Introduction
Adenoid cystic carcinoma (ACC) is an uncommon
malignant neoplasm consisting of epithelial and myoepithelial cells
arranged in variable patterns, including tubular, cribriform, and
solid architecture. This tumor is thought to progress slowly but
remains on a relentless clinical course (1,2). The
clinicopathologic features and behaviors of ACC are quite distinct,
illustrated by frequent perineural invasion, local recurrence and
late distant metastasis (3). The
5-year overall survival (OS) rate for ACC patients ranges from
64–91% (1–6). Factors that influence survival include
age, tumor size, tumor site, clinical stage, lymph node
involvement, status of surgical margins and distant metastasis
(1,2,6,7). While
radiotherapy has been reported to improve survival in cases with
microscopic residual disease/positive surgical margins (8), the value of chemotherapy in this tumor
appears to be limited (4,9).
Although it mainly affects the major and minor
salivary glands, ACC may also occur in a number of other locations
including the uterine cervix and vulva (10,11).
Lower female genital tract tumors with adenoid cystic
differentiation are very rare, accounting for less than 1% of all
lower female genital tract malignancies (10). These tumors are thought to originate
from the major vestibular (Bartholin) glands of the vulva and minor
secretory glands or reserve cells of the uterine cervix, displaying
similar histopathological features to ACC of non-genital tract
sites. It has been proposed that ACCs of the uterine cervix and
vulva can be sub-classified into two distinct groups based on the
presence or absence of high-risk human papillomavirus (HPV)
(12). The oncogenic mechanisms
that underlie the development of HPV-unrelated ACC are thought to
be similar to those of ACCs in other sites. In fact, nuclear factor
NFIB-associated gene rearrangement is a frequent genetic event in
vulvar ACCs, conferring a driving force to transform the cells
(13). Unlike vulvar ACCs, our
previous results demonstrated that cervical carcinomas with mixed
differentiation, including adenoid cystic carcinomatous component,
are etiologically associated with high-risk HPV and can be
identified by diffuse p16 expression (12).
Adenoid basal tumors (epitheliomas/carcinomas) of
the uterine cervix are also rare lower female genital tract
neoplasms that display a mixed configuration of basaloid, squamous,
and glandular morphology (11,14,15).
Similar to cervical ACC, adenoid basal tumors are also derived from
high-risk HPV-infected reserve cells, sharing many morphologic
features with ACC (14,16). In fact, the two tumors were
previously regarded as a single entity (15,17).
Lacking destructive infiltrative growth, adenoid basal epithelioma
is usually an incidental finding in patients treated for a
high-grade squamous intraepithelial lesion and usually behaves in a
benign fashion (16,17). The terms ‘adenoid basal carcinoma
(ABC)’ and ‘adenoid basal epithelioma’ are considered synonymous in
the 2014 WHO Classification of the tumors of the uterine cervix
(10). The presence of any invasive
carcinoma subtype with ABC needs to be reported as a ‘mixed
carcinoma’.
Due to the rarity of ACC and ABC in the lower female
genital tract, the majority of published literature comprises case
reports or small case series studies with limited sample sizes
(14,15,17).
Currently, the clinicopathologic features, treatment modalities and
clinical outcomes of these tumors remain largely unknown, thus
hampering the establishment of standard treatment protocols to
guide clinical management. The Surveillance, Epidemiology and End
Results (SEER) program of the National Cancer Institute is a public
source of epidemiologic information on the cancer incidence and
survival data from population-based cancer registries covering ~28%
of the population of the United States (18). Using data from the SEER program, the
clinicopathologic features and survival outcomes of ACC and ABC of
the uterine cervix were investigated. These features were also
compared with those of vulvar ACC.
Materials and methods
Representative histologic images
The images of uterine cervical ACCs and ABCs and
vulvar ACCs were taken from consultation cases at the Johns Hopkins
Hospital (Baltimore, MD, USA). Details of methods for
immunohistochemical analysis of p16 expression have been previously
reported (12). In brief,
formalin-fixed, paraffin-embedded tissue sections were used.
Immunoperoxidase labeling was done with anti-p16(INK4a) (cat. no.
705-4793; Ventana Medical Systems, Inc., Tucson, AZ, USA) at a
dilution of 1:500. All images were captured at a magnification of
×200.
Patient selection
The SEER November 18, 2016 submission (18) is a public-use database that includes
updated cancer incidence and population data associated by age,
sex, ethnicity, year of diagnosis, geographic areas and cause of
death. Data on patients with ACC and ABC of the lower female
genital tract were obtained. All patients with a diagnosis of
primary ACC and ABC of the uterine cervix and ACC of the vulva from
1973–2014 were included in the present study. A signed Research
Data Agreement was obtained to access these data. The present study
was approved by the Institutional Review Board at the Johns Hopkins
University School of Medicine (Baltimore, MD, USA).
Variables
The following International Classification of
Diseases for Oncology (ICD-O) site codes were used: C53 for cervix
uteri and C51 for vulva. The following ICD-O histology codes were
used: 8200/3 for ACC and 8098/3 for ABC. Ethnicity was recorded in
the SEER database as ‘White,’ ‘Black,’ ‘Other: American Indian, AK
Native, Asian/Pacific Islander’ or ‘Unknown’. Marital status was
grouped as ‘Married’ (including common law), ‘Single’ (single-never
married, separated, divorced or widowed), or ‘Unknown’. All
diagnoses were microscopically confirmed by the contributing
agency. Therapy was coded as surgery, radiotherapy, surgery with
radiotherapy and no/unknown. SEER staging was based on the theory
of cancer growth: ‘Localized’ tumor was confined to the organ of
origin without extension beyond the primary organ; ‘Regional
extension’ of tumor referred to direct extension to adjacent organs
or structures or spread to regional lymph nodes; the ‘Distant’
stage was defined when the cancer had spread to parts of the body
remote from the primary tumor. The tumor size, lymph node
involvement and the International Federation of Gynecology and
Obstetrics (FIGO) stage information were available for the cases
that were recorded from 1988–2014.
Statistical analysis
A χ2 test or Fisher's exact test was used
to evaluate the differences between categorical data. The Wilcoxon
signed-rank test was used to compare continuous data. Prognostic
factors predictive of CSS and OS were analyzed using univariate and
multivariate Cox proportional hazards models. CSS and OS were
calculated using the Kaplan-Meier method and compared using the
log-rank test. Statistical analysis was performed with SAS version
9.4 (SAS Institute, Inc., Cary, NC, USA). P<0.05 was considered
to be statistically significant. SEER*Stat (version 8.3.4; National
Cancer Institute; National Institutes of Health, Bethesda, MD, USA)
software was used for incidence rates calculation. All rates were
age-adjusted to the 2000 US standard (18).
Results
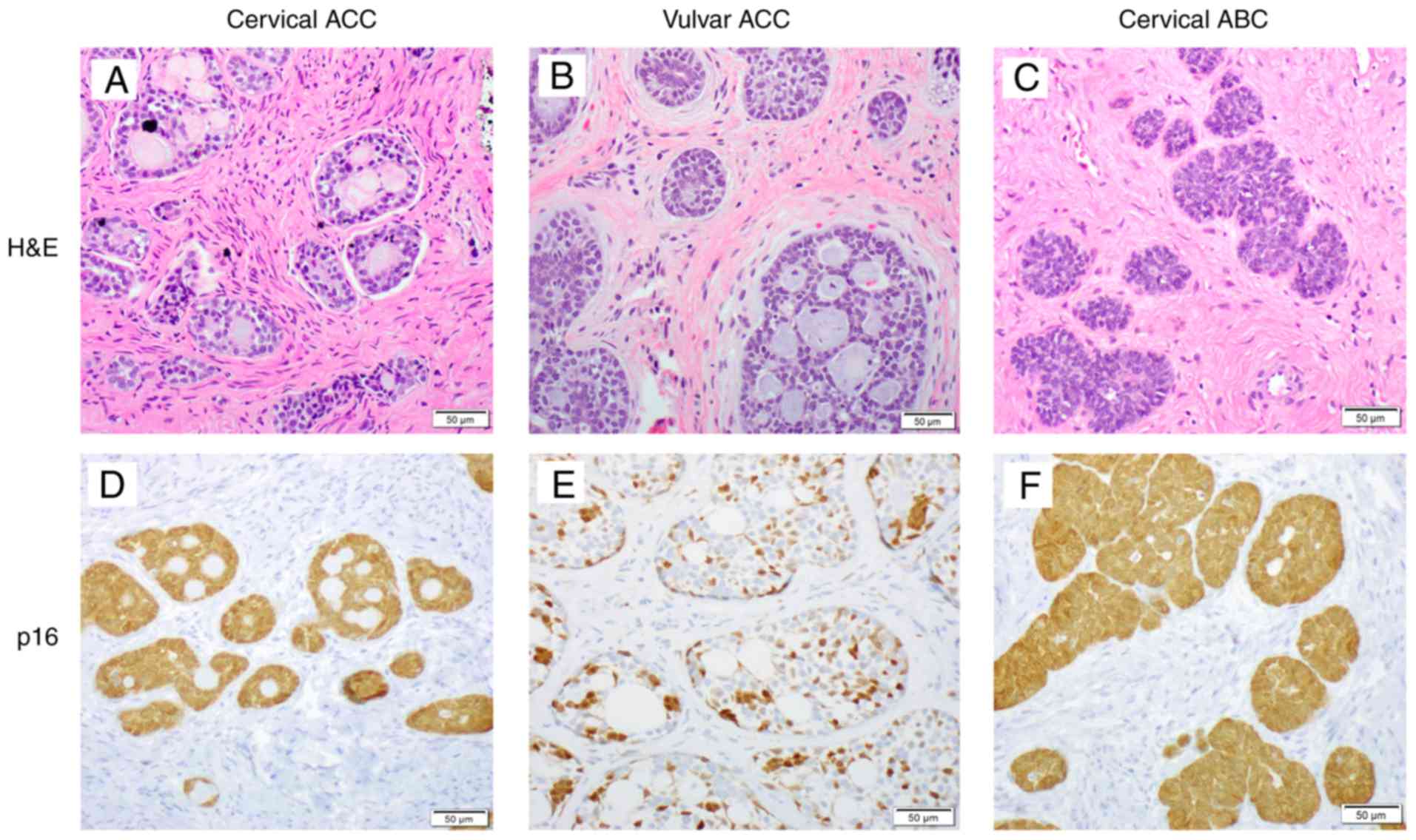
Histologic features
Representative histologic images of uterine cervical
ACCs and ABCs and vulvar ACCs are presented in Fig. 1. Whereas certain cervical ACCs
(Fig. 1A) had morphologic features
similar to those of the vulva (Fig.
1B), others displayed features of higher grades in appearance,
characterized by larger, less uniform nuclei with evident nucleoli
and readily identified mitotic figures and apoptotic bodies. The
cervical ABCs contained low-grade adenoid basal epithelioma
components characterized by discrete nests of tumor in which the
surrounding stroma lacked a desmoplastic reaction and the cytologic
features were uniform/bland and basaloid in appearance (Fig. 1C). The cervical ACCs exhibited a
diffuse p16 staining pattern, consistent with high-risk
HPV-associated etiology (Fig. 1D).
The vulvar ACCs usually displayed classic morphologic features
characterized by uniform, small cells arranged in cords and nests
with a cribriform pattern and the cystic lumens commonly filled
with acellular basement membrane-like material (Fig. 1B). p16, a surrogate marker for
high-risk HPV infection, mostly exhibited a focal and patchy
staining pattern (Fig. 1E). Nearly
all of the cervical ABCs exhibited a diffuse p16 staining pattern
(Fig. 1F) associated with high-risk
HPV infection.
Clinicopathologic and therapeutic
characteristics
Incidence
The age-adjusted incidence of ACC of the uterine
cervix was 0.025 (white, 0.02; black, 0.087) per million with a
white-to-black ratio of 1:4.35. Compared with cervical ACC, the
age-adjusted incidence of ABC was slightly higher (total
population, 0.064 per million; white 0.057; black 0.078). The
incidence of vulvar ACC was 0.036 (white, 0.038; black 0.032) per
million. Black people thus appeared to be more susceptible to
cervical ACC and ABC.
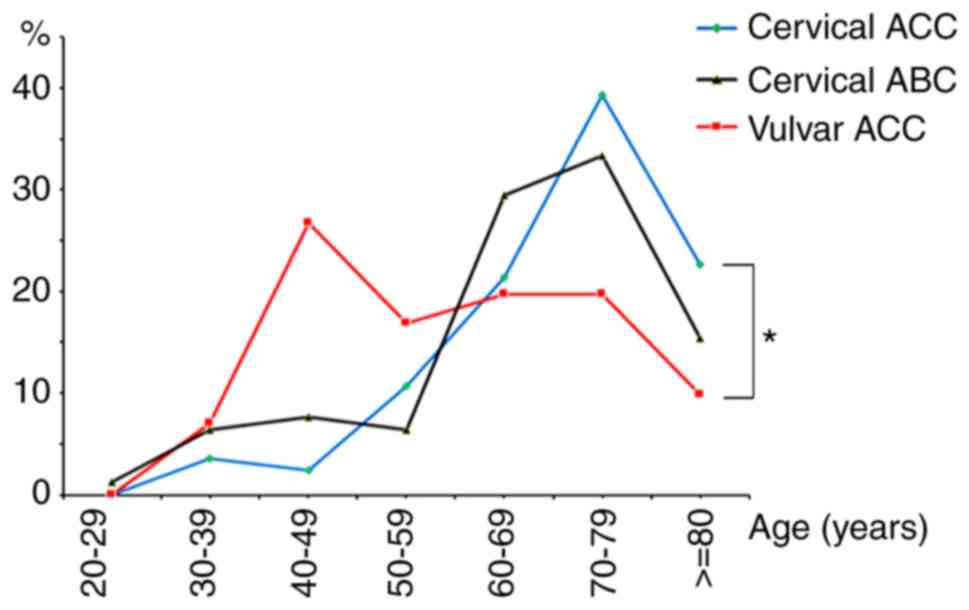
Age distribution
From 1973–2014, the SEER database identified a total
of 233 patients in the present study, including 84 cervical ACC
patients, 78 with cervical ABC and 71 with vulvar ACC. The age
distribution of these cases is summarized in Table I and illustrated in Fig. 2. The ages of the patients with
cervical ACC ranged from 30–90 years (median, 72 years) and ABC
from 28–89 years (median, 69 years). With a similar age
distribution pattern, the peak incidence of these two tumors was
observed in the seventh and eighth decades and had no statistical
difference. In contrast, the patients with vulvar ACC were
significantly younger (range, 31–95 years; median, 59 years) than
those with cervical ACC (P<0.0001). The peak incidence of vulvar
ACC was observed in the fifth decade of life (Fig. 2).
 | Table I.Clinicopathological
characteristics. |
Table I.
Clinicopathological
characteristics.
| Characteristic | Cervical ACC | Cervical ABC |
P-valuea | Vulvar ACC |
P-valuea |
|---|
| Total patients
(n) | 84 | 78 |
| 71 |
|
| Median age at
diagnosis, years (range) | 72 (30–90) | 69 (28–89) | 0.1236 | 59 (31–95) | <0.0001 |
| Ethnicity, n
(%) |
|
| 0.0002 |
| 0.0002 |
|
White | 48 (57.1) | 55 (70.5) |
| 60 (84.5) |
|
|
Black | 32 (38.1) | 9 (11.5) |
| 6 (8.5) |
|
|
Others | 4 (4.8) | 14 (18.0) |
| 5 (7.0) |
|
| Marital status, n
(%) |
|
| 0.0094 |
| <0.0001 |
|
Married | 15 (17.9) | 29 (37.2) |
| 40 (56.3) |
|
|
Singleb | 68 (81.0) | 45 (57.7) |
| 28 (39.5) |
|
|
Unknown | 1 (1.1) | 4 (5.1) |
| 3 (4.2) |
|
| SEER stage, n
(%) |
|
| 0.0002 |
| 0.2638 |
|
Localized | 46 (54.8) | 68 (87.2) |
| 45 (63.4) |
|
|
Regional | 29 (34.5) | 8 (10.3) |
| 19 (26.7) |
|
|
Distant | 3 (3.6) | 0 (0.0) |
| 6 (8.5) |
|
|
Unknown | 6 (7.1) | 2 (2.5) |
| 1 (1.4) |
|
| Surgery, n (%) |
|
| <0.0001 |
| <0.0001 |
|
Yes | 55 (65.4) | 77 (98.7) |
| 68 (95.8) |
|
| No | 24 (28.6) | 1 (1.3) |
| 3 (4.2) |
|
|
Unknown | 5 (6.0) | 0 (0.0) |
| 0 (0.0) |
|
| Radiation, n
(%) |
|
| <0.0001 |
| 0.0381 |
|
Yes | 49 (58.3) | 12 (15.4) |
| 28 (39.4) |
|
|
No/unknown | 35 (41.7) | 66 (84.6) |
| 43 (60.6) |
|
| Surgery and
radiation, n (%) |
|
| 0.1830 |
| 0.4469 |
|
Yes | 22 (26.2) | 12 (15.4) |
| 25 (35.2) |
|
|
No/Unknown | 62 (73.8) | 66 (84.6) |
| 46 (64.8) |
|
| Available FIGO
stage, n (%)c | 56 (100) | 76 (100) | <0.0001 | 55 (100) | 0.3041 |
| I | 33 (58.9) | 74 (97.4) |
| 29 (52.7) |
|
| II | 14 (25.0) | 1 (1.3) |
| 8 (14.5) |
|
|
III | 6 (10.7) | 1 (1.3) |
| 14 (25.5) |
|
| IV | 3 (5.4) | 0 (0.0) |
| 4 (7.3) |
|
| Tumor
sizec |
|
| <0.0001 |
| 0.9344 |
|
Available tumor size, n | 33 | 49 |
| 48 |
|
| Median
tumor size, cm (range) | 3.3 (0.7–8.0) | 1.0 (0.1–7.0) |
| 3.4 (0.3–9.0) |
|
| Lymph node
involvement, n (%)c | 60 | 78 | 0.0049 | 63 | 0.1143 |
| No | 45 (75.0) | 74 (94.8) |
| 56 (88.9) |
|
|
Yes | 1 (1.7) | 1 (1.3) |
| 2 (3.2) |
|
|
Unknown | 14 (23.3) | 3 (3.9) |
| 5 (7.9) |
|
Ethnicity and marital status
As presented in Table
I, 32 (38.1%) of 84 patients who had cervical ACC were black.
In contrast, only 11.5% (9/78) of patients with cervical ABC and
8.5% (6/71) of patients with vulvar ACC were black. Overall, 15
(17.9%) of 84 patients were married at the time of cervical ACC
diagnosis. The proportion of married patients significantly
increased in both the cervical ABC patients (37.2%; P=0.0094) and
the patients with vulvar ACC (56.3%; P<0.0001) compared with
those with cervical ACC (Table
I).
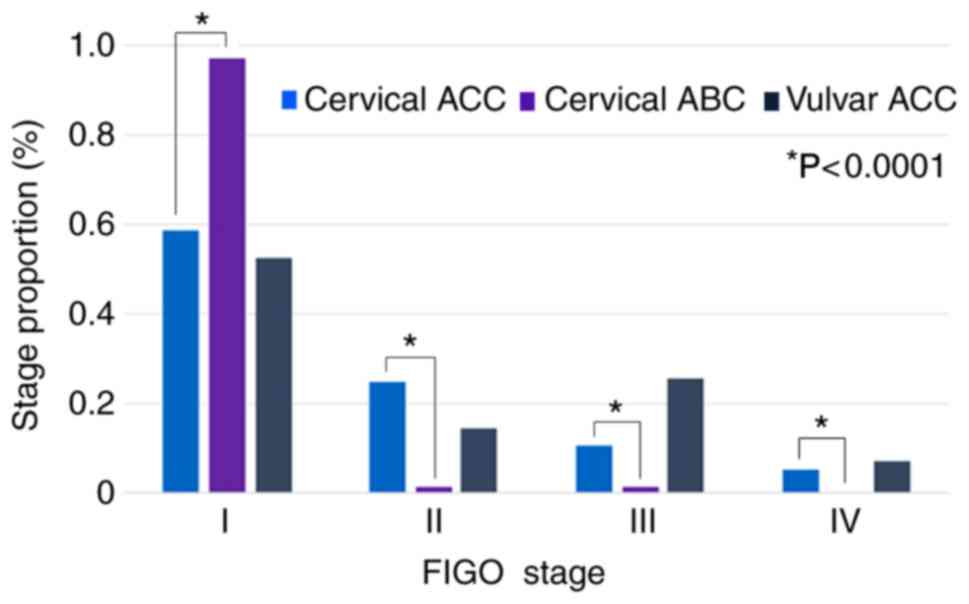
Stage
Of 233 patients in the present study, 224 with SEER
stage were available and are detailed in Table I. Of the patients with cervical ACC,
46 (54.8%) had localized disease, 29 (34.5%) had regional stage,
and 3 (3.6%) had distant metastatic disease. Whereas the patients
with vulvar ACC displayed a similar stage distribution (63.4%
localized, 26.7% regional and 8.5% distant) to those with cervical
ACC, the vast majority of patients with cervical ABC had localized
disease (87.2% localized, 10.3% regional, and 0% distant;
P=0.0002). A total of 187 patients (cervical ACC, 56; cervical ABC,
76; and vulvar ACC, 55) with FIGO stage were available for
analysis. The distribution of FIGO stage in different types of
tumor is presented in Fig. 3.
Overall, 33 (58.9%) of 56 patients with cervical ACC had FIGO stage
I disease, 14 (25.0%) had stage II, 6 (10.7%) had stage III and 3
(5.4%) had stage IV. Similar to the stage distribution of the
patients with cervical ACC (P=0.3041), the stage distribution of
the patients with vulvar ACC was 52.7% (29/55) stage I, 14.5%
(8/55) stage II, 25.5% (14/55) stage III and 7.3% (4/55) stage IV.
Only 2 (2.6%) of 76 cervical ABC patients were stage II and above
(97.4% stage I, 1.3% stage II, 1.3% stage III and 0% stage IV),
which was statistically significant compared with cervical ACC
(P<0.0001).
Tumor size and lymph node
involvement
A total of 33 cervical ACC patients had known tumor
sizes ranging from 0.7–8.0 cm (median, 3.3 cm; Table I). The median tumor size of 48
vulvar ACCs was 3.4 cm (range, 0.3–9.0 cm), similar to that of the
cervical ACCs. Commonly discovered as an incidental finding, the
known tumor size of 49 patients with cervical ABCs ranged from
0.1–7.0 cm (median, 1.0 cm), significantly smaller than the
cervical ACCs (P<0.0001). Lymph node association is a very
uncommon event in these tumors, with 1 case in the patients with
cervical ACC, 1 case in the patients with cervical ABC and 2 cases
in the patients with vulvar ACC, respectively.
Treatment
Whereas 77 (98.7%) of 78 patients with cervical ABC
and 68 (95.8%) of 71 patients with vulvar ACC underwent surgery,
only 55 (65.4%) of 84 cervical ACC patients were treated with
surgery (P<0.0001). In contrast to a low surgery rate, more
cervical ACC patients received radiation therapy (49/84; 58.3%)
compared with the cervical ABC patients (12/78; 15.4%; P<0.0001)
and vulvar ACC patients (28/71; 39.4%; P=0.0381). The number of
patients receiving both surgery and radiation was 22 (26.2%) of 84
with cervical ACC, 12 (15.4%) of 78 with cervical ABC and 25
(35.2%) of 71 with vulvar ACC, respectively.
Prognostic factors
Among all clinicopathologic variables analyzed in
the cervical ACCs, the factors significantly associated with 5-year
cause-specific survival (CSS) by univariate analysis were age,
ethnicity and SEER stage (Table
II). Increased age, black patients and higher stages were
associated with an adverse outcome. Tumor SEER stage remained an
independent prognostic factor upon multivariate analysis [hazard
ratio (HR) and 95% confidence interval (CI), 3.80 (1.23–11.77)].
Over a long-term follow-up, age and tumor SEER stage remained the
independent prognostic factors associated with 10-year CSS of the
cervical ACC patients by both univariate and multivariate analysis
(data not shown). As for the 5- and 10-year OS in this group of ACC
patients, increased age, as a continuous variable, was the only
significant factor associated with a worse prognosis by both
univariate and multivariate analysis (Table III and unpublished data).
 | Table II.Univariate and multivariate analysis
of the 5-year hazard ratio of cause-specific survival. |
Table II.
Univariate and multivariate analysis
of the 5-year hazard ratio of cause-specific survival.
|
| Cervical ACC | Cervical ABC | Vulvar ACC |
|---|
|
|
|
|
|
|---|
| Characteristic | Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate |
|---|
| Age at
diagnosis | 1.07 | 1.06 | 1.08 | 1.21 | 1.07 | 1.10 |
|
|
(1.02–1.13)a | (0.99–1.12) | (0.99–1.18) | (0.95–1.56) |
(1.01–1.12)a |
(1.02–1.19)a |
| Ethnicity |
|
White | Reference |
| Reference |
| Reference |
|
|
Black | 2.35 | 2.14 | – | – | – | – |
|
|
(1.03–5.36)a | (0.89–5.18) |
|
|
|
|
|
Other | – | – | 4.23 | 9.14 | 2.28 | 1.79 |
|
|
|
| (0.26–67.8) | (0.51–165.7) | (0.27–19.6) | (0.17–19.31) |
| Marital status |
|
Married | Reference |
| Reference |
| Reference |
|
|
Singleb | 0.89 | 1.20 | – | – | 0.25 | 0.03 |
|
| (0.30–2.62) | (0.30–4.84) |
|
| (0.03–2.04) |
(<0.01–0.55)a |
|
Unknown | – | – | – | – | – | – |
| SEER stage |
|
Localized | Reference |
| Reference |
| Reference |
|
|
Regional | 5.40 | 3.80 | – | – | 3.79 | 2.51 |
|
|
(1.92–15.20)a |
(1.23–11.77)a |
|
| (0.63–22.74) | (0.30–21.26) |
|
Distant | 3.63 | 4.31 | – | – | 10.9 | 29.8 |
|
| (0.42–31.06) | (0.44–41.90) |
|
|
(1.54–77.60)a |
(2.82–314.90)a |
|
Unknown | – | – | – | – | – | – |
| Surgery |
|
Yes | Reference |
| Reference |
| Reference |
|
| No | 2.38 | 1.53 | – | – | – | – |
|
| (0.97–5.88) | (0.56–4.19) |
|
|
|
|
|
Unknown | – | – | – | – | – | – |
 | Table III.Univariate and multivariate analysis
of the 5-year hazard ratio of overall survival. |
Table III.
Univariate and multivariate analysis
of the 5-year hazard ratio of overall survival.
|
| Cervical ACC | Cervical ABC | Vulvar ACC |
|---|
|
|
|
|
|
|---|
| Characteristic | Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate |
|---|
| Age at
diagnosis | 1.06 | 1.05 | 1.08 | 1.08 | 1.09 | 1.16 |
|
|
(1.02–1.11)a |
(1.00–1.10)a | (0.99–1.18) | (0.97–1.20) |
(1.04–1.14)a |
(1.07–1.26)a |
| Ethnicity |
|
White | Reference |
| Reference |
| Reference |
|
|
Black | 1.95 | 1.72 | – | – | – | – |
|
| (0.96–3.94) | (0.82–3.60) |
|
|
|
|
|
Other | 0.98 | 1.48 | 3.41 | 2.27 | 1.52 | 1.67 |
|
| (0.13–7.43) | (0.18–12.04) | (0.76–15.28) | (0.44–11.81) | (0.19–12.1) | (0.17–16.59) |
| Marital status |
|
Married | Reference |
| Reference |
| Reference |
|
|
Singleb | 1.28 | 1.45 | 1.61 | 1.62 | 0.83 | 0.13 |
|
| (0.45–3.67) | (0.42–5.08) | (0.31–8.29) | (0.30–8.66) | (0.24–2.82) |
(0.02–0.79)a |
|
Unknown | – | – | – | – | – | – |
| SEER stage |
|
Localized | Reference |
| Reference |
| Reference |
|
|
Regional | 2.29 | 1.88 | 5.82 | 5.18 | 2.05 | 1.07 |
|
|
(1.08–4.90)a | (0.80–4.43) |
(1.30–26.03)a |
(1.06–25.37)a | (0.55–7.64) | (0.21–5.49) |
|
Distant | 1.38 | 1.71 | – | – | 4.28 | 4.96 |
|
| (0.18–10.52) | (0.21–14.19) |
|
| (0.83–22.1) | (0.72–33.97) |
|
Unknown | – | – | – | – | – | – |
| Surgery |
|
Yes | Reference |
| Reference |
| Reference |
|
| No | 1.49 | 1.18 | – | – | 4.14 | 0.07 |
|
| (0.69–3.24) | (0.51–2.72) |
|
| (0.52–33.0) |
(<0.01–3.29) |
|
Unknown | – | – | – | – | – | – |
Subsequently, the prognostic factors of the patients
with vulvar ACC were analyzed. Age was significantly associated
with a 5- and 10-year CSS by both univariate and multivariate
analysis (Table II and unpublished
data). Notably, the patients who were single appeared to have a
favorable prognosis upon multivariate analysis (5-year CSS, HR
0.03, 95% CI 0.002–0.55; 10-year CSS, HR 0.17, 95% CI 0.03–0.86).
Higher SEER stage was adversely correlated with CSS and OS survival
when univariate and multivariate analyses were performed over a
10-year follow-up (multivariate CSS, HR 5.71, 95% CI 1.11–29.37;
multivariate OS, HR 4.01, 95% CI 1.10–14.67).
Clinicopathologic factors associated with CSS and OS
in the patients with cervical ABC were further analyzed. SEER stage
remained as the only significant factor associated with 5-year OS
by both univariate and multivariate analysis (univariate OS, HR
5.82, 95% CI 1.30–26.03; multivariate OS, HR 5.18, 95% CI
1.06–25.37; Table III). None of
the other clinicopathologic factors reached statistical
significance by all types of survival analysis.
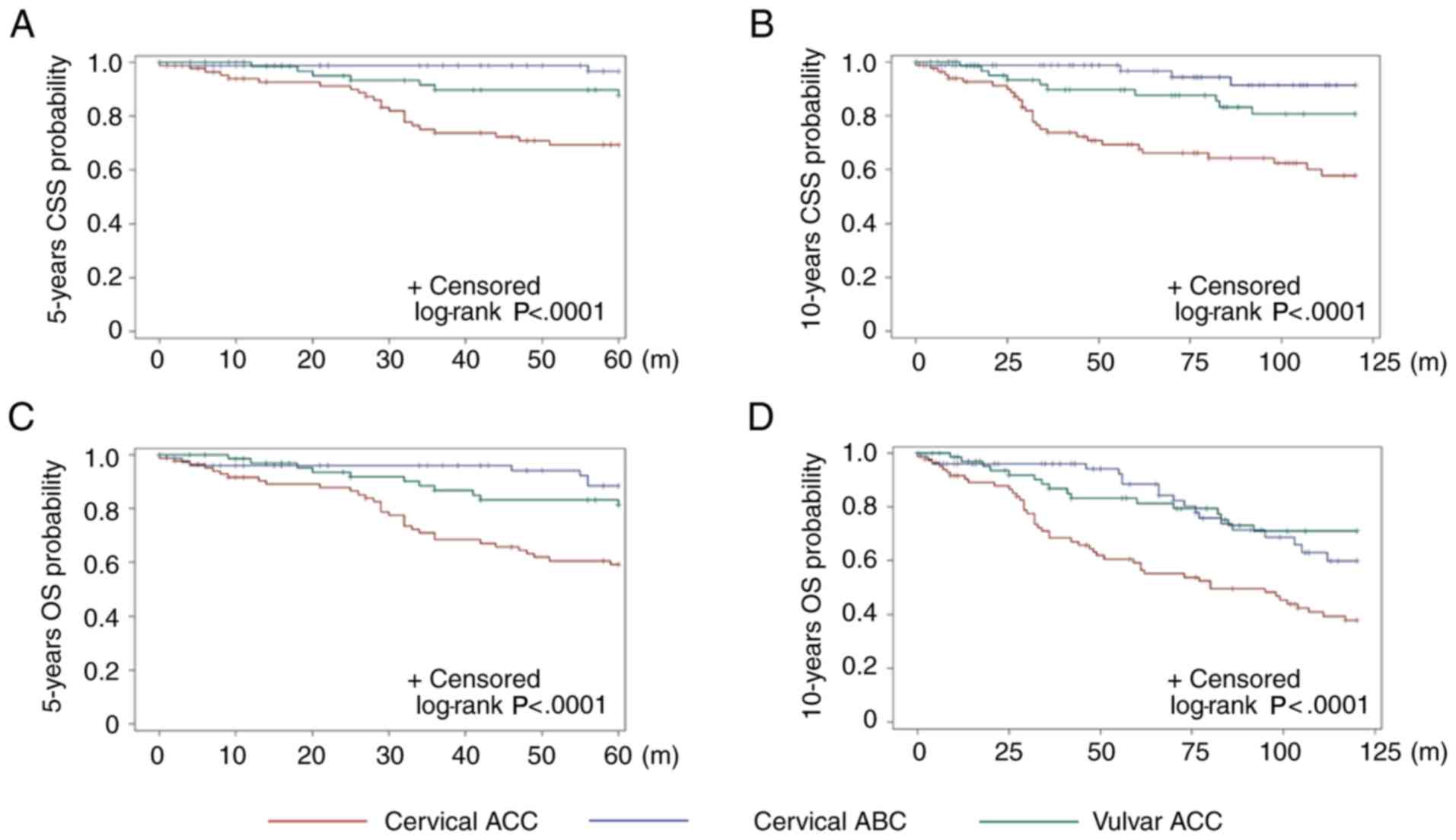
Comparative survival outcomes
The Kaplan-Meier plots (Fig. 4) illustrate a significant difference
in CSS and OS between the different types of tumor. The 5- and
10-year CSS rates in the patients with cervical ACC were 69.3 and
57.9%, respectively. Compared with cervical ACC, the patients with
cervical ABC had a much better prognosis, with 5- and 10-year CSS
rates of 96.6 and 91.4% (P<0.0001), respectively. The prognosis
of the vulvar ACC patients was between cervical ACC and ABC, with
5- and 10-year CSS rates of 87.7 and 80.7%, respectively, which
were significantly different from that of cervical ACC (5-year CSS;
10-year CSS, P=0.01), but comparable with that of ABC (5-year CSS,
P=0.1; 10-year CSS, P=0.08). The 5- and 10-year OS rate for
patients with cervical ACC was 59.2 and 37.7%, respectively. The
5-year OS rates for the patients with cervical ABC (88.3%) and
vulvar ACC (81.2%) were similar, but more favorable compared with
cervical ACC (cervical ABC vs. cervical ACC, P=0.002; vulvar ACC
vs. cervical ACC, P=0.01). The 10-year OS for the patients with
cervical ABC appeared to be worse than that of vulvar ACC (59.9 vs.
71.1%), but statistically insignificant.
Discussion
ACC and ABC in the lower female genital tract are
extremely rare, illustrated by markedly low incidence rates
described in the present study. Given their rarity, the majority of
knowledge about these tumors is limited to case reports and small
case series at a single institution. In this population-based
study, clinicopathologic characteristics and prognostic factors of
ACC and ABC in the lower female genital tract were systemically
investigated. Consistent with previous findings, the results of the
present study demonstrated distinct age distribution among patients
with these lesions. The previously reported median ages of patients
with both cervical ACC and ABC were mostly in the 60s and 70s
(range, 50–86 years) (12,16,19).
Similarly, the median age of the patients with these tumors were 72
and 69 years, respectively, in the present study. It is of interest
that, unlike cervical ACC and ABC, other high-risk HPV-associated
tumors, such as squamous cell carcinoma or adenocarcinoma, usually
occurred in the 40s and 50s (10,20,21).
The reason for this difference remains elusive. In a small case
series study, it was demonstrated that the median age of patients
with vulvar ACC was 52 years old, similar to the results of the
current study (median, 59) (13).
Our previous results also demonstrated that cervical carcinomas
with mixed differentiation including an adenoid cystic component
are high-risk HPV-associated, whereas pure ACCs of vulvar and
cervical origin appear to be unrelated to high-risk HPV (12). Consistent with this finding, it has
been demonstrated that 66.7% of vulvar ACCs harbored NFIB
rearrangement (13). The difference
in the etiology may be attributed to the distinct age distribution
in these tumors.
It has been well accepted that both cervical ACC and
ABC have a predilection to affect elderly, non-Caucasian women
(11,19). The present study revealed a low
proportion of black patients with these tumors (38.1% ACC and 11.5%
ABC, respectively) that appeared to contradict documented
literature. Notably, the absolute number of patients of different
ethnicities reflects proportionally registered patients based on
the whole population selection. When the incidence rate is
considered, cervical ACCs in the black population occur much more
frequently compared with the Caucasian population. Similarly, the
occurrence of cervical ABCs in the black population has increased
1.4 times compared with the Caucasian population. Increased
occurrence of cervical ACC and ABC in the black population is
postulated to be due to a relatively high HPV infection rate in
this population (22). To the best
of our knowledge, the ethnicity distribution of patients with
vulvar ACC, an HPV-unrelated tumor, has not yet been systemically
investigated. In the present study, the occurrence of vulvar ACC
appeared to have no racial predilection. It is also notable that
there was a remarkable marital status difference among the three
types of tumors. The patients with cervical ACC and ABC tended to
be unmarried compared with those with vulvar ACC. While this
observation may reflect the socioeconomic and/or racial difference,
the precise reason remains unknown.
Cervical ABC is usually an incidental finding in
patients undergoing hysterectomy or cone biopsy for a coexistent
high-grade squamous intraepithelial lesion or other reasons
(10,16,19).
Thus, these patients are usually asymptomatic without grossly
detectable masses. Not surprisingly, it was demonstrated that the
median size of the cervical ABC was 1 cm, significantly smaller
than the cervical and vulvar ACCs. In the present study, the
clinical presentations of cervical and vulvar ACCs included pain,
abnormal bleeding, discharge and palpable masses that can be
ulcerated or friable with a median size of 3.3 and 3.4 cm,
respectively. Metastasis to the lymph nodes is a very rare event
for all three types of tumor, evidenced by the fact that only 4
patients had lymph node involvement among 181 patients for whom
information on nodal status was available. Accordingly, the value
of lymph node dissection is obscure for these tumors.
In keeping with previous studies (19,23),
it was demonstrated that nearly all cervical ABCs (97.4%) were FIGO
stage I. Notably, only 2 patients in the present study had a higher
stage of disease; 1 patient presented with stage II and the other
with stage III. In an early study, 13 of 14 women with ABC of the
cervix had either stage IA or stage IB disease and all pursued a
benign clinical course (19).
Patients with typical histologic features of ABC typically have
excellent prognosis, evidenced by 5- and 10-year CCS rates of 96.6
and 91.4%, respectively, in the present study. Accordingly, the
present authors suggest that the term ‘adenoid basal carcinoma’
does not reflect its biologic behavior and clinical outcome. It has
been proposed that pure low-grade adenoid basal tumors lacking
appreciable cytological atypia, mitotic activity and an
infiltrative pattern in a desmoplastic stroma are designated as
‘adenoid basal epitheliomas’; tumors composed of both typical
low-grade adenoid basal tumor (epithelioma) and an invasive,
cytologically malignant component exhibiting adenoid
basal/squamous, pure squamous, and/or adenoid cystic
differentiation can be diagnosed as invasive carcinomas (16,23,24).
Thus, the majority of adenoid basal tumors can be classified as
either epithelioma or carcinoma depending on whether microscopic
features of malignancy are present.
Current standard treatment protocols are not
available for ABC and ACC tumors in the female genital tract.
Typical cervical ABCs can be treated conservatively in that the
tumor has not been associated with metastasis or tumor-related
death. In the present study, 99% of ABC patients received surgery,
and of these, 12 patients also received radiation therapy. The
patients who received both surgery and radiation therapy may also
have a component of invasive and destructive carcinoma that was not
uncommonly coexistent with typical ABC (16). Because of benign behavior, the
survival of patients with cervical ABC is thought to not differ
significantly from the general population (19). Consistently, the only significant
factor associated with 5-year OS by both univariate and
multivariate analysis was the SEER stage. This finding may be
confounded by undefined factors as a similar result was not
obtained in CSS studies.
Originally regarded as a single entity derived from
progenitor reserve cells, ACC and ABC of the uterine cervix share
many morphologic features, occur in older women, and are high-risk
HPV-associated (11). As proposed
by previous investigators, ABCs should be distinguished from ACCs
in that the latter are associated with a distinctly unfavorable
prognosis (19,25–28).
Whereas only 2 patients (2.6%) with cervical ABC had a high stage
of disease in the present study, 41.1% of cervical ACCs displayed
aggressive behavior (FIGO stage II and higher). Notably, 3 patients
had tumors that invaded the mucosa of the bladder or rectum or
extended beyond the pelvis, pathologically defined as FIGO stage
IV. Correlated with stratified pathologic features, the present
results demonstrated that the 5- and 10-year CSS rates for patients
with cervical ACC were 69.3 and 57.9%, respectively. It is not
surprising that a worse OS rate profile (5-year OS 59.2%; 10-year
OS 37.7%) for these patients is the consequence of combined disease
and elderly status (median age, 72 years). Furthermore, the
prognosis of cervical ACCs appeared worse than that of the ACCs of
the head and neck (6).
Regardless of anatomical site, the most common
treatment modality of ACC is surgical resection with postoperative
radiotherapy (9,29). Specifically, cervical ACC cases
usually follow the guidelines that are established for similarly
staged patients with squamous cell carcinoma of cervix and include
surgery and radiation therapy, either alone or in a combined
setting (30). In the present
study, 29% of the patients with cervical ACC did not receive
surgery, probably due to inoperable disease in the elderly
(28). Similar to ACC in other
locations, cervical ACC is also thought to be a radiosensitive
tumor, and radiotherapy is commonly applied to these patients
(19,29,31–33).
Although undefined as an adjuvant or primary treatment,
chemotherapy may also benefit patients with high stage or recurrent
disease (30,34,35).
With regard to survival, it was demonstrated that an advanced age
and a high stage remained constant factors that were associated
with a poor prognosis. In fact, these factors also affected the
clinical outcomes of ACCs of the head and neck (6).
To the best of our knowledge there have been no
previous studies that aimed to systemically characterize
clinicopathologic features and survival outcomes in patients with
vulvar ACC, largely due to its rarity. The present results
demonstrated that, similar to cervical ACC but unlike cervical ABC,
almost half of the patients had stage II and higher disease (stage
I, 52.7%; stage II, 14.5%; stage III, 25.5%; and stage IV, 7.3%).
Notably, even frequently present with high stage, the prognosis of
vulvar ACC patients appeared to be similar to that of cervical ABC
and more favorable compared with that of cervical ACC. A previous
study comparatively investigated the demographics and clinical
features of patients with ACC by disease site including the female
genital tract (7). The study
demonstrated that patients with localized ACCs of the female
genital system had a 5-year disease-specific survival rate of 87.2%
and a 10-year rate of 76.8%. The limitation of that study was that
ACCs of the uterine cervix and vulva were analyzed as a single
entity. As the oncogenic basis of cervical ACC and vulvar ACC is
different (12), the interpretation
of clinicopathologic features in that setting may be biased.
Similar to cervical ACC and ABC, there is no current
consensus regarding the optimal treatment of vulvar ACC. Surgical
resection of the neoplasm with clear margins is the primary
treatment and local recurrence can be managed with radiotherapy
(36–38). Adjuvant chemotherapy is also
recommended prior to surgery or when the margins are positive/local
invasion is present (39,40). Unlike patients with cervical ACC, it
was demonstrated that almost all patients with vulvar ACC underwent
surgery and more than one-third received radiotherapy. Similar to
cervical ACC, age and tumor stage were prognostic factors
associated with both OS and CSS, but patients with vulvar ACC
appeared to have a better prognosis compared with those with
cervical ACC.
The major limitation of the present study is the
lack of a centralized pathologic review. From a pathologic point of
view, ABC of the cervix is an epithelial tumor composed solely of
small, well-differentiated, rounded nests of basaloid cells that
have scanty cytoplasm and resemble basal cell carcinomas. ABC is
often associated with squamous intraepithelial lesions or other
carcinoma subtypes (16,23,41–44). A
retrospective study at our institution indicated that ~30% of ABCs
were associated with another type of carcinoma, including squamous
cell carcinoma, ACC, and/or small cell neuroendocrine carcinoma
(data not shown). Similarly, our previous study and documented
literature demonstrated that cervical ACC was frequently admixed
with other invasive tumors (12,45–47).
Accordingly, the precise diagnosis of these tumors is extremely
important for the guidance of clinical management. Unfortunately,
centralized pathologic review of the SEER cases to obtain a second
opinion for the diagnosis is not possible. Furthermore, critical
pathologic factors associated with the prognosis such as
lymphovascular space invasion, resection margin status,
histopathologic variability, and details of the treatment
information are not available. Nevertheless, the current
population-based study, rather than case reports or small case
series, allows the systemic investigation of the
clinicopathological features and survival outcomes of these
tumors.
In conclusion, the present data demonstrated that
the distinctive clinicopathologic features and survival outcomes
differ significantly among cervical ABCs, cervical ACCs, and vulvar
ACCs, thus providing a rationale for location/pathologic type-based
management strategies. Despite dozens of case reports and small
case series on clinicopathologic features in these rare tumors, the
present study systemically explored the prognosis, clinical
outcomes, and related pathologic factors based on a population
study. The present study may lead to a prospective clinical trial
to improve the management of patients with cervical ABCs, cervical
ACCs and vulvar ACCs according to stratified prognostic
factors.
Acknowledgements
The present study used the Surveillance,
Epidemiology, and End Results (SEER) program database. The authors
acknowledge the efforts of the National Cancer Institute in the
creation of this database.
Funding
The present study was supported by the Career
Development Award by the Cervical Cancer SPORE program (grant no.
5P50CA098252) at Johns Hopkins (DX).
Availability of data and materials
All original data and statistical code are available
upon request.
Authors' contributions
DX and JL contributed to the design and
implementation of the research, the analysis of the results and the
writing of the manuscript. Both authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board at the Johns Hopkins University School of Medicine
(Baltimore, MD, USA).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Khan AJ, DiGiovanna MP, Ross DA, Sasaki
CT, Carter D, Son YH and Haffty BG: Adenoid cystic carcinoma: A
retrospective clinical review. Int J Cancer. 96:149–158. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ellington CL, Goodman M, Kono SA, Grist W,
Wadsworth T, Chen AY, Owonikoko T, Ramalingam S, Shin DM, Khuri FR,
et al: Adenoid cystic carcinoma of the head and neck: Incidence and
survival trends based on 1973–2007 surveillance, epidemiology, and
end results data. Cancer. 118:4444–4451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Weert S, Bloemena E, van der Waal I,
de Bree R, Rietveld DH, Kuik JD and Leemans CR: Adenoid cystic
carcinoma of the head and neck: A single-center analysis of 105
consecutive cases over a 30-year period. Oral Oncol. 49:824–829.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iseli TA, Karnell LH, Graham SM, Funk GF,
Buatti JM, Gupta AK, Robinson RA and Hoffman HT: Role of
radiotherapy in adenoid cystic carcinoma of the head and neck. J
Laryngol Otol. 123:1137–1144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lloyd S, Yu JB, Wilson LD and Decker RH:
Determinants and patterns of survival in adenoid cystic carcinoma
of the head and neck, including an analysis of adjuvant radiation
therapy. Am J Clin Oncol. 34:76–81. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jang S, Patel PN, Kimple RJ and McCulloch
TM: Clinical outcomes and prognostic factors of adenoid cystic
carcinoma of the head and neck. Anticancer Res. 37:3045–3052.
2017.PubMed/NCBI
|
|
7
|
Li N, Xu L, Zhao H, El-Naggar AK and
Sturgis EM: A comparison of the demographics, clinical features,
and survival of patients with adenoid cystic carcinoma of major and
minor salivary glands versus less common sites within the
surveillance, epidemiology, and end results registry. Cancer.
118:3945–3953. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Silverman DA, Carlson TP, Khuntia D,
Bergstrom RT, Saxton J and Esclamado RM: Role for postoperative
radiation therapy in adenoid cystic carcinoma of the head and neck.
Laryngoscope. 114:1194–1199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coca-Pelaz A, Rodrigo JP, Bradley PJ,
Vander Poorten V, Triantafyllou A, Hunt JL, Strojan P, Rinaldo A,
Haigentz M Jr, Takes RP, et al: Adenoid cystic carcinoma of the
head and neck-An update. Oral Oncol. 51:652–661. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kurman RJ, Carcangiu ML, Herrington CS and
Young RH: WHO Classification of Tumors of Female Reproductive
Organs. 6. 4th. IARC Press; Lyon: 2014
|
|
11
|
Grayson W, Taylor LF and Cooper K: Adenoid
cystic and adenoid basal carcinoma of the uterine cervix:
Comparative morphologic, mucin, and immunohistochemical profile of
two rare neoplasms of putative ‘reserve cell’ origin. Am J Surg
Pathol. 23:448–458. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xing D, Schoolmeester JK, Ren Z, Isacson C
and Ronnett BM: Lower female genital tract tumors with adenoid
cystic differentiation: P16 expression and high-risk HPV detection.
Am J Surg Pathol. 40:529–536. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xing D, Bakhsh S, Melnyk N, Isacson C, Ho
J, Huntsman DG, Gilks CB, Ronnett BM and Horlings HM: Frequent
NFIB-associated gene rearrangement in adenoid cystic carcinoma of
the vulva. Int J Gynecol Pathol. 36:289–293. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jones MW, Kounelis S, Papadaki H, Bakker
A, Swalsky PA and Finkelstein SD: The origin and molecular
characterization of adenoid basal carcinoma of the uterine cervix.
Int J Gynecol Pathol. 16:301–306. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grayson W, Taylor LF and Cooper K: Adenoid
basal carcinoma of the uterine cervix: Detection of integrated
human papillomavirus in a rare tumor of putative ‘reserve cell’
origin. Int J Gynecol Pathol. 16:307–312. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Parwani AV, Smith Sehdev AE, Kurman RJ and
Ronnett BM: Cervical adenoid basal tumors comprised of adenoid
basal epithelioma associated with various types of invasive
carcinoma: Clinicopathologic features, human papillomavirus DNA
detection, and P16 expression. Human pathology. 36:82–90. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grayson W and Cooper K: Adenoid basal
epithelioma versus adenoid basal carcinoma. Am J Surg Pathol.
24:313–314. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
National Cancer Institute Surveillance E,
and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat
Database: Incidence-SEER 9 Regs Research Data, Nov 2016 Sub
(1973–2014) <Katrina/Rita Population Adjustment> - Linked To
County Attributes-Total U.S., 1969–2015 Counties, National Cancer
Institute DCCPS, Surveillance Research Program, released April
2017, based on the November 2016 submission. https://seer.cancer.gov/data-software/documentation/seerstat/nov2016/November
18–2016
|
|
19
|
Ferry JA and Scully RE: ‘Adenoid cystic’
carcinoma and adenoid basal carcinoma of the uterine cervix. A
study of 28 cases. Am J Surg Pathol. 12:134–144. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Small W Jr, Bacon MA, Bajaj A, Chuang LT,
Fisher BJ, Harkenrider MM, Jhingran A, Kitchener HC, Mileshkin LR,
Viswanathan AN, et al: Cervical cancer: A global health crisis.
Cancer. 123:2404–2412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou J, Wu SG, Sun JY, Li FY, Lin HX, Chen
QH and He ZY: Comparison of clinical outcomes of squamous cell
carcinoma, adenocarcinoma, and adenosquamous carcinoma of the
uterine cervix after definitive radiotherapy: A population-based
analysis. J Cancer Res Clin Oncol. 143:115–122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Viens LJ, Henley SJ, Watson M, Markowitz
LE, Thomas CC, Thompson TD, Razzaghi H and Saraiya M: Human
papillomavirus-associated cancers-United States, 2008–2012. MMWR
Morb Mortal Wkly Rep. 65:661–666. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brainard JA and Hart WR: Adenoid basal
epitheliomas of the uterine cervix: A reevaluation of distinctive
cervical basaloid lesions currently classified as adenoid basal
carcinoma and adenoid basal hyperplasia. Am J Surg Pathol.
22:965–975. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Russell MJ and Fadare O: Adenoid basal
lesions of the uterine cervix: Evolving terminology and
clinicopathological concepts. Diagn Pathol. 1:182006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Albores-Saavedra J, Manivel C, Mora A,
Vuitch F, Milchgrub S and Gould E: The solid variant of adenoid
cystic carcinoma of the cervix. Int J Gynecol Pathol. 11:2–10.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Dinh T and Woodruff JD: Adenoid cystic
and adenoid basal carcinomas of the cervix. Obstet Gynecol.
65:705–709. 1985.PubMed/NCBI
|
|
27
|
Chen TD, Chuang HC and Lee LY: Adenoid
basal carcinoma of the uterine cervix: Clinicopathologic features
of 12 cases with reference to CD117 expression. Int J Gynecol
Pathol. 31:25–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dixit S, Singhal S, Vyas R, Murthy A and
Baboo HA: Adenoid cystic carcinoma of the cervix. J Postgrad Med.
39:211–215. 1993.PubMed/NCBI
|
|
29
|
Bjorndal K, Krogdahl A, Therkildsen MH,
Charabi B, Kristensen CA, Andersen E, Schytte S, Primdahl H,
Johansen J, Pedersen HB, et al: Salivary adenoid cystic carcinoma
in Denmark 1990–2005: Outcome and independent prognostic factors
including the benefit of radiotherapy. Results of the Danish Head
and Neck Cancer Group (DAHANCA). Oral Oncol. 51:1138–1142. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kaur P, Khurana A, Chauhan AK, Singh G,
Kataria SP and Singh S: Adenoid cystic carcinoma of cervix:
Treatment strategy. J Clin Diagn Res. 7:2596–2597. 2013.PubMed/NCBI
|
|
31
|
Prempree T, Villasanta U and Tang CK:
Management of adenoid cystic carcinoma of the uterine cervix
(cylindroma): Report of six cases and reappraisal of all cases
reported in the medical literature. Cancer. 46:1631–1635. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Koyfman SA, Abidi A, Ravichandran P,
Higgins SA and Azodi M: Adenoid cystic carcinoma of the cervix.
Gynecol Oncol. 99:477–480. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nishida M, Nasu K, Takai N, Miyakawa I and
Kashima K: Adenoid cystic carcinoma of the uterine cervix. Int J
Clin Oncol. 10:198–200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
King LA, Talledo OE, Gallup DG, Melhus O
and Otken LB: Adenoid cystic carcinoma of the cervix in women under
age 40. Gynecol Oncol. 32:26–30. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Phillips GL Jr and Frye LP: Adenoid cystic
carcinoma of the cervix: A case report with implications for
chemotherapeutic treatment. Gynecol Oncol. 22:260–262. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nomura H, Nagashima M, Aoki Y and
Takeshima N: Resection of the inferior pubic ramus to completely
remove locally advance adenoid cystic carcinoma of Bartholin's
gland. Gynecol Oncol. 147:723–724. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Woida FM and Ribeiro-Silva A: Adenoid
cystic carcinoma of the Bartholin gland: An overview. Arch Pathol
Lab Med. 131:796–798. 2007.PubMed/NCBI
|
|
38
|
Yoon G, Kim HS, Lee YY, Kim TJ, Choi CH,
Song SY, Kim BG, Bae DS and Lee JW: Analysis of clinical outcomes
of patients with adenoid cystic carcinoma of Bartholin glands. Int
J Clin Exp Pathol. 8:5688–5694. 2015.PubMed/NCBI
|
|
39
|
Mousa A, Rahimi K and Warkus T:
Neoadjuvant chemoradiotherapy followed by radical vulvectomy for
adenoid cystic carcinoma of Bartholin's gland: A case report and
review of the literature. Eur J Gynaecol Oncol. 37:113–116.
2016.PubMed/NCBI
|
|
40
|
Yang SY, Lee JW, Kim WS, Jung KL, Lee SJ,
Lee JH, Bae DS and Kim BG: Adenoid cystic carcinoma of the
Bartholin's gland: Report of two cases and review of the
literature. Gynecol Oncol. 100:422–425. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ferry JA: Adenoid basal carcinoma of the
uterine cervix: Evolution of a distinctive clinicopathologic
entity. Int J Gynecol Pathol. 16:299–300. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wincewicz A, Lewitowicz P, Urbaniak-Wasik
S, Kanczuga-Koda L, Koda M, Adamczyk-Gruszka O and Sulkowski S:
Adenoid basal carcinoma-like tumor combined with invasive squamous
cell carcinoma foci of uterine cervix-A case report of 55-year-old
woman with literature review. Rom J Morphol Embryol. 55 Suppl
3:S1225–S1230. 2014.
|
|
43
|
Teramoto N, Nishimura R, Saeki T, Nogawa T
and Hiura M: Adenoid basal carcinoma of the uterine cervix: Report
of two cases with reference to adenosquamous carcinoma. Pathology
international. 55:445–452. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Takeshima Y, Amatya VJ, Nakayori F, Nakano
T, Iwaoki Y, Daitoku K and Inai K: Co-existent carcinosarcoma and
adenoid basal carcinoma of the uterine cervix and correlation with
human papillomavirus infection. Int J Gynecol Pathol. 21:186–190.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shi X, Wu S, Huo Z, Ling Q, Luo Y and
Liang Z: Co-existing of adenoid cystic carcinoma and invasive
squamous cell carcinoma of the uterine cervix: A report of 3 cases
with immunohistochemical study and evaluation of human
papillomavirus status. Diagn Pathol. 10:1452015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yannacou N, Gerolymatos A, Parissi-Mathiou
P, Chranioti S and Perdikis T: Carcinosarcoma of the uterine cervix
composed of an adenoid cystic carcinoma and an homologous stromal
sarcoma. A case report. Eur J Gynaecol Oncol. 21:292–294.
2000.PubMed/NCBI
|
|
47
|
Mathoulin-Portier MP, Penault-Llorca F,
Labit-Bouvier C, Charafe E, Martin F, Hassoun J and Jacquemier J:
Malignant mullerian mixed tumor of the uterine cervix with adenoid
cystic component. Int J Gynecol Pathol. 17:91–92. 1998. View Article : Google Scholar : PubMed/NCBI
|