Introduction
More than 70,000 adolescent and young adult (AYA)
patients, with ages between 15 and 39 years, are diagnosed with
cancer every year only in the United States. The incidence of
cancer in children younger than 15 years of age is also
approximately 10,000 cases per year (1).
In Europe, >15,000 AYAs are diagnosed with cancer
each year, according to the International Society of Paediatric
Oncology (https://siop-online.org/). In 2004,
the Spanish Registry of Childhood Tumors (RETI-SEHOP) concluded
that approximately 1,100 children in Spain are diagnosed with
cancer annually (https://www.uv.es/rnti/index.html). The incidence of
cancer in children in Spain is similar to that in other European
countries, with approximately 160 new cases per million children
each year, aged between zero and 14 years, according to the Spanish
Federation of Parents of Children with Cancer (FEPNC; http://cancerinfantil.org/cancer-infantil-cifras/).
The survival rates of these patients have been steadily increasing
in recent years due to the greater efficacy of novel oncological
treatments. In fact, the 5-year survival rate for pediatric cancer
patients approaches 80% (2).
However, treatments entailing chemotherapy or radiotherapy, often
result in impaired or absent reproductive ability. Moreover, only
approximately half of patients report any reproductive issues,
having previously been informed about the therapeutic options of
fertility preservation (FP), which has been identified as the
second most important aspect among young patients, following
survival. In order to improve the quality of life and the survival
of patients, the improvement of FP techniques has become an
important topic in the field of research over the past years
(3). In fact, the concept of
oncofertility has recently appeared in the form of an
interdisciplinary integrated network, based on medical methods,
which is designed to maximize the reproductive future of
oncological patients by offering various FP techniques.
Furthermore, a standardization of the FP healthcare for oncological
patients is required. Currently, two major international networks
have been created for this purpose: The International Network on
Cancer, Infertility and Pregnancy (INCIP) and the European Society
of Gynecological Oncology (ESGO) (2).
Fertility preservation techniques in females
and males
A literature review was conducted by searching for
publications in PubMed between September, 2017 and May, 2018 with
the following keywords and their combinations: ‘fertility’,
‘preservation’, ‘women’, ‘male’, ‘prepuberal’, ‘young adults’,
‘gonadotoxic treatment’, ‘cancer treatment’ and
‘oncofertility’.
At the 20th week of embryogenesis, women reach the
maximal number of germ cells in the genital rides, with
approximately 6–7 million potential oocytes, known as the
primordial follicles. However, only 1 million of these will remain
at birth and only approximately 400,000 oocytes will survive to
puberty. This number, known as the ovarian reserve, will decrease,
reaching 1,000 oocytes at the time of menopause, due to the
approximately 450 monthly ovulatory cycles, during which process
most oocytes undergo atresia (degeneration and reabsorption)
(4). The preservation of the
ovarian reserve is necessary to maintain overall women's health, as
it plays a role not only in oocyte development and fertility, but
also in other systems, such as the cardiovascular system and
osseous system (5).
The degree of the depletion of the ovarian reserve
differs between chemotherapy and radiotherapy. As regards
chemotherapy, it varies depending on the age of the patient (the
younger the patient, the lesser the risk of ovarian failure), the
chemotherapy agent used (alkylating agents being of greatest risk)
and the duration of the treatment. Oocytes are very sensitive to
radiation. Exposure to 20–30 Gy of radiation or total body
radiation of 15 Gy lead to the loss of ovarian function [premature
ovarian failure (POF)] (3).
The following techniques are available to women who
are willing to preserve their fertility prior to, or during chemo-
and radiotherapy: Embryo cryopreservation, immature or mature
oocyte cryopreservation, ovarian tissue cryopreservation (OTC) and
ovarian transposition. In this review, we specifically focus on the
oocyte and OTC. Other experimental techniques, such as the
activation of ovarian follicles, in vitro follicle culture,
artificial ovaries and novel fertoprotective agents, may appear to
be promising, although further research is still required (6).
In males, the onset of production of spermatozoa
begins at puberty and it is known as spermarche. Unlike women, from
the moment of the spermarche, spermatogenesis is maintained during
the entire duration of a man's life, on account of the
spermatogonia type A, among others (7). Testicular stem cells differentiate
into spermatogonia, which will eventually become spermatozoa under
the process of spermatogenesis. Spermatogonia in the testes are
extremely sensitive to radiation, regardless of age. Leydig cells,
on the other hand, are more sensitive to radiation prior to the
onset of puberty, whereas in adulthood, they become more resistant
to radiation (8). Consequently,
adult patients may preserve Leydig cell function and testosterone
production following radiotherapy despite being azoospermic.
Furthermore, if a population of spermatogonial stem cells (SSCs)
remains after cancer treatment, as the effect is dose-dependent,
the regeneration of spermatozoa may continue for years (9). Those at the highest risk of developing
permanent sterility are children and adolescents with testicular
cancer, leukemia and Ewing's sarcoma. Sperm banking is the
recommended FP technique for males, although the cryopreservation
of SSCs is also available.
Fertility preservation techniques in
females
Embryo cryopreservation
This technique has established success rates and is
a widely used and reliable method. It is like an in vitro
fertilization (IVF) protocol, which has been performed for over 30
years. Women undergo controlled ovarian stimulation (COS) with
gonadotropin injections to promote multifollicular growth. After
10–14 days, oocyte retrieval is performed, normally under conscious
sedation and with transvaginal ultrasound-guided needle aspiration
(10). The oocytes are then
fertilized in the laboratory and are cryopreserved for future use,
commonly in their blastocyst phase (4).
The disadvantages of this technique are mainly three
as follows: The need of a stable male partner, ethical issues
regarding embryo disposition and the time required for ovarian
stimulation. COS normally begins during the early follicular phase.
When a patient is diagnosed in her early follicular phase, ovarian
stimulation with gonadotropin-releasing hormone (GnRH) antagonist
begins immediately. However, if the patient is in any other phase,
the IVF standard protocols require the patient to wait up to 3
weeks before the process begins (10). Therefore, this method is not a
viable option for women whose aggressive cancer treatment is of
highest priority, as the IVF standard protocols require a wait of
up to 3 weeks before the process begins (10). It is also not recommended in women
with hormone-sensitive cancers and is not possible for prepubertal
girls. It is also not recommended in women with hormone-sensitive
cancers and not possible for prepubertal girls.
There are three main cryopreservation techniques:
Slow-freezing, ultra-rapid and vitrification. Slow-freezing
involves a step-wise programmed decrease in temperature (11), achieving a freezing equilibrium due
to the exchange of the extra- and intracellular fluids without
causing meaningful osmotic and deformation cellular effects.
However, ice crystals can be formed within the cells, which can
result in extremely harmful effects (12). The procedure is long-lasting
(approximately 1 or 2 h) and requires expensive instrumentation and
large quantities of liquid nitrogen, among others. Vitrification
converts water into solid glass-like cells, avoiding ice crystal
formation, both intracellular and extracellular (13). Expensive instrumentation it is not
required, and only several minutes are needed. Furthermore, a
meta-analysis in 2013 revealed that the rates of oocyte survival,
fertilization and implantation where higher in vitrification than
in slow-freezing methods (14). For
these reasons, vitrification is nowadays the preferred
technique.
Data on pregnancy and live both rates in cancer
patients after frozen embryo transfer are limited. Live birth rates
in non-oncological patients <35 years of age amount to 38.7% per
frozen embryo transfer and to 34,8% for oocyte donor cycles
(15).
Oocyte cryopreservation
As an alternative to embryo cryopreservation, this
technique is the preferred option for postpuberal and adolescent
females, women without a stable partner, and for those who do not
wish to use a sperm donor. It overcomes the ethical and religious
issues that emerge from the embryo preservation. Clinical outcomes
in the oocyte vitrification strategy are superior to slow-freezing
and thawing (16). With oocyte
vitrification, women are able to conceive in the future and
maintain their reproductive autonomy. However, it is not
appropriate for patients who are in urgent need of treatment or
patients with hormone-sensitive cancers, as the procedure also
includes COS. The oocytes can be cryopreserved as mature eggs or as
immature germinal vesicle oocytes. Mature oocyte cryopreservation
is performed with the oocytes whose development is terminated in
metaphase II. Nowadays, this is the preferred method for
postpuberal patients and for patients whose chemotherapy and
radiotherapy can be delayed. Immature oocytes obtained by
aspiration and followed by in vitro maturation (IVM)
techniques is a suitable option for prepubertal girls and women
with hormone-sensitive cancers or with polycystic ovarian syndrome
(PCOS), since COS is not required. This also allows the possibility
of immediate cancer treatment. Oocytes will be matured in
vitro (through IVM) as the cryopreservation of mature oocytes
has yielded better survival outcomes than immature cryopreserved
oocytes (17). The retrieval of
immature oocytes can also be achieved during an OTC procedure.
OTC
Although this technique is still considered
experimental, it is currently the only option for pediatric
patients and for patients with hormone-dependent diseases, as it is
COS-independent and does not delay the oncological treatment. It
does not require a male partner or a sperm donor.
OTC is an invasive procedure, as it requires general
anesthesia to surgically remove the ovarian tissue. This tissue,
with a high content on follicles, is cryopreserved and can then be
used as for the following procedures: i) Orthotopic implantation
(reimplantation into the pelvic cavity; e.g., remaining ovarian
tissue or peritoneum) or heterotopic implantation outside of the
ovaries (e.g., rectum, pectoralis muscle, abdominal wall and chest
wall); ii) isolation of follicles from the thawed tissue for in
vitro growth, maturation and fertilization. During OTC, it is
possible to aspirate immature oocytes from antral follicles of the
ovarian tissue. Isolated oocytes can be cryopreserved or matured
in vitro (through IVM) for later vitrification (18).
Either ovarian cortical tissue cryopreservation
(slow-freezing) or whole ovary cryopreservation can be performed.
All egg-containing follicles are in the outer one-millimeter layer
of the ovary, and thus the removal of this layer of tissue is
sufficient for cryopreservation. The success rate of live-birth
after reimplantation is approximately 30% (6). The cryopreservation of the whole ovary
remains a technical challenge due to the bigger size of the tissue,
which hinders a homogeneous and adequate dispersion of
cryoprotectant, and the vascular damage in form of ice crystals.
Further studies are required in order for this technique to be used
in standard clinical practice.
Up to 2015, 60 live birth cases had been reported
with OTC in adult patients. However, the total number of
re-implantation performed in each center until that time was
unknown; thus, no success rates could be concluded (19). Prior to menarche, only one live
birth following the autografting of cryopreserved tissue has been
published (20), at least to the
best of our knowledge. In 2015, Donnez et al (19) published a large case series (n=111)
which revealed a pregnancy rate proportion of 29% (n=32). Two women
delivered 3 babies each, proving the efficacy of the technique and
the possibility of conceiving naturally after only one
procedure.
The most worrisome concern of OTC is the possibility
of the re-introduction of carcinogenic cells into the cured patient
or the subsequent malignant transformation of the ovarian tissue,
which has been already reported (21). For this reason, a thorough
examination of the ovarian tissue prior to cryopreservation and
reimplantation is required.
Ovarian transposition
(oophoropexy)
This procedure aims to prevent ovarian damage during
radiation therapy by relocating the ovaries away from the radiation
field. Therefore, it will be of use in women who will undergo
pelvic or low abdominal radiation therapy without additional
gonadotoxic chemotherapy (22).
According to the radiation field outlined by the radiation
oncologist, the surgeon will decide the optimal location in the
abdominal wall for ovarian transposition. Altogether, the ovaries
will not be harmed by the therapy and ovarian failure will be
prevented. The procedure is normally performed laparoscopically
before the commencement of radiation. Success rates are not
conclusive, as they vary from 16 to 90% (23).
Fertoprotective adjuvant agents
Another approach to preserving fertility is to
protect the follicles during oncological treatment by
administrating fertoprotective agents. One example is the use of
GnRHa agonists, which are administrated 10 days prior to the
commencement of thechemotherapy. GnRH analogues interfere with the
hypothalamic-pituitary-gonadal axis and inhibit the ovarian
function by suppressing gonadotrophin levels to prepubertal levels
(3). Two meta-analysis of
randomized trials concluded a reduced risk of POF in young breast
cancer patients (24,25), whereas its use was unclear in
ovarian cancer and lymphoma (25).
Another study demonstrated no effect in young patients with
lymphoma (26). The quality of the
evidence is insufficient to draw meaningful conclusions;
high-quality studies are required to examine the long-term effects
of the use of GnRHa on premature ovarian insufficiency (POI).
Emerging techniques
Activation of ovarian follicles
Cryopreserved ovarian tissue from prepubertal
patients and patients with POF contains immature primordial
follicles, which need to be activated in order to begin developing.
This can be induced either in vivo [by interrupting the
Hippo signaling pathway (27)] or
in vitro, prior to autotransplantation, by activating the
phosphatidylinositol 3-kinase (PI3K)/phosphatase and tensin homolog
(PTEN)/protein kinase B (AKT)/Forkhead box O3 (FOXO3) pathway,
which regulates primordial follicle activation in oocytes (27). This pathway also plays a crucial
role in the follicle-stimulating hormone (FSH) stimulation of
granulosa cell differentiation in antral follicles and in oocyte
maturation of preovulatory follicles (27). This may be a promising fertility
option for prepubertal patients and patients with primary ovarian
insufficiency, whose cryopreserved tissue contains immature
primordial follicles suitable for this technique. In vitro
protocols involving the PTEN/AKT pathway are being developed in
order to increase the pool of viable activated follicles available
for in vitro growth (IVG) procedures (28).
In vitro follicle culture
This technique may be an option for patients who
require urgent oncological treatment, and therefore are not good
candidates for oocyte or embryo cryopreservation, such as patients
with acute leukemia or acute myeloblastic leukemia (AML). OTC is
the available option momentarily for these patients. However, the
possibility of re-seeding original cancer cells from the ovarian
tissue exists, and therefore other alternatives need to be
raised.
The ovarian follicle culture in vitro, aims
to mitigate the risk of re-implanting malignant cells from the
cryopreserved ovarian tissue. It is therefore useful in patients
with cancers whose metastasis appear often in the ovary or patients
with BRAC1 and BRAC2 mutations, due to the increased risk of an
ovarian cancer, which would not make possible the transplantation
of cryopreserved ovarian cortex (29). However, the complete maturation of
primordial follicles has not been achieved in humans yet (30).
In this procedure, individual follicles are isolated
from the patient's bank tissue, which will afterwards be matured
in vitro to become a functioning oocyte. These will be
fertilized, and the embryos will be transferred to the uterus. The
follicles can be cultured in two-dimensional (2D) or
three-dimensional (3D) systems. These 3D culture methods are the
most successful in maintaining the sphericity and the
communications between cells (29)
and have also shown greater follicular viability, follicle and
oocyte diameters and hormone production (3).
Artificial ovaries
The creation of an artificial ovary for
transplantation is a very promising fertility-restoring technique.
Isolated preantral follicles obtained from ovarian cryopreserved
tissue, together with other ovarian cells in a 3D-matrix, or
scaffold, result in a ovary-like environment, which could allow the
growth of follicles and therefore could restore both fertility and
endocrine function of the ovary once they are transplanted
(3). Luyckx et al (31) achieved the survival and growth of
murine ovarian follicles (primary, secondary and antral follicles)
within 1 week following the transplantation of ovarian cells in a
fibrin matrix. Moreover, Laronda et al (32) accomplished the initiation of puberty
in ovariectomized mice following an artificial ovary
transplant.
Specific target tissue drugs
Both nanoparticles and fertopotective agents share
the aim of protecting ovarian cells during gonadotoxic oncological
treatments. These are discussed below:
i) Nanoparticles
This procedure entails the encapsulation of the
therapeutic agent in order to reduce its plasma clearance and
therefore its toxicity. For such a purpose, a nanoparticulate
formulation of the therapeutic agent is developed and encapsulated
within liposomal vesicles or ‘nanobins’ (NB) (33). Ahn et al (34) demonstrated a superior antitumor
efficacy of the nanoparticulate formulation of arsenic trioxide
(As2O3) in nanobins [NB(Ni, As)] in a murine
model of lymphoma as well as a reduced fertotoxicity.
ii) Novel fertoprotective agents
Current research focus on two different pathways: a)
Anti-apoptotic agents, such as imatinib, sphingosine-1-phosphatase
(AS101), granulocyte colony-stimulating factor (G-CSF), thyroid
hormone (T3) and tamoxifen (28),
and they have shown to diminish follicle loss in animal models
(35); and on b) agents which
prevent follicle activation, such as AS101, an immunomodulator
interacting with the PI3K/PTEN/AKT follicle activation pathway
(36) and the anti-Mullerian
hormone (35). In summary, a number
of novel fertoprotectives agents to protect oocytes against
gonadotoxic treatments are being investigated and may be available
soon (3).
Fertility preservation techniques in
males
In males undergoing gonadotoxic treatment, both
sperm cryopreservation or testicular tissue cryopreservation are
currently available (3,6). The American Society of Clinical
Oncology (ASCO) guidelines recommend that oncologists inform about
the risk of infertility in patients with cancer during their
reproductive stages of life, as well as to refer them to
specialists in fertility treatment.
Cryopreservation of spermatozoa
The cryopreservation of ejaculated semen is the
recommended FP technique for adult males and pubertal boys
producing sperm in the ejaculate, who will be undergoing
gonadotoxic treatment (37). For
patients receiving radiation therapy only, gonadal shielding may be
an option if sperm collection is not possible.
The spermarche begins at puberty, but it is not
exactly known when this onset begins, since clinical parameters,
such as Tanner stage or increase on reproductive hormones, do not
always correlate with spermermatogenesis onset, according to some
data from urine examination and electro-ejaculation in pubertal
boys (38,39). The successful sperm collection
following masturbation has been reported for boys aged 12 years and
older (40,41).
The procedure includes the collection of, ideally,
at least 3 semen samples, with an abstinence period of at least 48
h in between samples, and the following cryopreservation of the
sperm samples, although often more than one semen sample must be
taken in the same day to avoid the oncological treatment delay
(7). In the case of ejaculation
failure or when no spermatozoa are found in the ejaculate, sperm
can be retrieved by epidydimal sperm aspiration, either
percutaneous (PESA) or with microsurgery (MESA), testicular sperm
extraction (TESE) or electro-ejaculation (42,43).
Assisted reproductive treatment such as IVF and
intracytoplasmic sperm injection (ICSI) are afterwards required.
ICSI has the advantage of also allowing reproduction when the semen
is of very poor quality or with only a few spermatozoa (7).
The pregnancy rates vary from 12% for intrauterine
insemination to 32% for ICSI. To date, no follow-up data for large
cohorts of children born after assisted reproductive treatment
using frozen-thawed sperm of men with cancer are available in the
literature, at least to the best of our knowledge.
It is worth mentioning that the European Germ Cancer
Consensus Group and ASCO strongly recommend informing patients
about the possibility of cryopreservation techniques before
undergoing orchiectomy or gonadotoxical treatment (30). Unfortunately, such recommendations
are oftentimes not followed by health-care professionals, and many
patients remain without counseling in the matter.
Cryopreservation of SSCs in
prepubertal children
Prepubertal children do not undergo spermatogenesis
yet, and therefore they do not have mature sperm in their testes.
Hence, the cryopreservation of spermatozoa is not possible. The
only possibility for them is to preserve testicular tissue, which
contains SSCs.
In an analogous manner to the cryopreservation of
ovarian tissue in women, the testicular tissue can be obtained
(through a testicular biopsy) and cryopreserved in form of
spermatogonia or in form of testicular tissue (using slow-freeze or
ultra-rapid techniques). This will be thereafter available to use
when the patient is free of oncological illness and desires to have
children. Once the tissue is thawed, it would allow in vitro
spermatogenesis (44) or
autotrasplantation of the cryopreserved tissue, either by infusion
of a cell suspension into the seminiferous tubules or
intratesticular grafting of the tissue (7).
The reintroduction of testicular stem cells into the
seminiferous tissue could restart the sperm production (7). Orthotopic transplantation entails the
risk of re-seeding malignant cancer cells (e.g., in patients with
leukemia), as occurs with ovarian autotransplantation. To mitigate
the problem, a decontaminated cell suspension could be a possible
solution (45). In vitro
culture of testicular cells to obtain mature spermatozoa also
circumvents the risk of reseeding malignant cells in the
auto-transplant of testicular tissue, being another branch of
research at the moment (46). It is
important to stress that fertility restoration strategies by
auto-transplantation of cryopreserved testicular tissue have not
been tested yet for safe clinical use in humans and therefore it is
still considered experimental (6).
More research is still needed regarding the use of frozen-thawed
tissue to obtain mature spermatozoa in vitro (9).
Conclusions and future perspectives
Oncological healthcare is nowadays far from being
solely the cure of cancer. Providing hope of future fertility
following oncological treatment, significantly increases the
quality of life of the patients and helps them to cope emotionally
with cancer (30). FP in both
female and male oncological patients is nowadays possible and
should be integrated as part of the oncological health care.
Different techniques exist and the most appropriate should be
chosen depending on the characteristics of the patients: Male,
female, prepubertal or postpubertal. Some of these have already
proven successful outcomes whereas others, newer and more
innovative, are still in need of further improvement and
development.
Sperm banking is now considered the first line FP
option for male patients; oocytes vitrification is currently
considered the first line option for postpuberal female patients in
which it is possible to delay chemotherapy and hormonal stimulation
is authorized. Embryo banking gets in ethical conflict when it
comes to preserving fertility, as healthcare's aim is to solely
preserve the woman's fertility, which is the reason why it is not
considered the first-line treatment anymore. Furthermore, growing
evidence of safety and efficiency success in oocyte vitrification,
upholds this technique to be the preferred one. When facing a
therapeutic emergency, or contraindication for hormonal stimulation
exists, OTC or puncture of immature oocytes are available. Immature
oocytes will then be cryopreserved, directly or after being matured
in vitro, to be vitrified as mature oocytes or as embryos
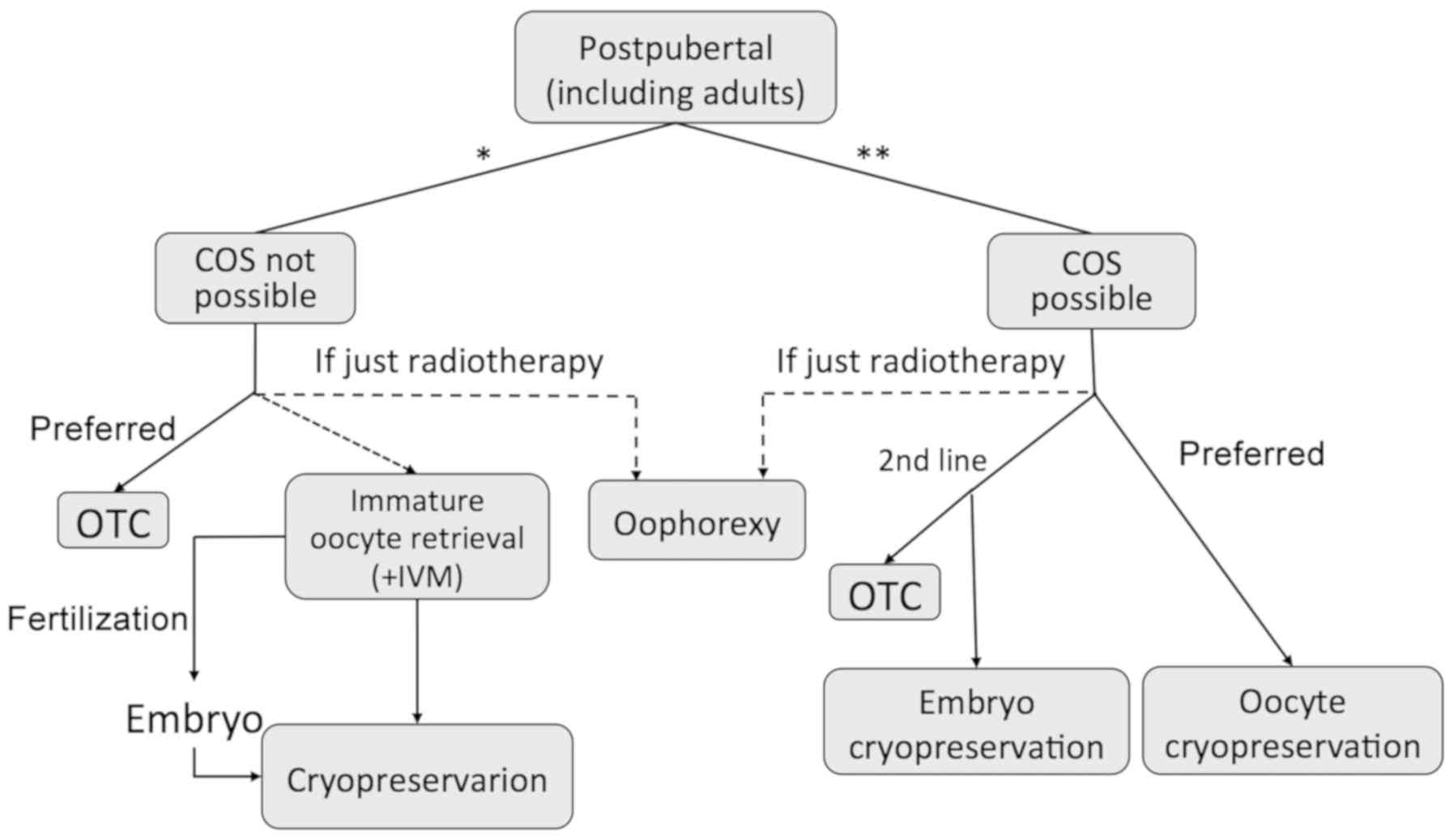
after a fertilization technique (Fig.
1).
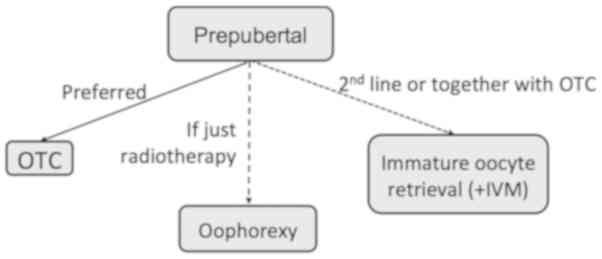
Among all the patients to whom these techniques
address, pediatric and adolescent patients are the ones with the
most restricted FP options (Fig.
2), higher survival rates, and thus those with the longest life
expectancy. Therefore, special effort should be made to improve
quality of life in this unique population and fulfill their
reproductive wish.
As oncofertility is a recent concept and it is
rapidly gaining importance, novel procedures involving emerging
technological advances are being developed. The in vitro
activation of ovarian follicles has proven itself to be a very
promising technique for future approaches, as it could be addressed
to patients with restricted FP options: Prepubertal children,
hormone-sensitive tumors and those at urge to start treatment. OTC
and subsequent transplantation, although still considered
experimental, is currently the only hope for prepubertal children
(Fig. 2). This technique has shown
encouraging results in adult patients, but literature regarding
pregnancy in prepubertal children is very scarce. Only one live
birth after autograft of cryopreserved tissue before menarche has
been published (20).
Development of specific target chemotherapeutical
treatment such as NB and the creation of artificial ovaries from
stem cells, would respectively avoid and completely restore the
ovarian function. Therefore, further development in these emerging
techniques may lead to ground breaking advances of this field in
the not so far future. Certainly, new FP techniques will continue
to develop in the following years. However, despite the growing
advances in the subject, optimal counselling from healthcare
professionals is lacking.
In conclusion, FP in both female and male
oncological patients is nowadays possible and should be integrated
as part of the oncological health care. Different techniques exist
and the most appropriate should be chosen depending on the
characteristics of the patients: male, female, prepubertal or
postpubertal. Many of the techniques are still in under
experimental trials, whereas some others are standardized and
established. Oocyte vitrification for female patients and sperm
banking for male patients are now considered the first line FP
option.
Acknowledgements
The authors would like to thank Mr. Aris Protopapas
(Ludwig Maximilian University of Munich, Munich, Germany) for his
helpful comments that greatly improved upon the manuscript.
Funding
The present study was supported grants from the
Instituto Carlos III (PI 11/01377; PI15/01763 and PI18/01484, and
ISCIII-RETICRD12/0036/0029), Fundación Vencer el Cancer (VEC), and
from the Government of Catalonia (2017SGR-1014).
Availability of data and materials
Not applicable.
Authors' contributions
SDPL and PGB conceived and designed the study. SDPL
collected the information (performed literature search) and wrote
the manuscript. CS, FMS, AT and PGB assisted with the collection
and selection of the information (literature search). FMS, AT and
MP contributed to the conception and design of the study and wrote
different sections, reviewed the manuscript and suggested some
changes. MP and PGB edited the manuscript up to the final form. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AKT
|
protein kinase B
|
|
As2O3
|
arsenic trioxide
|
|
ASCO
|
American Society of Clinical
Oncology
|
|
AYAs
|
adolescent and young adults
|
|
COS
|
controlled ovarian stimulation
|
|
ESGO
|
European Society of Gynecological
Oncology
|
|
FEPNC
|
Spanish Federation of Parents of
Children with Cancer
|
|
FOXO3
|
Forkhead box O3
|
|
FP
|
fertility preservation
|
|
G-CSF
|
granulocyte colony-stimulating
factor
|
|
GnRH
|
gonadotropin-releasing hormone
|
|
IVF
|
in vitro fertilization
|
|
IVM
|
in vitro maturation
|
|
ICSI
|
intracytoplasmic sperm injection
|
|
INCIP
|
International Network on Cancer,
Infertility and Pregnancy
|
|
NB
|
nanobins
|
|
OTC
|
ovarian tissue cryopreservation
|
|
PCOS
|
polycystic ovarian syndrome
|
|
PGD
|
pre-implantation genetic diagnosis
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
POF
|
premature ovarian failure
|
|
POI
|
primary ovarian insufficiency
|
|
PTEN
|
phosphatase and tensin homolog
|
References
|
1
|
Coccia PF, Pappo AS, Altman J, Bhatia S,
Borinstein SC, Flynn J, Frazier AL, George S, Goldsby R, Hayashi R,
et al: Adolescent and young adult oncology, version 2.2014. J Natl
Compr Canc Netw. 12:20–32. 2014. View Article : Google Scholar
|
|
2
|
Melan K, Amant F, Veronique-Baudin J,
Joachim C and Janky E: Fertility preservation healthcare circuit
and networks in cancer patients worldwide: What are the issues? BMC
Cancer. 18:1922018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim SY, Kim SK, Lee JR and Woodruff TK:
Toward precision medicine for preserving fertility in cancer
patients: Existing and emerging fertility preservation options for
women. J Gynecol Oncol. 27:e222016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Donnez J and Dolmans MM: Fertility
preservation in women. Nat Rev Endocrinol. 9:735–749. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marder W, Fisseha S, Ganser MA and Somers
EC: Ovarian damage during chemotherapy in autoimmune diseases:
Broad health implications beyond fertility. Clin Med Insights
Reprod Heal. 2012:9–18. 2012.
|
|
6
|
Martinez F; International Society for
Fertility Preservation-ESHRE-ASRM Expert Working Group, : Update on
fertility preservation from the Barcelona International Society for
Fertility Preservation-ESHRE-ASRM 2015 expert meeting: Indications,
results and future perspectives. Fertil Steril. 108:407–415.e11.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tournaye H, Dohle GR and Barratt CLR:
Fertility preservation in men with cancer. Lancet. 384:1295–1301.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shalet SM, Tsatsoulis A, Whitehead E and
Read G: Vulnerability of the human Leydig cell to radiation damage
is dependent upon age. J Endocrinol. 120:161–165. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rodriguez-Wallberg K and Oktay K:
Fertility preservation during cancer treatment: Clinical
guidelines. Cancer Manag Res. 6:105–117. 2014.PubMed/NCBI
|
|
10
|
Cakmak H and Rosen MP: Ovarian stimulation
in cancer patients. Fertil Steril. 99:1476–1484. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abdel Hafez FF, Desai N, Abou-Setta AM,
Falcone T and Goldfarb J: Slow freezing, vitrification and
ultra-rapid freezing of human embryos: A systematic review and
meta-analysis. Reprod Biomed Online. 20:209–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rezazadeh Valojerdi M, Eftekhari-Yazdi P,
Karimian L, Hassani F and Movaghar B: Vitrification versus slow
freezing gives excellent survival, post warming embryo morphology
and pregnancy outcomes for human cleaved embryos. J Assist Reprod
Genet. 26:347–354. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vajta G and Kuwayama M: Improving
cryopreservation systems. Theriogenology. 65:236–244. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cil AP, Bang H and Oktay K: Age-specific
probability of live birth with oocyte cryopreservation: An
individual patient data meta-analysis. Fertil Steril. 100:492–9.e3.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mahajan N: Fertility preservation in
female cancer patients: An overview. J Hum Reprod Sci. 8:3–13.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Donnez J and Dolmans M-M: Fertility
Preservation in Women. N Engl J Med. 377:1657–1665. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lim JH and Chian RC: In vitro maturation
of human immature oocytes. J Reprod Stem Cell Biotechnol.
doi.org/10.1177/205891581000100205.
|
|
18
|
Meirow D, Levron J, Eldar-Geva T, Hardan
I, Fridman E, Zalel Y, Schiff E and Dor J: Pregnancy after
transplantation of cryopreserved ovarian tissue in a patient with
ovarian failure after chemotherapy. N Engl J Med. 353:318–321.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Donnez J, Dolmans MM, Diaz C and Pellicer
A: Ovarian cortex transplantation: Time to move on from
experimental studies to open clinical application. Fertil Steril.
104:1097–1098. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Demeestere I, Simon P, Dedeken L, Moffa F,
Tsépélidis S, Brachet C, Delbaere A, Devreker F and Ferster A: Live
birth after autograft of ovarian tissue cryopreserved during
childhood. Hum Reprod. 30:2107–2109. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ernst EH, Offersen BV, Andersen CY and
Ernst E: Legal termination of a pregnancy resulting from
transplanted cryopreserved ovarian tissue due to cancer recurrence.
J Assist Reprod Genet. 30:975–978. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tulandi T: Ovarian transposition before
pelvic radiation. UpToDate. 2018, https://www.uptodate.com/contents/ovarian-transposition-before-pelvic-radiation
|
|
23
|
Sonmezer M and Oktay K: Fertility
preservation in female patients. Hum Reprod Update. 10:251–266.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lambertini M, Ceppi M, Poggio F, Peccatori
FA, Azim HA Jr, Ugolini D, Pronzato P, Loibl S, Moore HC, Partridge
AH, et al: Ovarian suppression using luteinizing hormone-releasing
hormone agonists during chemotherapy to preserve ovarian function
and fertility of breast cancer patients: A meta-analysis of
randomized studies. Ann Oncol. 26:2408–2419. 2015.PubMed/NCBI
|
|
25
|
Del Mastro L, Ceppi M, Poggio F, Bighin C,
Peccatori F, Demeestere I, Levaggi A, Giraudi S, Lambertini M,
D'Alonzo A, et al: Gonadotropin-releasing hormone analogues for the
prevention of chemotherapy-induced premature ovarian failure in
cancer women: Systematic review and meta-analysis of randomized
trials. Cancer Treat Rev. 40:675–683. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Demeestere I, Brice P, Peccatori FA,
Kentos A, Dupuis J, Zachee P, Casasnovas O, Van Den Neste E,
Dechene J, De Maertelaer V, et al: No Evidence for the Benefit of
Gonadotropin-Releasing Hormone Agonist in Preserving Ovarian
Function and Fertility in Lymphoma Survivors Treated With
Chemotherapy: Final Long-Term Report of a Prospective Randomized
Trial. J Clin Oncol. 34:2568–2574. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hsueh AJW, Kawamura K, Cheng Y and Fauser
BCJM: Intraovarian control of early folliculogenesis. Endocr Rev.
36:1–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Novella-Maestre E, Herraiz S,
Rodríguez-Iglesias B, Díaz-García C and Pellicer A: Short-term PTEN
inhibition improves in vitro activation of primordial
follicles, preserves follicular viability, and restores AMH levels
in cryopreserved ovarian tissue from cancer patients. PLoS One.
10:e01277862015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Colgan TJ, Murphy J, Cole DE, Narod S and
Rosen B: Occult carcinoma in prophylactic oophorectomy specimens:
Prevalence and association with BRCA germline mutation status. Am J
Surg Pathol. 25:1283–1289. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ben-Aharon I, Abir R, Perl G, Stein J,
Gilad G, Toledano H, Elitzur S, Avrahami G, Ben-Haroush A, Oron G,
et al: Optimizing the process of fertility preservation in
pediatric female cancer patients - a multidisciplinary program. BMC
Cancer. 16:6202016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Luyckx V, Dolmans M-M, Vanacker J, Legat
C, Fortuño Moya C, Donnez J and Amorim CA: A new step toward the
artificial ovary: Survival and proliferation of isolated murine
follicles after autologous transplantation in a fibrin scaffold.
Fertil Steril. 101:1149–1156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Laronda MM, Jakus AE, Whelan KA, Wertheim
JA, Shah RN and Woodruff TK: Initiation of puberty in mice
following decellularized ovary transplant. Biomaterials. Feb
14–2015.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ahn RW, Chen F, Chen H, Stern ST, Clogston
JD, Patri AK, Raja MR, Swindell EP, Parimi V, Cryns VL, et al: A
novel nanoparticulate formulation of arsenic trioxide with enhanced
therapeutic efficacy in a murine model of breast cancer. Clin
Cancer Res. 16:3607–3617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ahn RW, Barrett SL, Raja MR, Jozefik JK,
Spaho L, Chen H, Bally MB, Mazar AP, Avram MJ, Winter JN, et al:
Nano-encapsulation of arsenic trioxide enhances efficacy against
murine lymphoma model while minimizing its impact on ovarian
reserve in vitro and in vivo. PLoS One. Mar
20–2013.(Epub ahead of print). View Article : Google Scholar
|
|
35
|
Roness H, Kashi O and Meirow D: Prevention
of chemotherapy-induced ovarian damage. Fertil Steril. 105:20–29.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kalich-Philosoph L, Roness H, Carmely A,
Fishel-Bartal M, Ligumsky H, Paglin S, et al: Cyclophosphamide
Triggers Follicle Activation and “Burnout”; AS101 Prevents Follicle
Loss and Preserves Fertility. Sci Transl Med. May 15;185ra622013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pacey AA and Eiser C: Banking sperm is
only the first of many decisions for men: What healthcare
professionals and men need to know. Hum Fertil (Camb). 14:208–217.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pedersen JL, Nysom K, Jørgensen M, Nielsen
CT, Müller J, Keiding N and Skakkebaek NE: Spermaturia and puberty.
Arch Dis Child. 69:384–387. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
van der Kaaij MAE, van Echten-Arends J,
Simons AHM and Kluin-Nelemans HC: Fertility preservation after
chemotherapy for Hodgkin lymphoma. Hematol Oncol. 28:168–179. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hagenäs I, Jørgensen N, Rechnitzer C,
Sommer P, Holm M, Schmiegelow K, Daugaard G, Jacobsen N and Juul A:
Clinical and biochemical correlates of successful semen collection
for cryopreservation from 12–18-year-old patients: A single-center
study of 86 adolescents. Hum Reprod. 25:2031–2038. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bahadur G, Ling KLE, Hart R, Ralph D, Wafa
R, Ashraf A, Jaman N, Mahmud S and Oyede AW: Semen quality and
cryopreservation in adolescent cancer patients. Hum Reprod.
17:3157–3161. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chan PT, Palermo GD, Veeck LL, Rosenwaks Z
and Schlegel PN: Testicular sperm extraction combined with
intracytoplasmic sperm injection in the treatment of men with
persistent azoospermia postchemotherapy. Cancer. 92:1632–1637.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Köhn FM, Schroeder-Printzen I, Weidner W,
Montag M, van der Ven H and Schill WB: Testicular sperm extraction
in a patient with metachronous bilateral testicular cancer. Hum
Reprod. 16:2343–2346. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ning L, Meng J, Goossens E, Lahoutte T,
Marichal M and Tournaye H: In search of an efficient injection
technique for future clinical application of spermatogonial stem
cell transplantation: Infusion of contrast dyes in isolated
cadaveric human testes. Fertil Steril. 98:1443–8.e1. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Goossens E, Van Saen D and Tournaye H:
Spermatogonial stem cell preservation and transplantation: From
research to clinic. Hum Reprod. 28:897–907. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Stukenborg J-B, Schlatt S, Simoni M, Yeung
C-H, Elhija MA, Luetjens CM, Huleihel M and Wistuba J: New horizons
for in vitro spermatogenesis? An update on novel
three-dimensional culture systems as tools for meiotic and
post-meiotic differentiation of testicular germ cells. Mol Hum
Reprod. 15:521–529. 2009. View Article : Google Scholar : PubMed/NCBI
|