Introduction
Apart from medullary carcinoma, all other types of
thyroid cancers originate from follicular cells, which form the
simple unicellular epithelium of the thyroid (1). In spite of the development of
preventive measures, the incidence of thyroid cancer has increased
more than 3-fold during the past 3 decades, and this disease tends
to affect younger patients (2). As
the most common type of thyroid cancer, papillary thyroid carcinoma
(PTC) accounts for more than 80% of the total cases of this disease
(3). With the development of
treatment strategies such as radiotherapy, chemotherapy, thyroid
hormone treatment, surgical resection and combined treatment, the
5-year survival rate of patients with PTC has now reached 95%
(4). However, due to the unclear
pathogenesis, the recurrence rate of this disease is still high
(5). More importantly, PTC may
occasionally dedifferentiate into more aggressive and lethal
thyroid cancers (1). Therefore, the
in-depth understanding of the pathogenesis of this disease is
highly needed.
Long non-coding RNA, which is also known as lncRNA,
is a class of non-coding RNAs with a length longer than 200
nucleotides, which is significantly longer than that of short
interfering RNAs, microRNAs and other short RNAs (6). Numerous studies have shown that
different lncRNAs have their specific functions in normal
biological or pathological processes (7). In addition to this, quite a few of
lncRNAs were proven to play essential roles in the development and
progression of a variety of human diseases including liver
diseases, heart diseases, and different types of human cancer
(8–10). A recent study showed that lncRNA
PAX8-AS1:28, or lnc-PSD4-1:14, is abnormally expressed in PTC
(11), indicating the involvement
of lncRNA PAX8-AS1:28 in this disease. However, the role of
lnc-PSD4-1:14 in PTC is still unknown.
In the present study, expression of lncRNA
PAX8-AS1:28 in PTC tissues and adjacent healthy tissues were
detected. The relationships between PAX8-AS1:28, PAX8 and MYC were
investigated.
Materials and methods
Patients
A total of 38 patients with PTC were enrolled in our
hospital from October 2016 to October 2017. These patients included
13 males and 25 females, and the ages ranged from 20 to 78 years
with an average age of 40.2 years. All patients were diagnosed by
the standards that have been established by the World Health
Organization. Patients with thyroid microcarcinoma were excluded.
All patients received surgical resections, and tumor tissues as
well as adjacent healthy tissues were collected during the surgical
operation. All patients had normal thyroid function before surgery,
and none of them received radiotherapy or chemotherapy before
admission. The Ethics Committee of the Second Affiliated Hospital
of Wenzhou Medical University approved this study. All patients
provided informed consent.
Cell lines and cell culture
Normal thyroid follicular epithelial cell line
Nthy-ori 3-1 and PTC cell line IHH-4 were purchased from the
American Type Culture Collection (ATCC, Manassas, VA, USA). Cells
were cultured with DMEM containing 10% FBS, 100 mg/ml penicillin G
and 100 U/ml streptomycin (all from Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) in an incubator at 37°C in 5%
CO2.
Real-time quantitative PCR
Trizol reagent (Invitrogen; Thermo Fisher
Scientific) was used for total RNA extraction. The quality of RNA
samples was tested using NanoDrop™ 2000 spectrophotometers (Thermo
Fisher Scientific), and only those with an A260/A280 ratio between
1.8 and 2.0 were used in reverse transcription to synthesize cDNA
by using Oligo(dT)15 (Sangon, Shanghai, China) and AMV
reverse transcriptase (Gibco; Thermo Fisher Scientific). TaqMan PCR
kit (Thermo Fisher Scientific) was used for PCR reaction. The PCR
reaction was performed on Bio-Rad iCycler (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The following primers were used in the
PCR reactions: 5′-GGCTTTGTGCTACTGCTTCA-3′ (forward) and
5′-TCTAACCCTCCTGGCTTCCT-3′ (reverse) for PAX8-AS1:28;
5′-GATCAGGATAGCTGCCGACT-3′ (forward) and 5′-GTTGTACCTGCTCGCCTTTG-3′
(reverse) for PAX8; 5′-CAGGAGGCATTGCTGATGAT-3′ (forward) and
5′-GAAGGCTGGGGCTCATTT-3′ (reverse) for GAPDH;
5′-GCCACGTCTCCACACATCAG-3′ (forward) and
5′-TCTTGGCAGCAGGATAGTCCTT-3′ (reverse) for MYC. PCR reaction
conditions were: 95°C for 1 min, followed by 40 cycles of 95°C for
15 sec and 56°C for 35 sec. Ct values were processed using the
2−ΔΔCT method, and relative expression level of each
gene was normalized to endogenous control GAPDH.
Construction of lncRNA PAX8-AS1:28 and
MYC expression vector and transfection
Cloning primers for PAX8-AS1:28 were
5′-CGTGTCGACCTCATTCATTTGTTC-3′ (forward) and
5′-CGAAAGCTTAAAAAACTGACACATGC-3′ (reverse). Cloning primers for MYC
were 5′-CGAAAGCTTGCCACCATGCTCGGAAGGACTATCCTGCTGCCAA-3′ (forward)
and 5′-CGTGGATCCGGCGCTCCAAGACGTTGTGTGTTCG-3′ (reverse). PCR
amplified fragments with the U6 promoter were inserted into the
pcDNA3.1 vector. IHH-4 cells were collected during the logarithmic
growth phase, and were seeded in 6-well plates with
4.5×105 cells per well. Cells were cultured in an
incubator (37°C, 5% CO2). Five micrograms of PAX8-AS1:28
and MYC expression plasmid was diluted in 250 µl of serum-free
medium and transfection was performed using Invitrogen™
Lipofectamine 2000 transfection reagent (cat. no. 11668-019, Thermo
Fisher Scientific). Medium was replaced 4–6 h after transfection.
After incubation for another 48 h, the cells were collected for
subsequent experiments. Expression of PAX8-AS1:28 and MYC was
detected after transfection to confirm the success of
transfection.
siRNA transfection
MYC siRNA (cat. no. AM16708) and control siRNA (cat.
no. AM4611) were purchased from Applied Biosystems/ Thermo Fisher
Scientific. The PAX8-AS1:28 siRNA target sequence was
GAGAGGTCATTATGTGAAGGCT. siRNA (final concentration, 50 nM) was
transfected into IHH-4 cells using Lipofectamine 2000 transfection
reagent (cat. no. 11668-019, Thermo Fisher Scientific) according to
the manufacturer's instructions. Medium was replaced 4–6 h after
transfection. After incubation for another 48 h, the cells were
collected for subsequent experiments.
Cell proliferation assay
Cell counting kit (CCK-8; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was used to measure cell proliferation ability
according to the manufacturers instructions. Briefly, 100 µl of
cell suspension containing 5×103 cells was transferred
to each well of 96-well plates. Cells were cultured and CCK-8
solution (10 µl) was added into each well 24, 48 and 72 h later.
After incubation at 37°C for another 4 h, optical density (OD)
values at 450 nm were measured using a microplate reader (Bio-Rad,
USA).
Western blot assay
Total protein was extracted using cell lysis buffer
(P0013K, Beyotime Institute of Biotechnology, Haimen, China), and
the concentration was determined by BCA assay. Then, 20 µg of
protein from each sample was subjected to 10% SDS-PAGE gel
electrophoresis, followed by transmembrane to PVDF. Membranes were
then blocked with 5% skimmed milk at room temperature for 1 h.
After washing with TBST, membranes were then incubated with primary
antibodies including rabbit anti-PAX8 (dilution 1:2,000, cat. no.
ab53490, Abcam), rabbit anti-c-Myc (dilution 1:2,000, cat. no.
ab32072, Abcam) and rabbit anti-β-actin (dilution 1:2,000, cat. no.
ab8227, Abcam) overnight at 4°C. After washing with PBS, the
membranes were further incubated with anti-rabbit IgG-HRP secondary
antibody (dilution 1:1,000, cat. no. MBS435036, MyBioSource) at
room temperature for 2 h. Then the membranes were washed again with
TBST, signals were detected using ECL (Sigma-Aldrich; Merck KGaA)
method. Relative expression level of each protein was normalized to
endogenous control β-actin using ImageJ software (National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Statistical analyses were performed using SPSS 19.0
(IBM Corp., Armonk, NY, USA). Normal distribution data were
recorded as mean ± SD), and comparisons between two groups were
performed by t-test. Correlation between the expression levels of
AX8-AS1:28 and PAX8 mRNAs was analyzed by Spearman's rank
correlation coefficient. P<0.05 was considered to be
statistically significant.
Results
Expression of PAX8-AS1:28 and PAX8
mRNAs in PTC tissues and adjacent normal tissues
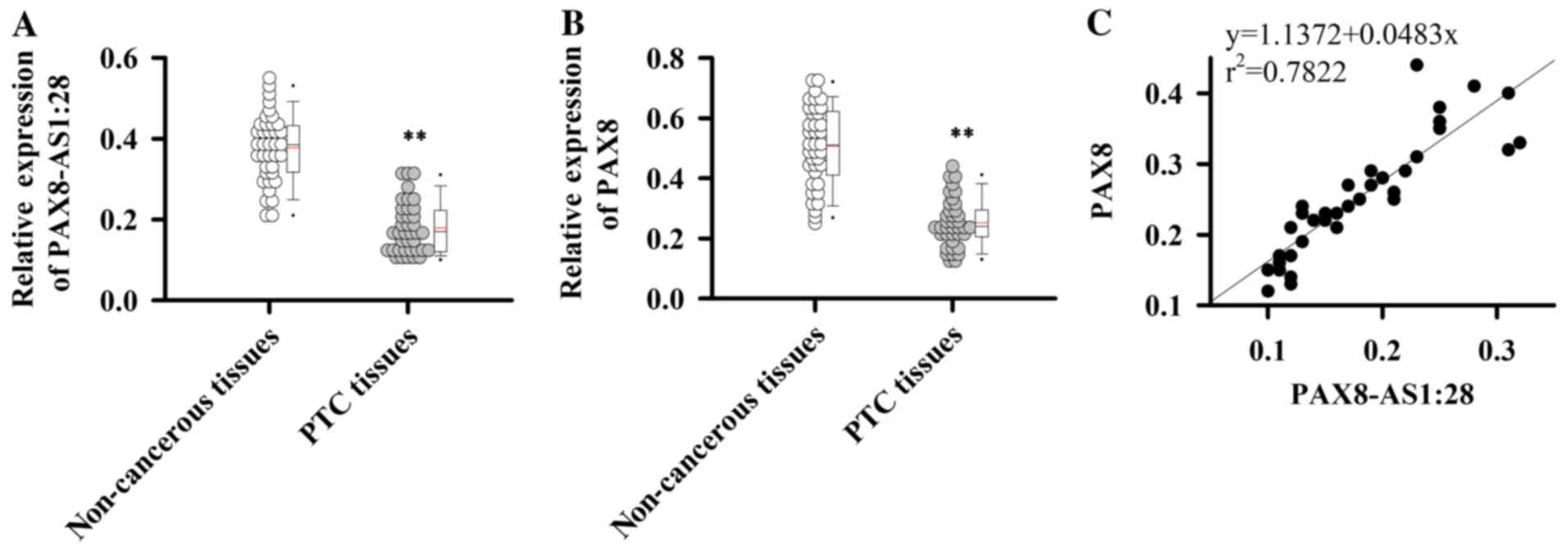
qRT-PCR was performed to detect the expression of
PAX8-AS1:28 and PAX8 mRNAs in PTC tissues and adjacent normal
tissues. Compared with adjacent normal tissues, expression levels
of PAX8-AS1:28 (Fig. 1A) and PAX8
(Fig. 1B) mRNAs were significantly
reduced in PTC tissues (P<0.01). In addition, the expression
level of PAX8-AS1:28 was positively correlated with expression of
PAX8 (Fig. 1C,
r2=0.7822). These data suggest that PAX8-AS1:28 and PAX8
are downregulated in PTC.
Expression of PAX8-AS1:28 and PAX8 in
normal and PTC cells
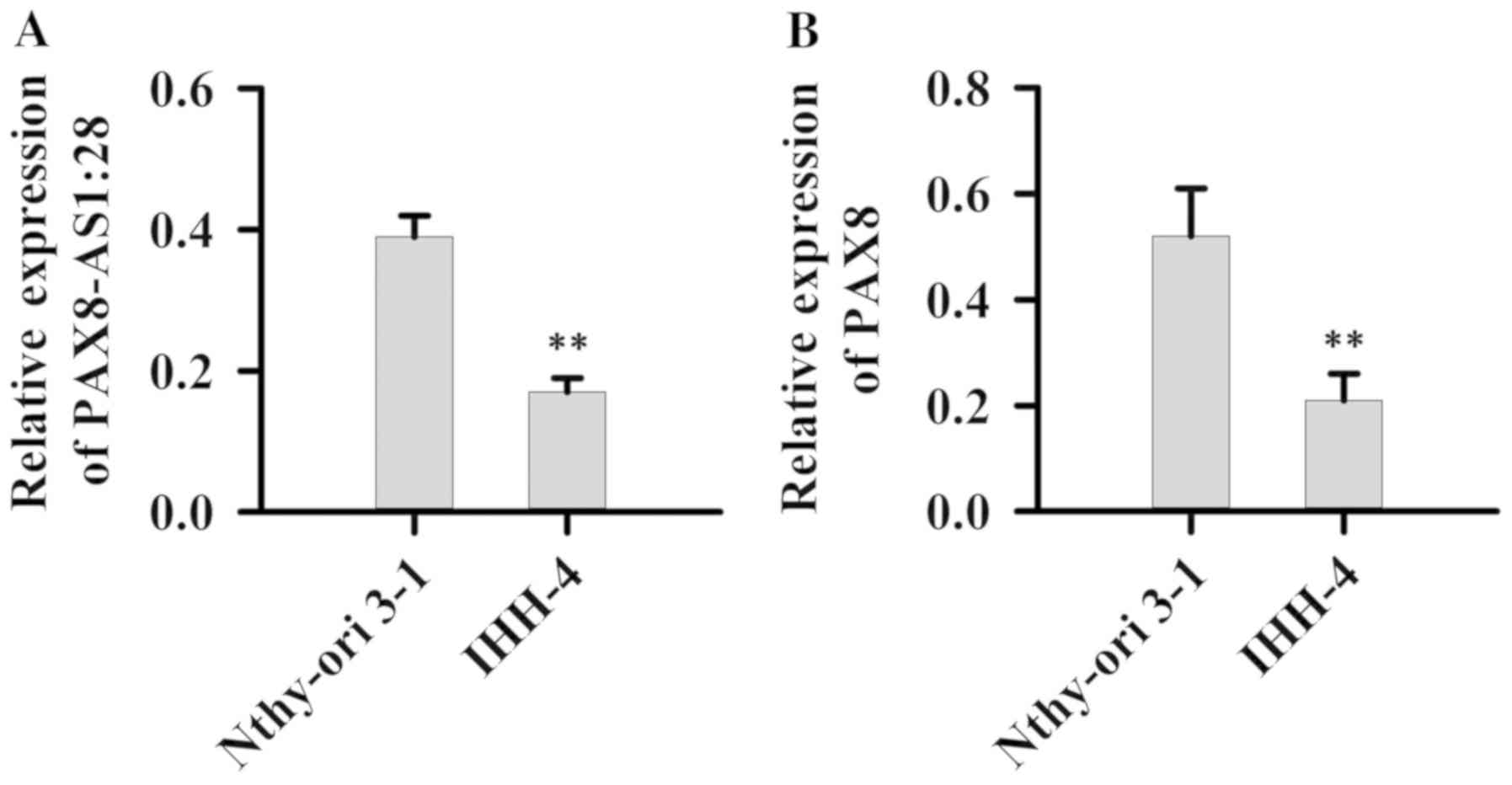
Expression of PAX8-AS1:28 and PAX8 in normal cell
line Nthy-ori 3-1 as well as PTC cell line IHH-4 was detected.
Compared with normal cell line Nthy-ori 3-1, expression levels of
PAX8-AS1:28 (Fig. 2A) and PAX8
mRNAs (Fig. 2B) were significantly
reduced in the IHH-4 cells (P<0.01).
Effect of PAX8-AS1:28 overexpression
and silencing on PAX8 expression and IHH-4 cell growth
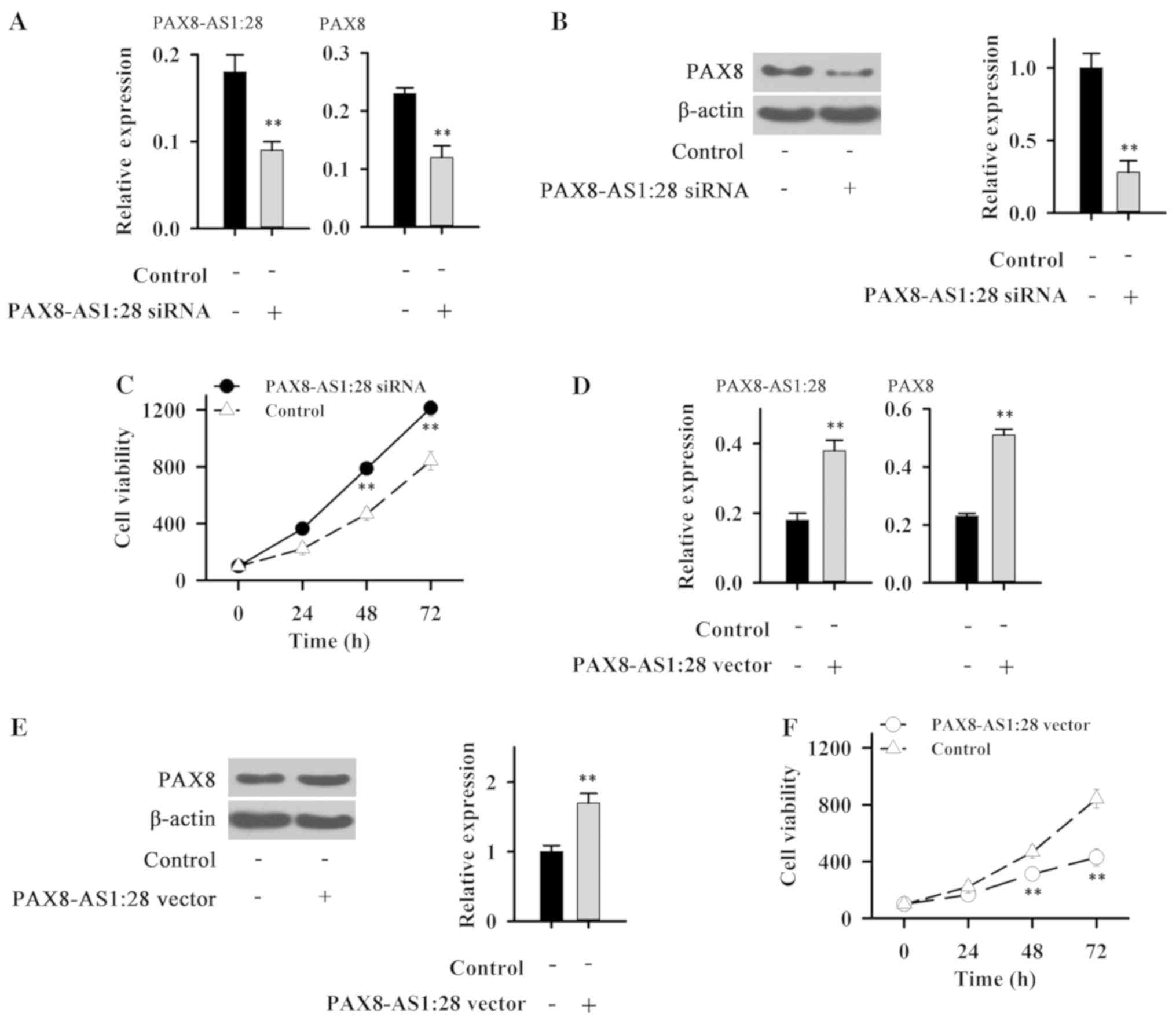
After siRNA silencing, the expression level of
PAX8-AS1 was significantly reduced (Fig. 3A), indicating the success of
transfection. Compared with control cells, expression levels of
PAX8 mRNA (Fig. 3A) and protein
(Fig. 3B) were significantly
reduced in IHH-4 cells following PAX8-AS1:28 siRNA silencing. In
addition, PAX8-AS1:28 siRNA silencing significantly promoted the
growth of IHH-4 cells (Fig.
3C).
Cells transfected with the PAX8-AS1:28 plasmid show
an elevated expression level of PAX8-AS1:28 (Fig. 3D), indicating the successfully
established PAX8-AS1 overexpression cell line. Compared with the
control cells, expression levels of PAX8 mRNA (Fig. 3D) and protein (Fig. 3E) were significantly increased in
IHH-4 cells with PAX8-AS1:28 overexpression. In addition,
PAX8-AS1:28 overexpression significantly inhibited the growth of
IHH-4 cells (Fig. 3F). These data
suggest that PAX8-AS1:28 can positively regulate the expression of
PAX8 to inhibit PTC.
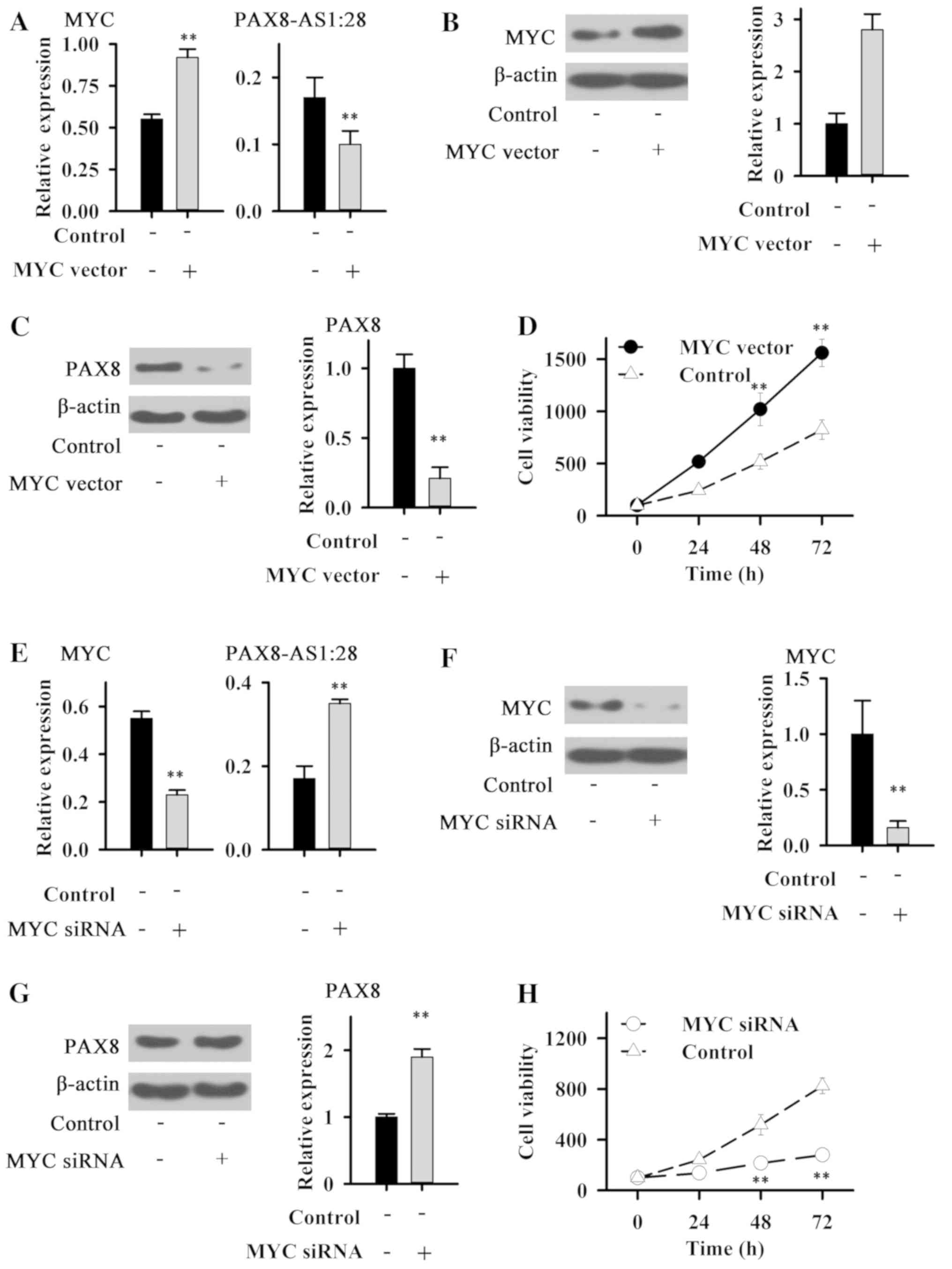
Expression of MYC in normal cell lines
and PTC cell lines
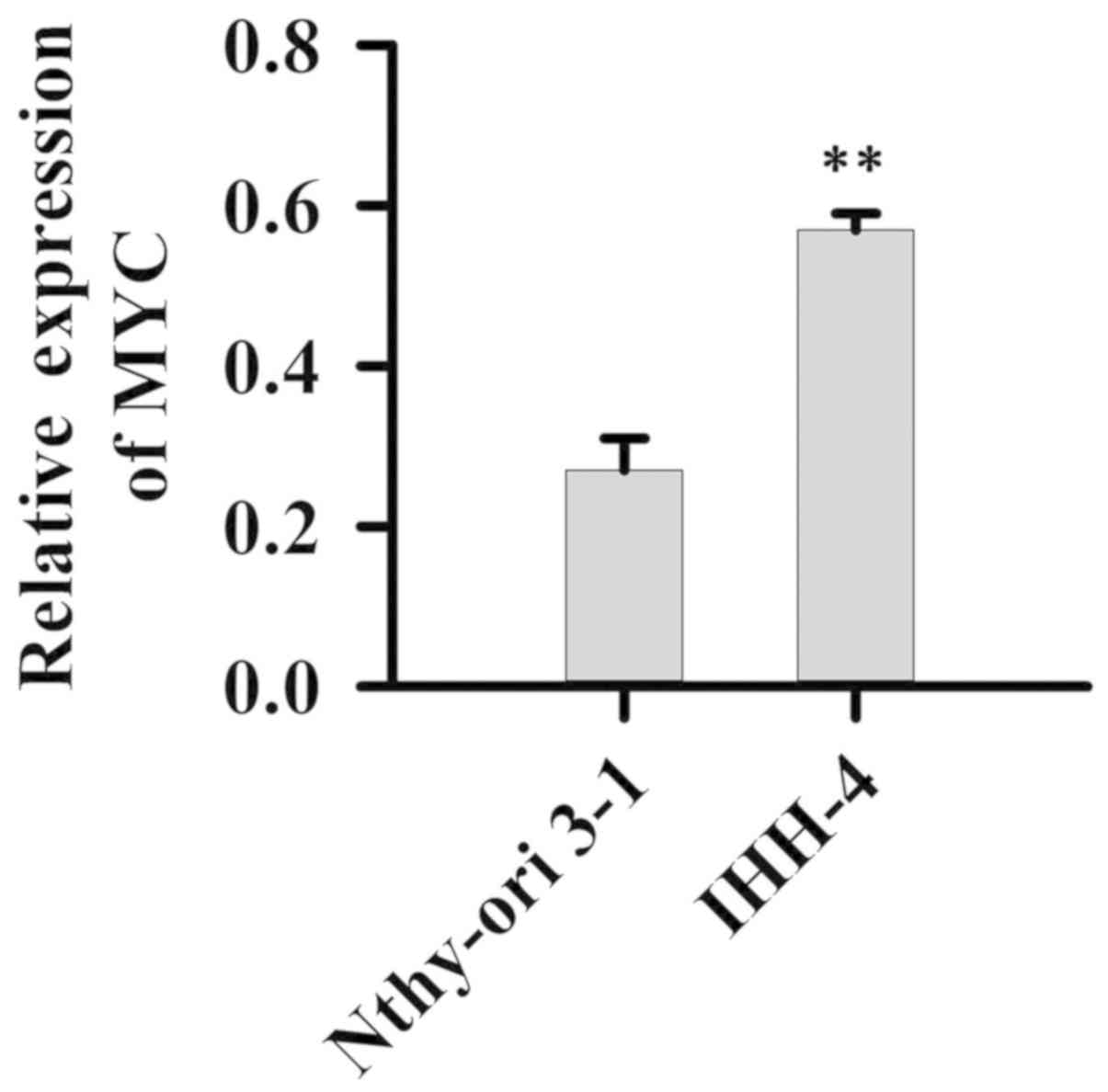
Expression of MYC in normal cell line Nthy-ori 3-1
and PTC cell line IHH-4 was detected. Compared with normal cell
line Nthy-ori 3-1, expression levels of MYC were significantly
increased in thr IHH-4 cells (P<0.01, Fig. 4).
Effects of MYC overexpression and
silencing on PAX8 and PAX8-AS1:28 expression and IHH-4 cell
growth
Cells transfected with the MYC plasmid showed
elevated expression level of MYC (Fig.
5A and B), indicating the successfully established MYC
overexpression cell line. Compared with the control cells,
expression levels of PAX8-AS1:28 (Fig.
5A) and PAX8 (Fig. 5A and C)
were significantly decreased in the IHH-4 cells with MYC
overexpression. In addition MYC overexpression significantly
promoted the growth of IHH-4 cells (Fig. 5D).
After siRNA silencing, expression level of MYC was
significantly reduced (Fig. 5E and
F), indicating the success of transfection. Compared with the
control cells, expression levels of PAX8-AS1:28 (Fig. 5E) and PAX8 protein (Fig. 5G) were significantly reduced in
IHH-4 cells following MYC siRNA silencing. In addition, MYC siRNA
silencing significantly inhibited the growth of IHH-4 cells
(Fig. 5H). These data suggest that
MYC can negatively regulate the expression of PAX8-AS1:28 and PAX8
to promote PTC.
Discussion
The pathogenesis of PTC is still unclear. Therefore,
understanding the molecular pathogenesis and mechanisms underlying
PTC is still a ‘hot research spot’ regarding the treatment of PTC
(12). Recent studies have
identified multiple pathways that are related to the development
and progression of PTC, such as the HIF-1α pathway (13), the thyroid-stimulating hormone
receptor signaling pathway (14)
and the WNT/β-catenin signaling pathway (15). Epigenetic and genetic alterations in
those pathways, including altered gene copy-number, gene mutation
and aberrant gene methylation play central roles in the
pathogenesis of PTC (12). A
variety of lnRNAs have also been proven to be involved in the
development of PTC. lncRNA SLC6A9 was found to be downregulated in
131I-resistant PTC accompanied by the inhibition of PARP-1, and a
high expression level of SLC6A9 was found to be positively
correlated with the overall survival rate and disease-free survival
rate of PTC patients who received 131I therapy, indicating that
SLC6A9 can potentially serve as a novel target for the treatment of
131I-resistant PTC (16). In
addition to the direct effects on PTC, lncRNAs can also interact
with key signal transduction pathways that are involved in the
pathogenesis of PTC. In a recent study, lncRNA PTCSC was proven to
significantly regulate the expression of SCAI and subsequently
alter the activity of Wnt/β-catenin signal transduction, which in
turn regulated the proliferation and migration of PTC cells
(17). All of these previous
studies suggest that lncRNA are key players in the pathogenesis of
PTC.
lnc-PSD4-1:14, or lncRNA PAX8-AS1:28, is a newly
discovered lncRNA. Based on our knowledge, the functionality of
lncRNA PAX8-AS1:28 is still unknown. It has been reported that the
expression level of lncRNA PAX8-AS1:28 is commonly decreased in
patients with neck squamous cell carcinoma, and a higher expression
level of lncRNA PAX8-AS1:28 is closely correlated with better
survival outcome (18). lncRNA
PAX8-AS1:28 expression is also downregulated in patients with PTC
(11), and the reduced expression
level of lncRNA PAX8-AS1:28 is closely correlated with the poor
survival of these patients (19).
Consistent with previous studies, in our study, the expression
level of PAX8-AS1:28 was found to be lower in PTC tissues and PTC
cells than that in adjacent healthy tissues and a normal cell line.
Our finding further confirmed the involvement of PAX8-AS1:28 in
PTC.
Paired box gene 8, or PAX8, is a transcription
factor that belongs to the paired box (PAX) family (20). Mutations in PAX8 have been proven to
be related with various thyroid diseases including thyroid
follicular carcinomas, thyroid dysgenesis and atypical follicular
thyroid adenomas (20). lncRNA
PAX8-AS1:28 overlaps with paired box 8 (PAX8) in an antisense
orientation (18), indicating the
possible interactions between PAX8 and lncRNA PAX8-AS1:28. In this
study, PAX8 expression was also significantly downregulated in PTC
tissues and PTC cells than that in adjacent healthy tissues and
normal cells. In addition, lncRNA PAX8-AS1:28 expression was found
to be positively correlated with the expression of PAX8. Moreover,
lncRNA PAX8-AS1:28 silencing reduced the expression level of PAX8
and promoted PTC cell growth. In contrast, lncRNA PAX8-AS1:28
overexpression increased the expression level of PAX8 and inhibited
PTC cell growth. Those data suggest that PAX8-AS1:28 may affect PTC
cell growth by positively regulating the expression of PAX8.
As an oncogene, MYC is overexpressed in various
tumor tissues, and MYC overexpression promotes the proliferation,
migration and invasion of tumor cells (21). An increased expression level of MYC
has also been detected in patients with PTC (22), indicating the involvement of MYC in
this disease. Consistent with previous studies, in our study, the
expression level of MYC in PTC cells was found to be significantly
higher than that in a normal cell line. In a recent study, protein
levels of MYC and PAX8 were found to be inversely correlated with
each other in thyroid tumors (23),
indicating the opposite roles of those 2 proteins in thyroid
cancer. In our study, MYC silencing increased expression levels of
PAX8-AS1:28 as well as PAX8 and inhibited tumor cell growth, while
MYC overexpression decreased expression levels of PAX8-AS1:28 as
well as PAX8 and promoted tumor cell growth. MYC is multifunctional
and nuclear phosphoprotein that is involved in the expression
regulation of a large set of genes. The downregulation of
PAX8-AS1:28 and PAX8 may be caused by the direct role of MYC or its
downstream targets. All these data suggest that MYC can promote
PTC, and this function may be correlated with the downregulation of
expression of PAX8-AS1:28 and PAX8. It is interesting that
antisense PAX8-AS1:28 elevated the expression of PAX8. The possible
explanation is that PAX8-AS1:28 may be involved in the
post-transcriptional process of PAX8 transcripts, which has been
observed in a recent study (24).
In conclusion, the expression level of PAX8-AS1:28
and PAX8 were lower in PTC tumor tissue and PTC cell lines than
that in healthy tissue and normal cell lines, while the expression
level of MYC was higher in PTC cell lines than that in normal cell
lines. PAX8-AS1:28 can positively regulate PAX8 expression and
inhibit PTC tumor cell growth. In contrast, MYC can negatively
regulate the expression of PAX8-AS1:28 as well as PAX8 and promote
tumor cell growth. Therefore, PAX8-AS1:28 and PAX8 may serve as
biomarkers for the diagnosis of PTC. They may also serve as targets
for the treatment of this disease. The present study was still
limited by the small sample size. Further studies with a larger
sample size are needed to further confirm the conclusions in the
present study.
Acknowledgments
Not applicable.
Funding
We thank the financial support from Natural Science
Foundation of Zhejiang Province (no. LY14H190003).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YZ and JC conceived and designed the study. YZ and
FL performed the experiments. JC wrote the paper. YZ and FL
reviewed and edited the manuscript. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The Ethics Committee of the Second Affiliated
Hospital of Wenzhou Medical University (Wenzhou, China) approved
this study and all patients provided informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Agrawal N, Akbani R, Aksoy BA, Ally A,
Arachchi H, Asa SL, Auman JT, Balasundaram M, Balu S, Baylin SB, et
al: Integrated genomic characterization of papillary thyroid
carcinoma. Cell. 159:676–690. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Olaleye O, Ekrikpo U, Moorthy R, Lyne O,
Wiseberg J, Black M and Mitchell D: Increasing incidence of
differentiated thyroid cancer in South East England: 1987–2006. Eur
Arch Otorhinolaryngol. 268:899–906. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Londero SC, Krogdahl A, Bastholt L,
Overgaard J, Trolle W, Pedersen HB, Bentzen J, Schytte S,
Christiansen P and Godballe C: Papillary thyroid microcarcinoma in
Denmark 1996–2008: A national study of epidemiology and clinical
significance. Thyroid. 23:1159–1164. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hay ID, Thompson GB, Grant CS, Bergstralh
EJ, Dvorak CE, Gorman CA, Maurer MS, McIver B, Mullan BP, Oberg AL,
et al: Papillary thyroid carcinoma managed at the Mayo Clinic
during six decades (1940–1999): Temporal trends in initial therapy
and long-term outcome in 2444 consecutively treated patients. World
J Surg. 26:879–885. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ito Y, Miyauchi A, Kudo T, Kihara M,
Fukushima M and Miya A: The effectiveness of prophylactic modified
neck dissection for reducing the development of lymph node
recurrence of papillary thyroid carcinoma. World J Surg.
41:2283–2289. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Perkel JM: Visiting ‘Noncodarnia’.
Biotechniques. 54:303–304. 2013. View Article : Google Scholar
|
|
7
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang K, Liu F, Zhou LY, Long B, Yuan SM,
Wang Y, Liu CY, Sun T, Zhang XJ and Li PF: The long noncoding RNA
CHRF regulates cardiac hypertrophy by targeting miR-489. Circ Res.
114:1377–1388. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takahashi K, Yan I, Haga H and Patel T:
Long noncoding RNA in liver diseases. Hepatology. 60:744–753. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prensner JR and Chinnaiyan AM: The
emergence of lncRNAs in cancer biology. Cancer Discov. 1:391–407.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lan X, Zhang H, Wang Z, Dong W, Sun W,
Shao L, Zhang T and Zhang D: Genome-wide analysis of long noncoding
RNA expression profile in papillary thyroid carcinoma. Gene.
569:109–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xing M: Molecular pathogenesis and
mechanisms of thyroid cancer. Nat Rev Cancer. 13:184–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zerilli M, Zito G, Martorana A, Pitrone M,
Cabibi D, Cappello F, Giordano C and Rodolico V:
BRAFV600E mutation influences hypoxia-inducible
factor-1α expression levels in papillary thyroid cancer. Mod
Pathol. 23:1052–1060. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boelaert K, Horacek J, Holder RL,
Watkinson JC, Sheppard MC and Franklyn JA: Serum thyrotropin
concentration as a novel predictor of malignancy in thyroid nodules
investigated by fine-needle aspiration. J Clin Endocrinol Metab.
91:4295–4301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiang C, Zhang ML, Zhao QZ, Xie QP, Yan
HC, Yu X, Wang P and Wang Y: LncRNA-SLC6A9-5:2: A potent sensitizer
in 131I-resistant papillary thyroid carcinoma with
PARP-1 induction. Oncotarget. 8:22954–22967. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Lu X, Geng Z, Yang G and Shi Y:
LncRNA PTCSC3/miR-574-5p governs cell proliferation and migration
of papillary thyroid carcinoma via Wnt/β-catenin signaling. J Cell
Biochem. 118:4745–4752. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu V, Singh P, Rahimy E, Zheng H, Kuo SZ,
Kim E, Wang-Rodriguez J and Ongkeko WM: RNA-seq analysis identifies
key long non-coding RNAs connected to the pathogenesis of
alcohol-associated head and neck squamous cell carcinoma. Oncol
Lett. 12:2846–2853. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo YH, Liang L, He RQ, Wen DY, Deng GF,
Yang H, He Y, Ma W, Cai XY, Chen JQ, et al: RNA-sequencing
investigation identifies an effective risk score generated by three
novel lncRNAs for the survival of papillary thyroid cancer
patients. Oncotarget. 8:74139–74158. 2017.PubMed/NCBI
|
|
20
|
Plachov D, Chowdhury K, Walther C, Simon
D, Guenet JL and Gruss P: Pax8, a murine paired box gene
expressed in the developing excretory system and thyroid gland.
Development. 110:643–651. 1990.PubMed/NCBI
|
|
21
|
Wolfer A and Ramaswamy S: MYC and
metastasis. Cancer Res. 71:2034–2037. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu YJ, Luo XY, Yang Y, Chen CY, Zhang ZY
and Guo X: Characterization and significance of MUC1 and c-myc
expression in elderly patients with papillary thyroid carcinoma.
Genet Mol Res. 14:15325–15330. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu X, Zhao L, Park JW, Willingham MC and
Cheng SY: Synergistic signaling of KRAS and thyroid hormone
receptor β mutants promotes undifferentiated thyroid cancer through
MYC up-regulation. Neoplasia. 16:757–769. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Van der Wal E, Bergsma AJ, Pijnenburg JM,
Van der Ploeg AT and Pijnappel WWMP: Antisense oligonucleotides
promote exon inclusion and correct the common c.-32-13T>G
GAA splicing variant in pompe disease. Mol Ther Nucleic
Acids. 7:90–100. 2017. View Article : Google Scholar : PubMed/NCBI
|