Introduction
Bladder cancer is the most common malignancy of the
urinary tract and is associated with a high rate of tumor
recurrence. At present, an estimated 79,000 new cases of bladder
cancer and 16,800 cases of bladder cancer-associated mortality are
recorded each year in the US (1).
Despite advances in bladder cancer therapies, ~50% of patients
develop metastasis (2); therefore,
it is important to investigate the mechanisms underlying metastasis
of bladder cancer cells.
Molecular studies on bladder cancer have largely
focused on mutations in fibroblast growth factor receptor 3, tumor
protein p53 (3,4) and other genes (5,6).
Furthermore, deletion of chromosome 9 is detected in the majority
of cases of non-muscle invasive bladder cancer (7). In addition, factors associated with
bladder cancer include the phosphatidylinositol-3-kinase/protein
kinase B/mechanistic target of rapamycin pathway, RAS, cell cycle
progression and receptor tyrosine kinases (8–10). Two
bladder cancer cell lines, 253J B-V and 253J, were used in the
present study. The 253J B-V cell line is derived from 253J, and
exhibits a propensity for tumor formation and metastasis. Using a
protein chip, we revealed that the protein expression levels of
zinc finger and BTB domain-containing 38 (ZBTB38) were higher in
253J B-V cells compared with in 253J cells (She et al,
unpublished data). However, the role of ZBTB38 in bladder cancer is
not well characterized; therefore, it is important to investigate
whether ZBTB38 can promote the migration and invasive growth of
bladder cancer cells.
ZBTB38 contains 10 C2H2-type
zinc fingers domains and an N-terminal BTB/POZ domain, namely
Kaiso-like protein (11,12). Few studies have investigated the
functional role of ZBTB38 in cancer. For example, ZBTB38 has been
reported to recognize and bind to genes harboring CpG islands,
particularly the promoters of tumor suppressors (11,13–15).
In addition, Kaiso is associated with metastasis in the context of
several types of cancer (16,17)
and has a close connection with the Wnt signaling pathway (18–20).
The Wnt/β-catenin signaling pathway, also known as the canonical
Wnt pathway, is closely associated with malignant transformation of
cancer cells and metastasis (21,22).
In addition, the Wnt/β-catenin pathway has been demonstrated to be
involved in the development of bladder cancer (23).
The present study aimed to investigate whether
ZBTB38 can promote the migration and invasive growth of bladder
cancer cells, and to identify the possible effects of ZBTB38 on the
Wnt/β-catenin signaling pathway. To the best of our knowledge, this
is the first study to investigate the role of ZBTB38 in bladder
cancer.
Materials and methods
Cell culture
The SV-HUC-1 human normal bladder cell line, and the
253J, 253J B-V, 5637, T24 and J82 bladder cancer cell lines were
obtained from Professor Junjun She (The First Affiliated Hospital
of Xi'an Jiaotong University, Xi'an, China). 253J B-V cells were
developed from 253J cells. Briefly, the 253J cells were injected
into the bladder of nude mice. After 6 months, a tumor appeared in
the bladder. The tumor was cultured and named 253J B-I, which was
again injected into the bladder of nude mice. By repeating this
process 4 times, the 253J B-V cells were obtained; these cells
possess a stronger ability for metastasis (24). All cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS; HyClone; GE Healthcare Life Sciences, Logan, UT, USA). The
cells were cultured in 100-mm dishes at 37°C in an atmosphere
containing 5% CO2 and saturated humidity.
Cell transfection and RNA
interference
Small interfering siRNAs were synthesized by
Shanghai GenePharma Co., Ltd., (Shanghai, China), and the plasmid
was synthesized by Shanghai GeneChem Co., Ltd. (Shanghai, China).
Plasmid and siRNA transfection was performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Briefly, 253J cells were transfected with
plasmids (4 µg; 2 ng/µl) once they reached 80–90% cell confluence
in a 6-well plate, whereas 253J B-V cells were transfected with
siRNAs (50 nM) once they reached 70–80% cell confluence in a 6-well
plate; ~6 h post-transfection, the supernatants were
replaced with fresh medium. After 48 h, the cells were harvested
for experiments. The siRNA sequences used in the present study were
as follows: ZBTB38-1, 5′-CACUAUCAUUGUGGAAGAUTT-3′; ZBTB38-2,
5′-CCUAGUGUCUAUCCGUAUATT-3′; control (non-targeting),
5′-UUCUCCGAACGUGUCACGUTT-3′. The plasmids were constructed by
cloning the ZBTB38 siRNA sequences into GV141 vectors (Shanghai
GeneChem Co., Ltd.), which contained the following information:
CMV-MCS-3FLAG-SV40-Neomycin; the restriction site was
XhoI/KpnI and the plasmid was ampicillin-resistant.
The negative control was an empty vector (pcDNA3.1–3FLAG; Shanghai
GeneChem Co., Ltd.).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using RNA fast200
(Fastagen, Shanghai, China). The 5X PrimeScript RT Master Mix
(Takara Biotechnology Co., Ltd., Dalian, China) was used to reverse
transcribe mRNA into cDNA, according to the manufacturer's
protocol. qPCR was performed using 2X SYBR-Green Mix (Takara
Biotechnology Co., Ltd.) and a Bio-Rad detection system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The reactions were
visualized according to the manufacturer's protocol, as follows:
95°C for 30 sec, followed by 40 cycles at 95°C for 5 sec and
60°C for 30 sec, and then one cycle at 65°C for 5 sec and
95°C for 50 sec, and were quantified using the
2−ΔΔCq method (25). The
primers used were as follows: ZBTB38, forward
5′-TGTCTTGAAGTGAGGCTCTGCTG-3′, reverse 5′-AGCAAGCCTTGTGGACCAAAC-3′;
E-cadherin, forward 5′-gAgTgCCAACTggACCATTCAgTA-3′, reverse
5′-AgTCACCCACCTCTAAggCCATC-3′; vimentin, forward
5′-TgACATTgAgATTgCCACCTACAg-3′, and reverse
5′-TCAACCGtcTTAATCAgAAgTgTCC-3′; and GAPDH, forward
5′-agaaggctggggctcatttg-3′ and reverse 5′-agg ggc cat cca cag tct
tc-3′. GAPDH was used as an internal control.
Patients and tissue specimens
The present study was conducted in accordance with
the Code of Ethics of the World Medical Association. A total of 6
pairs of bladder cancer tissues (each pair consisted of one tumor
sample and one paracarcinoma sample) were obtained from 6 patients,
and two normal bladder tissues were also obtained from two of the 6
patients. All of the tissues were obtained from the Pathology
Department of the First Affiliated Hospital of Xi'an Jiaotong
University; the present study was approved by the Ethical Committee
on Human Research of the First Affiliated Hospital of Xi'an
Jiaotong University. Patients provided written informed consent for
the use of their tissues. Images of the tissues were captured using
a Leica SCN400 slide scanner (Leica Microsystems, Inc., Buffalo,
Grove, IL, USA).
Western blotting
Proteins were extracted from cells for western
blotting using radioimmunoprecipitation assay lysis buffer [0.05 M
Tris-HCl (pH 7.4), 0.15 mM NaCl, 1% Triton X-100, 0.1% SDS, 0.001 M
EDTA, 1% sodium deoxycholate and protease inhibitors]. Protein
concentration was then quantified using the bicinchoninic acid
method. Subsequently, proteins (50 µg, 3 µg/µl) were
separated by 10% SDS-PAGE and transferred to polyvinylidene
fluoride membranes (0.22 µm; EMD Millipore, Billerica, MA,
USA). The membranes were blocked with 5% skimmed milk containing
Tris-buffered saline and 0.1% Tween-20 (TBST) for 2 h at 37°C, and
were probed with respective antibodies at 4°C overnight. After
washing with TBST (0.1%), the membranes were incubated with
anti-mouse or anti-rabbit secondary antibodies. Immunoblots were
detected using the Bio-Rad ChemiDoc™ XRS+ system (Bio-Rad
Laboratories, Inc.) through enhanced chemiluminescence (Immobilon
western HRP substrate; Merck KGaA, Darmstadt, Germany). The blots
were semi-quantified by Image Lab software, version 3.0 (Bio-Rad
Laboratories, Inc.)
Antibodies
ZBTB38 (1:100, cat. no. ab50664; Abcam, Cambridge,
MA, USA) was used for immunohistochemistry (IHC). The following
primary antibodies were used for western blotting: ZBTB38 (1:500,
cat. no. ab112051; Abcam), E-cadherin (1:1,000, cat. no. 14472:
Cell Signaling Technology, Inc., Danvers, MA, USA); zonula
occludens (ZO)-1 (1:1,000, cat. no. 13663; Cell Signaling
Technology, Inc.); vimentin (1:1,000, cat. no. 5741; Cell Signaling
Technology, Inc.); β-catenin (1:1,000, cat. no. 8480; Cell
Signaling Technology, Inc.); Wnt/β-catenin activated targets
antibody sampler kit (1:1,000, cat. no. 8655; Cell Signaling
Technology, Inc.); GAPDH (1:10,000, cat. no. HRP-60004; ProteinTech
Group, Inc., Chicago, IL, USA); and β-actin (1:10,000, cat. no.
HRP-60008; ProteinTech Group, Inc.). Anti-rabbit and anti-mouse
horseradish peroxidase-conjugated secondary antibodies (1:5,000;
cat. nos. sc-2004 and sc-2005) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA).
Cell proliferation
The MTT assay (Amresco, LLC, Solon, OH, USA) was
used to assess cell proliferation. Briefly, 253J and 253J B-V cells
were transfected with plasmid or siRNAs. After 24 h, cells were
transferred from 6-well plates to 48-well plates (~30% cell
confluence). Subsequently, 50 µl MTT was added to each well for 4 h
at 37°C, after which, 375 µl dimethyl sulfoxide was added.
Subsequently, cells were measured at an optical density of 490 nm
every day for 3 successive days.
Wound-healing and Transwell
assays
Prior to the wound- healing assay, 253J and 253J-B-V
cells were transfected with plasmid or siRNAs in 6-well plates,
respectively. The cells (90–100% confluence) were starved of serum
for 12 h prior to experimentation and pipette tips were used
to generate wounds in the cell layer; subsequently, the cells were
washed with PBS and placed in fresh medium. Microscopic images of
the wound were obtained at 0, 8 and 24 h (magnification,
×10; Leica DMi8; Leica Microsystems, Inc.). For the Transwell
assay, a total of 24 h post-transfection, 2×104
cells were placed in 24-well Transwell chambers with polycarbonate
filters (pore size, 8 µm; Corning Incorporated, Corning, NY,
USA). For the invasion assay, 2×105 cells suspended in
medium without FBS were seeded in the upper chambers, which were
coated with Matrigel (1:10; BD Biosciences, Franklin Lakes, NJ,
USA). For the migration assay, 2×104 cells were seeded
in the upper chambers, which were uncoated. The insert was
incubated in 600 µl DMEM supplemented with 10% FBS at 37°C
in an atmosphere containing 5% CO2. Subsequently, the
cells were fixed in 95% ethanol at 25°C for 20 min,
stained with 0.3% crystal violet at 25°C for 15 min
and scrubbed gently to remove the non-migratory and non-invasive
cells. Images were captured under a microscope (magnification, ×20;
Leica DMi8; Leica Microsystems, Inc.).
Bladder cancer survival analysis
Gepia survival analysis for clinical outcomes
[disease-free survival (DFS)] of patients with bladder cancer was
performed using web tool survival plots (http://gepia.cancer-pku.cn/). The data (402 samples)
used by Gepia were obtained from The Cancer Genome Atlas database.
The percentiles of patients were auto selected based on the best
performing thresholds.
Xenograft model of bladder cancer lung
metastasis
All animal procedures complied with the Guidelines
of the Institutional Animal Use and Care Committee of Xi'an
Jiaotong University. The animal procedures were approved by the
Institutional Animal Use and Care Committee of Xi'an Jiaotong
University (no. XJTULAC2018-505). A total of 10 4-week-old SCID
male mice (n=5 mice/group) were purchased from the Centre of
Laboratory Animals (The Medical College of Xi'an Jiaotong
University) and were maintained according to the guidelines of the
Centre of Laboratory Animals. The mice were maintained as follows:
Temperature, 21±2°C; humidity, 40–70%; 12-h light/dark
cycle; ad libitum access to food and water. All mice were
randomly assigned into experimental groups (control group weight,
20.81±0.45 g; ZBTB38 knock down group weight,
21.10±0.37 g). The siRNA sequence used in this experiment
was ZBTB38-2 (5′-CCUAGUGUCUAUCCGUAUATT-3′). Briefly, 253J B-V
control cells and ZBTB38 siRNA-transfected 253J B-V cells
(1×106, diluted in 100 µl PBS) were injected into
the lateral tail vein of SCID mice; mice were monitored daily. When
the first mice began to suffer from serious tumor burden, at 4
weeks, and the other mice exhibited various levels of emaciation,
all animals were sacrificed. Both scientific and humane endpoints
were taken into account (26).
Notably, the body condition of the mice was observed daily;
although there were no significant alterations in body weight, the
loss of muscle and subcutaneous fat led to reduced body condition.
This could be detected through feeling the pelvis and backbone of
the mice. These alterations indicated the possibility of serious
tumor burden. A total of 4 weeks after cell injection, all mice
were sacrificed, and the lungs were dissected, weighed,
photographed and fixed in 4% paraformaldehyde for 48 h at 4°C.
IHC and hematoxylin and eosin
(H&E) staining
Paraffin-embedded sections were deparaffinized in
xylene and rehydrated in a decreasing concentration gradient of
ethanol (100, 95, 80 and 75%). After 3 washes (5 min/wash) with
PBS, the sections were incubated with 3% hydrogen peroxide for 10
min at 37°C. For antigen retrieval, the sections were placed in
sodium citrate (pH 9.0) in a microwave oven on high heat for 5 min,
and on medium heat for 13 min. After cooling to room temperature,
sections were washed with PBS and were incubated with bovine serum
albumin (Amresco, LLC) for 30 min at 37°C. Subsequently, the
sections were incubated overnight with the primary antibody in a
moist box at 4°C. After rinsing with PBS, the sections were
incubated with the secondary antibody (1:100) at 37°C for 15 min
included in the kit (cat. no. SP-9001; OriGene Technologies, Inc.,
Beijing, China), according to the manufacturer's protocol. Finally,
DAB (1:1,000; cat. no. ZLI-9017; OriGene Technologies, Inc.) was
used to stain sections at 25°C for 2 min, followed by
counterstaining with hematoxylin (at 25°C for 10 min. The
sections were then dehydrated in a graded ethanol series (50, 75,
95 and 100%).
The protocol for H&E staining was similar to
that for IHC. Following immersion of the sections in xylene and
ethanol (100, 95, 80 and 75%), they were counterstained with
hematoxylin at 25°C for 10 min. Subsequently, the sections were
placed in eosin for 2 min and dehydrated with ethanol. All sections
were examined using a microscope slide scanner (Leica MP SCN400;
Leica Microsystems, Inc.).
Drug treatment
Following transfection for 24 h, the cells (80–90%
confluence) were treated with 20 µM SKL2001 (Selleck Chemicals,
Houston, TX, USA) for 24 h, or with 20 µM XAV939 (Selleck
Chemicals) for 24 h. The cells then underwent western blotting.
Statistical analysis
All experiments were repeated at least 3 times and
the results are expressed as the means ± standard error of the
mean; conversely, the animal experiments were performed only
once. Statistical analysis was performed using GraphPad Prism 5
(GraphPad Software, Inc., La Jolla, CA, USA). Comparisons between
two groups were conducted using unpaired Student's t-test. One-way
analysis of variance followed by the Student-Newman-Keuls test was
used for multiple comparisons. All statistical tests were
two-sided. P<0.05 was considered to indicate a statistically
significant difference.
Results
ZBTB38 is associated with poor
prognosis of patients with bladder cancer
It has been reported that ~50% of patients with
bladder cancer exhibit local or distant recurrence, which is
associated with a high rate of mortality (2). The web tool Gepia (http://gepia.cancer-pku.cn) (27) was used to investigate the
association between ZBTB38 expression and the survival rate of
patients with bladder cancer. ZBTB38 expression exhibited a
significantly negative association with DFS (Fig. 1A). This finding indicated that
patients with high levels of ZBTB38 expression have a poor
prognosis; therefore, ZBTB38 expression may be considered a marker
of poor prognosis. The results of mass spectrometry (She et
al, unpublished data) revealed that the protein expression
levels of ZBTB38 in 253J B-V cells were ~5.8 times higher than
those in 253J cells (data not shown). Therefore, the present study
aimed to further investigate the role of ZBTB38 in bladder cancer
progression and metastasis. Six cases of paired bladder cancer
tissue samples were detected using IHC (Fig. 1B). Positive staining of ZBTB38 was
frequently detected in highly aggressive bladder cancer;
conversely, the expression levels of ZBTB38 were very low in normal
bladder tissues and less aggressive bladder cancer. Subsequently,
the mRNA expression levels of ZBTB38 were examined in various human
bladder cancer cells distinguished by their metastatic ability
(Fig. 1C). The results revealed
that the mRNA expression levels of ZBTB38 in the highly aggressive
bladder cancer cells were slightly higher compared with in the less
aggressive bladder cancer cells. Based on the results of mass
spectrometry and the results presented in Fig. 1B, 253J and 253J B-V cells were
selected for in vitro experiments. As shown in Fig. 1D, the protein expression levels of
ZBTB38 did not differ between the SV-HUC-1 normal bladder cells and
253J bladder cancer cells. However, ZBTB38 expression was
significantly higher in highly aggressive 253J B-V bladder cancer
cells; ZBTB38 expression increased by ~5.8-fold in 253J B-V cells
compared with in 253J cells. Therefore, the present study focused
on the effects of ZBTB38 on highly aggressive bladder cancer.
Although the mRNA expression levels of ZBTB38 in normal and cancer
tissues are interesting and require further investigation, the
present study considered the results of DFS and protein expression
analysis sufficient enough to reflect the significant increase of
ZBTB38 expression in highly aggressive bladder cancer.
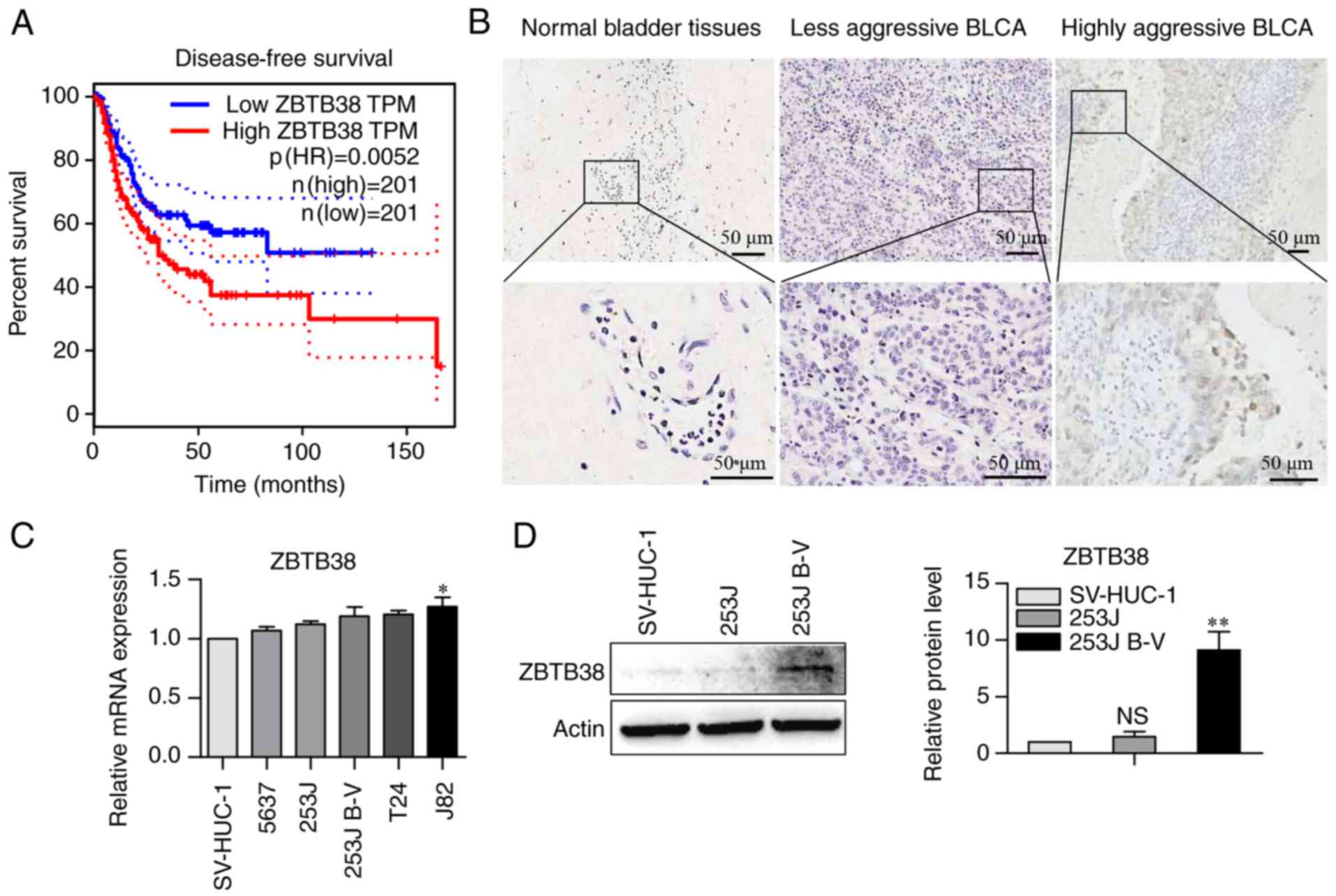 | Figure 1.ZBTB38 expression is associated with
poor prognosis of patients with bladder cancer. (A) Using The
Cancer Genome Atlas database, the association between the mRNA
expression levels of ZBTB38 and disease-free survival was
determined in patients with bladder cancer. (B) Representative
immunohistochemical staining images of ZBTB38 expression in human
bladder cancer and non-tumor tissues; scale bar, 50 µm. (C) mRNA
expression levels of ZBTB38 in SV-HUC-1, 5637, 253J, 253J B-V, T24
and J82 cells lines, as evaluated using reverse
transcription-quantitative polymerase chain reaction. SV-HUC-1
cells were used as a control. (D) Protein expression levels of
ZBTB38 in SV-HUC-1 normal bladder cells, and 253J and 253J B-V
bladder cancer cells. SV-HUC-1 cells were used as a control. Data
are presented as the means ± standard error of the mean.
*P<0.05, **P<0.01 vs. SV-HUC-1 cells. NS, not significant;
ZBTB28, zinc finger and BTB domain-containing 38. |
ZBTB38 inhibits cell proliferation,
and promotes migration and invasion of bladder cancer cells
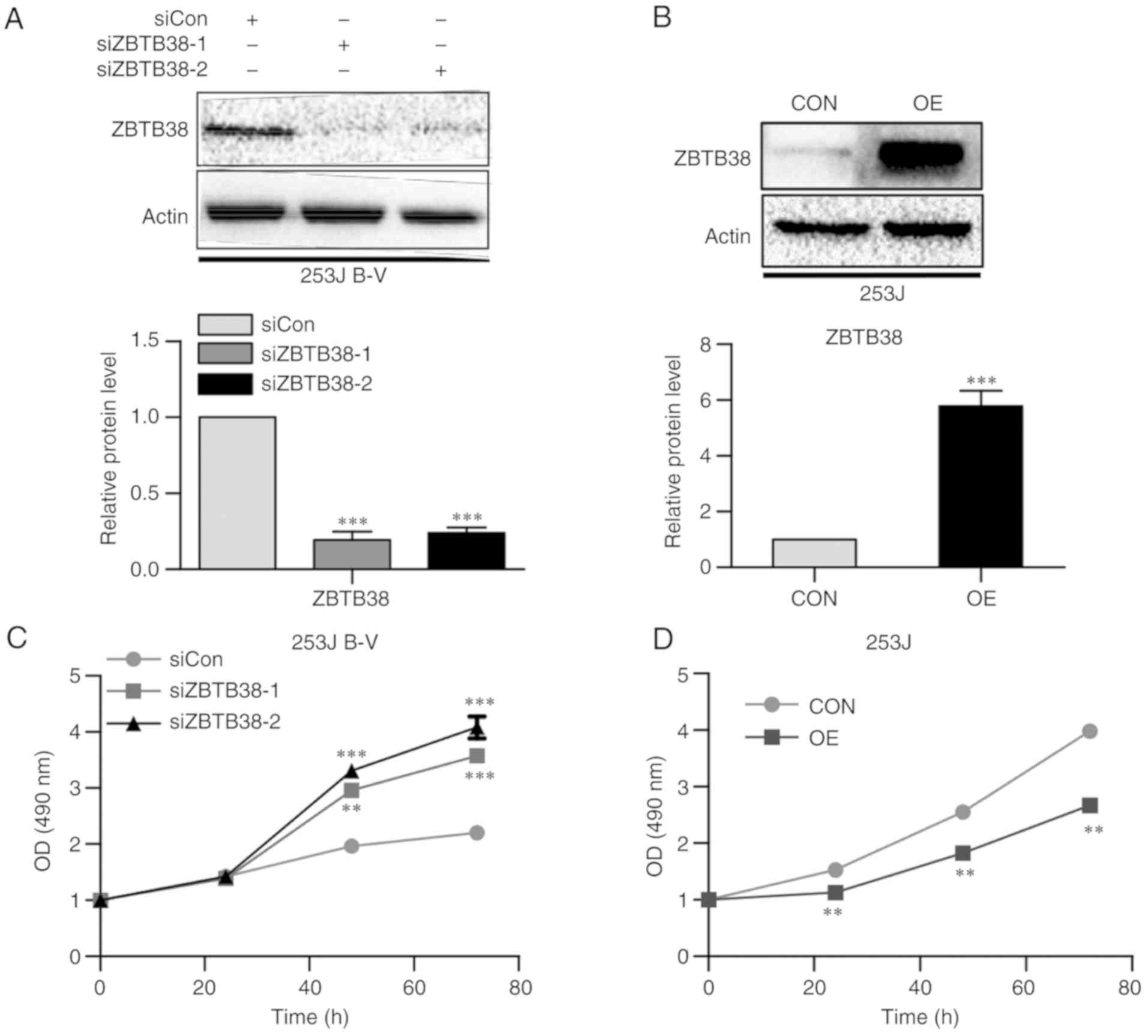
Using siRNA, ZBTB38 was knocked down in 253J B-V
cells (Fig. 2A). In addition, 253J
cells were transfected with a plasmid, in order to induce
overexpression of ZBTB38 (Fig. B). After knocking down ZBTB38 in
253J B-V cells, cell proliferation was promoted (Fig. 2C); conversely, the opposite results
were obtained following overexpression of ZBTB38 in 253J cells
(Fig. 2D). These results indicated
that ZBTB38 may inhibit proliferation of bladder cancer cells.
In view of the different expression patterns of
ZBTB38 in 253J and 253J B-V cells, the present study assessed
whether ZBTB38 may induce cancer progression and enhances
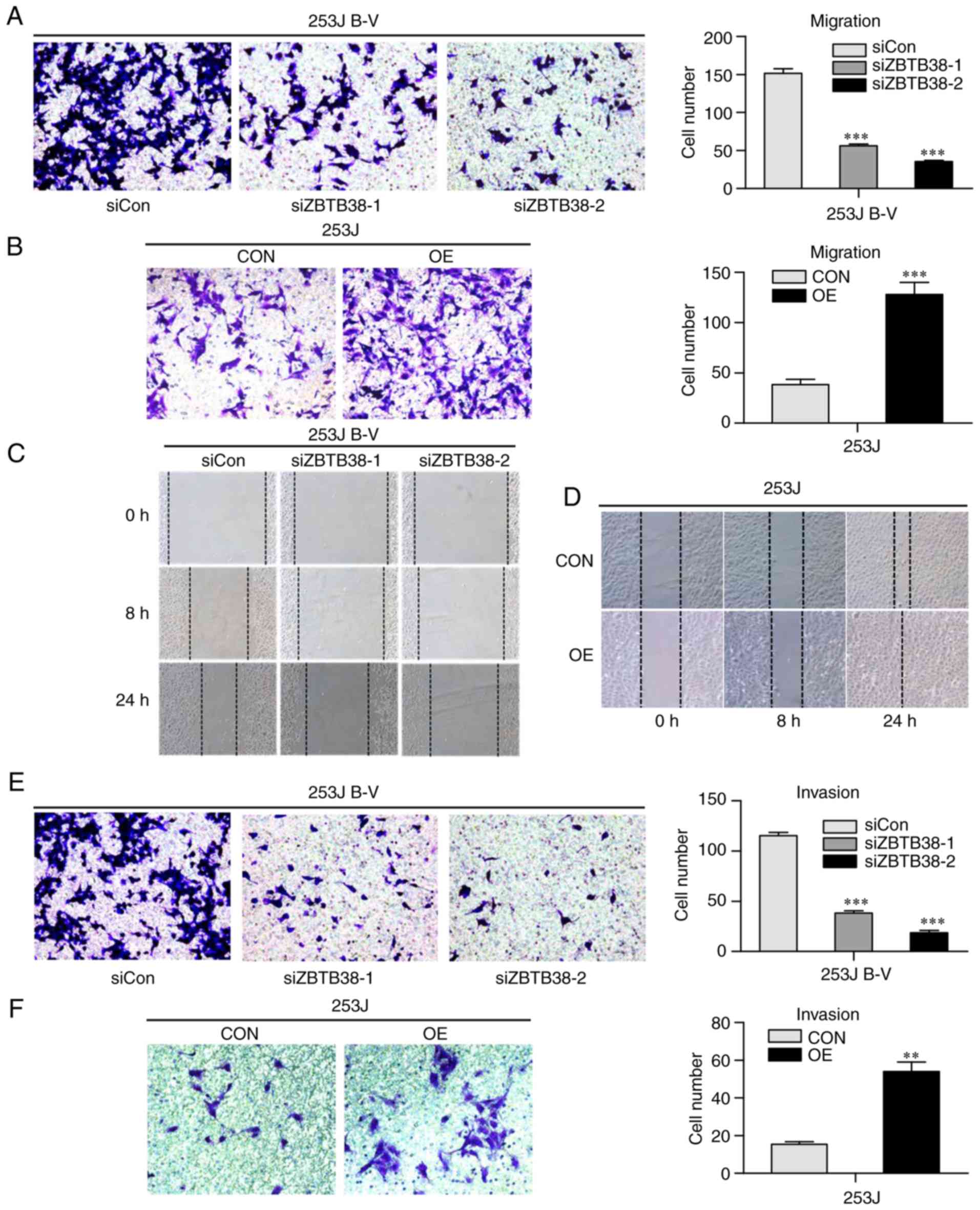
metastasis of bladder cancer cells. Transwell assay was performed
to assess the migratory ability of 253J and 253J B-V cells. The
results revealed that ZBTB38 knockdown reduced the migratory
ability of highly aggressive bladder cancer cells, whereas
migration was increased in the less aggressive bladder cancer cells
overexpressing ZBTB38 (Fig. 3A and
B). The results of the wound-healing (Fig. 3C and D) and Transwell assays
(Fig. 3E and F) were consistent
with the results presented in Fig. 3A
and B. These findings suggested that ZBTB38 may promote the
migration and invasive growth of bladder cancer cells, whereas it
inhibited cell proliferation.
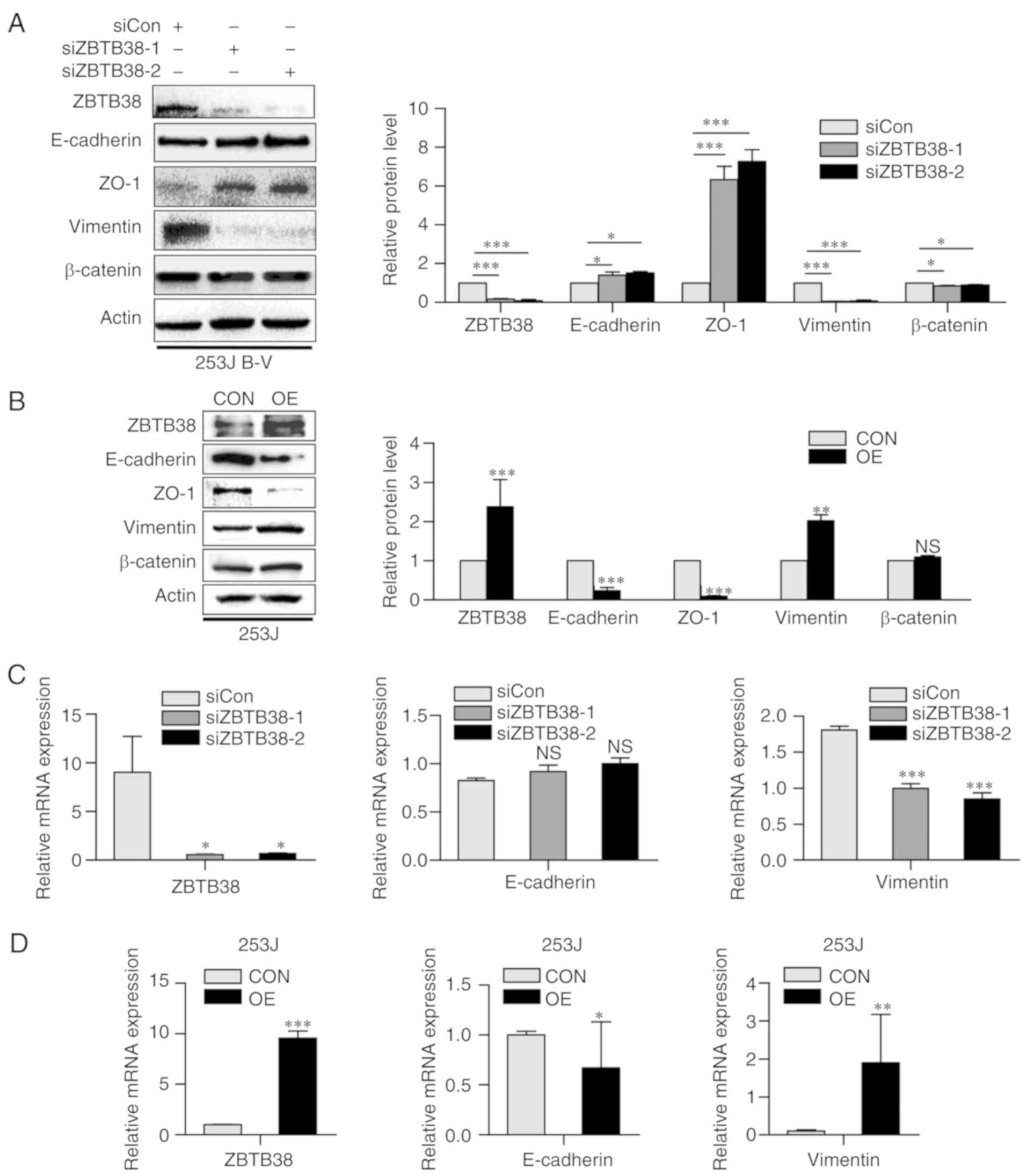
ZBTB38 accelerates the progression of
epithelial-mesen-chymal transition (EMT)
The close association of EMT with cell migration and
invasion is well documented (27).
Advanced cancer often undergoes partial or full EMT; this process
disrupts epithelial junction formation, and may contribute to
invasion and metastasis. EMT is also a hallmark of malignant tumor
progression, and the main cause of cancer-associated mortality.
E-cadherin and ZO-1 are main epithelial markers, whereas vimentin
and N-cadherin are commonly used mesenchymal markers. β-catenin is
a common transcription factor in the progression of EMT (27). Therefore, the present study detected
the expression levels of E-cadherin, ZO-1, vimentin and β-catenin
by western blotting. Alterations in the expression levels of
EMT-associated proteins were detected in ZBTB38 knockdown 253J B-V
cells and in ZBTB38-overexpressing 253J cells. Loss of ZBTB38 in
253J B-V cells enhanced the expression levels of E-cadherin and
ZO-1, whereas the expression levels of vimentin and β-catenin were
decreased. Opposing results were observed in 253J cells
overexpressing ZBTB38. Overexpression of ZBTB38 in 253J cells
decreased the expression levels of E-cadherin and ZO-1, and
increased the expression levels of vimentin. In addition, β-catenin
expression was not significantly altered. Notably, ZBTB38 knockdown
in 253J B-V cells induced a decrease in mesenchymal traits and an
increase in epithelial features (Fig.
4A). Conversely, ZBTB38 overexpression in 253J cells induced an
increase in mesenchymal traits and a decrease in epithelial
features (Fig. 4B). Similar results
were obtained with regards to the mRNA expression levels of EMT
components (Fig. 4C and D).
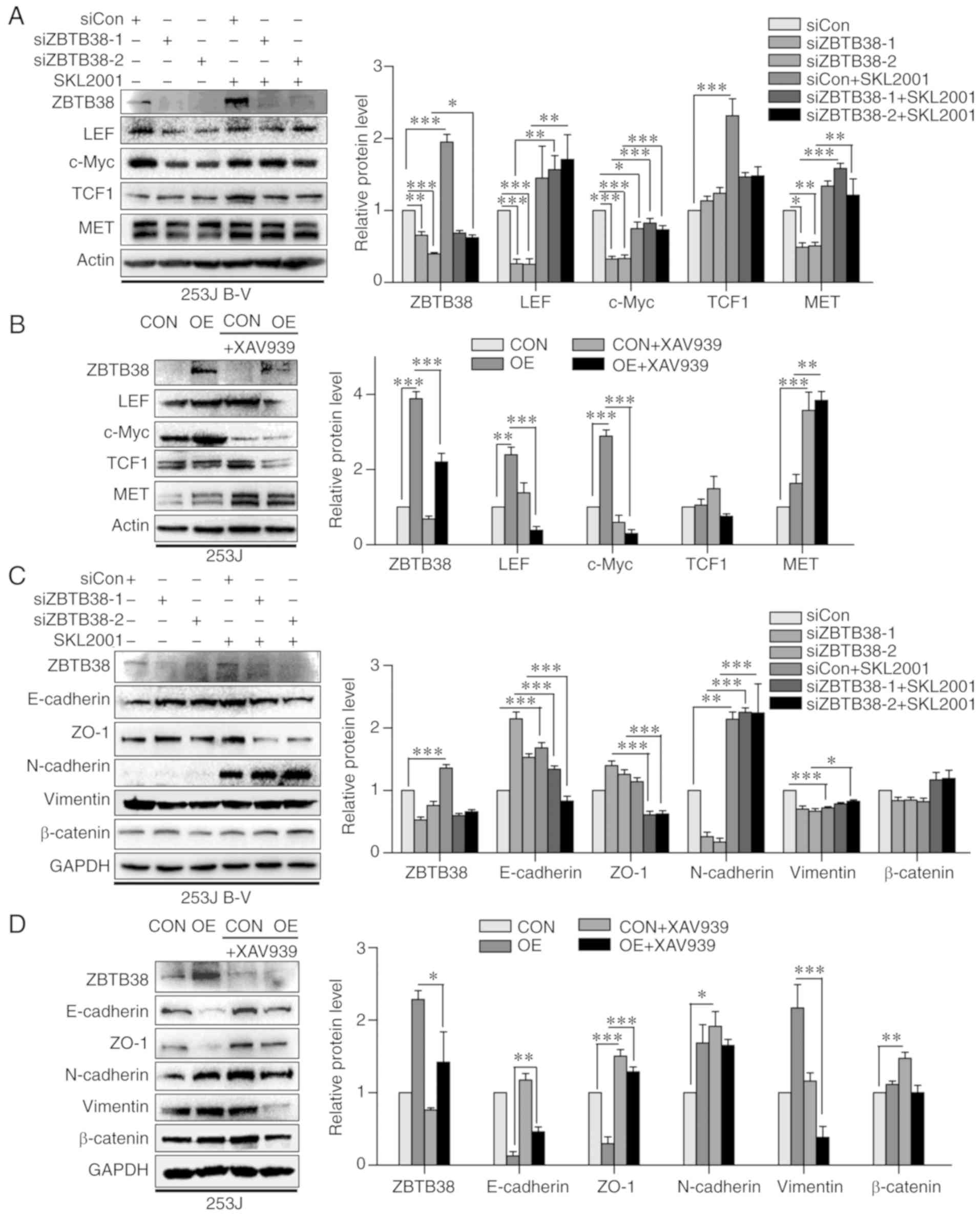
ZBTB38 promotes Wnt signaling pathway
activity
ZBTB38 belongs to the Kaiso family, which all have
similar domains and functions. It has previously been reported that
Kaiso has a close connection with the Wnt signaling pathway
(11). Therefore, it may be
hypothesized that ZBTB38 is associated with Wnt; however, to the
best of our knowledge, this has yet to be demonstrated. The present
study detected alterations in downstream proteins of the Wnt
signaling pathway, such as c-Myc and MET. Knockdown of ZBTB38 led
to inhibition of the Wnt signaling pathway. Conversely,
overexpression of ZBTB38 led to activation of the Wnt signaling
pathway. In order to further investigate this phenomenon, cells
were treated with an activator or inhibitor of the Wnt signaling
pathway, respectively (Fig. 5A and
B). XAV939, which can specifically inhibit the Wnt/β-catenin
signaling pathway via inhibition of tankyrase 1/2, was used as an
inhibitor. SKL2001 is a novel activator of the Wnt/β-catenin
pathway, which can abolish the interaction between Axin/β-catenin.
Following treatment with SKL2001, the decreased expression levels
of lymphoid enhancer binding factor 1 (LEF), c-Myc and MET in the
knockdown group were significantly increased. In addition, TCF1
expression exhibited a similar trend; however, the findings were
not statistically significant. As shown in Fig. 5A, treatment of ZBTB38 knockdown
cells with SKL2001 led to an increase in the protein expression
levels of downstream proteins of the Wnt/β-catenin signaling
pathway. Similarly, treatment of ZBTB38-overexpressing cells with
XAV939 induced alterations in the expression levels of these
downstream proteins (Fig. 5B).
Following treatment with XAV939, the increased expression levels of
LEF and c-Myc in the overexpression group were significantly
decreased. The expression levels of TCF1 exhibited a similar trend;
however, the findings were not statistically significant. Western
blotting was conducted to examine whether EMT markers were affected
by inhibition or activation of Wnt. Following treatment of the
knockdown group with SKL2001, the increased expression levels of
E-cadherin and ZO-1 were decreased, whereas the decreased
expression levels of N-cadherin and vimentin were increased. The
expression levels of β-catenin were not markedly altered. As
shown in Fig. 5C, epithelial
features decreased and mesenchymal traits increased following
treatment of ZBTB38 knockdown cells with SKL2001. Similarly,
changes in EMT marker expression were observed following treatment
of ZBTB38-overexpressing cells with XAV939 (Fig. 5D). Following treatment with XAV939,
the decreased expression levels of E-cadherin and ZO-1 were
increased, and the increased expression levels of vimentin were
decreased. N-cadherin expression exhibited a similar same trend;
however, the findings were not statistically significant. There was
also no significant alteration in β-catenin expression.
These findings suggested that ZBTB38 may regulate activity of the
Wnt/β-catenin signaling pathway.
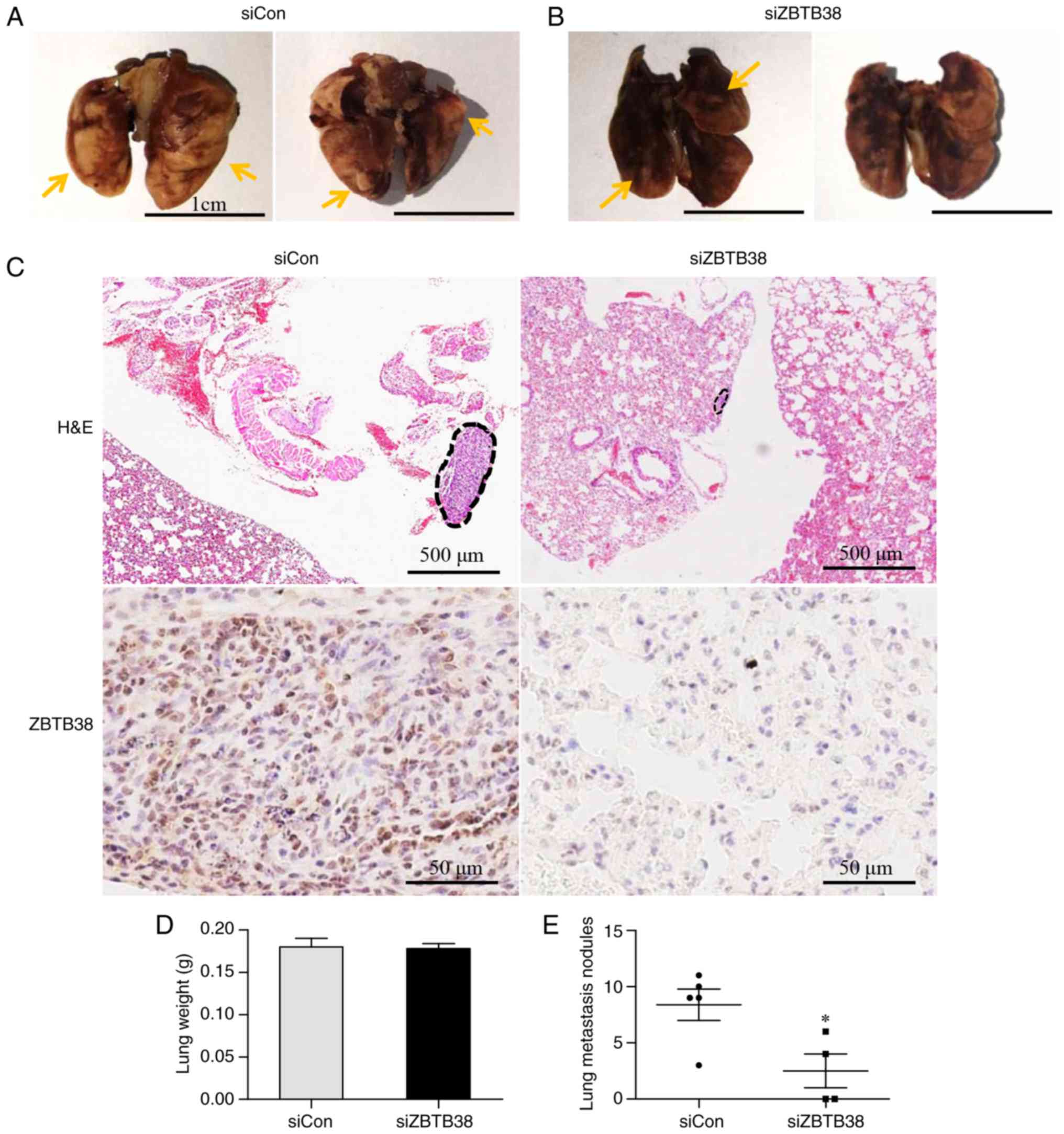
ZBTB38 promotes bladder cancer lung
metastasis
Consistent with the database results, ZBTB38
expression exhibited a positive association with the metastatic
capacity of bladder cancer cell lines. To elucidate the role of
ZBTB38 in bladder cancer metastasis, ~1×105 ZBTB38
knockdown 253J B-V cells and negative control cells were injected
into the caudal veins of male SCID mice. After 4 weeks, all mice in
the negative control group exhibited metastatic lesions in lungs
(5/5), whereas only two mice in the experimental group developed
metastatic lesions in the lungs caused by ZBTB38 knockdown cells
(2/4). Lung metastatic nodules in the control group were much
larger than in the ZBTB38-knockdown group (Fig. 6A-C). The wet lung weight in the
ZBTB38 knockdown group was slightly lower than that in the negative
control group; however, the difference was not statistically
significant (Fig. 6D). Furthermore,
the average number of metastatic nodules in the negative control
group was 8.4±1.4, whereas it was only 2.5±1.5 in the ZBTB38
knockdown group; the number of lung metastatic nodules in the
control group was significantly higher compared with in the ZBTB38
knockdown group (Fig. 6E).
Collectively, these results suggested that ZBTB38 may promote
bladder cancer lung metastasis.
Discussion
The present study demonstrated that ZBTB38 may
promote the migration and invasion of bladder cancer cells via
facilitating the Wnt/β-catenin signaling pathway. Notably, to the
best of our knowledge, the present study is the first to assess the
role of ZBTB38 in bladder cancer. In our previous study (She et
al, unpublished data), it was reported that the protein
expression levels of ZBTB38 were higher in the highly aggressive
bladder cancer cell line 253J B-V compared with in the less
aggressive bladder cancer cell line 253J. Therefore, the present
study investigated whether ZBTB38 promotes bladder cancer cell
migration and invasion.
ZBTB38 is a Kaiso-like protein, which contains 10
C2H2-type zinc fingers domains and an
N-terminal BTB/POZ domain (11).
High protein expression levels of ZBTB38 have been reported to be
associated with unfavorable DFS of patients. In the present study,
transfection of 253J B-V cells with siRNA, in order to inhibit
ZBTB38 expression, resulted in decreased migration and invasive
growth compared with in the negative control cells. Subsequently,
253J cells overexpressing ZBTB38 exhibited an increase in the
migration and invasive growth of cells compared with in the control
cells. These results indicated that ZBTB38 expression may promote
the migration and invasive growth of bladder cancer cells.
EMT is a vital step in cancer progression that
enhances cell motility and invasion (28,29).
In ZBTB38 knockdown 253J B-V cells, the expression levels of
E-cadherin were increased, whereas those of vimentin were
decreased. Conversely, when ZBTB38 was overexpressed, the
epithelial features decreased and the mesenchymal traits increased.
Advanced cancer is often associated with metastasis and cancer cell
death, and these cancer cells exhibit partial or full EMT (28). The present study revealed that
patients with bladder cancer and increased ZBTB38 expression had a
lower DFS. Therefore, overexpression of ZBTB38 may promote EMT,
contribute to invasion and metastasis, and cause poor DFS of
patients with bladder cancer. ZBTB38 belongs to the Kaiso family
and loss of Kaiso has been reported to inhibit metastasis of
triple-negative breast cancer (30,31).
Furthermore, Kaiso can promote migration and invasive growth of
prostate cancer cells by regulating microRNA-31 (32). However, transfection of 253J B-V
cells with siRNA, in order to decrease ZBTB38 expression, resulted
in an increase in cell proliferation compared with in the negative
control cells. Subsequently, 253J cells with ZBTB38 overexpression
exhibited a decrease in proliferation compared with in the control
cells. These results suggested that whether ZBTB38 functions as a
potential oncogene or tumor suppressor remains unclear.
The Wnt/β-catenin signaling pathway serves an
important role in cell fate determination and may promote cancer
progression (33–35). Kaiso, which is also named ZBTB33 and
is a member of the ZBTB family, has been revealed to possess
oncogenic activity; in addition, Kaiso can regulate
TCF/LEF1-activity and formation of the β-catenin complex (36). ZBTB38 not only belongs to the ZBTB
family, but is also termed a Kaiso-like protein, together with
ZBTB4. Therefore, the present study aimed to investigate the
association between ZBTB38 and the Wnt/β-catenin signaling pathway.
In the present study, inhibition of ZBTB38 expression suppressed
the Wnt/β-catenin pathway, whereas overexpression of ZBTB38
activated it. Following treatment with an activator or inhibitor of
the Wnt/β-catenin pathway, respectively, suppression and activation
of the Wnt/β-catenin pathway were reversed. In addition, the
effects of ZBTB38 on EMT markers were also reversed upon treatment
of the cells with the Wnt/β-catenin pathway activator or inhibitor.
These results indicated that ZBTB38 promotes the invasion and
migration of bladder cancer cells by regulating the Wnt/β-catenin
pathway.
In conclusion, these findings suggested that ZBTB38
may be closely associated with bladder cancer. To the best of our
knowledge, this is the first study to demonstrate that ZBTB38 may
promote the migration and invasion of bladder cancer cells by
affecting activity of the Wnt/β-catenin signaling pathway.
Collectively, these findings indicated that ZBTB38 may be a
biomarker of poor prognosis for patients with bladder cancer. Based
on the literature regarding Kaiso, it may be hypothesized that
ZBTB38 affects the Wnt/β-catenin pathway by regulating TCF/LEF1
activity. However, further studies are required to determine the
definitive mechanism by which ZBTB38 affects the Wnt/β-catenin
pathway.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81272342, 81502620
and 81502413).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
PL and JS initiated, conceived and designed the
research. JJ participated in the design of the study, performed the
experiments, and collected data. JL and YW participated in the MTT
and invasion assays, and assisted with the drafting of the
manuscript. MZ and LY assisted with the animal experiments. FS
analyzed the data and performed the statistical analysis. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal procedures were approved by the
Institutional Animal Use and Care Committee of the Xi'an Jiaotong
University (no. XJTULAC2018-505). The use of human bladder tissues
was approved by the Ethical Committee on Human Research of the
First Affiliated Hospital of Xi'an Jiaotong University (no.
XJTU1AF2018LSK-101). Patients provided written informed consent for
the use of their tissues.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Massari F, Santoni M, di Nunno V, Cheng L,
Lopez-Beltran A, Cimadamore A, Gasparrini S, Scarpelli M, Battelli
N and Montironi R: Adjuvant and neoadjuvant approaches for
urothelial cancer: Updated indications and controversies. Cancer
Treat Rev. 68:80–85. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mertens LS, Neuzillet Y, Horenblas S and
van Rhijn BW: Landmarks in non-muscle-invasive bladder cancer. Nat
Rev Urol. 11:476–480. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van Rhijn BW, Vis AN, van der Kwast TH,
Kirkels WJ, Radvanyi F, Ooms EC, Chopin DK, Boevé ER, Jöbsis AC and
Zwarthoff EC: Molecular grading of urothelial cell carcinoma with
fibroblast growth factor receptor 3 and MIB-1 is superior to
pathologic grade for the prediction of clinical outcome. J Clin
Oncol. 21:1912–1921. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang X and Zhang Y: Bladder cancer and
genetic mutations. Cell Biochem Biophys. 73:65–69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Knowles MA and Hurst CD: Molecular biology
of bladder cancer: New insights into pathogenesis and clinical
diversity. Nature Rev Cancer. 15:25–41. 2015. View Article : Google Scholar
|
|
7
|
Knowles MA: Molecular subtypes of bladder
cancer: Jekyll and Hyde or chalk and cheese? Carcinogenesis.
27:361–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mukherjee N, Houston TJ, Cardenas E and
Ghosh R: To be an ally or an adversary in bladder cancer: The
NF-kappaB story has not unfolded. Carcinogenesis. 36:299–306. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim J, Akbani R, Creighton CJ, Lerner SP,
Weinstein JN, Getz G and Kwiatkowski DJ: Invasive bladder cancer:
Genomic insights and therapeutic promise. Clin Cancer Res.
21:4514–4524. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Houede N and Pourquier P: Targeting the
genetic alterations of the PI3K-AKT-mTOR pathway: Its potential use
in the treatment of bladder cancers. Pharmacol Ther. 145:1–18.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Filion GJ, Zhenilo S, Salozhin S, Yamada
D, Prokhortchouk E and Defossez PA: A family of human zinc finger
proteins that bind methylated DNA and repress transcription. Mol
Cell Biol. 26:169–181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kiefer H, Chatail-Hermitte F, Ravassard P,
Bayard E, Brunet I and Mallet J: ZENON, a novel POZ Kruppel-like
DNA binding protein associated with differentiation and/or survival
of late postmitotic neurons. Mol Cell Biol. 25:1713–1729. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miotto B, Chibi M, Xie P, Koundrioukoff S,
Moolman-Smook H, Pugh D, Debatisse M, He F, Zhang L and Defossez
PA: The RBBP6/ZBTB38/MCM10 axis regulates DNA replication and
common fragile site stability. Cell Rep. 7:575–587. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kote-Jarai Z, Olama AA, Giles GG, Severi
G, Schleutker J, Weischer M, Campa D, Riboli E, Key T, Gronberg H,
et al: Seven prostate cancer susceptibility loci identified by a
multi-stage genome-wide association study. Nat Genet. 43:785–791.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miotto B, Marchal C, Adelmant Guinot N,
Xie P, Marto JA, Zhang L and Defossez PA: Stabilization of the
methyl-CpG binding protein ZBTB38 by the deubiquitinase USP9X
limits the occurrence and toxicity of oxidative stress in human
cells. Nucleic Acids Res. 46:4392–4404. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun Z, Cao Y, Hu G, Zhao J, Chen M, Wang
S, Ye Z, Chen H, Wang W and Wang Y: Jinfu'an decoction inhibits
invasion and metastasis in human lung cancer cells (H1650) via
p120ctn-mediated induction and Kaiso. Med Sci Monit. 24:2878–2886.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jones J, Wang H, Zhou J, Hardy S, Turner
T, Austin D, He Q, Wells A, Grizzle WE and Yates C: Nuclear Kaiso
indicates aggressive prostate cancers and promotes migration and
invasiveness of prostate cancer cells. Am J Pathol. 181:1836–1846.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kourtidis A, Lu R, Pence LJ and
Anastasiadis PZ: A central role for cadherin signaling in cancer.
Exp cell Res. 358:78–85. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kourtidis A, Ngok SP and Anastasiadis PZ:
P120 catenin: An essential regulator of cadherin stability,
adhesion-induced signaling, and cancer progression. Prog Mol Biol
Transl Sci. 116:409–432. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iioka H, Doerner SK and Tamai K: Kaiso is
a bimodal modulator for Wnt/beta-catenin signaling. FEBS Lett.
583:627–632. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Logan CY and Nusse R: The Wnt signaling
pathway in development and disease. Annu Rev Cell Dev Biol.
20:781–810. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pierzynski JA, Hildebrandt MA, Kamat AM,
Lin J, Ye Y, Dinney CP and Wu X: Genetic variants in the
Wnt/beta-catenin signaling pathway as indicators of bladder cancer
risk. J Urol. 194:1771–1776. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Colin P, Fishbeck R, Singh RK, Eve B,
Pathak S, Brown N, Xie B, Fan D, Bucana CD and Fidler IJ: Isolation
and characterization of metastatic variants from human transitional
cell carcinoma passaged by orthotopic implantation in athymic nude
mice. J Urol. 154:1532–1538. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wallace J: Humane endpoints and cancer
research. ILAR J. 41:87–93. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gilles C, Polette M, Mestdagt M,
Nawrocki-Raby B, Ruggeri P, Birembaut P and Foidart JM:
Transactivation of vimentin by beta-catenin in human breast cancer
cells. Cancer Res. 63:2658–2664. 2003.PubMed/NCBI
|
|
30
|
Kwiecien JM, Bassey-Archibong BI,
Dabrowski W, Rayner LG, Lucas AR and Daniel JM: Loss of Kaiso
expression in breast cancer cells prevents intra-vascular invasion
in the lung and secondary metastasis. PLoS One. 12:e01838832017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bassey-Archibong BI, Kwiecien JM,
Milosavljevic SB, Hallett RM, Rayner LG, Erb MJ, Crawford-Brown CJ,
Stephenson KB, Bédard PA, Hassell JA, et al: Kaiso depletion
attenuates transforming growth factor-beta signaling and metastatic
activity of triple-negative breast cancer cells. Oncogenesis.
5:e2082016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang H, Liu W, Black S, Turner O, Daniel
JM, Dean-Colomb W, He QP, Davis M and Yates C: Kaiso, a
transcriptional repressor, promotes cell migration and invasion of
prostate cancer cells through regulation of miR-31 expression.
Oncotarget. 7:5677–5689. 2016.PubMed/NCBI
|
|
33
|
Takebe N, Miele L, Harris PJ, Jeong W,
Bando H, Kahn M, Yang SX and Ivy SP: Targeting Notch, Hedgehog, and
Wnt pathways in cancer stem cells: Clinical update. Nat Rev Clin
Oncol. 12:445–464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xi Y and Chen Y: Wnt signaling pathway:
Implications for therapy in lung cancer and bone metastasis. Cancer
Lett. 353:8–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Prokhortchouk A, Sansom O, Selfridge J,
Caballero IM, Salozhin S, Aithozhina D, Cerchietti L, Meng FG,
Augenlicht LH, Mariadason JM, et al: Kaiso-deficient mice show
resistance to intestinal cancer. Mol Cell Biol. 26:199–208. 2006.
View Article : Google Scholar : PubMed/NCBI
|