Introduction
Ovarian cancer is regarded as one of the most
dangerous gynecological malignancies in the United States, as a
consequence to its insidious onset, late presentation, and limited
advances in therapy (1–3). As of 2016, a woman living in the
United States carries a 1 in 75 lifetime risk of the disease, as
well as a 1 in 100 risk of associated death; a statistic which over
the last 40 years, has only changed by small increments, while
other cancers have experienced large breakthroughs both in
screening and in treatment (4,5).
Interleukin (IL)-33 was identified in 2005. Its
associated signaling, which occurs through the receptor IL-1
receptor like 1 (ST2), was revealed to activate mitogen activated
protein kinase and nuclear factor (NF)-κB responses, eventually
leading to an in vitro T helper cell 2 polarized type
response (6,7). Mechanistic investigation of IL-33 and
its role in carcinogenesis has only recently begun (6). Various studies have indicated that
IL-33 has a pro-tumor effect in cholangiocarcinoma, colon and
breast cancer (8–10). IL-33 has been identified as a useful
biomarker in the diagnosis or prediction of prognosis in non-small
cell lung cancer (11).
Interestingly, other studies have suggested that IL-33 has an
anti-tumor effect in colon cancer (12,13)
and our previous study on pancreatic and colon cancer echoes these
findings (14). This suggests the
complex role of IL-33 in the pathogenesis of neoplasia. In ovarian
cancer, increased levels of IL-33 and ST2 have been found in
neoplastic lesions (15). However,
the direct effects of IL-33 on ovarian cancer are still unknown,
therefore the present study addressed the lack of research through
the use of a widely studied ovarian cancer cell line, A2780.
Materials and methods
Tumor cell line
A2780, a widely used human ovarian cancer cell line
(ATCC; Manassas, VA, USA) was maintained for ~5 days in Dulbecco's
modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 37°C in a humidified 5%
CO2 incubator. Medium supplements included 10%
heat-inactivated fetal bovine serum and 1% penicillin-streptomycin
(Gibco; Thermo Fisher Scientific, Inc.). Cells were grown to 70%
confluence, then subjected to treatment with IL-33 or medium
alone.
Treatment of ovarian carcinoma cells
with IL-33
Once reaching 70% confluence, ovarian carcinoma
cells were treated for 3 days with IL-33 (50 ng/ml; Shenandoah
Biotechnology, Inc., Warwick, PA, USA) or medium alone at 37°C. The
concentration determined for experimental treatment was previously
derived from our pilot experiments and previous cytokine studies
(16–20).
Clonogenic survival assay
A total of 3 days post-incubation with IL-33, A2780
cells were detached and counted in a hemocytometer. A clonogenic
survival assay was performed as described in our previous studies
(16–18). The number of treatment colonies were
then expressed as a percentage of total control colonies.
Determination of cell
proliferation
Cell proliferation was quantified using the Quick
Cell Proliferation Colorimetric Assay Kit (BioVision, Inc.,
Milpitas, CA, USA), which assesses for the degree of which cellular
mitochondria cleave tetrazolium salts into formazan dye. The
resulting concentration of formazan dye, detected at an absorbance
of 440 nm, is thus positively associated with the degree of
mitochondrial dehydrogenase activity. Higher activity is positively
associated with proliferation potential (16,17,21,22).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Experimental and control cells were washed with PBS
and homogenized in TRIzol® (Invitrogen; Thermo Fisher
Scientific, Inc.). RNA extraction was performed, and concentration
was measured by NanoDrop (Thermo Fisher Scientific, Inc.,
Wilmington, DE, USA). RT-PCR was carried out using 1 µg of A2780
mRNA, yielding cDNA aliquots proportional to mRNA expression
profiles of our original cultures. Primer sequences specific for
pro and anti-proliferative/apoptotic molecules, as well as for
GAPDH were then amplified. GAPDH, a housekeeping gene, was used to
internally control for differences in amounts of cDNA amplified. A
more detailed description of our RT-PCR process, including
semi-quantification methods and primer sequences, can be found in
our previous cytokine studies (16–20).
Immunohistochemistry (IHC)
Using a Cytopro cytocentrifuge (Wescor, Inc., Logan,
UT, USA), A2780 ovarian cancer cells were spun into positively
charged slides. IHC was used to stain for the proteins Fas cell
surface death receptor (Fas) and p27. Cells were first incubated
with 0.1% saponin in 1.0% BSA (Thermo Fisher Scientific, Inc.) for
30 min at room temperature, and then with the anti-Fas (cat. no.
sc-716; Santa Cruz Biotechnology Inc.) and anti-p27 (cat. no.
sc-528; Santa Cruz Biotechnology, Inc.) polyclonal antibodies for
60 min at 1 µg/ml at room temperature. Primary antibody incubation
was followed by incubation with a biotinylated secondary antibody
(711-065-152; Jackson ImmunoResearch Laboratories, Inc., West
Grove, PA, USA) at 0.5 µg/ml for 30 min. The avidin-biotin complex
immunoperoxidase system (Vector Laboratories, Inc., Burlingame, CA,
USA) was then used to detect immunoreactivity. Slides were
developed using NovaRED (vector) as a chromogen. Hematoxylin was
then applied as a counterstain for 30 min at room temperature. As a
negative control, the primary antibody was replaced with an equal
amount of rabbit IgG. Staining was observed under a Genco
microscope (JC-311; magnification, ×400; Jenco International Inc.,
Portland, OR) and MetaMorph version 6.3r6 (Molecular Devices, LLC,
Sunnyvale, CA, USA) was used to measure the average intensity for
p27 and Fas within the areas covered. Results are reported as an
average staining intensity of 3 slides ± the standard error of the
mean relative to that in controls.
Caspase-3 activity
Caspase-3 activity was assayed using a
caspase-3/CPP32 colorimetric assay kit (BioVision, Inc.), and
increased activity of caspase-3 is positively associated with an
increased propensity for apoptosis (16–20).
Statistical analysis
All experiments were performed in triplicate. Data
are presented as the means + standard error of the means.
Statistical analysis was conducted using an unpaired, two-tailed
Student's t-test (Excel 2007; Microsoft Corporation, Redmond, WA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
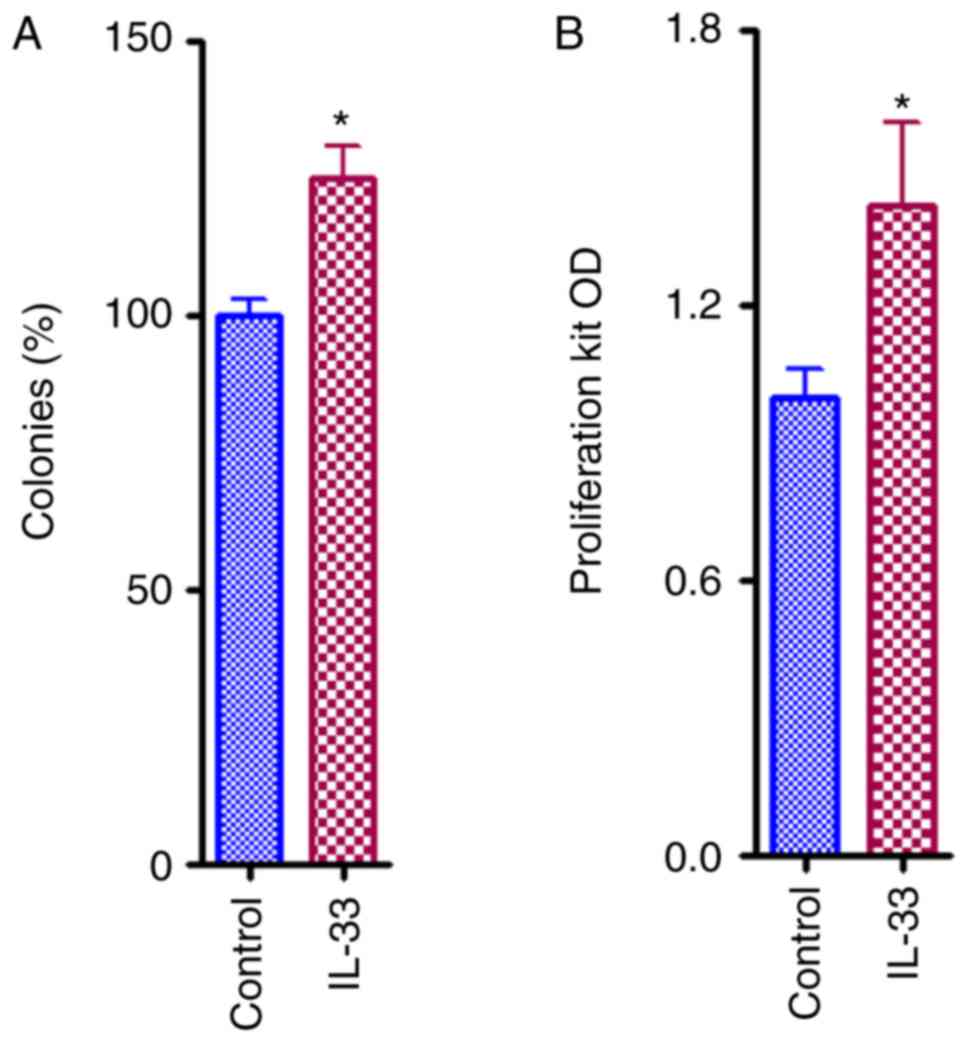
IL-33 favors proliferation and growth
of ovarian cancer cells
When compared with controls, direct IL-33 (50 ng/ml)
treatment in A2780 ovarian cancer cells induced a statistically
significant increase in proliferation (P<0.05), as indicated by
the results of the clonogenic survival assay (Fig. 1A) and the quick cell proliferation
assay (Fig. 1B). These results
clearly indicated that IL-33 has an oncogenic effect.
IL-33 downregulates the expression
level of p27 of ovarian cancer cell
To investigate the direct effect of IL-33 on the
expression of pro and anti-proliferative molecules, cell cultures
of A2780 at 70% confluence were treated with IL-33 for 3 days at 50
ng/ml. mRNA expression alteration for pro-proliferative molecules
[Cyclins B, D, E, cyclin-dependent kinase (Cdk) 2 and Cdk4] and
anti-proliferative molecules (p18, p21, p27, p53) was evaluated by
RT-PCR. The results revealed a significant increase and a
significant decease were in p21 and p27 expression levels,
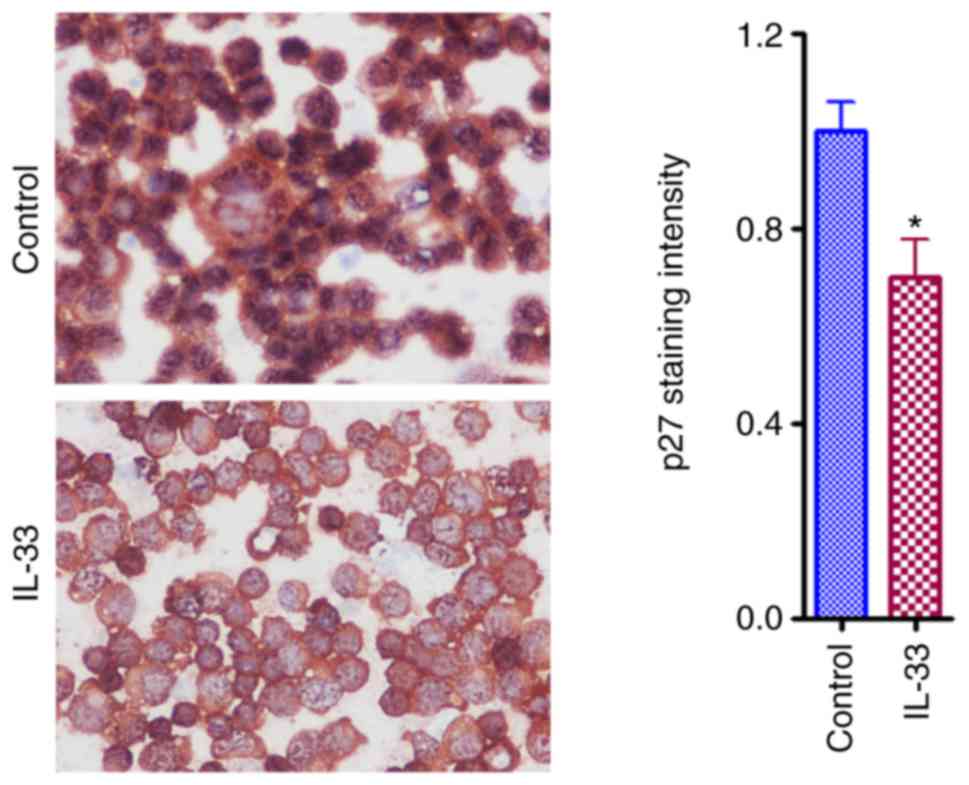
respectively, when compared with controls (Fig. 2). A decreased mRNA expression of p27
was further demonstrated by the IHC results (Fig. 3). A decreased expression of p27
induced by IL-33 may contribute to the effect of IL-33 on cell
growth because p27 is an anti-proliferative molecule in the cell
cycle. An increased expression of p21 is unexpected and may be a
compensatory effect.
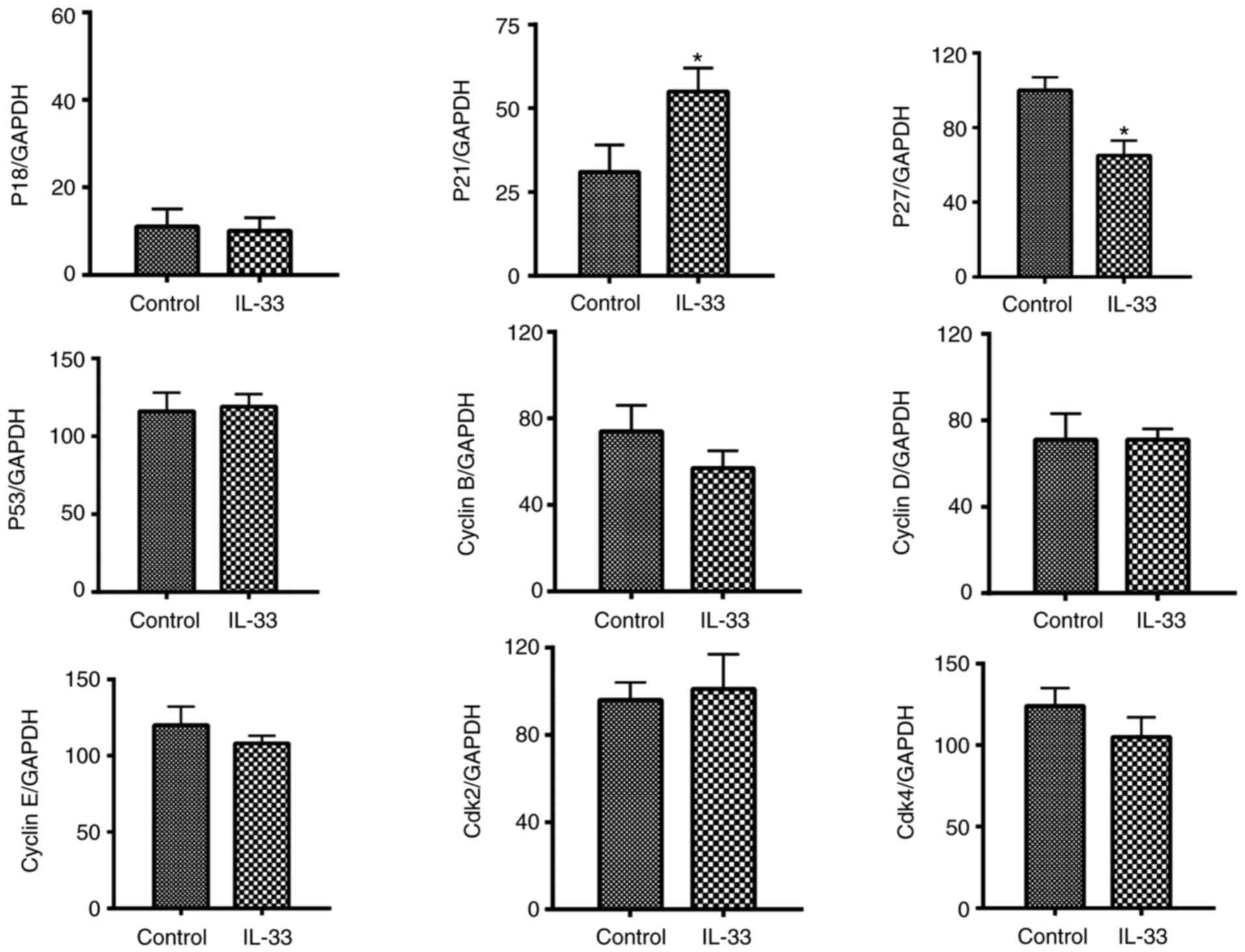 | Figure 2.IL-33 downregulates the mRNA
expression of p27 in ovarian cancer cells. mRNA expression levels
of for pro-proliferative molecules (Cyclins B, D, E, Cdk 2 and
Cdk4) and anti-proliferative molecules (p18, p21, p27, p53),
evaluated by reverse transcription-polymerase chain reaction.
Results are expressed as the mean ratio of molecule densitometric
Units/GAPDH + standard error of the mean (magnification, ×100).
*P<0.05 vs. control. Cdk, cyclin-dependent kinase; IL-33,
interleukin-33. |
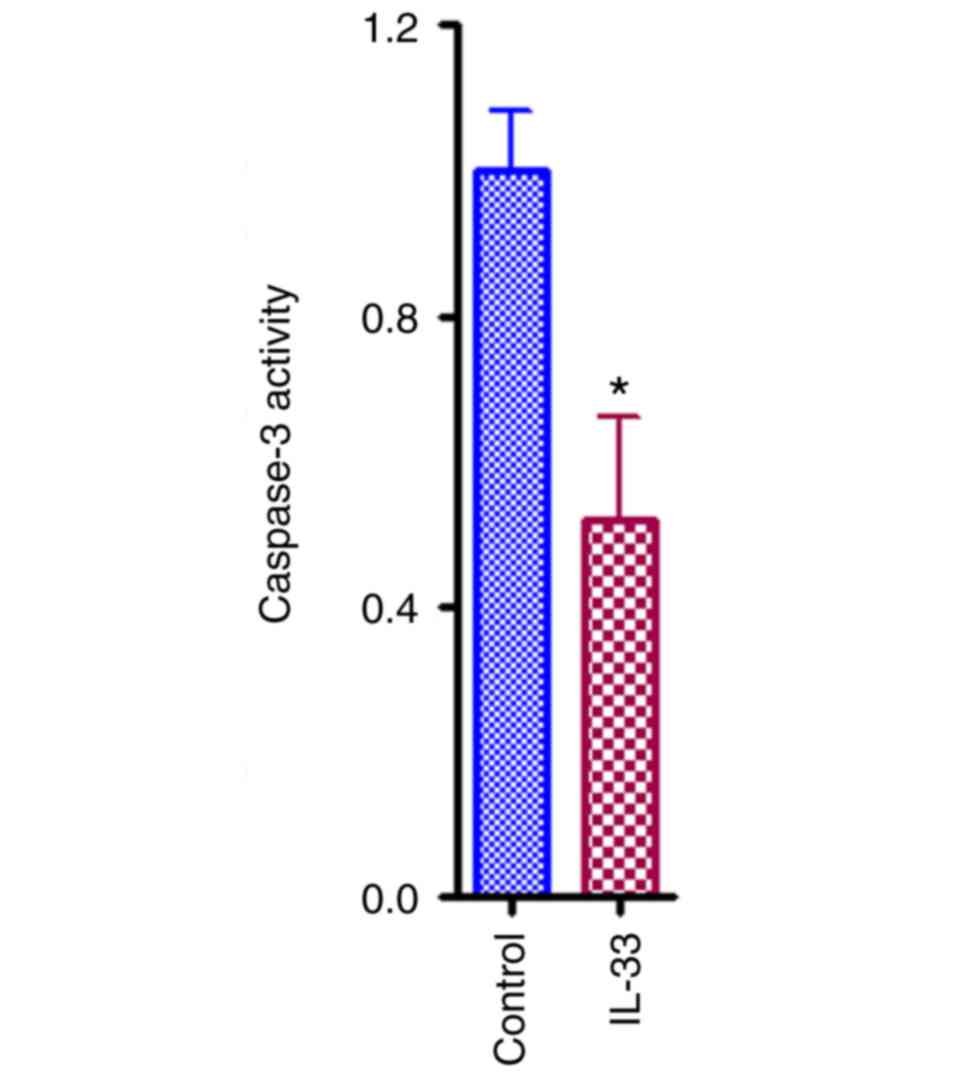
IL-33 inhibits apoptosis of ovarian
cancer cells
Apoptosis also contributes to the cellular
population of ovarian cancer. To address if IL-33 has any effect on
cellular apoptosis of A2780 ovarian cancer cells, a
well-established apoptosis kit was used. As presented in Fig. 4, the relative caspase-3 activity in
IL-33 treated group decreased by ~50% compared with control group
(Fig. 4; P<0.05).
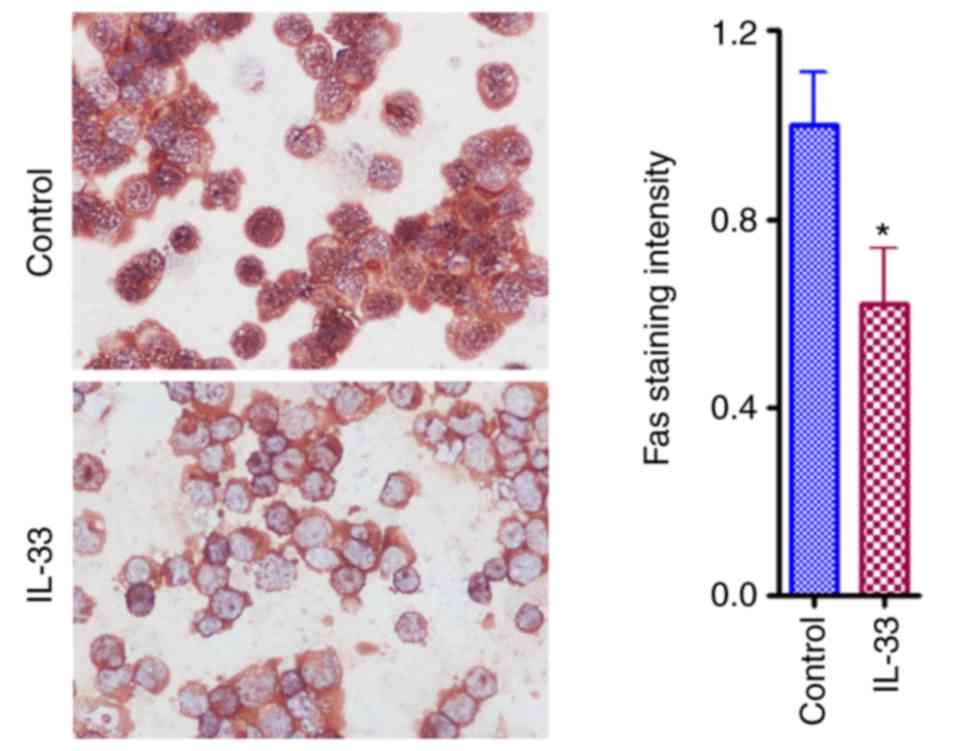
IL-33 downregulates the expression
levels of Fas and tumor necrosis factor-related apoptosis-inducing
ligand receptor 1 (TRAILR1) in ovarian cancer cells
mRNA expression of pro-apoptotic molecules [Fas, Fas
ligand, TRAIL, TRAILR1 and B cell lymphoma (Bcl)-2 associated X,
apoptosis regulator] as well as anti-apoptotic molecules
(FLICE-inhibitory protein, Bcl-2, and Survivin) were
semi-quantified and compared between IL-33 treatment and control
groups. It was demonstrated that mRNA levels of Fas and TRAILR1
significantly decreased, and levels of TRAIL significantly
increased (Fig. 5; P<0.05).
Consistent with decreased levels of mRNA detected by RT-PCR, IHC
results for Fas protein levels were also significantly decreased
(Fig. 6).
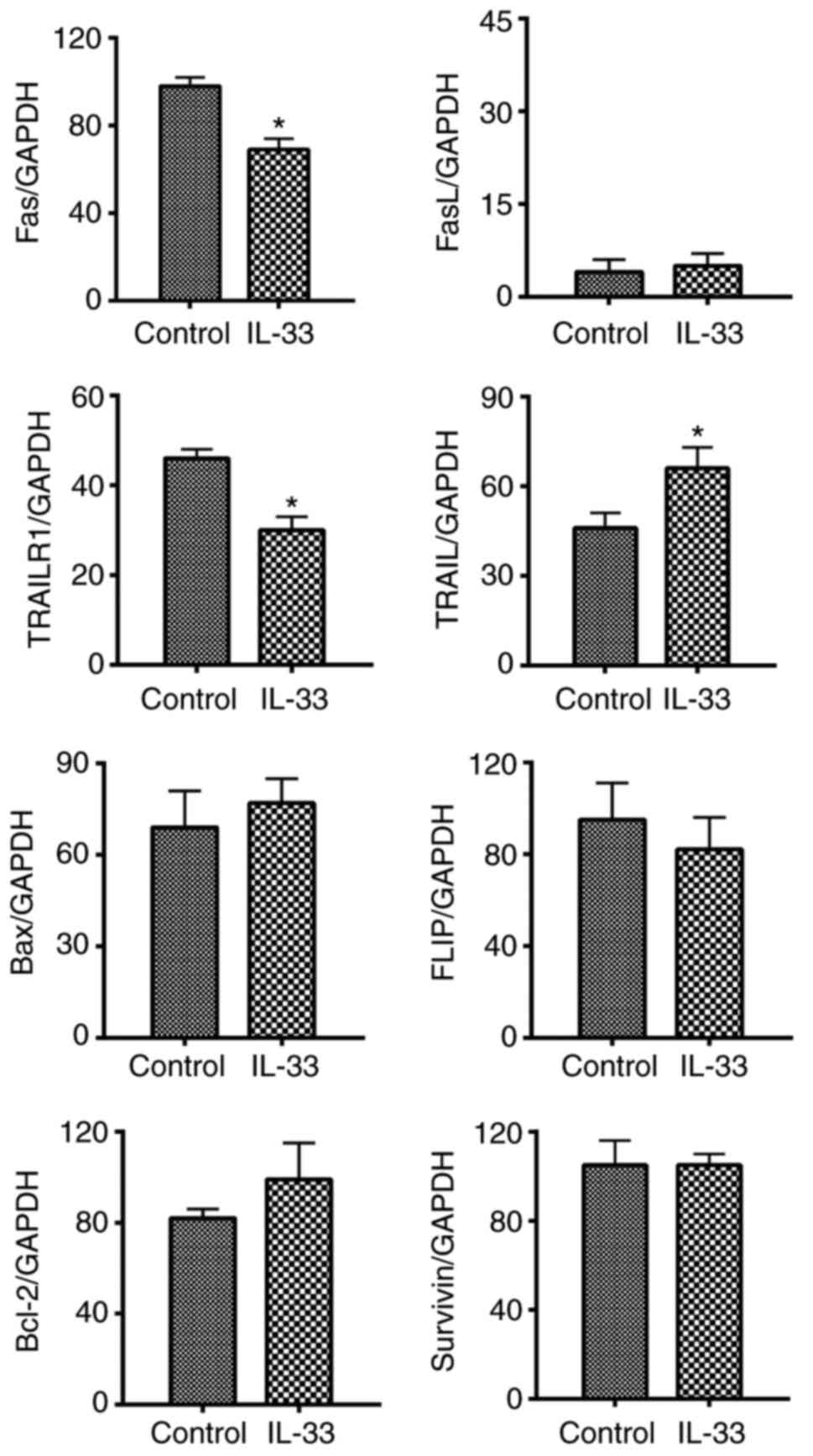 | Figure 5.IL-33 downregulates the mRNA
expression levels of Fas and TRAILR1 in ovarian cancer cells. mRNA
expression levels of pro-apoptotic molecules (Fas, FasL, TRAIL,
TRAILR1 and Bax) as well as anti-apoptotic molecules (FLIP, Bcl-2,
and Survivin) wereevaluated by reverse transcription-polymerase
chain reaction. Results are expressed as the mean ratio of molecule
densitometric Units/GAPDH + standard error of the mean
(magnification, ×100). *P<0.05 vs. control. FasL, Fas ligand;
TRAIL, tumor necrosis factor-related apoptosis-inducing ligand;
TRAILR1, TRAIL receptor 1; Bcl-2, B cell lymphoma-2; Bax, Bcl-2
associated X, apoptosis regulator; FLIP, FLICE-inhibitory
protein. |
Discussion
Ovarian cancer is the most fatal gynecological
malignancy and the fifth leading cause of cancer
associated-mortality for women in the United States (23). Annually in the USA there are ~22,000
newly diagnosed cases, and 14,000 associated fatalities (24). However, its pathogenesis still
remains poorly understood (25,26). A
lack of knowledge of its pathogenesis and molecular mechanisms has
made any endeavors into significantly reducing risk and mortality
largely ineffective (27).
A report published in 2016 from the American Cancer
Society highlights that if ovarian cancer is diagnosed while it is
still inside the ovary (stages 1A or 1B) a 92% 5-year survival rate
can be expected-however, only 15% of all ovarian cancers are
detected at this early stage (28).
The remaining 85% of diagnoses are considerably less treatable, and
they account for a severe descent in the 5-year survival rate;
~45%. Currently, ovarian cancer is treated upfront with surgery,
followed by a chemotherapy regimen appropriate for its stage
(28–30). However, with the further development
and promise of immunomodulatory approaches to treatment, the
mainstay will likely be guided towards more favorable remission
rates, with less accompanying side effects throughout the treatment
process (31). Of the possible
pathways which may eventually be targeted in an immunologically
orientated approach to treatment, the IL-33/ST2 axis provides
initial promise. The present study demonstrated that IL-33
exhibited a pro-tumor effect by promoting growth and inhibiting
apoptosis in ovarian cancer cells. The underlying potential
molecular mechanisms may be due to downregulation of p27, Fas and
TRAILR1. The study clearly indicated that the inhibition of IL-33
and its associated effects in the IL-33/ST2 signaling pathway may
serve as a promising strategy to treat ovarian cancer. It is
well-known that the IL-33/ST2 pathway activates NF-κB and
mitogen-activated protein kinases (MAPK) (8–10,15,32).
This is the upstream of the effect of the IL-33/ST2 pathway which
has been well studied. The present study focused on the downstream
effects of the IL-33/ST2 pathway, including molecules directly
associated with proliferation and apoptosis which are influenced by
activation of the NF-κB and MAPK.
Consistent with the results of the present study,
previous studies in cholangiocarcinoma, colon cancer and breast
cancer have all identified IL-33/ST2 signaling to be positively
associated with tumor growth and metastasis (8–10).
Interestingly, another study on ovarian cancer using an in
vivo model found that IL-33 and ST2 are both highly upregulated
in ovarian tumors, with an even higher level of expression in
metastatic lesions (15). To
further implicate IL-33 and ST2, this group also investigated and
found expression of these two to be positively associated with
extracellular signal-regulated kinase and c-Jun N-terminal kinase
signaling pathways, both of which are known to promote metastasis
(15,32).
Of the known anti- and pro-proliferative molecules
investigated in the present study, only p21 and p27 showed
significant results, an increase and a decrease, respectively. p21
is a typical anti-proliferative molecule, a higher level of p21
does not favor cell growth and p21 may function as a negative
regulator for cancer metastasis (33). Thus, it is possible that the
increased level of p21 was a compensatory mechanism for ovarian
cancer cells to avoid marked changes to their environment to keep
the balance of pro- and anti-proliferative molecules, particularly
when another anti-proliferative molecule p27 decreased. As a
result, it may be possible that the downregulation of the
anti-proliferative molecule p27 dominated and shifted the balance
to favor cancer cell growth.
Decreased apoptosis is a well-known characteristic
of cancer cells. In the present study, there was a notable decrease
in apoptosis among ovarian cancer cells in the presence of IL-33,
which suggested that IL-33 inhibited apoptosis of ovarian cancer
cells. The intricacies of the molecular mechanism may be rather
complex. Here, the expression of Fas and TRAILR1 was decreased in
the presence of IL-33, which may elucidate the appearance of
decreased apoptosis in the tissue, since both Fas and TRAILR1 are
known pro-apoptotic molecules. Notably, another pro-apoptotic
molecule TRAIL was unexpectedly upregulated in the presence of
IL-33. This could also be due to a compensatory effort. When
considering the alterations in p21 following the addition of IL-33,
the seemingly paradoxical changes for these anti-proliferative and
pro-apoptotic molecules indicated that it is not the change of one
specific molecule, but the balance between pro- and
anti-proliferative as well as pro- and anti-apoptotic molecules
which determines the fate of ovarian cancer cells.
The pilot experiments from our lab indicated that
IL-33 was not detected in A2780 ovarian cancer cells by
immunohistostaining. We further checked online information
regarding this issue our finding was consistent with the
information in the RNA and protein expression database (http://www.proteinatlas.org/) which indicated that
little RNA of IL-33 was detectable and IL-33 protein cannot be
detected in 90% of ovarian cancer samples. Since no or little IL-33
is expressed in ovarian cancer cells, this may make knockdown of
IL-33 unnecessary. If IL-33 was found highly/moderately expressed
in a cancer cell line, then it would be critical to knock out or
block endogenous IL-33 to further confirm our initial findings.
Despite the fact that little or no IL-33 is expressed on ovarian
cancer cells, exogenous IL-33 from stroma cells of ovarian cancer
could possibly be the source of IL-33 that promotes growth of
ovarian cancer (34).
In conclusion, A2780 ovarian cancer cells undergo
pro-proliferative and anti-apoptotic changes in the presence of
IL-33, which lead to increased culture number and proliferative
potential. The downregulation of p27 as well as Fas and TRAILR1 is
very likely to be involved in these changes. The present study
suggests that IL-33 may play a role in the promotion of ovarian
cancer tumorigenesis, and consequently, may be a therapeutic target
for better management of ovarian cancer.
Acknowledgements
The authors would like to thank Miss Mikayla M.
Brockmeyer (Des Moines University) and Mr. Dylan Weir (University
of Missouri) for their constructive comments regarding the revision
of this manuscript.
Funding
The present study was supported by Des Moines
University (grant no. IOER 112-3749 to YF).
Availability of data and materials
Data and materials are available upon request.
Authors' contributions
YF conceived and designed the study. XL, DMH, NJT,
ZZ, AA, CQ, QB, XC and YF performed the experiments. YF, XL, DMH,
NJT, ZZ, GW, MBN and MRW analyzed and interpreted the data. XL, NJT
and YF wrote the draft. YF, MBN, MRW, GW, AA, CQ, QB and XC made
critical revisions. All authors read and approved the final version
of the manuscript.
Ethical approval and consent to
participate
Ethical approval was obtained from Des Moines
University and the University of Missouri IRB for the use of human
cell lines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lataifeh I, Marsden DE, Robertson G,
Gebski V and Hacker NF: Presenting symptoms of epithelial ovarian
cancer. Aust N Z J Obstet Gynaecol. 45:211–214. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vine MF, Calingaert B, Berchuck A and
Schildkraut JM: Characterization of prediagnostic symptoms among
primary epithelial ovarian cancer cases and controls. Gynecol
Oncol. 90:75–82. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Orsulic S, Li Y, Soslow RA, Vitale-Cross
LA, Gutkind JS and Varmus HE: Induction of ovarian cancer by
defined multiple genetic changes in a mouse model system. Cancer
Cell. 1:53–62. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moss HA, Berchuck A, Neely ML, Myers ER
and Havrilesky LJ: Estimating cost-effectiveness of a multimodal
ovarian cancer screening program in the United States: Secondary
analysis of the UK collaborative trial of ovarian cancer screening
(UKCTOCS). JAMA Oncol. 4:190–195. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sporn MB: The war on cancer. Lancet.
347:1377–1381. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schmitz J, Owyang A, Oldham E, Song Y,
Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, et
al: IL-33, an interleukin-1-like cytokine that signals via the IL-1
receptor-related protein ST2 and induces T helper type 2-associated
cytokines. Immunity. 23:479–490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sanada S, Hakuno D, Higgins LJ, Schreiter
ER, McKenzie AN and Lee RT: IL-33 and ST2 comprise a critical
biomechanically induced and cardioprotective signaling system. J
Clin Invest. 117:1538–1549. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li J, Razumilava N, Gores GJ, Walters S,
Mizuochi T, Mourya R, Bessho K, Wang YH, Glaser SS, Shivakumar P
and Bezerra JA: Biliary repair and carcinogenesis are mediated by
IL-33-dependent cholangiocyte proliferation. J Clin Invest.
124:3241–3251. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu X, Zhu L, Lu X, Bian H, Wu X, Yang W
and Qin Q: IL-33/ST2 pathway contributes to metastasis of human
colorectal cancer. Biochem Biophys Res Commun. 453:486–492. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jovanovic IP, Pejnovic NN, Radosavljevic
GD, Pantic JM, Milovanovic MZ, Arsenijevic NN and Lukic ML:
Interleukin-33/ST2 axis promotes breast cancer growth and
metastases by facilitating intratumoral accumulation of
immunosuppressive and innate lymphoid cells. Int J Cancer.
134:1669–1682. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu LA, Fu Y, Zhang DN and Zhang J: Serum
IL-33 as a diagnostic and prognostic marker in non-small cell lung
cancer. Asian Pac J Cancer Prev. 14:2563–2566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao X, Wang X, Yang Q, Zhao X, Wen W, Li
G, Lu J, Qin W, Qi Y, Xie F, et al: Tumoral expression of IL-33
inhibits tumor growth and modifies the tumor microenvironment
through CD8+ T and NK cells. J Immunol. 194:438–445.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng W, Shi P, Lei B, Chai X and Tan C:
IL-33 influenced the development of colorectal cancer via
regulating Fra-1. Int J Clin Exp Pathol. 10:467–472. 2017.
|
|
14
|
Fang Y, Zhao L, Xiao H, Cook KM, Bai Q,
Herrick EJ, Chen X, Qin C, Zhu Z, Wakefield MR, et al: IL-33 acts
as a foe to MIA PaCa-2 pancreatic cancer. Med Oncol. 34:232017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tong X, Barbour M, Hou K, Gao C, Cao S,
Zheng J, Zhao Y, Mu R and Jiang HR: Interleukin-33 predicts poor
prognosis and promotes ovarian cancer cell growth and metastasis
through regulating ERK and JNK signaling pathways. Mol Oncol.
10:113–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fang Y, DeMarco VG and Nicholl MB:
Resveratrol enhances radiation sensitivity in prostate cancer by
inhibiting cell proliferation and promoting cell senescence and
apoptosis. Cancer Sci. 103:1090–1098. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fang Y, Herrick EJ and Nicholl MB: A
possible role for perforin and granzyme B in resveratrol enhanced
radiosensitivity of prostate cancer. J Androl. 33:752–760. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fang Y, Chen X, Bai Q, Qin C, Mohamud AO,
Zhu Z, Ball TW, Ruth CM, Newcomer DR and Herrick EJ: IL-9 inhibits
HTB-72 melanoma cell growth through upregulation of p21 and TRAIL.
J Surg Oncol. 111:969–974. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fang Y, Sharp GC, Yagita H and
Braley-Mullen H: A critical role for TRAIL in resolution of
granulomatous experimental autoimmune thyroiditis. J Pathol.
216:505–513. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fang Y, Wei Y, Demarco V, Chen K, Sharp GC
and Braley-Mullen H: Murine FLIP transgene expressed on thyroid
epithelial cells promotes resolution of granulomatous experimental
autoimmune thyroiditis in DBA/1 mice. Am J Pathol. 170:875–887.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fang Y, Bradley MJ, Cook KM, Herrick EJ
and Nicholl MB: A potential role for resveratrol as a radiation
sensitizer for melanoma treatment. J Surg Res. 183:645–653. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fang Y, Bradley MJ, Bai Q, Cook KM,
Herrick EJ and Nicholl MB: Hydrogen peroxide enhances
radiation-induced apoptosis and inhibition of melanoma cell
proliferation. Anticancer Res. 33:1799–1807. 2013.PubMed/NCBI
|
|
23
|
Brucks JA: Ovarian cancer. The most lethal
gynecologic malignancy. Nurs Clin North Am. 27:835–845.
1992.PubMed/NCBI
|
|
24
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Daniilidis A and Karagiannis V: Epithelial
ovarian cancer. Risk factors, screening and the role of
prophylactic oophorectomy. Hippokratia. 11:63–66. 2007.PubMed/NCBI
|
|
26
|
Landen CN Jr, Birrer MJ and Sood AK: Early
events in the pathogenesis of epithelial ovarian cancer. J Clin
Oncol. 26:995–1005. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kurman RJ and Shih IeM: The origin and
pathogenesis of epithelial ovarian cancer-a proposed unifying
theory. Am J Surg Pathol. 34:433–443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Roett MA and Evans P: Ovarian cancer: An
overview. Am Fam Physician. 80:609–616. 2009.PubMed/NCBI
|
|
29
|
Lalrinpuii E, Bhageerathy PS, Sebastian A,
Jeyaseelan L, VinothaThomas, Thomas A, Chandy R and Peedicayil A:
Ovarian cancer in young women. Indian J Surg Oncol. 8:540–547.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pignata S, Cannella L, Leopardo D, Pisano
C, Bruni GS and Facchini G: Chemotherapy in epithelial ovarian
cancer. Cancer Lett. 303:73–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Patil US, Jaydeokar AV and Bandawane DD:
Immunomodulators: A pharmacological review. Int J Pharm Pharm Sci.
4 Suppl 1:S30–S36. 2012.
|
|
32
|
Fu H, Hu Z, Wen J, Wang K and Liu Y:
TGF-beta promotes invasion and metastasis of gastric cancer cells
by increasing fascin1 expression via ERK and JNK signal pathways.
Acta Biochim Biophys Sin. 41:648–656. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Deryabin PI, Borodkina AV, Nikolsky NN and
Burova EB: Relationship between p53/p21/Rb and MAPK signaling
pathways in human endometrium-derived stem cells under oxidative
stress. Tsitologiia. 57:788–795. 2015.PubMed/NCBI
|
|
34
|
Chen X, Lu K, Timko NJ, Weir DM, Zhu Z,
Qin C, Mann JD, Bai Q, Xiao H, Nicholl MB, et al: IL-33 notably
inhibits the growth of colon cancer cells. Oncol Lett. 16:769–774.
2018.PubMed/NCBI
|