Introduction
Lung cancer is the most common malignant tumor and
one of the main causes of cancer-related mortality worldwide
(1). The 5-year survival rate is
~15%, and only 15–17% of patients diagnosed with distant metastasis
survive for 1 year (2,3). Adenocarcinoma is one of the major
subtypes of non-small cell lung cancer (NSCLC), and lung
adenocarcinoma patients frequently develop metastases (4). It is estimated that >35% of
patients with advanced lung adenocarcinoma develop distant
metastases, resulting in shorter survival and poor quality of life
(5). Conventional treatments,
including chemotherapy, radiotherapy and bisphosphonates, have been
shown to have limited efficacy (1).
Hence, it is crucial to elucidate the mechanisms underlying lung
adenocarcinoma progression.
Estrogen and progesterone receptors have been shown
to play an important role in NCSLC, particularly lung
adenocarcinoma (6). The incidence
and mortality of lung cancer were found to be higher among women
who receive hormone replacement therapy (7). The effects of estrogen are mediated
via estrogen receptors (ERα and ERβ) (8). Estrogen receptors are consistently
found in lung cancer tissues and cell lines (particularly
adenocarcinoma), mostly in the form of Erβ (9). Hsu et al reported that estrogen
promoted lung adenocarcinoma cell proliferation and migration via
ERβ, and high expression of ERβ was identified as an adverse
prognostic factor in patients with lung adenocarcinoma (9). However, the detailed mechanism
underlying ERβ-mediated lung adenocarcinoma progression remains
unclear.
The aim of the present study was to determine
whether the expression of ERβ is higher in lung adenocarcinoma
tissues, as well as observe the effects of its knockdown by
lentivirus interference RNA on lung adenocarcinoma cell growth and
invasion in vitro and in vivo.
Materials and methods
Immunohistochemical staining of
ERβ
Tissue microarray (TMA) assays were obtained from
Superchip (Shanghai, China) and included 75 cases of tissues from
lung adenocarcinoma and adjacent normal tissues (array ID:
HLug-Ade150Sur-02). The recorded clinicopathological information
included age, sex, tumor size, tumor location and TNM stage.
Experiments were performed as described previously (10,11).
The tissue sections were de-paraffinized, rehydrated, and treated
according to standard protocols (12). Polyclonal rabbit anti-ERβ antibody
(dilution 1:50; cat. no. ab3577) was purchased from Abcam
(Cambridge, UK) (13) and incubated
at 4°C overnight.
Immunohistochemical assay
The stained tissue arrays were defined as one of
nine degrees, according to the immunohistochemical scores reported
(10,11). Primarily, six degrees of
proportional score for positive staining were assigned according to
the proportion of positive tumor cells (0, none; 1, <1/100; 2,
1–10/100; 3, 10–30/100; 4, 30–60/100; and 5, >60/100).
Thereafter, four degrees of intensity score were assigned according
to the intensity of staining (0, none; 1, weak; 2, intermediate;
and 3, strong). The proportion and intensity scores were then added
to yield a total score, which ranged from 0 to 8. According to the
total score, the cases were classified as low/negative expression
(total score, 0–4) and high expression (total score, 5–8). Final
scores were confirmed in a double-blind manner by two independent
pathologists.
Drugs and chemicals
The ERβ agonist diarylpropionitrile (DPN;
CAS1428-67-7) and the ERα agonist propylpyrazoletriol (PPT;
CAS263717-53-9) were purchased from Tocris Bioscience (Bristol,
UK); 17β-estradiol (E2; MFCD01074033) was purchased from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Cell lines and cell culture
The human lung adenocarcinoma cell line A549, the
human breast cancer cell line MCF-7 and human bronchial epithelial
(HBE) cells were preserved in our laboratory. The A549, MCF-7 and
HBE cells were maintained in RPMI-1640 medium (C11875500B; Gibco,
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (FBS; Biochrom GmbH, Berlin, Germany). The
cells were incubated at 37°C in a humidified atmosphere of 5%
CO2/95% air.
Western blot analysis
Cells were lysed for protein extraction. After being
quantified, 25 µg of protein was subjected to 10%
SDS-polyacrylamide gel electrophoresis and transferred to
polyvinylidene fluoride membranes. The membranes were then blocked
in 5% fat-free milk and incubated overnight at 4°C with rabbit
polyclonal antibodies against ERβ (dilution 1:2,000; cat. no.
ab3577), extracellular signal-regulated kinase (ERK)1/2 (dilution
1:500; cat. no. ab176640), pERK1/2 (dilution 1:500; cat. no.
ab76299), matrix metalloproteinase (MMP)-2 (dilution 1:2,000; cat.
no. ab37150), MMP-9 (dilution 1:2,000; cat. no. ab38898) and mouse
monoclonal antibody against GAPDH (dilution 1:8,000; cat. no.
ab8245), all purchased from Abcam. Next, the membranes were
incubated with anti-rabbit/mouse secondary antibodies (cat. nos.
GTX213110-10/GTX213111-01; GeneTex, Inc., Irvine, CA, USA).
Finally, the content of the target proteins was determined by
chemiluminescence (Pierce; Thermo Fisher Scientific, Inc.).
Cell proliferation assay
The MTT assay (Sigma-Aldrich; Merck KGaA) was
conducted to determine cell proliferation. Briefly, A549 cells were
seeded into 96-well plates (Corning Inc., Corning, NY, USA) at a
density of 1×104 cells/well and treated with E2 (10 nM),
DPN (10 nM) or PPT (10 nM) for 0, 24, 48 and 72 h, and added the
same volume of phosphate-buffered saline (PBS) as the control
group. At each time-point, 20 µl MTT (10 mg/ml) was added to each
well and successively incubated for another 4 h at 37°C. After
removing the supernatant, 150 µl dimethylsulfoxide (S7020;
Invitrogen; Thermo Fisher Scientific, Inc.) was added for 10 min to
dissolve the formazan crystals. The absorbance was measured at 490
nm with a microplate reader (Multiskan MK3; Thermo Fisher
Scientific, Inc.). Each experiment was performed in triplicate and
repeated three times.
Cell infection
Short hairpin RNAs (shRNA1: GCATGGAACATCTGCTCAA;
shRNA2: GCTGAATGCCCACGTGCTT; shRNA3: GCAAAGAGGGCTCCCAGAA) targeting
ERβ (ERβ-GV248-RNAi NM_001437, target sequence:
GCAAAGAGGGCTCCCAGAA) and control shRNA (NC-GV248, target sequence:
TTCTCCGAACGTGTCACGT) were obtained from Shanghai GeneChem Co., Ltd.
(Shanghai, China). A549 cells were infected with the ERβ-shRNA
lentivirus to knock down ERβ expression, and NC-shRNA was used at
the same time as a negative control group, according to the
manufacturer's instructions. After 72 h, green fluorescent protein
indicated that the rate of infection was ~90% at a multiplicity of
infection of 10. Stably transfected cells were then selected with
puromycin for 2 weeks.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA of infected A549 cells was isolated from
cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's instructions. Total RNA (500
ng) was then reverse-transcribed into cDNA (Takara Bio, Inc., Otsu,
Japan). RT-PCR was performed using SYBR Premix Ex
Taq™ II (Takara Bio, Inc.) and gene expression was
quantified using the cycle quantification (Cq) method. The PCR
primers were as follows: ERβ forward, GATCATTGCTCCTCCTGAGC and
reverse, CACCTTCACCGTTCCAGTTT; GAPDH forward,
AGCACGGCTCCATATACATACC and reverse, TGGACCACTAAAGGAGAAAGGT.
Colony formation assay
The infected A549 and control cells in the
logarithmic growth phase were harvested and plated into 6-well
plates (cat. no. A1098201; BioExcellence International Tech Co.,
Ltd.) at 500 cells/well. After incubation for 8 days, cell colonies
(>50 cells) were stained with 0.25% crystal violet solution and
their number was manually counted. Each experiment included three
independent biological replicates and each was performed in
triplicate.
Cell invasion assay
The invasive potential of the cells was measured
using 8-µm pore size Transwell inserts (Corning, Inc.). The
infected A549 and control cells were resuspended in serum-free
RPMI-1640 and then seeded in triplicates in the upper chamber
covered with 70 µl Matrigel (diluted in 1:8; Corning, Inc.). Medium
(500 µl) containing 10% FBS was added to the bottom chamber to
serve as the chemoattractant. After 24 h, cells that had migrated
to the lower chamber were fixed with 95% ethyl alcohol and then
stained with 0.5% crystal violet solution. Finally, the number of
invading cells was counted in five random fields per sample and the
mean was calculated. Each experiment included three independent
biological replicates and each was performed in triplicate.
In vivo experiments
An experimental model of A549 cell lung metastasis
was constructed to study the effects of ERβ on lung adenocarcinoma
in vivo. A total of 18 female NOD-SCID mice, aged 4 weeks
and weighing 20–25 g, were purchased from Beijing HFK Bioscience
Co. (Beijing, China). They were housed in a specific pathogen-free
(SPF) laboratory animal environment (temperature, 22°C; ventilation
rate, 15/h; light/dark cycle, 12/12 h; food was sterilized with
Cobalt-60 irradiation and water was autoclaved, and access to the
food was ad libitum; tumor size not exceed 2.0 cm) by
professional breeders and randomly divided into three groups (6
mice/group). A549, A549-ERβ-shRNA and A549-NC-shRNA cells
(1×106/200 µl) were harvested, resuspended in PBS and
injected via the tail vein. After 3 days, E2 (0.1 mg/kg) was
subcutaneously injected once a week in a volume of 100 µl per
mouse. When the experimental mice developed symptoms such as
lameness, joint stiffness, decreased exercise capacity, paraplegia,
or an experiment for 42 days, the experiment required termination.
The mice were sacrificed humanely in a transparent euthanasia
device (ventilated 10% of isoflurane for 1 min before laying the
mice, and constantly ventilated isoflurane for another 3 min after
the mice were dead). The lungs were then excised and weighed; the
number of the metastatic lesions larger than 0.5 mm in diameter on
the surface of the lungs was counted, fixed in 10% formalin,
embedded in paraffin, and sectioned for H&E staining.
All animal studies strictly abided by the
Regulations on Animal Experimentation formulated by the Laboratory
Animal Center of the Fourth Military Medical University (The Air
Force Medical University) (Xi'an, China) and the present study was
approved by the Animal Experimental Ethical Inspection Committee of
this Center (no. 20170803).
Statistical analysis
Statistical analyses were conducted using SPSS 16.0
(SPSS Inc., Chicago, IL, USA). Wilcoxon rank sum test was used for
immunohistochemical total scores analysis and the p-ERK/ERK ratio
analysis was conducted by t-test. The other data were analyzed by
one-way analysis of variance (ANOVA) with least significant
difference (LSD) test as the post hoc test. P-values <0.05 were
considered to indicate statistically ignificant differences.
Results
Expression of ERβ in lung
adenocarcinoma patient samples and cell lines
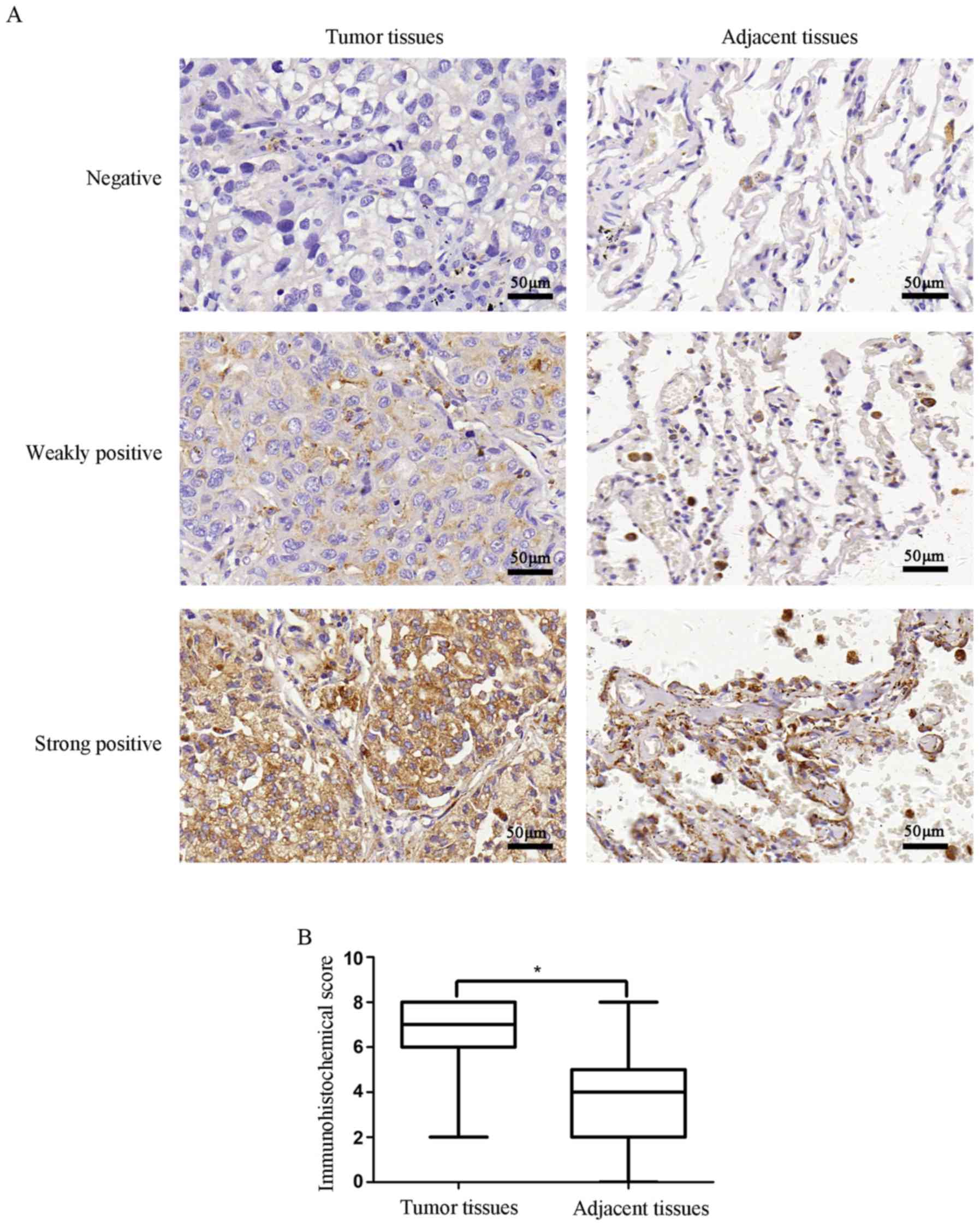
Immunohistochemistry was used to evaluate the
association of ERβ expression and pathological characteristics with
a TMA that consisted of 75 paired lung adenocarcinoma specimens and
corresponding normal samples. The expression of ERβ was observed in
the cytoplasm. The correlations of ERβ expression with
clinicopathological characteristics in the lung adenocarcinoma
patients are presented in Table I
and Fig. 1. We observed that the
protein expression of ERβ was higher in lung adenocarcinoma tissues
compared with that in adjacent non-cancerous tissues (P<0.001).
Notably, ERβ protein expression was significantly correlated with
tumor size (P=0.018), lymph node metastasis (P=0.041), clinical
stage (P=0.041) and tumor differentiation (P<0.001). The results
indicated that the expression of ERβ may be associated with the
occurrence and progression of lung adenocarcinoma.
 | Table I.Correlation of ERβ with the
clinicopathological characteristics of the lung adenocarcinoma
patients. |
Table I.
Correlation of ERβ with the
clinicopathological characteristics of the lung adenocarcinoma
patients.
|
| ERβ expression |
|
|---|
|
|
|
|
|---|
| Characteristics | N | Low, n (%) | High, n (%) | P-value |
|---|
| Tissue source |
|
|
| <0.0001 |
|
Tumor | 75 | 6 (11.5) | 69 (70.4) |
|
|
Adjacent | 75 | 26 (88.5) | 49 (29.6) |
|
| Age (years) |
|
|
| 0.352 |
|
<60 | 32 | 11 (37.9) | 22 (45.7) |
|
| ≥60 | 43 | 19 (62.1) | 25 (58.1) |
|
| Sex |
|
|
| 0.200 |
| M | 40 | 3 (33.3) | 25 (56.1) |
|
| F | 35 | 6 (66.7) | 24 (43.9) |
|
| Tumor size (mm) |
|
|
| 0.018 |
| ≤30 | 31 | 7 (77.8) | 24 (36.4) |
|
|
>30 | 44 | 2 (22.2) | 42 (63.6) |
|
| Location |
|
|
| 0.356 |
| Left | 31 | 5 (55.6) | 26 (39.4) |
|
|
Right | 44 | 4 (44.4) | 40 (60.6) |
|
|
Differentiation |
|
|
| <0.0001 |
|
Well | 8 | 5 (55.6) | 3 (4.5) |
|
|
Moderate | 51 | 3 (33.3) | 48 (72.7) |
|
|
Poor | 16 | 1 (11.1) | 15 (22.7) |
|
| Lymph node
metastasis |
|
|
| 0.041 |
| No | 27 | 6 (66.7) | 21 (31.8) |
|
|
Yes | 48 | 3 (33.3) | 45 (68.2) |
|
| Stage |
|
|
| 0.041 |
|
I–II | 43 | 8 (88.9) | 35 (53.0) |
|
|
III | 32 | 1 (11.1) | 31 (47.0) |
|
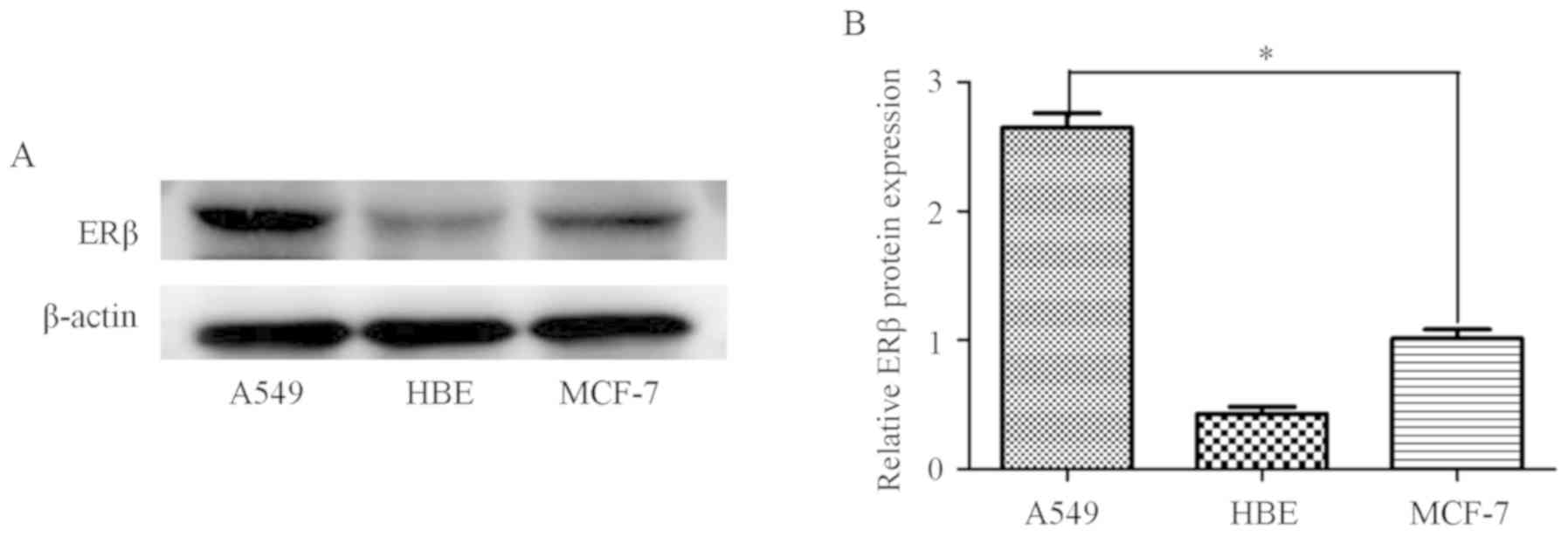
Furthermore, ERβ expression was compared among lung
adenocarcinoma cells (A549), breast cancer cells (MCF-7) and HBE
cells. MCF-7 cells were used as the positive control, and ERβ
expression was found to be higher in A549 compared with that in
MCF-7 and HBE cells (Fig. 2). The
results indicated that ERβ is an important functional ER subtype in
A549 cells.
E2 and DPN promote A549 cell
proliferation
The effects of E2, DPN and PPT on A549 and MCF-7
cells were investigated. MTT assay was conducted to evaluate cell
proliferation. As shown in Fig. 3A,
the proliferation of cells treated with E2 and DPN was markedly
increased compared with that in the control groups while PPT which
was effective for the (AMCF-7 cells (Fig. 3B) did not obviously promote A549
cell proliferation; the E2-induced A549 cell proliferation may be
mediated via ERβ more than ERα.
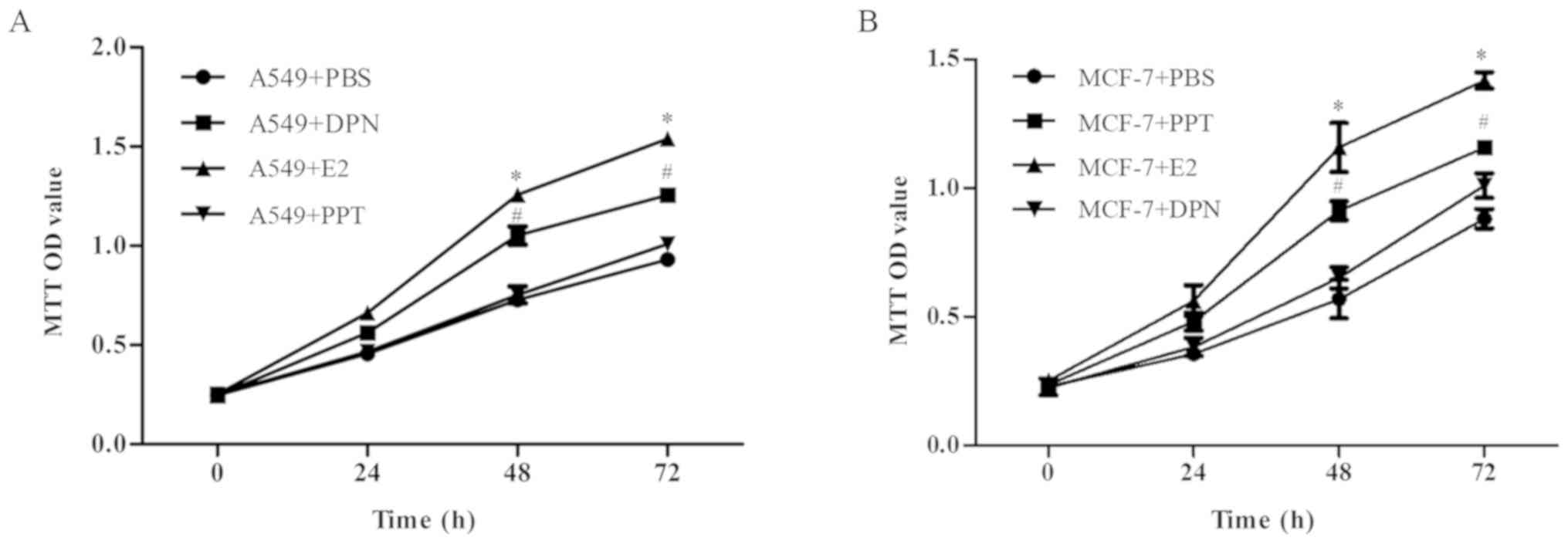 | Figure 3.E2 and DPN promote A549 cell
proliferation. Cells were treated with E2, DPN, PPT or PBS for 0,
24, 48 and 72 h, and cell proliferation was evaluated using the MTT
assay. (A) Effect of E2, DPN and PPT on the A549 cell
proliferation. (B) Effect of E2, DPN and PPT on the MCF-7 cell
proliferation. Data are presented as the mean ± standard deviation.
*P<0.05 (E2 group vs. control group) and #P<0.05
(DPN group vs. control group). DPN, diarylpropionitrile; PPT,
propylpyrazoletriol; E2, 17β-estradiol; PBS, phosphate-buffered
saline. |
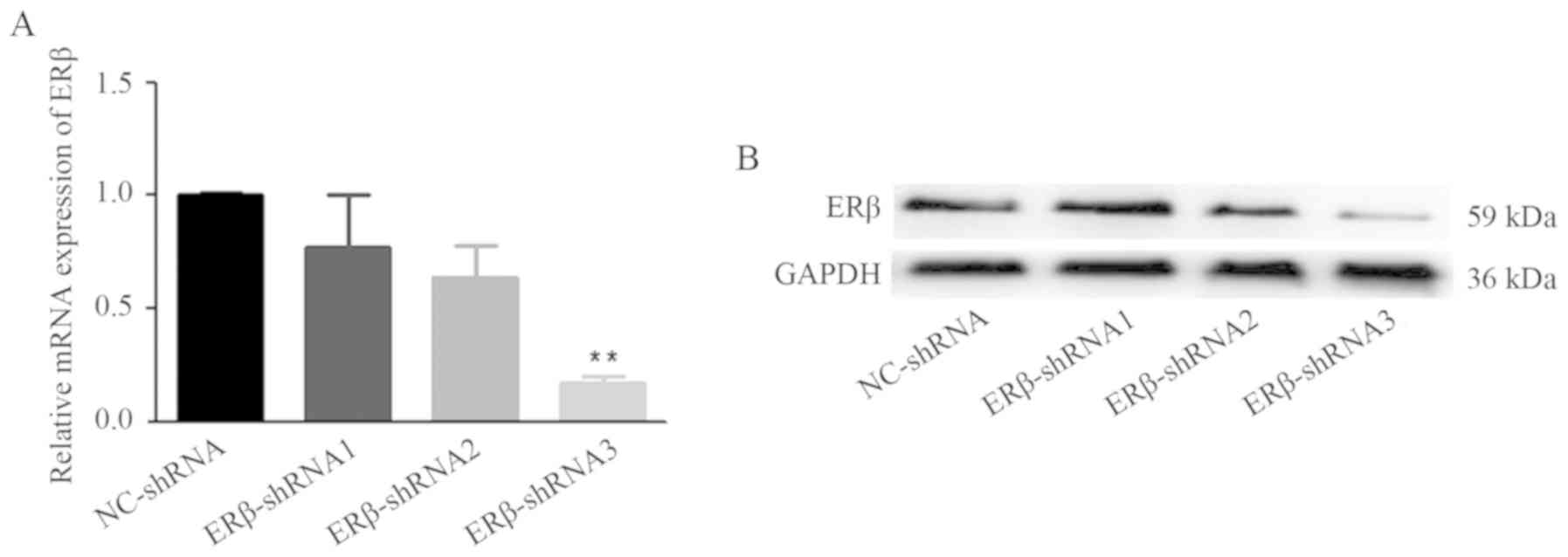
Expression of ERβ decreases in cells
stably transfected by lentivirus RNA interference
The lentivirus RNA interference technique was used
to downregulate the expression of ERβ. Following cell infection and
antibiotic screening for 2 weeks, the infection efficacy was
confirmed by RT-PCR (Fig. 4A) and
western blot analysis (Fig. 4B and
C). ERβ was stably decreased by shRNA and the ERβ-GV248-RNAi#3
was the most effective one, thus we named it as ERβ-shRNA for the
following tests. The results indicated that ERβ expression was
suppressed by ERβ-shRNA.
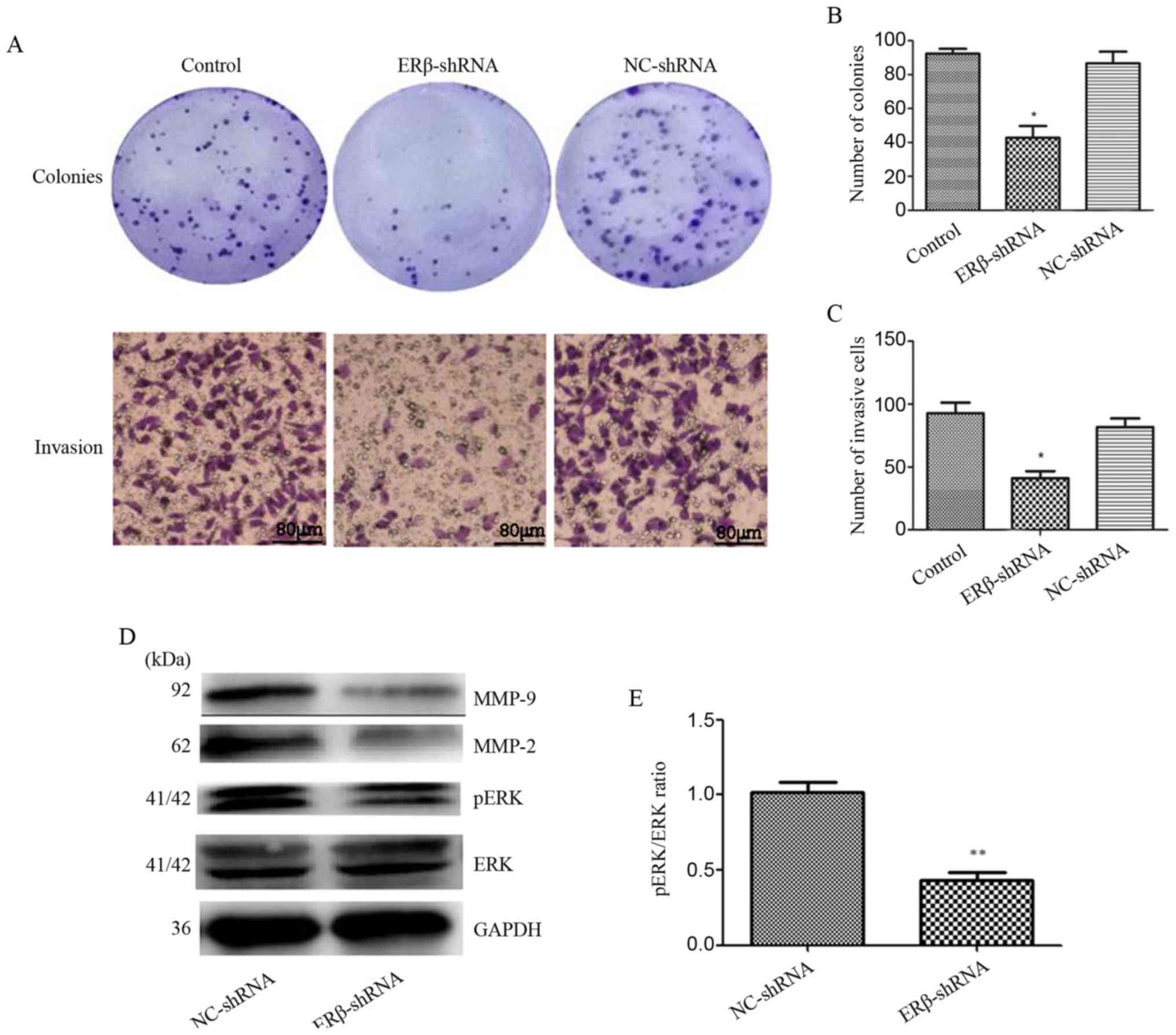
Downregulation of ERβ expression
inhibits colony formation and invasion of A549 cells in vitro
The effects of ERβ-shRNA on A549 cells were
examined. As shown in Fig. 5A-D,
colony formation and cell invasion assays demonstrated that
ERβ-shRNA inhibited A549 cell proliferation and invasion compared
with NC-shRNA and control (P<0.05). Mechanistically, ERβ
knockdown suppressed the expression of pERK, MMP-2 and MMP-9
(Fig. 5E; P<0.05). These
findings indicate that ERβ is a functional mediator. Therefore,
knockdown of ERβ expression inhibited A549 cell proliferation and
invasion via downregulation of pERK, MMP-2 and MMP-9.
Downregulation of ERβ expression
suppresses lung metastasis of A549 cells in vivo
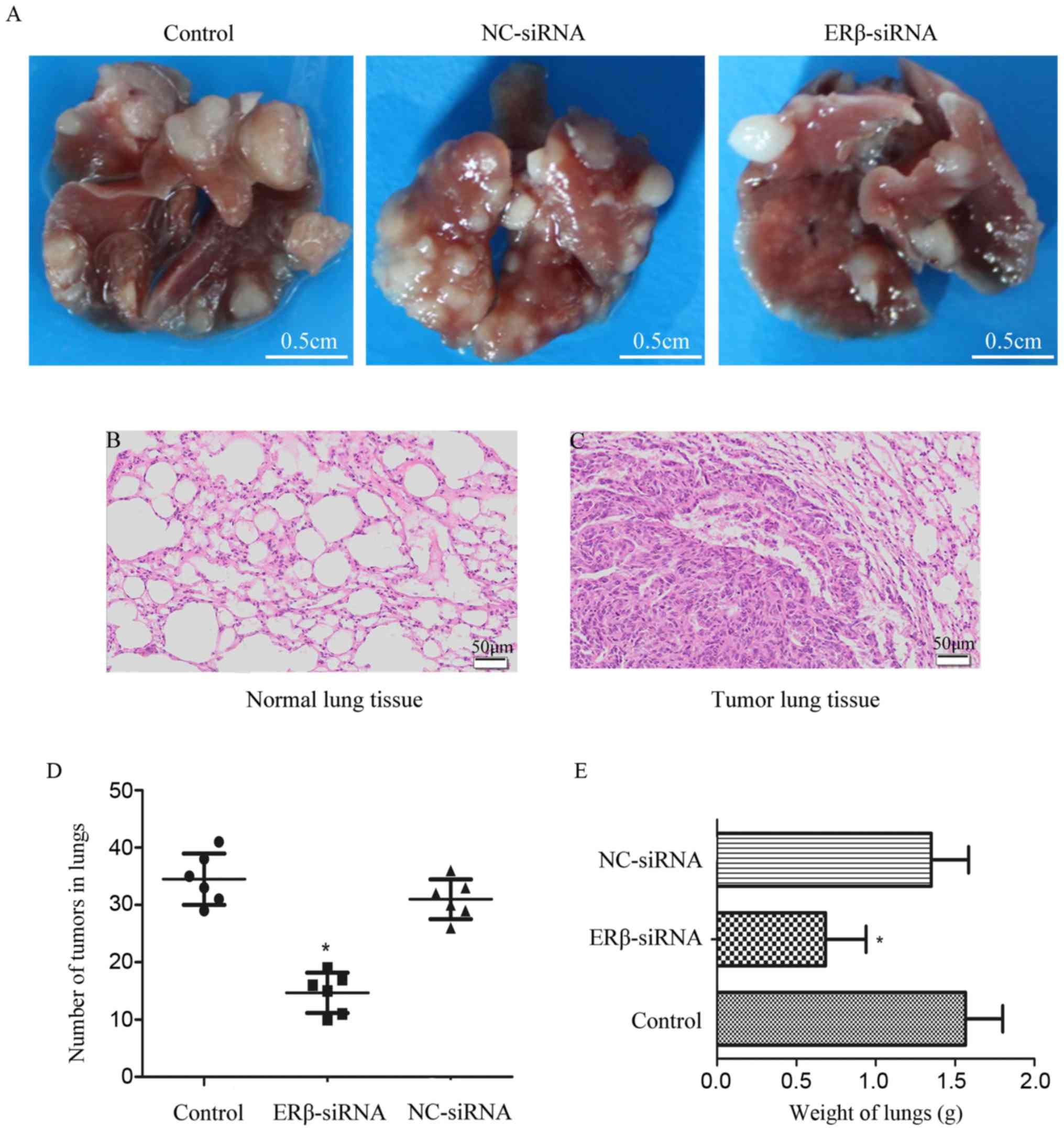
To investigate whether downregulation of ERβ
expression may serve as a therapeutic target for lung
adenocarcinoma, an experimental lung metastatic mouse model was
constructed. In vivo lung metastasis assay demonstrated that
downregulation of ERβ expression was associated with fewer
metastatic tumors and lower lung weight compared with the control
(Fig. 6). These results indicated
that downregulation of ERβ may inhibit tumor growth and lung
metastasis in vivo.
Discussion
Estrogen and ERs are considered to play an important
role in lung carcinogenesis (14).
Several studies have demonstrated that ERβ is the predominant ER in
lung cancer tissue and tumor cell lines, particularly
adenocarcinoma (15,16). It was previously reported that
estrogen promotes lung adenocarcinoma cell proliferation and
migration via ERβ (11). However,
the detailed mechanism underlying ERβ-mediated lung adenocarcinoma
progression remains unclear. The present study was designed to
investigate the biological effects and mechanism of action of ERβ
in lung adenocarcinoma.
To determine the association between ERβ and
clinicopathological characteristics in lung adenocarcinoma,
immunohistochemistry was used to evaluate the expression of ERβ in
TMA, which included 75 tumor and adjacent normal tissues from
patients with lung adenocarcinoma. A higher ERβ expression was
detected in lung adenocarcinoma specimens compared with adjacent
non-cancerous tissues. Notably, ERβ protein expression was found to
be significantly correlated with tumor size (P=0.018), lymph node
metastasis (P=0.041), clinical stage (P=0.041) and tumor
differentiation (P<0.001), suggesting that ERβ plays an
important role in the occurrence and development of lung
adenocarcinoma. Our findings were similar to those of Luo et
al, who reported that ERβ overexpression promotes the
progression of NSCLC (16).
Our findings in vitro were in agreement with
the TMA results. ERβ expression was higher in the A549 cell line
compared with that in HBE cells. ERβ was identified as the
important ER subtype in lung adenocarcinoma cells. MTT assay was
used to investigate whether the effects of E2 are mediated via ERα
or ERβ. The results indicated that E2 induced lung adenocarcinoma
cell proliferation via ERβ. These results are supported by the
findings of Fan et al (17)
and Warner and Gustafsson (18),
who reported that ERα was not the main mediator of transcriptional
responses to E2 in NSCLC cells, but ERβ was more likely to be the
primary type in lung tumor tissues and cell lines, and that
estrogen-dependent responses in NSCLC cells are principally
mediated by ERβ. Furthermore, we demonstrated that downregulation
of ERβ expression inhibited colony formation and invasion of A549
cells. In vivo, we constructed an experimental lung
metastatic mouse model. The lung metastasis assay demonstrated that
downregulation of ERβ expression was associated with fewer
metastatic tumors and lower lung weight compared with the control
group. Our results are consistent with those of Fan et al,
who reported that estrogen and DPN promote lung metastasis of A549
cells (17). Fan et al's
study revealed that estrogen induced lung cancer metastasis through
the ERβ/MMP-2 axis. However, in the present study, MMP-9 was
identified as a novel target gene of ERβ. Thus, ERβ may promote
lung cancer metastasis not only through MMP-2, but also through
MMP-9. The development of lung adenocarcinoma is a complex
multistep process. The ERK signaling pathway plays an important
role in lung cancer cell proliferation and invasion. Therefore, the
protein expression of ERK and pERK was determined in A549-ERβ-shRNA
cells. The results revealed that downregulation of ERβ expression
decreased pERK expression levels. This finding suggested that
downregulation of ERβ expression inhibited the proliferation and
invasion of A549 cells through suppressing the phosphorylation of
ERK. ERs also regulate gene expression through binding to other
transcription factors, such as the activator protein 1 (AP-1)
(19), the most important
structural components of which are c-Jun and c-Fos. In addition,
MMP is crucial for malignant tumor metastasis (20,21),
particularly MMP-2 and MMP-9.
Taken together, the findings of the present study
indicate that ERβ may promote lung adenocarcinoma growth and
metastasis through the MEK/ERK signaling pathway and MMP-2/MMP-9
expression, and therefore, hold promise as a novel therapeutic
target for lung adenocarcinoma.
Acknowledgements
The authors would like to thank all the colleagues
of the Oncology Research Center for their comments on earlier
versions of this manuscript.
Funding
The present study was supported by the National
Natural Science Foundation of China (nos. 81272348, 81572814,
81572251) and the Natural Science Foundation of Shanxi, China (no.
2018JM7095).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HZ designed the study. WC and BX performed the
immunohistochemical assay and all the in vitro experiments
and collected the data. HP, LH and WS conducted the animal
experiments and collected the data. PC, LD, ZZ and LL analyzed the
data and performed the relative statistical analysis. ZZ and LL
provided guidance during the study. WC contributed to the writing
of the manuscript. All authors have read and approved the final
version of this manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
All animal studies strictly abide by the Regulations
on Animal Experimentation formulated by the Laboratory Animal
Center of the Fourth Military Medical University (The Air Force
Medical University) (Xi'an, China) and this study was approved by
the Animal Experimental Ethical Inspection Committee of this Center
(no. 20170803).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zamay TN, Zamay GS, Kolovskaya OS, Zukov
RA, Petrova MM, Gargaun A, Berezovski MV and Kichkailo AS: Current
and prospective protein biomarkers of lung cancer. Cancers.
9:E1552017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fiorentino FP, Macaluso M, Miranda F,
Montanari M, Russo A, Bagella L and Giordano A: CTCF and BORIS
regulate Rb2/p130 gene transcription: A novel mechanism and
a new paradigm for understanding the biology of lung cancer. Mol
Cancer Res. 9:225–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Blandin Knight S, Crosbie PA, Balata H,
Chudziak J, Hussell T and Dive C: Progress and prospects of early
detection in lung cancer. Open Biol. 7:1700702017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ren G, Esposito M and Kang Y: Bone
metastasis and the metastatic niche. J Mol Med. 93:1203–1212. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zang L, Ma M, Hu J, Qiu H, Huang B and Chu
T: The effects of lung and prostate cancer bone metastasis on serum
osteoprotegerin levels: A meta-analysis. Sci Rep. 5:183242015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siegfried JM and Stabile LP: Estrongenic
steroid hormones in lung cancer. Semin Oncol. 41:5–16. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chlebowski RT, Schwartz AG, Wakelee H,
Anderson GL, Stefanick ML, Manson JE, Rodabough RJ, Chien JW,
Wactawski-Wende J, Gass M and Women's Health Initiative
Investigators: Oestrogen plus progestin and lung cancer in
postmenopausal women (Women's Health Initiative trial): A post-hoc
analysis of a randomised controlled trial. Lancet. 374:1243–1251.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sims NA, Clément-Lacroix P, Minet D,
Fraslon-Vanhulle C, Gaillard-Kelly M, Resche-Rigon M and Baron R: A
functional androgen receptor is not sufficient to allow estradiol
to protect bone after gonadectomy in estradiol receptor-deficient
mice. J Clin Invest. 111:1319–1327. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hsu LH, Liu KJ, Tsai MF, Wu CR, Feng AC,
Chu NM and Kao SH: Estrogen adversely affects the prognosis of
patients with lung adenocarcinoma. Cancer Sci. 106:51–59. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawai H, Ishii A, Washiya K, Konno T, Kon
H, Yamaya C, Ono I, Minamiya Y and Ogawa J: Estrogen receptor alpha
and beta are prognostic factors in non-small cell lung cancer. Clin
Cancer Res. 11:5084–5089. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Allred DC, Harvey JM, Berardo M and Clark
GM: Prognostic and predictive factors in breast cancer by
immunohistochemical analysis. Mod Pathol. 11:155–168.
1998.PubMed/NCBI
|
|
12
|
Liu Y, Yan X, Liu N, Zhou J, Liu J, Pang
H, Cao J, Liu Y, Wang Y, Liu L and Zhang H: Lentivirus-delivered
ZEB-1 small interfering RNA inhibits lung adenocarcinoma cell
growth in vitro and in vivo. J Cancer Res Clin Oncol.
138:1329–1338. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Andersson S, Sundberg M, Pristovsek N,
Ibrahim A, Jonsson P, Katona B, Clausson CM, Zieba A, Ramström M,
Söderberg O, et al: Insufficient antibody validation challenges
oestrogen receptor beta research. Nat Commun. 8:158402017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hsu LH, Chu NM and Kao SH: Estrogen,
estrogen receptor and lung cancer. Int J Mol Sci. 18:17132017.
View Article : Google Scholar :
|
|
15
|
Mah V, Marquez D, Alavi M, Maresh EL,
Zhang L, Yoon N, Horvath S, Bagryanova L, Fishbein MC, Chia D, et
al: Expression levels of estrogen receptor beta in conjunction with
aromatase predict survival in non-small cell lung cancer. Lung
Cancer. 74:318–325. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo Z, Wu R, Jiang Y, Qiu Z, Chen W and Li
W: Overexpression of estrogen receptor beta is a prognostic marker
in non-small cell lung cancer: A meta-analysis. Int J Clin Exp Med.
8:8686–8697. 2015.PubMed/NCBI
|
|
17
|
Fan S, Liao Y, Liu C, Huang Q, Liang H, Ai
B, Fu S and Zhou S: Estrogen promotes tumor metastasis via estrogen
receptor beta-mediated regulation of matrix-metalloproteinase-2 in
non-small cell lung cancer. Oncotarget. 8:56443–56459.
2017.PubMed/NCBI
|
|
18
|
Warner M and Gustafsson JA: The role of
estrogen receptor beta (ERbeta) in malignant diseases--a new
potential target for antiproliferative drugs in prevention and
treatment of cancer. Biochem Biophys Res Commun. 396:63–66. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Prusty BK and Das BC: Constitutive
activation of transcription factor AP-1 in cervical cancer and
suppression of human papillomavirus (HPV) transcription and AP-1
activity in HeLa cells by curcumin. Int J Cancer. 113:951–960.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao XZ, Liu Y, Zhou LJ, Wang ZQ, Wu ZH
and Yang XY: Role of estrogen in lung cancer based on the estrogen
receptor-epithelial mesenchymal transduction signaling pathways.
OncoTargets Ther. 8:2849–2863. 2015. View Article : Google Scholar
|
|
21
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|