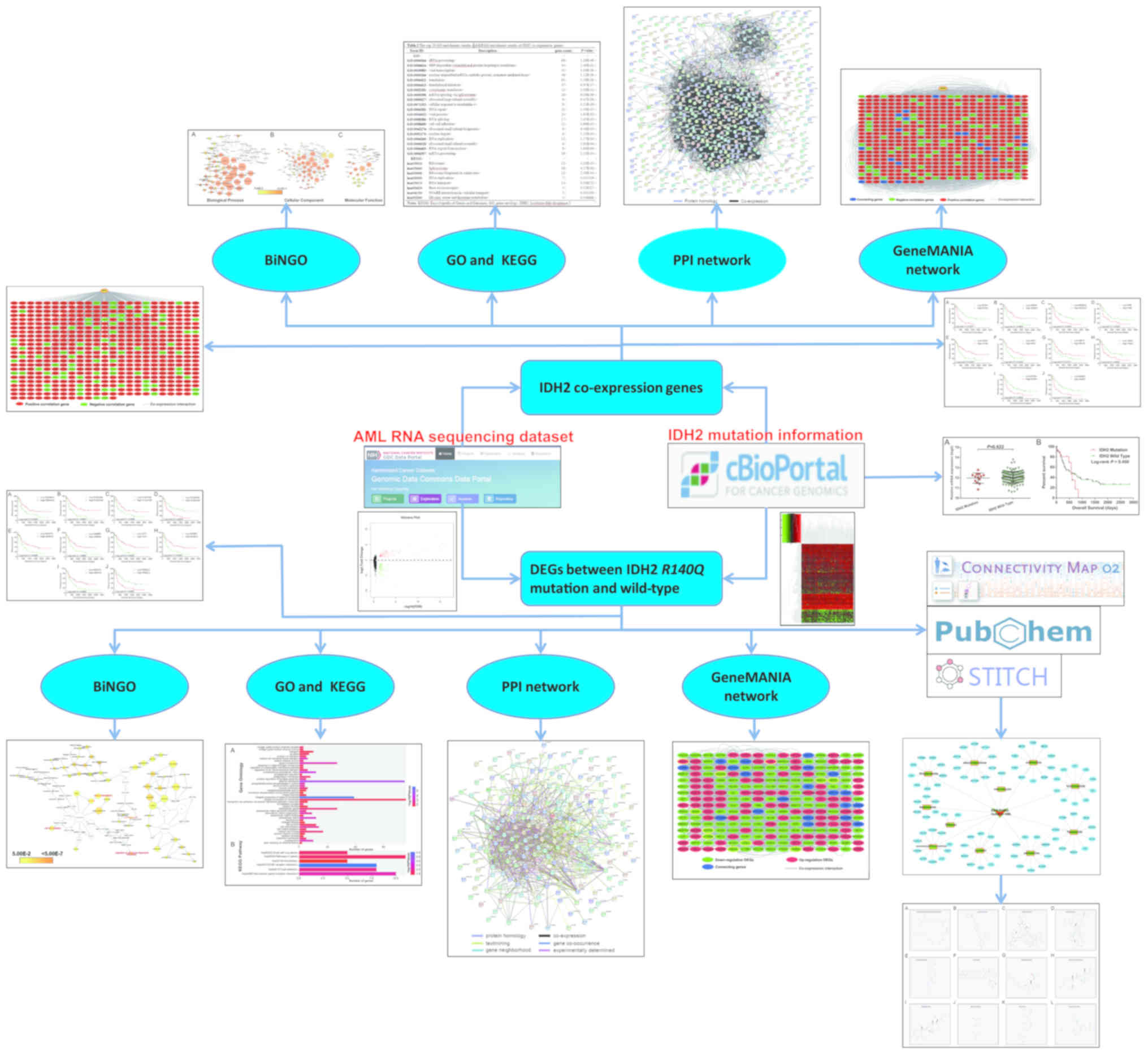
Introduction
Acute myeloid leukemia (AML) is a cancer of the
myeloid lineage hematopoietic cells, and is clonally heterogeneous
since all myeloid precursors and progenitors can potentially
undergo malignant transformation (1,2). The
uncontrolled proliferation and accumulation of immature myeloid
cells or blasts impair normal hematopoiesis, thereby increasing the
risk of severe infections and hemorrhage (1,2). The
genomic landscape of AML is highly diverse, and some mutations can
be predictive of prognosis, as well as potential therapeutic
targets. Most leukemias are characterized by specific oncoproteins
encoded by fusion genes resulting from chromosomal translocations.
Therefore, these oncoproteins not only serve as diagnostic markers
of specific types of leukemia but also as potential therapeutic
targets. In addition, some chromosomal abnormalities are associated
with characteristic histopathological and clinical features, which
form the cornerstone of the accurate diagnosis and treatment of
leukemia (1). However, the
heterogeneity of AML has stymied its targeted treatment, with
current mortality rates well over 50% (1).
Isocitrate dehydrogenase 2 (IDH2) mutations
are frequently observed in AML (3)
and are not only potential targets for personalized therapies
(4,5), but also indicators of AML prognosis
(6,7). R140Q is the most frequent
IDH2 mutation in AML, and is correlated to advanced age,
normal karyotype, and the French-American-British (FAB)
classification M2 at diagnosis (8).
Wiseman et al observed that multi-lineage hematopoiesis from
IDH2 R140Q clones was frequently reconstituted after
chemotherapy in AML patients (9).
However, the key genes and pathways related to IDH2
mutations in AML are not completely clear. In a previous study, we
used RNA sequencing datasets from The Cancer Genome Atlas (TGCA)
database and multiple bioinformatic analyses to identify potential
molecular mechanisms in tumor protein p53 (TP53)-mutated
AML, as well as potential prognostic biomarkers (10,11).
The aim of the present study was to identify the potential
molecular mechanisms and genes associated with IDH2 R140Q
mutation in adult de novo AML using a similar approach, in
addition to potential targeted therapeutic drugs using the
Connectivity Map (CMap).
Materials and methods
RNA-seq data of adult de novo AML
patients
The RNA-seq dataset of the bone marrow tissues of an
adult de novo AML patient cohort collected at diagnosis, as
well as the corresponding survival information, were obtained from
TCGA (https://gdc-portal.nci.nih.gov/;
accessed August 10, 2018) database (12). The corresponding information on
IDH2 R140Q mutation status was obtained from the cBioPortal
for Cancer Genomics website (http://www.cbioportal.org/index.do; accessed August
10, 2018) (13,14). Since all the data in this study were
retrieved from TCGA, the present study did not require the approval
of the ethics committee. The RNA-seq data are available on a public
domain, and were acquired and analyzed according to the published
guidelines of TCGA (https://cancergenome.nih.gov/publications/publicationguidelines).
Identification of differentially
expressed genes (DEGs) and their prognostic value in AML
DEGs between the IDH2 R140Q-mutated and
wild-type adult de novo AML were identified by edgeR using
the following criteria: |log2 fold change (FC) | ≥1, and
both P-value and false discovery rate (FDR) <0.05 (15,16).
The heat map and volcano plot of the DEGs were generated using the
‘gplots’ package in R platform, and their prognostic value in AML
was determined using the ‘survival’ package in the R platform
(17,18).
Functional assessment
Functional assessment of the DEGs, in terms of Gene
Ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes
(KEGG) pathway enrichment, was performed by Database for
Annotation, Visualization, and Integrated Discovery (DAVID) v6.8
(https://david.ncifcrf.gov/tools.jsp;
accessed August 10, 2018) (19,20)
and those with P-values <0.05 were considered statistically
significant. The directed acyclic graph of GO terms was drawn using
the Biological Networks Gene Ontology (BiNGO) tool in
Cytoscape_v3.6.1, a plugin used to assess the overrepresentation of
GO categories in biological networks (21).
Construction of protein-protein and
gene-gene interaction networks
The Search Tool for the Retrieval of Interacting
Genes/Proteins (STRING) database (http://string.embl.de/; accessed August 10, 2018) was
used to construct the protein-protein interaction (PPI) networks
(22–24), and GeneMANIA (http://genemania.org/; accessed August 10, 2018) was
used for gene-gene interaction (GGI) networks (25,26).
Connectivity Map analysis
Connectivity Map (CMap, https://portals.broadinstitute.org/cmap/; accessed
August 10, 2018) is an online tool and data source for analyzing
the mechanism of action and localization of drugs based on
transcriptome data (27,28). A positive score indicates an
inducement effect of a small-molecule drug on the query signatures,
and a negative score reflects a repression effect. CMap was used to
screen for putative small-molecule drugs against IDH2
R140Q-mutated AML, and those with a connective score < −0.2
were identified as the potential therapeutic drugs. The chemical
structures of these drugs were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/; accessed
August 10, 2018) (29,30) and the Search Tool for Interacting
Chemicals (STITCH: http://stitch.embl.de/; accessed August 10, 2018) was
used to construct protein-chemical interaction networks based on
the existing literature and databases (31–33).
Genome-wide co-expression network
analysis
To assess the biological relevance of the IDH2
R140Q mutation in AML, the IDH2 co-expressing genes were
screened using the Pearson correlation test. The genome-wide
co-expression analysis was performed using the ‘cor’ function in
the R platform, and the genes with a |Pearson correlation
coefficient| >0.75 and P<0.05 were identified as the IDH2
R140Q co-expressing genes. In addition, the prognostic values
of these genes was evaluated by the ‘survival’ package in the R
platform (17,18).
Statistical analysis
The IDH2 mRNA expression levels in the
R140Q-mutated and wild-type AML bone marrow tissues was
compared by the Student's t-test. The clinical outcomes of the
R140Q-mutated and wild-type AML patients was compared by the
Kaplan-Meier method and log-rank test. Hazard ratio (HR) and 95%
confidence interval (CI) were calculated using the Cox proportional
hazards regression model. FDR in edgeR was calculated by multiple
testing with the Benjamini-Hochberg procedure (34–36).
P-value <0.05 was regarded as statistically significant. All
statistical analyses were conducted using the SPSS version 20.0
(IBM Corp., Armonk, NY, USA) and R 3.5.0 (https://www.r-project.org/; accessed June 21,
2018).
Results
Patient data
RNA-seq datasets from 151 AML patients were
downloaded from TCGA (12), and
data of 3 patients with a second IDH2 mutation was excluded.
Thirteen of the remaining 148 patients had the IDH2 R140Q
mutation and 135 patients were IDH2 wild-type. Their
corresponding survival information, including overall survival
duration and survival status, was also download from TCGA. The
survival information for 11 patients (10 IDH2 wild-type and
1 IDH2 R140Q-mutated) was missing. The bioinformatic
analysis flowchart for the present study is shown in Fig. 1.
DEG screening
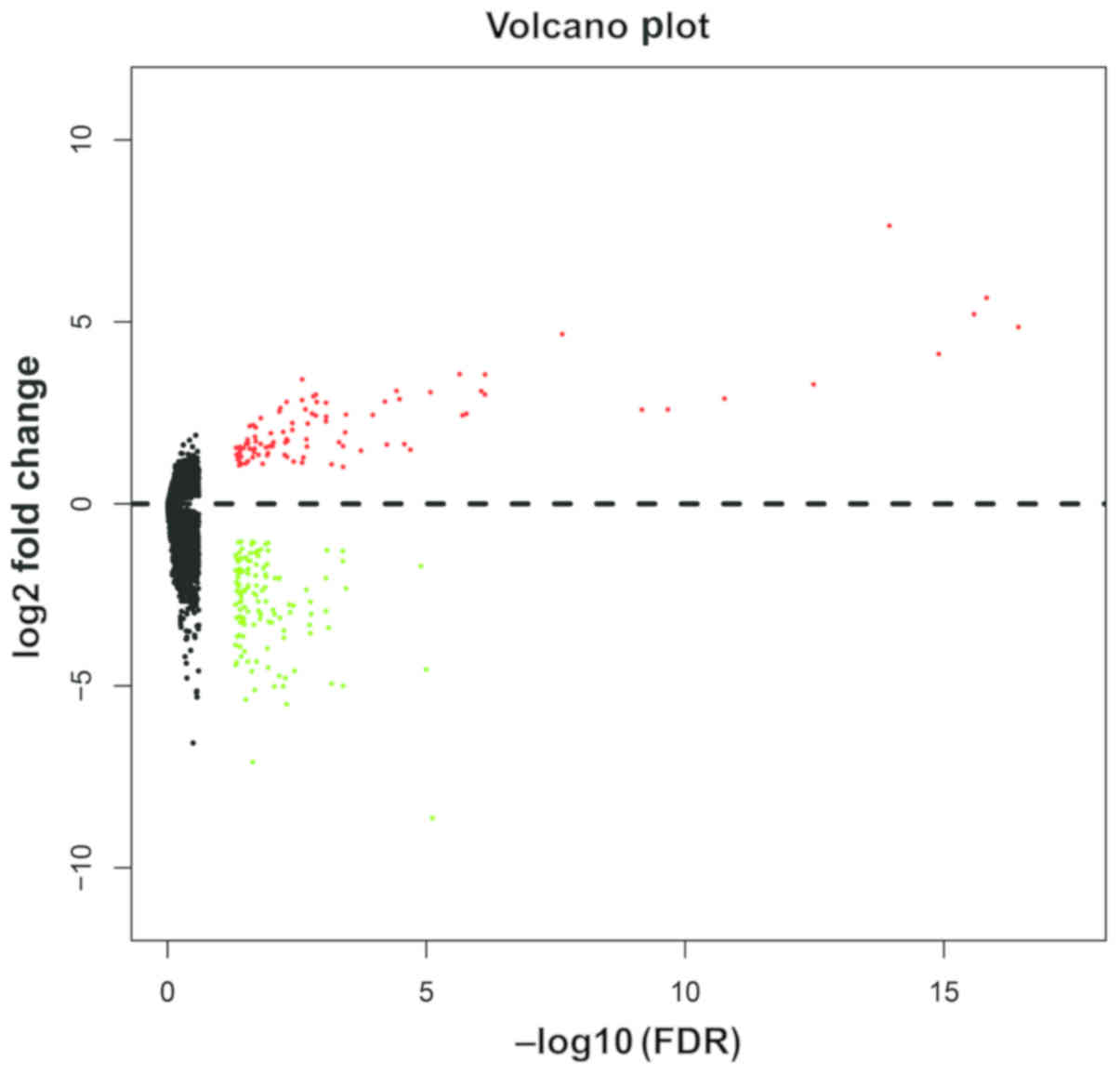
A total of 230 DEGs were identified between the
IDH2 wild-type and R140Q-mutated AML samples
(Table SI), of which 133 were
downregulated and 97 were upregulated in the bone marrow of the
IDH2 R140Q-mutated AML patients. The heat map and volcano
plot of the DEGs are shown in Fig.
S1 and Fig. 2,
respectively.
Association of the DEGs with
survival
To investigate the prognostic value of the IDH2
R140Q-specific DEGs, we stratified the patients into a high-
and low-expression groups according to the median values of each of
these DEGs, and compared their survival status. Due to the
incomplete survival information, we could not perform a
multivariate Cox proportional risk regression model, and therefore
assessed their prognostic values through a univariate analysis.
Thirty-one DEGs were significantly associated with the OS (Table SII), and the top 10 significant
prognostic DEGs were tetraspanin 10 (TSPAN10), pleckstrin
homology domain containing A5 (PLEKHA5), coiled-coil domain
containing 198 (CCDC198, also known as C14orf105),
pleckstrin homology domain containing A6 (PLEKHA6), A-kinase
anchoring protein 12 (AKAP12), gamma-aminobutyric acid type
A receptor delta subunit (GABRD), fucosyltransferase 1
(FUT1), potassium voltage-gated channel interacting protein
3 (KCNIP3), immunoglobulin superfamily DCC subclass member 4
(IGDCC4) and podocalyxin like 2 (PODXL2) (Fig. 3A-J).
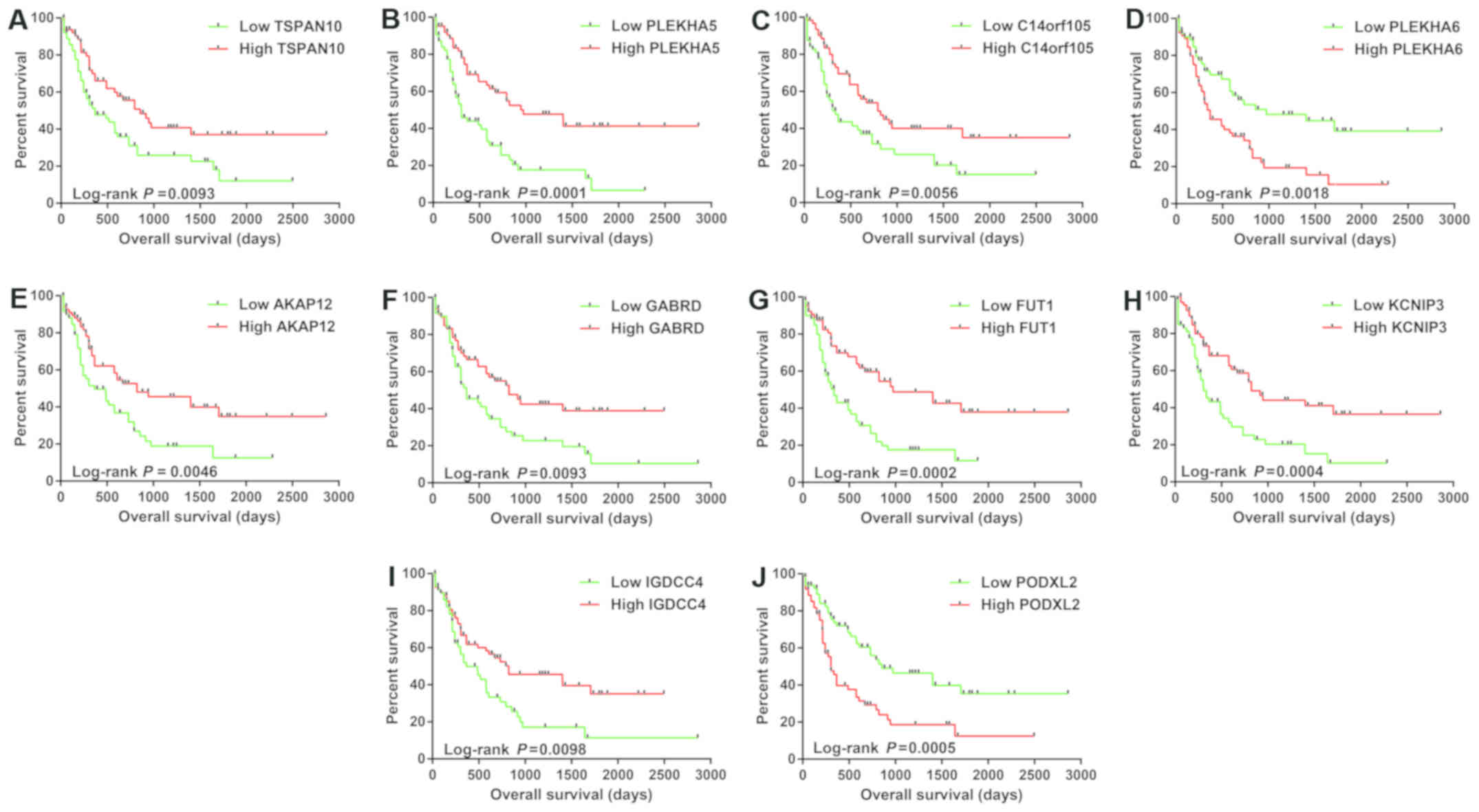 | Figure 3.The Kaplan-Meier curves of the top 10
significantly prognostic DEGs. (A) Overall survival stratified by
TSPAN10, (B) PLEKHA5, (C) C14orf105, (D)
PLEKHA6, (E) AKAP12, (F) GABRD, (G)
FUT1, (H) KCNIP3, (I) IGDCC4 and (J)
PODXL2. DEGs, differentially expressed genes;
TSPAN10, tetraspanin 10; PLEKHA5, pleckstrin homology
domain containing A5; C14orf105, coiled-coil domain
containing 198 (also known as C14orf105); PLEKHA6,
pleckstrin homology domain containing A6; AKAP12, A-kinase
anchoring protein 12; GABRD, gamma-aminobutyric acid type A
receptor delta subunit; FUT1, fucosyltransferase 1;
KCNIP3, potassium voltage-gated channel interacting protein
3; IGDCC4, immunoglobulin superfamily DCC subclass member 4;
PODXL2, podocalyxin like 2. |
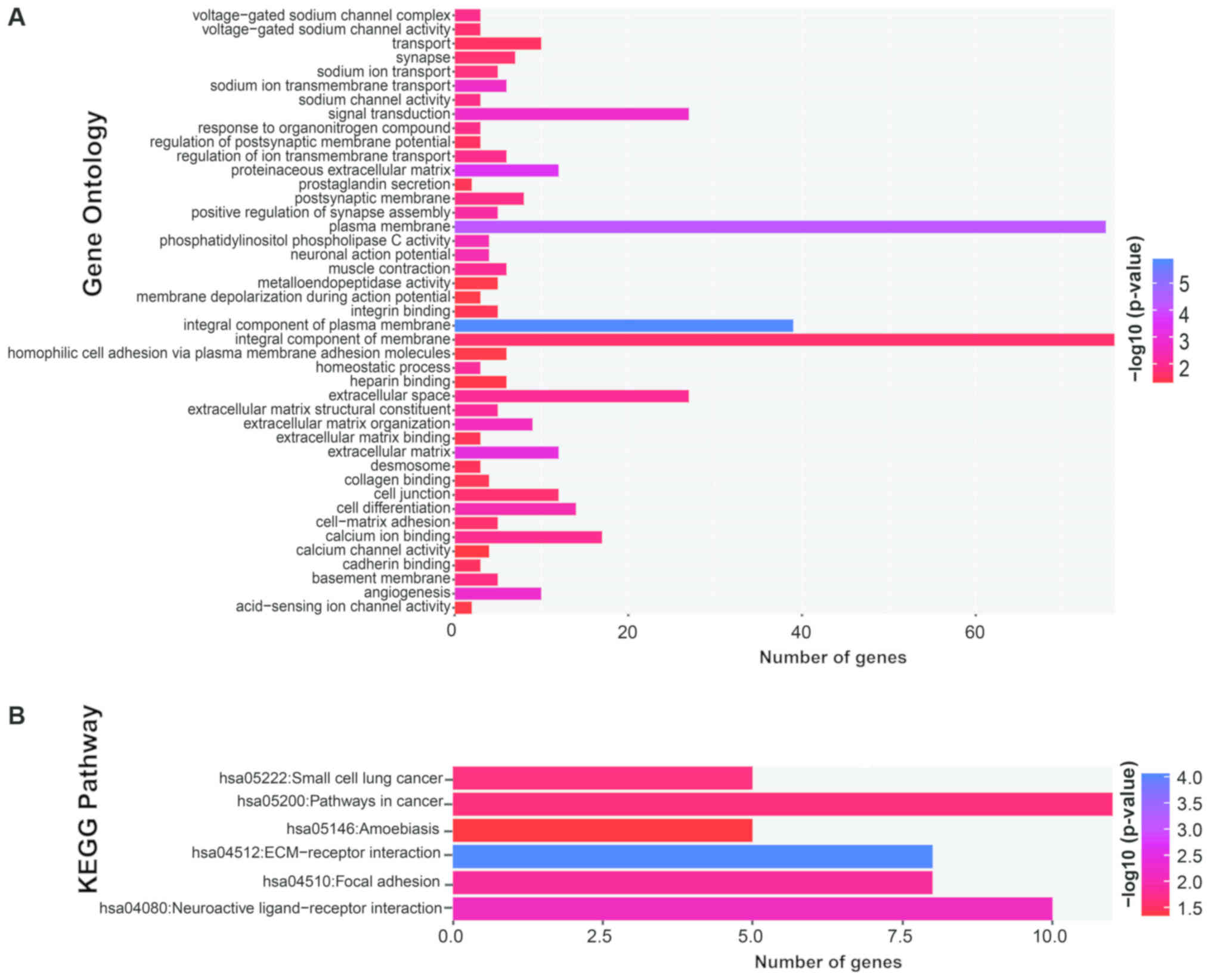
Functional assessment of DEGs
The DEGs were functionally assessed using the GO
terms and KEGG pathways. The significantly enriched GO terms were
angiogenesis, cell differentiation, cell-matrix adhesion,
homophilic cell adhesion via plasma membrane adhesion molecules,
cell junction, signal transduction, phosphoinositide phospholipase
C activity and integrin binding (Fig.
4A). KEGG analysis showed that the DEGs were significantly
enriched in ECM-receptor interaction, focal adhesion and pathways
in cancer (Fig. 4B). The BiNGO
analysis also demonstrated that these DEGs were significantly
enriched in regulation of calcium ion-dependent exocytosis, cell
differentiation, signal transmission, signaling process and
phosphoinositide phospholipase C activity (Fig. S2).
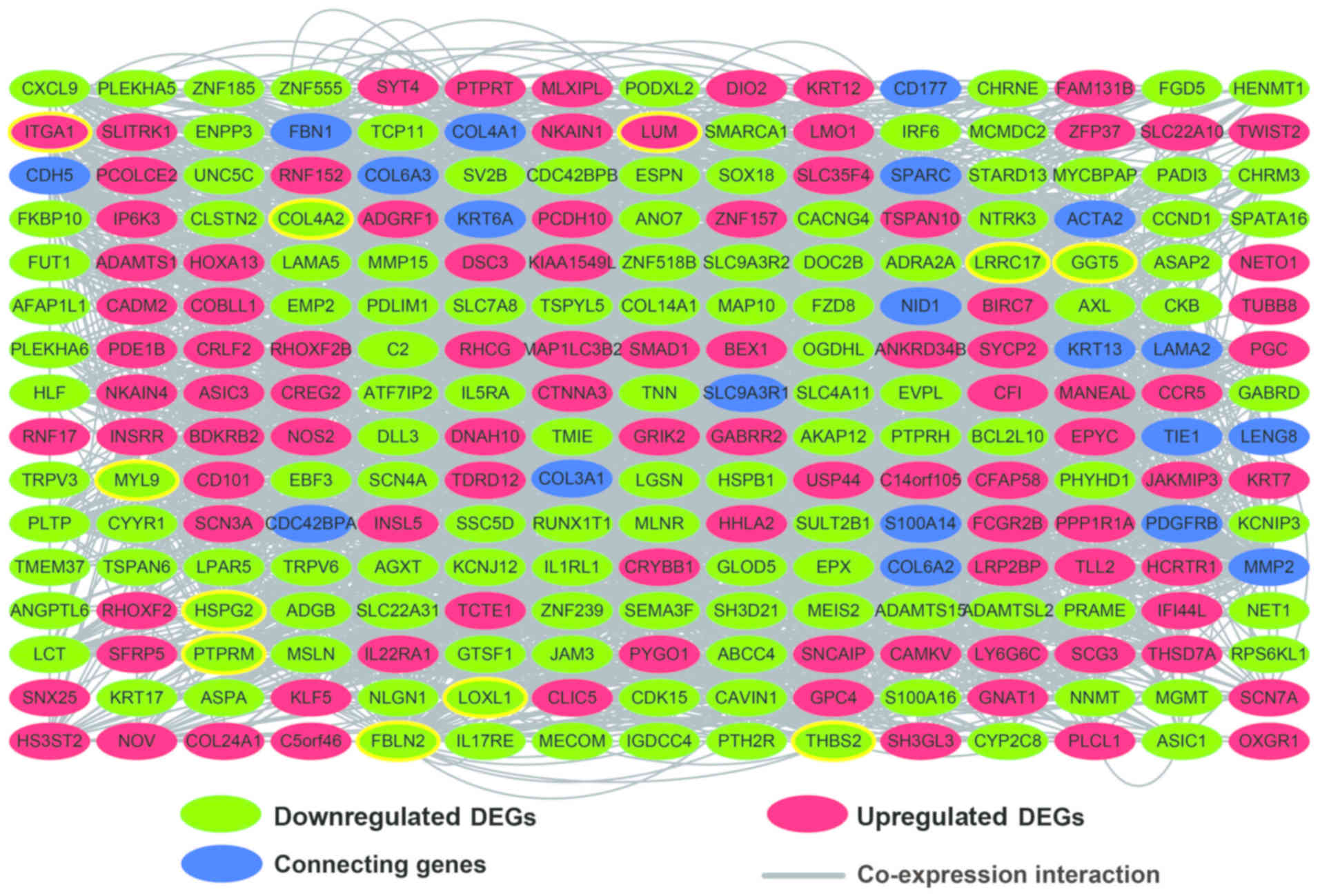
GGI and PPI interaction network
construction of DEGs
The GGI network indicated complex interactions
between the DEGs (Fig. 5), and the
top 10 degree genes that were identified as the hub genes were
collagen type IV α2 chain (COL4A2), lumican (LUM),
fibulin 2 (FBLN2), thrombospondin 2 (THBS2), lysyl
oxidase like 1 (LOXL1), myosin light chain 9 (MYL9),
heparan sulfate proteoglycan 2 (HSPG2),
gamma-glutamyltransferase 5 (GGT5), integrin subunit alpha 1
(ITGA1), protein tyrosine phosphatase, receptor type M
(PTPRM), and leucine rich repeat containing 17
(LRRC17); the highest degree was that of COL4A2
(degree=127). The PPI network supported the GGI results, and
substantiated complex interactions among these DEGs at the protein
level as well (Fig. S3). The top
10 degree genes identified as hub genes in the PPI networks were
insulin receptor related receptor (INSRR), LUM, AXL receptor
tyrosine kinase (AXL), cyclin D1 (CCND1), CDC42
binding protein kinase beta (CDC42BPB), cyclin dependent
kinase 15 (CDK15), nitric oxide synthase 2 (NOS2),
t-complex-associated-testis-expressed 1 (TCTE1), SWI/SNF
related, matrix associated, actin dependent regulator of chromatin,
subfamily a, member 1 (SMARCA1) and espin (ESPN); the
highest degree was that of INSRR (degree=39). The LUM
gene was common to both GGI and PPI networks, indicating its
critical role in IDH2 R140Q-mutated AML.
CMap analysis
The CMap analysis was conducted to screen for
small-molecule drugs targeting the IDH2 R140Q mutation.
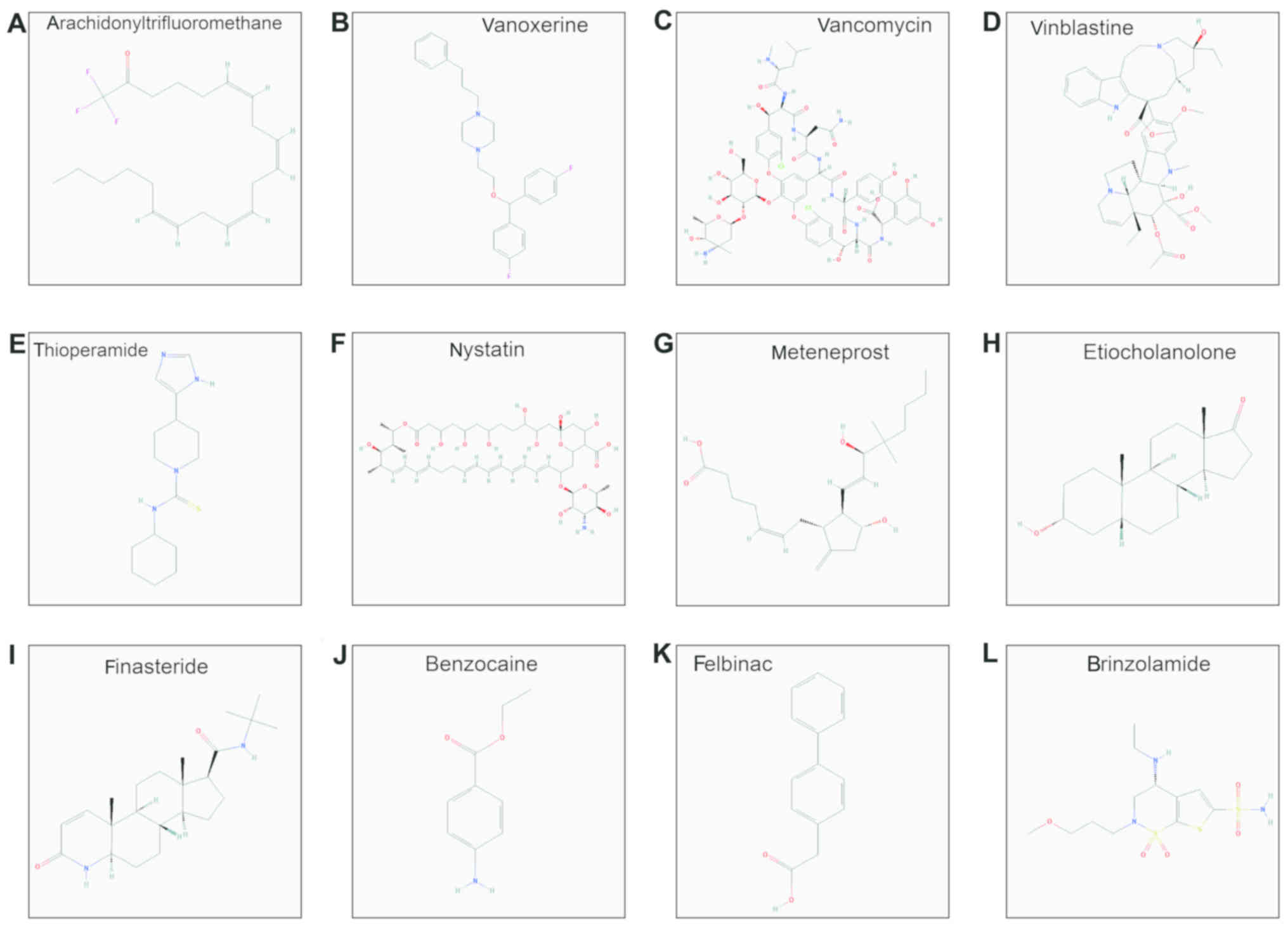
Thirteen drugs were identified: arachidonyltrifluoromethane,
vanoxerine, vancomycin, vinblastine, thioperamide, nystatin,
meteneprost, etiocholanolone, finasteride, PF-00539745-00,
benzocaine, felbinac and brinzolamide (Table I and Fig. 6). (PF-00539745-00 does not appear in
the figure as its chemical structure is not available in PubChem).
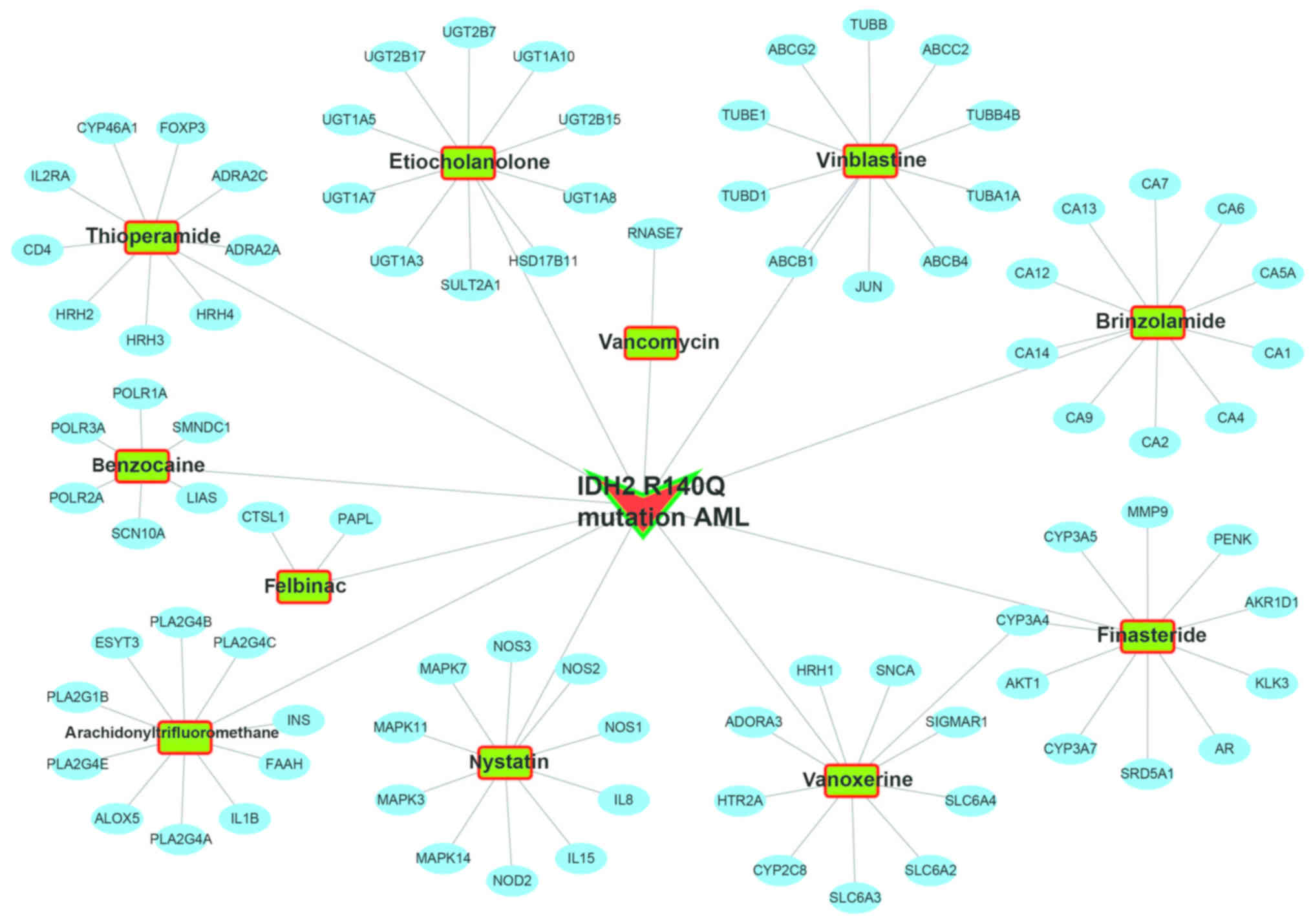
The protein-chemical interaction networks of 11 drugs were obtained
from STITCH (Fig. 7), and
cytochrome P450 family 2 subfamily C member 8 (CYP2C8), NOS2
and adrenoceptor α 2A (ADRA2A) were common to the DEGs and
protein-chemical interaction networks. In addition, KEGG analysis
showed that ADRA2A was involved in the neuroactive
ligand-receptor interaction pathway, and NOS2 in pathways in cancer
and amoebiasis pathway. Based on these findings, we hypothesized
that nystatin is a potential therapeutic agent against IDH2
R140Q-mutated AML that acts through the nystatin-NOS2
pathway to regulate cancer/amoebiasis pathways. Similarly,
thioperamide possibly functions through the
thioperamide-ADRA2A-neuroactive ligand-receptor interaction
pathway axis, and vanoxerine through the vanoxerine-CYP2C8
axis.
 | Table I.Potential targeted therapeutic drugs
for IDH2 R140Q-mutated AML based on Connectivity Map analysis. |
Table I.
Potential targeted therapeutic drugs
for IDH2 R140Q-mutated AML based on Connectivity Map analysis.
| CMap name | Mean connective
score | n | Enrichment | P-value | Specificity | Percent
non-null |
|---|
|
Arachidonyltrifluoromethane | −0.616 | 2 | −0.92 | 0.01296 | 0.0261 | 100 |
| Vanoxerine | −0.586 | 4 | −0.718 | 0.01281 | 0.025 | 75 |
| Vancomycin | −0.48 | 4 | −0.71 | 0.01428 | 0.0552 | 75 |
| Vinblastine | −0.436 | 3 | −0.87 | 0.00433 | 0.0534 | 66 |
| Thioperamide | −0.386 | 5 | −0.697 | 0.00555 | 0.0373 | 60 |
| Nystatin | −0.381 | 3 | −0.797 | 0.01711 | 0.0216 | 66 |
| Meteneprost | −0.364 | 4 | −0.648 | 0.0366 | 0.1797 | 50 |
|
Etiocholanolone | −0.351 | 6 | −0.678 | 0.00302 | 0.0844 | 50 |
| Finasteride | −0.348 | 6 | −0.664 | 0.00407 | 0.0854 | 50 |
| PF-00539745-00 | −0.345 | 3 | −0.855 | 0.00603 | 0.0593 | 66 |
| Benzocaine | −0.34 | 4 | −0.829 | 0.00163 | 0.0134 | 50 |
| Felbinac | −0.337 | 4 | −0.68 | 0.02316 | 0.2199 | 50 |
| Brinzolamide | −0.282 | 4 | −0.651 | 0.03503 | 0.0541 | 50 |
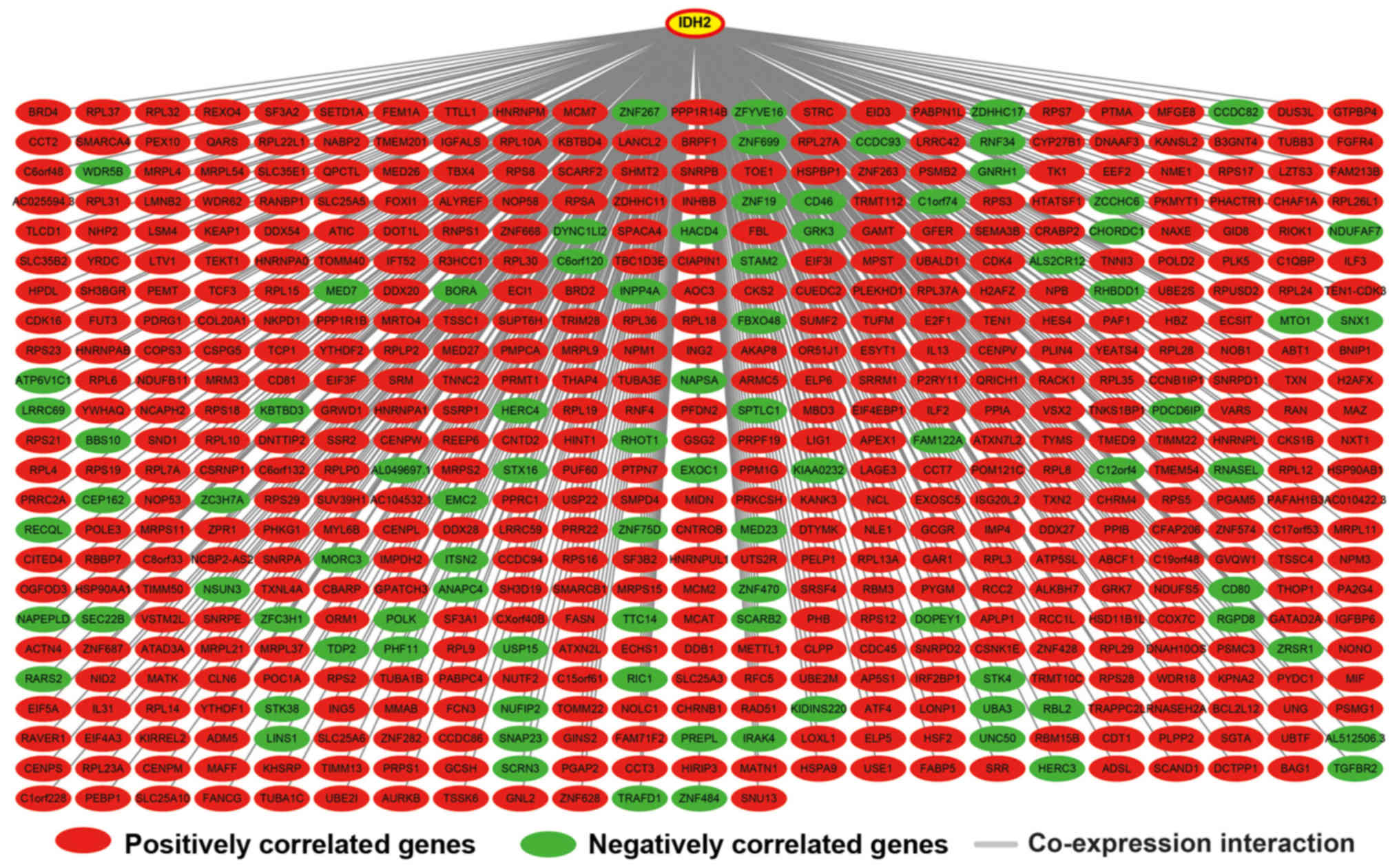
Genome-wide co-expression network
analysis
Genome-wide co-expression network analysis showed
that 542 genes were co-expressed with IDH2 in the IDH2
R140Q-mutated AML bone marrow tissues, of which 455 were
positively correlated and 87 were negatively correlated with
IDH2 R140Q (Fig. 8 and
Table SIII). GO enrichment
analysis showed that IDH2 and the co-expressed genes were
significantly enriched in DNA replication, DNA repair, cell-cell
adhesion, cell division and cell cycle-related biological processes
(Table II and Table SIV), while KEGG analysis showed
significant enrichment in DNA replication, SNARE interactions in
vesicular transport and base excision repair pathway (Table II and Table SV). Furthermore, these results were
verified by BiNGO, which indicated significant enrichment in the
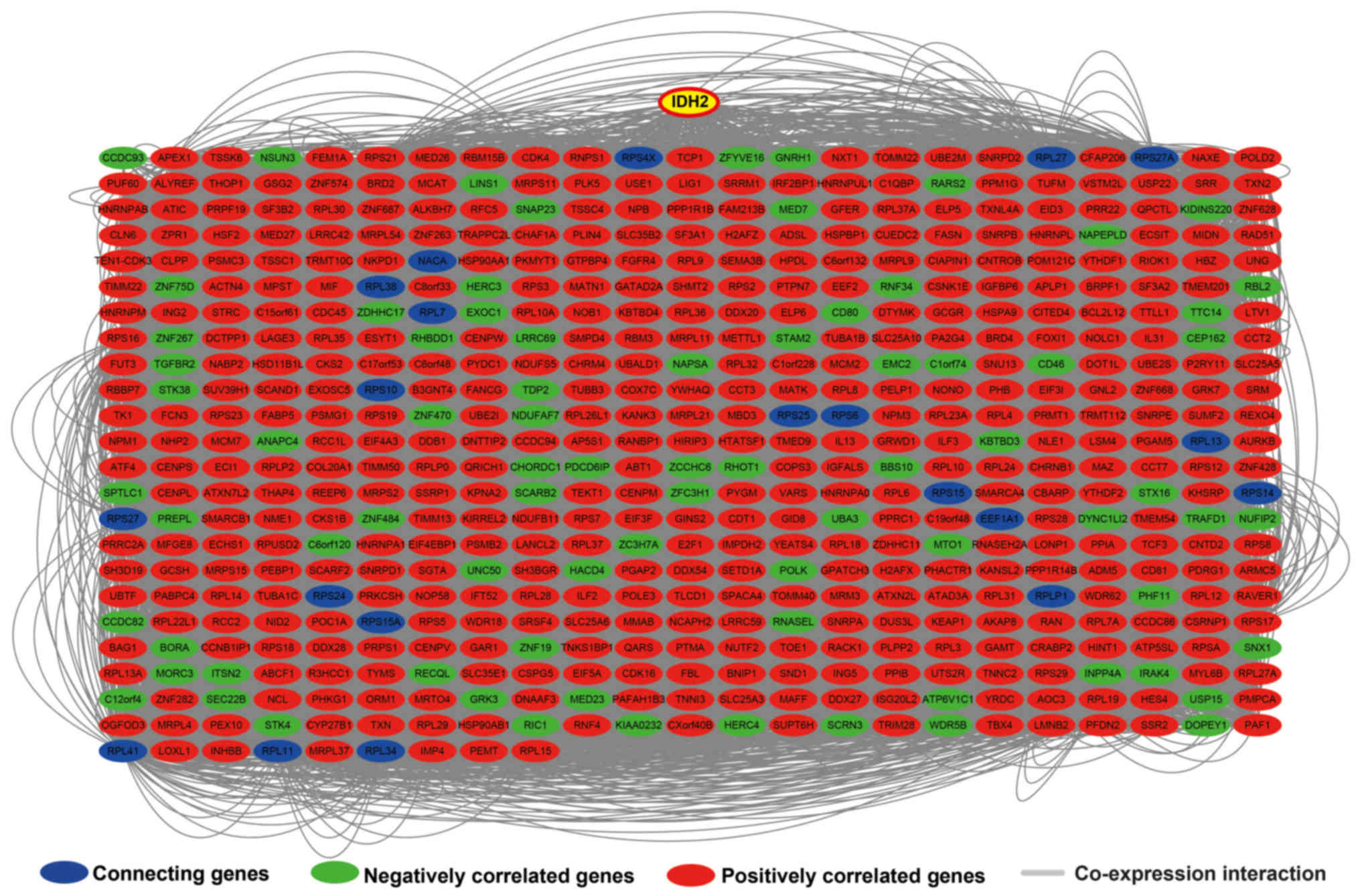
cell cycle process and DNA replication (Fig. S4). GGI (Fig. 9) and PPI (Fig. S5) interaction networks were
respectively constructed using geneMANIA and STRING, and further
verified the relationship between IDH2 and the co-expressing
genes. The prognostic value of these co-expressing genes in AML was
determined using the ‘survival’ package in the R platform, and 56
genes were significantly associated with the OS (Table SVI). The top ten significantly
prognostic genes were splicing factor 3a subunit 1 (SF3A1),
centromere protein V (CENPV), interferon stimulated
exonuclease gene 20 like 2 (ISG20L2), prohibitin
(PHB), glycine cleavage system protein H (GCSH), heat
shock transcription factor 2 (HSF2), zinc finger protein 19
(ZNF19), EARP complex and GARP complex interacting protein 1
(EIPR1, also known as TSSC1), enoyl-CoA hydratase,
short chain 1 (ECHS1), and nucleic acid binding protein 2
(NABP2). The Kaplan-Meier curves of the patients stratified
into the low-and high-expressing groups are shown in Fig. 10A-J.
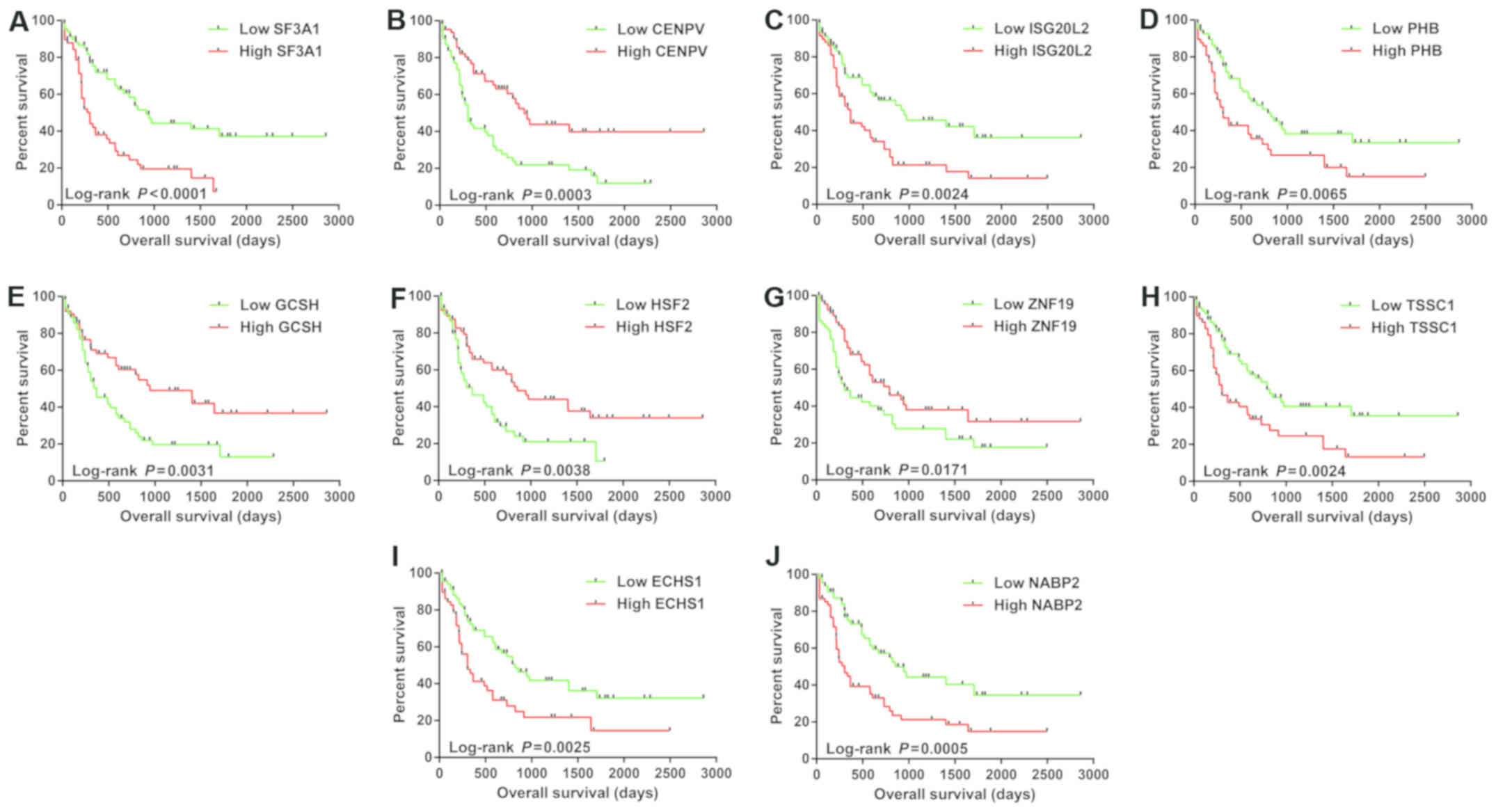 | Figure 10.The Kaplan-Meier curves of the top 10
significantly prognostic IDH2 co-expression genes in IDH2
R140Q-mutated AML. Overall survival stratified by (A)
SF3A1, (B) CENPV, (C) ISG20L2, (D) PHB,
(E) GCSH, (F) HSF2, (G) ZNF19, (H)
TSSC1, (I) ECHS1 and (J) NABP2. IDH2,
isocitrate dehydrogenase 2; SF3A1, splicing factor 3a
subunit 1; CENPV, centromere protein V; ISG20L2,
interferon stimulated exonuclease gene 20 like 2; PHB,
prohibitin; GCSH, glycine cleavage system protein H;
HSF2, heat shock transcription factor 2; ZNF19, zinc
finger protein 19; TSSC1, EARP complex and GARP complex
interacting protein 1; ECHS1, enoyl-CoA hydratase, short
chain 1; NABP2, nucleic acid binding protein 2. |
 | Table II.The top 20 GO enriched and KEGG
enriched IDH2 co-expression genes. |
Table II.
The top 20 GO enriched and KEGG
enriched IDH2 co-expression genes.
| Term ID | Description | Gene count | P-value |
|---|
| GO |
|
|
|
|
GO:0006364 | rRNA
processing | 66 | 1.29E-48 |
|
GO:0006614 | SRP-dependent
cotranslational protein targeting to membrane | 44 | 2.46E-41 |
|
GO:0019083 | Viral
transcription | 45 | 1.06E-38 |
|
GO:0000184 | Nuclear-transcribed
mRNA catabolic process, nonsense-mediated decay | 46 | 1.12E-38 |
|
GO:0006412 | Translation | 61 | 5.18E-38 |
|
GO:0006413 | Translational
initiation | 47 | 8.97E-37 |
|
GO:0002181 | Cytoplasmic
translation | 12 | 3.09E-11 |
|
GO:0000398 | mRNA splicing, via
spliceosome | 26 | 6.08E-09 |
|
GO:0000027 | Ribosomal large
subunit assembly | 9 | 6.47E-08 |
|
GO:0071353 | Cellular response
to interleukin-4 | 8 | 3.53E-06 |
|
GO:0006281 | DNA repair | 21 | 1.59E-05 |
|
GO:0016032 | Viral process | 24 | 1.94E-05 |
|
GO:0008380 | RNA splicing | 17 | 2.45E-05 |
|
GO:0098609 | Cell-cell
adhesion | 22 | 3.99E-05 |
|
GO:0042274 | Ribosomal small
subunit biogenesis | 6 | 6.48E-05 |
|
GO:0051170 | Nuclear import | 6 | 1.21E-04 |
|
GO:0006260 | DNA
replication | 15 | 1.57E-04 |
|
GO:0000028 | Ribosomal small
subunit assembly | 6 | 1.61E-04 |
|
GO:0006405 | RNA export from
nucleus | 9 | 1.64E-04 |
|
GO:0006397 | mRNA
processing | 16 | 2.13E-04 |
| KEGG |
|
|
|
|
hsa03010 | Ribosome | 52 | 4.30E-40 |
|
hsa03040 | Spliceosome | 18 | 4.17E-06 |
|
hsa03008 | Ribosome biogenesis
in eukaryotes | 12 | 2.38E-04 |
|
hsa03030 | DNA
replication | 7 | 0.001519 |
|
hsa03013 | RNA transport | 14 | 0.008172 |
|
hsa03410 | Base excision
repair | 5 | 0.028527 |
|
hsa04130 | SNARE interactions
in vesicular transport | 5 | 0.031456 |
|
hsa00260 | Glycine, serine and
threonine metabolism | 5 | 0.048668 |
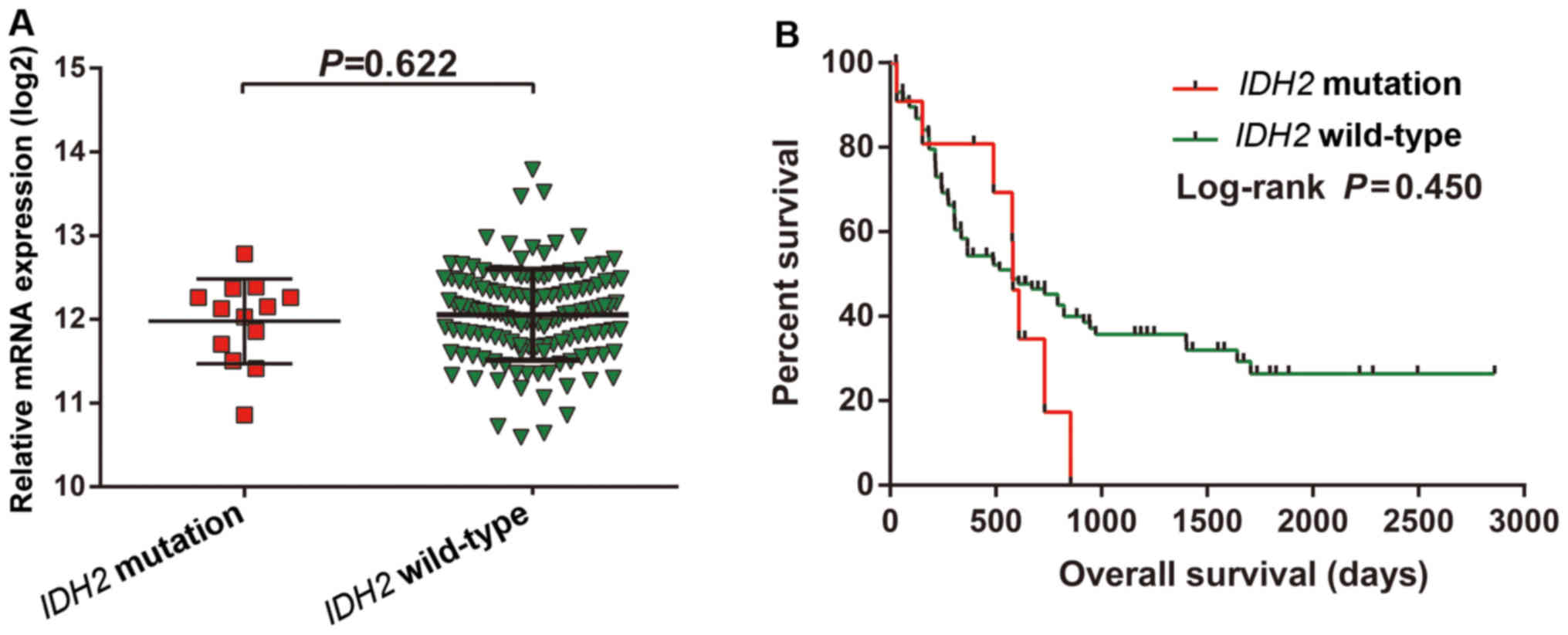
Survival analysis of IDH2
To better understand the role of the IDH2
R140Q mutation in AML, we compared the expression levels of the
wild-type and mutated IDH2, and observed no significant
differences (Fig. 11A).
Furthermore, the OS of the AML patients harboring the wild-type or
IDH2 R140Q mutant was also not significantly different
(Fig. 11B).
Discussion
Although studies have reported fewer mutations in
acute myeloid leukemia (AML) compared to other cancers, those
specifically related to AML pathogenesis and prognoses have not
been fully elucidated. An integrative analysis based on
multi-genomic data is necessary to determine the relationship
between specific gene mutations and cancer progression. Isocitrate
dehydrogenase (IDH) is a family of enzymes that catalyze the
oxidative decarboxylation of isocitrate to α-ketoglutarate and
NADPH in the tricarboxylic acid (TCA) cycle. The human genome has
five IDH genes that encode three isozymes, IDH1, IDH2 and IDH3 of
which IDH2 is mitochondrial and frequently mutated in
different cancers, including AML, brain tumors and gliomas
(4). The most common mutations in
IDH are located at R132 (IDH1), R140 and R172 (IDH2)
(3,8,37). In
the present study, the RNA-seq dataset from The Cancer Genome Atlas
(TCGA) was utilized to identify the key genes and pathways
associated with the IDH2 R140Q mutation in adult de
novo AML for the first time using multiple bioinformatic
methods. In addition, potential small-molecule drugs targeting the
mutated IDH2 were identified.
Functional assessment of the IDH2
R140Q-specific differentially expressed genes (DEGs) showed
significant association with angiogenesis, cell differentiation,
cell-matrix adhesion, homophilic cell adhesion via plasma membrane
adhesion molecules and cell junction. Previous studies have shown a
prognostic role of marrow angiogenesis-associated factors in AML
patients, and a promising therapeutic role of angiogenesis
inhibition (38–40). IDH mutations induce tumorigenesis by
epigenetic alterations, as well as by disrupting the tricarboxylic
acid (TCA) cycle and activating hypoxia-related signaling pathways,
which increase anabolic processes and angiogenesis (41,42).
Functional assessment of IDH2 co-expressing genes showed
enrichment in DNA replication, DNA repair, cell-cell adhesion, cell
division, and cell cycle-related processes. Therefore, the
biological and clinical manifestations of IDH2 R140Q
mutation are distinct from wild-type AML at the cellular level.
Further studies are needed to validate the functions of these
IDH2 co-expressing genes.
Among the hub differentially expressed genes (DEGs)
identified by protein-protein interaction (PPI) and gene-gene
interaction (GGI), several have been correlated to AML, especially
in patients with numerous mutations. Previous studies have
identified MYH9 as a direct target of RUNX1, an
important transcription factor that regulates the differentiation
of hematopoietic stem cells into mature blood cells, and is
frequently mutated in AML (43,44).
Zhang et al reported downregulation of CCND1 in an AML cell
line treated with amifostine, and may therefore be its direct
target (45). AXL is
essential for the constitutive Fms-like tyrosine kinase-3
(FLT3) phosphorylation in FLT3-internal tandem
duplication AML, which is responsible for the aberrant blast cell
proliferation and the clinical outcome of AML, and its inhibition
decreases FLT3 phosphorylation (46). Therefore, AXL is a potential
target for treating FLT3-mutated AML, and its specific
inhibitor BGB324 has been tested against leukemia cell
proliferation and therapy resistance (47,48).
AKAP12 is a tumor-suppressor gene that is inactivated in
childhood myeloid malignancies via epigenetic silencing through
promoter DNA methylation (49).
SF3A1 is a core spliceosomal gene that plays a fundamental
role in the processing of nascent RNA transcripts. Although a
somatic mutation of SF3A1 has been reported in AML, its
rarity has precluded analysis of its function and molecular
mechanism (50,51). In the present study, the prognostic
DEGs and IDH2 co-expressing genes were identified based on a
single cohort of TCGA. Therefore, our results have to be verified
in additional larger cohorts with complete clinical parameters.
Although we identified 13 candidate drugs targeting
the IDH2 R140Q mutation, we could not find any evidence of
their application against AML that were based on previous studies.
Vinblastine is one of the most widely used plant-derived
chemotherapeutic agents used to treat cancers, including AML. The
combination of cytosine arabinoside, VP 16–213, vincristine and
vinblastine (A-triple-V) is used to treat AML relapse, and results
in complete remission in most cases (52,53).
Finasteride is a 5-a-reductase inhibitor used for the treatment of
alopecia and prostate cancer. Chau et al demonstrated that
while finasteride may not have a dose-dependent effect on prostate
cancer, it may decrease the risk (54). One of the potential mechanisms of
finasteride action in prostate cancer is the inhibition of cancer
cell invasion and metastasis by downregulation of matrix
metalloproteinase (MMP)2 and MMP9 (55). Wiebe et al reported that
progesterone-induced stimulation of mammary tumorigenesis can also
be inhibited by finasteride (56).
In addition, it also decreased melanogenesis in both melanocytes
and melanoma cells by inhibiting adenylate cyclase and the
melanocortin 1 receptor (57). The
potential therapeutic effects of these drugs on AML need to be
studied further.
The present study has some limitations that need to
be clarified. First, our results are from a single cohort of TCGA
and generated by bioinformatic analysis, and thus need to be
verified in additional cohorts, as well as in experimental studies,
such as in vitro validation. Second, due to the relatively
small sample size of our cohort, and only 13 patients with the
IDH2 R140Q mutation, the results generated in this study
still need to be further verified using a larger sample size in
future research. Third, we did not observe any significant
differences in the IDH2 mRNA expression levels and OS
duration between the IDH2 R140Q mutant and wild-type AML
patients, which may be due to the small sample size. Despite these
limitations, we identified multiple genes associated with the
IDH2 R140Q mutation, and its potential molecular mechanisms
in adult de novo AML. In addition, we also identified 13
drugs specific for IDH2 R140Q-mutated AML, which once
verified can be used for AML patients harboring this mutation.
In conclusion, we identified several prognostic
biomarkers and potential molecular mechanisms, that may play an
essential role in IDH2 R140Q-mutated adult de novo
AML and improve prognosis, as well as 13 candidate drugs for
IDH2 R140Q-mutated adult de novo AML. However, our
findings need to be verified by further experimental and clinical
studies for future clinical application.
Supplementary Material
Supporting Data
Acknowledgements
The authors thank the contributors of The Cancer
Genome Atlas (https://portal.gdc.cancer.gov/) and the cBioPortal
for Cancer Genomics (http://www.cbioportal.org/index.do) for their
contribution to share the AML dataset on open access. In addition,
we also would like to acknowledge the helpful comments on this
paper received from our reviewers.
Funding
The present study was supported in part by the
National Natural Science Foundation of China (no. 81160075), the
Natural Science Foundation of Guangxi (no. 0728124) and the
Self-raised Scientific Research Fund of the Ministry of Health of
Guangxi Province (Z2014035).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable request.
All raw IDH2 mutation and RNA-seq dataset of AML, which were
included in the present study, can be downloaded from TCGA
(https://portal.gdc.cancer.gov/) and
cBioPortal for Cancer Genomics (http://www.cbioportal.org/index.do).
Authors' contributions
RH, XiL and QL acquired the data and created a
draft of the manuscript; RH, XiL, JL, JW, XS, XiaL, BL, FZ, YH and
QL conducted and further performed the study, processed and
analyzed the data; RH and QL revised and approved the final version
of the manuscript. All authors read and approved the manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
This study does not contain any studies with human
participants or animals performed by any of the authors. Since all
AML dataset included in this manuscript were obtained from The
Cancer Genome Atlas and cBioPortal for Cancer Genomics, therefore,
additional approval by an Ethics Committee is not necessary. In
addition, the procedures of this manuscript were in accordance with
the Helsinki declaration of 1964 and its later amendments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Professor Qiaochuan Li: ORCID: https://orcid.org/0000-0001-6255-8155.
References
|
1
|
Short NJ, Rytting ME and Cortes JE: Acute
myeloid leukaemia. Lancet. 392:593–606. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Estey E and Döhner H: Acute myeloid
leukaemia. Lancet. 368:1894–1907. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Paschka P, Schlenk RF, Gaidzik VI, Habdank
M, Krönke J, Bullinger L, Späth D, Kayser S, Zucknick M, Götze K,
et al: IDH1 and IDH2 mutations are frequent genetic alterations in
acute myeloid leukemia and confer adverse prognosis in
cytogenetically normal acute myeloid leukemia with NPM1 mutation
without FLT3 internal tandem duplication. J Clin Oncol.
28:3636–3643. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dang L, Yen K and Attar EC: IDH mutations
in cancer and progress toward development of targeted therapeutics.
Ann Oncol. 27:599–608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shih AH, Meydan C, Shank K,
Garrett-Bakelman FE, Ward PS, Intlekofer AM, Nazir A, Stein EM,
Knapp K, Glass J, et al: Combination targeted therapy to disrupt
aberrant oncogenic signaling and reverse epigenetic dysfunction in
IDH2- and TET2-mutant acute myeloid leukemia. Cancer Discov.
7:494–505. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Green CL, Evans CM, Zhao L, Hills RK,
Burnett AK, Linch DC and Gale RE: The prognostic significance of
IDH2 mutations in AML depends on the location of the mutation.
Blood. 118:409–412. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
DiNardo CD, Ravandi F, Agresta S,
Konopleva M, Takahashi K, Kadia T, Routbort M, Patel KP, Mark
Brandt, Pierce S, et al: Characteristics, clinical outcome, and
prognostic significance of IDH mutations in AML. Am J Hematol.
90:732–736. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chao HY, Jia ZX, Chen T, Lu XZ, Cen L,
Xiao R, Jiang NK, Ying JH, Zhou M and Zhang R: IDH2 mutations are
frequent in Chinese patients with acute myeloid leukemia and
associated with NPM1 mutations and FAB-M2 subtype. Int J Lab
Hematol. 34:502–509. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wiseman DH, Williams EL, Wilks DP, Sun
Leong H, Somerville TD, Dennis MW, Struys EA, Bakkali A, Salomons
GS and Somervaille TC: Frequent reconstitution of IDH2(R140Q)
mutant clonal multilineage hematopoiesis following chemotherapy for
acute myeloid leukemia. Leukemia. 30:1946–1950. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang R, Liao X and Li Q: Identification
of key pathways and genes in TP53 mutation acute myeloid leukemia:
Evidence from bioinformatics analysis. OncoTargets Ther.
11:163–173. 2017. View Article : Google Scholar
|
|
11
|
Huang R, Liao X and Li Q: Identification
and validation of potential prognostic gene biomarkers for
predicting survival in patients with acute myeloid leukemia.
OncoTargets Ther. 10:5243–5254. 2017. View Article : Google Scholar
|
|
12
|
Ley TJ, Miller C, Ding L, Raphael BJ,
Mungall AJ, Robertson A, Hoadley K, Triche TJ Jr, Laird PW, Baty
JD, et al Cancer Genome Atlas Research Network, : Genomic and
epigenomic landscapes of adult de novo acute myeloid leukemia. N
Engl J Med. 368:2059–2074. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6:pl12013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A Bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McCarthy DJ, Chen Y and Smyth GK:
Differential expression analysis of multifactor RNA-Seq experiments
with respect to biological variation. Nucleic Acids Res.
40:4288–4297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liao X, Yang C, Huang R, Han C, Yu T,
Huang K, Liu X, Yu L, Zhu G, Su H, et al: Identification of
potential prognostic long non-coding rna biomarkers for predicting
survival in patients with hepatocellular carcinoma. Cell Physiol
Biochem. 48:1854–1869. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei HT, Guo EN, Liao XW, Chen LS, Wang JL,
Ni M and Liang C: Genome-scale analysis to identify potential
prognostic microRNA biomarkers for predicting overall survival in
patients with colon adenocarcinoma. Oncol Rep. 40:1947–1958.
2018.PubMed/NCBI
|
|
19
|
Huang W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiao X, Sherman BT, Huang W, Stephens R,
Baseler MW, Lane HC and Lempicki RA: DAVID-WS: A stateful web
service to facilitate gene/protein list analysis. Bioinformatics.
28:1805–1806. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maere S, Heymans K and Kuiper M: BiNGO: A
Cytoscape plugin to assess overrepresentation of gene ontology
categories in biological networks. Bioinformatics. 21:3448–3449.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Szklarczyk D, Morris JH, Cook H, Kuhn M,
Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, et al:
The STRING database in 2017: Quality-controlled protein-protein
association networks, made broadly accessible. Nucleic Acids Res.
45(D1): D362–D368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
von Mering C, Jensen LJ, Snel B, Hooper
SD, Krupp M, Foglierini M, Jouffre N, Huynen MA and Bork P: STRING:
Known and predicted protein-protein associations, integrated and
transferred across organisms. Nucleic Acids Res. 33:D433–D437.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Warde-Farley D, Donaldson SL, Comes O,
Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT,
et al: The GeneMANIA prediction server: Biological network
integration for gene prioritization and predicting gene function.
Nucleic Acids Res. 38 (Suppl 2):W214–W220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mostafavi S, Ray D, Warde-Farley D,
Grouios C and Morris Q: GeneMANIA: A real-time multiple association
network integration algorithm for predicting gene function. Genome
Biol. 9 (Suppl 1):S42008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lamb J, Crawford ED, Peck D, Modell JW,
Blat IC, Wrobel MJ, Lerner J, Brunet JP, Subramanian A, Ross KN, et
al: The Connectivity Map: Using gene-expression signatures to
connect small molecules, genes, and disease. Science.
313:1929–1935. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lamb J: The Connectivity Map: A new tool
for biomedical research. Nat Rev Cancer. 7:54–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim S, Thiessen PA, Bolton EE, Chen J, Fu
G, Gindulyte A, Han L, He J, He S, Shoemaker BA, et al: PubChem
substance and compound databases. Nucleic Acids Res.
44:D1202–D1213. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Bryant SH, Cheng T, Wang J,
Gindulyte A, Shoemaker BA, Thiessen PA, He S and Zhang J: PubChem
BioAssay: 2017 update. Nucleic Acids Res. 45:D955–D963. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuhn M, von Mering C, Campillos M, Jensen
LJ and Bork P: STITCH: Interaction networks of chemicals and
proteins. Nucleic Acids Res. 36:D684–D688. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kuhn M, Szklarczyk D, Franceschini A,
Campillos M, von Mering C, Jensen LJ, Beyer A and Bork P: STITCH 2:
An interaction network database for small molecules and proteins.
Nucleic Acids Res. 38 (Suppl 1):D552–D556. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Szklarczyk D, Santos A, von Mering C,
Jensen LJ, Bork P and Kuhn M: STITCH 5: Augmenting protein-chemical
interaction networks with tissue and affinity data. Nucleic Acids
Res. 44:D380–D384. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Benjamini Y and Hochberg Y: Controlling
the false discovery rate: A practical and powerful approach to
multiple testing. J R Stat Soc Series B Stat Methodol. 57:289–300.
1995.
|
|
35
|
Reiner A, Yekutieli D and Benjamini Y:
Identifying differentially expressed genes using false discovery
rate controlling procedures. Bioinformatics. 19:368–375. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Benjamini Y, Drai D, Elmer G, Kafkafi N
and Golani I: Controlling the false discovery rate in behavior
genetics research. Behav Brain Res. 125:279–284. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ahmad F, Mohota R, Sanap S, Mandava S and
Das BR: Molecular evaluation of DNMT3A and IDH1/2 gene mutation:
Frequency, distribution pattern and associations with additional
molecular markers in normal karyotype Indian acute myeloid leukemia
patients. Asian Pac J Cancer Prev. 15:1247–1253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee CY, Tien HF, Hu CY, Chou WC and Lin
LI: Marrow angiogenesis-associated factors as prognostic biomarkers
in patients with acute myelogenous leukaemia. Br J Cancer.
97:877–882. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Trujillo A, McGee C and Cogle CR:
Angiogenesis in acute myeloid leukemia and opportunities for novel
therapies. J Oncol. 2012:1286082012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hussong JW, Rodgers GM and Shami PJ:
Evidence of increased angiogenesis in patients with acute myeloid
leukemia. Blood. 95:309–313. 2000.PubMed/NCBI
|
|
41
|
Krell D, Mulholland P, Frampton AE, Krell
J, Stebbing J and Bardella C: IDH mutations in tumorigenesis and
their potential role as novel therapeutic targets. Future Oncol.
9:1923–1935. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schaap FG, French PJ and Bovée JV:
Mutations in the isocitrate dehydrogenase genes IDH1 and IDH2 in
tumors. Adv Anat Pathol. 20:32–38. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bluteau D, Glembotsky AC, Raimbault A,
Balayn N, Gilles L, Rameau P, Nurden P, Alessi MC, Debili N,
Vainchenker W, et al: Dysmegakaryopoiesis of FPD/AML pedigrees with
constitutional RUNX1 mutations is linked to myosin II deregulated
expression. Blood. 120:2708–2718. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Okuda T, Nishimura M, Nakao M and Fujita
Y: RUNX1/AML1: A central player in hematopoiesis. Int J Hematol.
74:252–257. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang F, Yang B, Zhang K, Hou ML, Lu XC
and Li YX: CCND1-BCL2 Gene Network: A direct target of Amifostine
in human acute megakaryocytic leukemia cells. Chem Biol Drug Des.
89:681–693. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Park IK, Mishra A, Chandler J, Whitman SP,
Marcucci G and Caligiuri MA: Inhibition of the receptor tyrosine
kinase Axl impedes activation of the FLT3 internal tandem
duplication in human acute myeloid leukemia: Implications for Axl
as a potential therapeutic target. Blood. 121:2064–2073. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Janning M, Ben-Batalla I and Loges S: Axl
inhibition: A potential road to a novel acute myeloid leukemia
therapy? Expert Rev Hematol. 8:135–138. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ben-Batalla I, Schultze A, Wroblewski M,
Erdmann R, Heuser M, Waizenegger JS, Riecken K, Binder M, Schewe D,
Sawall S, et al: Axl, a prognostic and therapeutic target in acute
myeloid leukemia mediates paracrine crosstalk of leukemia cells
with bone marrow stroma. Blood. 122:2443–2452. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Flotho C, Paulun A, Batz C and Niemeyer
CM: AKAP12, a gene with tumour suppressor properties, is a target
of promoter DNA methylation in childhood myeloid malignancies. Br J
Haematol. 138:644–650. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yoshida K, Sanada M, Shiraishi Y, Nowak D,
Nagata Y, Yamamoto R, Sato Y, Sato-Otsubo A, Kon A, Nagasaki M, et
al: Frequent pathway mutations of splicing machinery in
myelodysplasia. Nature. 478:64–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Larsson CA, Cote G and Quintás-Cardama A:
The changing mutational landscape of acute myeloid leukemia and
myelodysplastic syndrome. Mol Cancer Res. 11:815–827. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sauter C, Fehr J, Frick P, Gmuer J,
Honegger H and Martz G: Acute myelogenous leukemia: Successful
treatment of relapse with cytosine arabinoside, VP 16–213,
vincristine and vinblastine (A-triple-V). Eur J Cancer Clin Oncol.
18:733–737. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Müller MR, Sauter C, Erni J and Martz G:
Influence of a new relapse treatment for acute myeloid leukemia
(AML) on in vitro granulopoiesis. Anticancer Res. 3:127–131.
1983.PubMed/NCBI
|
|
54
|
Chau CH, Price DK, Till C, Goodman PJ,
Chen X, Leach RJ, Johnson-Pais TL, Hsing AW, Hoque A, Tangen CM, et
al: Finasteride concentrations and prostate cancer risk: Results
from the Prostate Cancer Prevention Trial. PLoS One.
10:e01266722015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Moroz A, Delella FK, Almeida R, Lacorte
LM, Fávaro WJ, Deffune E and Felisbino SL: Finasteride inhibits
human prostate cancer cell invasion through MMP2 and MMP9
downregulation. PLoS One. 8:e847572013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wiebe JP, Rivas MA, Mercogliano MF,
Elizalde PV and Schillaci R: Progesterone-induced stimulation of
mammary tumorigenesis is due to the progesterone metabolite,
5α-dihydroprogesterone (5αP) and can be suppressed by the
5α-reductase inhibitor, finasteride. J Steroid Biochem Mol Biol.
149:27–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Seo JO, Yumnam S, Jeong KW and Kim SY:
Finasteride inhibits melanogenesis through regulation of the
adenylate cyclase in melanocytes and melanoma cells. Arch Pharm
Res. 41:324–332. 2018. View Article : Google Scholar : PubMed/NCBI
|