Introduction
Liver cancer is the most common cancer and the
second leading cause of cancer-related deaths worldwide. In China
in 2015, there were 466,000 new cases of liver cancer, 422,000
patients succumbed to liver cancer (1), and the 5-year survival rate of liver
cancer was only 10.1% (2).
Traditional treatments such as surgery, radiotherapy and
chemotherapy are not effective. Some preclinical progress has been
made in the development of targeted therapies for liver cancer,
however sorafenib, a Raf kinase inhibitor, is the only targeted
drug for treatment of advanced liver cancer in clinical use
(3). Therefore, it is necessary to
explore the molecular mechanisms of development and progression of
liver cancer to identify additional therapeutic targets.
Cancer-testis antigens (CTAs) are a family of
tumor-associated antigens that are encoded by germ line-associated
genes with expression restricted to testis in healthy subjects but
ectopic expression in various tumors (4). Sperm-associated antigen 9
(SPAG9) is a member of the CTA family located on human
chromosome 17q21.33 and normally expressed in the equatorial plate
of sperm acrosome. It can be overexpressed in tumors of the ovary,
breast, lung and bone (5–7), and the expression of SPAG9 in tumor
tissues is correlated with progression and prognosis of cancer
patients (8,9). We previously demonstrated that SPAG9
was overexpressed in liver cancer (10), however, how SPAG9 influences liver
cancer progression is not yet clear. In the present study, we
analyzed the function of SPAG9 in the liver cancer-derived HepG2
cells. Our data indicated that SPAG9 interacts with the MAPK
pathway in liver cancer cells to enhance proliferation, providing
evidence that SPAG9 and the MAPK pathway are potential liver cancer
therapeutic targets.
Materials and methods
Patient samples
A total of 20 liver cancer patients were enrolled
between January 2005 and January 2015 at Hunan Provincial Brain
Hospital (Changsha, China). The experiments were performed
according to the Medical Ethics Committee of Hunan Provincial Brain
Hospital (no. L2017003). Written informed consent was obtained from
all patients prior to the collection of liver cancer tissue
samples. The exclusion criteria were as follows: i) patients had
distant metastasis; ii) patients had received previous radiotherapy
or chemotherapy prior to hepatectomy; iii) patients had a serious
infection or other malignant diseases. Cancerous tissues and
adjacent non-cancerous tissues were obtained from 20 patients with
liver cancer during surgical tumor resections in accordance with
informed consent. The diagnosis of liver cancer was confirmed by
pathobiology (11). All clinical
and biological data of the patients are presented in Table I.
 | Table I.Characteristics of patients with
liver cancer. |
Table I.
Characteristics of patients with
liver cancer.
| Sample | Age (years) | Sex | Clinical
pathological diagnosis (11) |
|---|
| 1 | 62 | Female | Moderately
differentiated liver cancer |
| 2 | 50 | Female | Moderate-poorly
differentiated liver cancer |
| 3 | 73 | Male | Moderately
differentiated liver cancer |
| 4 | 51 | Male | Moderate-highly
differentiated liver cancer |
| 5 | 49 | Female | Highly
differentiated liver cancer |
| 6 | 32 | Male | Moderately
differentiated liver cancer |
| 7 | 62 | Male | Moderately
differentiated liver cancer |
| 8 | 34 | Male | Highly
differentiated liver cancer |
| 9 | 50 | Male | Highly
differentiated liver cancer |
| 10 | 42 | Male | Moderately
differentiated liver cancer |
| 11 | 64 | Male | Moderately
differentiated liver cancer |
| 12 | 41 | Male | Highly
differentiated liver cancer |
| 13 | 52 | Male | Moderately
differentiated liver cancer |
| 14 | 49 | Male | Moderately
differentiated liver cancer |
| 15 | 66 | Male | Moderately
differentiated liver cancer |
| 16 | 52 | Female | Moderately
differentiated liver cancer |
| 17 | 40 | Male | Moderately
differentiated liver cancer |
| 18 | 49 | Male | Highly
differentiated liver cancer |
| 19 | 45 | Male | Highly
differentiated liver cancer |
| 20 | 59 | Male | Moderately
differentiated liver cancer |
Cell lines and cell culture
HepG2 cells were purchased from the Cell Bank of
Type Culture Collection of Chinese Academy of Sciences (Shanghai,
China). The cells were cultured in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.),
100 IU/ml penicillin and 100 IU/ml streptomycin in an atmosphere
containing 5% CO2 at 37°C.
Immunohistochemistry (IHC)
The expression of SPAG9 protein in liver cancer
tissues was analyzed by IHC as previously described (8). Paraffin-embedded blocks were prepared
from the tissue specimens and serial sections of 4 µm were cut.
Sections of tumor tissues and adjacent non-cancer tissues were
incubated with anti-SPAG9 antibody (dilution 1:100; cat. no.
ab12331; Abcam, Cambridge, MA, USA). Subsequently, sections were
incubated with alkaline phosphatase-conjugated mouse anti-rabbit
immunoglobulin G (dilution 1:1,000; cat. no. sc-2358; Santa Cruz
Biotechnology, Santa Cruz, CA, USA). The immunoreactivity was
visualized using 0.05% 3,3′-diaminobenzidine. Images of tissue
sections were captured using an Olympus light microscope (Olympus
Corp., Tokyo, Japan) after staining with hematoxylin. The
assessment criteria were described in a previous study (8).
Cell immunofluorescence
Cells were washed three times with
phosphate-buffered saline (PBS) and were then fixed with 4%
pre-cooled paraformaldehyde for 15 min. The cells were washed with
PBS and then permeabilized with PBS containing 0.1% Triton X-100
for 20 min at room temperature. The cells were washed with PBS
containing 0.1% Triton X-100 and were then incubated with 5% bovine
serum albumin (BSA) for 30 min at room temperature. The coverslips
were incubated with anti-SPAG9 antibody (dilution 1:100; cat. no.
ab12331; Abcam) overnight at 4°C. The cells were washed with PBS
containing 0.1% Triton X-100, incubated with donkey anti-rabbit
secondary antibody (dilution 1:2,000; cat. no. ab150073; Abcam) at
37°C for 1 h in the dark, and then washed with PBS containing 0.1%
Triton X-100. The cells were then incubated with DAPI for 90 sec,
washed with PBS, and observed under a confocal microscope (Olympus
Corp.).
Western blotting
Cells were lysed for 30 min on ice with RIPA lysis
buffer (Beyotime Institute of Biotechnology, Haimen, China; cat.
no. P0013B). The cell lysate was then centrifuged at 12,000 × g for
5 min at 4°C. The supernatant was carefully collected following
centrifugation. Total protein in the cell lysate was quantified
with a BCA protein assay kit (CWBio, Beijing, China). Proteins (50
µg) were separated by 6 and 8% SDS-PAGE and transferred onto
polyvinylidene difluoride (PVDF) membranes (0.45 µm; Millipore,
Billerica, MA, USA). PVDF membranes were blocked with 5% non-fat
dry milk for 2 h at room temperature, and then membranes were
incubated for 24 h at 4°C with antibodies against SPAG9 (dilution
1:1,000; cat. no. ab12331; Abcam), JNK (dilution 1:1,000; cat. no.
ab179461; Abcam), p38 (dilution 1:1,500; cat. no. ab170099; Abcam),
MKK3 (dilution 1:5,000; cat. no. ab195037; Abcam), MKK6 (dilution
1:5,000; cat. no. ab33866; Abcam) and GAPDH (dilution 1:3,000; cat.
no. LCA04; Auragene, Changsha, China). After washing with PBS
containing 0.05% Tween-20, the membranes were incubated with
HRP-conjugated AffiniPure goat anti-rabbit IgG (H+L) (dilution
1:4,000; cat. no. SA00001-2; ProteinTech Group, Inc., Chicago, IL,
USA). The bands were visualized using SuperECL Plus Western
Blotting Substrate (Pierce; Thermo Fisher Scientifc, Inc.) and were
analyzed using Gel Automated Digitizing System software (version
4.0; Silk Scientific, Orem, UT, USA). GAPDH levels were used as an
internal standard.
Immunoprecipitation and immunoblotting
analyses
To prepare cell lysates, cells were washed in PBS,
lysed in cell lysis buffer for 20 min, and centrifuged at 12,000 ×
g for 15 min at 4°C to remove insoluble debris. A 50-µl aliquot of
supernatant was analyzed by western blot analysis. The remainder
was incubated with anti-SPAG9 antibody (dilution 1:1,000; cat. no.
ab12331; Abcam) or anti-JNK antibody (dilution 1:1,000; cat. no.
ab179461; Abcam) at 4°C overnight with slow shaking. To these
samples 10 µl of resuspended protein A/G-agarose beads
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added. After
incubation for 4 h at 4°C, the immobilized proteins were collected
by 10,000 × g centrifugation for 1 min, washed three times with the
cell lysis buffer, and solubilized by boiling for 5 min in SDS-PAGE
loading buffer (10 mmol/l Tris-HCl, 2% SDS, 2 mmol/l EDTA, 0.02%
bromophenol blue and 6% glycerol; pH 6.8). After separation on
SDS-PAGE, proteins were transferred onto a PVDF membrane
(Millipore), blocked with 5% non-fat dry milk, washed briefly, and
incubated with anti-JNK antibody (dilution 1:1,000; cat. no.
ab179461; Abcam) or anti-SPAG9 antibody (dilution 1:1,000; cat. no.
ab12331; Abcam). Blots were washed three times with PBS containing
0.05% Tween-20 and incubated with HRP-conjugated AffiniPure goat
anti-mouse IgG(H+L) (dilution 1:4,000; cat. no. SA00001-1;
Proteintech Group, Inc.) or HRP-conjugated AffiniPure goat
anti-rabbit IgG(H+L) (dilution 1:4,000; cat. no. SA00001-2;
Proteintech Group, Inc.). The bands were analyzed using Gel
Automated Digitizing System software (version 4.0; Silk
Scientific).
Cell proliferation analysis
Cell proliferation was quantified using the Cell
Counting Kit-8 (CCK-8) assay (Dojindo Laboratories, Kumamoto,
Japan). Briefly, cells were plated in 96-well plates
(5×104 cells/well). At 24 and 48 h after transfection
with SPAG9-targeted siRNA or the control siRNA, SPAG9 siRNA
sequences or control siRNA were constructed: SPAG9 siRNA,
TCTGGAAACGACATTTATGG; control siRNA, TGAAGGTCGGAGTCAACGGATT. The
cell proliferation assay was performed by the addition of 10 µl
CCK-8 solution to each well, followed by incubation at 37°C for 1.5
h. Absorbance was measured at a wavelength of 450 nm using a
Bio-Rad microplate reader (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Cell cycle assay
After transfection with SPAG9-targeted siRNA
or the control siRNA, HepG2 cells were harvested, washed twice with
PBS, and fixed in 70% ethanol at 4°C overnight. Cells were
incubated with propidium iodide (PI) at room temperature for 1 h
and were analyzed by flow cytometry using a BD Biosciences flow
cytometer (BD Biosciences, San Jose, CA, USA).
Cell apoptosis assay
Apoptotic cells were distinguished from normal cells
using an Annexin V-FITC/PI apoptosis kit for flow cytometry
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. After transfection with SPAG9
siRNA or siRNA control, HepG2 cells were harvested and washed twice
with cold PBS and then incubated with 5 µl FITC-Annexin V and 1 µl
PI working solution (100 µg/ml) for 15 min in the dark at room
temperature. Cellular fluorescence was measured by flow
cytometry.
Statistical analyses
Statistical analyses were performed using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA). Data are presented as the means ±
standard deviations (SD). Statistical analyses were performed with
one-way ANOVA, two-way ANOVA and repeated measures ANOVA as
appropriate. Bonferroni was used as a post hoc test in one-way
ANOVA. The statistical significance level was set at P<0.05
(two-sided).
Results
SPAG9 is overexpressed in cancer
tissues of liver patients and is located in the cytoplasm and
nucleus of HepG2 cells
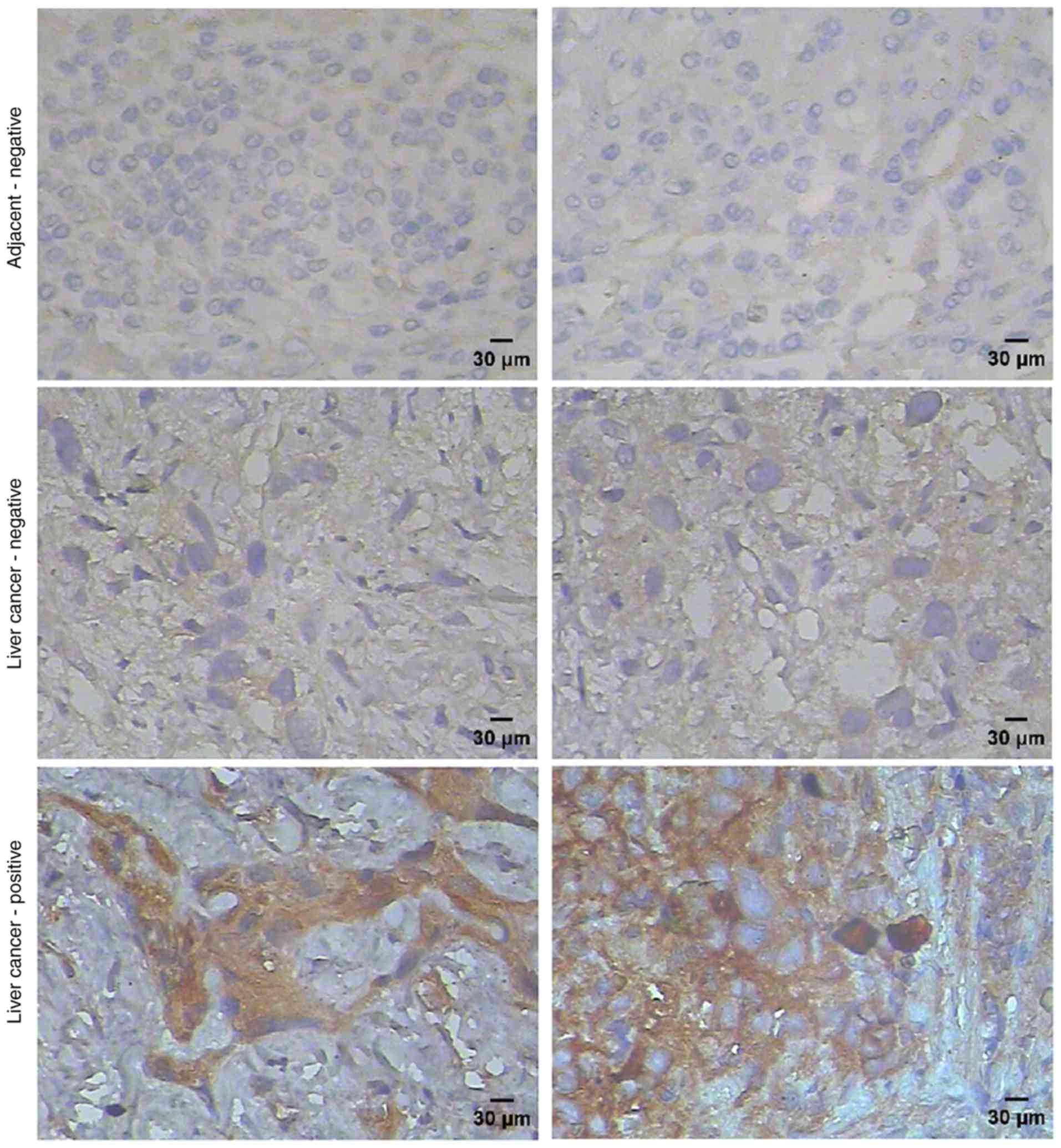
We analyzed the expression of SPAG9 protein in 20
liver cancer specimens (without consideration of the type or stage
of cancer) and 10 adjacent non-cancerous tissues by
immunohistochemistry. We observed the overexpression of SPAG9 in 16
out of 20 (80%) liver cancer specimens; no or weak staining was
observed in adjacent non-cancerous tissues (Fig. 1). Immunofluorescence revealed that
SPAG9 was expressed in the liver cancer-derived HepG2 cells. As
displayed in Fig. 2, SPAG9 protein
was localized in cytoplasmic and nuclear compartments of HepG2
cells.
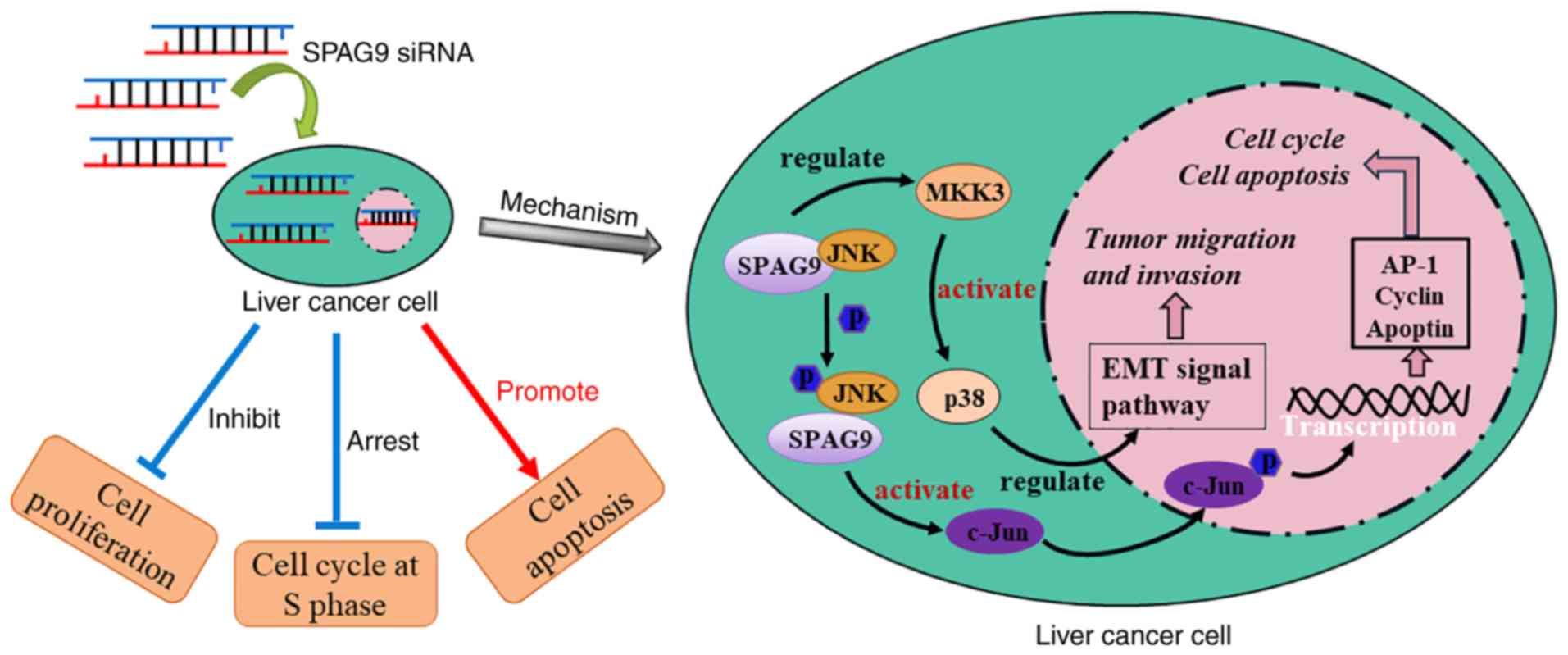
siRNA-mediated SPAG9 depletion
inhibits HepG2 cell proliferation
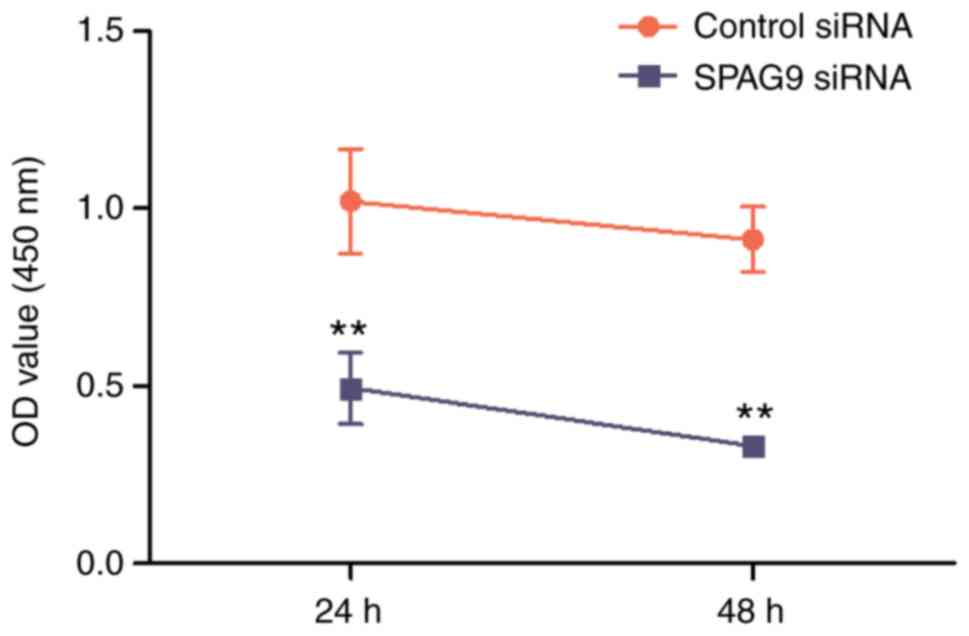
To explore the role of SPAG9 in cell proliferation,
HepG2 cells were transfected with a SPAG9-specific siRNA or
with a control siRNA. A CCK-8 assay was performed to evaluate the
proliferation of HepG2 cells. The results revealed that the
silencing of SPAG9 expression significantly decreased
proliferation compared to cells transfected with the control siRNA
after 24 and 48 h (P<0.01 and P<0.01, respectively; Fig. 3).
HepG2 cells deficient in SPAG9 undergo
apoptosis after cell cycle arrest
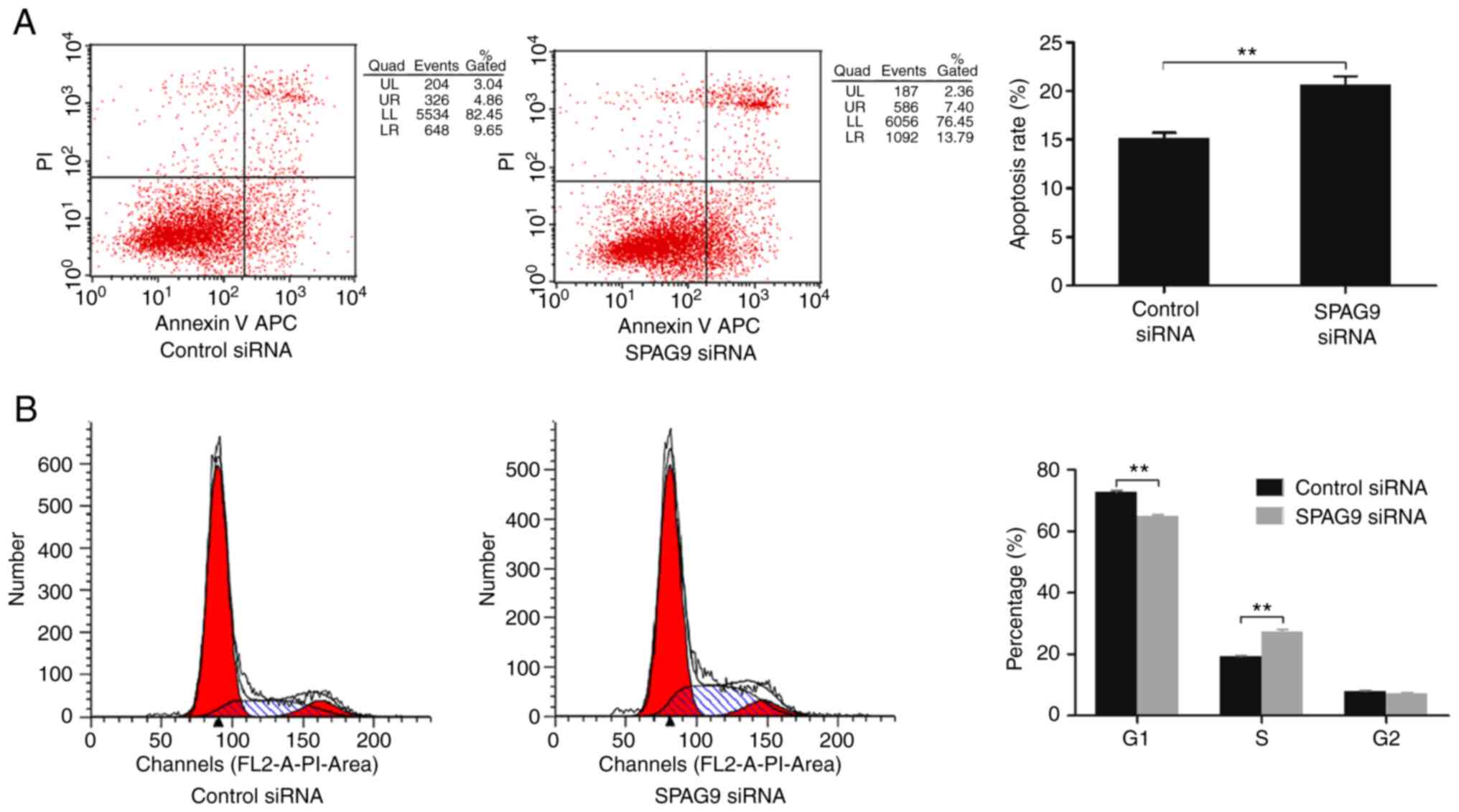
Flow cytometry was used to assess the effect of
SPAG9 on cell apoptosis and the cell cycle. Compared with cells
treated with control siRNA, apoptosis of SPAG9-depleted cells was
significantly increased (P=0.003; Fig.
4A). Moreover, there was a significant increase in the
proportion of the cell population in the S phase (P=0.000) and a
significant decrease in the proportion of the cells in the G1 phase
(P=0.004) in cells treated with SPAG9-targeted siRNA
relative to control cells (Fig.
4B).
SPAG9 deficiency results in reduction
in JNK levels
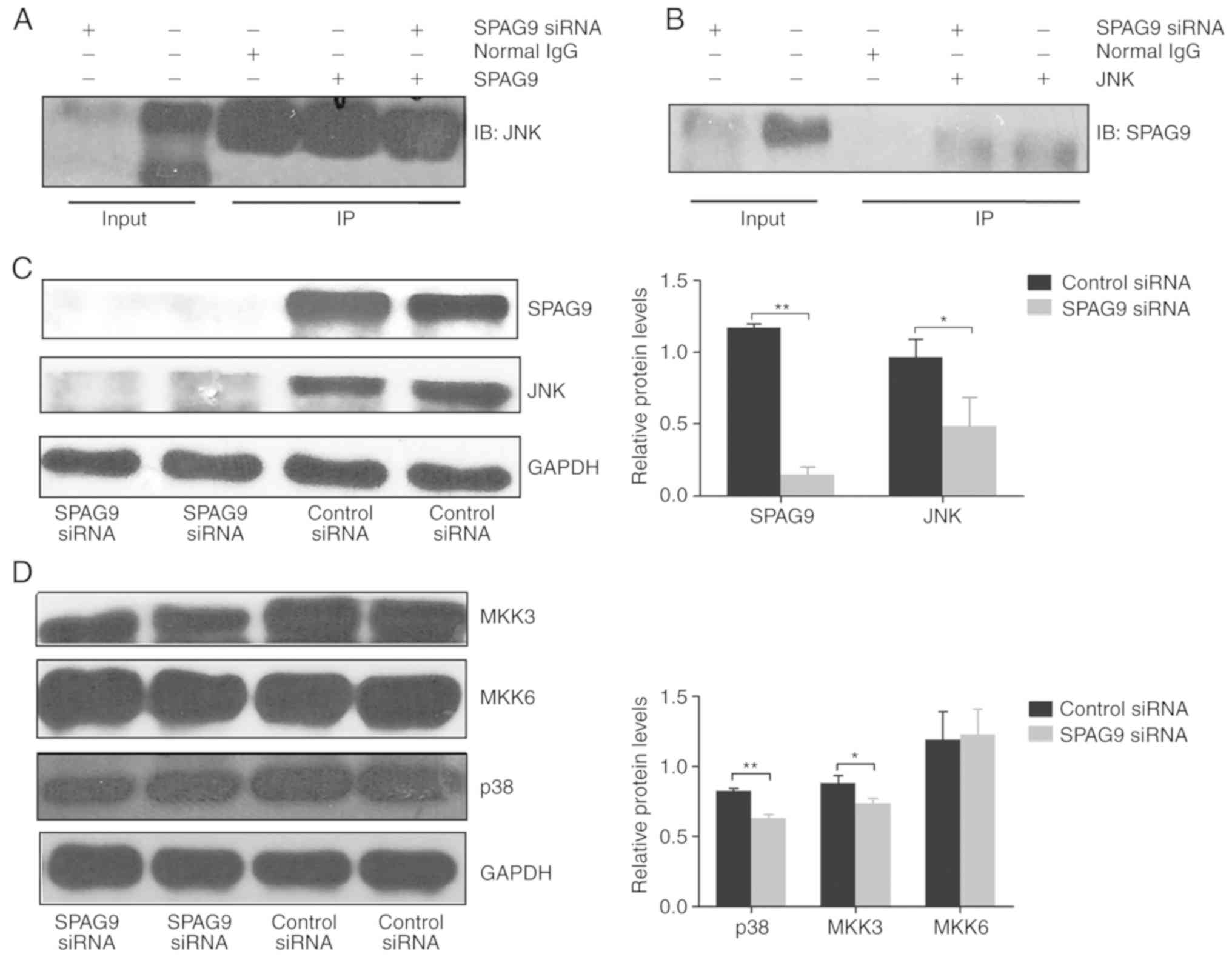
SPAG9 has a JNK-binding domain (12) that is predicted to regulate
JNK-mediated signaling. We detected an interaction between SPAG9
and JNK in HepG2 cells by co-immunoprecipitation (Fig. 5A and B). Furthermore, the levels of
SPAG9 and JNK were significantly decreased in cells transfected
with SPAG9-targeted siRNA compared to control cells
(P<0.001, P=0.024; Fig. 5C).
These results indicated that SPAG9 acts upstream of JNK.
SPAG9 silencing inhibits p38
activation
p38 is another member of the MAPK signaling pathway,
and MKK3 and MKK6 are kinases that act upstream of p38 (13). To determine whether SPAG9 regulates
p38-mediated signaling in liver cancer cells, we examined the
expression of p38, MKK3 and MKK6 in HepG2 cells transfected with
SPAG9-targeted siRNA and control siRNA. Expression of p38
and MKK3 were significantly reduced in cells with reduced SPAG9
expression (P<0.001, P=0.016), but there was no significant
change in levels of MKK6 (P=0.824; Fig.
5D).
Discussion
Numerous studies have indicated that the progression
of liver cancer involves enhanced cell proliferation and resistance
to apoptosis (14). Despite
evidence pointing to a role for sperm-associated antigen 9 (SPAG9)
as a tumor promoter in various types of cancer (15), mechanisms are still unclear. In the
present study we explored the mechanism by which SPAG9 promoted
liver cancer. Firstly, we observed that SPAG9 was overexpressed in
16 of the 20 (80%) liver cancer samples tested. The protein was not
expressed or was expressed only weakly in adjacent non-cancerous
tissues as revealed by immunohistochemistry. This finding confirms
a previously proposed role for SPAG9 in liver cancer progression
(16,17). It has been reported that SPAG9 is
located in the cytoplasm of tumor cells (6,18);
however, we observed SPAG9 in both the cytoplasm and nucleus of
HepG2 cells using both immunohistochemistry and cellular
immunofluorescence. Secondly, we found that silencing of
SPAG9 expression inhibited proliferation of HepG2 cells and
promoted apoptosis and cell cycle arrest (Fig. 6, left panel). Notably, our previous
study discovered that SPAG9 depletion caused cell cycle arrest in
the G1-G2 phase in QGY-7703 cells (10); however, in HepG2 cells, the cell
cycle was arrested in the S phase. We speculated that this
difference is due to the different types of liver cancer from which
these two cell lines were derived: QGY-7703 cells were derived from
a hepatocellular carcinoma, but HepG2 cells were derived from a
hepatoblastoma (19), and the
specific regulatory mechanism of SPAG9 in the cell cycle and
apoptosis requires further exploration. Thus, SPAG9 was highly
expressed in tissues of patients with liver cancer and in cell
lines derived from liver tumors; therefore, the biological function
and potential clinical value of SPAG9 warrants further study.
Mitogen-activated protein kinase (MAPK) is a
signaling pathway associated with cell proliferation,
differentiation, migration, invasion and apoptosis (20). Scaffold proteins can recruit MAPK
cascade components to direct phosphorylation and pathway activation
(21), SPAG9 can act as a scaffold
protein by tethering JNK and other p38 signaling modules through
its JNK-binding domain (22), and
it has been revealed to participate in the activation of the MAPK
signaling pathway (23). In a
previous study, SPAG9 was revealed to be involved in osteosarcoma
proliferation and invasion by positive regulation of JNK-mediated
signaling (7). Our results
demonstrated the involvement of SPAG9 in liver cancer. We also
revealed that silencing of SPAG9 significantly downregulated
the expression of JNK. In the liver cancer-derived HepG2 cells,
SPAG9 interacted with JNK; this interaction was previously
demonstrated in an ovarian cancer cell line (5). JNK is a kinase that phosphorylates
scaffold proteins and protein kinases, and its upregulation
contributes to cancer growth and apoptosis (24,25).
Our data suggest that SPAG9 directly interacts with JNK to regulate
the cell growth and apoptosis of liver cancer cells.
p38 is a MAP kinase that regulates a
stress-activated signaling pathway. It can be activated by various
extracellular stimuli such as oxidative stress, ischemia hypoxia,
UV irradiation and cytokines, and it regulates cell survival and
death during normal development and during tumorigenesis (26). p38 plays a role in the progression
of liver cancer, and increased phosphorylation of p38 is a
predictor of poor survival of patients with liver cancer, and
inactivation of p38 can lead to apoptosis in liver cancer cells
(27,28). SPAG9 may regulate tumor cell
survival and apoptosis through p38 signaling (29), and SPAG9/p38 signaling pathway
activation has been shown to cause changes in expression of
proteins that regulate the cell cycle and that activate the
epithelial-mesenchymal transition (30,31).
The activation of p38 requires three-tiered signaling that involves
MAP kinase kinase kinases (MAPKKKs), MAP kinase kinases (MKKs) and
MAP kinases (MAPKs). MKK3 and MKK6 are kinases that act upstream of
p38 (32).
In the present study that focused on liver cancer,
we found that silencing of SPAG9 significantly decreased the
expression of p38 and MKK3 but did not cause a significant change
in the expression of MKK6. Stramucci et al proposed that
blocking upstream kinases could interfere with p38-mediated
signaling resulting in blocking of pro-tumorigenic signals and
leaving tumor suppressive signals unaffected (32). Indeed, MKK3 is regarded as a target
for tumor therapy (33). Our
results indicated that in liver cancer SPAG9 regulated the
activation of p38 through the SPAG9/MKK3/p38 axis (Fig. 6, right panel), and factors involved
in this novel pathway are possible therapeutic targets for liver
cancer.
Acknowledgements
We thank the Department of Pathology at Hunan
Provincial Brain Hospital for providing patient samples.
Funding
The present study was supported by grants from the
Graduate Innovative Project of Hunan Province (no. CX2016B381).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
SL conceived and designed the study, performed the
experiments and drafted the manuscript. BR analyzed the data,
revised the manuscript, gave final approval of the version to be
published, and agreed to be accountable for all aspects of the
work. GZ provided experimental guidance, analyzed the data and
revised the manuscript. JL, WC, YH, XC and YF performed the
experiments, acquired and interpreted the data, and revised the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The experiments were performed according to the
Medical Ethics Committee of Hunan Brain Hopsital (no. L2017003).
Written informed consent was obtained from all patients prior to
the collection of liver cancer tissues.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SPAG9
|
sperm-associated antigen 9
|
|
CTA
|
cancer testis antigen
|
|
MAPKs
|
mitogen-activated protein kinases
|
|
CCK-8
|
Cell Counting Kit-8
|
|
MAPKKKs
|
MAP kinase kinases kinases
|
|
MKKs
|
MAP kinase kinases
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zeng H, Zheng R, Guo Y, Zhang S, Zou X,
Wang N, Zhang L, Tang J, Chen J, Wei K, et al: Cancer survival in
China, 2003–2005: A population-based study. Int J Cancer.
136:1921–1930. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fu J and Wang H: Precision diagnosis and
treatment of liver cancer in China. Cancer Lett. 412:283–288. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Whitehurst AW: Cause and consequence of
cancer/testis antigen activation in cancer. Annu Rev Pharmacol
Toxicol. 54:251–272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ha JH, Yan M, Gomathinayagam R, Jayaraman
M, Husain S, Liu J, Mukherjee P, Reddy EP, Song YS and Dhanasekaran
DN: Aberrant expression of JNK-associated leucine-zipper protein,
JLP, promotes accelerated growth of ovarian cancer. Oncotarget.
7:72845–72859. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jagadish N, Gupta N, Agarwal S, Parashar
D, Sharma A, Fatima R, Topno AP, Kumar V and Suri A:
Sperm-associated antigen 9 (SPAG9) promotes the survival and tumor
growth of triple-negative breast cancer cells. Tumour Biol.
37:13101–13110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xiao C, Fu L, Yan C, Shou F, Liu Q, Li L,
Cui S, Duan J, Jin G, Chen J, et al: SPAG9 is overexpressed in
osteosarcoma, and regulates cell proliferation and invasion through
regulation of JunD. Oncol Lett. 12:2674–2679. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ren B, Wei X, Zou G, He J, Xu G, Xu F,
Huang Y, Zhu H, Li Y, Ma G, et al: Cancer testis antigen SPAG9 is a
promising marker for the diagnosis and treatment of lung cancer.
Oncol Rep. 35:2599–2605. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miao ZF, Wang ZN, Zhao TT, Xu YY, Wu JH,
Liu XY, Xu H, You Y and Xu HM: Overexpression of SPAG9 in human
gastric cancer is correlated with poor prognosis. Virchows Arch.
467:525–533. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ren B, Zou G, He J, Huang Y, Ma G, Xu G,
Li Y and Yu P: Sperm-associated antigen 9 is upregulated in
hepatocellular carcinoma tissue and enhances QGY cell proliferation
and invasion in vitro. Oncol Lett. 15:415–422.
2018.PubMed/NCBI
|
|
11
|
Edmondson HA and Steiner PE: Primary
carcinoma of the liver: A study of 100 cases among 48,900
necropsies. Cancer. 7:462–503. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shankar S, Mohapatra B, Verma S, Selvi R,
Jagadish N and Suri A: Isolation and characterization of a haploid
germ cell specific sperm associated antigen 9 (SPAG9) from the
baboon. Mol Reprod Dev. 69:186–193. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cuadrado A and Nebreda AR: Mechanisms and
functions of p38 MAPK signalling. Biochem J. 429:403–417. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu W, Xu C, Wan H, Liu C, Wen C, Lu H and
Wan F: MicroRNA-206 overexpression promotes apoptosis, induces cell
cycle arrest and inhibits the migration of human hepatocellular
carcinoma HepG2 cells. Int J Mol Med. 34:420–428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pan J, Yu H, Guo Z, Liu Q, Ding M, Xu K
and Mao L: Emerging role of sperm-associated antigen 9 in
tumorigenesis. Biomed Pharmacother. 103:1212–1216. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan Q, Lou G, Qian Y, Qin B, Xu X, Wang Y,
Liu Y and Dong X: SPAG9 is involved in hepatocarcinoma cell
migration and invasion via modulation of ELK1 expression. Onco
Targets Ther. 9:1067–1075. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie C, Fu L, Liu N and Li Q:
Overexpression of SPAG9 correlates with poor prognosis and tumor
progression in hepatocellular carcinoma. Tumour Biol. 35:7685–7691.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ren B, Luo S, Xu F, Zou G, Xu G, He J,
Huang Y, Zhu H and Li Y: The expression of DAMP proteins HSP70 and
cancer-testis antigen SPAG9 in peripheral blood of patients with
HCC and lung cancer. Cell Stress Chaperones. 22:237–244. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hsiao CC, Chen PH, Cheng CI, Tsai MS,
Chang CY, Lu SC, Hsieh MC, Lin YC, Lee PH and Kao YH: Toll-like
receptor-4 is a target for suppression of proliferation and
chemoresistance in HepG2 hepatoblastoma cells. Cancer Lett.
368:144–152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rohrabaugh S, Kesarwani M, Kincaid Z,
Huber E, Leddonne J, Siddiqui Z, Khalifa Y, Komurov K, Grimes HL
and Azam M: Enhanced MAPK signaling is essential for CSF3R induced
leukemia. Leukemia. 31:1770–1778. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Raman M, Chen W and Cobb MH: Differential
regulation and properties of MAPKs. Oncogene. 26:3100–3112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee CM, Onésime D, Reddy CD, Dhanasekaran
N and Reddy EP: JLP: A scaffolding protein that tethers JNK/p38MAPK
signaling modules and transcription factors. Proc Natl Acad Sci
USA. 99:14189–14194. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jagadish N, Rana R, Selvi R, Mishra D,
Garg M, Yadav S, Herr JC, Okumura K, Hasegawa A, Koyama K and Suri
A: Characterization of a novel human sperm-associated antigen 9
(SPAG9) having structural homology with c-Jun N-terminal
kinase-interacting protein. Biochem J. 389:73–82. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Win S, Than TA, Zhang J, Oo C, Min RWM and
Kaplowitz N: New insights into the role and mechanism of
c-Jun-N-terminal kinase signaling in the pathobiology of liver
diseases. Hepatology. 67:2013–2024. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seki E, Brenner DA and Karin M: A liver
full of JNK: Signaling in regulation of cell function and disease
pathogenesis, and clinical approaches. Gastroenterology.
143:307–320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grossi V, Peserico A, Tezil T and Simone
C: p38α MAPK pathway: A key factor in colorectal cancer therapy and
chemoresistance. World J Gastroenterol. 20:9744–9758. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang SN, Lee KT, Tsai CJ, Chen YJ and Yeh
YT: Phosphorylated p38 and JNK MAPK proteins in hepatocellular
carcinoma. Eur J Clin Invest. 42:1295–1301. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hsieh SC, Huang MH, Cheng CW, Hung JH,
Yang SF and Hsieh YH: α-Mangostin induces mitochondrial dependent
apoptosis in human hepatoma SK-Hep-1 cells through inhibition of
p38 MAPK pathway. Apoptosis. 18:1548–1560. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jilg CA, Ketscher A, Metzger E, Hummel B,
Willmann D, Rüsseler V, Drendel V, Imhof A, Jung M, Franz H, et al:
PRK1/PKN1 controls migration and metastasis of androgen-independent
prostate cancer cells. Oncotarget. 5:12646–12664. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang L, Yan L, Cao M, Zhang H, Li C, Bai
Y, Yu P, Li M and Zhao X: SPAG9 promotes endometrial carcinoma cell
invasion through regulation of genes related to the
epithelial-mesenchymal transition. Eur J Gynaecol Oncol.
37:312–319. 2016.PubMed/NCBI
|
|
31
|
Sinha A, Agarwal S, Parashar D, Verma A,
Saini S, Jagadish N, Ansari AS, Lohiya NK and Suri A: Down
regulation of SPAG9 reduces growth and invasive potential of
triple-negative breast cancer cells: Possible implications in
targeted therapy. J Exp Clin Cancer Res. 32:692013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stramucci L, Pranteda A and Bossi G:
Insights of crosstalk between p53 protein and the MKK3/MKK6/p38
MAPK signaling pathway in cancer. Cancers. 10:E1312018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Baldari S, Ubertini V, Garufi A, D'Orazi G
and Bossi G: Targeting MKK3 as a novel anticancer strategy:
Molecular mechanisms and therapeutical implications. Cell Death
Dis. 6:e16212015. View Article : Google Scholar : PubMed/NCBI
|