Introduction
Malignant glioma is the most frequent and lethal
type of primary tumor of the central nervous system (1–3).
Despite improvements in therapeutic technologies, the current
treatment strategies remain poorly effective. Increasing evidence
indicated that mesenchymal stem cells (MSCs) preferentially migrate
to and engraft into tumor sites and interact with tumor
microenvironments, which, in addition to their availability,
immunological compatibility and relative ease of in vitro
manipulation without the need for immortalization, indicates these
cells as the most attractive candidates for tumor therapy (4–6).
Although MSCs have high potential for application in
tumor therapy, a number of adverse effects have been demonstrated
in the context of their direct and indirect involvement in the
tumor microenvironment (6–9). In the tumor niche, MSCs interact with
tumor cells and may promote angiogenesis, tumor growth, migration,
invasion and metastasis (6–9). MSCs can also undergo malignant
transformation following long-term in vitro culture
(10). Furthermore, in tumor
microenvironment, MSCs can undergo malignant transformation,
through increased migration and invasion abilities, increased
proliferating capacity, and form tumors in immunocompromised mice
(7–9).
In our previous studies, it was demonstrated that
MSCs can undergo malignant transformation through migration and
invasion abilities, in vivo tumorigenesis and growth, with
S100B/advanced glycosylation end-product specific receptor serving
a role by activating the interleukin 6 (IL6)/signal transducer and
activator of transcription 3 (STAT3) signaling pathway (7–9).
However, in addition to tumor cells, numerous tumor immune cells,
including monocytes, macrophages, mast cells, microglia and
neutrophils, serve indispensable roles in the initiation and
progression of glioblastoma in the tumor microenvironment (10–12).
In the central nervous system, the presence of human
T helper (Th)17 lymphocytes and their deleterious role were
described in multiple sclerosis lesions (13). Liu et al (13) reported the expression of IL17 and
IL22 receptors on blood-brain barrier endothelial cells during
multiple sclerosis lesions and in experimental autoimmune
encephalomyelitis, a mouse model of multiple sclerosis. IL22, a
member of the IL10 cytokine family, is produced by a number of
subsets of lymphocytes, including γδ T cells, Th22 cells, Th17
cells, natural killer T cells, innate lymphoid cells and
CD8+ lymphocytes (14).
IL22 appears to act exclusively on non-hematopoietic cells,
expressing a heterodimer transmembrane complex composed of IL22RA1
and IL10RB subunits (15). IL22RA1
is almost entirely expressed on cells of non-hematopoietic origin
(16). The primary signaling
pathway downstream of IL22RA1 is the STAT3 cascade, which mediates
the majority of IL22-induced effects, including promotion of tumor
growth and metastasis, as well as inhibition of apoptosis (14). Furthermore, Seki et al
(17) demonstrated that IL22
attenuates double-stranded RNA-induced upregulation of programmed
death-ligand 1 in airway epithelial cells via a STAT3-dependent
mechanism. Thus, it has been concluded that in the glioma
microenvironment, the occurrence and development of glioma is not
only associated with glioma cells, but also involves IL22 secreted
by Th17 lymphocytes and other immune cells. It was hypothesized
that IL22 produced by immune cells would activate the STAT3 cascade
through interaction with IL22RA1, to promote the malignant
transformation of MSCs. Therefore, the characteristics of
transformed malignant MSCs and the mechanism underlying their
transformation were evaluated, thereby highlighting the safety
issues to be addressed prior to the clinical application of
MSCs.
Materials and methods
MSC isolation, culture, and
transfection
Male Sprague Dawley rats (n=40; 4-week-old; 40±10 g
each; from the Experimental Animal Center of Chongqing Medical
University, Chongqing, China) were kept at 23±3°C and 55±5%
humidity, with normal diet and regular drinking water. A 12/12 h
light/dark cycle used for all rats. The rats were euthanized
through intraperitoneal injection of a mixture solution of ketamine
(87.5 mg/kg) and xylazine (12.5 mg/kg), and the bone marrow
aspirates were separated and cultivated by the plastic adherence
method (18). All experiments using
rats were approved by the Medical Research Ethics Committee of
Chongqing Medical University for the Ethics of Animal Experiments
of Chongqing Medical University. MSCs were cultured in Dulbecco's
modified Eagle's medium (DMEM)/F12 containing 10% fetal bovine
serum (FBS; both from Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and incubated at 37°C in an atmosphere containing
5% CO2 and 95% humidity. At 70–80% confluence, the cells
were subcultured for four passages and harvested for phenotypic
characterization and differentiation as described previously
(13). MSC phenotypes were analyzed
with a flow cytometer (BD FACSCanto; BD Biosciences; Becton,
Dickinson and Company, Franklin Lakes, NJ, USA), according to the
subsequent protocol. Cells were trypsinized and incubated with
fluorescein isothiocyanate (FITC)-conjugated monoclonal anti-rat
cluster of differentiation 71 (CD71; 1:100; cat. no. 554890; BD
Biosciences; Becton, Dickinson and Company), CD90 (1:100; cat. no.
554894; BD Biosciences; Becton, Dickinson and Company), CD45
(1:100; cat. no. 561867; BD Biosciences; Becton, Dickinson and
Company) and phycoerythrin-conjugated mouse anti-rat CD34 (1:200;
cat. no. AM20322RP-N; OriGene Technologies, Inc., Rockville, MD,
USA).
Small-interfering RNA (siRNA) targeting rat STAT3
(si-STAT3) and the corresponding negative control (si-NC) were
designed and synthesized by Shanghai GeneBio Co., Ltd. (Shanghai,
China). For the transfection procedure, MSCs were grown to 80–90%
confluence and transfected with 25 nM si-STAT3 and 25 nM negative
control siRNA using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocols. The target mRNA sequence for si-STAT3 was
(5′-GGCAUAUGCAGCCAGCAA-3′) and si-NC was
(5′-UUUGCUGGCUGCAUAUGCC-3′). After incubation for 48 h, cells were
washed with PBS, harvested and subjected to proliferation, western
blot analysis, and invasion and migration assays.
Indirect co-culture of MSCs with rat
glioma C6 cells
Rat C6 glioma cells were obtained from Children's
Hospital Affiliated to Chongqing Medical University. MSCs were
indirectly co-cultured with glioma cells using Transwell chambers
(EMD Millipore, Billerica, MA, USA). A total of 3×105 C6
cells (in 200 µl DMEM/F12 supplemented with 10% FBS) were seeded
into the upper chamber of the Transwell plate (0.4 mm). An equal
number of MSCs (in 200 µl DMEM/F12 supplemented with 10% FBS) was
seeded in the lower part of the chamber. After 7 days of indirect
co-culture with C6 cells, the MSCs were collected for analysis.
MSCs cultured alone served as the control. The cells were imaged
under a confocal microscope (magnification, ×100; Eclipse Ti2;
Nikon Corporation, Tokyo, Japan).
Cell Counting Kit-8 (CCK-8) detection
of viability
Cell viability was assayed with CCK-8 (Chongqing
ATGene Pharmaceutical Technology Co., Ltd., Chongqing, China),
following the manufacturer's protocols. MSCs and C6 glioma cells
were seeded in 96-well plates (1×104 cells/well) and
cultured in 100 µl serum-free DMEM/F12 medium for 1, 2, 3, 4, 5, 6
and 7 days, or a 24 and 48 h incubation of MSCs with exogenous IL22
(Abcam, Cambridge, MA, USA) at 37°C. Subsequently, the cells were
incubated with 10% CCK-8 solution for 2 h at 37°C and the
absorbance value was measured at 450 nm using a microplate reader
(BioTek Instruments, Inc., Winooski, VT, USA). All measurements
were conducted with eight replicates and each experiment was
repeated at least three times.
Cell migration and invasion
For invasion and migration assays, 1×105
cells in serum-free DMEM/F12 medium were placed into the upper
chamber of a Transwell plate (24-well; 8-mm pore size) coated with
or without Matrigel, respectively. DMEM/F12 medium containing 10%
FBS was added to the lower chamber. After incubation for 24 h, the
cells remaining on the upper membrane were removed with cotton
wool. The cells that had migrated or invaded through the membrane
were fixed with 4% paraformaldehyde for 30 min at 37°C and stained
with 0.1% crystal violet for 4 h at 37°C (Beyotime Institute of
Biotechnology, Shanghai, China), imaged and counted using an
inverted microscope (magnification, ×100; Eclipse Ti2; Nikon
Corporation).
Cell cycle and flow cytometry
Cell cycle distribution was analyzed using a flow
cytometer (BD FACSCanto). Cells were pooled and placed in tubes at
a density of 1.6×105 cells/tube. The cells were fixed in
70% ethanol for 24 h at 4°C and washed three times with PBS.
Finally, the cell pellets were tested by Cell Cycle kit (Thermo
Fisher Scientific, Inc.), incubated with RNase (1 mg/ml; Thermo
Fisher Scientific, Inc.) and 400 µl propidium iodide solution (100
µl/ml; Thermo Fisher Scientific, Inc.) for 30 min at 37°C in the
dark and analyzed by flow cytometry using ModFit LT software
(version 3.2; Verity Software House, Inc., Topsham, ME, USA). Each
experiment was repeated at least three times.
ELISA
Secreted IL22 was measured in the supernatant of the
C6 glioma cell and MSC culture medium using an ELISA. The ELISA
(cat. no. E-EL-R2440c; Elabscience Biotechnology Co., Ltd., Wuhan,
China) was performed according to the manufacturer's protocols, and
the results were analyzed with SoftMax® Pro-5 (Molecular
Devices, LLC, Sunnyvale, CA, USA). Concentrations below the
detection limit (5 pg/ml) were considered undetectable.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted using an RNA extraction kit
(BioTeke Corporation, Beijing, China), and cDNA synthesis was
conducted with a PrimeScript™ RT Master Mix kit, according to the
manufacturer's protocols (Takara Biotechnology Co., Ltd., Dalian,
China). RT-qpcr was performed in three replicates using a
SYBR® Premix Ex Taq Kit (Takara Bio, Inc., Tokyo,
Japan), according to the manufacturer's protocol using
gene-specific primers, and products were measured on a CFX96
Real-time PCR system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The primers used were as follows: IL22RA1, forward,
5′-CTGTGGAGACCCGAAAC-3′, and reverse, 5′-GCACCCGAGAAGGAGT-3′. GAPDH
(forward, 5′-ACCACAGTCCATGCCATCAC-3′; and reverse,
5′-TCCACCACCCTGTTGCTGTA-3′) was used as the endogenous housekeeping
gene for normalization of mRNA levels. The results are expressed as
the mean of 2−∆∆Cq ± standard deviation (19). The PCR amplification was performed
as follows: 95°C for 5 min; 35 cycles of 95°C for 1 min, 60°C for 1
min and 72°C for 1 min; and then 72°C for 7 min.
Immunofluorescence staining
Cells were seeded in 24-well plates
(4×104 cells/well) DMEM/F12 medium containing 10% FBS
for 24 h at 37°C. Subsequently, they were then fixed with 4%
paraformaldehyde for 1 h at 37°C, permeabilized with 0.5% Triton
X-100, and blocked for 1 h at 37°C with 5% bovine serum albumin
(Gibco; Thermo Fisher Scientific, Inc.). Following this, the cells
were incubated overnight at 4°C with the primary antibodies.
Primary antibodies against STAT3 (cat. no. 12640; 1:200),
phospho(p)-STAT3 (cat. no. 9134; 1:200), B-cell lymphoma-extra
large (Bcl-xL, cat. no. 2764; 1:200) and cyclin D1 (cat. no. 2922;
1:200) were obtained from Cell Signaling Technology, Inc. (Danvers,
MA, USA). Following a PBS wash, the cells were incubated with FITC
(cat. no. S0008) or Cy3 (cat. no. S0011) conjugated goat
anti-rabbit IgG secondary antibodies for an additional 2 h at 37°C
before being washed with PBS again at 37°C three times in the dark.
Secondary antibodies were also obtained from Affinity (1:200;
Affinity Biosciences, Cambridge, UK). Subsequently, 20 µl
4′,6-diamidino-2-phenylindole (DAPI; Beyotime Institute of
Biotechnology) was added to stain the nuclei at 37°C for 1 h.
Images were obtained with a A1R Confocal microscopy system
(magnification, ×100; Nikon Corporation) and analyzed with Nikon
NIS-element AR 4.0 software (Nikon Corporation).
Western blot analysis
Cell lysates (Whole Protein Extraction kit; Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China) were collected and a
Bicinchoninic Acid protein assay (Nanjing KeyGen Biotech Co., Ltd.)
was performed to determine protein concentrations. Proteins (40 µg)
were separated on 8% SDS-PAGE and then transferred onto
polyvinylidene fluoride membranes (EMD Millipore). Subsequently,
the polyvinylidene fluoride membranes were blocked for 1 h in TBS
containing 0.1% Tween-20 at room temperature with 5% fat-free milk.
The following commercial antibodies were used to incubate membranes
overnight at 4°C: STAT3 (cat. no. 12640; 1:1,000; Cell Signaling
Technology, Inc.), p-STAT3 (cat. no. 9134; 1:1,000; Cell Signaling
Technology, Inc.), cyclin D1 (cat. no. 2922; 1:1,000; Cell
Signaling Technology, Inc.), and Bcl-xL (cat. no. 2764; 1:1,000;
Cell Signaling Technology, Inc.) and GAPDH (cat. no. T0004;
1:5,000; Affinity Biosciences). Subsequently, membranes were
incubated with goat anti-rabbit IgG horseradish
peroxidase-conjugated secondary antibodies (1:5,000; cat. no.
SA00001-2; ProteinTech Group, Inc., Chicago, IL, USA) for 2 h at
37°C. Blots were developed with the chemiluminescent detection
method by enhanced chemiluminescence Prime Western Blotting
Detection reagent (cat. no. KGP1127; Nanjing KeyGen Biotech Co.,
Ltd). The band intensity of western blotting was measured by
densitometry using the Quantity One software v4.6.7 (Bio-Rad
Laboratories). The protein levels were normalized to the protein
level of GAPDH, which was used as a loading control.
Bioinformatic analysis of the
expression of IL22 and IL22RA1 in glioblastoma
Heat maps of IL22 and IL22RA1 expression were
identified using The Cancer Genome Atlas-Glioblastoma Multiforme
(TCGA-GBM) data with the University of California, Santa Cruz
(UCSC) Cancer Genomics Browser (http://xena.ucsc.edu/) (20–24).
Statistical analysis
SPSS 20.0 software (IBM Corp., Armonk, NY, USA) was
used to analyze data with independent sample Student's t-test or
one-way analysis of variance analysis of variance test with post
hoc contrasts by Student-Newman-Keuls test. All values are reported
as mean ± standard deviation of the mean. P<0.05 was considered
to indicate a statistically significant difference.
Results
Identification of MSCs
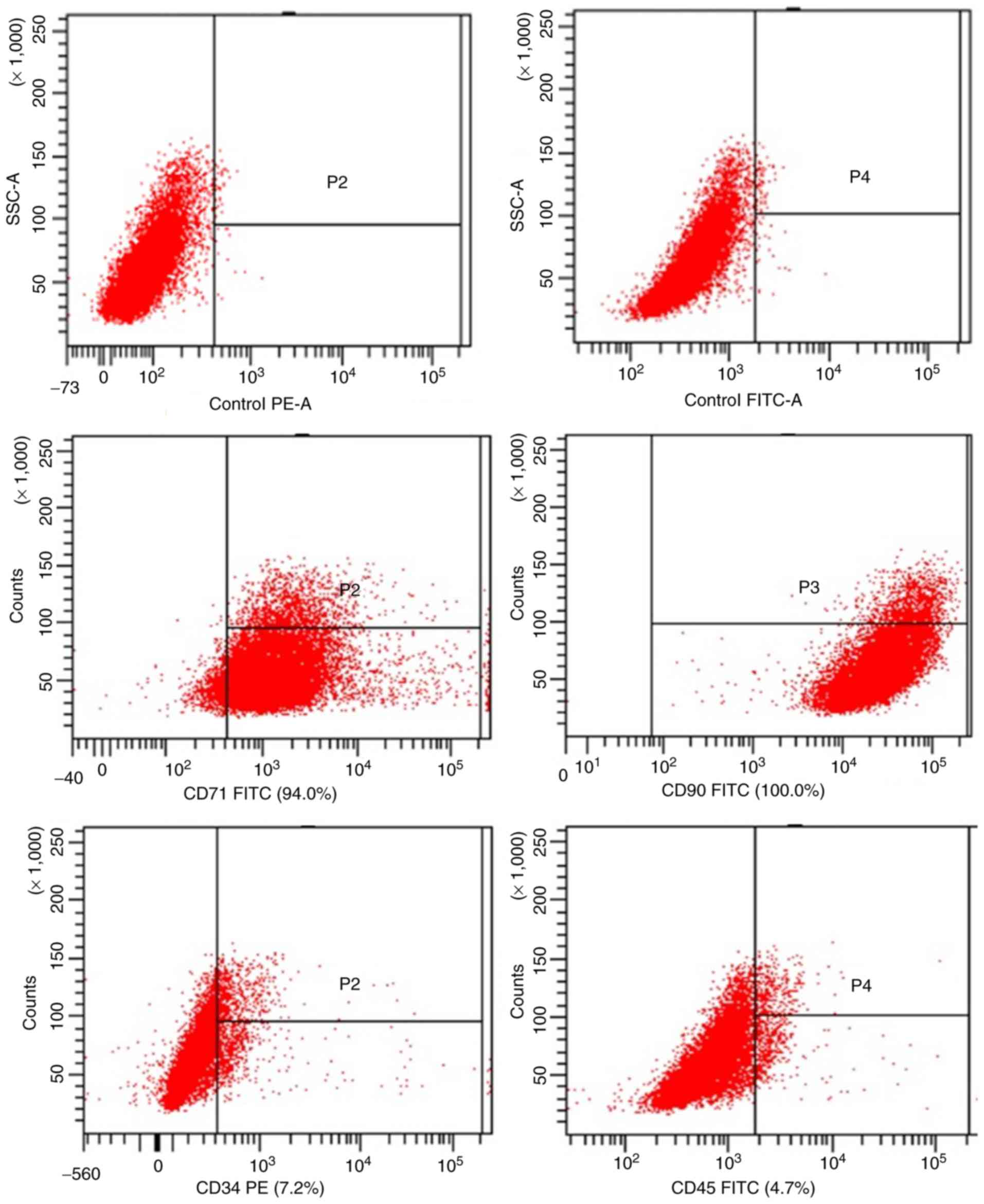
To confirm that the cells used in the present study
were MSCs, their phenotype was determined by measuring the specific
cell surface markers CD71, CD90, CD34 and CD45. As depicted in
Fig. 1, flow cytometric analyses
revealed that the primary cultured MSCs were positive for CD7 and
CD90 while negative for CD34 and CD45, indicating that they were
MSCs.
Malignant transformation of MSCs in
the simulated tumor microenvironment
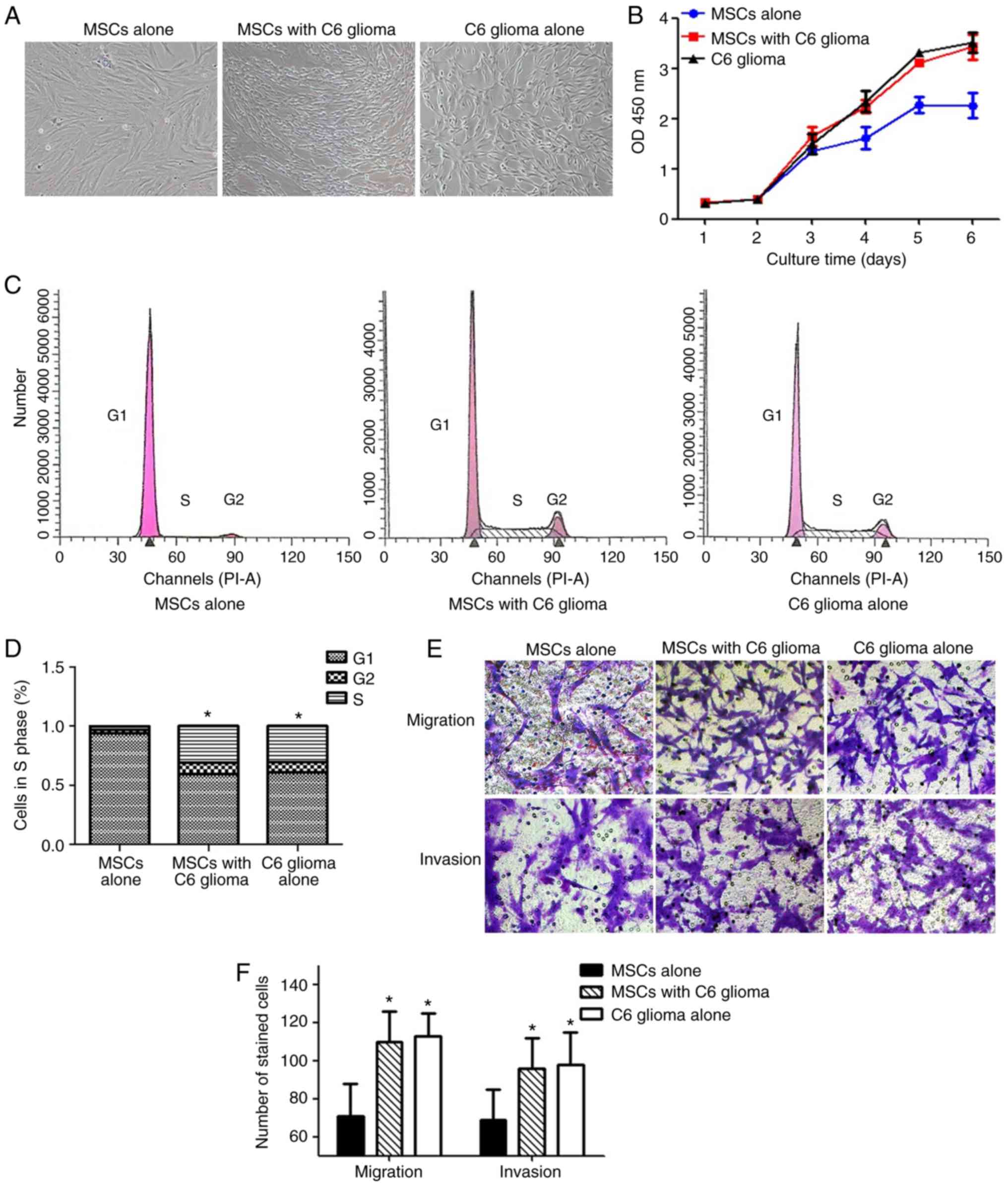
Following long-term exposure to the tumor
microenvironment, the MSCs exhibited numerous biological
characteristics of C6 glioma cells, including altered cell
morphology, and increased proliferation and invasion/migration
ability. As depicted in Fig. 2A,
the MSCs exhibited a typical long fusiform morphology, whirlpool
and orderly arrangement. However, following long-term indirect
co-culture with C6 glioma cells, the MSCs became thinner and longer
with reduced cytoplasm around the nucleus, and an increased
nuclear/cytoplasmic ratio. Therefore, they exhibited a similar
shape to that of C6 glioma cells. Furthermore, MSCs co-cultured
with C6 glioma cells exhibited an increased proliferation rate,
compared with normal MSCs from day 4–6 (Fig. 2B). The flow cytometry results
demonstrated that the percentage of MSCs in the G2/S phase was
significantly increased following co-culture with C6 glioma cells,
compared with the control group, indicating that cell proliferation
was increased (P<0.05; Fig. 2C and
D). Furthermore, MSCs co-cultured with C6 glioma cells
exhibited a significantly increased migration and invasion
capacity, compared with normal MSCs (both P<0.05; Fig. 2E and F).
Bioinformatics analysis of the
expression of IL22 and IL22RA1 in glioblastoma
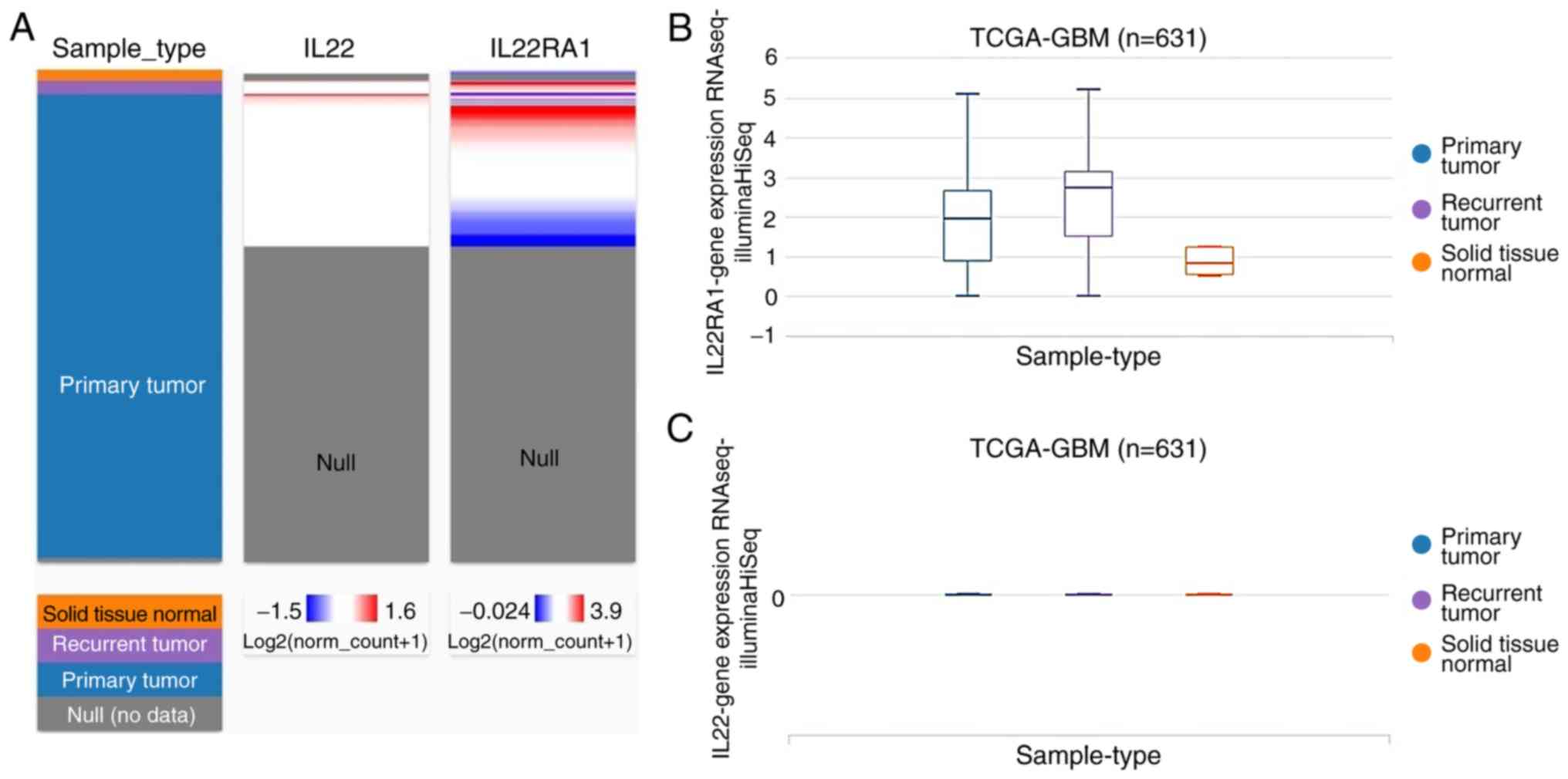
The expression profile of IL22 and IL22RA1 in
TCGA-GBM was analyzed using the UCSC Cancer Genomics Browser
(Fig. 3). The heatmap and the
corresponding box plots depict that primary and recurrent
glioblastomas have increased IL22RA1, expression compared with
normal tissue (Fig. 3A and B),
whereas the expression of IL22 was low in glioblastoma and normal
tissues (Fig. 3A and C).
Expression of IL22RA1, not IL22, is
increased in MSCs co-cultured with C6 glioma cells
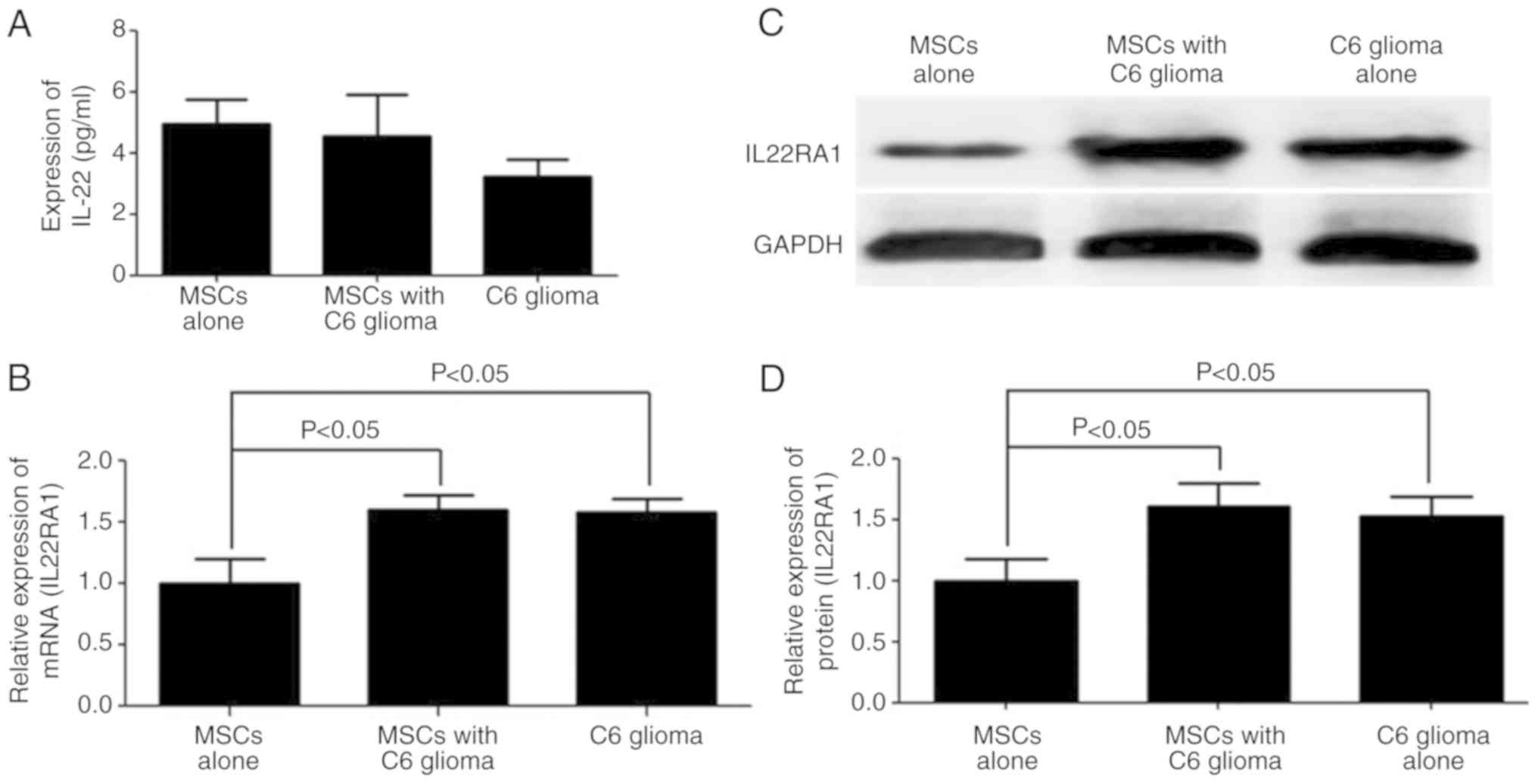
Hepatocyte growth factor, IL6 and S100B are highly
secreted in MSCs co-cultured with C6 glioma cells, as demonstrated
in previous studies (7–9). However, other cytokines may affect the
MSCs phenotype. Thus, an ELISA was performed to detect the
expression level of IL22, which is primarily produced by immune
cells (17). IL22 was not detected
(<5 pg/ml) in the culture supernatant of C6 glioma cells or
MSCs, which is consistent with previous studies (25,26)
(Fig. 4A). However, the mRNA and
protein expression levels of IL22RA1 were significantly increased
in the MSCs co-cultured with C6 glioma cells (Fig. 4B-D).
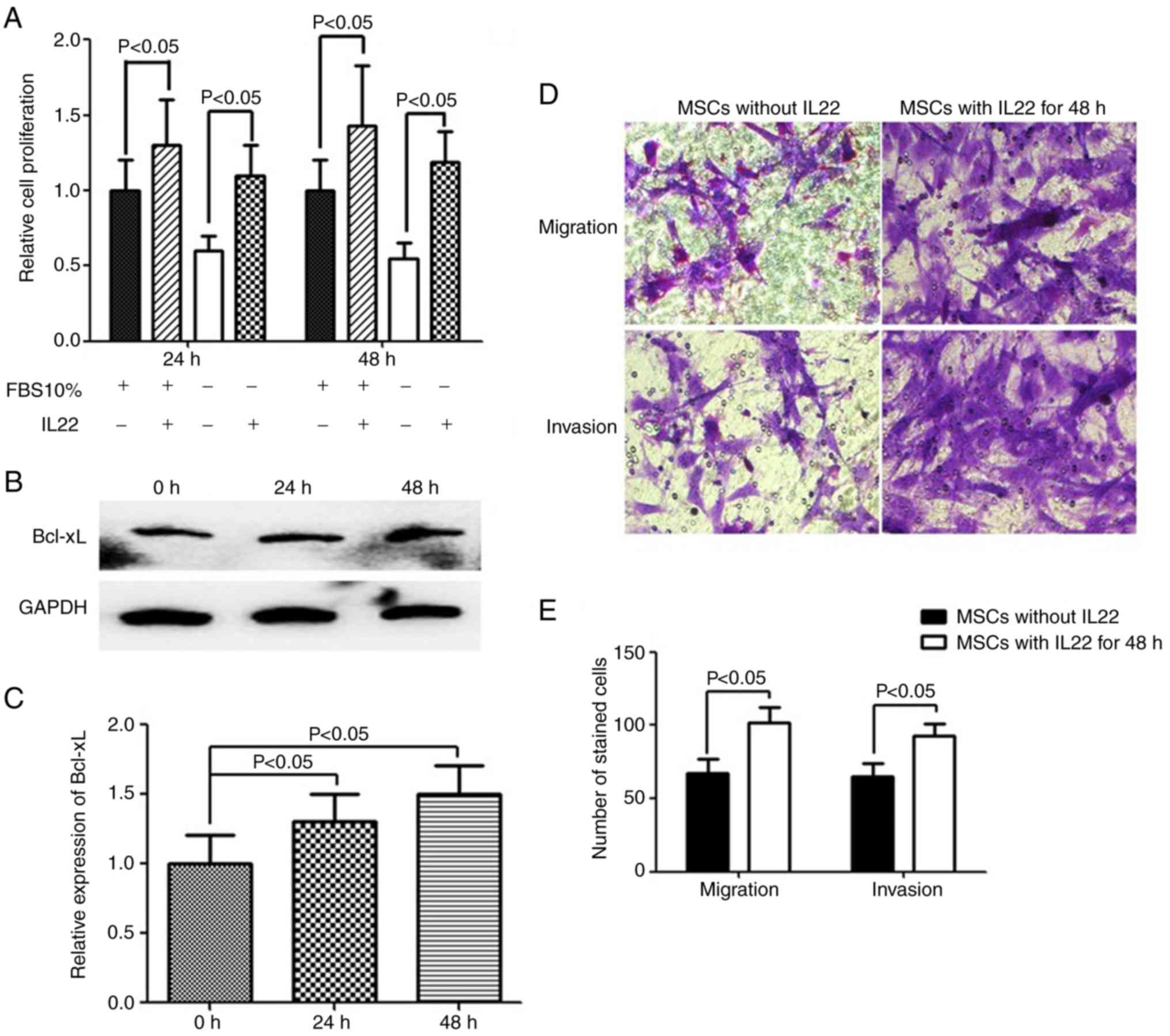
IL22 induces MSC proliferation,
migration and invasion
Since IL22RA1 was expressed by MSCs and its
expression was significantly increased following co-culture with C6
glioma cells, the biological functions of IL22 were investigated.
Therefore, proliferation assays were performed with exogenous IL22
in FBS-free or 10% FBS DMEM/F12 medium. A 24 and 48 h incubation of
MSCs with exogenous IL22 induced cell proliferation, as assessed
with a CCK-8 assay (Fig. 5A).
Previous studies revealed activation of the anti-apoptotic protein
Bcl-xL by IL22 in glioblastoma (26), hepatocarcinoma (27) and lung cancer (20) cells. Therefore, the association
between Bcl-xL protein and IL22 in MSCs was examined. The results
revealed that IL22 increased the expression of Bcl-xL (Fig. 5B and C), which is consistent with
previous studies. Furthermore, MSCs incubated with IL22 exhibited
significantly increased migration and invasion capacity, compared
with normal MSCs (P<0.05; Fig. 5D
and E).
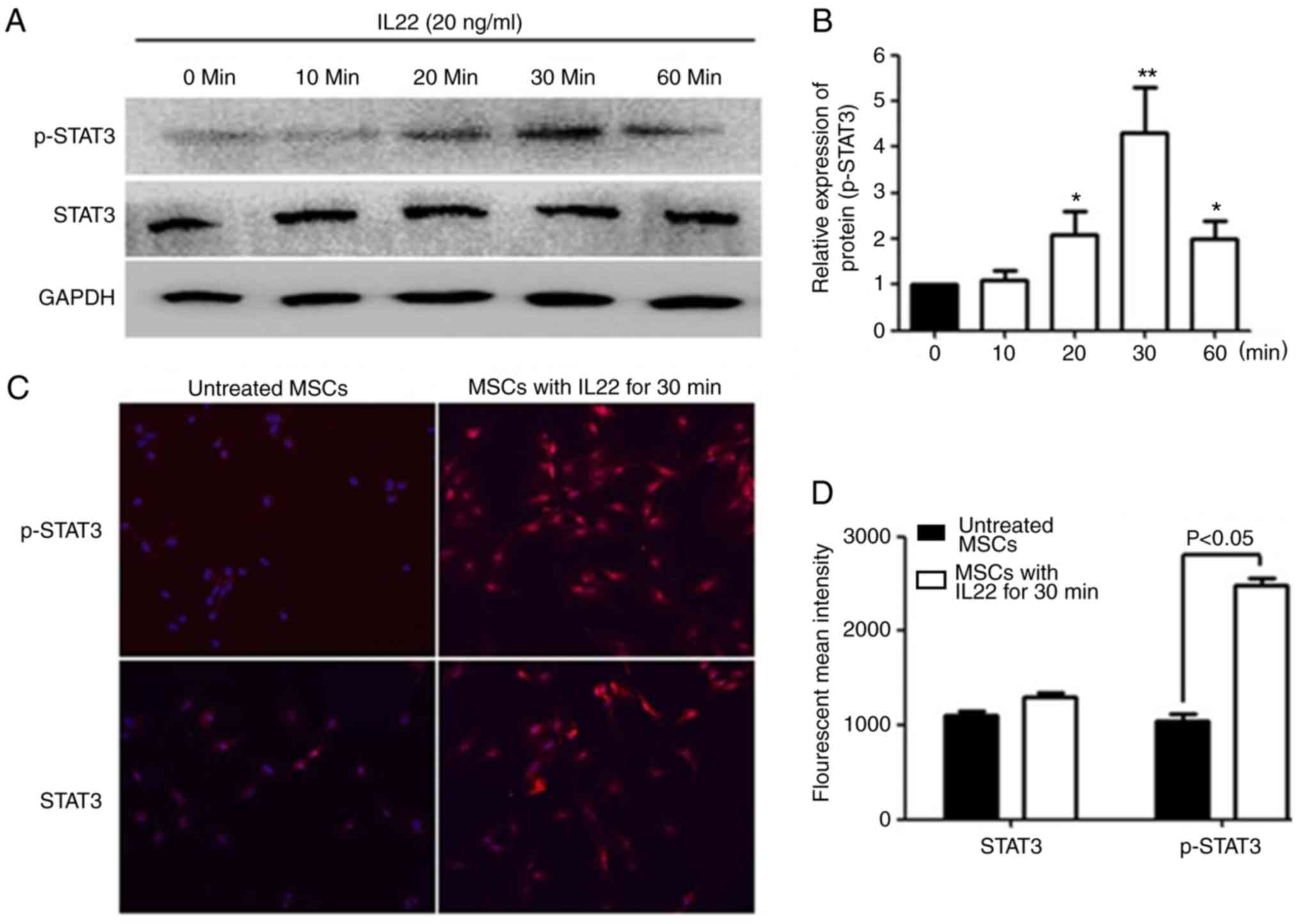
IL22 triggers phosphorylation of STAT3
in MSCs
To determine the signal transduction pathway induced
by IL22R activation, western blot analysis was performed to measure
the normal and phosphorylated forms of STAT3 (Tyr-705) in MSCs
following IL22 simulation. The results demonstrated that IL22
activated STAT3 phosphorylation in MSCs, with the peak detected at
30 min (4.3-fold increase) (Fig. 6A and
B). To further confirm the IL22 induction of STAT3
phosphorylation, the normal and phosphorylated forms of STAT3 were
examined with immunofluorescence staining (Fig. 6C and D). The results indicated
p-STAT3 nuclear localization in MSCs after 30 min of treatment,
demonstrating that IL22 induced STAT3 activation and nuclear
translocation.
Effect of inhibition of STAT3
To investigate the role of STAT3 pathway activation
in MSCs under IL22 treatment, MSCs treated with IL22 were
transfected with si-STAT3 or non-targeting control siRNA. Analyses
using western blotting (Fig. 7A and
B) and immunofluorescence (Fig. 7C
and D) indicated that the expression levels of STAT3, p-STAT3,
cyclin D1 and Bcl-xL were significantly decreased in the si-STAT3
treatment group, compared with the control siRNA treatment group or
the without siRNA treatment group (P<0.05). Furthermore,
inhibition of STAT3 signaling by si-STAT3 significantly decreased
the IL22 simulation-induced increase in MSC proliferation,
demonstrating that IL22 promoted the proliferation ability of MSCs
via STAT3 signaling (Fig. 7E).
Additionally, si-STAT3 significantly inhibited the migration and
invasion ability of MSCs under IL22 stimulation, indicating that
IL22 also promotes MSC migration and invasion through STAT3
signaling (Fig. 7F and G).
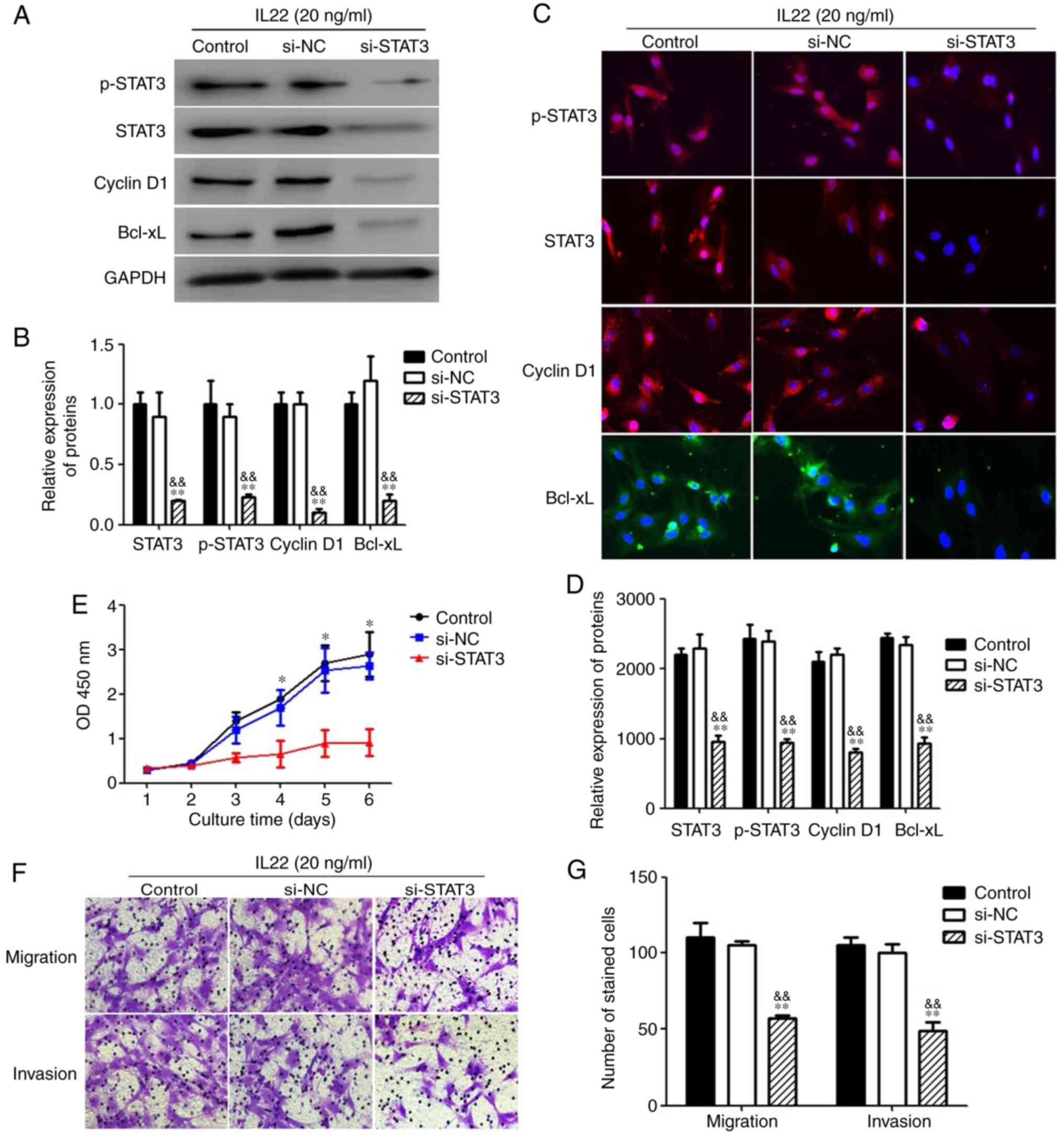 | Figure 7.The suppression of upregulation of
proliferation, migration and invasion by IL22 via STAT3 in MSCs.
(A) MSCs treated with IL22 (20 ng/ml) were transfected with
si-STAT3 (20 nM/well), non-specific siRNA (si-NC) or vehicle alone
for 48 h. After transfection, total cell lysates were assayed for
STAT3, p-STAT3, Cyclin D1 and Bcl-xL western blot analysis. (B)
Histogram of STAT3, p-STAT3, Cyclin D1 and Bcl-xL protein
expression in three groups. (C) The immunofluorescence staining
depicts the significant downregulation of STAT3, p-STAT3, Cyclin D1
and Bcl-xL in MSCs treated with IL22 after transfection with
si-STAT3. (D) The proliferation ability was measured with a Cell
Counting Kit-8 assay. (E) The migration and invasion abilities were
measured with Transwell and Matrigel assays. (F) Histogram of
migration and invasion in two groups. **P<0.01, compared with
the si-NC group; &&P<0.01, compared with the
control group. MSCs, mesenchymal stem cells; p-STAT3,
phospho-signal transducer and activator of transcription 3; IL22,
interleukin 22; siRNA, small interfering RNA; Bcl-xL, B-cell
lymphoma-extra large; NC, negative control; OD, optical
density. |
Discussion
Bone marrow-derived mesenchymal stem cells (MSCs)
exhibit potent tumoritropic migratory properties that render them
attractive for use as targeted-delivery vehicles in tumor treatment
(6,28–35).
Numerous experimental studies have confirmed the antitumor
potential of MSCs modified with therapeutic genes and/or loaded
with chemotherapeutic drugs (21–23).
Thus, modified MSCs are a promising approach to deliver therapeutic
agents to tumor niches. However, a number of contradictory reports
and arguments indicated that MSCs can exert various adverse effects
when they enter the tumor microenvironment (7–9,24).
Chen et al (7) determined
that MSCs can promote tumor progression on bladder cancer model. Xu
et al (8) demonstrated that
mesenchymal-stem-cell-secreted interleukin (IL)-6 enhances
resistance to cisplatin via the STAT3 pathway in breast cancer.
Furthermore, mesenchymal stem cell-derived IL-8 could promote
osteosarcoma cell anoikis resistance and pulmonary metastasis
(9). Thus, further research is
required to improve the safety of this approach. Previously, it was
demonstrated that MSCs directly or indirectly co-cultured with C6
glioma cells have a risk of malignant transformation and that this
process may be mediated by S100B and IL6 secreted by C6 glioma
cells through activation of the STAT3 pathway (7–9).
However, the mechanism underlying the transformation of MSCs
remains poorly understood.
IL22 is an effector cytokine that serves a major
role in the regulation of inflammatory responses in a variety of
tissues, including colorectal carcinogenesis, lung cancer and
gastric cancer (36–39). The majority of cancer types,
including gliomas, are associated with inflammation (25). Furthermore, a functional role of
IL22 in carcinogenesis has been reported in colorectal, stomach and
lung carcinoma, glioblastoma and hepatocarcinoma (25–29).
In the present study, a gene expression database was investigated
with clearly defined parameters distinguishing cancer and normal
tissues. Analysis using the TCGA-GBM database revealed increased
IL22RA1 mRNA expression in glioblastoma, compared with
corresponding normal tissue. Investigation of the expression of
IL22 and IL22RA1 in MSCs and C6 glioma cells failed to determine
IL22 in the culture supernatant of C6 glioma cells or MSCs.
However, the expression of IL22RA1 mRNA and protein was
significantly increased in MSCs co-cultured with C6 glioma cells,
compared with MSCs alone, indicating that the malignant
transformation of MSCs is associated with the IL22/IL22RA1
signaling axis and that IL22 may be derived from immune cells in
the tumor microenvironment. Thus, to clarify whether IL22 promotes
the malignant transformation of MSCs, MSCs were stimulated with
exogenous IL22 in vitro. The results demonstrated that MSCs
incubated with IL22 had increased Bcl-xL expression, proliferation,
migration and invasion, compared with normal MSCs.
Previous studies indicated that IL22 may activate
STAT3 signaling in different types of cells (26,30,31).
In this regard, it was determined that STAT3 signaling pathway is
activated by IL22 in MSCs and revealed that STAT3 phosphorylation
is enhanced in MSCs following IL22 simulation. Accumulating
evidence indicates that IL22 can promote cell proliferation and
anti-apoptosis via STAT3 signaling (26,37–43).
Inhibition of STAT3 signaling by si-STAT3 suppressed the
IL22-induced increase of cell proliferation, migration and
invasion. Based on the aforementioned results, it was considered
that the extracellular IL22/IL22RA1 interaction is responsible for
the activation of STAT3 in the malignant transformation of MSCs,
with activated STAT3 serving an important role in the malignant
transformation of MSCs in the tumor microenvironment.
In contrast, C6 glioma cells and MSCs did not
secrete IL22 but did express a functional IL22 receptor (IL22RA1).
The expression of IL22 was significantly increased during the
malignant transformation of MSCs, indicating that IL2 could be
provided by microenvironmental cells. In addition to tumor cells,
immune cells, extracellular matrix, blood vessels and cytokines
constitute key parts of the glioblastoma tumor microenvironment,
which can be advantageous for tumor proliferation (44). Furthermore, Th17 cell invasion has
been reported in an experimental mouse model of malignant glioma as
well as in human glioma (45).
Therefore, it may be considered that immunocompetent cells can
interact with MSCs and secrete IL22 to promote the malignant
transformation of MSCs. Combined with our previous findings
(17,26,27),
the present study indicates that IL6 secreted by C6 glioma cells
may share signaling pathways with IL22, which serve a synergistic
role in promoting the malignant transformation of MSCs.
In the present study, the aim was to investigate the
effect and mechanism of IL22 upregulation in cell-based experiments
in vitro as a preliminary investigation. However, animal
studies on IL22RA1 upregulation and knockout or inhibition could
better reveal the association between IL22/IL22RA1 and the
malignant transformation of MSCs, and thus should be performed in
future studies.
Acknowledgements
The authors would like to thank Professor Yi Luo of
the Department of Urology, University of Iowa (Iowa, USA) for his
technical support.
Funding
The present study was partially supported by a grant
from the National Natural Science Foundation of China (grant no.
81670270) to JZ and the Scientific Research Project of Shanxi
Provincial Department of health (grant no. 201601070) to XC.
Availability of data and materials
All data generated or analyzed during this study are
included in the published article.
Authors' contributions
XC and JZ conducted the data gathering, data
analyses and figure/table preparations. XJ, QY, ZX, BT, JT and JZ
provided material input, data analysis and assisted with revising
the manuscript. JZ supervised the experimental design and
manuscript writing. All authors read and approved the final
manuscript and agree to be accountable for all aspects of the
research in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
All experiments using rats were approved by the
Medical Research Ethics Committee of Chongqing Medical University
for the Ethics of Animal Experiments of Chongqing Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Noh H, Zhao Q, Yan J, Kong LY,
Gabrusiewicz K, Hong S, Xia X, Heimberger AB and Li S: Cell surface
vimentin-targeted monoclonal antibody 86C increases sensitivity to
temozolomide in glioma stem cells. Cancer Lett. 433:176–185. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yan L, Cai K, Sun K, Gui J and Liang J:
MiR-1290 promotes proliferation, migration, and invasion of glioma
cells by targeting LHX6. J Cell Physiol. 233:6621–6629.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang K, Huang R, Li G, Zeng F, Zhao Z, Liu
Y, Hu H and Jiang T: CKAP2 expression is associated with glioma
tumor growth and acts as a prognostic factor in highgrade glioma.
Oncol Rep. 40:2036–2046. 2018.PubMed/NCBI
|
|
4
|
Sun Z, Wang S and Zhao RC: The roles of
mesenchymal stem cells in tumor inflammatory microenvironment. J
Hematol Oncol. 7:142014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Razmkhah M, Abtahi S and Ghaderi A:
Mesenchymal stem cells, immune cells and tumor cells cross talk: A
sinister triangle in the tumor microenvironment. Curr Stem Cell Res
Ther. 14:43–51. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu S, De Veirman K, De Becker A,
Vanderkerken K and Van Riet I: Mesenchymal stem cells in multiple
myeloma: A therapeutical tool or target? Leukemia. 32:1500–1514.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen J, Ma L, Zhang N, Zhu Y, Zhang K, Xu
Z and Wang Q: Mesenchymal stem cells promote tumor progression via
inducing stroma remodeling on rabbit VX2 bladder tumor model. Int J
Biol Sci. 14:1012–1021. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu H, Zhou Y, Li W, Zhang B, Zhang H, Zhao
S, Zheng P, Wu H and Yang J: Tumor-derived
mesenchymal-stem-cell-secreted IL-6 enhances resistance to
cisplatin via the STAT3 pathway in breast cancer. Oncol Lett.
15:9142–9150. 2018.PubMed/NCBI
|
|
9
|
Du L, Han XG, Tu B, Wang MQ, Qiao H, Zhang
SH, Fan QM and Tang TT: CXCR1/Akt signaling activation induced by
mesenchymal stem cell-derived IL-8 promotes osteosarcoma cell
anoikis resistance and pulmonary metastasis. Cell Death Dis.
9:7142018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rosland GV, Svendsen A, Torsvik A, Sobala
E, McCormack E, Immervoll H, Mysliwietz J, Tonn JC, Goldbrunner R,
Lønning PE, et al: Long-term cultures of bone marrow-derived human
mesenchymal stem cells frequently undergo spontaneous malignant
transformation. Cancer Res. 69:5331–5339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tan B, Shen L, Yang K, Huang D, Li X, Li
Y, Zhao L, Chen J, Yi Q, Xu H, et al: C6 glioma-conditioned medium
induces malignant transformation of mesenchymal stem cells:
Possible role of S100B/RAGE pathway. Biochem Biophys Res Commun.
495:78–85. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cui X, Liu J, Bai L, Tian J and Zhu J:
Interleukin-6 induces malignant transformation of rat mesenchymal
stem cells in association with enhanced signaling of signal
transducer and activator of transcription 3. Cancer Sci. 105:64–71.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu J, Zhang Y, Bai L, Cui X and Zhu J:
Rat bone marrow mesenchymal stem cells undergo malignant
transformation via indirect co-cultured with tumour cells. Cell
Biochem Funct. 30:650–656. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morisse MC, Jouannet S, Dominguez-Villar
M, Sanson M and Idbaih A: Interactions between tumor-associated
macrophages and tumor cells in glioblastoma: Unraveling promising
targeted therapies. Expert Rev Neurother. 18:729–737. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Broekman ML, Maas SL, Abels ER, Mempel TR,
Krichevsky AM and Breakefield XO: Multidimensional communication in
the microenvirons of glioblastoma. Nat Rev Neurol. 14:482–495.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mukherjee S, Fried A, Hussaini R, White R,
Baidoo J, Yalamanchi S and Banerjee P: Phytosomal curcumin causes
natural killer cell-dependent repolarization of glioblastoma (GBM)
tumor-associated microglia/macrophages and elimination of GBM and
GBM stem cells. J Exp Clin Cancer Res. 37:1682018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He W, Wu J, Shi J, Huo YM, Dai W, Geng J,
Lu P, Yang MW, Fang Y, Wang W, et al: IL22RA1/STAT3 signaling
promotes stemness and tumorigenicity in pancreatic cancer. Cancer
Res. 78:3293–3305. 2018.PubMed/NCBI
|
|
18
|
Xie XJ, Di TT, Wang Y, Wang MX, Meng YJ,
Lin Y, Xu XL, Li P and Zhao JX: Indirubin ameliorates
imiquimod-induced psoriasis-like skin lesions in mice by inhibiting
inflammatory responses mediated by IL-17A-producing gd T cells. Mol
Immunol. 101:386–395. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Endam LM, Bossé Y, Filali-Mouhim A,
Cormier C, Boisvert P, Boulet LP, Hudson TJ and Desrosiers M:
Polymorphisms in the interleukin-22 receptor alpha-1 gene are
associated with severe chronic rhinosinusitis. Otolaryngol Head
Neck Surg. 140:741–747. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shall G, Menosky M, Decker S, Nethala P,
Welchko R, Leveque X, Lu M, Sandstrom M, Hochgeschwender U,
Rossignol J, et al: Effects of passage number and differentiation
protocol on the generation of dopaminergic neurons from rat bone
marrow-derived mesenchymal stem cells. Int J Mol Sci. 19(pii):
E7202018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li H and Chen C: Quercetin Has
Antimetastatic Effects on Gastric Cancer Cells via the Interruption
of uPA/uPAR Function by Modulating NF-κb, PKC-δ, ERK1/2, and AMPKα.
Integr Cancer Ther. 17:511–523. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen D, Zou J, Zong Y, Meng H, An G and
Yang L: Anti-human CD138 monoclonal antibodies and their bispecific
formats: Generation and characterization. Immunopharmacol
Immunotoxicol. 38:175–183. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liakou E, Mavrogonatou E, Pratsinis H,
Rizou S, Evangelou K, Panagiotou PN, Karamanos NK, Gorgoulis VG and
Kletsas D: Ionizing radiation-mediated premature senescence and
paracrine interactions with cancer cells enhance the expression of
syndecan 1 in human breast stromal fibroblasts: The role of TGF-β.
Aging. 8:1650–1669. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Boniface K, Guignouard E, Pedretti N,
Garcia M, Delwail A, Bernard FX, Nau F, Guillet G, Dagregorio G,
Yssel H, et al: A role for T cell-derived interleukin 22 in
psoriatic skin inflammation. Clin Exp Immunol. 150:407–415. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Akil H, Abbaci A, Lalloué F, Bessette B,
Costes LM, Domballe L, Charreau S, Guilloteau K, Karayan-Tapon L,
Bernard FX, et al: IL22/IL-22R pathway induces cell survival in
human glioblastoma cells. PLoS One. 10:e01198722015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang R, Tan Z, Deng L, Chen Y, Xia Y, Gao
Y, Wang X and Sun B: Interleukin-22 promotes human hepatocellular
carcinoma by activation of STAT3. Hepatology. 54:900–909. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang W, Chen Y, Wei H, Zheng C, Sun R,
Zhang J and Tian Z: Antiapoptotic activity of autocrine
interleukin-22 and therapeutic effects of interleukin-22-small
interfering RNA on human lung cancer xenografts. Clin Cancer Res.
14:6432–6439. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Corsten MF and Shah K: Therapeutic
stem-cells for cancer treatment: Hopes and hurdles in tactical
warfare. Lancet Oncol. 9:376–384. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qian J, Hu Y, Zhao L, Xia J, Li C, Shi L
and Xu F: Protective role of adipose-derived stem cells in
Staphylococcus aureus-induced lung injury is mediated by
RegIIIγ secretion. Stem Cells. 34:1947–1956. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu F, Hu Y, Zhou J and Wang X: Mesenchymal
stem cells in acute lung injury: Are they ready for translational
medicine? J Cell Mol Med. 17:927–935. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ahn Jo, Lee Hw, Seo Kw, Kang Sk, Ra Jc and
Youn Hy: Anti-tumor effect of adipose tissue derived-mesenchymal
stem cells expressing interferon-β and treatment with cisplatin in
a xenograft mouse model for canine melanoma. PLoS One.
8:e748972013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu G, Guo Y, Seng Z, Cui G and Qu J: Bone
marrow-derived mesenchymal stem cells co-expressing interleukin-18
and interferon-β exhibit potent antitumor effect against
intracranial glioma in rats. Oncol Rep. 34:1915–1922. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Studeny M, Marini FC, Champlin RE,
Zompetta C, Fidler IJ and Andreeff M: Bone marrow-derived
mesenchymal stem cells as vehicles for interferon-beta delivery
into tumors. Cancer Res. 62:3603–3608. 2002.PubMed/NCBI
|
|
35
|
He X, Li B, Shao Y, Zhao N, Hsu Y, Zhang Z
and Zhu L: Cell fusion between gastric epithelial cells and
mesenchymal stem cells results in epithelial-to-mesenchymal
transition and malignant transformation. BMC Cancer. 15:242015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Leyva-Castillo JM, Yoon J and Geha RS:
IL-22 promotes allergic airway inflammation in epicutaneously
sensitized mice. J Allergy Clin Immunol. Jun 18–2018.(Epub ahead of
print). pii: S0091-6749(18)30856-X. doi:
10.1016/j.jaci.2018.05.032. PubMed/NCBI
|
|
37
|
Khare V, Paul G, Movadat O, Frick A,
Jambrich M, Krnjic A, Marian B, Wrba F and Gasche C: IL10R2
overexpression promotes IL22/STAT3 signaling in colorectal
carcinogenesis. Cancer Immunol Res. 3:1227–1235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shen Z, Ye Y, Kauttu T, Seppänen H,
Vainionpää S, Wang S, Mustonen H and Puolakkainen P: The novel
focal adhesion gene kindlin-2 promotes the invasion of gastric
cancer cells mediated by tumor-associated macrophages. Oncol Rep.
29:791–797. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Khosravi N, Caetano MS, Cumpian AM, Unver
N, De la Garza Ramos C, Noble O, Daliri S, Hernandez BJ, Gutierrez
BA, Evans SE, et al: IL22 promotes Kras-Mutant lung cancer by
induction of a protumor immune response and protection of stemness
properties. Cancer Immunol Res. 6:788–797. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen E, Cen Y, Lu D, Luo W and Jiang H:
IL-22 inactivates hepatic stellate cells via downregulation of the
TGF-β1/Notch signaling pathway. Mol Med Rep. 17:5449–5453.
2018.PubMed/NCBI
|
|
41
|
Yeste A, Mascanfroni ID, Nadeau M, Burns
EJ, Tukpah AM, Santiago A, Wu C, Patel B, Kumar D and Quintana FJ:
IL-21 induces IL-22 production in CD4+ T cells. Nat Commun.
5:37532014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guo X, Qiu J, Tu T, Yang X, Deng L, Anders
RA, Zhou L and Fu YX: Induction of innate lymphoid cell-derived
interleukin-22 by the transcription factor STAT3 mediates
protection against intestinal infection. Immunity. 40:25–39. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang B, Xie S, Su Z, Song S, Xu H, Chen
G, Cao W, Yin S, Gao Q and Wang H: Heme oxygenase-1 induction
attenuates imiquimod-induced psoriasiform inflammation by negative
regulation of Stat3 signaling. Sci Rep. 6:211322016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu VF, Yang J, Lebrun DG and Li M:
Understanding the role of cytokines in Glioblastoma Multiforme
pathogenesis. Cancer Lett. 316:139–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wainwright DA, Sengupta S, Han Y, Ulasov
IV and Lesniak MS: The presence of IL-17A and T helper 17 cells in
experimental mouse brain tumors and human glioma. PLoS One.
5:e153902010. View Article : Google Scholar : PubMed/NCBI
|