Introduction
Human urinary bladder cancer (UBC) is the most
common urological tumor, which is frequently metastatic and
recurrent (1). According to an
epidemiologic study, the UBC incidence of 2015 in China is
8.05/100,000, and the mortality is 3.29/100,000 (2). However, the mechanisms of development
of UBC remain unclear.
The rapid growth of cancer cells requires a lot of
energy, and relying only on the aerobic oxidation of mitochondria
is not sufficient (3). Therefore,
cancer cells also acquire energy for growth by anaerobic
glycolysis, a phenomenon known as the Warburg effect (4). Metabolomics analysis demonstrated that
cancer cells exhibit an increased energy metabolic phenotype for
glycolysis under aerobic and anaerobic conditions (5). It has been reported that glucose
transporter, lactate dehydrogenase A (LDHA) and pyruvate kinase are
highly expressed in numerous tumor types, including lung and
gastric cancer, and 18-fluoro-deoxyglucose positron emission
tomography/computerized tomography had been used to confirm that
tumor cells are more capable of using glucose, compared with normal
cells (6,7).
Phospholipase Cε (PLCε) is a member of the
phospholipase C family, which catalyzes polyphosphoinositol, such
as phosphatidylinositol 4,5-diphosphate, and produces the second
messenger, including 1,4,5-triphosphate and diacylglycerol
(8). Our previous studies
demonstrated that PLCε may serve an important role in UBC growth
(9,10) and activation of the signal
transducer and activator of transcription 3 (STAT3) pathway
(11). STAT3 is involved in the
development and progression of a variety of tumor types, including
colon, gastric and liver cancer (12–14),
which may be partially achieved by regulating anaerobic glycolysis
(15). Since our previous results
demonstrated that PLCε may act upstream of STAT3, it may be
considered that PLCε participates in cancer cell growth by
regulating anaerobic glycolysis. Therefore, in order to further
investigate the role and mechanisms of PLCε in bladder cancer, the
T24 cell line and a control strain that stably knocked down PLCε
were established using short hairpin RNA (shRNA) targeting PLCε and
non-target control. Subsequently, the mRNA expression levels of
tumor associated molecules were examined with gene chip and
multiple signaling pathways, including P53, mitogen-activated
protein kinase, pyruvate metabolism, tryptophan metabolism, and
cysteine and methionine metabolism, and they were significantly
decreased in PLCε-deficient T24 cells, compared with control cells.
As an important functional gene in aerobic glycolysis (16,17),
LDHA was also determined to be regulated by PLCε in T24 cells
(unpublished data), indicating the role of PLCε in glycolysis.
In the present study, the correlation among PLCε,
STAT3 and LDHA in UBC growth was confirmed. Additionally, these
data may provide insights into mechanisms and potential treatments
of UBC.
Materials and methods
Tissue specimens
A total of 64 UBC tissue samples and 42 adjacent
tissue samples were obtained from 64 patients (male:female, 53:11;
age range, 34–88 years; median age, 65 years) who underwent surgery
at the Department of Urology in the First Affiliated Hospital of
Chongqing Medical University (Chongqing, China) from January 2017
to December 2017. The histological grade and stage were determined
according to the UICC guidelines (18). The patients provided informed
consent. All samples were stored at −80°C until required. The
present study was approved by the Ethics and Research Committees of
Chongqing Medical University (approval no. 2016-152).
Cell culture
T24 and HeLa cells were purchased from the Shanghai
Cell Bank (Shanghai, China). Subsequently, T24 cells was cultured
in RPMI-1640 medium and HeLa cells was cultured in Dulbecco's
modified Eagle's medium, and both were supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) at 37°C in an atmosphere containing 5% CO2. The
PLCε stable knockdown T24 cell line was described in our previous
study (9). A total of
5×104 T24 cells/well were cultured in a 6-well plate at
37°C overnight until ~60% confluence. The cells were then
transfected with a mixture of 0.4 µg PLCε shRNA (sh-PLCε) or 0.4 µg
non-target control shRNA (sh-NC), and 8 µl Effectene transfections
reagent (Qiagen China Co., Ltd., Shanghai, China) in 1 ml of fresh
serum-free RPMI-1640 medium. The transfected cells were selected
using G418 (400 µg/ml). Monoclonal cells were collected after 4
weeks of exposure to selective pressure and were further cultured
at 37°C for subsequent experimentation.
Cell proliferation
Cell viability was analyzed via Cell Counting Kit-8
(CCK-8; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany; cat. no.
96992) assays. For small interfering RNA (siRNA) transfections,
cells were plated and in a 96-well plate at a density of
1×104 cells/well and cultured overnight at 37°C.
Subsequently, 0.5 µl gene-specific siRNAs (20 µM) each well were
transfected in the presence of Opti-MEM medium (cat. no. 51985034;
Gibco; Thermo Fisher Scientific, Inc.) using X-tremeGENE siRNA
transfection reagent (cat. no. 4476093001; Roche Applied Science,
Penzberg, Germany), according to the manufacturer's protocols.
siRNA targeting LDHA (5′-CGAACTGGGCAGTATAAAC-3′) and negative
control (5′-UUCUCCGAACGUGUCACGUTT-3′) were designed and synthesized
by Shanghai Genepharma Co., Ltd. (Shanghai, China). After 48 h post
transfection, 100 µl CCK-8 solution was added to each well of the
plate after various time points (12, 24, 48, 72 and 96 h), and the
plate was incubated at 37°C for 4 h. Subsequently, the absorbance
of each well was measured at 450 nm.
Reagents and antibodies
Stattic (cat. no. S7024) and recombinant human
interleukin-6 (IL-6; cat. no. CTP0061) were purchased from Selleck
Chemicals (Houston, TX, USA) and Gibco (Thermo Fisher Scientific,
Inc.), respectively. Antibodies against β-actin (cat. no. sc-47778)
and PLCε (cat. no. sc-28402) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA), and antibodies against LDHA
(cat. no. 3582), total STAT3 (cat. no. 4904) and phospho-STAT3
(cat. no. 9145) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Anti-HA antibody (cat. no. ab9110) was
purchased from Abcam (Cambridge, UK).
Immunochemistry
Sections were observed using an upright phase
contrast light microscope (Nikon Corporation, Tokyo, Japan) at
magnifications of ×200. The tissue specimens were fixed with 10%
neutral formalin at room temperature for 15 min, embedded in
paraffin and 5 µm thick sections were prepared. Paraffin
wax-embedded tissue sections were dewaxed, rehydrated (incubated in
100, 95, 80 and 75% series gradient ethanol at room temperature for
3 min each and then in distilled water for 10 min) at room
temperature and microwaved at 95°C for 30 min in sodium citrate
buffer (0.01 M, pH 6.0; Anhui Leagene Biotechnology Co., Ltd,
Huaibei, Anhui China, http://www.leagene.cn) to repair antigen epitopes. The
tissue sections were incubated at 37°C for 10 min with 3%
H2O2 and blocked by 5% normal goat serum
(cat. no. AR0009; Wuhan Boster Biological Technology, Ltd., Wuhan,
China) at 37°C for 10 min in order to eliminate endogenous
peroxidase activity. Following this, tissue sections were incubated
with primary monoclonal antibodies targeting PLCε (dilution 1:50)
and LDHA (dilution 1:200) at 4°C overnight. Subsequently, sections
were incubated with biotinylated goat anti-rat (cat. no. 31830) or
rabbit anti-goat IgG antibody (cat. no. 31732) (dilution, 1:5,000;
Invitrogen; Thermo Fisher Scientific, Inc.) for 45 min at 37°C,
followed by incubation with streptavidin peroxidase at 37°C for 15
min. Sections were stained with the chromogen diaminobenzidine at
room temperature for 2 min (OriGene Technologies, Inc., Beijing,
China) until a brown color developed. The cell nucleus was
counterstained with hematoxylin at room temperature for 2 min.
After rinsing twice with running water, the sections were immersed
in 95% ethanol for 5 sec and then stained with eosin for 1 min at
room temperature. All images were quantified using two parameters,
staining positive rate and positive stating intensity. The positive
staining intensity criteria were as follows: 0 points, no staining;
1 point, light yellow; 2 points, brownish yellow; and 3 points,
brown. The cell positive staining rate was as follows: 0%, no
positive staining; 1, <5% positive staining; 2, 5–50% positive
staining; 3, >50% positive staining. The total score is the sum
of the positive staining intensity score and the cell positive
staining rate score. For statistical analysis, the slice tissue
with a total score of 0–2 was judged to be negative, and the slice
with a total score of 3–6 was judged to be positive.
Protein isolation and western
blotting
Whole-cell lysates were prepared from cells that had
been washed with PBS 3 times and harvested by centrifugation at 500
× g for 10 min at 4°C. Cell pellets were resuspended in
Radioimmunoprecipitation Assay lysis buffer, containing 50 mM Tris,
pH 7.5, 150 mM sodium chloride, 1% NP-40, 0.2% SDS, 0.5% sodium
deoxycholate, 0.1 mM ethylenediaminetetraacetic acid, and 1%
protease and phosphatase inhibitors (Sigma-Aldrich; Merck KGaA), on
ice for 30 min with occasional vortex. The lysates were then
centrifuged at 14,000 × g for 15 min at 4°C. Subsequently,
supernatants were collected, and protein concentrations were
measured using a Bio-Rad protein assay kit (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Cell lysates (40 µg) were separated by
10% SDS-PAGE and transferred to polyvinylidene difluoride membranes
(Immobilon-P membranes; EMD Millipore, Billerica, MA, USA).
Membranes were blocked with blocking buffer (5% non-fat milk and
0.1% Tween-20 in TBS) for 1 h at room temperature. Following
incubation with appropriate primary antibodies (β-actin, 1:1,000
dilution; PLCε, 1:500 dilution; LDHA, 1:1,000 dilution; total
STAT3, 1:1,000 dilution; phospho-STAT3, 1:1,000 dilution) overnight
at 4°C, membranes were then incubated with horseradish
peroxidase-conjugated secondary antibodies (1:5,000; cat. no.
A16096; Invitrogen; Thermo Fisher Scientific, Inc.) at room
temperature for 1 h, and protein bands were detected using a
Electrochemiluminescence Plus Western Blotting Detection system
(cat. no. RPN2133; GE Healthcare Life Sciences, Little Chalfont,
UK) with a ChemiDoc XRS+ imaging system (Bio-Rad Laboratories,
Inc.).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
RNA was extracted using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). cDNA was generated
using a High-Capacity cDNA Reverse Transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.). RT-qPCR was performed
using a CFX96 Real-Time System (Bio-Rad Laboratories, Inc.) with
specific sense and antisense primers in a 20 µl reaction volume
containing 10 µl SYBR®-Green PCR Master mix (Bio-Rad
Laboratories, Inc.), 10 µl 1 µM primer stock and 40 ng cDNA. The
conditions of RT-qPCR were as follows: 10 min at 95°C, followed by
40 cycles of 95°C for 15 sec and 60°C for 30 sec. Gene relative
expression was analyzed using the method of comparative
2−ΔΔCq (19) and then
normalized by β-actin. The primers were as follows: β-actin,
forward, 5′-GTCTGCCTTGGTAGTGGATAATG-3′, and reverse,
5′-TCGAGGACGCCCTATCATGG-3′. PLCε, forward,
5′-GCTTCTTAACACGGGACTTGG-3′, and reverse,
5′-CTTCAAGGGCATTGTGCTCTC-3′. LDHA, forward,
5′-ATGGCAACTCTAAAGGATCAGC-3′, and reverse,
5′-CCAACCCCAACAACTGTAATCT-3′.
Glucose consumption and lactate
production
A total of 1×105 cells were incubated in
each well of a 6-well plate at 37°C with RPMI-1640 medium
overnight. Following the corresponding treatment, medium was
collected by centrifugation at 500 × g for 5 min at room
temperature to remove the cells, and glucose consumption was
measured using a Glucose (HK) Assay kit (Sigma-Aldrich; Merck KGaA;
cat. no. GAHK20). Lactate levels and pH values in the culture media
were measured using a Lactate Assay kit (BioVision, Inc., Milpitas,
CA, USA).
Chromatin immunoprecipitation (ChIP)
assay
HeLa cells were co-transfected with pcDNA-HA-STAT3
and pGL4-pLDHA plasmids (OBio Technology Corp., Ltd., Shanghai,
China). Mammalian expression vector pcDNA-HA-STAT3 was purchased
from Sino Biological, Inc. (Beijing, China). Briefly, complete
coding sequence of STAT3 (2,313 bp; reference sequence: NM_139276)
was cloned into pcDNA3.1 (+) using restriction site HindIII
and NotI. HA tag (5′-TATCCTTACGACGTGCCTGACTACGCC-3′) was
fused to the N-terminus of STAT3 open reading frame. The LDHA
promoter sequence (−2,000 to +200 bp; NM_005566) was artificially
synthesized by OBio Technology and cloned into the pGL4.10 plasmid
(Promega Corporation, Madison, WI, USA). After 48 h post
transfection, cells were treated with formaldehyde at a final
concentration of 1% at room temperature for 10 min to crosslink DNA
and proteins. The crosslinking reaction was stopped by adding
glycine at 0.125 mol/l final concentration for 5 min at room
temperature. Cells were rinsed twice with ice-cold 1X PBS and
resuspended in cell lysis buffer (10 mM Tris-HCL (pH 8.0), 10 mM
NaCl, 3 mM MaCl2, 0.5% NP-40 and protease inhibitors)
and incubated on ice for 15 min. The cell suspension was vortexed
at 1,000 × g for 5 sec every 5 min to aid the release of the
nuclei. Nuclei were collected by centrifugation at 800 × g at 4°C
for 5 min, resuspended in nuclei lysis buffer [1% SDS, 5 mmol/l
EDTA, 50 mmol/l Tris-HCl (pH 8.0) and protease inhibitors] and
sonicated to generate chromatin to length of 200–500 bp (10×15 sec
at 55% maximum potency). Following centrifugation at 12,000 × g for
10 min at 4°C, samples (400 mg of protein extracts) were
immunoprecipitated overnight at 4°C with 2 µg anti-HA antibody
(dilution 1:200; cat. no. ab9110; Abcam). Subsequently, 1%
supernatant from the immunoprecipitation was saved as total input
of chromatin and was processed with the eluted immunoprecipitates
beginning at the crosslink reversal step. Following this, 20 µl
magnetic beads (Dynabeads M-280 Sheep anti Mouse IgG; Invitrogen;
Thermo Fisher Scientific, Inc.) were added into each sample and
incubated at 4°C for 4 h with rotation. Immunoprecipitates were
washed once with each of the ChIP Low Salt buffer (0.1% SDS, 1%
Triton-100, 2 mM EDTA, 50 mM Hepes and 150 mM NaCl; pH 7.5), ChIP
High Salt buffer (0.1% SDS, 1% Triton Χ-100, 2 mM EDTA, 50 mM Hepes
and 500 mM NaCl; pH 7.5), ChIP LiCl buffer (0.25 M LiCl, 0.5%
NP-40, 0.5% sodium deoxycholated, 1 mM EDTA and 10 mM Tris-HCl; pH
8.0) and TE buffer (10 mM Tris-HCl and 1 mM EDTA). Immunocomplexes
were eluted with 90 µl elution buffer (1% SDS and 50 mmol/l
NaHCO3) and 10 g RNase A was added to the pooled
eluates. Crosslinks were reverted by incubation at 65°C for at
least 6 h. Samples were added with 1 l 20 g/l proteinase K and
incubated for 2 h at 45°C. After incubation at 95°C for 10 min,
samples were purified with a Qiaquick PCR purification kit (28104;
Qiagen GmbH, Hilden, Germany). Based on the predicted binding sites
by the JASPAR database (http://jaspar.genereg.net/), corresponding ChIP
primers (ChIP1, 2 and 3) were designed for three predicted possible
binding sites as follows: ChIP1, forward,
5′-CCCCAACCCAAGCCTTTCAG-3′, and reverse,
5′-ACCTCAGGGCAGGGCAGATT-3′; ChIP2, forward,
5′-CCCCATTTCAGAACCTAGAGTG-3′, and reverse,
5′-GTGCAGCTTTGAGATAGATCCATAA-3′; and ChIP3, forward,
5′-TTCCCTAATCATTTGGTCTTTCC-3′, and reverse,
5′-CTGGGCCTGTATTCTTGCTG-3′. DNA samples were amplified with target
promoter-specific primers using RT-qPCR, as aforementioned, except
for the number of cycles being adjusted to 50 cycles, and
normalized to normal IgG control.
Luciferase reporter assay
Construction of eukaryotic expression plasmid
pcDNA-HA-STAT3 and luciferase reporter plasmid pGL4-pLDHA [-2,000
to +200 bp of LDHA (reference sequence: NM_005566) promoter
inserted into pGL4.10 luciferase reporter plasmid] was
aforementioned. HeLa cells were co-transfected with pcDNA-HA-STAT3,
pGL4-pLDHA and pGL4.7-hRluc (cat. no. E6881; Promega Corporation)
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After 48 h post transfection, the
Dual-Luciferase Reporter assay system (cat. no. E1910; Promega
Corporation) was used to detect luciferase activity, according to
the manufacturer's protocol. All experiments were performed in
triplicate and normalized to Renilla luciferase
activity.
Xenograft tumor model in vivo
Male BALB/c-nude mice (3–5 weeks old; weighing 16–20
g) were used to establish the T24 ×enograft tumor model. A total of
15 mice were purchased from Hufukang Bioscience Inc. (Beijing,
China) and housed in individual ventilated cage systems in
Experimental Animal Center of Chongqing Medical University at
constant temperature (22°C) and humidity (50–60%), and with a 12 h
light-dark cycle. All the mice had free access to food and water
throughout the experiments. The experimental procedures were
approved by the Chongqing Medical University Institutional Animal
Care and Use Committee. The T24 cells (5×106) were
suspended in Matrigel (BD Biosciences; Becton-Dickinson and
Company, Franklin Lakes, NJ, USA) and subcutaneously implanted into
the left flank of nude mice. Following implantation, tumor volumes
were measured every 6 days until the mice were sacrificed by
CO2 at day 30.
Statistics
Each experiment was repeated at least three times
with two technical replicates each unless indicated otherwise, as
this was generally sufficient to achieve statistical significance
for differences. Statistical significance between groups was
calculated by using one-way analysis of variance, followed by
Tukey's test and statistical significance between the two groups
was calculated by two-tailed unpaired Student's t-test using
commercially available statistical software (SigmaPlot 11.0 for
Windows; Systat Software, Inc., San Jose, CA, USA). Data are
presented as means ± standard deviations. Correlation analysis was
determined using Pearson's correlation analysis and χ2
test was used for enumeration data. P<0.05 was considered to
indicate a statistically significant difference.
Results
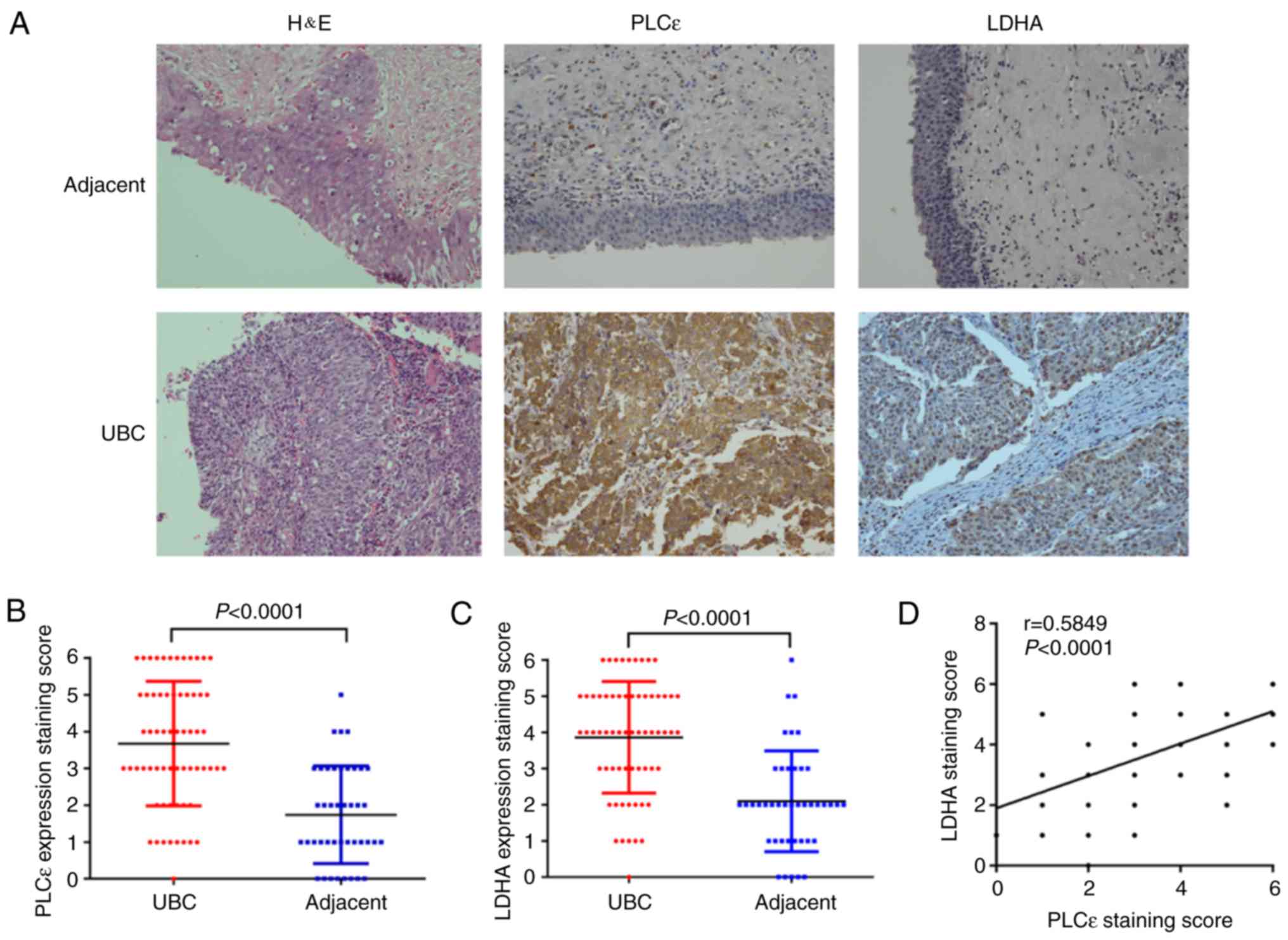
PLCε and LDHA are overexpressed in
UBC
To examine the expression profile of PLCε and LDHA
in UBC, the expression of PLCε and LDHA in UBC specimens (n=64) and
adjacent specimens (n=42) was analyzed using immunochemistry.
Positive rates of PLCε (76.6%) and LDHA (79.7%) in UBC specimens
were significantly increased, compared with adjacent tissue samples
(31.0 and 28.6% respectively; χ2 test; P<0.001;
Table I).
 | Table I.The association between PLCε and LDHA
expression levels and clinical pathological parameters. |
Table I.
The association between PLCε and LDHA
expression levels and clinical pathological parameters.
|
|
|
|
| P-value |
|---|
|
|
|
|
|
|
|---|
| Variables | No. (%) | PLCε Positive
(%) | LDHA Positive
(%) | PLCε | LDHA |
|---|
| Specimens |
|
|
|
<0.001a |
<0.001a |
|
UBC | 64 (100.0) | 49 (76.6) | 51 (79.7) |
|
|
|
Adjacent | 42 (65.6) | 13 (31.0) | 12 (28.6) |
|
|
| Sex |
|
|
| 0.651 | 0.309 |
|
Male | 53 (82.8) | 40 (75.5) | 41 (77.4) |
|
|
|
Female | 11 (17.2) | 9 (81.8) | 10 (90.9) |
|
|
| Age (years) |
|
|
| 0.369 | 0.654 |
|
≥60 | 52 (81.3) | 41 (78.8) | 42 (80.8) |
|
|
|
<60 | 12 (18.7) | 8 (66.7) | 9 (75.0) |
|
|
| Histologic stage
(18) |
|
|
| 0.18 | 0.759 |
|
Ta-T1 | 42 (65.6) | 30 (71.4) | 33 (78.6) |
|
|
|
T2-T4 | 22 (34.4) | 19 (86.4) | 18 (81.8) |
|
|
| Histologic grade
(18) |
|
|
| 0.819 | 0.574 |
| Low
grade | 24 (37.5) | 18 (75.0) | 20 (83.3) |
|
|
| High
grade | 40 (62.5) | 31 (77.5) | 31 (77.5) |
|
|
Furthermore, the results of immunochemistry staining
were quantified. Additionally, the arithmetic mean of staining
scores of PLCε (3.672±0.211; n=64) and LDHA (3.859±0.193; n=64) in
UBC were also significantly increased, compared with adjacent
specimens (PLCε, 1.738±0.205; and LDHA, 2.095±0.215, respectively;
n=42) (Fig. 1A-C), indicating the
role of PLCε and LDHA in UBC development. Additionally, the
staining scores of PLCε and LDHA were positively correlated
(Fig. 1D). Subsequently, a total of
21 pairs of UBC and adjacent tissue samples were collected to
perform protein and mRNA detection for PLCε and LDHA, and the
identical results as immunochemical staining were obtained. Both
protein (Fig. 1E-H) and mRNA
(Fig. 1I-K) relative expression of
PLCε and LDHA were significantly increased in UBC, compared with
adjacent tissues, and also positively correlated.
PLCε and LDHA knockdown inhibits cell
proliferation, glucose consumption and lactate production in T24
cells
As PLCε and LDHA expression levels are positively
correlated in human UBC, how these two proteins regulated each
other were further investigated. Firstly, the expression of LDHA
was detected in PLCε-deficient T24 cells, compared with sh-NC. A
decrease of LDHA in T24 cells following PLCε knockdown indicated
that PLCε may act upstream of LDHA (Fig. 2A). Consistent with our previous data
(7), PLCε deficiency significantly
inhibited T24 cells proliferation from 48 h post seeding (Fig. 2B). While LDHA is involved in
anaerobic glycolysis, the effects of PLCε deficiency on glucose
consumption and lactate production were also investigated. PLCε
knockdown significantly inhibited glucose consumption (2.422±0.184
nmol/min/1×106 cells), compared with the sh-NC control
group (3.559±0.179 nmol/min/1×106 cells) (Fig. 2C). Additionally, lactate production
was also decreased by PLCε deficiency in T24 cells (3.919±0.291 vs.
6.210±0.323 nmol/min/1×106; Fig. 2D), compared with the sh-NC control
group. To further elucidate the role of LDHA in PLCε mediated
inhibition of cells proliferation and glycolysis, LDHA was knocked
down by siRNA (Fig. 3A) to compare
the effect of LDHA with PLCε on cells proliferation and glycolysis.
As depicted in Fig. 3B-D, LDHA
knockdown could achieve the identical inhibition to cell growth and
glycolysis as PLCε, and these two genes had synergistic
effects.
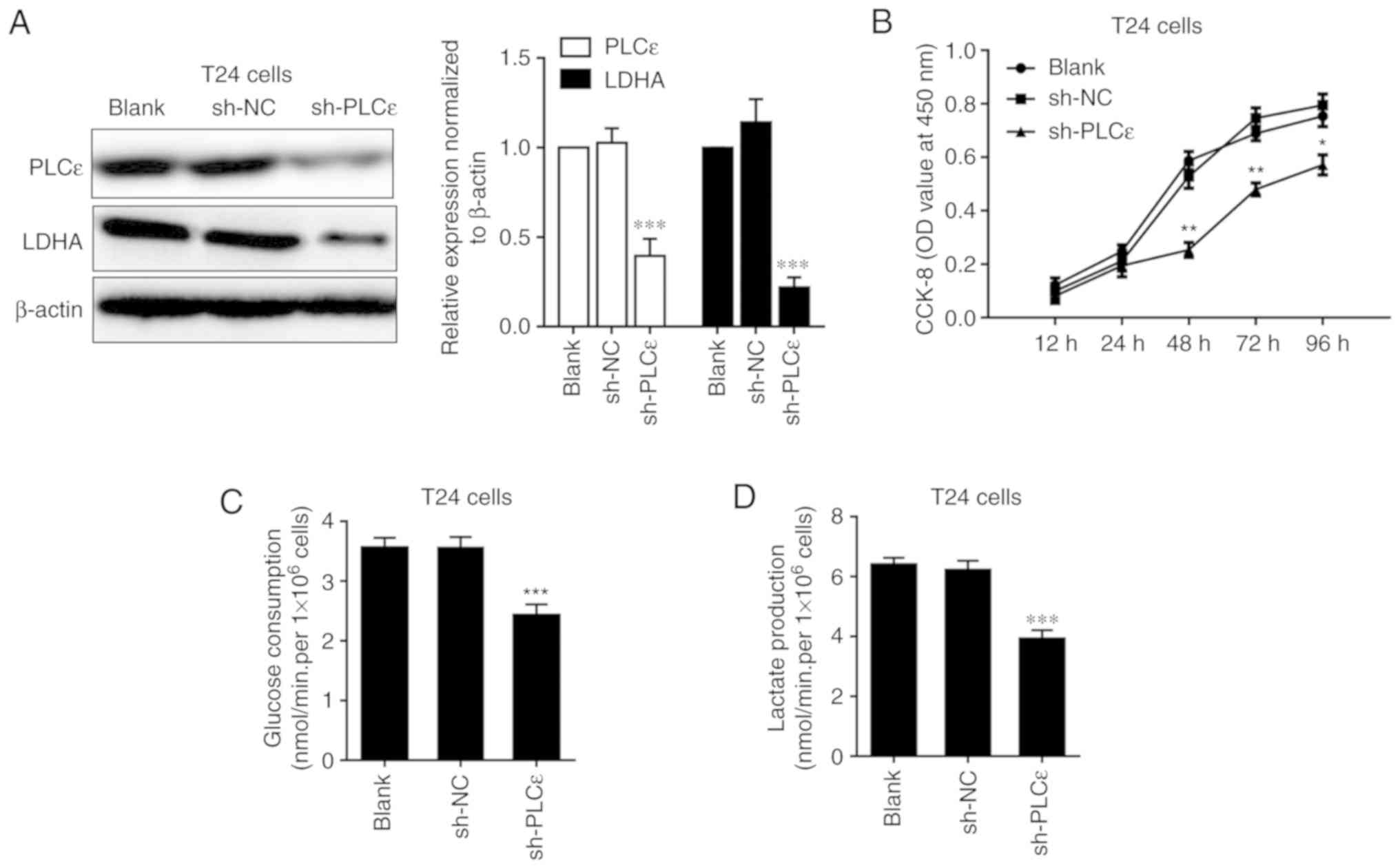 | Figure 2.LCε kncokdown decreases LDHA
expression and inhibits cell proliferation, glucose consumption and
lactate production in T24 cells. Impact of PLCε difiecncity on (A)
LDHA expression, (B) cell proliferation, (C) glucose consumption
and (D) lactate production in T24 cells. Values were presented as
means ± standard deviations of three independent experiments.
*P<0.05, **P<0.01 and ***P<0.001, compared with the sh-NC
group. PLCε, phosphatidylinositol-specific phospholipase Cε; LDHA,
lactate dehydrogenase; CCK-8, Cell Counting Kit-8; OD, optical
density; NC, negative control; shRNA, short hairpin RNA. |
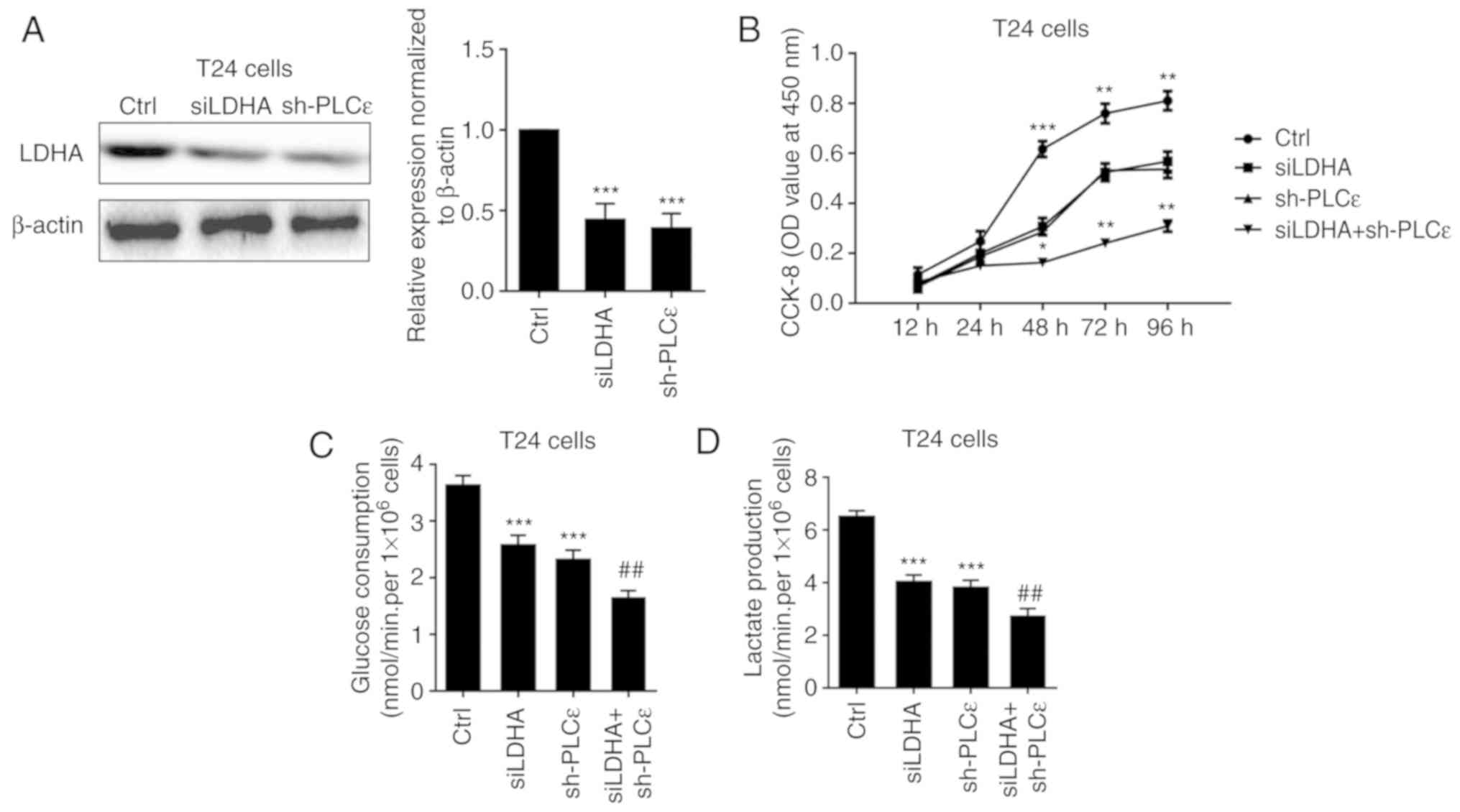 | Figure 3.Synergistic effects of LDHA and PLCε
deficiency on cell proliferation, glucose consumption and lactate
production. (A) LDHA and PLCε knockdown (B) inhibited cell
proliferation, (C) glucose consumption and (D) lactate production
in T24 cells. Values were presented as means ± standard deviations
of three independent experiments. *P<0.05, **P<0.01 and
***P<0.001, compared with the Ctrl group.
##P<0.01, compared with the sh-PLCε group. PLCε,
phosphatidylinositol-specific phospholipase Cε; LDHA, lactate
dehydrogenase; si, small interfering; sh, short hairpin; CCK-8,
Cell Counting Kit-8; Ctrl, control; OD, optical density. |
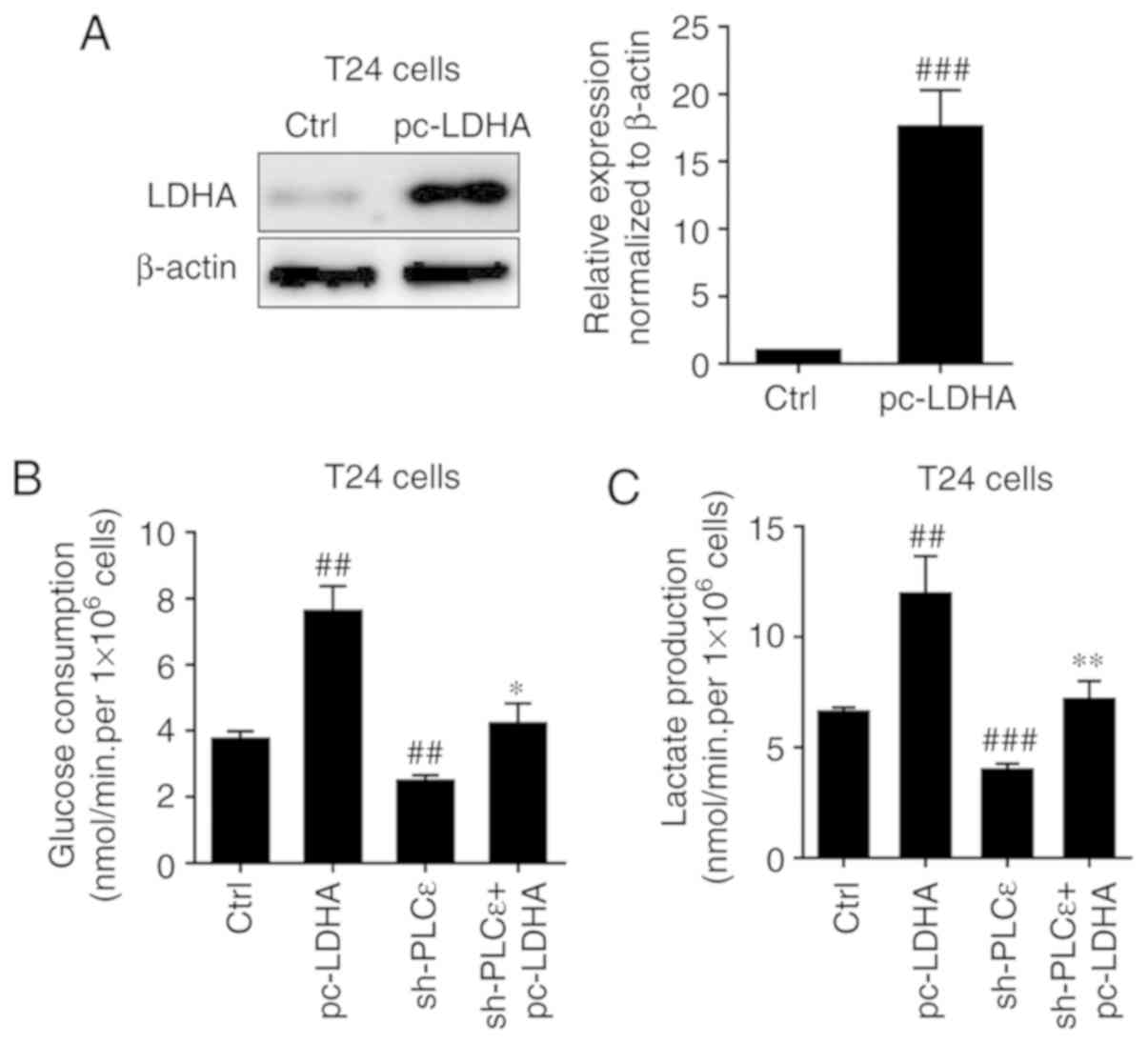
Subsequently, LDHA was overexpressed in T24 cells
(Fig. 4A) to determine whether it
could rescue cells glucose consumption and lactate production
decline caused by PLCε deficiency. Overexpression of LDHA in T24
cells significantly upregulated glucose consumption (Fig. 4B) and lactate production (Fig. 4C) of bladder cancer cells, and
completely blocked PLCε knockdown induced a decrease of glucose
consumption and lactate production.
STAT3 is involved in LDHA regulation
by PLCε
Our previous study determined that in bladder cancer
cells, PLCε could participate in the regulation of STAT3
phosphorylation (11), and STAT3
activation can promote the growth of tumor cells by promoting
anaerobic glycolysis (15,20). Therefore, it was speculated that
PLCε regulated LDHA by affecting the activation of STAT3. As
depicted in Fig. 5A, PLCε knockdown
was able to downregulate the phosphorylation of STAT3 in T24 cells.
Furthermore, the STAT3 inhibitor stattic (5 and 10 µM for 24 h)
used to inhibit the phosphorylation of STAT3 also downregulated the
expression of LDHA in UBC cells (Fig.
5B). Additionally, the STAT3 activator IL-6 could upregulate
LDHA expression and blocked PLCε knockdown mediated a decrease of
LDHA expression (Fig. 5C).
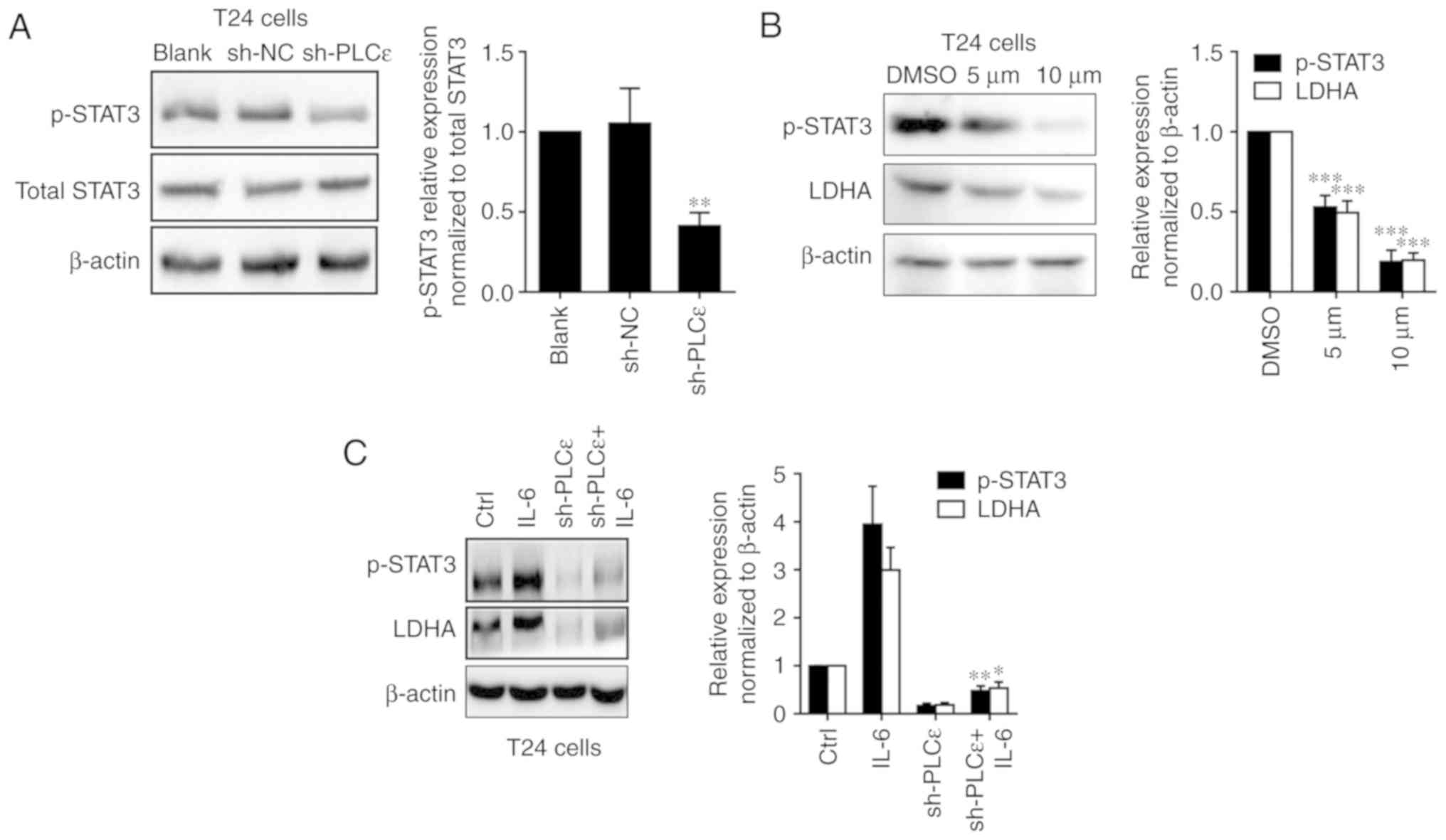 | Figure 5.PLCε regulates LDHA through the STAT3
pathway. (A) PLCε knockdown downregulated phosphorylation of STAT3
in T24 cells. (B) After 24 h post treatment of STAT3 inhibitor
stattic (5 and 10 µM dissolved in DMSO), LDHA expression was
downregulated in T24 cells with STAT3 phosphorylation inhibition.
(C) IL-6 (20 ng/ml for 24 h) rescued PLCε knockdown mediated STAT3
phosphorylation and LDHA downregulation in T24 cells. Values were
presented as means ± standard deviations of three independent
experiments. *P<0.05, **P<0.01 and ***P<0.001, compared
with the (A) sh-NC group, (B) DMSO group or (C) sh-PLCε group.
PLCε, phosphatidylinositol-specific phospholipase Cε; LDHA, lactate
dehydrogenase; STAT3, signal transducer and activator of
transcription 3; sh, short hairpin; NC, negative control; DMSO,
dimethyl sulfoxide; p-, phospho-; IL, interleukin. |
The aforementioned results indicate that PLCε may
regulate the expression of LDHA by affecting the activation of
STAT3. To further clarify the regulatory mechanism of the
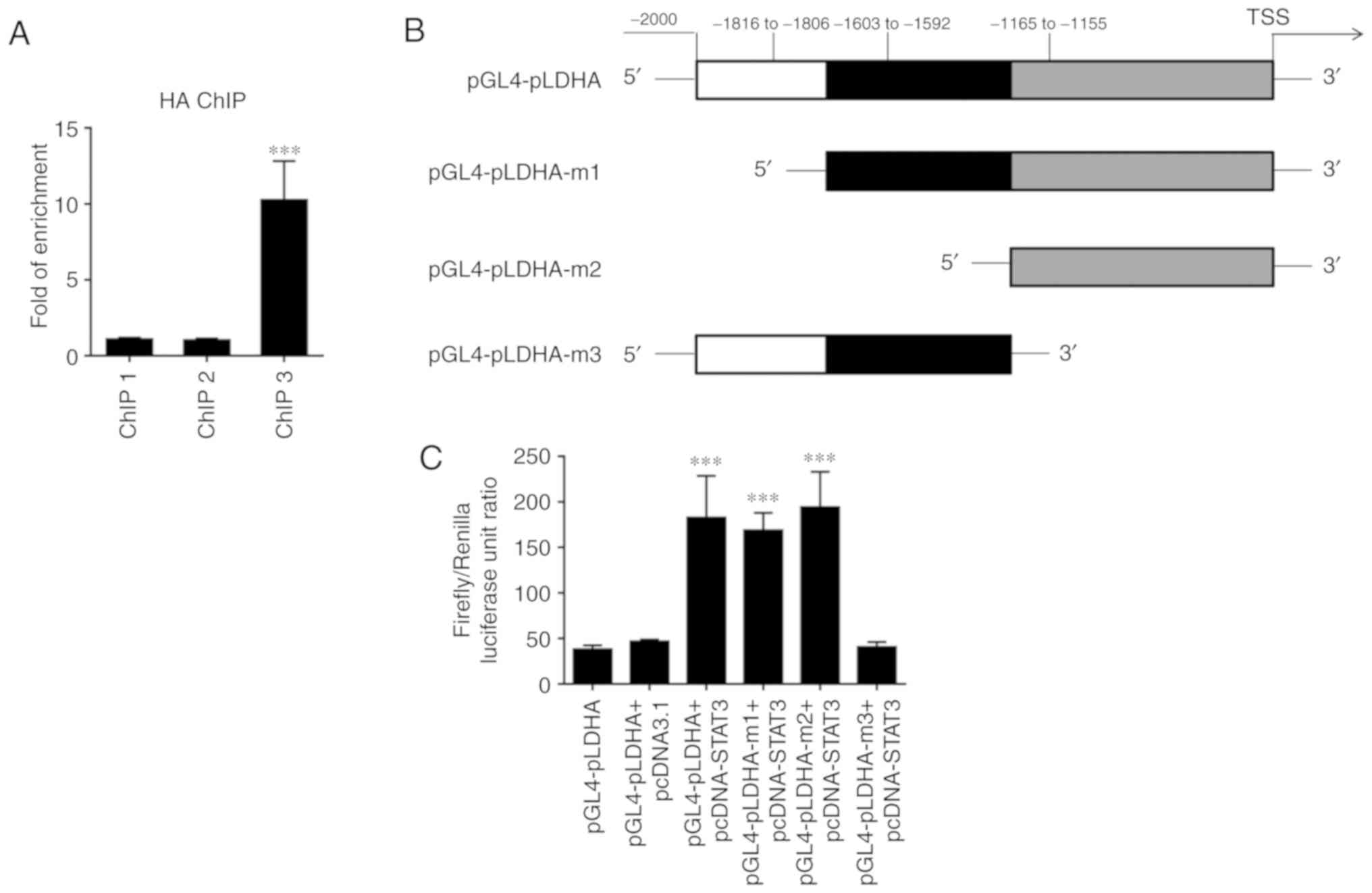
transcription factor STAT3 on LDHA, ChIP experiments were
performed. The ChIP results revealed that STAT3 could bind to the
promoter of LDHA at the site corresponding to ChIP3 primers due to
a significant enrichment being observed in samples
immunoprecipitated by HA-antibody (Fig.
6A). The dual luciferase reporter assay further confirmed that
binding of this site of the LDHA promoter to STAT3 enhanced LDHA
expression (Fig. 6B and C).
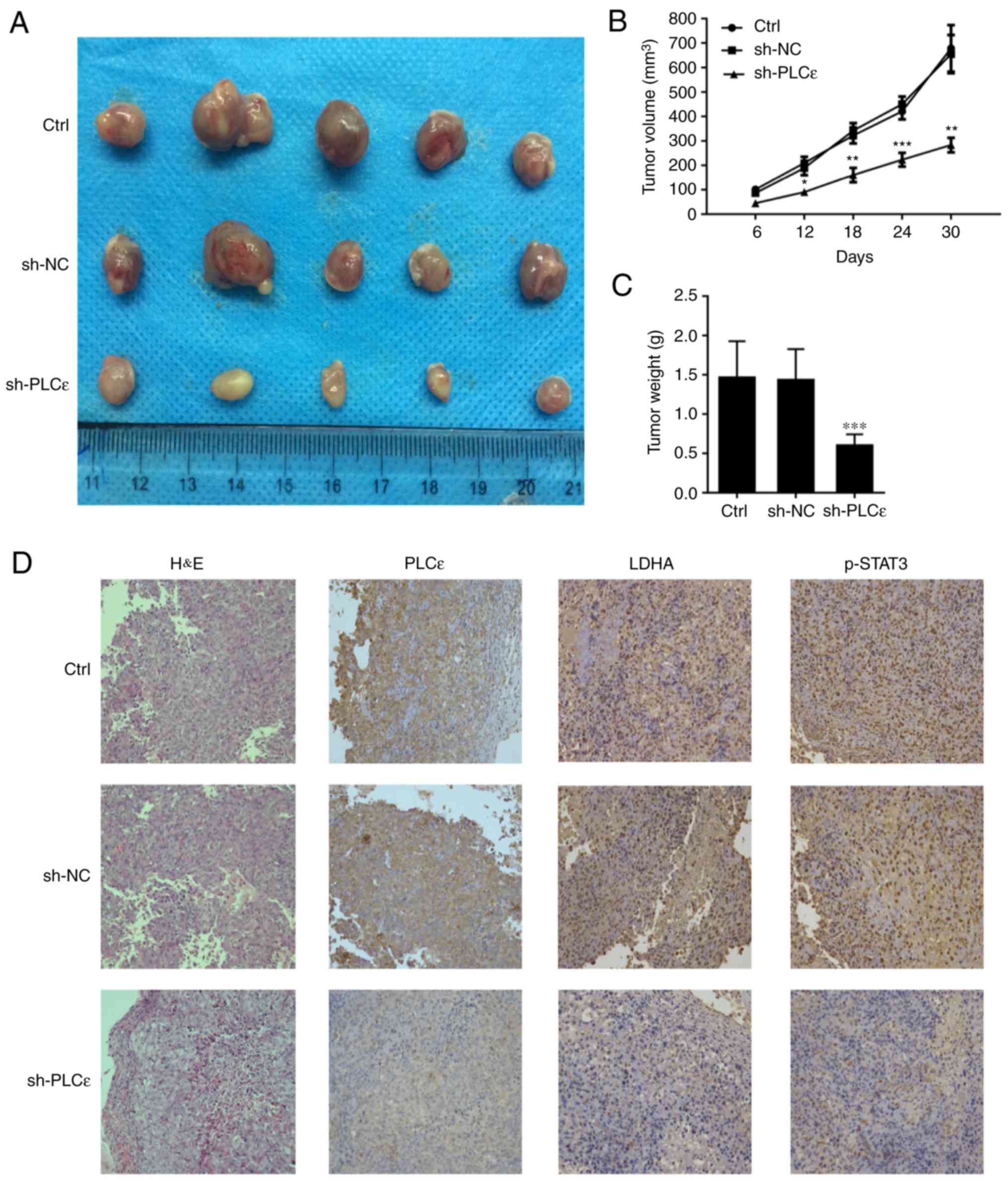
Knockdown of PLCε inhibits bladder
cancer cell growth in vivo
In the present study, BALB/c nude mice were injected
with T24 cells without any treatment, T24 cells infected with sh-NC
and T24 cells infected with sh-PLCε, in order for nude mice to be
subcutaneously tumorigenic, and observed at the same time. As
depicted in Fig. 7A and B,
PLCε-deficient cancer cells growth was significantly reduced,
compared with the control. At day 30 post cells injection, the
weight of the tumor in the PLCε knockdown group was also reduced,
compared with the control groups (Fig.
7C). The expression of PLCε, LDHA and phosphorylation of STAT3
was confirmed, which demonstrated similar results as the in
vitro experiments (Fig.
7D).
Discussion
PLCε is a member of the PLC family (21). In addition to the typical catalytic
X and Y, and C2 domains, PLCε has two carboxy-terminal Ras-binding
domains and a guanine nucleotide exchange factor domain CDC25
(22,23), compared with other PLC family
members. These special domains activate multiple signaling pathways
to promote the development of tumors (24). Previous studies demonstrated that
high expression of PLCε is associated with the development of a
variety of cancer types, including gastric cancer and esophageal
squamous cell carcinoma (25,26).
Previously, numerous studies demonstrated that the high expression
of PLCε is associated with the development, invasion and metastasis
of bladder cancer and prostate cancer in urinary system (9–11,27,28),
but the mechanisms are not completely understood.
The Warburg effect has been demonstrated to provide
energy for tumor initiation, invasion and metastasis in the
majority of malignant tumor types, including pancreatic cancer and
melanoma (29). The Warburg effect
occurs when cancer cells grow too fast for them to survive under
the condition of hypoxia and mitochondrial function gets damaged
(30). Following glucose
metabolizing to pyruvate, it no longer undergoes aerobic oxidation
through the mitochondrial pathway and is converted into lactate by
LDHA (31,32). In UBC, LDHA overexpression has
already been demonstrated to promote progression by stimulating
epithelial-mesenchymal transition (33). In the present study, it was
demonstrated that LDHA and PLCε were overexpressed in human UBC
tissue specimens at the mRNA and protein level, and both of them
are positively correlated. When PLCε was knocked down in T24 cells,
LDHA expression, cells proliferation, glucose consumption and
lactate production were also downregulated in the present study.
These results indicated that PLCε may affect cell metabolisms to
facilitate UBC progression. To clarify the role of LDHA in the
regulation of bladder cancer cell growth by PLCε, cell growth or
glucose metabolism of T24 cells was compared by knockdown of PLCε
and LDHA. The knockdown of PLCε or LDHA inhibited cell growth and
the Warburg effect to a similar extent, while synergistic effects
were demonstrated when both were deficient. This may be associated
with PLCε also promoting the growth of UBC cells through other
pathways.
Ever since STATs were first identified in 1988
(34), this group of transcription
factors has been demonstrated to be involved in multiple cellular
processes, including cell proliferation (35,36),
metabolism (37), immune response
(38–40), autophagy (41) and apoptosis (42). Among them, the role of STAT3 in
tumors is the most widely studied (14). Since PLCε was determined to regulate
lipopolysaccharides-mediated STAT3 phosphorylation in UBC cells
(11), and STAT3 has been confirmed
to participate in the Warburg effect (37,43–45).
Additionally, the effect of STAT3 phosphorylation on LDHA
expression was investigated in the present study. As depicted in
Fig. 5, inhibition of STAT3
phosphorylation downregulated LDHA, and STAT3 activator IL-6 could
rescue STAT3 inactivation mediated LDHA downregulation, indicating
that STAT3 may be a transcription factor targeting LDHA. To date,
LDHA have been determined to be regulated by a number of
transcription factors in various cells, including cyclic adenosine
3′,5′-monophosphate (cAMP), cAMP response element binding protein
(46), specificity protein 1
(47), hypoxia-inducible factor 1
(48), c-Myc (49), heat shock factor 1 (50), forkhead box M1 (51) and Kruppel like factor 4 (52). However, whether STAT3 participates
in the regulation of LDHA expression has not been reported. In the
present study, the results indicated that STAT3 may be involved in
the regulation of LDHA transcription levels; therefore, whether
LDHA promoter sequence has theoretical binding sites for STAT3 was
investigated. According to the analysis from JASPAR, three
predicted possible STAT3 binding sites were speculated and
subsequent ChIP and luciferase reporter experiments also confirmed
the binding of STAT3 to the LDHA promoter.
In conclusion, the present study determined for the
first time that PLCε may regulate the expression of LDHA by
affecting the activation of STAT3, and thus participate in the
anaerobic glycolysis of cancer cells, affecting tumorigenesis.
Subsequently, how PLCε regulates the activation of STAT3 requires
further clarification. The present data enrich the understanding of
the pathogenesis of UBC and may provide novel therapeutic targets
for the treatment of UBC.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant (grant
no. cstc2018jcyjAX0188) from Chongqing science and technology
commission.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
XW and CL supervised and directed the present study.
HC performed the majority of the experiments. XW, CL and HC drafted
the manuscript. XW, CL, YHa and YG contributed to the project
design. YHe, MY and WS collected the tumor samples and the clinical
data. All authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Written informed consent for research purposes was
obtained from each patient in the present study. Additionally, all
applicable international, national and/or institutional guidelines
for the care and use of animals were followed. The study protocol
regarding patients and animals was approved by the Ethics Committee
of the First Affiliated Hospital of Chongqing Medical University
(approval no. 2016-152).
Patients consent for publication
All patients were provided informed consent as part
of a clinical protocol to participate in the present study and data
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pashos CL, Botteman MF, Laskin BL and
Redaelli A: Bladder cancer: Epidemiology, diagnosis, and
management. Cancer Pract. 10:311–322. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gwangwa MV, Joubert AM and Visagie MH:
Crosstalk between the Warburg effect, redox regulation and
autophagy induction in tumourigenesis. Cell Mol Biol Lett.
23:202018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Warburg O: On the origin of cancer cells.
Science. 123:309–314. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cantor JR and Sabatini DM: Cancer cell
metabolism: One hallmark, many faces. Cancer Discov. 2:881–898.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cairns RA, Harris IS and Mak TW:
Regulation of cancer cell metabolism. Nat Rev Cancer. 11:85–95.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Henry J, Guillotte A, Luberto C and Del
Poeta M: Characterization of inositol
phospho-sphingolipid-phospholipase C 1 (Isc1) in Cryptococcus
neoformans reveals unique biochemical features. FEBS Lett.
585:635–640. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng H, Luo C, Wu X, Zhang Y, He Y, Wu Q,
Xia Y and Zhang J: shRNA targeting PLCε inhibits bladder cancer
cell growth in vitro and in vivo. Urology. 78:474 e477–e411. 2011.
View Article : Google Scholar
|
|
10
|
Jiang T, Liu T, Li L, Yang Z, Bai Y, Liu D
and Kong C: Knockout of phospholipase Cε attenuates
N-butyl-N-(4-hydroxybutyl) nitrosamine-induced bladder
tumorigenesis. Mol Med Rep. 13:2039–2045. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang X, Ou L, Tang M, Wang Y, Wang X, Chen
E, Diao J, Wu X and Luo X: Knockdown of PLCε inhibits inflammatory
cytokine release via STAT3 phosphorylation in human bladder cancer
cells. Tumour Biol. 36:9723–9732. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grivennikov SI and Karin M: Dangerous
liaisons: STAT3 and NF-kappaB collaboration and crosstalk in
cancer. Cytokine Growth Factor Rev. 21:11–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ihle JN: STATs: Signal transducers and
activators of transcription. Cell. 84:331–334. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu H, Lee H, Herrmann A, Buettner R and
Jove R: Revisiting STAT3 signalling in cancer: New and unexpected
biological functions. Nat Rev Cancer. 14:736–746. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li M, Jin R, Wang W, Zhang T, Sang J, Li
N, Han Q, Zhao W, Li C and Liu Z: STAT3 regulates glycolysis via
targeting hexokinase 2 in hepatocellular carcinoma cells.
Oncotarget. 8:24777–24784. 2017.PubMed/NCBI
|
|
16
|
Le A, Cooper CR, Gouw AM, Dinavahi R,
Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL and Dang
CV: Inhibition of lactate dehydrogenase A induces oxidative stress
and inhibits tumor progression. Proc Natl Acad Sci USA.
107:2037–2042. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Augoff K, Hryniewicz-Jankowska A and
Tabola R: Lactate dehydrogenase 5: An old friend and a new hope in
the war on cancer. Cancer Lett. 358:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Magers MJ, Lopez-Beltran A, Montironi R,
Williamson SR, Kaimakliotis HZ and Cheng L: Staging of bladder
cancer. Histopathology. 74:112–134. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wake MS and Watson CJ: STAT3 the
oncogene-still eluding therapy? FEBS J. 282:2600–2611. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kelley GG, Reks SE, Ondrako JM and Smrcka
AV: Phospholipase C(epsilon): A novel Ras effector. EMBO J.
20:743–754. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hicks SN, Jezyk MR, Gershburg S, Seifert
JP, Harden TK and Sondek J: General and versatile autoinhibition of
PLC isozymes. Mol Cell. 31:383–394. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wing MR, Snyder JT, Sondek J and Harden
TK: Direct activation of phospholipase C-epsilon by Rho. J Biol
Chem. 278:41253–41258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cullen PJ: Ras effectors: Buying shares in
Ras plc. Curr Biol. 11:R342–R344. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Abnet CC, Freedman ND, Hu N, Wang Z, Yu K,
Shu XO, Yuan JM, Zheng W, Dawsey SM, Dong LM, et al: A shared
susceptibility locus in PLCE1 at 10q23 for gastric adenocarcinoma
and esophageal squamous cell carcinoma. Nat Genet. 42:764–767.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Smrcka AV, Brown JH and Holz GG: Role of
phospholipase Cε in physiological phosphoinositide signaling
networks. Cell Signal. 24:1333–1343. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ou L, Guo Y, Luo C, Wu X, Zhao Y and Cai
X: RNA interference suppressing PLCE1 gene expression decreases
invasive power of human bladder cancer T24 cell line. Cancer Genet
Cytogenet. 200:110–119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ling Y, Chunli L, Xiaohou W and Qiaoling
Z: Involvement of the PLCε/PKCα pathway in human BIU-87 bladder
cancer cell proliferation. Cell Biol Int. 35:1031–1036. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liberti MV and Locasale JW: The Warburg
effect: How does it benefit cancer cells? Trends Biochem Sci.
41:211–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ristic B, Bhutia YD and Ganapathy V:
Cell-surface G-protein- coupled receptors for tumor-associated
metabolites: A direct link to mitochondrial dysfunction in cancer.
Biochim Biophys Acta Rev Cancer. 1868:246–257. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Alfarouk KO, Verduzco D, Rauch C,
Muddathir AK, Adil HH, Elhassan GO, Ibrahim ME, David Polo Orozco
J, Cardone RA, Reshkin SJ and Harguindey S: Glycolysis, tumor
metabolism, cancer growth and dissemination. A new pH-based
etiopathogenic perspective and therapeutic approach to an old
cancer question. Oncoscience. 1:777–802. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim JW and Dang CV: Cancer's molecular
sweet tooth and the Warburg effect. Cancer Res. 66:8927–8930. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jiang F, Ma S, Xue Y, Hou J and Zhang Y:
LDH-A promotes malignant progression via activation of
epithelial-to-mesenchymal transition and conferring stemness in
muscle-invasive bladder cancer. Biochem Biophys Res Commun.
469:985–992. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Levy DE, Kessler DS, Pine R, Reich N and
Darnell JE Jr: Interferon-induced nuclear factors that bind a
shared promoter element correlate with positive and negative
transcriptional control. Genes Dev. 2:383–393. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Williams JG: STAT signalling in cell
proliferation and in development. Curr Opin Genet Dev. 10:503–507.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
La Fortezza M, Schenk M, Cosolo A,
Kolybaba A, Grass I and Classen AK: JAK/STAT signalling mediates
cell survival in response to tissue stress. Development.
143:2907–2919. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Demaria M, Giorgi C, Lebiedzinska M,
Esposito G, D'Angeli L, Bartoli A, Gough DJ, Turkson J, Levy DE,
Watson CJ, et al: A STAT3-mediated metabolic switch is involved in
tumour transformation and STAT3 addiction. Aging (Albany NY).
2:823–842. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Roca Suarez AA, Van Renne N, Baumert TF
and Lupberger J: Viral manipulation of STAT3: Evade, exploit, and
injure. PLoS Pathog. 14:e10068392018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Villarino AV, Kanno Y and O'Shea JJ:
Mechanisms and consequences of Jak-STAT signaling in the immune
system. Nat Immunol. 18:374–384. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nan Y, Wu C and Zhang YJ: Interplay
between janus kinase/signal transducer and activator of
transcription signaling activated by type I interferons and viral
antagonism. Front Immunol. 8:17582017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Maycotte P, Gearheart CM, Barnard R, Aryal
S, Mulcahy Levy JM, Fosmire SP, Hansen RJ, Morgan MJ, Porter CC,
Gustafson DL and Thorburn A: STAT3-mediated autophagy dependence
identifies subtypes of breast cancer where autophagy inhibition can
be efficacious. Cancer Res. 74:2579–2590. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fathi N, Rashidi G, Khodadadi A, Shahi S
and Sharifi S: STAT3 and apoptosis challenges in cancer. Int J Biol
Macromol. 117:993–1001. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li J, Liu T, Zhao L, Chen W, Hou H, Ye Z
and Li X: Ginsenoside 20(S)Rg3 inhibits the Warburg effect through
STAT3 pathways in ovarian cancer cells. Int J Oncol. 46:775–781.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Akiyama Y, Iizuka A, Kume A, Komiyama M,
Urakami K, Ashizawa T, Miyata H, Omiya M, Kusuhara M and Yamaguchi
K: Effect of STAT3 inhibition on the metabolic switch in a highly
STAT3-activated lymphoma cell line. Cancer Genomics Proteomics.
12:133–142. 2015.PubMed/NCBI
|
|
45
|
Darnell JE Jr: STAT3, HIF-1, glucose
addiction and Warburg effect. Aging (Albany NY). 2:890–891. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kwast-Welfeld J, Soong CJ, Short ML and
Jungmann RA: Identification of rat ovarian nuclear factors that
interact with the cAMP-inducible lactate dehydrogenase A subunit
promoter. J Biol Chem. 264:6941–6947. 1989.PubMed/NCBI
|
|
47
|
Short ML, Huang D, Milkowski DM, Short S,
Kunstman K, Soong CJ, Chung KC and Jungmann RA: Analysis of the rat
lactate dehydrogenase A subunit gene promoter/regulatory region.
Biochem J. 304:391–398. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Semenza GL, Jiang BH, Leung SW, Passantino
R, Concordet JP, Maire P and Giallongo A: Hypoxia response elements
in the aldolase A, enolase 1, and lactate dehydrogenase A gene
promoters contain essential binding sites for hypoxia-inducible
factor 1. J Biol Chem. 271:32529–32537. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lewis BC, Prescott JE, Campbell SE, Shim
H, Orlowski RZ and Dang CV: Tumor induction by the c-Myc target
genes rcl and lactate dehydrogenase A. Cancer Res. 60:6178–6183.
2000.PubMed/NCBI
|
|
50
|
Zhao YH, Zhou M, Liu H, Ding Y, Khong HT,
Yu D, Fodstad O and Tan M: Upregulation of lactate dehydrogenase A
by ErbB2 through heat shock factor 1 promotes breast cancer cell
glycolysis and growth. Oncogene. 28:3689–3701. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cui J, Shi M, Xie D, Wei D, Jia Z, Zheng
S, Gao Y, Huang S and Xie K: FOXM1 promotes the Warburg effect and
pancreatic cancer progression via transactivation of LDHA
expression. Clin Cancer Res. 20:2595–2606. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shi M, Cui J, Du J, Wei D, Jia Z, Zhang J,
Zhu Z, Gao Y and Xie K: A novel KLF4/LDHA signaling pathway
regulates aerobic glycolysis in and progression of pancreatic
cancer. Clin Cancer Res. 20:4370–4380. 2014. View Article : Google Scholar : PubMed/NCBI
|