Introduction
Chronic lymphocytic leukaemia (CLL) is the most
common form of leukaemia and involves the clonal expansion of
abnormal B cells in the lymph nodes and/or blood. B cells, part of
the adaptive immune response, function to produce antibodies
against antigens; however in CLL, B cells fail to mature and
differentiate correctly, eventually crowding out healthy blood
cells (1). CLL is a disease which
mainly affects adults and can clinically be subdivided into two
major types depending on the mutational status of the
immunoglobulin heavy chain gene (IGVH): Patients with CLL cells
harbouring unmutated IGVH (UM-IGVH) have an average survival of 8
years, whereas patients with CLL with mutated IGVH (M-IGVH) have an
average survival of >25 years. In addition, acquired chromosomal
abnormalities involving deletions in chromosomes 11q, 13q and 17p
and trisomy 12 are common in CLL, and these abnormalities predict
the time to first treatment and CLL-specific survival rate. The
genes principally responsible for an adverse prognosis, associated
with deletions (del) in 17p13 and del11q22, have been recognised as
TP53 and ATM, respectively (2). Other poor prognostic markers have been
identified, including the lack of expression of CD38, expression of
cytoplasmic marker z-associated protein 70 and, more recently,
mutations in p53, Notch-1, splicing factor 3B subunit 1 and
baculoviral IAP repeat-containing protein 3 (2).
Current treatment paradigms for CLL involve use of
fludarabine phosphate, cyclophosphamide and monoclonal antibodies,
including rituximab (known as the FCR regime) (3). Fludarabine phosphate, the gold
standard treatment for CLL, was originally synthesised in 1969
(4), and received US Food and Drug
Administration (FDA)-approval for the treatment of CLL in 1991. It
is a synthetic adenine nucleoside pro-drug, which is activated to a
triphosphate form, F-ara-ATP. This activated form of fludarabine
phosphate interferes with DNA synthesis by inhibiting
ribonucleotide reductase, DNA polymerases, DNA ligase I and DNA
primase. It is indicated for the first- and second-line treatment
of CLL, and leads to more complete remissions (CR; 7–40%) than
other conventional chemotherapies, including
cyclophosphamide/doxorubicin/prednisone (CAP) and
cyclophosphamide/hydroxydaunorubicin (doxorubicin)/oncovin
(vincristine)/prednisone treatment (CHOP) regimens (5). Fludarabine phosphate is also
successfully used in combination with several other
chemotherapeutics.
A number of more recent agents have also received
European Medicines Agency (EMA) and FDA approval for the treatment
of CLL: Ibrutinib, as a frontline treatment, which targets B-cell
receptor signalling through the inhibition of Bruton's tyrosine
kinase (Btk) (6); obinutuzumab
approved in combination with chlorambucil, which is a type-2
monoclonal anti-CD20 antibody that results in direct and
antibody-dependent cell-mediated cytotoxicity of leukaemia cells
(7); and idelalisib approved in
combination with rituximab for relapsed CLL, which inhibits B-cell
receptor signalling through the inhibition of phosphatidylinositol
3-kinase (PI3K) δ molecules (8).
The most recently approved CLL drug, venetoclax, is a B-cell
lymphoma 2 (Bcl-2) inhibitor. However, there are significant issues
associated with these drugs, including the development of
resistance, and there is an unmet need for alternative drug
therapies for these diseases.
Antidepressants are a class of compounds which mimic
the effects of naturally occurring neurotransmitters. They inhibit
reuptake transporters, maintaining high concentrations of
neurotransmitters in the synapse, and are used to treat the
symptoms of depression, anxiety and several other psychological
illnesses (9,10).
The finding of the serotonin reuptake transporter
(SERT) in B cell malignancies (11)
led to the investigation of antidepressants and their analogues as
potential anti-lymphoma/leukaemic agents. Activity has been
demonstrated by tricyclic antidepressants citalopram, imipramine
and clomipramine in HL-60 human acute myeloid leukemia cells and
normal lymphocytes (12–14); and fluoxetine (11,15,16),
MDMA and its analogues (11,17),
fenfluramine (11), clomipramine
(11), and maprotiline and its
analogues (15,16,18) in
Burkitt's lymphoma cell lines. In addition, our previous studies
reported on the synthesis and SERT inhibitory activity of a number
of structurally related 1,3-bis(aryl)-2-nitro-1-propene and
1,3-bis(aryl)-2-propanamine compounds and identified their
antiproliferative activity against Burkitt's lymphoma (19,20).
However, antidepressants have been shown to exhibit toxicity
against a wide range of cancer cell lines (21–24),
other than those of lymphatic origin, and toxicity has been
reported to be independent of SERT and other transporters (15).
The drug 4-methylthioamphetamine (4-MTA) is an
illegal analogue of the antidepressant amphetamine. Nitrostyrenes
[(2-nitrovinyl)benzenes)] are a family of compounds based on the
scaffold structure of 4-MTA. Nitrostyrene derivatives have also
been reported to induce potent anticancer effects in a range of
cancer cell lines; for example, nitrostyrenes have demonstrated
pronounced anticancer activity in oral (25) and colon cancer (26), osteosarcoma (27) and Erlich ascetic tumour cell lines
(28). Nitrostyrene derivatives
have also been shown to possess a diverse range of other biological
activities, including antimicrobial (29), anti-inflammatory (30) and immunosuppressive properties
(31). Various mechanisms of action
have been identified for nitrostyrene analogues, including tubulin
inhibition (29,32), telomerase inhibition (33), protein tyrosine phosphatase (PTP)
inhibition (34) and phospholipase
A2 inhibition (35).
Our previous study reported on the synthesis of a
library of novel analogues and structural derivatives of 4-MTA;
1,3-bis(aryl)-2-nitro-1-propene derivatives (n=193), which contain
a classic nitrostyrene structure, and evaluated their potential
activity as antiproliferative agents against Burkitt's lymphoma
(36). This screen allowed for the
development of a structure-activity relationship (SAR) and an
optimised nitrostyrene structure for predicted antiproliferative
activity. The aim of the present study was to evaluate
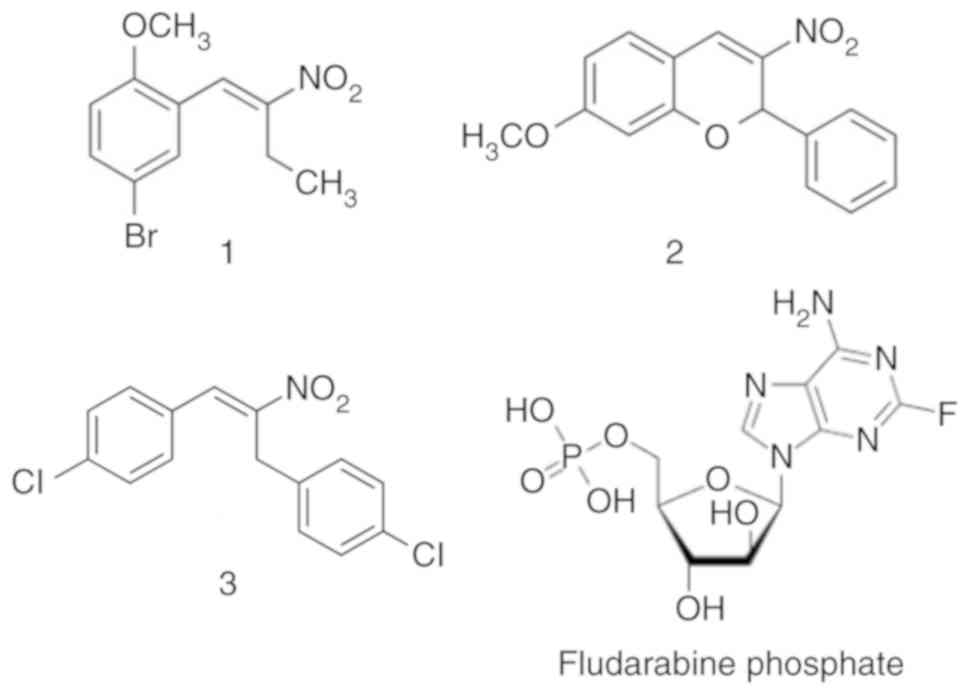
representative nitrostyrene compounds (Fig. 1) in a range of cancer types, with a
focus on CLL.
Materials and methods
Materials
Alamar blue was obtained from BioSource Europe S.A
(Nivelles, Belgium) and foetal bovine serum (FBS) was from
Invitrogen; Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
RPMI-1640 and DMEM were sourced from Biosciences, Ltd. (Dublin,
Ireland) and Lymphoprep from Axis-Shield (Oslo, Norway). Cell
culture consumables were from Greiner Bio-One, Ltd. (Stonehouse,
UK), and all other reagents used were from Sigma; Merck KGaA
(Darmstadt, Germany). Compounds 1, 2 and 3 were prepared as
previously reported (36).
Cell culture
The DG-75, BJAB and Ramos Burkitt's lymphoma cell
lines were provided by Dr Dermot Walls (School of Biotechnology,
Dublin City University, Dublin, Ireland). The MUTU-I (c179) cell
line was provided by Professor Martin Rowe (Division of Cancer
Studies, The University of Birmingham, Birmingham, UK). The
EBV-transformed CLL PGA-1, I83 (M-IGVH, good prognosis), HG-3 and
CII (UM-IGVH, poor prognosis) cell lines were provided by Professor
Anders Rosén (Linköping University, Linköping, Sweden) (37). Other cell lines were sourced as
follows: HL-60 promyelocytic leukaemia cells (cat. no. 98070106;
ECACC, Salisbury, UK), HeLa cervical cancer cells (cat. no.
93021013; ECACC), MCF-7 breast cancers (cat. no. 86012803; ECACC)
and HS-5 bone marrow stromal cells [cat. no. CRL-11882; American
Type Culture Collection (ATCC) Manassas, VA, USA].
The CLL and HL-60 Burkitt's lymphoma cell lines were
grown in RPMI-1640 (Glutamax; Thermo Fisher Scientific, Inc.)
medium supplemented with 10% (v/v) FBS and 50 µg/ml
penicillin/streptomycin and seeded at a density of 2×105
cells/ml. The MUTU-I cell line also required the additional
supplements of a-thioglycerol [5 mM in phosphate-buffered saline
(PBS) with 20 µM bathocuprione disulfonic acid], sodium pyruvate
(100 mM) and HEPES (1 mM). The HS-5 bone marrow stromal, HeLa
cervical cancer and MCF-7 breast cancer cell lines were grown in
DMEM supplemented with 10% (v/v) FBS and 50 µg/ml
penicillin/streptomycin and seeded at a density of 5×104
cells/ml. All cells were grown in a humidified environment of 37°C
maintained at 95% O2 and 5% CO2 and passaged
at least twice weekly depending on their levels of confluency.
Alamar blue viability assay
The cells were seeded in 200 µl medium in a 96-well
plate and were treated and incubated as required (up to 72 h).
Alamar blue (20 µl) was then added to each well and incubated at
37°C in the dark for 4 h. The plates were then read on a
fluorescence plate reader (SpectraMax Gemini; Molecular Devices
LLC, Sunnyvale, CA, USA) with excitement and emission wavelengths
of 544 and 590 nm, respectively. The experiments were performed in
triplicate. A sample containing only reagent and medium (without
any cells) was used as a blank control. The vehicle samples were
set as 100% viability, from which any decrease in viability was
calculated. To determine the IC50 values, the cells were
treated with seven concentrations of nitrostyrene compounds over a
range of 0.5–20 µM (0.5, 1, 2.5, 5, 7.5, 10 and 20 µM) or five
concentrations of fludarabine phosphate over a range of 1–50 µM (1,
5, 10, 20 and 50 µM).
Flow cytometry
The CLL cells (5×104 cells/ml) were
treated 37°C with the nitrostyrene compounds for 48 h, harvested by
centrifugation at 400 × g and rinsed with 0.5 ml of Ca2+
Annexin V-binding buffer (0.1 M HEPES, pH 7.4; 0.14 M NaCl; 25 mM
CaCl2). The samples were resuspended in 50 µl FITC
Annexin V (diluted 1:33 in Ca2+ Annexin V-binding
buffer), incubated on ice for 10 min, washed with Annexin V-binding
buffer and resuspended in 500 µl of propidium iodide (PI) solution
(0.5 µg/ml). The samples were analysed within 1 h using a CyAn ADP
flow cytometer (Beckman Coulter, Inc., Brea, CA, USA) counting
10,000 cells and analysed using the FlowJo software (FlowJo LLC,
Ashland, OR, USA) package. Early apoptotic cells were Annexin
V-positive and PI-negative, and late apoptotic cells were Annexin
V-positive and PI-positive.
LDH assay
The CLL cells were seeded in 200 µl medium in a
96-well plate, following treatment with 5 µM nitrostyrene compounds
for 48 h, 50 µl of cell supernatants were placed in a fresh 96 well
plate and 100 µl lactate dehydrogenase (LDH) assay mixture (Promega
Corp., Madison, WI, USA) was added to each sample. The plate was
covered with tin foil and incubated at room temperature for 30 min.
The reaction was terminated by the addition of 15 µl of 1 N HCl to
each well. The absorbances of each sample were then obtained at 490
and 690 nm. The lysis solution wells were set at 100% lysis, from
which the LDH release of the treated samples was calculated.
Inhibitor experiments
The cells (5×104 cells/ml) were
pretreated at 37°C with either 5 mM N-acetyl-cysteine (Nac) for 1 h
or 40 µM caspase inhibitor (Z-VAD; MBL International, Co., Woburn,
MA, USA) for 4 h prior to 5 µM compound treatment for 48 h.
Co-treatment analysis
Drug interactions were determined by median dose
effect analysis using CalcuSyn version 2.0 software (BioSoft,
Cambridge, UK). This method is based on the drug effect equation of
Chou and Talalay and can determine the degree of synergism or
antagonism between two compounds by generating a combination index
(CI) value. CI values of <1, =1 and >1 indicate synergism, an
additive effect and antagonism, respectively (38).
Ex vivo CLL patient samples
Fresh peripheral blood (10 ml) was collected with
written informed consent from patients with CLL (n=14) in
EDTA-anticoagulant tubes. Ethical approval for all procedures
performed on ex vivo CLL patient samples was obtained from
the St. James's Hospital and Adelaide and Meath incorporating the
National Children's Hospital Joint Ethics Committee (Dublin,
Ireland). Informed consent for the use of tissues and publication
of patient data was obtained from each subject in line with the
Declaration of Helsinki. Patients were included if they were
diagnosed with CLL, consented to the trial and were >18 years of
age. Patients were excluded if the spontaneous background levels of
apoptosis were ≥70%. Patients were recruited between July 2013 and
August 2014. The peripheral blood was diluted with an equal volume
of RPMI medium and peripheral blood mononuclear cells (PBMCs) were
isolated via lymphoprep ficoll gradient centrifugation at 600 × g
for 30 min at 4°C. The pelleted cells were resuspended in freezing
medium (10% DMSO, 90% FBS) and frozen until required. When
required, the frozen stocks were resuspended in RPMI-1640
(Glutamax) medium supplemented with 10% (v/v) FBS and 50 µg/ml
penicillin/streptomycin, and seeded at a density of
1×106 cells/ml. The cells were then treated for 48 h
with the compounds or the required vehicles and positive
controls.
Statistical analysis
GraphPad Prism 5 software (GraphPad Software, Inc.,
La Jolla, CA, USA) was used to perform statistical analysis on all
samples. For comparisons in datasets containing multiple groups,
one way analysis of variance and Tukey's post hoc test was used,
and P<0.05 was considered to indicate a statistically
significant difference. For comparisons in datasets containing two
groups, Student's paired t-test was used.
Results and Discussion
Lead nitrostyrene compounds potently
reduce cellular viability and induce apoptosis in a range of cancer
cell lines
Representative nitrostyrene compounds, 1 (previously
named 145) (36) and lead compound
2 (previously named 66) (36),
together with the previous lead compound 3 (previously named 85)
(20) were evaluated for their
ability to reduce the viability of a range of cancerous cell lines
via Alamar blue viability assays. Fludarabine phosphate was used as
a comparative control for CLL cell lines, whereas taxol was used as
a comparative control for all other cancer cell lines. The results
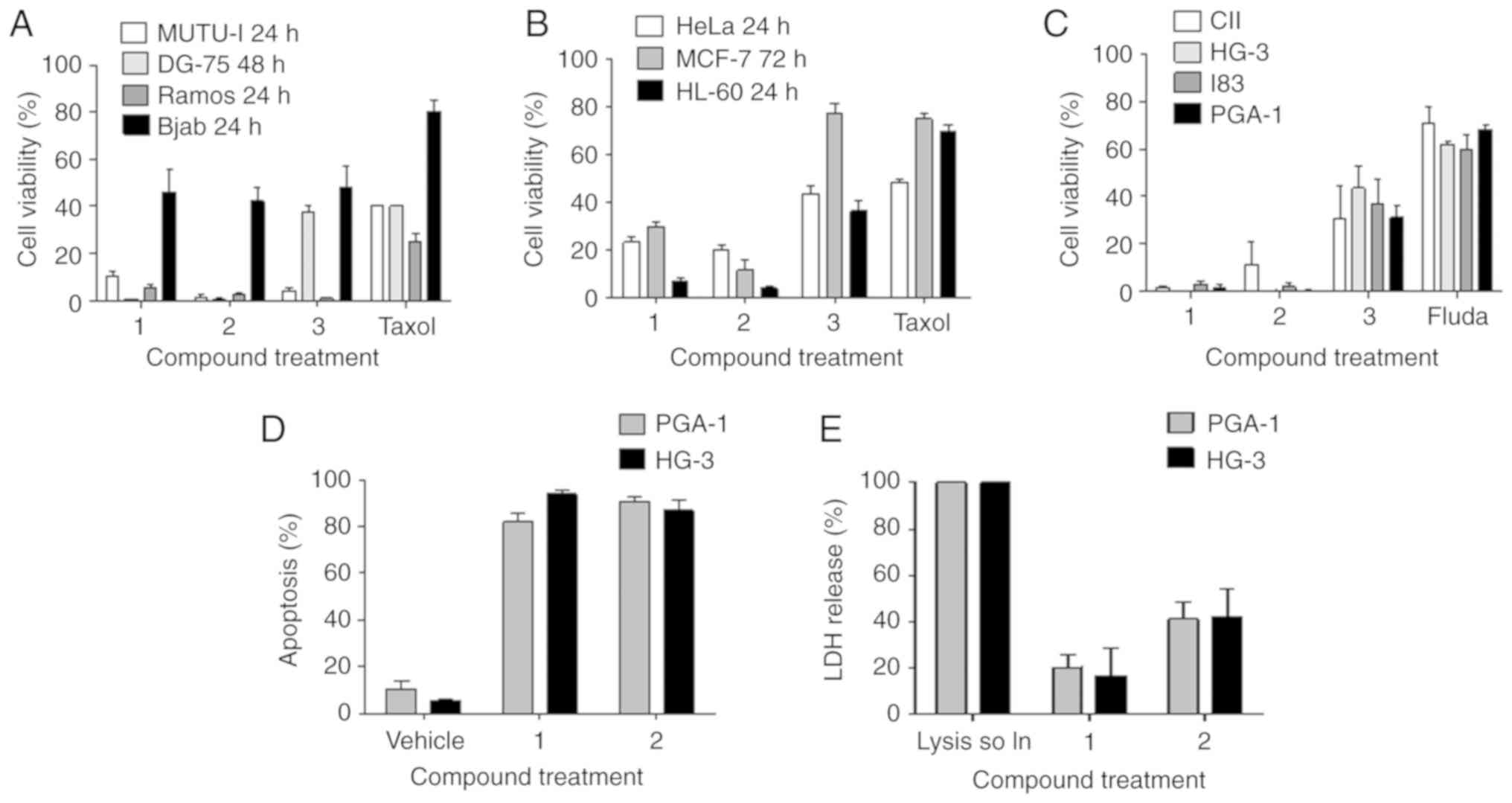
demonstrated that compounds 1 and 2 potently reduced the viability
of a range of Burkitt's lymphoma (Fig.
2A); HeLa, MCF-7 and HL-60 (Fig.
2B) and CLL cell lines (Fig.
2C) at a concentration of 10 µM. The results in Fig. 2A are in agreement with previously
published results (36). Of note,
compounds 1 and 2 reduced the viability of all cell lines to a
greater extent than either comparator compound, fludarabine
phosphate or taxol.
In the CLL cell lines, the IC50 values
obtained were in the low micromolar range for compounds 1 and 2
(2–5 µM) and were ~2-3-fold lower than the IC50 values
obtained for compound 3. Fludarabine phosphate, the current
frontline treatment for CLL, was significantly less active than the
nitrostyrene compounds, with IC50 values of between 20
and 50 µM (4–15-fold higher than for 1 and 2. The IC50
values for the nitrostyrene compounds were similar across all four
cell lines, irrespective of IGVH mutational status (I83 and PGA-1:
M-IGVH; HG-3 and CII: UM-IGVH) (Table
I).
 | Table I.IC50 values following
treatment with the indicated compounds for 48 h in chronic
lymphocytic leukaemia cell lines. |
Table I.
IC50 values following
treatment with the indicated compounds for 48 h in chronic
lymphocytic leukaemia cell lines.
|
| IC50
(µM) |
|---|
|
|
|
|---|
| Compound | CII | HG-3 | I83 | PGA-1 |
|---|
| Fluda | 49.9 | 28.1 | 20.7 | 32 |
| 1 | 3.46 | 2.54 | 4.74 | 2.9 |
| 2 | 3.46 | 3.08 | 4.13 | 2.17 |
| 3 | 8.05 | 8.69 | 8.43 | 6.74 |
The PGA-1 and HG-3 cell lines (39) were selected for subsequent
experiments to investigate the ability of the compounds to induce
apoptosis in these two cell lines. The cells underwent a
significant increase in apoptosis, as determined using Annexin V
and PI staining (Fig. 2D). In
agreement with these results, compound 1 induced only a low
percentage of LDH release, indicative of necrosis, an alternative
but less desirable form of cell death from a clinical point of view
due to its inflammatory effects. Compound 2 induced a higher, but
moderate percentage of LDH release (Fig. 2E).
Lead nitrostyrene-induced cell death
is reactive oxygen species (ROS)- and caspase-dependent
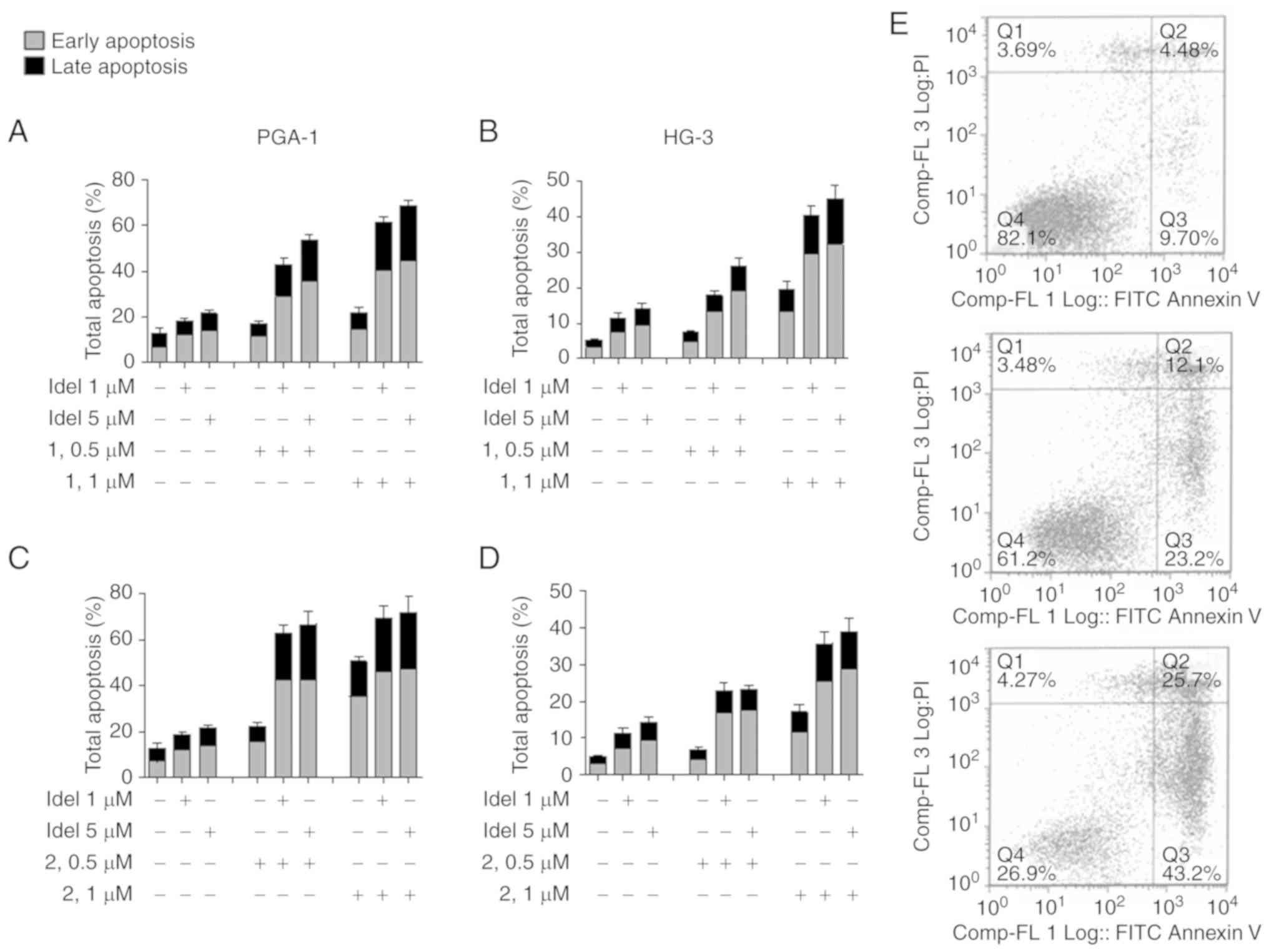
Pretreatment of the cells with N-acetyl-cysteine
(Nac), a ROS inhibitor, caused a significant reduction in
nitrostyrene-induced apoptosis compared with that in cells treated
with the nitrostyrene compounds alone (Fig. 3A and B). ROS are normally produced
in low number as a toxic by-product following oxidative
phosphorylation in the mitochondria; the results suggested that the
target of nitrostyrene compounds 1 and 2 may be the mitochondria.
Further experiments revealed that the inhibition of caspases, which
are intracellular cysteine proteases commonly associated with
apoptosis, significantly reduced the percentage of cells undergoing
apoptosis following treatment with nitrostyrene compounds 1 and 2,
suggesting a caspase-dependent form of cell death may be occurring
(Fig. 3C and D). Representative dot
plots are shown for illustrative purposes (Fig. 3E). These results are in agreement
with those of previous studies on nitrostyrene analogues and the
involvement of caspases. For example, caspase activation was
observed with trans-β-nitrostyrene in LoVo adenocarcinoma cells
(40), two nitrostyrene derivative
compounds (NTS1 and NTS2) in Ehrlich ascitic tumour cells (28) and 2-aryl-3-nitro-2H-chromenes
in breast cancer cells (41).
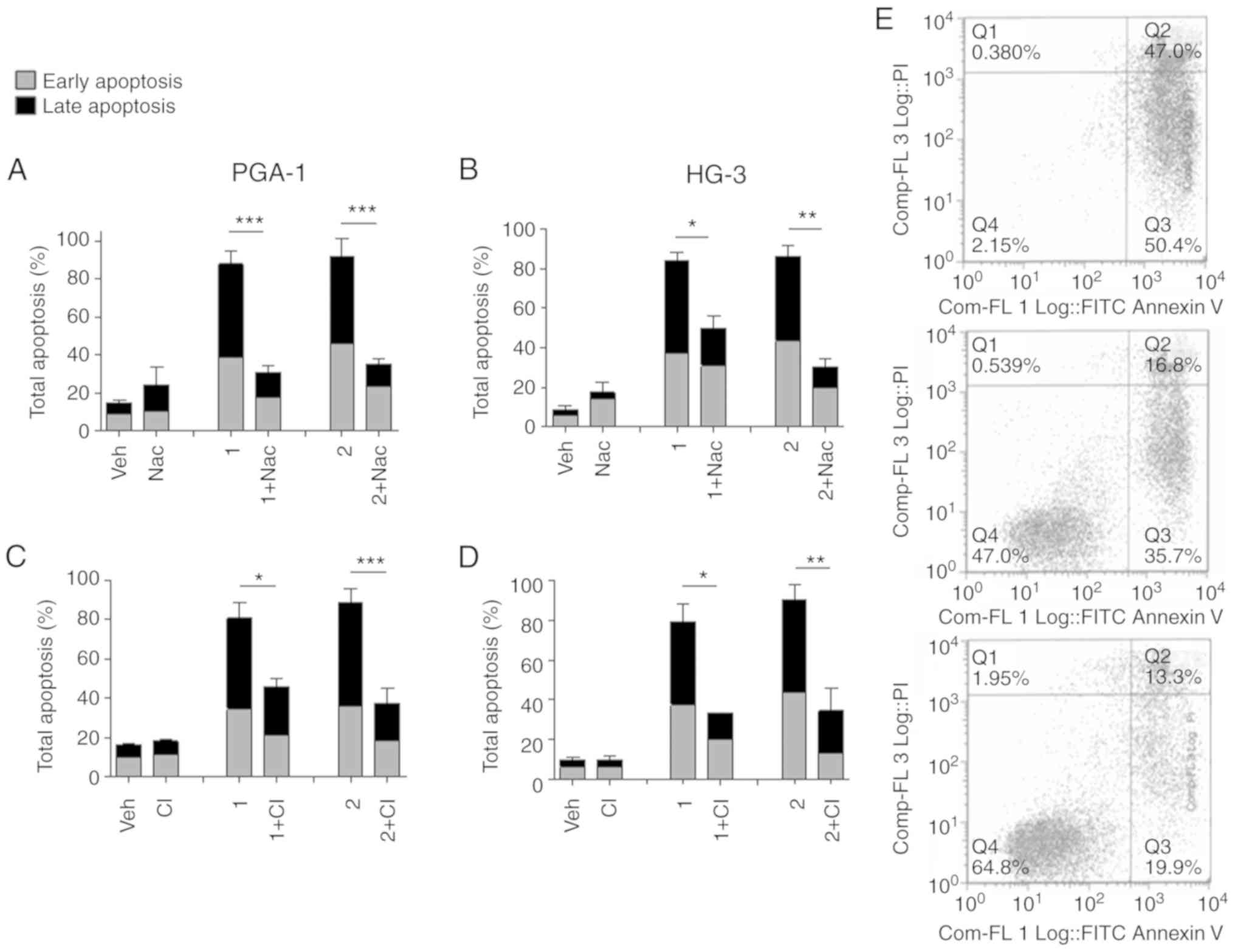 | Figure 3.Nitrostyrene analogue-induced
apoptosis is caspase- and ROS-dependent. CLL cells were pretreated
with the ROS inhibitor (5 mM Nac) for 1 h or the CI (40 µM Z-VAD)
for 4 h, followed by treatment for a further 48 h with 5 µM of the
indicated nitrostyrene compounds. Cells were subsequently analysed
for apoptosis by Annexin V and PI flow cytometry. Early apoptotic
cells were Annexin V-positive, PI-negative, late apoptotic cells
were Annexin V- and PI-positive. (A) PGA-1 cells, ROS inhibition,
(B) HG-3 cells, ROS inhibition, (C) PGA-1 cells, caspase inhibition
and (D) HG-3 cells, caspase inhibition. Values are presented as the
mean ± standard error of the mean of three independent experiments.
*P<0.05; **P<0.01; ***P<0.001. (E) Representative dot
plots of HG-3 cells treated with (top) 5 µM compound 1, (middle)
Nac + 5 µM compound 1 and (bottom) CI + 5 µM compound 1. CLL,
chronic lymphocytic leukaemia; ROS, reactive oxygen species; CI,
caspase inhibitor; PI, propidium iodide; Veh, vehicle; Nac,
N-acetyl-cysteine. |
Lead nitrostyrene compounds
synergistically enhance the effect of idelalisib
Few cancer treatments exist as a standalone therapy,
so it is important to develop small molecule inhibitors that can
potentiate the effects of other treatments. Idelalisib, a
PI3K-inhibitor, has recently been FDA-approved for the treatment of
relapsed CLL in combination with rituximab and EMA-approved in
combination with rituximab or ofatumumab (8). Therefore, the present study aimed to
determine whether idelalisib augments the apoptotic effect of the
lead nitrostyrene compounds, or vice versa. The results
demonstrated that treatment with 1 µM of compound 1 alone caused
22.0±7.5% of cells to undergo apoptosis, however, following
co-treatment with 5 µM idelalisib, increased this apoptosis to a
mean of 68.4±8.0% in the PGA-1 cells (Fig. 4A). Similar increases in apoptosis
were also obtained in the HG-3 cells treated with compound 1
(Fig. 4B) and in the PGA-1 and HG-3
cells treated with compound 2, respectively (Fig. 4C and D). Representative dot plots
are shown for illustrative purposes (Fig. 4E). The median dose analysis showed
that, for all combinations assessed in both CLL cell lines, a CI
value of <1 was obtained, indicating synergism between the
compounds (Table II). These
results are in agreement with a variety of other anticancer agents
that have been shown to synergise with idelalisib, including the
Syk inhibitor GS-9973 (42),
histone deacetylase inhibitor panobinostat (LBH589) and
suberoylanilide hydroxamic acid (43) and ibrutinib (44). Reports of nitrostyrenes in
combination with other anticancer agents are limited; however,
nitrostyrenes have previously been documented to synergise with
another anticancer agent, 5-fluorouracil, in colon cancer cells
in vitro (40).
 | Table II.Median dose effect analysis of CLL
cell lines. |
Table II.
Median dose effect analysis of CLL
cell lines.
| Treatment | Idelalisib
(µM) | Nitrostyrene
(µM) | Fa | CI |
|---|
| HG-3 |
| Idelalisib-1
combination | 1 | 0.5 | 0.179 | 0.774 |
|
| 1 | 1 | 0.403 | 0.759 |
|
| 5 | 0.5 | 0.259 | 0.588 |
|
| 5 | 1 | 0.452 | 0.680 |
| Idelalisib-2
combination | 1 | 0.5 | 0.228 | 0.482 |
|
| 1 | 1 | 0.357 | 0.639 |
|
| 5 | 0.5 | 0.231 | 0.545 |
|
| 5 | 1 | 0.391 | 0.589 |
| PGA-1 |
| Idelalisib-1
combination | 1 | 0.5 | 0.429 | 0.416 |
|
| 1 | 1 | 0.616 | 0.475 |
|
| 5 | 0.5 | 0.537 | 0.301 |
|
| 5 | 1 | 0.684 | 0.380 |
| Idelalisib-2
combination | 1 | 0.5 | 0.627 | 0.284 |
|
| 1 | 1 | 0.693 | 0.445 |
|
| 5 | 0.5 | 0.667 | 0.246 |
|
| 5 | 1 | 0.720 | 0.400 |
Lead nitrostyrene compounds potently
induce apoptosis in ex vivo CLL patient samples, including those
with poor prognostic indicators
The present study investigated whether these novel
nitrostyrene compounds were also capable of inducing apoptosis in
cancer cells isolated from patients with CLL. For this, informed
consent was obtained from a cohort of patients with CLL (n=14); the
details of these patients are outlined in Table III. Of these 14 patients, three
were women and 11 were men, and the cohort ranged in age between 49
and 78 years.
 | Table III.List of patient details. |
Table III.
List of patient details.
| Patient no. | Age (years) | Sex | Binet stage | Karyotype | CD38 | IGVH |
|---|
| 1 | 78 | M | A | ND | Negative | UM |
| 2 | 60 | M | C | Del13q, Del
11q | Positive | UM |
| 3 | 68 | M | A | ND | ND | ND |
| 4 | 66 | M | A | Del13q, Del17p | Negative | ND |
| 5 | 72 | M | B | Del11q | Negative | UM |
| 6 | 78 | F | A | Normal | Positive | UM |
| 7 | 49 | F | A | Del13q | Negative | M |
| 8 | 78 | M | A | ND | Negative | ND |
| 9 | 72 | M | A | ND | Negative | ND |
| 10 | 61 | F | A | Del17p | Negative | UM |
| 11 | 59 | M | C | Normal | Negative | UM |
| 12 | 57 | M | B | Del13q | Negative | M |
| 13 | 69 | M | A | ND | Negative | ND |
| 14 | 73 | M | A | Normal | ND |
|
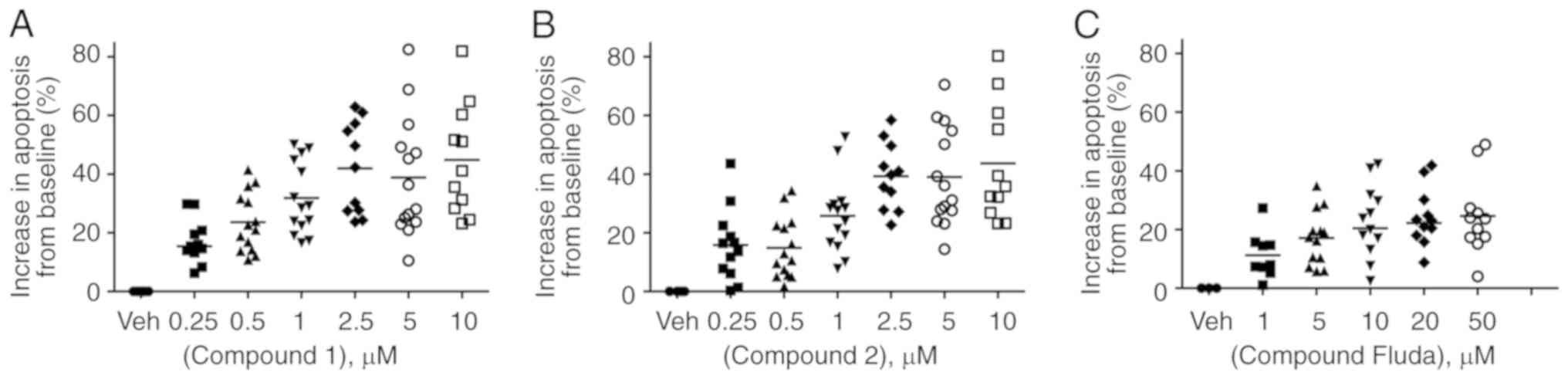
The PBMCs were isolated from the blood samples of
patients with CLL by Ficoll gradient and stored in liquid nitrogen
in biobanking facilities until required. Primary CLL cells are
known to die rapidly in culture and as a result underwent a high
and varied background level of spontaneous apoptosis in the present
study. Therefore, the results are presented as the increase in
apoptosis observed following treatment with compound 1 or 2 over
vehicle-treated cells. The cells were incubated with a range of
concentrations of compounds 1 and 2 for 48 h. The results
demonstrated CLL cells underwent a significant dose-dependent
increase in apoptosis when treated with representative lead
nitrostyrene compounds. Apoptosis increased, on average by
15.4±2.6% when treated with 250 nM compound 1 (range, −2.6–29.8%).
This increased to 44.9±5.7% apoptosis when treated with 10 µM
compound 1 (range, 23.2–81.9%) (Fig.
5A). Similarly, cells underwent an average increase in
apoptosis of 15.8±3.6% (range, 0.3–43.6%) and 43.7±6.0% (range,
23.2–80.3%) following treatment with 250 and 10 µM compound 2,
respectively (Fig. 5B). By
contrast, patient samples treated with fludarabine phosphate
underwent a lower increase in apoptosis of 24.6±4.0% (range,
4.0–49.0%) at the highest concentration of 50 µM (Fig. 5C).
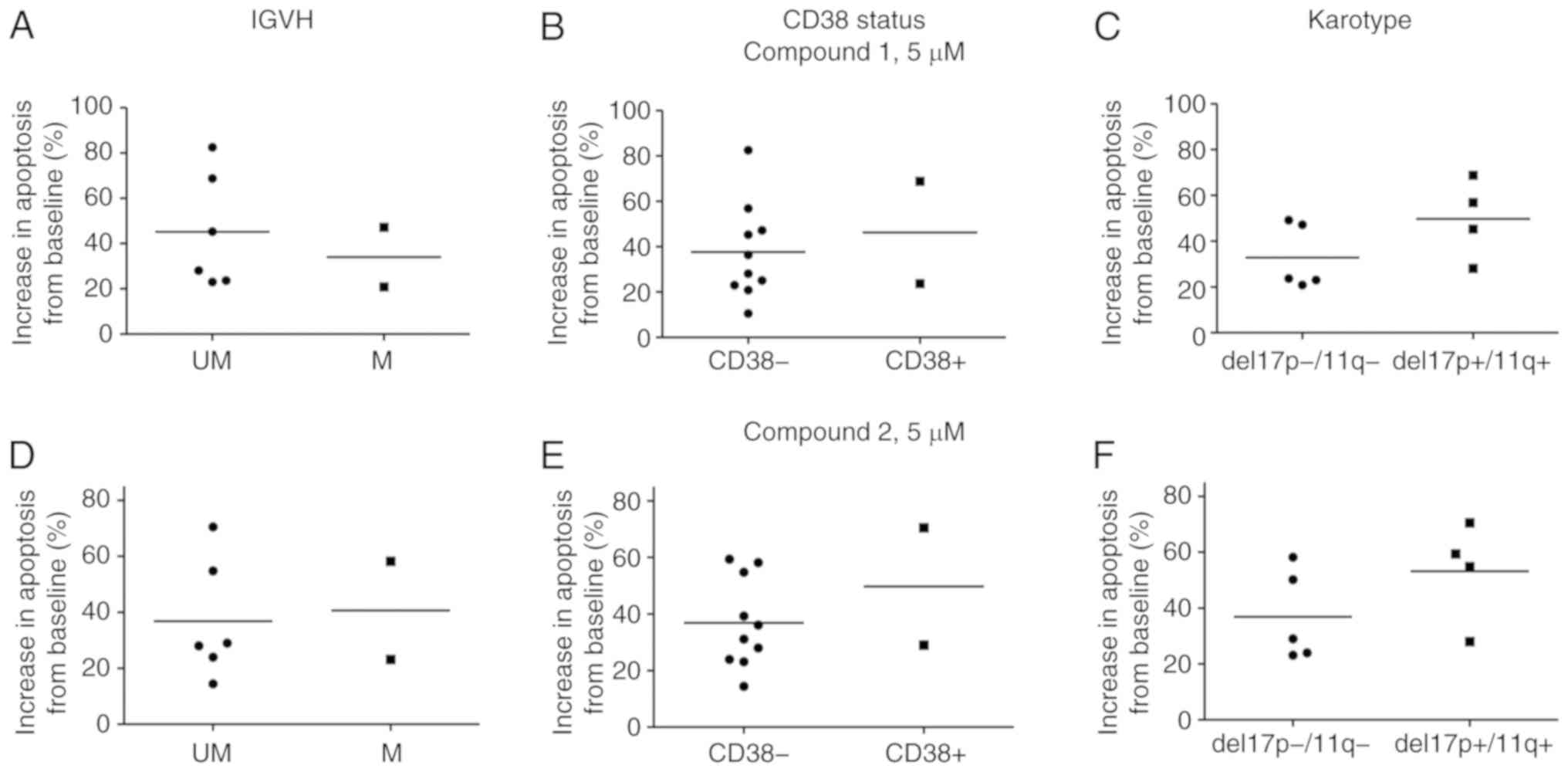
The samples treated with compound 1 demonstrated
similar levels of apoptosis when harbouring poor clinical markers
(UM-IGVH, CD38-, del17p and del11q) compared with samples with
favourable clinical markers (M-IGVH, CD38+) (Fig. 6A-C). Similar results were obtained
for compound 2 (Fig. 6D-F). These
chromosomal abnormalities are associated with clinically
progressive disease, shorter remission durations and shorter
overall survival rates following standard chemotherapy (2).
CLL cells in vivo are known to be resistant
to apoptosis, assisted by interactions with lymph node and bone
marrow microenvironments. CLL cells adhering to bone marrow stromal
cells through integrins show decreased protein levels of the
anti-apoptotic Bcl-2 and are influenced by stromal cell secretion
of interleukin IL-4 and interferon-α/λ (45,46).
In order to mimic this microenviromental-mediated resistance of
cancer cells to apoptosis, primary CLL cells (n=8) were co-cultured
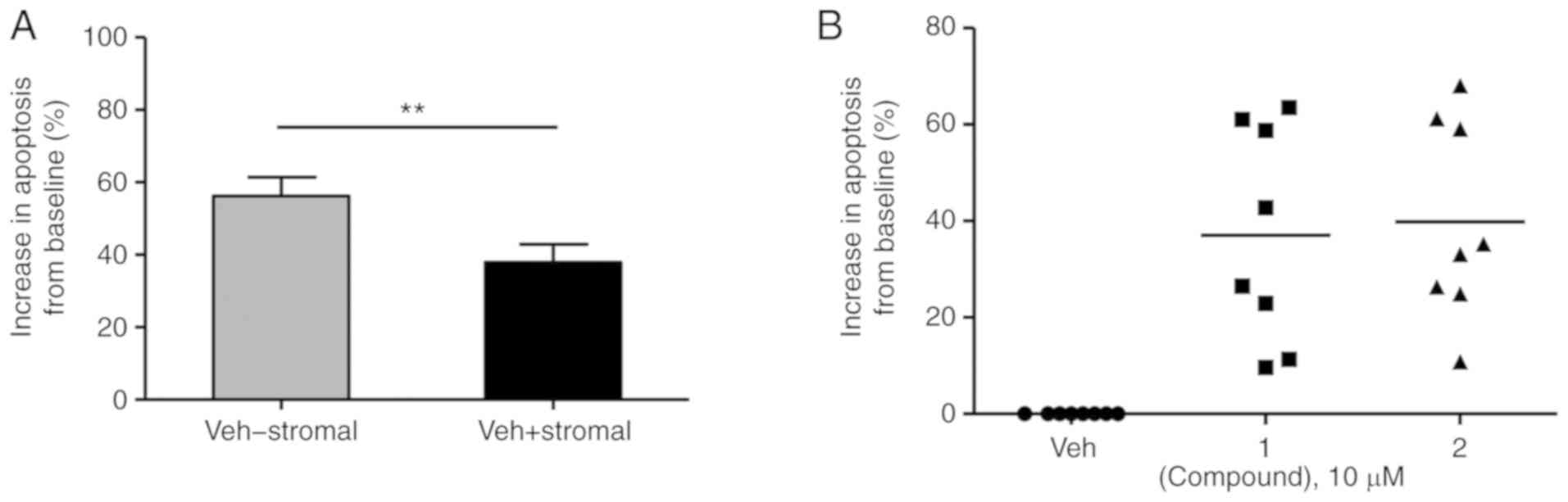
with bone marrow-derived stromal HS5 cells. As a result, primary
untreated CLL cells developed a greater ability to survive in
culture, with spontaneous background apoptotic levels dropping
significantly by almost 20% from 56.2±5.1 to 37.9±5.0% (Fig. 7A). The co-cultured ex vivo
primary CLL cells also underwent a significant increase in
apoptosis following co-treatment with 10 µM compound 1 and 2 of
37.0±7.9 (range, 9.6–63.5%) and 39.9±7.2% (range, 10.8–68.1%),
respectively (Fig. 7B). This
suggests the ability of nitrostyrene compounds to overcome the drug
resistance afforded to primary CLL cells by their in vivo
microenvironment.
Although nitrostyrene derivatives have been
confirmed to be potent at inducing apoptosis in a wide variety of
cell types, reports on clinical development are limited; however,
Bartels et al demonstrated the successful in vivo
application of two nitrostyrene derivatives in a BALB/c
myelopoiesis model mouse model (47). The results outlined above suggest
the clinical potential for the development of novel nitrostyrene
compounds as a treatment for CLL, and the study by Bartels et
al suggests that nitrostyrene compounds are well tolerated
in vivo.
Drugs used to treat CLL include fludarabine
phosphate, rituximab, the PI3K inhibitor idelalisib and the Btk
inhibitor ibrutinib (1). However,
there are significant issues associated with these drugs, including
the development of resistance, and an unmet requirement is emerging
for alternative drug therapies for this disease. In the present
study, it was demonstrated that lead nitrostyrene compounds offer
potential for the treatment of CLL (compounds 1, 2 and 3). Although
the original target of nitrostyrene compounds was considered to be
SERT, which is overexpressed in a number of cancer types, it is now
known that SERT is not connected with the compound mechanism of
action. As a result, the molecular target of these compounds
remains to be elucidated. However, numerous studies on structurally
similar compounds have pointed to a diverse range of potential
targets, including tubulin (29,32),
telomerases (33), protein tyrosine
phosphatases (34) and
phospholipase A2 (35).
Follow-up investigations into these target/s are ongoing.
In the present study, two lead nitrostyrene
compounds with aryl substitutions, compounds 1 and 2, potently
reduced cell viability in a broad range of cancer cell lines,
including CLL cells, associated with a poor prognosis. These lead
compounds demonstrated significantly higher potent
antiproliferative activity than the conventional drug treatment
fludarabine phosphate and demonstrated IC50 values of
<10 µM for all CLL cells. The apoptotic cell death was caspase
and ROS-dependent and was significantly and synergistically
enhanced by idelalisib. The compounds also successfully induced
apoptosis in primary cells from patients with CLL, including those
with poor prognostic markers. The compounds also overcame stromal
cell-induced chemoresistance in CLL cells. These results
demonstrate the potential of nitrostyrene analogues for the
treatment of CLL.
Acknowledgements
Not applicable.
Funding
Funding was provided by Trinity College Dublin.
Availability of data and materials
The corresponding author can be contacted for more
information.
Authors' contributions
SAB performed the majority of the experiments and
wrote the manuscript; AJB performed some experiments and
synthesized the compounds; EV provided access to the patient
samples; EV, PVB, AMM, MJM and DCW contributed to the design of the
study and supervised the project. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Ethical approval for all procedures performed on
ex vivo CLL patient samples was obtained from the St.
James's Hospital and Adelaide and Meath incorporating the National
Children's Hospital Joint Ethics Committee (Dublin, Ireland).
Informed consent for the use of tissues was obtained from each
subject in line with the Declaration of Helsinki.
Patient consent for publication
Informed consent for publication of patient data was
obtained from each subject in line with the Declaration of
Helsinki.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Btk
|
Bruton's tyrosine kinase
|
|
CAP
|
cyclopho-sphamide/doxorubicin/prednisone
|
|
CHOP
|
cyclophosphamide/hydroxydaunorubicin
(doxorubicin)/oncovin (vincristine)/prednisone
|
|
CLL
|
chronic lymphocytic leukaemia
|
|
EBV
|
Epstein Barr virus
|
|
EMA
|
European Medicines Agency
|
|
FBS
|
foetal bovine serum
|
|
FDA
|
US Food and Drug Administration
|
|
LDH
|
lactate dehydrogenase
|
|
M-IGVH
|
mutated immunoglobulin heavy chain
gene
|
|
4-MTA
|
4-methylthioamphetamine
|
|
Nac
|
N-acetyl-cysteine
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
PI
|
propidium iodide
|
|
PTP
|
protein tyrosine phosphatase
|
|
ROS
|
reactive oxygen species
|
|
SAR
|
structure activity relationship
|
|
SEM
|
standard error of the mean
|
|
SERT
|
serotonin reuptake transporter
|
|
UM-IGVH
|
unmutated immunoglobulin heavy chain
gene
|
|
ZAP-70
|
serum marker z-associated protein
70
|
References
|
1
|
Nabhan C and Rosen ST: Chronic lymphocytic
leukemia: A clinical review. JAMA. 312:2265–2276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Puiggros A, Blanco G and Espinet B:
Genetic abnormalities in chronic lymphocytic leukemia: Where we are
and where we go. Biomed Res Int. 2014:4359832014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Skarbnik AP and Faderl S: The role of
combined fludarabine, cyclophosphamide and rituximab
chemoimmunotherapy in chronic lymphocytic leukemia: Current
evidence and controversies. Ther Adv Hematol. 8:99–105. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Montgomery JA and Hewson K: Nucleosides of
2-fluoroadenine. J Med Chem. 12:498–504. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hallek M: Chronic lymphocytic leukemia:
2017 update on diagnosis, risk stratification, and treatment. Am J
Hematol. 92:946–965. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aalipour A and Advani RH: Bruton's
tyrosine kinase inhibitors and their clinical potential in the
treatment of B-cell malignancies: focus on ibrutinib. Ther Adv
Hematol. 5:121–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shah A: New developments in the treatment
of chronic lymphocytic leukemia: role of obinutuzumab. Ther Clin
Risk Manag. 11:1113–1122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shah A and Mangaonkar A: Idelalisib: A
novel PI3Kδ inhibitor for chronic lymphocytic leukemia. Ann
Pharmacother. 49:1162–1170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duman RS: Neurobiology of stress,
depression, and rapid acting antidepressants: remodeling synaptic
connections. Depress Anxiety. 31:291–296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gold PW, Machado-Vieira R and Pavlatou MG:
Clinical and biochemical manifestations of depression: Relation to
the neurobiology of stress. Neural Plast. 2015:5819762015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meredith EJ, Holder MJ, Chamba A, Challa
A, Drake-Lee A, Bunce CM, Drayson MT, Pilkington G, Blakely RD,
Dyer MJ, et al: The serotonin transporter (SLC6A4) is present in
B-cell clones of diverse malignant origin: probing a potential
antitumor target for psychotropics. FASEB J. 19:1187–1189. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xia Z, Bergstrand A, DePierre JW and
Nassberger L: The antidepressants imipramine, clomipramine, and
citalopram induce apoptosis in human acute myeloid leukemia HL-60
cells via caspase-3 activation. J Biochem Mol Toxicol. 13:338–347.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xia Z, DePierre JW and Nassberger L:
Modulation of apoptosis induced by tricyclic antidepressants in
human peripheral lymphocytes. J Biochem Mol Toxicol. 12:115–123.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xia Z, Karlsson H, De Pierre JW and
Nassberger L: Tricicylic antidepressants induce apoptosis in human
T-lymphocytes. Int J Immunopharmacol. 19:645–654. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cloonan SM, Drozgowska A, Fayne D and
Williams DC: The antidepressants maprotiline and fluoxetine have
potent selective antiproliferative effects against Burkitt lymphoma
independently of the norepinephrine and serotonin transporters.
Leuk Lymphoma. 51:523–539. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cloonan SM and Williams DC: The
antidepressants maprotiline and fluoxetine induce Type II
autophagic cell death in drug-resistant Burkitt's lymphoma. Int J
Cancer. 128:1712–1723. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gandy MN, McIldowie M, Lewis K, Wasik AM,
Salomonczyk D, Wagg K, Millar ZA, Tindiglia D, Huot P, Johnston T,
et al: Redesigning the designer drug ecstasy: non-psychoactive MDMA
analogues exhibiting Burkitt's lymphoma cytotoxicity. Med Chem
Commun. 1:287–293. 2010. View Article : Google Scholar
|
|
18
|
McNamara YM, Bright SA, Byrne AJ, Cloonan
SM, McCabe T, Williams DC and Meegan MJ: Synthesis and
antiproliferative action of a novel series of maprotiline
analogues. Eur J Med Chem. 71:333–353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cloonan SM, Keating JJ, Butler SG, Knox
AJ, Jørgensen AM, Peters GH, Rai D, Corrigan D, Lloyd DG, Williams
DC, et al: Synthesis and serotonin transporter activity of
sulphur-substituted alpha-alkyl phenethylamines as a new class of
anticancer agents. Eur J Med Chem. 44:4862–4888. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McNamara YM, Cloonan SM, Knox AJ, Keating
JJ, Butler SG, Peters GH, Meegan MJ and Williams DC: Synthesis and
serotonin transporter activity of 1,3-bis(aryl)-2-nitro-1-propenes
as a new class of anticancer agents. Bioorg Med Chem. 19:1328–1348.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kinjo T, Kowalczyk P, Kowalczyk M,
Walaszek Z, Slaga TJ and Hanausek M: Effects of desipramine on the
cell cycle and apoptosis in Ca3/7 mouse skin squamous carcinoma
cells. Int J Mol Med. 25:861–867. 2010.PubMed/NCBI
|
|
22
|
Parker KA, Glaysher S, Hurren J, Knight
LA, McCormick D, Suovouri A, Amberger-Murphy V, Pilkington GJ and
Cree IA: The effect of tricyclic antidepressants on cutaneous
melanoma cell lines and primary cell cultures. Anticancer Drugs.
23:65–69. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jeon SH, Kim SH, Kim Y, Kim YS, Lim Y, Lee
YH and Shin SY: The tricyclic antidepressant imipramine induces
autophagic cell death in U-87MG glioma cells. Biochem Biophys Res
Commun. 413:311–317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu T, Chou CT, Liang WZ, Yu CC, Chang HT,
Kuo CC, Chen WC, Kuo DH, Ho CM, Shieh P and Jan CR: Effect of
antidepressant doxepin on Ca2+ homeostasis and viability
in PC3 human prostate cancer cells. Chin J Physiol. 58:178–187.
2015.PubMed/NCBI
|
|
25
|
Wang YY, Chen YK, Hsu YL, Chiu WC, Tsai
CH, Hu SC, Hsieh PW and Yuan SF: Synthetic β-nitrostyrene
derivative CYT-Rx20 as inhibitor of oral cancer cell proliferation
and tumor growth through glutathione suppression and reactive
oxygen species induction. Head Neck. 39:1055–1064. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsai CH, Hung AC, Chen YY, Chiu YW, Hsieh
PW, Lee YC, Su YH, Chang PC, Hu SC and Yuan SF:
3′-hydroxy-4′-methoxy-β-methyl-β-nitrostyrene inhibits
tumorigenesis in colorectal cancer cells through ROS-mediated DNA
damage and mitochondrial dysfunction. Oncotarget. 8:18106–18117.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Messerschmitt PJ, Rettew AN, Schroeder NO,
Brookover RE, Jakatdar AP, Getty PJ and Greenfield EM: Osteosarcoma
phenotype is inhibited by 3,4-methylenedioxy-β-nitrostyrene.
Sarcoma. 2012:4797122012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Calgarotto AK, da Silva Pereira GJ,
Bechara A, Paredes-Gamero EJ, Barbosa CM, Hirata H, de Souza
Queiroz ML, Smaili SS and Bincoletto C: Autophagy inhibited Ehrlich
ascitic tumor cells apoptosis induced by the nitrostyrene
derivative compounds: Relationship with cytosolic calcium
mobilization. Eur J Pharmacol. 678:6–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pettit RK, Pettit GR, Hamel E, Hogan F,
Moser BR, Wolf S, Pon S, Chapuis JC and Schmidt JM:
E-Combretastatin and E-resveratrol structural modifications:
Antimicrobial and cancer cell growth inhibitory β-E-nitrostyrenes.
Bioorg Med Chem. 17:6606–6612. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He Y, Varadarajan S, Muñoz-Planillo R,
Burberry A, Nakamura Y and Núñez G:
3,4-Methylenedioxy-β-nitrostyrene inhibits NLRP3 inflammasome
activation by blocking assembly of the inflammasome. J Biol Chem.
289:1142–1150. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Carter KC, Finnon YS, Daeid NN, Robson DC
and Waddell R: The effect of nitrostyrene on cell proliferation and
macrophage immune responses. Immunopharmacol Immunotoxicol.
24:187–197. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jain N, Yada D, Shaik TB, Vasantha G,
Reddy PS, Kalivendi SV and Sreedhar B: Synthesis and antitumor
evaluation of nitrovinyl biphenyls: anticancer agents based on
allocolchicines. Chem Med Chem. 6:859–868. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim JH, Kim JH, Lee GE, Lee JE and Chung
IK: Potent inhibition of human telomerase by nitrostyrene
derivatives. Mol Pharmacol. 63:1117–1124. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Park J and Pei D: trans-Beta-nitrostyrene
derivatives as slow-binding inhibitors of protein tyrosine
phosphatases. Bioche-mistry. 43:15014–15021. 2004. View Article : Google Scholar
|
|
35
|
Villar JA, Lima FT, Veber CL, Oliveira AR,
Calgarotto AK, Marangoni S and da Silva SL: Synthesis and
evaluation of nitrostyrene derivative compounds, new snake venom
phospholipase A2 inhibitors. Toxicon. 51:1467–1478. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Byrne AJ, Bright SA, Fayne D, McKeown JP,
McCabe T, Twamley B, Williams C and Meegan MJ: Synthesis,
antiproliferative and pro-apoptotic effects of nitrostyrenes and
related compounds in Burkitt's lymphoma. Med Chem. 14:181–199.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lanemo Myhrinder A, Hellqvist E, Sidorova
E, Söderberg A, Baxendale H, Dahle C, Willander K, Tobin G, Bäckman
E, Söderberg O, et al: A new perspective: molecular motifs on
oxidized LDL, apoptotic cells, and bacteria are targets for chronic
lymphocytic leukemia antibodies. Blood. 111:3838–3848. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
McElligott AM, Maginn EN, Greene LM,
McGuckin S, Hayat A, Browne PV, Butini S, Campiani G, Catherwood
MA, Vandenberghe E, et al: The novel tubulin-targeting agent
pyrrolo-1,5-benzoxazepine-15 induces apoptosis in poor prognostic
subgroups of chronic lymphocytic leukemia. Cancer Res.
69:8366–8375. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Werner JM, Eger K and Jürgen Steinfelder
H: Comparison of the rapid pro-apoptotic effect of
trans-beta-nitrostyrenes with delayed apoptosis induced by the
standard agent 5-fluorouracil in colon cancer cells. Apoptosis.
12:235–246. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rahmani-Nezhad S, Safavi M, Pordeli M,
Ardestani SK, Khosravani L, Pourshojaei Y, Mahdavi M, Emami S,
Foroumadi A and Shafiee A: Synthesis, in vitro cytotoxicity and
apoptosis inducing study of 2-aryl-3-nitro-2H-chromene derivatives
as potent anti-breast cancer agents. Eur J Med Chem. 86:562–569.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Burke RT, Meadows S, Loriaux MM, Currie
KS, Mitchell SA, Maciejewski P, Clarke AS, Dipaolo JA, Druker BJ,
Lannutti BJ and Spurgeon SE: A potential therapeutic strategy for
chronic lymphocytic leukemia by combining Idelalisib and GS-9973, a
novel spleen tyrosine kinase (Syk) inhibitor. Oncotarget.
5:908–915. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bodo J, Zhao X, Sharma A, Hill BT, Portell
CA, Lannutti BJ, Almasan A and his ED: The phosphatidylinositol
3-kinases (PI3K) inhibitor GS-1101 synergistically potentiates
histone deacetylase inhibitor-induced proliferation inhibition and
apoptosis through the inactivation of PI3K and extracellular
signal-regulated kinase pathways. Br J Haematol. 163:72–80. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
de Rooij MF, Kuil A, Kater AP, Kersten MJ,
Pals ST and Spaargaren M: Ibrutinib and idelalisib synergistically
target BCR-controlled adhesion in MCL and CLL: A rationale for
combination therapy. Blood. 125:2306–2309. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Panayiotidis P, Jones D, Ganeshaguru K,
Foroni L and Hoffbrand AV: Human bone marrow stromal cells prevent
apoptosis and support the survival of chronic lymphocytic leukaemia
cells in vitro. Br J Haematol 92. 92:97–103. 1996. View Article : Google Scholar
|
|
46
|
Lagneaux L, Delforge A, De Bruyn C,
Bernier M and Bron D: Adhesion to bone marrow stroma inhibits
apoptosis of chronic lymphocytic leukemia cells. Leuk Lymphoma.
35:445–453. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bartels M, Calgarotto AK, Martens AC, Maso
V, da Silva SL, Bierings MB, de Souza Queiroz ML and Coffer PJ:
Differential effects of nitrostyrene derivatives on myelopoiesis
involve regulation of C/EBPα and p38MAPK activity. PLoS One.
9:e905862014. View Article : Google Scholar : PubMed/NCBI
|