Introduction
Human cytomegalovirus (HCMV) is a member of the
Herpesviridae family with a genome size of 236 kb, the
largest of any known human virus (1,2). The
HCMV genome encodes 750 proteins, of which ~50 are involved in the
construction of new viral particles. The vast majority of viral
proteins function in regulating important host functions that
assist the virus to co-exist with its host (3). During evolution, HCMV has developed
unique mechanisms to adapt to the human immune system, allowing it
to maintain a latent phase in CD34+ myeloid progenitor
cells (4). Originally it was
thought that HCMV is completely inactive during latency. However,
various viral transcripts and proteins appear to be induced during
this phase, including latency unique natural antigen (LUNA)
(5), G-protein coupled receptor
homolog US28 (US28), viral interleukin (IL)-10 homolog (UL111A) and
membrane glycoprotein UL144 (UL144) (6). These viral proteins affect the immune
system. For example, LUNA stimulates the immune response (5), US28 is associated with inflammation,
and UL111A and UL144 are involved in HCMV immune evasion (7). Furthermore, different biological
conditions were shown to be central in the reactivation of latent
HCMV, including inflammation, immune suppression, other infections
and epigenetic modifications (8,9). The
critical factor in transcriptional reactivation of latent HCMV is
terminal differentiation of latently infected monocytes into
macrophages or dendritic cells (8,10).
Accumulating evidence suggests an association between histone
modification, chromatin modulation, HCMV genome transcription and
replication, both during latency and lytic infection (9,11,12).
Additionally, previous studies have shown that histone deacetylases
(HDACs) are bound to the HCMV promoter and HDAC activity results in
repression of viral gene expression and latency (13–15).
However, the involvement of host cell epigenetic alterations in
HCMV reactivation remains poorly understood.
Previous studies have revealed the frequent presence
of HCMV nucleic acid sequences and proteins in malignant brain
tumors, including glioblastoma multiforme (GBM), medulloblastoma
(MB) and neuroblastoma (16–18).
GBM is the most aggressive brain tumor in adults, with fatal
outcomes. Despite aggressive surgery and advanced therapy, the
median overall survival of these patients is ~14 months (19). The risk factors for GBM are unknown
but environmental exposures to radiation, vinyl chloride and
pesticides have been considered in the initiation of GBM (20,21).
Traumatic brain injury and consequent inflammation have also been
suggested in the etiology of GBM, although this is still
controversial (22).
HCMV has not been classified as an oncogenic virus
as it does not have the ability to transform cells, but the term
oncomodulation has been used to describe the actions of HCMV in
tumors (23). HCMV may modulate
cancer progression through interactions with potential oncogenic
factors within the host cells, such as p53, retinoblastoma protein
(Rb) and cyclins, resulting in uncontrolled cell proliferation
(24). Our recent report found that
HCMV replication significantly increased in HCMV infected MB cells
treated with 5-azacytidine (5AZA) (25). Since epigenetic drugs are suggested
as agents of cancer therapy (26)
and GBM tissues have been shown to be frequently positive for HCMV
proteins and nucleic acids, the present study investigated the
viral replication, proliferation and invasion capacity of
5AZA-treated HCMV-infected GBM cells (U343MG), and examined the
expression of DNMT-1 and HCMV proteins in GBM tissue specimens.
Materials and methods
Cell culture and HCMV infection
U343MG and MRC-5 cells were obtained from American
Type Culture Collection (Manassas, VA, USA). All cells
(1.5–3.0×105) were cultured under the following
conditions, unless otherwise stated: Dulbecco's modified Eagle's
medium containing 10% heat-inactivated fetal bovine serum (FBS),
100 U/ml penicillin and 100 µg/ml streptomycin (all from Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a
humidified 5% CO2. Cells were infected with HCMV
clinical isolate (strain VR1814; a kind gift from Dr Giuseppe
Gerna, University of Pavia, Pavia, Italy) at a multiplicity of
infection (MOI) of 5 for U343MG cells and a MOI of 1 for MRC-5
cells, for 3 days. In all experiments, cells cultured without HCMV
were used as the negative control. Transcripts from HCMV infected
MRC-5 cells were used as positive control [with an infection
efficiency for HCMV-Major Immediate-Early gene equal to
Ct = 21.2 from quantitative TaqMan polymerase chain
reaction (qPCR); protocol described below], since MRC-5 cells are
permissive for infection using the HCMV strain VR1814 (>80%
infected cells). The number of cells used in different treatment
experiments was 1.5–3.0×105. All experiments were
performed with three independent repeats.
Immunofluorescence staining
Uninfected and HCMV infected U343MG cells
(1.5–3.0×105) were fixed in ice-cold acetone:methanol
(1:1) for 10 min (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Immunofluorescence staining of different proteins was performed as
described previously, with minor modifications (25). Endogenous non-specific binding was
blocked using Protein Block, Serum-Free (cat. no. X0909; Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA). The cells were
incubated with the following primary antibodies: Mouse monoclonal
antibody to immediate early (IE-1 and IE-2; 1:100; cat. no. 11–003;
ARGENE; bioMérieux, Verniolle, France); mouse monoclonal antibody
to glycoprotein gB (gB; 1:50; cat. no. P1216; EastCoast Bio, Inc.,
North Berwick, ME, USA); rabbit polyclonal antibody to DNMT-1
(1:500; cat. no. ab19905; Abcam). Binding of primary antibodies to
specific proteins was detected by incubation with Alexa Fluor
488-conjugated goat anti-mouse (1:500; cat no: A-11001; Molecular
Probes; Thermo Fisher Scientific, Inc.) and Texas red-conjugated
goat anti-rabbit (1:500, cat no: T-6391, Molecular Probes; Thermo
Fisher Scientific, Inc.) as secondary antibodies for 45 min at room
temperature. Incubation with PBS instead of primary antibodies
served as negative control. Ready to use fluorescence mounting
medium (with DAPI; Vectashield; Vector Laboratories, Inc.,
Burlingame, CA, USA) was used for 1 min for nuclear staining and
mounting. Staining was evaluated by confocal microscopy (scale bar,
50 µm; Leica TCS SP5; Leica Microsystems GmbH, Wetzlar,
Germany).
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
A RNeasy kit (Qiagen AB, Sollentuna, Sweden) was
used to extract the total RNA from cells according to the
manufacturer's instructions. The RNA concentration was measured
using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific,
Inc.). RNA (500 ng) was converted to cDNA using the SuperScript III
First-Strand Synthesis system (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
TaqMan qPCR with specific target primers and probes (FAM
fluorophore) was performed using a 7900HT Fast Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Target
primers and probes used in this study were as follows, as described
previously (25): HCMV–IE (forward
primer, 5′-TGACGAGGGCCCTTCCT-3′ and reverse primer,
3′-CCTTGGTCACGGGTGTCT-5′; probes, FAM-AAGGTGCCACGGCCCG-NFQ) and
HCMV-gB (forward primer, 5′-GCTACCGCCCTACCTCAAG-3′ and reverse
primer, 3′-CGCCAACGGCCTTTCC-5′; probes, FAM-CCCAGGCCGCTCATG-NFQ)
and DNMT-1 (assay ID, Hs00945875_m1; Life Technologies; Thermo
Fisher Scientific, Inc.). RNase P (housekeeping gene; assay ID,
4316844; Life Technologies; Thermo Fisher Scientific, Inc.) was
used for normalization. All assays were performed according to the
manufacturer's protocols.
The qPCR thermocycler was initiated with polymerase
activation at 95°C for 20 sec followed by 40 cycles of denaturation
at 95°C for 1 sec and annealing/extension at 60°C for 20 sec. PCR
results were analyzed with SDS version 2.4 software (Thermo Fisher
Scientific, Inc.). The ΔCt method was used for calculation of Ct
values for different transcripts. The 2−ΔΔCt method was
used to quantify relative fold changes (27).
5AZA treatment
U343MG cells were treated with 5AZA
(5-Aza-2′-deoxycytidine; 10 µM; Sigma-Aldrich; Merck KGaA) for 3
days and subsequently infected with HCMV–VR1814 at a MOI of 5 for 3
days, whilst maintaining the 5AZA concentration. Cells were fixed
in ice-cold acetone:methanol (1:1) for 10 min 3 days post-infection
(dpi) and were kept at −20°C until further experimentation.
Ganciclovir® treatment
Ganciclovir® (2 mM; cymevene; Apoteket
AB, Stockholm, Sweden) was used to treat U343MG cells at the time
of HCMV infection. Cells were fixed as described above.
Invasion assay
Cells were plated on 6-well plates (day 0),
untreated (2×105) or treated with 5-AZA
(3×105) from days 0–5. On day 2, cells were infected (or
not) with HCMV (MOI of 5). On day 5, cells were detached from the
plates and used for the invasion assay. Invasion assays were
performed using CytoSelect™ 6-Well Cell Migration and Invasion
assay kit (8 µm; Colorimetric Format; Cell Biolabs, Inc., San
Diego, CA, USA) according to the manufacturer's instructions.
Briefly, under sterile conditions, untreated or 5AZA treated
uninfected or HCMV infected cells in serum free culturing medium
were plated on each insert and culturing media containing 10% FBS
was added in the lower well of the migration plate. The plates were
incubated at 37°C (5% CO2) for 24 h. Non-migrated cells
on the upper side of the inserts were gently removed by wet
cotton-tipped swabs. The inserts were transferred to a new wells
and 200 µl extraction solution (provided in the kit) was added
followed by a 10 min incubation on an orbital shaker. Finally, 100
µl from each sample was transferred to a 96-well microtiter plate.
The optical density of the resulting extracts was measured at λ=560
nm and normalization was performed with the proliferation values at
time 0 before treatment.
Proliferation assay
Uninfected and HCMV infected U343MG cells, untreated
or treated with 5AZA, were seeded in 96-well culture plates
(1,000/well) and allowed to attach. At 3 days post-treatment, cell
viability was assessed using the CellTiter 96® AQueous
Non-Radioactive Cell Proliferation assay (Promega Corporation,
Madison, WI, USA) according to the manufacturer's instructions. The
absorbance was recorded at λ=490 and normalization was performed
with the proliferation values at day 2, prior to treatment.
Immunohistochemical staining
(IHC)
Paraffin embedded human GBM tumor tissue sections
from five patients (four patients with primary and one patient with
secondary GBM; one primary GBM patient had chromosome 1p19q
deletion; Table I) were
retrospectively and randomly obtained between October 2009 and
November 2010 from the Department of Pathology at Karolinska
University Hospital (Stockholm, Sweden). The inclusion criterion
was GBM tumor tissue. No recruitment period or other control
samples/groups were defined. GBM tumor tissue samples were obtained
at surgery at Karolinska University Hospital from adult GBM
patients with written informed consent. Samples were stored in a
biorepository and anonymized. Ethical permission was obtained from
the Ethics Committee at the Karolinska Institutet (Stockholm,
Sweden; Dnr. 2008/628-31).
 | Table I.GBM patient characteristics. |
Table I.
GBM patient characteristics.
| Patient | GBM | Sex | Age | P53 mutations | KI-67 (%) | TTP (months) | OS (months) |
|---|
| 1 | Primary | Female | 62 | Negative | 80 | NR | 3 |
| 2 |
Primarya | Male | 53 | ND | 25 | 8 | 14.5 |
| 3 | Primary | Male | 75 | Positive | 25 | 3 | 11 |
| 4 | Primary | Male | 62 | Negative | 35 | 9 | 15.5 |
| 5 |
Secondaryb | Female | 34 | Positive | 70 | NR | 38c, 8d |
Immunohistochemical staining of the tissues was
performed as described previously, with some minor modifications
(16,17). Following deparaffinization of the
tissue specimens in xylene and rehydration in a series of
decreasing ethanol concentrations, tissue sections were
permeabilized with pepsin (Nordic BioSite AB, Täby, Sweden) and
citrate buffer (Bio-Genex Laboratories, Fremont, CA, USA).
Endogenous peroxidase activity was blocked with 3%
H2O2 (Sigma-Aldrich; Merck KGaA) and the
sections were treated with an avidin/biotin blocking kit (cat. no.
X0590; Dako; Agilent Technologies, Inc.), Fc receptor blocker (cat.
no. NB309; Innovex Biosciences, Richmond, CA, USA) and Background
Buster (cat. no. NB306; Innovex Biosciences) to eliminate
non-specific binding. All blocking steps were performed according
to the kit protocols. ImmPRESS reagent kits (cat. no: MP-7401 and
MP-7402; Vector Laboratories, Inc.) and diaminobenzidine were used
to detect binding of primary antibodies to targeted proteins. The
primary antibodies used in IHC were as follows: HCMV–IE (cat. no.
MAB810R; Merck KGaA), HCMV-Late (cat. no. MAB8127; Merck KGaA),
HCMV-gB (a kind gift from Dr William Britt, University of Alabama,
USA), DNMT-1 (cat. no. ab19905, Abcam). For the negative control
group, primary antibodies were omitted. For scanning of IHC
sections, a Hamamatsu Nano Zoomer-XR Digital slide scanner C12000
(Hamamatsu Phototonics, Hamamatsu, Japan) was employed, with
visualization using the Nano Zoomer Digital Pathology (NDP) viewer
software (U12388-01; NDP.view2 Viewing; Hamamatsu Phototonics).
Statistical analysis
All analyses were performed using GraphPad Prism
version 6 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05
was considered to indicate a statistically significant difference.
Unpaired Student's t-test or one-way analysis of variance followed
by Dunnett's multiple comparisons test were used to assess the
statistical significance between different variables. Data are
presented as the mean ± standard error of the mean. All experiments
were performed with three independent repeats.
Results
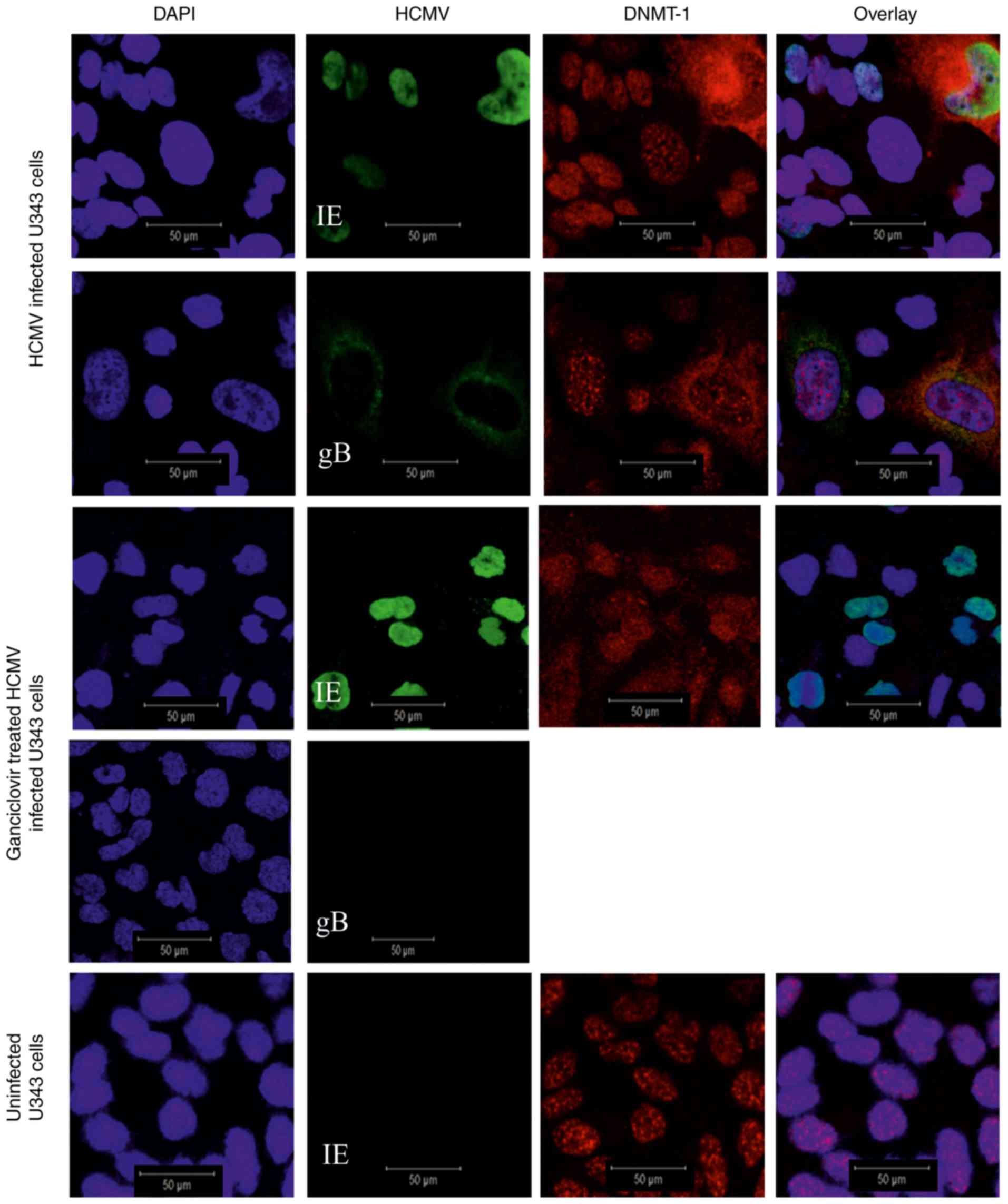
Cytoplasmic expression of DNMT-1 in
HCMV infected U343MG cells expressing viral late genes
U343MG GBM cells were infected with HCMV for 3 days
in order to observe full length viral replication. Expression of
DNMT-1 was detected in the nucleus of uninfected and HCMV–IE
expressing cells (i.e. cells displaying immediate early phases of
HCMV infection), but was evidently expressed exclusively in the
extranuclear space of HCMV-gB positive cells (i.e. cells displaying
later phases of HCMV infection) (Fig.
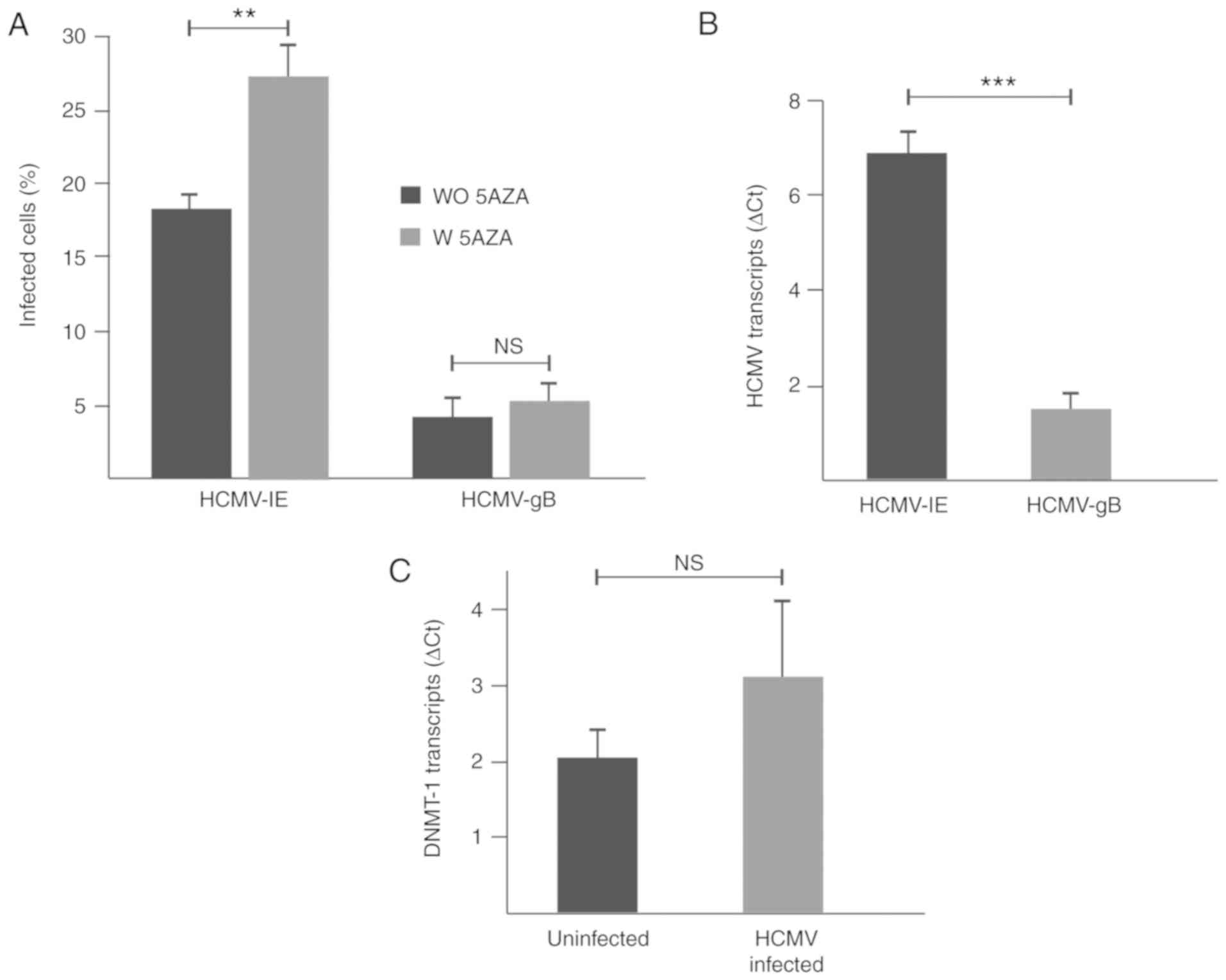
1). At 3 dpi, 18 and 4% of the U343MG cells expressed HCMV–IE
and HCMV-gB proteins, respectively, (P=0.002) and the HCMV–IE
transcript was present at higher levels than HCMV-gB transcripts in
the infected cells (P=0.0007; Fig. 2A
and B). DNMT-1 transcript levels did not change significantly
in HCMV-infected cells compared to uninfected cells (Fig. 2C).
5AZA treatment does not modulate
transcription levels of viral genes
U343MG GBM cells were treated with 5AZA and
subsequently infected with HCMV for 3 days. Untreated and/or
uninfected cells were used as the controls. In 5AZA-treated
HCMV-infected cells, HCMV–IE and HCMV-gB proteins were detected in
27 and 5% of the cells, respectively (P=0.002; Fig. 2A). No significant differences were
observed in the levels of HCMV–IE and HCMV-gB viral transcripts, as
well as DNMT-1 transcripts in 5AZA-treated HCMV-infected cells
compared with untreated HCMV-infected cells (data not shown;
HCMV–IE, P=0.2; HCMV-gB, P=0.4; DNMT-1, P=0.1).
Antiviral treatment prevents
extranuclear localization of DNMT-1
Uninfected and HCMV-infected U343MG GBM cells were
treated with the antiviral drug Ganciclovir® in order to
inhibit the expression of late viral genes. As expected, HCMV-gB
protein expression was not detected in the
Ganciclovir®-treated cells (Fig. 1). DNMT-1 expression was retained in
the nuclei of HCMV-infected antiviral treated cells (Fig. 1).
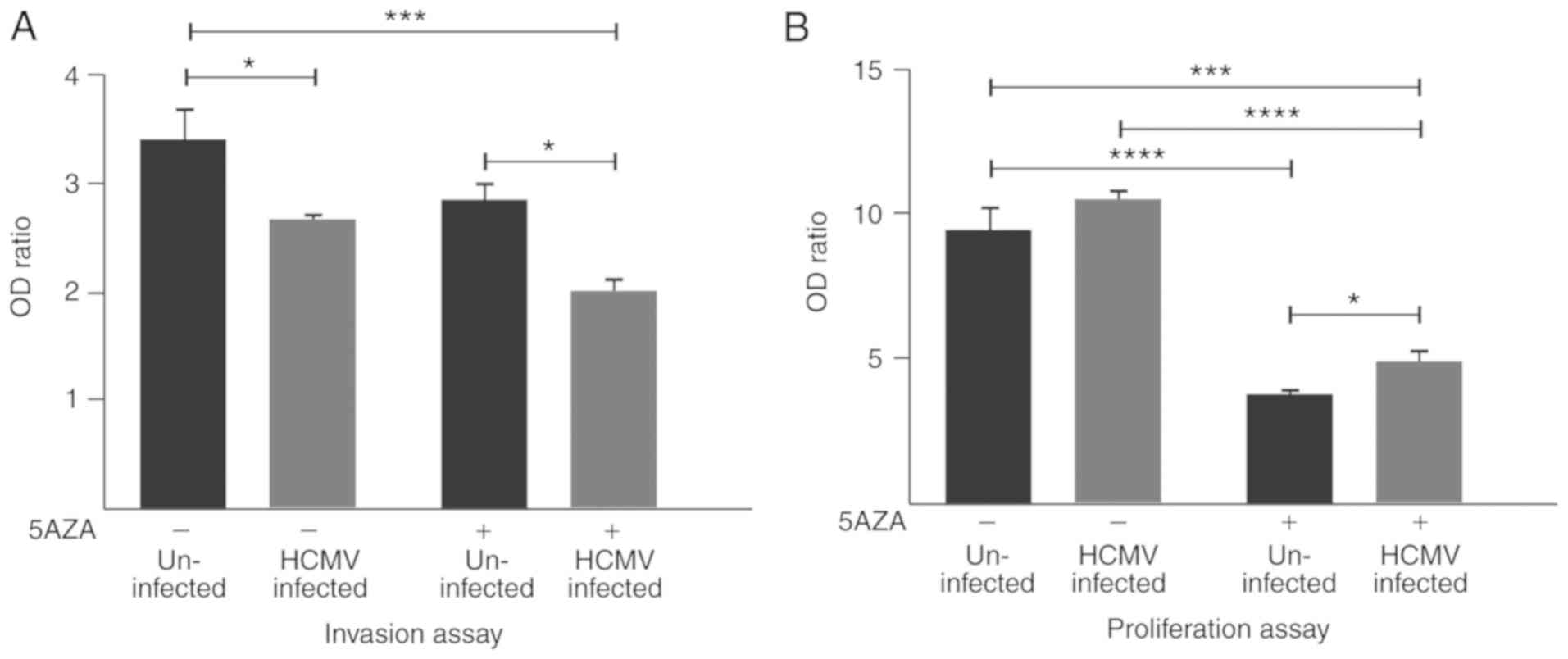
U343MG cell invasion is significantly
decreased by HCMV infection
Untreated HCMV-infected U343MG cell invasion was
significantly decreased compared with the untreated uninfected
cells (P=0.03). Cell invasion was further decreased with 5AZA
treatment in infected cells, compared with the untreated uninfected
cells (P=0.0009). However, a borderline significant decrease in
invasive ability was observed in treated infected cells compared
with the untreated infected cells (P=0.05). A significant
difference in invasion was observed between the 5AZA-treated
uninfected and HCMV-infected cells (P=0.02; Fig. 3A).
5AZA treatment significantly decreases
the proliferation of uninfected and HCMV-infected U343MG GBM
cells
The proliferation of 5AZA-treated uninfected and
HCMV-infected cells was significantly decreased compared with the
untreated uninfected cells (P<0.0001) and untreated HCMV
infected cells (P<0.0001), respectively (Fig. 3B). No significant differences in
proliferation were detected between the untreated uninfected cells,
compared with the untreated infected cells (P=0.4). The
proliferation ability of 5AZA-treated uninfected cells was
significantly decreased compared with the 5AZA-treated
HCMV-infected cells (P=0.02). Furthermore, the proliferation of
5AZA-treated HCMV-infected cells was significantly decreased
compare to untreated uninfected cells (P=0.0001; Fig. 3B).
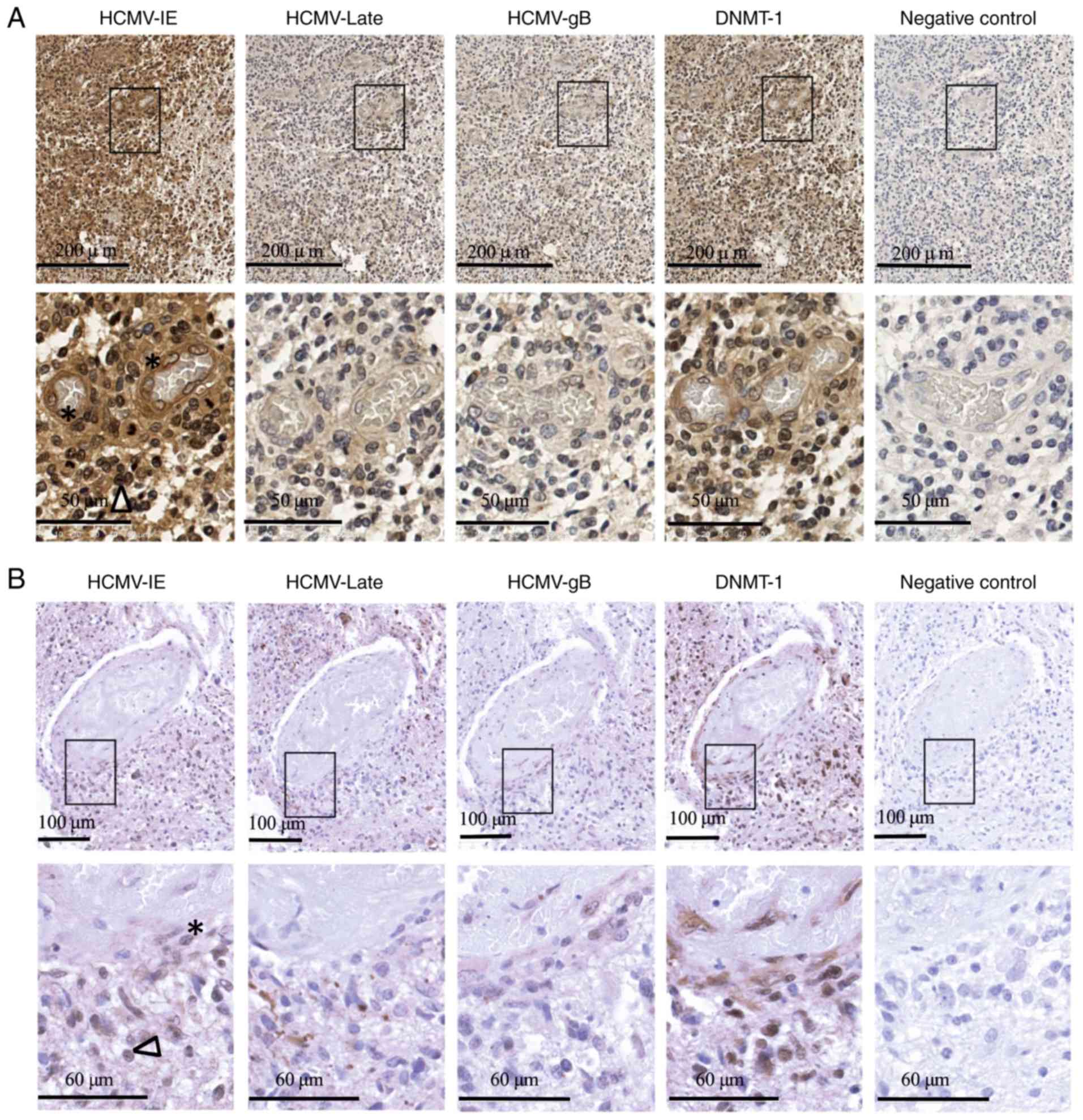
Cytoplasmic expression of DNMT-1 in
the cells of vessel walls within the GBM
Expression of HCMV–IE, HCMV-gB, HCMV-late and DNMT-1
proteins was examined in available GBM tissues sections obtained
from 5 patients by IHC (Table II).
While the expression of HCMV-gB and late proteins was low and
rarely detected in GBM tissues, the expression of HCMV–IE was
frequently detected at different levels in tumor cells and in the
cells of blood vessel walls within the tumors. Furthermore, while
DNMT-1 was expressed in the nuclei of tissue tumor cells, it was
also present in the extranuclear space of cells in the vessel wall
within the tumors (Fig. 4A and B
depict two patients with primary GBM). In one GBM patient (patient
number 2) who had deletion in chromosome 1p19q, DNMT-1 was
expressed in the extranuclear space in the majority of tumor cells
as well as in the vessel walls.
 | Table II.MGMT methylation status and detection
of HCMV-proteins and DNMT-1 in GBM tissues by immunohistochemical
staining. |
Table II.
MGMT methylation status and detection
of HCMV-proteins and DNMT-1 in GBM tissues by immunohistochemical
staining.
|
| HCMV | MGMT | DNMT-1 |
|---|
|
|
|
|
|
|---|
| Patient | IE | Late | gB | Methylation | Nucleus | Cytoplasmic |
|---|
| 1 | 4+ | 2+ | 1+ | ND | Tumor cells | Vessel cells |
| 2 | 4+ | 2+ | 1+ | Unmethylated | Tumor cells | Tumor and vessel
walls |
| 3 | 4+ | 1+ | 1+ | Unmethylated | Tumor cells | Vessel walls |
| 4 | 3+ | 2+ | 1+ | Methylated | Tumor cells | Vessel walls |
| 5 | 4+ | 2+ | 1+ | Methylated | Tumor cells | Vessel walls |
Discussion
HCMV latency has been unambiguously linked to
epigenetic states by viral histone deacetylation, and viral
reactivation to histone acetylation (9,11,12,15).
We previously reported that HCMV replication increased in DNA
methylation-inhibited non-tumor (human umbilical vein endothelial
cells) and tumor cells (MB) (25).
However, in the present study, 5AZA treatment of HCMV-infected GBM
cells did not significantly affect viral replication, although the
treatment appeared to increase the number of cells expressing
HCMV–IE proteins by 10%. This effect is likely due to the decreased
number of cells as a result of 5AZA treatment, as untreated and
5AZA-treated cells were infected with an equal MOI of virus. As
DNMT-1 and viral DNA transcript levels were not significantly
altered by HCMV infection in U343 cells, the western blot analysis
was not included. The opposite results on HCMV replication upon
5AZA treatment in MB (25) and GBM
tumors suggests that the different biology and epigenetic
mechanisms involved may affect the oncogenic signaling pathways.
This distinction may be highly important when considering
epigenetic therapies of cancers that harbor HCMV, such as MB and
GBM. Furthermore, as we have previously reported for non-cancer
cells and MB cells (25), infection
of GBM cells by HCMV causes DNMT-1 accumulation in the
cytoplasmic/extranuclear space, rather than the nucleus. This was
found to be associated with the presence of HCMV late protein(s),
as demonstrated by IHC in the present study.
As DNMT-1 is likely to only function in the nucleus
(28), the DNMT inhibitor 5AZA was
used to investigate the effect of reduced DNMT-1 activity on the
cell proliferation and invasion. Both uninfected and HCMV-infected
cells had evidently reduced proliferation following 5AZA treatment.
HCMV infection of U343MG cells alone did not alter cell
proliferation, which has been previously reported in GBM cell lines
(29). In contrast, the infection
alone significantly decreased cell invasion, which was further
reduced by 5AZA treatment. However, we have previously reported an
increase in invasion by HCMV infection in U373MG cells (29). The differences in the invasion
capacity of HCMV-infected GBM cells may be explained by different
genetic background, global gene methylation status and infection
rate, as cells were infected for 5 days in our previous study,
compared to 3 days in the present study.
Whether or not the observed effects are related to
DNMT relocalization or to other mechanisms requires further
investigation. This information may be of importance when
considering treatment of GBM patients with methylation inhibitors.
However, since a majority of GBM tumors are likely to be infected
by HCMV, a limitation of the present study is that there was no
access HCMV negative GBM tissue sections. In our laboratory, the
expression of various HCMV proteins in hundreds of GBM tissues have
been detected, and only a limited number are HCMV protein negative.
Therefore, HCMV-protein negative tissue specimens could not be
examined for the current study.
We have previously reported extended overall
survival in GBM patients receiving continued treatment with the
antiviral drug Valcyte® as an add-on for routine
treatment (30,31). Hypothetically, based on the findings
of the present study, GBM patients may benefit from a combination
therapy including epigenetic drugs and Valcyte® with the
aim of reducing the proliferation and invasion of tumor cells. This
must, however, be thoroughly investigated prior to clinical trial
investigation. Notably, cytoplasmic localization of DNMT-1 was
predominantly observed in vessel walls, with or without HCMV-gB or
pan late protein expression within the GBM tumor tissues. Further,
in one GBM patient who had a co-deletion in chromosome 1p/19q, also
known as oligodendroglioma, DNMT-1 was detected in the extranuclear
space in the majority of tumor cells as well as in the vessel walls
within the tumors. The contribution of the 1p/19q deletion in
oligodendroglioma oncogenesis is still unknown. GBM patients with
1p/19q deletions are known to be chemosensitive with longer overall
survival (OS) and progression free survival (PFS) regardless of the
treatment they receive (32–36).
Furthermore, the link between the 1p/19q co-deletion and epigenetic
alterations in the course of demethylation/hypomethylation has
previously been studied. A positive association between isocitrate
dehydrogenase (IDH) promoter mutations and oncometabolite
R-2-hydroxyglutarate (R-2-HG) production was reported (37,38).
Various enzymes, including epigenetic regulatory methylcytosine
dioxygenase TET (TET)2, are inhibited by R-2-HG, causing
hypermethylation of DNA (37,38).
However, in the present study, DNMT-1 was localized in the
cytoplasm of many tumor cells and the cells of vessel walls within
the tumor in the patient with 1p/19q co-deletion. Additionally, in
this patient, the methylated-DNA-protein-cysteine methyltransferase
(MGMT) promoter was unmethylated and tumor regrowth occurred at 8
months, resulting in an overall survival of only 14.5 months. Of
note, HCMV–IE protein was frequently expressed at various levels
both in the tumor cells and in the cells of vessel walls within the
GBM tumors examined in this study. In an in vivo scenario in
GBM tumors where HCMV–IE is frequently expressed while HCMV-late
genes are rarely expressed, a direct or indirect effect of HCMV–IE
expression on DNMT-1 localization, increased tumor cell
proliferation, and invasion cannot be excluded. Hypothetically, the
absence of nuclear and presence of extranuclear localization of
DNMTs in HCMV-infected vessel cells in the GBM tissues may lead to
demethylation/hypomethylation of DNA and subsequent alteration of
cellular characteristics, as well as potential induction of factors
that impact angiogenesis and tumorigenesis. Furthermore, exposure
of the cells to inflammatory factors and cytokines such as
prostaglandin E2 (PGE2), interleukin (IL)-1, IL-6 and IL-8, alters
levels and/or activity of enzymes involved in DNA and histone
methylation, resulting in global and potentially gene-specific
changes which could in turn lead to changes in cellular phenotypes
over time, resulting in increased invasion and proliferation
(39,40). Furthermore, increased production of
inflammatory factors and cytokines such as PGE2, IL-2, IL-6 and
IL-8 in response to HCMV infection have previously been reported
(41,42).
In the present study, the proliferation and invasion
ability of uninfected and HCMV infected U343MG cells with or
without 5AZA treatment was investigated. A significant decrease in
the proliferation of both treated uninfected as well as infected
cells was detected. Conversely, invasion capacity was significantly
decreased both by infection alone, as well as by 5AZA treatment in
uninfected and infected cells. Based on this observation, the
potential benefits of this treatment in HCMV-infected GBM patients
to inhibit tumor invasion and proliferation should be further
investigated.
The importance of DNA methylation in GBM is
suggested by previous studies, which have reported hypomethylation
of oncogenes and hypermethylation of tumor suppressor genes in GBM
(43–46). Nagarajan and Costello (44) reported hypomethylation in 76
promoters including TERT and tumor protein 73, with subsequent
increased transcript expression, suggesting that alterations in the
gene regulatory mechanisms in GBM lead to the expression of
oncogenic proteins. Hypermethylated suppressor genes in GBM include
RB transcriptional corepressor 1, epithelial membrane protein 3,
Ras association domain family member 1 isoform A (RASSF1A),
cadherin 1 (CDH1) and zinc finger MYND-type containing 10, cell
cycle regulators [cyclin dependent kinase inhibitor 2 (CDKN2)A and
CDKN2B], DNA repair genes (MGMT, MutL homolog 1), and genes
involved in tumor invasion and apoptosis [death-associated protein
kinase 1 (DAPK) and TIMP metallopeptidase inhibitor 3] (44,46–54).
However, gene specific methylation analysis should be performed in
order to clarify the effects of HCMV infection on hyper- or
hypomethylation of these genes. We performed gene specific
methylation analyses of CDKNA, RASSF1A, MGMT, DAPK and CDH1 with a
multiplex methylation specific PCR (MMSP) (55) assay in untreated, 5AZA treated,
uninfected, and HCMV infected U343 MG cells. It was shown that all
examined genes under the different conditions were hypermethylated,
excluding CDKNA and DAPK, which were hypomethylated under all
different conditions (data not shown). Optimization of a more
accurate quantification method of gene hypermethylation is
required. It is likely that there are heterogeneities in the
methylation for several of these genes, but the method used is not
linear with the amount of methylation, and therefore very small
amounts of specific methylation may be revealed as greater than
they are. In the future, these experiments will be performed in
further detail.
As previously mentioned, extranuclear localization
of DNMT-1 was observed in the cells of vessel walls within the GBM
tumor tissues, as well as in the tumor cells of GBM tissues
examined from a patient with co-deletion on chromosome 1p/19q. This
may lead to altered cellular functions by demethylation of certain
genes, resulting in a more oncogenic phenotype of these cells. It
is of note that temozolomide treatment (alkylating agent) is more
effective in GBM patients with a hypermethylated (downregulated)
MGMT gene, which codes for a DNA repair enzyme (56,57).
In GBM patients, MGMT promoter methylation is used as a biomarker
for clinical temozolomide therapy management. Furthermore,
hypermethylation and mutations in IDH1/2 with functional
consequences for TET enzymes (involved in the demethylation of
histones and DNA) as well as glial differentiation and
establishment of glioma, have been reported (58). Since hypermethylation and silencing
of tumor suppressors are frequently observed in cancer (59,60),
and due to the reversible nature of epigenetic mechanisms, these
are potential therapeutic cancer targets (61).
In conclusion, it was demonstrated that
HCMV-infected GBM cells relocalize their DNMT-1 from the nucleus to
the extranuclear space, which coincided with viral late protein
production. The treatment of GBM cells with 5AZA resulted in
reduced cell proliferation. In addition, cell invasion was
decreased by infection. In vivo, most GBM tumors exhibited
cytoplasmic localization of DNMT-1, predominantly in blood vessel
cells, whereas cancer cells retained DNMT-1 expression in the
nuclei. These findings may be of importance in further
investigations into using DNA methylation and viral inhibitors in
GBM therapy.
Acknowledgements
Not applicable.
Funding
The Swedish Cancer Foundation, The Children's Cancer
Foundation of Sweden, Swedish Society for Medical Research (SLS),
Goljes Memory Foundation, Magnus Bergvall Foundation, Swedish
Society for Medical Research (SSMF), Karolinska Institute and Tore
Nilsson Foundation for Medical Research.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AR and TJE designed the study; AE, NL, BD and AR
performed the experiments; GS provide clinical samples and data; AR
and AE analyzed and generated figures; AR and TJE wrote the
manuscript. MRP, IN and LFH performed methylation experiments. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethical approval and consent to
participate
Ethical permission was approved by local ethical
committee at Karolinska Institute, Sweden (Dnr. 2008/628-31).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dziurzynski K, Chang SM, Heimberger AB,
Kalejta RF, McGregor Dallas SR, Smit M, Soroceanu L, Cobbs CS; HCMV
and Gliomas Symposium: Consensus on the role of human
cytomegalovirus in glioblastoma. Neuro Oncol. 14:246–255. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cunningham C, Gatherer D, Hilfrich B,
Baluchova K, Dargan DJ, Thomson M, Griffiths PD, Wilkinson GW,
Schulz TF and Davison AJ: Sequences of complete human
cytomegalovirus genomes from infected cell cultures and clinical
specimens. J Gen Virol. 91:605–615. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dunn W, Chou C, Li H, Hai R, Patterson D,
Stolc V, Zhu H and Liu F: Functional profiling of a human
cytomegalovirus genome. Proc Natl Acad Sci USA. 100:14223–14228.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Slobedman B and Mocarski ES: Quantitative
analysis of latent human cytomegalovirus. J Virol. 73:4806–4812.
1999.PubMed/NCBI
|
|
5
|
Bego MG, Keyes LR, Maciejewski J and St
Jeor SC: Human cytomegalovirus latency-associated protein LUNA is
expressed during HCMV infections in vivo. Arch Virol.
156:1847–1851. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wills MR, Poole E, Lau B, Krishna B and
Sinclair JH: The immunology of human cytomegalovirus latency: Could
latent infection be cleared by novel immunotherapeutic strategies?
Cell Mol Immunol. 12:128–138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goodrum F: Human cytomegalovirus latency:
Approaching the gordian knot. Annu Rev Virol. 3:333–357. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soderberg-Naucler C, Fish KN and Nelson
JA: Reactivation of latent human cytomegalovirus by allogeneic
stimulation of blood cells from healthy donors. Cell. 91:119–126.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu XF, Wang X, Yan S, Zhang Z, Abecassis
M and Hummel M: Epigenetic control of cytomegalovirus latency and
reactivation. Viruses. 5:1325–1345. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sinclair J: Human cytomegalovirus: Latency
and reactivation in the myeloid lineage. J Clin Virol. 41:180–185.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gan X, Wang H, Yu Y, Yi W, Zhu S, Li E and
Liang Y: Epigenetically repressing human cytomegalovirus lytic
infection and reactivation from latency in THP-1 model by targeting
H3K9 and H3K27 histone demethylases. PLoS One. 12:e01753902017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sinclair J: Chromatin structure regulates
human cytomegalovirus gene expression during latency, reactivation
and lytic infection. Biochim Biophys Acta. 1799:286–295. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meier JL: Reactivation of the human
cytomegalovirus major immediate-early regulatory region and viral
replication in embryonal NTera2 cells: Role of trichostatin A,
retinoic acid, and deletion of the 21-base-pair repeats and
modulator. J Virol. 75:1581–1593. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ioudinkova E, Arcangeletti MC, Rynditch A,
De Conto F, Motta F, Covan S, Pinardi F, Razin SV and Chezzi C:
Control of human cytomegalovirus gene expression by differential
histone modifications during lytic and latent infection of a
monocytic cell line. Gene. 384:120–128. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Murphy JC, Fischle W, Verdin E and
Sinclair JH: Control of cytomegalovirus lytic gene expression by
histone acetylation. EMBO J. 21:1112–1120. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Baryawno N, Rahbar A, Wolmer-Solberg N,
Taher C, Odeberg J, Darabi A, Khan Z, Sveinbjörnsson B, FuskevÅg
OM, Segerström L, et al: Detection of human cytomegalovirus in
medulloblastomas reveals a potential therapeutic target. J Clin
Invest. 121:4043–4055. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rahbar A, Orrego A, Peredo I, Dzabic M,
Wolmer-Solberg N, Strååt K, Stragliotto G and Söderberg-Nauclér C:
Human cytomegalovirus infection levels in glioblastoma multiforme
are of prognostic value for survival. J Clin Virol. 57:36–42. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wolmer-Solberg N, Baryawno N, Rahbar A,
Fuchs D, Odeberg J, Taher C, Wilhelmi V, Milosevic J, Mohammad AA,
Martinsson T, et al: Frequent detection of human cytomegalovirus in
neuroblastoma: A novel therapeutic target? Int J Cancer.
133:2351–2361. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Johnson DR, Fogh SE, Giannini C, Kaufmann
TJ, Raghunathan A, Theodosopoulos PV and Clarke JL: Case-based
review: Newly diagnosed glioblastoma. Neuro Oncol Pract. 2:106–121.
2015. View Article : Google Scholar
|
|
21
|
Alifieris C and Trafalis DT: Glioblastoma
multiforme: Pathogenesis and treatment. Pharmacol Ther. 152:63–82.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tyagi V, Theobald J, Barger J, Bustoros M,
Bayin NS, Modrek AS, Kader M, Anderer EG, Donahue B, Fatterpekar G,
et al: Traumatic brain injury and subsequent glioblastoma
development: Review of the literature and case reports. Surg Neurol
Int. 7:782016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Herbein G: The human cytomegalovirus, from
oncomodulation to oncogenesis. Viruses. 10(pii): E4082018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Michaelis M, Baumgarten P, Mittelbronn M,
Driever PH, Doerr HW and Cinatl J Jr: Oncomodulation by human
cytomegalovirus: Novel clinical findings open new roads. Med
Microbiol Immunol. 200:1–5. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Estekizadeh A, Landazur N, Bartek J Jr,
Beltoft Brøchner C, Davoudi B, Broholm H, Karimi M, Ekström TJ and
Rahbar A: Increased cytomegalovirus replication by 5-Azacytidine
and viral-induced cytoplasmic expression of DNMT1 in
medulloblastoma and endothelial cells. Int J Oncol. 52:1317–1327.
2018.PubMed/NCBI
|
|
26
|
Di Costanzo A, Del Gaudio N, Migliaccio A
and Altucci L: Epigenetic drugs against cancer: An evolving
landscape. Arch Toxicol. 88:1651–1668. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
(Human protein Atlas: https://www.proteinatlas.org/ENSG00000130816-DNMT1/cell)Version.
18.1, Atlas updated. 2018 11 15
|
|
29
|
Khan Z, Yaiw KC, Wilhelmi V, Lam H, Rahbar
A, Stragliotto G and Söderberg-Nauclér C: Human cytomegalovirus
immediate early proteins promote degradation of connexin 43 and
disrupt gap junction communication: Implications for a role in
gliomagenesis. Carcinogenesis. 35:145–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stragliotto G, Rahbar A, Solberg NW, Lilja
A, Taher C, Orrego A, Bjurman B, Tammik C, Skarman P, Peredo I, et
al: Effects of valganciclovir as an add-on therapy in patients with
cytomegalovirus-positive glioblastoma: A randomized, double-blind,
hypothesis-generating study. Int J Cancer. 133:1204–1213. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Soderberg-Naucler C, Rahbar A and
Stragliotto G: Survival in patients with glioblastoma receiving
valganciclovir. N Engl J Med. 369:985–986. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bettegowda C, Agrawal N, Jiao Y, Sausen M,
Wood LD, Hruban RH, Rodriguez FJ, Cahill DP, McLendon R, Riggins G,
et al: Mutations in CIC and FUBP1 contribute to human
oligodendroglioma. Science. 333:1453–1455. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bibikova M, Barnes B, Tsan C, Ho V,
Klotzle B, Le JM, Delano D, Zhang L, Schroth GP, Gunderson KL, et
al: High density DNA methylation array with single CpG site
resolution. Genomics. 98:288–295. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brennan CW, Verhaak RG, McKenna A, Campos
B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ,
Berman SH, et al: The somatic genomic landscape of glioblastoma.
Cell. 155:462–477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ahluwalia MS, Xie H, Dahiya S,
Hashemi-Sadraei N, Schiff D, Fisher PG, Chamberlain MC, Pannullo S,
Newton HB, Brewer C, et al: Efficacy and patient-reported outcomes
with dose-intense temozolomide in patients with newly diagnosed
pure and mixed anaplastic oligodendroglioma: A phase II multicenter
study. J Neurooncol. 122:111–119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vogelbaum MA, Hu C, Peereboom DM,
Macdonald DR, Giannini C, Suh JH, Jenkins RB, Laack NN, Brachman
DG, Shrieve DC, et al: Phase II trial of pre-irradiation and
concurrent temozolomide in patients with newly diagnosed anaplastic
oligodendrogliomas and mixed anaplastic oligoastrocytomas: Long
term results of RTOG BR0131. J Neurooncol. 124:413–420. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aihara K, Mukasa A, Nagae G, Nomura M,
Yamamoto S, Ueda H, Tatsuno K, Shibahara J, Takahashi M, Momose T,
et al: Genetic and epigenetic stability of oligodendrogliomas at
recurrence. Acta Neuropathol Commun. 5:182017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Suvà ML: Genetics and epigenetics of
gliomas. Swiss Med Wkly. 44:w140182014.
|
|
39
|
Li X, Zhang Q, Shi Q, Liu Y, Zhao K, Shen
Q, Shi Y, Liu X, Wang C, Li N, et al: Demethylase Kdm6a
epigenetically promotes IL-6 and IFN-β production in macrophages. J
Autoimmun. 80:85–94. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Winfield J, Esbitt A, Seutter SF, Desai B,
Abdo M, Vasconez M, Laidlaw W, Green K, Shamseddin SM and Borghaei
RC: Effect of inflammatory cytokines on DNA methylation and
demethylation. FASEB J. 30 (Suppl 1):S10532016.
|
|
41
|
Cheeran MC, Hu S, Yager SL, Gekker G,
Peterson PK and Lokensgard JR: Cytomegalovirus induces cytokine and
chemokine production differentially in microglia and astrocytes:
Antiviral implications. J Neurovirol. 7:135–147. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kline JN, Hunninghake GM, He B, Monick MM
and Hunninghake GW: Synergistic activation of the human
cytomegalovirus major immediate early promoter by prostaglandin E2
and cytokines. Exp Lung Res. 24:3–14. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gong M, Shi W, Qi J, Shao G, Shi Z, Wang
J, Chen J and Chu R: Alu hypomethylation and MGMT hypermethylation
in serum as biomarkers of glioma. Oncotarget. 8:76797–76806. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nagarajan RP and Costello JF: Epigenetic
mechanisms in glioblastoma multiforme. Semin Cancer Biol.
19:188–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cadieux B, Ching TT, VandenBerg SR and
Costello JF: Genome-wide hypomethylation in human glioblastomas
associated with specific copy number alteration,
methylenetetrahydrofolate reductase allele status, and increased
proliferation. Cancer Res. 66:8469–8476. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Costello JF, Berger MS, Huang HS and
Cavenee WK: Silencing of p16/CDKN2 expression in human
gliomas by methylation and chromatin condensation. Cancer Res.
56:2405–2410. 1996.PubMed/NCBI
|
|
47
|
Alaminos M, Davalos V, Ropero S, Setién F,
Paz MF, Herranz M, Fraga MF, Mora J, Cheung NK, Gerald WL, et al:
EMP3, a myelin-related gene located in the critical 19q13.3
region, is epigenetically silenced and exhibits features of a
candidate tumor suppressor in glioma and neuroblastoma. Cancer Res.
65:2565–2571. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nakamura M, Watanabe T, Yonekawa Y,
Kleihues P and Ohgaki H: Promoter methylation of the DNA repair
gene MGMT in astrocytomas is frequently associated with G:C ->
A:T mutations of the TP53 tumor suppressor gene. Carcinogenesis.
22:1715–1719. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hesson L, Bieche I, Krex D, Criniere E,
Hoang-Xuan K, Maher ER and Latif F: Frequent epigenetic
inactivation of RASSF1A and BLU genes located within
the critical 3p21.3 region in gliomas. Oncogene. 23:2408–2419.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Uhlmann K, Rohde K, Zeller C, Szymas J,
Vogel S, Marczinek K, Thiel G, Nürnberg P and Laird PW: Distinct
methylation profiles of glioma subtypes. Int J Cancer. 106:52–59.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nakamura M, Yonekawa Y, Kleihues P and
Ohgaki H: Promoter hypermethylation of the RB1 gene in
glioblastomas. Lab Invest. 81:77–82. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hegi ME, Diserens AC, Gorlia T, Hamou MF,
de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani
L, et al: MGMT gene silencing and benefit from temozolomide
in glioblastoma. N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gonzalez-Gomez P, Bello MJ, Arjona D,
Lomas J, Alonso ME, De Campos JM, Vaquero J, Isla A, Gutierrez M
and Rey JA: Promoter hypermethylation of multiple genes in
astrocytic gliomas. Int J Oncol. 22:601–608. 2003.PubMed/NCBI
|
|
54
|
Alonso ME, Bello MJ, Gonzalez-Gomez P,
Arjona D, Lomas J, de Campos JM, Isla A, Sarasa JL and Rey JA:
Aberrant promoter methylation of multiple genes in
oligodendrogliomas and ependymomas. Cancer Genet Cytogenet.
144:134–142. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nawaz I, Qiu X, Wu H, Li Y, Fan Y, Hu LF,
Zhou Q and Ernberg I: Development of a multiplex methylation
specific PCR suitable for (early) detection of non-small cell lung
cancer. Epigenetics. 9:1138–1148. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Paz MF, Yaya-Tur R, Rojas-Marcos I, Reynes
G, Pollan M, Aguirre-Cruz L, García-Lopez JL, Piquer J, Safont MJ,
Balaña C, et al: CpG island hypermethylation of the DNA repair
enzyme methyltransferase predicts response to temozolomide in
primary gliomas. Clin Cancer Res. 10:4933–4938. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Caren H, Pollard SM and Beck S: The good,
the bad and the ugly: Epigenetic mechanisms in glioblastoma. Mol
Aspects Med. 34:849–862. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Flanagan S, Lee M, Li CC, Suter CM and
Buckland ME: Promoter methylation analysis of IDH genes in human
gliomas. Front Oncol. 2:1932012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hughes LA, Melotte V, de Schrijver J, de
Maat M, Smit VT, Bovée JV, French PJ, van den Brandt PA, Schouten
LJ, de Meyer T, et al: The CpG island methylator phenotype: What's
in a name? Cancer Res. 73:5858–5868. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Dong Y, Zhao H, Li H, Li X and Yang S: DNA
methylation as an early diagnostic marker of cancer (Review).
Biomed Rep. 2:326–330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Fardi M, Solali S and Farshdousti Hagh M:
Epigenetic mechanisms as a new approach in cancer treatment: An
updated review. Genes Dis. 5:304–311. 2018. View Article : Google Scholar : PubMed/NCBI
|