Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
sixth most common malignant tumor worldwide, accounting for 7% of
all malignant tumors (1).
Hypopharyngeal squamous cell carcinoma (HSCC) is one of the
subtypes of HNSCC, accounting for ~2–6% of HNSCC cases (2–4). The
most common pathological site of HSCC is the pyriform sinus,
followed by the postcricoid region and the posterior pharyngeal
wall. Since the primary tumor location of HSCC is rather hidden,
most of the cancer cells grow in an infiltrative manner and can
easily invade into the submucosal layer; thus, early symptoms in
HSCC patients are not typical. The majority of HSCC patients are
diagnosed at an advanced stage, after cervical lymph node
metastasis, which makes HSCC the malignant tumor with the worst
treatment outcome among HNSCC subtypes. Although the treatment
efficacy of surgery and chemoradiotherapy for HSCC has improved in
recent years, 50% of patients still experience relapse 1 year later
(3,5); additionally, the 5-year survival rate
of patients has not significantly improved (2,6). To
date, no specific and/or sensitive biomarker can accurately predict
HSCC prognosis after surgery or chemoradiotherapy. Therefore, it is
necessary to develop new biomarkers with better specificity and
sensitivity for the clinical assessment of HSCC prognosis.
Evidence shows that chronic inflammation is closely
associated with many cancers, and ‘abnormal inflammation in cancer’
or ‘cancer-related inflammation’ has recently been identified as a
marker of ‘category VII cancer’. As a key factor in inflammatory
reactions, the inflammasome is a multiprotein complex. Its
activation can facilitate the maturity and secretion of the
proinflammatory cytokines IL-1β and IL-18 (7). Recent studies have shown that AIM2 is
also a natural immunosensor in the cytoplasm and that it can
identify double-strand DNA (dsDNA) derived from microorganisms and
the host. The multiprotein complex assembled by AIM2 after its
binding with dsDNA is called the AIM2 inflammasome (8,9). The
AIM2 inflammasome is one of the important inflammasomes in the
body. To date, AIM2 has been found to play an important role in the
development and progression of colon cancer, nasopharyngeal
carcinoma, melanoma, and prostate cancer. Decreased expression of
AIM2 and microsatellite instability of frequent frameshift
mutations have been observed in the tumor tissues of patients with
colorectal cancer (10–13). In colorectal cancer (10) and nasopharyngeal carcinoma (14), patients with decreased levels of
AIM2 expression had a poorer prognosis than patients with elevated
levels of AIM2 expression. AIM2 expression was also found to be
decreased in prostate cancer (15).
In contrast, its expression levels were increased in nasopharyngeal
carcinoma (15,16), lung adenocarcinoma (17), and oral squamous cell carcinoma
(18). However, the relationship
between AIM2 expression and prognosis in HSCC is unclear.
STAT3 is a protein encoded by a proto-oncogene, and
p-STAT3 is the activated state of STAT3. p-STAT3 promotes the
occurrence and development of tumors through various aspects and
pathways, and it participates in many mechanisms that interact with
one another. STAT3 is constitutively activated in many human
primary malignant tumors, such as liver (19), breast (20), lung (21) and prostate cancer (22), HNSCC (23), pancreatic (24) and kidney cancer (25), leucocythemia (26), rectal cancer (27), lymphoma (28), melanoma (29) and neurospongioma (30). High expression of p-STAT3 has been
detected in almost all human malignant tumors, particularly
malignant solid tumors. Activated STAT3 has been detected in
>50% of liver cancer, lung cancer and breast cancer cells and in
>95% of HNSCC cells (31).
p-STAT3 is also an important indicator of prognosis in HNSCC
patients (32) and is considered to
be an ideal therapeutic target (33,34).
Although several studies have shown that p-STAT3 is
a key factor in the transformation of chronic inflammatory diseases
to malignant tumors and that p-STAT3 expression is predictive of
prognosis in HSCC patients (35),
the association of both AIM2 and p-STAT3 expression with
clinicopathological characteristics is unknown. Therefore, the
present study was performed to investigate the clinical and
prognostic influence of the expression of AIM2 and p-STAT3 in HSCC
patients. Immunohistochemistry (IHC) and western blotting were
performed to detect the expression levels of AIM2 and p-STAT3 in
111 HSCC specimens and normal hypopharyngeal tissues as controls.
We found that low expression of AIM2 and high expression of p-STAT3
were associated with poor prognosis in HSCC patients.
Materials and methods
Patients and tissue specimens
Paraffin-embedded tissue samples from 111 patients
with HSCC were obtained from dissected tissues in the archives of
the Department of Otolaryngology-Head and Neck Surgery, Bethune
International Peace Hospital (Shijiazhuang, China), between 2003
and 2015. Tissue samples from corresponding adjacent normal
hypopharyngeal tissues from 20 cases were used as a control. The
inclusion criteria of the patients were as follows: i) A definite
pathological diagnosis of HSCC; ii) no anticancer treatment
(including chemoradiotherapy or biotreatment) before hypopharyngeal
resection; iii) absence of common diseases such as diabetes,
hypertension, coronary heart disease (CHD), and no history of
long-term drug use; iv) availability of formalin-fixed,
paraffin-embedded tissues after the excision of lesion with no
<250 tumor cells in the fixed tissues; and v) availability of
complete clinicopathological and follow-up data. The 111 patients
with HSCC were aged from 37 to 82 years, with a mean age of 61
years, and the average follow-up duration was 45.8 months (range,
4–170 months). The clinicopathological characteristics of the HSCC
patients are summarized in Table I.
The clinical stage of tumors was evaluated on the basis of the
pharyngeal cancer staging system of the American Joint Committee on
Cancer (AJCC) in 2016. The surgical procedures for HSCC involved
the resection of HSCC with or without preservation of laryngeal
function. Both procedures were accompanied by routine neck lymph
node dissection with ipsilateral lesion or bilateral neck lymph
node dissection beyond the midline of the lesion. Survival time was
defined as the interval from surgery to death or the interval from
surgery to the last follow-up date for surviving patients.
 | Table I.Association of AIM2 expression with
the clinicopathological characteristics of patients with HSCC. |
Table I.
Association of AIM2 expression with
the clinicopathological characteristics of patients with HSCC.
|
|
| AIM2 protein |
|---|
|
|
|
|
|---|
| Variable | All patients | Low expression | High
expression | χ2 |
P-valuea |
|---|
| Age at surgery |
|
|
| 0.086 | 0.7698 |
|
<60 | 48 | 20 | 28 |
|
|
|
≥60 | 63 | 28 | 35 |
|
|
| Sex |
|
|
| Fisher's exact
test | 0.0684 |
|
Male | 93 | 44 | 49 |
|
|
|
Female | 18 | 4 | 14 |
|
|
| Side |
|
|
| 0.721 | 0.3957 |
|
Left | 55 | 26 | 29 |
|
|
|
Right | 56 | 22 | 34 |
|
|
| Site of primary
tumor |
|
|
| 4.871 | 0.0875 |
|
Postcricoid region | 6 | 0 | 6 |
|
|
|
Pyriform sinus | 84 | 38 | 46 |
|
|
|
Posterior pharyngeal wall | 21 | 10 | 11 |
|
|
| Histological
grade |
|
|
| 2.137 | 0.3435 |
| I
(Well) | 18 | 5 | 13 |
|
|
| II
(Moderate) | 68 | 31 | 37 |
|
|
| III
(Poor) | 25 | 12 | 13 |
|
|
| Tumor size
(cm2) |
|
|
| Fisher's exact
test | 0.8315 |
|
<6 | 29 | 12 | 17 |
|
|
| ≥6 | 82 | 36 | 46 |
|
|
| Growth pattern |
|
|
| Fisher's exact
test | 0.4134 |
|
Ulcerative | 46 | 23 | 23 |
|
|
|
Protruding | 58 | 23 | 35 |
|
|
|
Mixed | 7 | 2 | 5 |
|
|
| Depth of
invasion |
|
|
| 0.2714 | 0.8731 |
|
Submucosa | 47 | 19 | 28 |
|
|
|
Muscular layer | 46 | 21 | 25 |
|
|
|
External laryngeal tissue | 18 | 8 | 10 |
|
|
| Tumor stage |
| T status |
|
|
| Fisher's exact
test | 0.9020 |
| T1 | 2 | 1 | 1 |
|
|
| T2 | 15 | 6 | 9 |
|
|
| T3 | 43 | 17 | 26 |
|
|
| T4 | 51 | 24 | 27 |
|
|
| N status |
|
|
| 4.483 | 0.0342 |
| N0 | 35 | 10 | 25 |
|
|
|
N1/N2/N3 | 76 | 38 | 38 |
|
|
| Clinical stage |
|
|
| Fisher's exact
test | 0.0934 |
| Stage
I | 2 | 1 | 1 |
|
|
| Stage
II | 8 | 4 | 4 |
|
|
| Stage
III | 17 | 3 | 14 |
|
|
| Stage
IV | 84 | 40 | 44 |
|
|
| Intravascular tumor
thrombus |
|
|
| 14.620 | 0.0001 |
|
Yes | 51 | 32 | 19 |
|
|
| No | 60 | 16 | 44 |
|
|
| Nerve invasion |
|
|
| 0.510 | 0.4750 |
|
Yes | 22 | 11 | 11 |
|
|
| No | 89 | 37 | 52 |
|
|
| Lymphatic
metastasis |
|
|
| 4.483 | 0.0342 |
|
Yes | 76 | 38 | 38 |
|
|
| No | 35 | 10 | 25 |
|
|
| Radiotherapy after
surgery |
|
|
| 1.627 | 0.2021 |
|
Yes | 83 | 33 | 50 |
|
|
| No | 28 | 15 | 13 |
|
|
| Complications after
surgery |
|
|
| 0.8811 | 0.3479 |
|
Yes | 21 | 11 | 10 |
|
|
| No | 90 | 37 | 53 |
|
|
| p-STAT3 |
|
|
| Fisher's exact
test | <0.0001 |
| Low
expression | 53 | 9 | 44 |
|
|
| High
expression | 58 | 39 | 19 |
|
|
The study was approved by the Medical Ethics
Institute of Bethune International Peace Hospital. All samples were
anonymous. Moreover, fresh tissue specimens from 5 HSCC and
corresponding adjacent normal hypopharyngeal tissues were collected
for western blotting at our institute in 2016. Corresponding
adjacent normal hypopharyngeal tissue with 1.5 cm of cancer margin
was selected during surgery. (Postoperative pathology confirmed
that this tissue was non-cancerous).
Western blot analysis
Total protein was isolated using RIPA lysis buffer
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China) from 5 fresh biopsy specimens of HSCC tissues and adjacent
normal hypopharyngeal tissues. Protein concentrations were
determined by the BCA method. Equal amounts of tissue lysates (20
µg) were resolved by 10% SDS-polyacrylamide gel electrophoresis
(PAGE) and electrotransferred onto a polyvinylidene fluoride (PVDF)
membrane (EMD Millipore, Billerica, MA, USA). After blocking with
TBS plus 5% non-fat milk for 2 h at room temperature, the membranes
were incubated with primary anti-human AIM2 (dilution 1:1,000; cat.
no. ab93015; Abcam, Cambrige, MA, USA), p-STAT3 (Tyr705; dilution
1:1,000; cat. no. Ab76315; Abcam), STAT3 (dilution 1:2,000; cat.
no. CST4904T; Cell Signaling Technology, Shanghai, China) and actin
(dilution 1:5,000; cat. no. BE0021; Shenzhen Bioeasy Biotechnology
Co., Ltd., Shenzhen, China). After washing for three times with
TBST, the membranes were incubated with a horseradish
peroxidase-conjugated secondary antibodies (goat anti-rabbit,
dilution 1:10,000; cat. no. BE0101; goat anti-mouse, dilution
1:10,000; cat. no. BE0102; both were from Shenzhen Bioeasy
Biotechnology Co., Ltd.) for 1 h at room temperature. The
immunoreactive signals were detected with an enhanced
chemiluminescence kit (EMD Millipore). All procedures were
conducted according to the manufacturer's instructions. Images were
captured using a UVP Gel imaging system (Junyi Corp., Beijing,
China). Strips were evaluated via gray value analysis using ImageJ
software (National Institutes of Health, Bethesda, MD, USA).
IHC
The expression of the AIM2 protein was detected by
immunohistochemistry (IHC). Five-micrometer-thick tissue sections
were deparaffinized and rehydrated conventionally with
dimethylbenzene and graded alcohol, and then rinsed with
phosphate-buffered saline (PBS) for 5 min. The tissue sections were
incubated with 3% H2O2 for 30 min to block
the activity of endogenous peroxidases. The sections were washed
with buffer for 5 min to deactivate H2O2. The
tissues were incubated in diluted normal serum at room temperature
for 20 min; the source of serum was the same as that of the
secondary antibody. The blocking liquid was decanted, and no
washing was performed. The tissue sections were incubated with a
1:100 dilution of anti-AIM2 polyclonal antibody and a 1:50 dilution
of anti-p-STAT3 polyclonal antibody for 30 min and then rinsed with
PBS for 5 min. The slides were incubated with 1:200 diluted
biotinylated goat-anti-rabbit solution (Vector Laboratories, Inc.,
San Francisco, CA, USA; cat. no. PK-4001) for 30 min, and then
washed with PBS for 5 min. The tissue sections were incubated in
Vectastain ABC reagent for 30 min and washed with PBS for 5 min.
The slides were incubated in peroxidase substrate until the desired
staining intensity was reached. Finally, the sections were washed
with tap water, counterstained with hematoxylin, differentiated,
dehydrated, hyalinized and sealed.
Measurement of AIM2 and p-STAT3
expression by IHC assay
Five random fields were selected from each slide for
scoring, and the mean score for each slide was used for the final
analysis. Positive staining was assessed using a four-point scoring
system: 0 (0–10% positive cells), 1 (11–35% positive cells), 2
(36–70% positive cells) and 3 (>70% positive cells). To ensure
the greatest objectivity, the intensity of positive staining was
also evaluated using a three-point scoring system: 0 (negative
staining), 1 (weak or light-yellow staining), and 2 (strong or
yellow-brown staining). The expression index of AIM2 and p-STAT3
was calculated as follows: Expression index = (intensity score) ×
(positive score). To obtain more accurate scores, 2 independent
senior observers (Jie An and Hui Li) who were blinded to the
available clinicopathological data and outcomes of HSCC patients
assessed all IHC samples (including tumor tissues and normal
control tissues). Discrepant results of the same tissue sections
were re-evaluated by both observes to obtain a consistent result.
The cut-off scores for high and low levels of AIM2 and p-STAT3
expression were obtained based on heterogeneity measurements, and
the survival rate of HSCC patients was analyzed by a log-rank test.
The optimal cut-off for this evaluation system was determined as
follows: high expression of AIM2 and p-STAT3 were indicated by an
expression index of >1, and low expression of AIM2 and p-STAT3
were indicated by an expression index of ≤1.
Selection of cut-off scores
Data were imported from our clinical research
datasets according to the method described by a previous study
(36). The optimal cut-off was
ascertained, and analysis charts were generated by R statistical
software (http://molpath.charite.de/cutoff) (36). X-tile charts were generated for the
evaluation of AIM2 and p-STAT3 expression and optimal cut-off
values based on outcomes.
Statistical analysis
The ratio of the western blotting gray value of the
target protein to that of the marker protein was used foR
statistical analysis. Since AIM2, p-STAT3 protein expression levels
and the p-STAT3/STAT3 ratio did follow a normal distribution,
statistical evaluation was performed via paired t-tests. The
optimal cut-off values for IHC-based expression associated with
survival were determined using the online version of X-tile
software (http://molpath.charite.de/cutoff). The χ2
or Fisher's exact tests was used to evaluate the association
between AIM2 expression and clinicopathological characteristics.
Survival curves were plotted using Kaplan-Meier survival analyses
and compared using a log-rank test. The predictive value of the
clinicopathological characteristics was evaluated using receiver
operating characteristic (ROC) curve analysis. The relative risk
(RR) of death associated with AIM2 expression and other variables
were assessed by univariate survival analysis (log-rank test) and
multivariate Cox proportional hazards regression models.
Correlations between variables, ROC curves, log-rank tests and
multiple Cox proportional hazards regression models were performed
using SPSS statistical software (SPSS standard version 13.0; SPSS,
Inc., Chicago, IL, USA). In all cases, a statistically significant
difference was considered if the P-value from a two-tailed test was
<0.05.
Results
Expression level of AIM2 in HSCC and
adjacent normal hypopharyngeal tissues by western blot assays
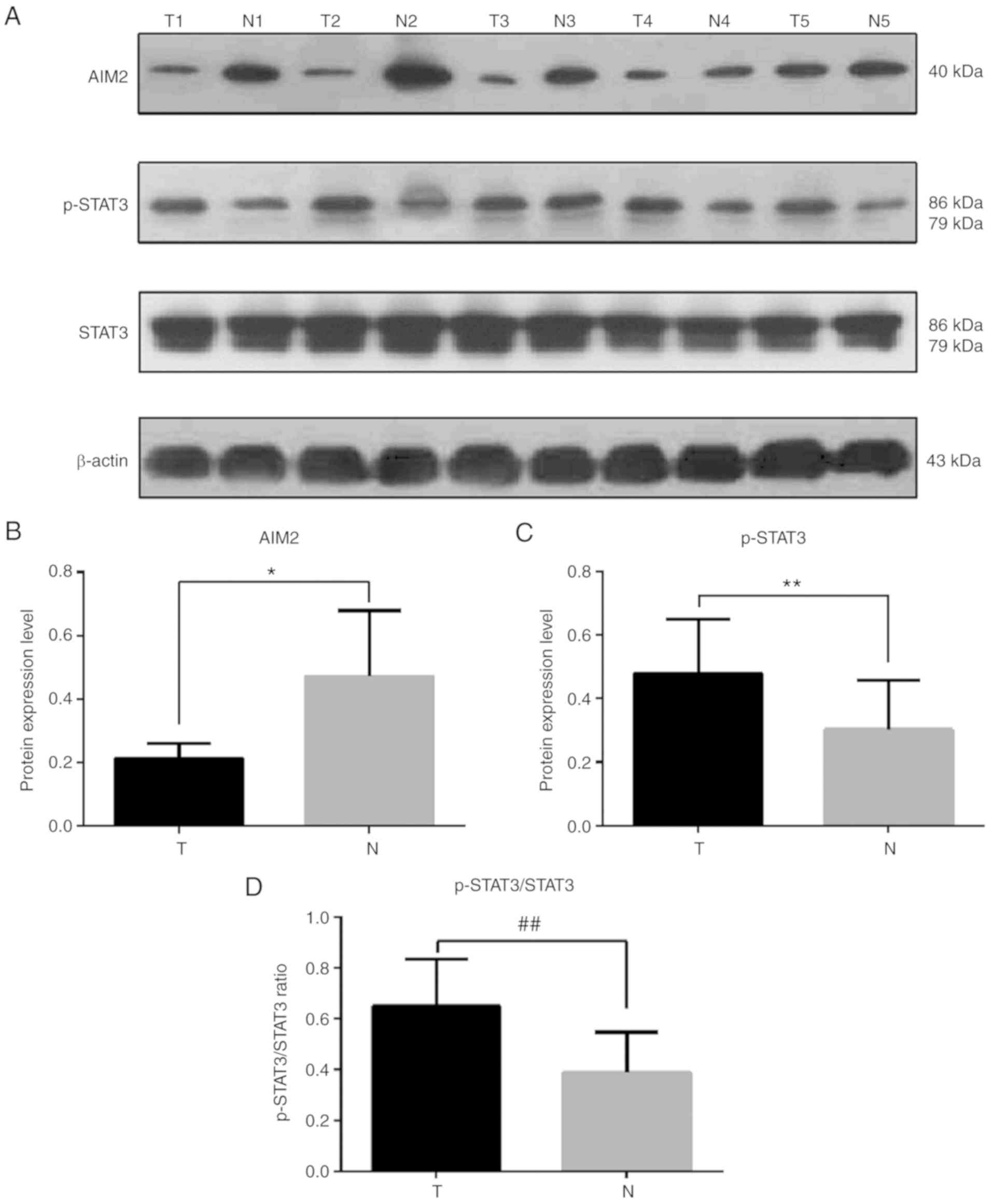
In the present study, the expression of AIM2,
p-STAT3 and total STAT3 proteins was detected by western blotting
in 5 pairs of primary HSCC and adjacent normal hypopharyngeal
tissues. Compared with those in adjacent hypopharyngeal tissues, an
apparent decrease in the expression of AIM2 protein and an increase
in the expression of p-STAT3 protein and p-STAT3/total STAT3 ratio
were detected in HSCC tissues (Fig.
1).
AIM2 and p-STAT3 expression in
hypopharyngeal tissues examined by IHC
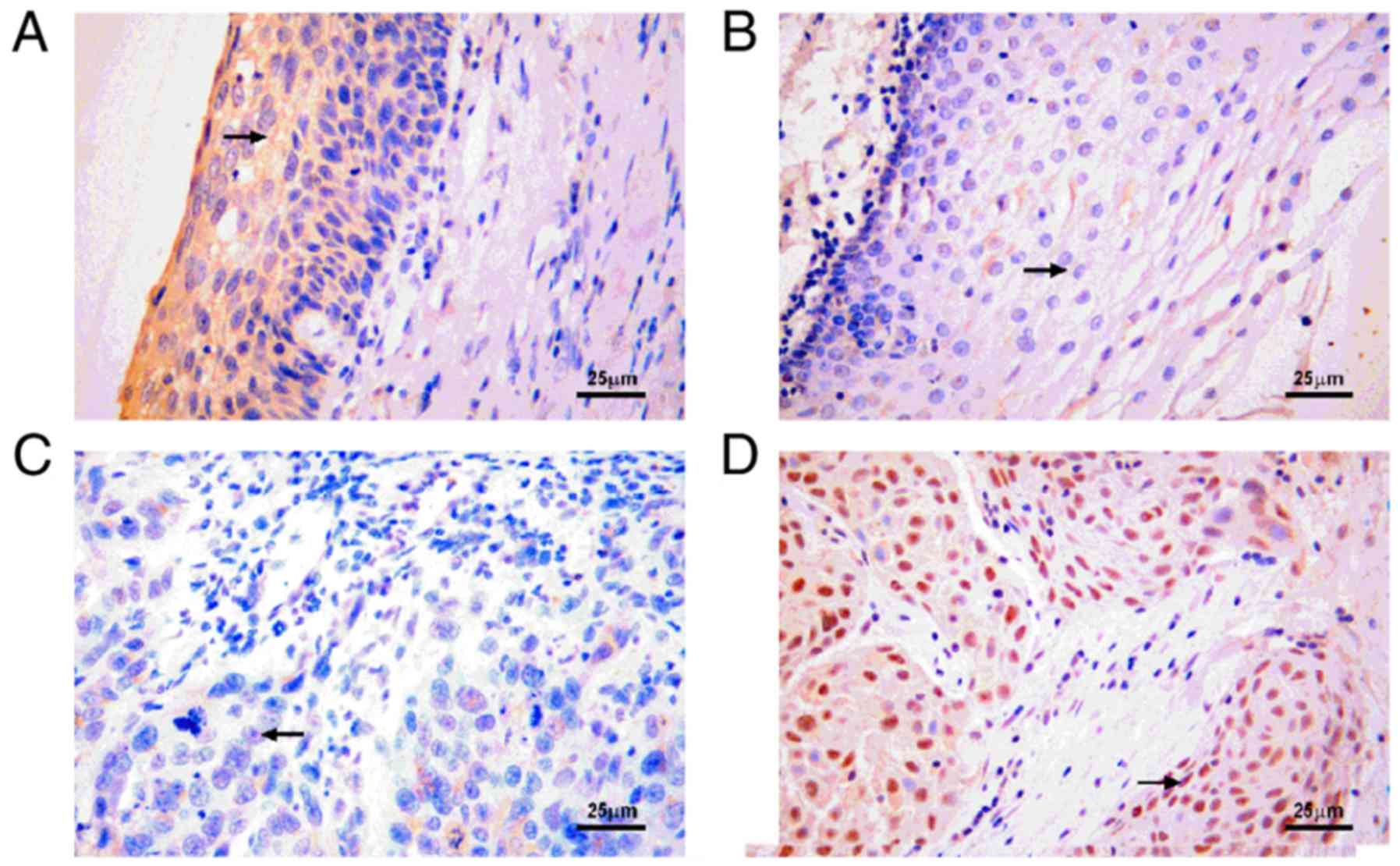
AIM2 and p-STAT3 expression could be successfully
and simultaneously detected by IHC in 111 HSCC and 20 normal
hypopharyngeal epithelial tissues (Fig.
2). AIM2 was primarily expressed in the cytoplasm (Fig. 2A), and p-STAT3 was primarily
expressed in the nucleus (Fig. 2D).
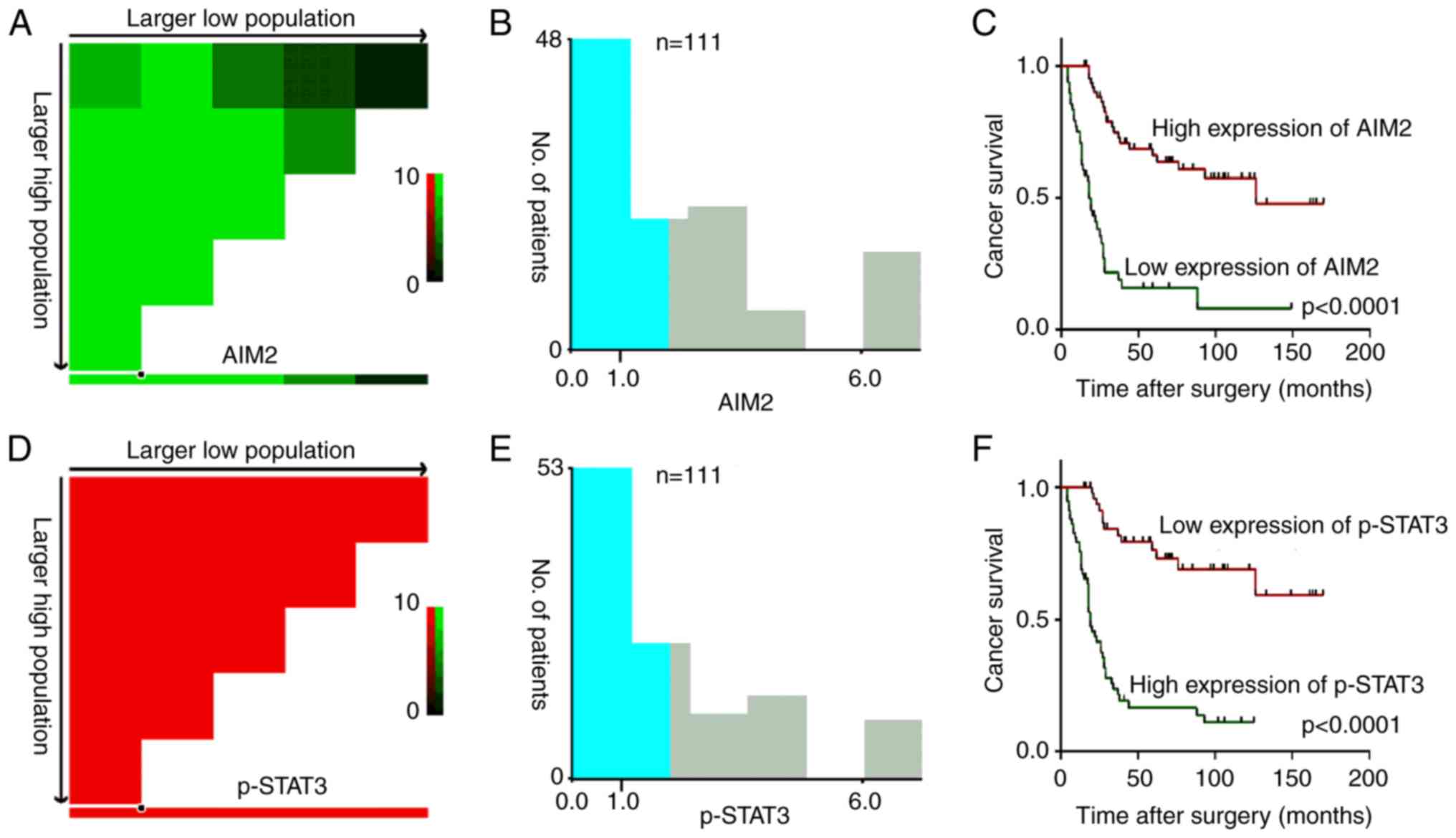
Based on the X-tile program, AIM2 expression higher than the
cut-off value of 1 was defined as high expression (Fig. 3A). Similarly, the cut-off score of 1
based on X-tile plots could also distinguish high or low p-STAT3
expression in the HSCC samples (Fig.
3D). In the present study, 63 (56.8%) HSCC cases had high
expression of AIM2, 48 (43.2%) cases had low expression of AIM2, 58
(52.3%) cases had high expression of p-STAT3, and 53 (47.7%) cases
had low expression of p-STAT3 as determined by IHC (Fig. 2). For the 20 samples of adjacent
normal hypopharyngeal tissues, high expression of AIM2 was detected
in 13 (75%) samples, and low expression of p-STAT3 was detected in
18 (90%) samples (Fig. 2A and B).
In addition, AIM2 expression was closely associated with
intravascular tumor thrombus and lymph node metastasis (P<0.05;
Table I).
Relationship between
clinicopathological characteristics, AIM2 expression and survival
rate in HSCC patients
Kaplan-Meier analysis revealed that
clinicopathological characteristics including growth pattern
(P=0.01), N status (P<0.0001), intravascular tumor thrombus
(P<0.0001), nerve invasion (P=0.0061), lymphatic metastasis
(P<0.0001) and radiotherapy after surgery (P=0.009), and
complications after surgery (P=0.031) had a significant impact on
patient survival (Table II).
Evaluation of patient survival revealed that low expression of AIM2
was closely associated with poor survival rates (P<0.0001;
Fig. 4C), and the mean and median
survival time of patients with low expression of AIM2 were 24 and
18 months, respectively. The mean and median survival time of
patients with high expression of AIM2 were 62.4 and 126 months,
respectively (Table II). Moreover,
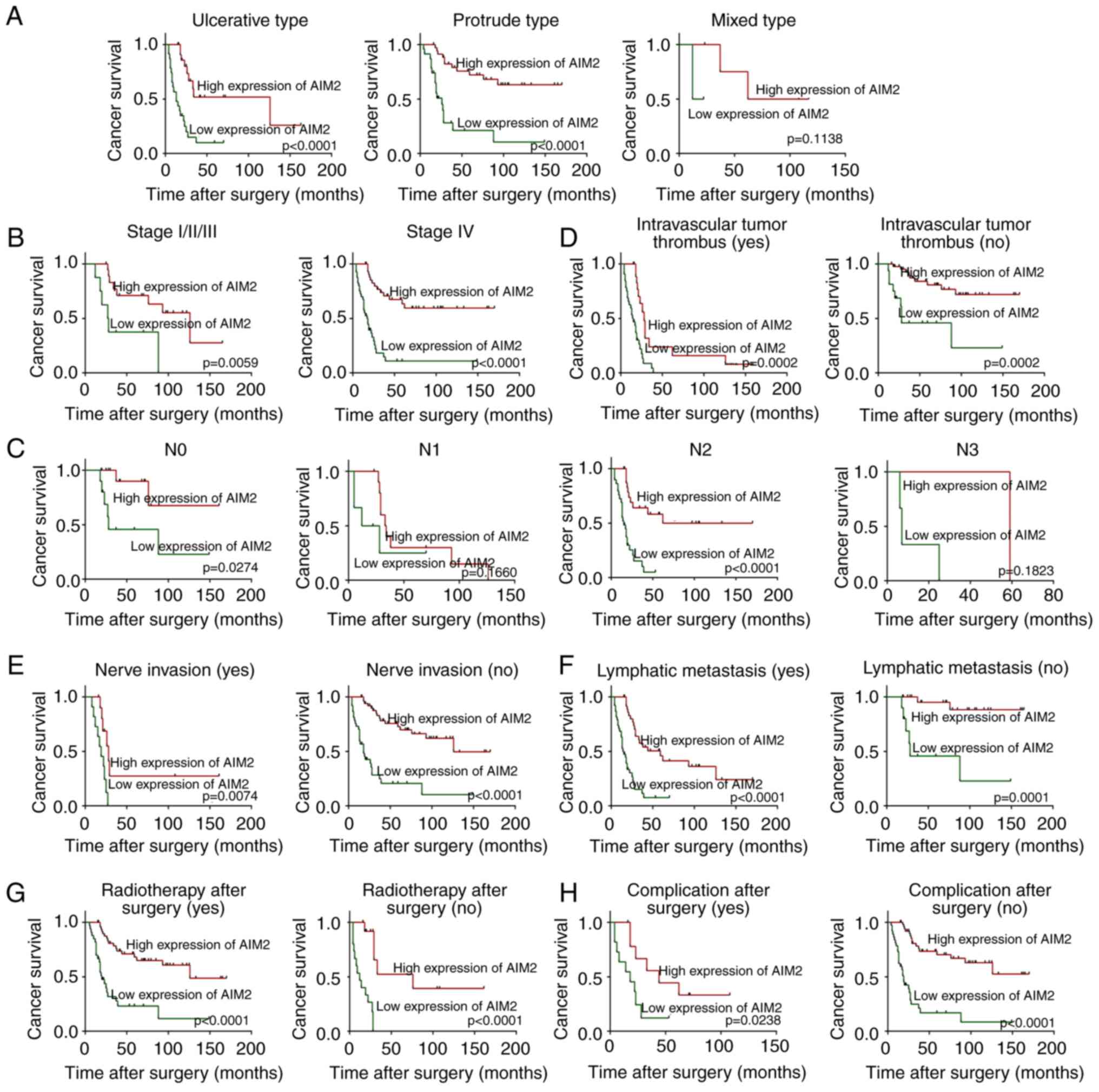
survival analysis in patients with high or low expression of AIM2
was performed based on the growth pattern, clinical stage, N
status, intravascular tumor thrombus, lymphatic metastasis, nerve
invasion, radiotherapy after surgery, and complications after
surgery. The results demonstrated that the factors associated with
poor prognosis in HSCC patients with low expression of AIM2
included ulcerative type growth (P<0.0001; Fig. 4A), protruding type growth
(P<0.0001; Fig. 4A), stage
I/II/III (P=0.0059; Fig. 4B), stage
IV (P<0.0001; Fig. 4B), N0
(P=0.0274; Fig. 4C), N2
(P<0.0001; Fig. 4C),
intravascular tumor thrombus (Yes) (P=0.0002; Fig. 4D), intravascular tumor thrombus (No)
(P=0.0002; Fig. 4D), nerve invasion
(Yes) (P=0.0074; Fig. 4E), nerve
invasion (No) (P<0.0001; Fig.
4E), lymphatic metastasis (Yes) (P<0.0001; Fig. 4F), lymphatic metastasis (No)
(P=0.0001; Fig. 4F), radiotherapy
after surgery (Yes) (P<0.0001; Fig.
4G), radiotherapy after surgery (No) (P<0.0001; Fig. 4G), complications after surgery (Yes)
(P=0.0238; Fig. 4H) and
complications after surgery (No) (P<0.0001; Fig. 4H).
 | Table II.Univariate and multivariate analyses
of different prognostic features in 111 HSCC patients. |
Table II.
Univariate and multivariate analyses
of different prognostic features in 111 HSCC patients.
|
| Univariate
analysisa | Multivariate
analysisb |
|---|
|
|
|
|
|---|
| Variable | All patients | Mean survival
(months) | Median survival
(months) | P-value | HR (95% CI) | P-value |
|---|
| Age at surgery |
|
|
| 0.408 |
|
|
|
<60 | 48 | 51.7 | 44 |
|
|
|
|
≥60 | 63 | 41.2 | 37 |
|
|
|
| Side |
|
|
| 0.432 |
|
|
|
Left | 55 | 43.7 | 38 |
|
|
|
|
Right | 56 | 47.8 | 37 |
|
|
|
| Sex |
|
|
| 0.004 | 0.830
(0.266–2.587) | 0.748 |
|
Male | 93 | 40.4 | 29 |
|
|
|
|
Female | 18 | 73.3 | UD |
|
|
|
| Site of primary
tumor |
|
|
| 0.060 |
|
|
|
Postcricoidregion | 6 | 79 | UD |
|
|
|
|
Posterior pharyngeal wall | 21 | 47.7 | 33 |
|
|
|
|
Pyriform sinus | 84 | 42.9 | 37 |
|
|
|
| Histological
grade |
|
|
| 0.202 |
|
|
| I
(well) | 18 | 62.9 | 88 |
|
|
|
| II
(moderate) | 68 | 46.5 | 29 |
|
|
|
| III
(poor) | 25 | 31.5 | 28 |
|
|
|
| Tumor size
(cm2) |
|
|
| 0.798 |
|
|
|
<6 | 29 | 40.4 | 39 |
|
|
|
| ≥6 | 82 | 47.7 | 37 |
|
|
|
| Growth pattern |
|
|
| 0.010 | 1.420
(0.779–2.591) | 0.252 |
|
Ulcerative | 46 | 29.4 | 25 |
|
|
|
|
Protruding | 58 | 45.8 | 88 |
|
|
|
|
Mixed | 7 | 54.4 | 62 |
|
|
|
| Tumor stage |
| T status |
|
|
| 0.830 |
|
|
| T1 | 2 | 52 | 52 |
|
|
|
| T2 | 15 | 45.5 | 62 |
|
|
|
| T3 | 43 | 46.4 | 34 |
|
|
|
| T4 | 51 | 45.0 | 37 |
|
|
|
| N status |
|
|
| <0.0001 | 1.636
(1.107–2.417) | 0.014c |
| N0 | 35 | 71.5 | UD |
|
|
|
| N1 | 17 | 39.5 | 29 |
|
|
|
| N2 | 55 | 32.9 | 22 |
|
|
|
| N3 | 4 | 24.3 | 16 |
|
|
|
| Clinical stage |
|
|
| 0.127 |
|
|
| Stage
I/II/III | 27 | 62.6 | 88 |
|
|
|
| Stage
IV | 84 | 40.4 | 29 |
|
|
|
| Depth of
invasion |
|
|
| 0.076 |
|
|
|
Submucosa | 47 | 53.5 | 88 |
|
|
|
|
Muscular layer | 46 | 46.4 | 34 |
|
|
|
|
External laryngeal tissue | 18 | 24 | 27 |
|
|
|
| Intravascular tumor
thrombus |
|
|
| <0.0001 | 0.225
(0.110–0.458) |
<0.0001c |
|
Yes | 51 | 23.6 | 19 |
|
|
|
| No | 60 | 64.6 | UD |
|
|
|
| Nerve invasion |
|
|
| 0.0061 | 1.253
(0.581–2.703) | 0.565 |
|
Yes | 22 | 30.5 | 23 |
|
|
|
| No | 89 | 49.5 | 62 |
|
|
|
| Lymphatic
metastasis |
|
|
| <0.0001 | 1.465
(0.277–7.761) | 0.654 |
|
Yes | 76 | 33.9 | UD |
|
|
|
| No | 35 | 71.5 | 26 |
|
|
|
| Radiotherapy after
surgery |
|
|
| 0.009 | 1.817
(0.804–4.107) | 0.151 |
|
Yes | 83 | 50.6 | 62 |
|
|
|
| No | 28 | 31.4 | 28 |
|
|
|
| Complications after
surgery |
|
|
| 0.031 | 0.735
(0.378–1.730) | 0.364 |
|
Yes | 21 | 31.8 | 23 |
|
|
|
| No | 90 | 49.0 | 59 |
|
|
|
| AIM2 |
|
|
| <0.0001 | 0.353
(0.188–0.663) | 0.0012c |
| Low
expression | 48 | 24.0 | 18 |
|
|
|
| High
expression | 63 | 62.4 | 126 |
|
|
|
| p-STAT3 |
|
|
| <0.0001 | 4.093
(2.076–8.071) |
<0.0001c |
| Low
expression | 53 | 65.2 | UD |
|
|
|
| High
expression | 58 | 28.0 | 19 |
|
|
|
Multivariate survival analysis of
independent prognostic factors of HSCC
Multivariate Cox proportional hazards regression
analysis was performed to assess the independent value of each
variable in predicting the survival of HSCC patients (Table II). After AIM2 expression and
clinicopathological characteristics (including sex, growth pattern,
N status, intravascular tumor thrombus, nerve invasion, lymphatic
metastasis, radiotherapy after surgery and complications after
surgery) were analyzed by univariate log-rank tests, the factors
that were significantly associated with the overall survival rate
were included in a multivariate Cox analysis (Table II). As anticipated, low expression
of AIM2 protein was an independent risk factor for HSCC patients
with poor prognosis [RR, 0.353; confidence interval (CI),
0.188–0.663; P=0.0012]. With respect to other features, only N
status (P=0.014; Table II) and
intravascular tumor thrombus (P<0.0001; Table II) were independent prognostic
predictors for the survival of HSCC patients.
Correlation between the expression
levels of AIM2 and p-STAT3 in HSCC
Western blot assays revealed an inverse association
between the expression levels of AIM2 and p-STAT3 proteins in HSCC
(Fig. 1A). Using the criteria
described earlier, high expression of p-STAT3 was detected in
58/111 (52.3%) cases in our HSCC cohort by IHC. Further analysis
revealed a significant inverse association between AIM2 and p-STAT3
expression in HSCC patients (P<0.0001, Fisher's exact test;
Table I and Fig. 2C and D).
Correlation of low expression of AIM2
combined with high expression of p-STAT3 with poor prognosis in
HSCC patients
To further confirm whether the expression of p-STAT3
can affect AIM2-related prognosis, we divided the HSCC cases into a
low p-STAT3 expression (low p-STAT3) group and a high p-STAT3
expression (high p-STAT3) group based on the optimal cut-off of the
p-STAT3 expression index. When all cases of HSCC were stratified by
the p-STAT3 expressioN status, patients with low AIM2 expression
had a significantly poorer prognosis than patients with high AIM2
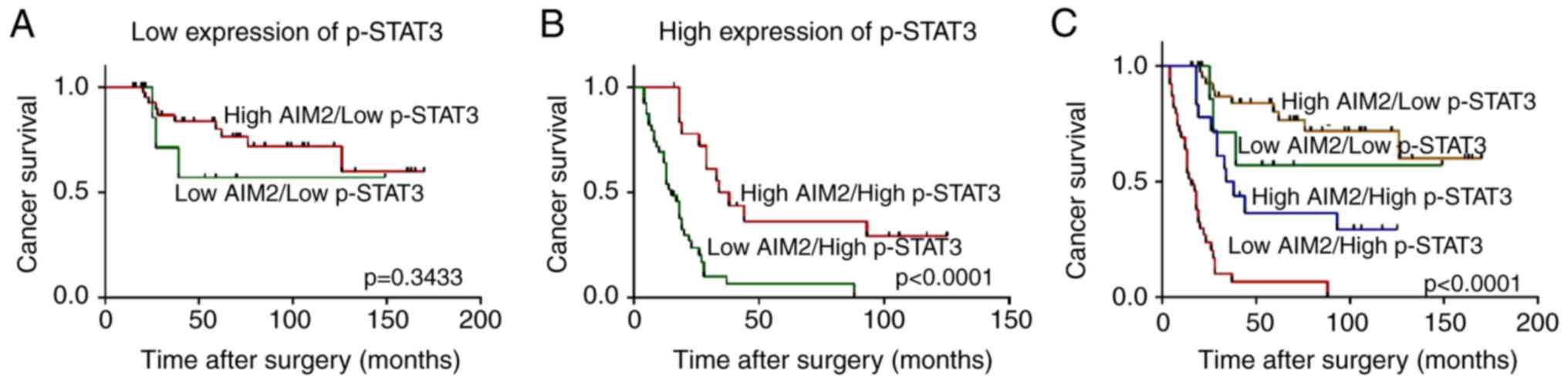
expression in the high p-STAT3 group (P<0.0001; Fig. 5B). However, no significant
difference in survival time was found between patients with low and
high AIM2 expression in the low p-STAT3 group (P=0.3433; Fig. 5A). In the combined analysis of AIM2
and p-STAT3 expression, the low AIM2/high p-STAT3 group had the
worst survival (mean survival time, 17.6 months; median survival
time, 15 months), the high AIM2/high p-STAT3 group and the low
AIM2/low p-STAT3 group had moderate survival (mean survival time,
49.5 and 51.4 months, respectively), and the high AIM2/low p-STAT3
group had the best survival (mean survival time, 68.0 months,
P<0.0001; Fig. 5C).
To evaluate the prognostic value of AIM2 expression
in all HSCC patients, we used ROC curves to assess the survival
rates of patients with high and low p-STAT3 expression. The results
confirmed the prospective predictive significance of AIM2 with
respect to specific survival in all HSCC patients (AUC=0.7160;
Fig. 6A). Upon further analysis in
the group with high p-STAT3 expression, AIM2 was identified as a
significant prognostic factor associated with the survival of HSCC
patients (AUC=0.7597, P<0.0001; Fig.
6B). In contrast, AIM2 had no statistical association with
survival in the group with low p-STAT3 expression (AUC=0.4856,
P=0.272; Fig. 6C).
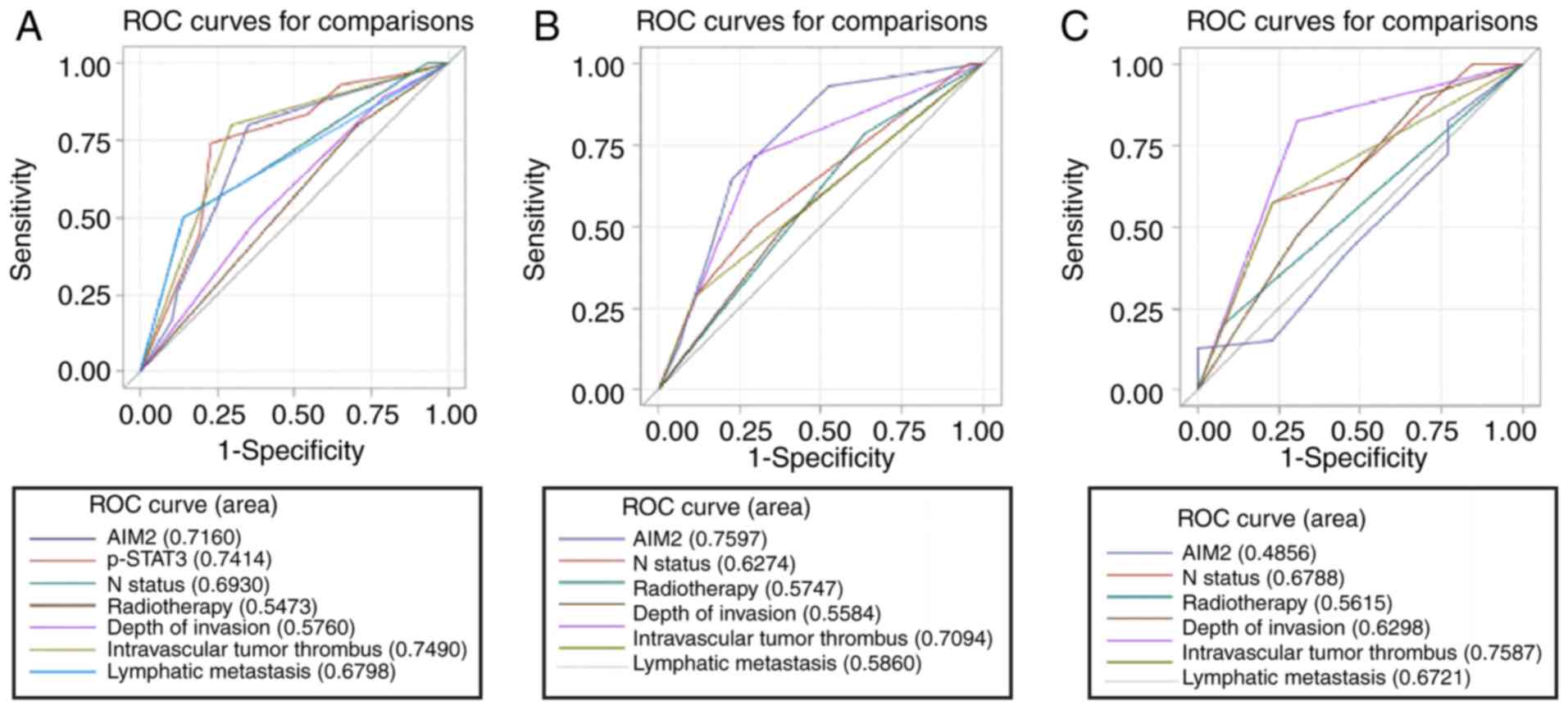 | Figure 6.ROC curves based on different
clinicopathological characteristics and AIM2 expression were
analyzed to assess the relationship between these features and
survival status. (A) For all patients, AIM2 expression [area under
the curve (AUC)=0.7160, P=0.0011), p-STAT3 (AUC=0.7414,
P<0.0001), N status (AUC=0.6930, P=0.0002), radiotherapy after
surgery (AUC=0.5473, P=0.2539), depth of invasion (AUC=0.5760,
P=0.1226), intravascular tumor thrombus (AUC=0.7490, P<0.0001),
and lymphatic metastasis (AUC=0.6798, P=0.0001) were significantly
associated with survival. (B) In the high p-STAT3 expression group,
AIM2 expression (AUC=0.7597, P=0.0202), N status (AUC=0.6274,
P=0.0907), radiotherapy after surgery (AUC=0.5747, P=0.3062), depth
of invasion (AUC=0.5584, P=0.4955), intravascular tumor thrombus
(AUC=0.7094, P=0.0084), and lymphatic metastasis (AUC=0.5860,
P=0.1337) were employed to predict survival. (C) In the low p-STAT3
expression group, AIM2 expression (AUC=0.4856, P=0.8953), N status
(AUC=0.6788, P=0.0388), radiotherapy after surgery (AUC=0.5615,
P=0.3238), depth of invasion (AUC=0.6298, P=0.1069), intravascular
tumor thrombus (AUC=0.7587, P=0.0012), and lymphatic metastasis
(AUC=0.6721, P=0.0396) were analyzed to assess survival durations.
ROC, receiver operating characteristic. |
Discussion
In HSCC, the biological effects and mechanisms of
AIM2 on tumor cells are unclear. Two theories have been put forward
to explain these effects. In one theory, AIM2 acts as a danger
signal receptor in the innate immune system and forms an
inflammatory complex, and, subsequently, the AIM2 inflammasome
promotes the maturation of cytokines mediated by caspase-1 and
triggers cell death in innate immune cells, resulting in pro-IL-1β
cleavage and IL-1β secretion (8,9).
Tumor-derived IL-1β can inhibit tumor growth and local recurrence
by recruiting neutrophils (14). In
another theory, AIM2 inhibits tumor growth in colon tumors
independent of the function of inflammatory proteins, but it can
inhibit tumor growth by suppressing the activity of Akt (37). AIM2 inhibits the growth of colon
tumors by reducing the proliferation of colon epithelial stem cells
(38) and possibly by controlling
the composition of the intestinal flora (39). These findings suggest that the loss
of AIM2 protein in tumor cells may provide favorable conditions for
the growth of cancer cells.
In the present study, 5 cases of fresh HSCC and
adjacent normal hypopharyngeal tissues were assessed by western
blotting, and the expression levels of AIM2 and p-STAT3 in 111
cases of surgically resected paraffin-embedded HSCC tissues were
analyzed by IHC. The western blot results revealed that AIM2 levels
were significantly lower in HSCC tissues than in adjacent normal
hypopharyngeal epithelial tissues. Reduced expression of AIM2 has
often been observed in other types of cancer, such as colon
(10) and prostate cancer (15), small cell lung cancer and
adenocarcinoma (17), and poorly
differentiated squamous cell carcinoma. These data suggest that
decreased expression of AIM2 may play an important role in certain
types of human cancers, including HSCC. However, the expression of
AIM2 is increased in nasopharyngeal carcinoma (14,16),
oral squamous cell carcinoma (18)
and lung adenocarcinoma (17). The
varying expression levels of AIM2 in different tumor tissues
suggest that AIM2 may play unique roles in different types of
cancers.
Our findings also revealed that the expression of
AIM2 in our HSCC cohort was associated with intravascular tumor
thrombus and lymph node metastasis. These results indicated that
the decreased expression of AIM2 in HSCC may contribute to an
increase in the malignant phenotype of tumors. Similar results were
obtained in other human malignancies such as colon cancer, prostate
cancer and nasopharyngeal carcinoma, and the decrease in or
deficiency of AIM2 expression was often correlated with more
aggressive phenotypes and poor prognosis (10,14,15).
However, in a study of cutaneous squamous cell carcinoma (cSCC),
the knockdown of AIM2 resulted in decrease in the viability of cSCC
cells, initiation of apoptosis, decrease in cell invasion and
downregulation of the invasion-associated proteases MMP1 and MMP13.
Moreover, AIM2 inhibited tumor growth and angiogenesis in skin
squamous cell carcinoma (SCC). Patsos et al found that the
overexpression of AIM2 can increase cellular adhesion and invasion
and promote migration (40). These
results clearly contradict our data. As suggested by studies from
numerous other research groups, AIM2 can be described as a
‘double-edged sword’, with both excessive expression and inhibition
of AIM2 promoting the occurrence and development of cancer
(38,40). Therefore, we speculate that the
regulation of AIM2 expression affects the expression of
metastasis-related proteins, but the specific mechanism is not very
clear and will be our future research direction.
In our study, we found that low expression of AIM2
was a robust and independent factor associated with poor prognosis
in HSCC patients. Notably, survival analysis-based growth pattern,
clinical stage, N status, intravascular tumor thrombus, lymphatic
metastasis, nerve invasion, radiotherapy after surgery, and
complications after surgery revealed that AIM2 expression was
closely related to the survival of different subsets of HSCC
patients. Therefore, AIM2 expression may be a potential factor for
predicting the clinical prognoses of patients with HSCC. Our data
suggested that the detection of AIM2 expression by IHC could serve
as an effective tool to determine the risk of invasion and/or
progression of HSCC.
IHC revealed that the high expression of p-STAT3 was
closely associated with short survival times in HSCC patients. This
result was consistent with previous findings (32). Further analysis revealed that the
expression of AIM2 in the HSCC cohort was negatively correlated
with p-STAT3 expression. To determine whether the changes in
p-STAT3 expression could affect the prognosis related to AIM2, we
evaluated the survival of HSCC patients stratified by p-STAT3
expression and found that low AIM2 expression was closely related
to poor prognosis in the group with high p-STAT3 expression but not
in the group with low p-STAT3 expression. This result was confirmed
by ROC curve analyses, which revealed that AIM2 was significantly
associated with the prognosis of patients in the group with high
p-STAT3 expression. In addition, when the expression of AIM2 was
analyzed in combination with that of p-STAT3, the survival rate of
the patients in the low AIM2/high p-STAT3 group was the lowest
among all groups. Previous studies have shown that the deletion of
AIM2 can promote the overexpression of IL-22 and enhance the
activation of STAT3 by regulating IL-18 production in drug-induced
colitis in Aim2−/− mice (41). Moreover, in Aim2−/− mice,
continued activation of STAT3 and Akt promoted the proliferation of
intestinal crypt cells and could contribute to the increase in the
susceptibility to colon cancer (41). In most cases, there is an
inflammatory environment around the HSCC tissues. Therefore, we
speculate that AIM2 may regulate the expression of p-STAT3 through
the IL-18/IL-22 pathways in HSCC, but the specific mechanism
requires further investigation.
In summary, the present study demonstrated the
pattern of AIM2 expression in normal hypopharyngeal tissues and
HSCC tissues. The results revealed that the reduced expression of
AIM2 may confer a malignant phenotype in HSCC cells. In addition,
our research indicated that the decreased expression of AIM2
protein in HSCC may be a new independent prognostic marker, and
more importantly, the expression of AIM2 combined with p-STAT3 in
tumor cells can predict the prognosis of tumor patients.
Although the specimens age ranged over a 12-year
period, we also believe that there is no correlation between
specimen age and antigen expression level. Furthermore, our study
design and protocols could minimize this correlation even if it
does exist. First, we have a standard protocol for paraffin
specimen preparation in which there is a very small and almost the
same time lag from specimen cutting to embedding for each tumor
sample. Second, antigen retrieval methods were routinely conducted
prior to immunohistochemical staining. Third, although samples were
obtained at different times from the patients enrolled in the
present study, these patients' clinical and pathological stages
were extremely similar and comparable. Finally, we found no
correlation between antigen expression level and specimen age, in
pre-experimental tests before we conducted formal and systematic
immunohistochemical study.
However, our study has some limitations. First, only
a small number of HSCC samples were used in our study. Second, AIM2
and p-STAT3 levels were not quantitatively analyzed. Third,
although our study provided important information on the
involvement of AIM2 in metastasis and invasion of tumors, further
experiments are required to determine the specific mechanisms
underlying the role of AIM2 in the metastasis and invasion of
HSCC.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by a grant
from the Research Foundation of Bethune International Peace
Hospital (201706) and the Key Research and Development Project Plan
of Hebei Provincial Science and Technology Department (no.
18277736D).
Availability of data and materials
The datasets analyzed during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
ZL and XL conceived and designed the study. ZL, XS
and XL contributed to the acquisition, the analysis and
interpretation of the data. ZL, HL and WW performed the experiments
and were involved in drafting the manuscript. All authors have read
and approved the final manuscript and agree to be responsible for
all aspects of the study to ensure that questions about any aspects
of the work are properly investigated or resolved.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee board of Bethune International Peace Hospital of PLA, and
the need for individual patient consent was waived.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang YL, Feng SH, Zhu J, Zhu GP, Li DS,
Wang Y, Zhu YX, Sun GH and Ji QH: Impact of lymph node ratio on the
survival of patients with hypopharyngeal squamous cell carcinoma: A
population-based analysis. PLoS One. 8:e566132013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hall SF, Groome PA, Irish J and O'Sullivan
B: The natural history of patients with squamous cell carcinoma of
the hypopharynx. Laryngoscope. 118:1362–1371. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brown JM and Wilson WR: Exploiting tumour
hypoxia in cancer treatment. Nat Rev Cancer. 4:437–447. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boyle P and Ferlay J: Cancer incidence and
mortality in Europe, 2004. Ann Oncol. 16:481–488. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takes RP, Strojan P, Silver CE, Bradley
PJ, Haigentz M Jr, Wolf GT, Shaha AR, Hartl DM, Olofsson J,
Langendijk JA, et al: Current trends in initial management of
hypopharyngeal cancer: The declining use of open surgery. Head
Neck. 34:270–281. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fernandes-Alnemri T, Yu JW, Datta P, Wu J
and Alnemri ES: AIM2 activates the inflammasome and cell death in
response to cytoplasmic DNA. Nature. 458:509–513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hornung V, Ablasser A, Charrel-Dennis M,
Bauernfeind F, Horvath G, Caffrey DR, Latz E and Fitzgerald KA:
AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating
inflammasome with ASC. Nature. 458:514–518. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dihlmann S, Tao S, Echterdiek F, Herpel E,
Jansen L, Chang- Claude J, Brenner H, Hoffmeister M and Kloor M:
Lack of Absent in Melanoma 2 (AIM2) expression in tumor cells is
closely associated with poor survival in colorectal cancer
patients. Int J Cancer. 135:2387–2396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schulmann K, Brasch FE, Kunstmann E, Engel
C, Pagenstecher C, Vogelsang H, Krüger S, Vogel T, Knaebel HP,
Rüschoff J, et al: HNPCC-associated small bowel cancer: Clinical
and molecular characteristics. Gastroenterology. 128:590–599. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Woerner SM, Kloor M, Schwitalle Y, Youmans
H, Doeberitz Mv, Gebert J and Dihlmann S: The putative tumor
suppressor AIM2 is frequently affected by different genetic
alterations in microsatellite unstable colon cancers. Genes
Chromosomes Cancer. 46:1080–1089. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim TM, Laird PW and Park PJ: The
landscape of microsatellite instability in colorectal and
endometrial cancer genomes. Cell. 155:858–868. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen LC, Wang LJ, Tsang NM, Ojcius DM,
Chen CC, Ouyang CN, Hsueh C, Liang Y, Chang KP, Chen CC, et al:
Tumour inflammasome-derived IL-1β recruits neutrophils and improves
local recurrence-free survival in EBV-induced nasopharyngeal
carcinoma. EMBO Mol Med. 4:1276–1293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ponomareva L, Liu H, Duan X, Dickerson E,
Shen H, Panchanathan R and Choubey D: AIM2, an IFN-inducible
cytosolic DNA sensor, in the development of benign prostate
hyperplasia and prostate cancer. Mol Cancer Res. 11:1193–1202.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang LJ, Hsu CW, Chen CC, Liang Y, Chen
LC, Ojcius DM, Tsang NM, Hsueh C, Wu CC and Chang YS:
Interactome-wide analysis identifies end-binding protein 1 as a
crucial component for the speck-like particle formation of
activated absence in melanoma 2 (AIM2) inflammasomes. Mol Cell
Proteomics. 11:1230–1244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kong H, Wang Y, Zeng X, Wang Z, Wang H and
Xie W: Differential expression of inflammasomes in lung cancer cell
lines and tissues. Tumour Biol. 36:7501–7513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kondo Y, Nagai K, Nakahata S, Saito Y,
Ichikawa T, Suekane A, Taki T, Iwakawa R, Enari M, Taniwaki M, et
al: Overexpression of the DNA sensor proteins, absent in melanoma 2
and interferon-inducible 16, contributes to tumorigenesis of oral
squamous cell carcinoma with p53 inactivation. Cancer Sci.
103:782–790. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sánchez A, Nagy P and Thorgeirsson SS:
STAT-3 activity in chemically-induced hepatocellular carcinoma. Eur
J Cancer. 39:2093–2098. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou T, Chao L, Rong G, Wang C, Ma R and
Wang X: Down-regulation of GRIM-19 is associated with STAT3
overexpression in breast carcinomas. Hum Pathol. 44:1773–1779.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim SM, Kwon OJ, Hong YK, Kim JH, Solca F,
Ha SJ, Soo RA, Christensen JG, Lee JH and Cho BC: Activation of
IL-6R/JAK1/STAT3 signaling induces de novo resistance to
irreversible EGFR inhibitors in non-small cell lung cancer with
T790M resistance mutation. Mol Cancer Ther. 11:2254–2264. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abdulghani J, Gu L, Dagvadorj A, Lutz J,
Leiby B, Bonuccelli G, Lisanti MP, Zellweger T, Alanen K, Mirtti T,
et al: Stat3 promotes metastatic progression of prostate cancer. Am
J Pathol. 172:1717–1728. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grandis JR, Drenning SD, Chakraborty A,
Zhou MY, Zeng Q, Pitt AS and Tweardy DJ: Requirement of Stat3 but
not Stat1 activation for epidermal growth factor receptor-mediated
cell growth in vitro. J Clin Invest. 102:1385–1392. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sahu RP and Srivastava SK: The role of
STAT-3 in the induction of apoptosis in pancreatic cancer cells by
benzyl isothiocyanate. J Natl Cancer Inst. 101:176–193. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo C, Yang G, Khun K, Kong X, Levy D, Lee
P and Melamed J: Activation of Stat3 in renal tumors. Am J Transl
Res. 1:283–290. 2009.PubMed/NCBI
|
|
26
|
Lin TS, Mahajan S and Frank DA: STAT
signaling in the pathogenesis and treatment of leukemias. Oncogene.
19:2496–2504. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Corvinus FM, Orth C, Moriggl R, Tsareva
SA, Wagner S, Pfitzner EB, Baus D, Kaufmann R, Huber LA, Zatloukal
K, et al: Persistent STAT3 activation in colon cancer is associated
with enhanced cell proliferation and tumor growth. Neoplasia.
7:545–555. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu CL, Jove R and Burakoff SJ:
Constitutive activation of the Janus kinase-STAT pathway in T
lymphoma overexpressing the Lck protein tyrosine kinase. J Immunol.
159:5206–5210. 1997.PubMed/NCBI
|
|
29
|
Niu G, Bowman T, Huang M, Shivers S,
Reintgen D, Daud A, Chang A, Kraker A, Jove R and Yu H: Roles of
activated Src and Stat3 signaling in melanoma tumor cell growth.
Oncogene. 21:7001–7010. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rahaman SO, Harbor PC, Chernova O, Barnett
GH, Vogelbaum MA and Haque SJ: Inhibition of constitutively active
Stat3 suppresses proliferation and induces apoptosis in
glioblastoma multiforme cells. Oncogene. 21:8404–8413. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Darnell JE: Validating Stat3 in cancer
therapy. Nat Med. 11:595–596. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Masuda M, Suzui M, Yasumatu R, Nakashima
T, Kuratomi Y, Azuma K, Tomita K, Komiyama S and Weinstein IB:
Constitutive activation of signal transducers and activators of
transcription 3 correlates with cyclin D1 overexpression and may
provide a novel prognostic marker in head and neck squamous cell
carcinoma. Cancer Res. 62:3351–3355. 2002.PubMed/NCBI
|
|
33
|
Leeman RJ, Lui VW and Grandis JR: STAT3 as
a therapeutic target in head and neck cancer. Expert Opin Biol
Ther. 6:231–241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lai SY and Johnson FM: Defining the role
of the JAK-STAT pathway in head and neck and thoracic malignancies:
Implications for future therapeutic approaches. Drug Resist Updat.
13:67–78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin WW and Karin M: A cytokine-mediated
link between innate immunity, inflammation, and cancer. J Clin
Invest. 117:1175–1183. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Budczies J, Klauschen F, Sinn BV, Győrffy
B, Schmitt WD, Darb-Esfahani S and Denkert C: Cutoff Finder: A
comprehensive and straightforward Web application enabling rapid
biomarker cut-off optimization. PLoS One. 7:e518622012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wilson JE, Petrucelli AS, Chen L,
Koblansky AA, Truax AD, Oyama Y, Rogers AB, Brickey WJ, Wang Y,
Schneider M, et al: Inflammasome-independent role of AIM2 in
suppressing colon tumorigenesis via DNA-PK and Akt. Nat Med.
21:906–913. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Man SM, Zhu Q, Zhu L, Liu Z, Karki R,
Malik A, Sharma D, Li L, Malireddi RK, Gurung P, et al: Critical
role for the DNA sensor AIM2 in stem cell proliferation and cancer.
Cell. 162:45–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
AIM2 blocks colon cancer in three ways.
Cancer Discov. 5:899–900. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Patsos G, Germann A, Gebert J and Dihlmann
S: Restoration of absent in melanoma 2 (AIM2) induces G2/M cell
cycle arrest and promotes invasion of colorectal cancer cells. Int
J Cancer. 126:1838–1849. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ratsimandresy RA, Indramohan M,
Dorfleutner A and Stehlik C: The AIM2 inflammasome is a central
regulator of intestinal homeostasis through the IL-18/IL-22/STAT3
pathway. Cell Mol Immunol. 14:127–142. 2017. View Article : Google Scholar : PubMed/NCBI
|