer. Ann NY Acad Sci. 1183:183–194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dong J, Liu Y, Liao W, Liu R, Shi P and
Wang L: miRNA-223 is a potential diagnostic and prognostic marker
for osteosarcoma. J Bone Oncol. 5:74–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Streppel MM, Pai S, Campbell NR, Hu C,
Yabuuchi S, Canto MI, Wang JS, Montgomery EA and Maitra A: MicroRNA
223 is upregulated in the multistep progression of Barrett's
esophagus and modulates sensitivity to chemotherapy by targeting
PARP1. Clin Cancer Res. 19:4067–4078. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kurashige J, Watanabe M, Iwatsuki M,
Kinoshita K, Saito S, Hiyoshi Y, Kamohara H, Baba Y, Mimori K and
Baba H: Overexpression of microRNA-223 regulates the ubiquitin
ligase FBXW7 in oesophageal squamous cell carcinoma. Br J Cancer.
106:182–188. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han J, Zhao F, Zhang J, Zhu H, Ma H, Li X,
Peng L, Sun J and Chen Z: miR-223 reverses the resistance of
EGFR-TKIs through IGF1R/PI3K/Akt signaling pathway. Int J Oncol.
48:1855–1867. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Inuzuka H, Shaik S, Onoyama I, Gao D,
Tseng A, Maser RS, Zhai B, Wan L, Gutierrez A, Lau AW, et al:
SCFFBW7 regulates cellular apoptosis by targeting MCL1
for ubiquitylation and destruction. Nature. 471:104–109. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wertz IE, Kusam S, Lam C, Okamoto T,
Sandoval W, Anderson DJ, Helgason E, Ernst JA, Eby M, Liu J, et al:
Sensitivity to antitubulin chemotherapeutics is regulated by MCL1
and FBW7. Nature. 471:110–114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu Y, Sengupta T, Kukreja L and Minella
AC: MicroRNA-223 regulates cyclin E activity by modulating
expression of F-box and WD-40 domain protein 7. J Biol Chem.
285:34439–34446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Minella AC and Clurman BE: Mechanisms of
tumor suppression by the SCFFbw7. Cell Cycle.
4:1356–1359. 2005. View Article : Google Scholar : PubMed/NCBI
|