Introduction
Glioblastomas (GBM, gliomas) are primary brain
tumours of glial origin. They are the most common central nervous
system neoplasms in adults. Each year, 5–6 of 100,000 individuals
are diagnosed with primary malignant brain tumours, of which ~80%
are malignant gliomas and more than half of these are glioblastomas
(1,2). There is a slight male predominance and
individuals between 45–70 years of age are mainly affected
(3). Despite the aggressiveness in
approach which includes surgical resection, irradiation and
chemotherapy, GBM is an aggressive neoplasm associated with high
mortality resulting from infiltrative growth and recurrence with a
uniformly fatal course.
Temozolomide (TMZ) is an alkylating agent used for
GBM treatment (4). The approved
dosage is 150–200 mg/square metre of body surface area, daily for 5
days of every 28-day cycle. A dosage of 75 mg/square metre for up
to 49 days is safe (5); this extent
of exposure to TMZ will damage the DNA repair enzyme encoded in the
human as O6-methylguanine-DNA methyltransferase (MGMT)
(4,6), but overexpression of MGMT in tumour
cells confers resistance to TMZ and impairs therapeutic
outcome.
The Hedgehog (Hh) signalling pathway was originally
discovered in Drosophila, and regulates embryonic segment
development (7). Hh signalling
plays a crucial role in GBM tumour progression and pathogenesis.
Its activation is mediated by sonic Hedgehog (Shh), which binds to
its receptor patched (PTCH) to promote GLI1 activation. Activation
of Hh/GLI1 thus promotes the resistance of glioma stem cells to TMZ
(8,9).
Arsenic trioxide (As2O3, ATO),
a Hh pathway inhibitor (10,11),
is used as a therapeutic agent for acute promyelocytic leukaemia
(APL) (12). It has also been
reported to show a substantial effect in a wide range of other
solid tumours including oesophageal (13), lung (14), liver (15), cervical cancer (16), prostate carcinoma (17) and osteosarcoma (18). Regardless of how sensitive different
types of tumour cells are to this drug, there is a limitation in
its clinical application in a wide range of haematological
malignancies and solid tumours (19,20).
Vismodegib (VIS) is a small molecule inhibitor of
smoothened (SMO). In the absence of PTCH1, VIS binds to SMO and
inhibits the atypical activation of the Hh pathway (21).
In clinical practice, combination therapy is often
used to enhance the cytotoxicity and reduce the adverse effects of
chemotherapeutic drugs (19,22).
In the present study, we demonstrated that the combination of VIS,
ATO and TMZ suppressed the growth of GBM.
Materials and methods
Cell line and reagents
Glioblastoma of unknown origin (GUO) [U-87MG
(ATCC® HTB-14™) (RRID:CVCL_0022)] and U138MG human
malignant GBM cell lines were purchased from the American Type
Culture Collection (ATCC; Manassas, VA, USA), while U251MG was
purchased from the Health Science Research Resource Bank (Osaka,
Japan). All cell lines grown as monolayer cultures in minimum
essential medium (MEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) were supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.) in
a humidified atmosphere containing 5% CO2 at 37°C.
Temozolomide (TMZ) was purchased from LKT
Laboratories Inc. (St. Paul, MN, USA), arsenic trioxide (ATO) was
from Nihon Shinyaku Co., Ltd. (Kyoto, Japan) and vismodegib (VIS)
was obtained from LC Laboratories (Woburn, MA, USA).
Cell viability assay
Cells were seeded at a density of 103
cells/well in 96-well plates and treated with vehicle, 1 or 3 µM of
ATO, 20 or 50 µM of VIS, and 300 or 1,000 µM of TMZ. Cell viability
was assessed by adding to each well 10 µl of a tetrazolium salt
(WST-1) (Roche Diagnostics, Basel, Switzerland) which was cleaved
by mitochondrial dehydrogenase activity (18). Fluorescence intensity was measured
after 2 h on a microplate reader.
Western blot assay
Briefly, cells were seeded at a density of
105 cells/well in 6-well plates with vehicle, or 1 µM
ATO, 30 µM VIS, or 300 and 600 µM TMZ in single or in combination
of ATO and TMZ and a combination of VIS and TMZ for 48 h, washed
with phosphate-buffered saline (PBS) and lysed using Mammalian
Protein Extraction reagent (Thermo Fisher Scientific, Inc.), 3 mM
p-APMSF (Wako Chemicals, Kanagawa, Japan) and 5 mg/ml aprotinin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The lysates were
centrifuged at 14,000 × g for 10 min at 4°C. Protein were
determined by bicinchoninic acid (BCA) reagents A and B at 50:1.
SDS-PAGE (4–15%) was conducted using 10 µg of each protein followed
by immunoblotting on polyvinylidene difluoride (PVDF) membrane
(Bio-Rad Laboratories, Hercules, CA, USA). Blocking was carried out
with 5% skim milk for 1 h followed by incubation at 4°C with the
following antibodies: γH2AX (cat. no. 2577), H2AX (cat. no. 2595),
cleaved caspase-3 (cat. no. 9664) and caspase-3 (cat. no. 9665; all
from Cell Signaling Technology Japan K.K., Tokyo, Japan) (23–26) at
a dilution of 1:1,000 overnight and alpha-tubulin (cat. no.
HRP-66031; ProteinTech Group, Inc., Rosemont, IL, USA) at a
dilution of 1:5,000 for 1 h. Incubation at room temperature with
horseradish peroxidase-conjugated anti-rabbit secondary antibodies
(cat. no. 7074) or anti-mouse (cat. no. 7076) (Cell Signaling
Technology Japan K.K., Tokyo, Japan) for 1 h at a dilution of
1:4,000. Signals were analyzed using ECL Western Blotting reagent
(Amersham; GE Healthcare Life Sciences, Little Chalfont, UK) and
LAS 4000 Mini image analyzer (Fujifilm, Tokyo, Japan).
Drug combination studies
GUO, U251MG and U138MG cells were seeded in 96-well
plates and treated with vehicle, single drug or a fixed drug ratio
of the combined drugs. ATO and TMZ was used at 1:320, VIS and TMZ
was used at 1:10. Cell viability was assessed by WST-1 assay. The
CalcuSyn (version 2.11; Biosoft, Ferguson, MO, USA) median effect
model was used to calculate the CI values and to analyse whether
the drug combinations were synergistic, antagonistic, or additive.
CI value of <1 indicates synergism, CI=1 indicates additivity,
and CI >1 indicates antagonism (27).
Animal studies
Four-week-old male nude mice weighing 20 g (Japan
SLC Inc., Hamamatsu, Japan) were used in the present study. Animal
care and experimental procedures were specifically approved and
carried out in accordance with the guidelines of the Institute of
Laboratory Animal Sciences, Graduate School of Medical and Dental
Sciences, Kagoshima University (Kagoshima, Japan) (no. MD
17101).
The animals were kept in a pathogen-free
environment, with 12-h light/dark cycle at 24°C, 40–70% relative
humidity and a free access to food and water ad libitum.
They were allowed to habituate for 7 days prior to tumour
inoculation. Briefly, 1×107 GUO tumour cells in 50 µl
MEM lacking FBS and antibiotics combined with 50 µl Matrigel
(Corning Life Sciences, Tewksbury MA, USA) were inoculated
subcutaneously into the flanks of nude mice. Tumours were allowed
to grow for 7 days, and then mice were randomly divided into the
control and the treatment groups (n=7 animals/group). They were
administered intraperitoneally (i.p) with either TMZ (10
mg/kg/daily), ATO (2.5 µg/g/daily), or VIS (25 mg/kg/day) or in
combination of ATO 2.5 µg/g/daily and TMZ 10 mg/kg/daily or VIS 25
mg/kg/daily and TMZ 10 mg/kg/daily, or with an equal volume of
vehicle as the control. These drug concentrations were selected
from published studies (8,14,15,22,28),
and after conducting a pilot study, we used the minimum effective
concentrations so as to be able to apply our results in clinical
settings. Injections were given 4 days a week for 2 weeks. Tumour
volumes were measured with callipers on alternative days with the
longest diameter being the length and the perpendicular diameter
being the width; volume was calculated using the formula (L ×
W2)/2. The maximum diameter exhibited by a single tumour
was 17 mm. Twenty four days after tumour inoculation, animals were
sacrificed by inhalation of CO2 at a rate of 10–30%/min
in an automatic euthanasia plastic chamber. The tumours were
excised, weighed, formalin-fixed and paraffin-embedded. Paraffin
sections (4 µm) were cut and stained with haematoxylin and eosin
(H&E) for light microscopic evaluation.
Statistical analysis
Statistical analyses were carried out using
Steel-Dwass test, as a post hoc test for pairwise comparisons
following a significant Kruskal-Wallis test with Excel Statistics
2013 (Microsoft Excel; Microsoft Corp., Redmond, WA, USA) and
KyPlot 5.0 (KyensLab Inc., Tokyo, Japan). P-values of <0.05 were
considered to indicate a statistically significant result.
Results
Single-agent efficacy of ATO, VIS and
TMZ on the growth of GBM cells
To examine the efficacy of ATO, VIS and TMZ on the
growth of GBM cells in vitro, GUO, U138MG and U251MG human
GBM cell lines, were used. WST-1 results showed that, there was a
dose-dependent inhibition in cell proliferation when all the cell
lines were treated with 1 or 3 µM of ATO although 1 µM ATO did not
show significant inhibition in the U251MG cell line (Fig. 1A). A concentration of 50 µM VIS was
significantly more effective in inhibiting the proliferation of the
GUO and U138MG and U251MG cells compared to 20 µM of VIS which did
not show a significant inhibitory effect when compared with the
vehicle control (Fig. 1B). TMZ when
used at a concentration of 300 or 1,000 µM significantly inhibited
the proliferation of GUO, U138MG and U251MG cells (Fig. 1C).
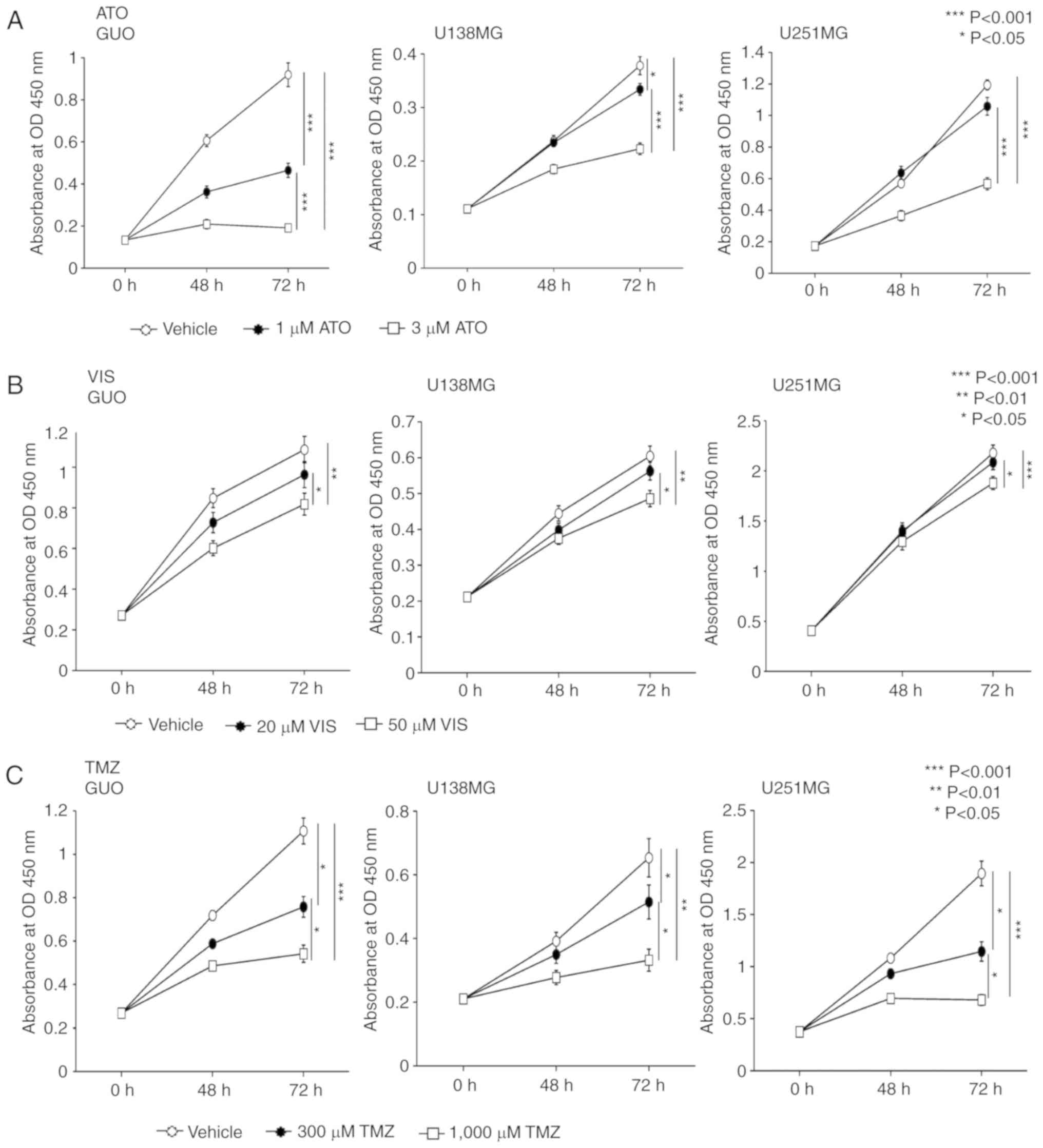 | Figure 1.ATO, VIS and TMZ hinder the growth of
human GBM cell lines, GUO, U138MG and U251MG. WST-1 assay
demonstrated that (A) 1 µM ATO significantly inhibited the growth
of GUO and U138MG cells but did not significantly inhibit the
proliferation of U251MG. Yet, 3 µM ATO significantly inhibited the
proliferation of all the cell lines used. WST-1 assay demonstrated
that (B) a concentration of 50 µM VIS was significantly more
effective in inhibiting the proliferation of the GUO and U138MG and
U251MG cells compared to 20 µM of VIS which showed only a slight
inhibitory effect when compared with the vehicle control. (C) WST-1
assay showed that treatment with 300 and 1,000 µM TMZ significantly
inhibited the growth of human GBM cells lines, GUO, U138MG and
U251MG cell lines in a dose-dependent manner. The experiment was
carried out in triplicate producing similar results. P<0.05 was
considered significant (Kruskal-Wallis test). Error bars represent
the mean ± SD. ATO, arsenic trioxide; VIS, vismodegib; TMZ,
temozolomide; GBM, glioblastoma; GUO, glioblastoma of unknown
origin; OD optical density. |
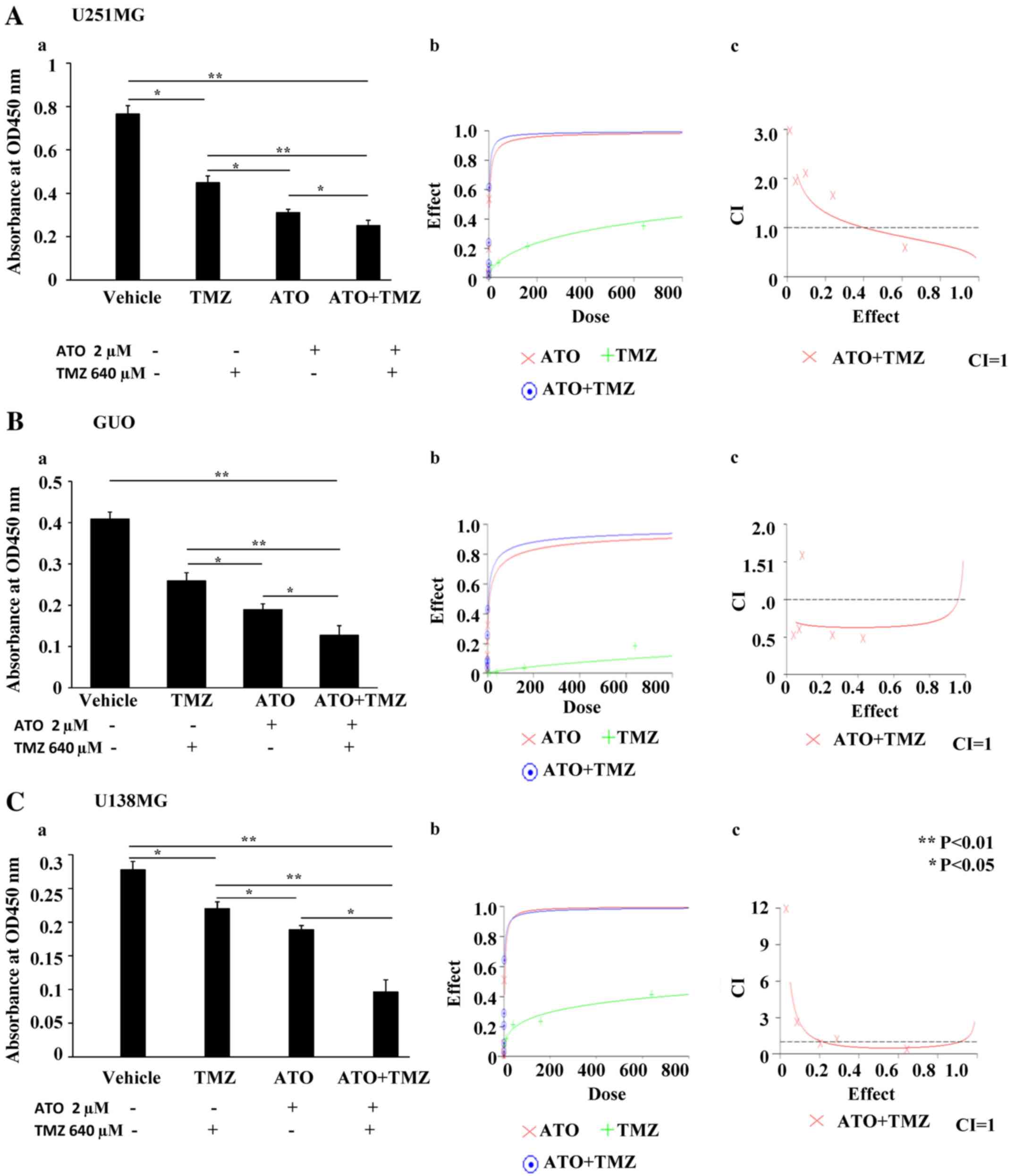
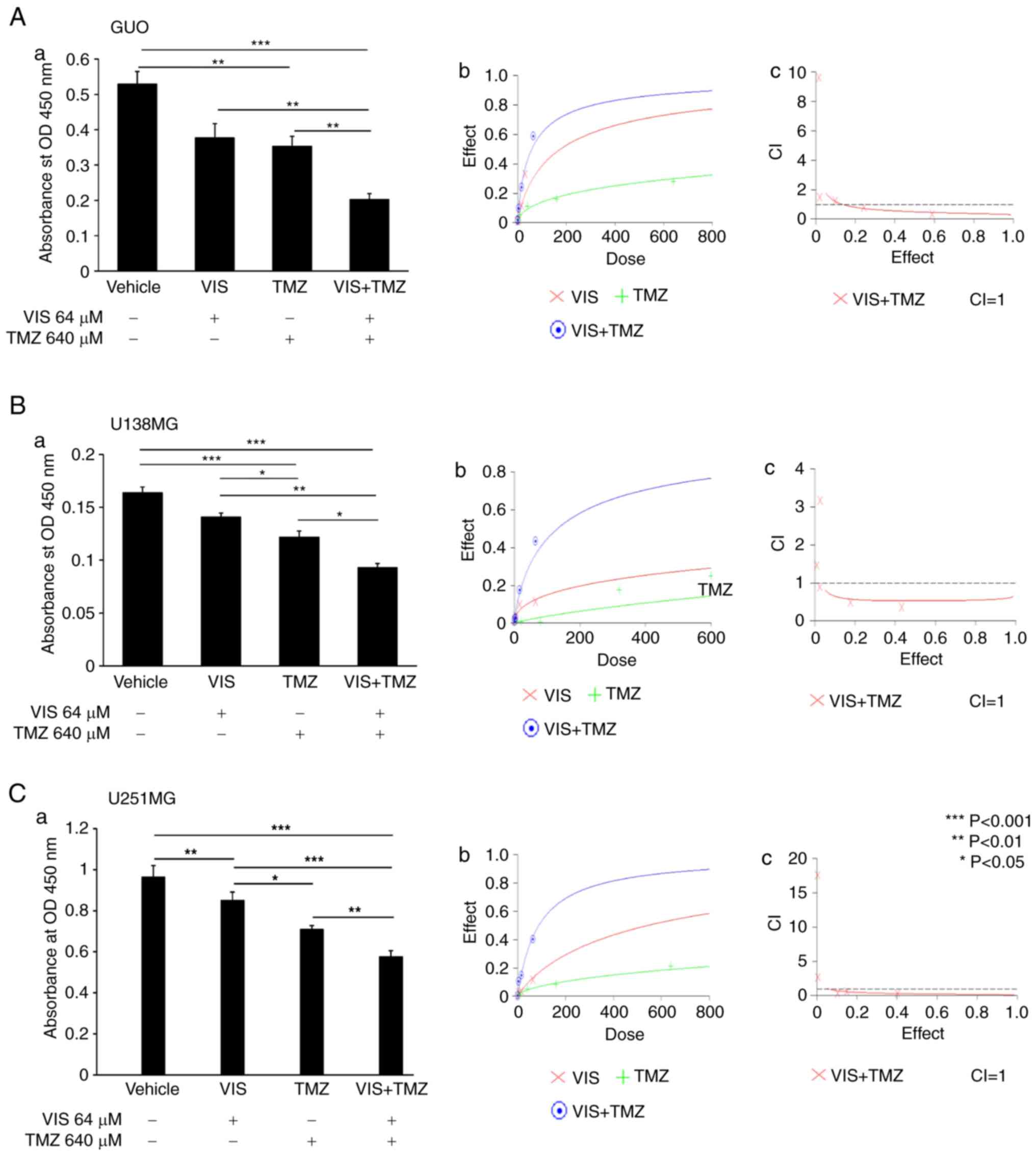
Combination of a Hedgehog (Hh)
inhibitor and a standard chemotherapeutic drug hinders the
proliferation of GBM cells in vitro
We then examined the effect of treating these cell
lines with Hh inhibitors VIS or ATO in combination with TMZ in a
dose-dependent manner. Five different concentrations were used at a
ratio of 1:320 for ATO:TMZ with the highest concentration being
2:640, and 1:10 for VIS:TMZ with the highest concentration being
64:640 (Table I). When combination
treatment was used, there was marked inhibition in the
proliferation of GBM cell lines, unlike with the use of the single
agents. This was shown by assessing the synergistic effect of these
drugs by CalcuSyn software version 2.11 (Figs. 2 and 3).
 | Table I.Combination index (CI) for a standard
anticancer drug when combined with a Hedgehog inhibitor. |
Table I.
Combination index (CI) for a standard
anticancer drug when combined with a Hedgehog inhibitor.
| Cell line | Drugs | CI
ED50 | CI
ED75 | CI
ED90 | Dm | m | r |
|---|
| U87MG | ATO+TMZ (CR
1:320) | 0.91 | 0.70 | 0.56 | 1.53 | 0.82 | 0.99 |
| U138MG | ATO+TMZ (CR
1:320) | 0.63 | 0.67 | 0.78 | 4.4 | 0.53 | 0.98 |
| U251MG | ATO+TMZ (CR
1:320) | 0.49 | 0.59 | 0.93 | 1.1 | 0.69 | 0.98 |
| U87MG | VIS+TMZ (CR
1:10) | 0.5 | 0.4 | 0.35 | 57.49 | 0.82 | 0.96 |
| U138MG | VIS+TMZ (CR
1:10) | 0.53 | 0.54 | 0.57 | 130.66 | 0.79 | 0.96 |
| U251MG | VIS+TMZ (CR
1:10) | 0.28 | 0.19 | 0.15 | 87.84 | 0.98 | 0.94 |
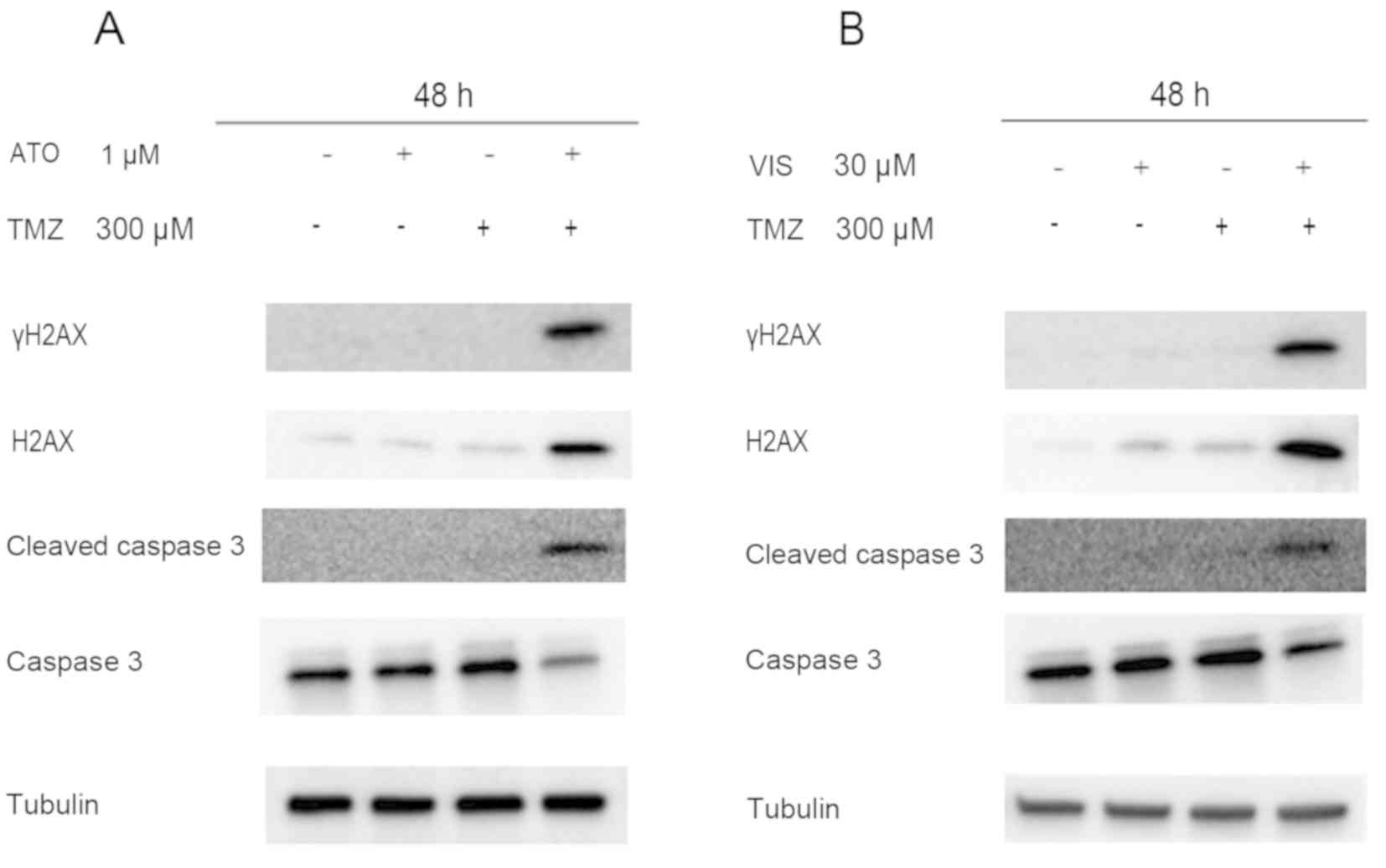
Combination of a Hedgehog (Hh)
inhibitor and standard anticancer drug triggered apoptosis of GBM
cells in vitro
We next examined the ability of 300 µM TMZ when
combined with 1 µM ATO/30 µM VIS to cause DNA damage and apoptosis
in GBM cells following treatment for 48 h. Western blot analyses
using γH2AX and cleaved caspase-3 revealed that there was higher
expression of γH2AX and cleaved caspase-3 when the drugs were
combined, unlike when they were used as single agents (Fig. 4).
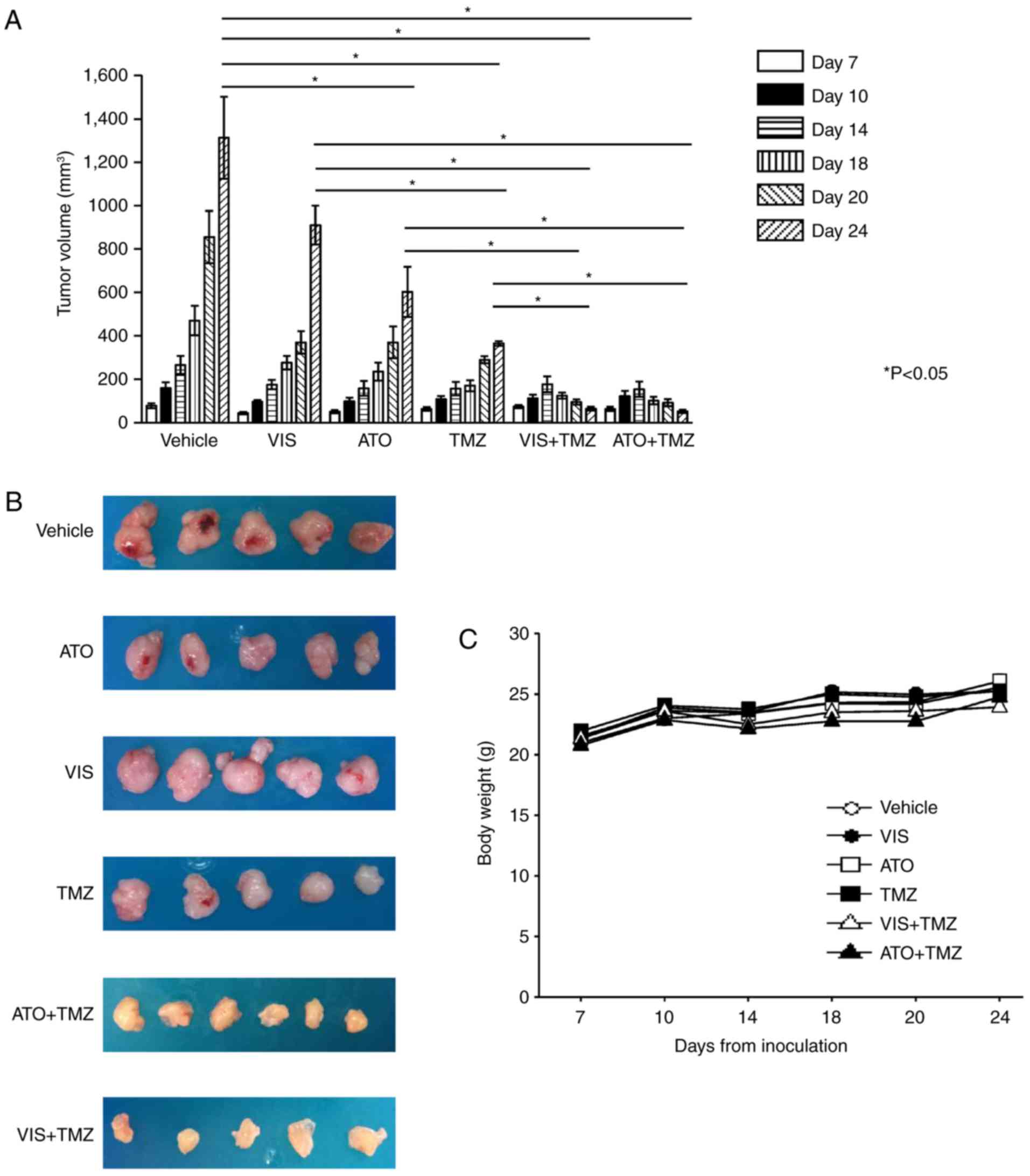
Combination of ATO/VIS and TMZ
prevents GBM proliferation in vivo
Mouse xenograft models showed that the combination
of ATO and TMZ, VIS and TMZ, significantly inhibited GBM
proliferation in vivo compared with the vehicle or single
drug administration (Fig. 5A and
B). We measured the body weight of the mice during the
treatments so as to assess the toxicity of these combination
treatments. We found that there was no significant difference
between the body weights of the control and the treatment groups
(Fig. 5C).
Discussion
The emergence of chemotherapeutic drug resistance is
a major limitation of therapy for glioblastoma (GBM) patients. In
spite of the fact that temozolomide (TMZ) is the standard regimen
for GBM, these tumours are highly resistant to chemotherapy
(3). On account of the mechanism
responsible for such resistance, several factors have been
stipulated, and DNA repair-related genes such as MGMT, MSH2
and MSH6 have been recognised as critical factors involved
in the survival of the tumour after treatment with alkylating
agents (29–31). MGMT expression is also
associated with GLI1 activity due to an apparent GLI1-binding site
in the MGMT gene promoter (32). Ulasov et al further
consolidated the possible link between Hedgehog (Hh) activity and
therapeutic resistance to TMZ by their experiments with
CD133+ glioma stem cells (33).
Arsenic trioxide (ATO) has been approved as an
anticancer agent for acute promyelocytic leukaemia (APL) by the
Food and Drug Administration (FDA) and Pharmaceutical and Medical
Devices Agency (PMDA) in Japan (34). Inhibition of the Hh pathway by ATO
could be a useful additional therapy to the standard chemotherapy
for GBM. Several mechanisms have been stipulated to account for the
inhibition of the Hh pathway by ATO. In a recent study, ATO was
shown to inhibit the transcription of GLI target genes and promote
apoptotic cell death in osteosarcoma cells due to increased DNA
damage (18). Other authors have
also reported inhibition of the expression of GLI2 and
downregulation of the expression of SMO and PTCH by
ATO (11,22,35).
Our findings delineate that ATO hindered the proliferation of GBM
cells both in vivo and in vitro.
Vismodegib (VIS) is the first Hh inhibitor to be
approved by the FDA for the treatment of basal cell carcinoma (BCC)
(36,37). Smoothened (SMO) inhibitors such as
VIS have been evaluated in recent clinical trials (37). Targeting the Hh pathway with VIS
blocks aberrant signalling caused by mutational inactivation of the
negative regulator PTCH1 or mutational activation of SMO (37,38).
Despite the impressive tumour regression achieved by
targeting the Hh pathway with ATO and VIS, resistance has also been
reported (39,40) thus, conferring the need for a
combination therapy (19).
The combination of ATO (Hh/GLI inhibitor) and
alkylating agents has been reported to synergistically inhibit the
proliferation of cells with inherited or acquired drug resistance
(41). Silencing of GLI1 in
GBM has also been reported to promote sensitivity to TMZ by broadly
reducing efflux behaviour attributed to multidrug transporters
(42). Our findings revealed that
combined treatment with either ATO and TMZ or VIS and TMZ was
better at inhibiting GBM growth in vitro and in vivo
than single-drug therapy. Among the two combination treatments, a
combination of ATO and TMZ has the most promising potential, due to
the effectiveness of ATO at a low concentration, compared to VIS.
We believe this is the first study to show the synergistic effect
of ATO/VIS with TMZ on GBM as determined by the CI-isobologram
method of Chou (43) and Chou and
Talalay (44).
Other authors including Nagao-Kitamoto et al
(22) and Saitoh et al
(27) also reported that combined
administration of VIS and ATO inhibited Hh pathway activation and
tumour growth compared with single-agent therapy. These
combinations could reduce the effective concentration of each drug
and hence decrease toxicity. In the present study, there was marked
inhibition of GBM growth when TMZ was combined with either ATO or
VIS.
GBM is a very heterogeneous and genomically unstable
tumour (45,46), hence posing the need to identify GBM
patients with activated Hh pathway before commencement of treatment
with Hh inhibitors. A recent study showed the usefulness of a
five-gene Hh signature that can strongly identify activated Hh in
medulloblastoma (47), and can thus
be used for screening patients who have high chances of benefiting
from Hh inhibitor therapies such as GBM patients. There is a high
likelihood that the pleiotropic effect of ATO and off-target
effects of SMO have a high possibility of affecting the growth
inhibition of GBM. However, combination of TMZ with either ATO or
VIS showed a promising therapeutic effect for GBM.
In conclusion, these findings denote that a
combination of Hh pathway inhibitors and TMZ may be an important
and safe therapeutic approach for the treatment of GBM.
Acknowledgements
We gratefully acknowledge the technical assistance
of Hui Gao from the Departments of Orthopaedic Surgery Graduate
School of Medical and Dental Sciences, Kagoshima University,
Kagoshima, Japan. We are also grateful to the Joint Research
Laboratory of Kagoshima University Graduate School of Medical and
Dental Sciences.
Funding
The present study was funded and supported by the
Japan's Ministry of Education, Culture, Sports, Science and
Technology (scholarship no. 150803).
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
CB and TS developed the experimental design,
conducted the experiments and drafted the manuscript. YS, HT, HS,
SM, SN, SK and NT collected, analysed and interpreted the data. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Animal care and experimental procedures were
specifically approved and carried out in accordance with the
guidelines of the Institute of Laboratory Animal Sciences, Graduate
School of Medical and Dental Sciences, Kagoshima University
(Kagoshima, Japan) (no. MD 17101).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stupp R, Tonn JC, Brada M and
Pentheroudakis G; ESMO Guidelines Working Group, : High-grade
malignant glioma: ESMO clinical practice guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 21 (Suppl 5):v190–v193. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schwartzbaum JA, Fisher JL, Aldape KD and
Wrensch M: Epidemiology and molecular pathology of glioma. Nat Clin
Pract Neurol. 2:494–503. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scorsetti M, Navarria P, Pessina F,
Ascolese AM, D'Agostino G, Tomatis S, De Rose F, Villa E, Maggi G,
Simonelli M, et al: Multimodality therapy approaches, local and
systemic treatment, compared with chemotherapy alone in recurrent
glioblastoma. BMC Cancer. 15:4862015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Newlands ES, Stevens MF, Wedge SR,
Wheelhouse RT and Brock C: Temozolomide: A review of its discovery,
chemical properties, pre-clinical development and clinical trials.
Cancer Treat Rev. 23:35–61. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brock CS, Newlands ES, Wedge SR, Bower M,
Evans H, Colquhoun I, Roddie M, Glaser M, Brampton MH and Rustin
GJ: Phase I trial of temozolomide using an extended continuous oral
schedule. Cancer Res. 58:4363–4367. 1998.PubMed/NCBI
|
|
6
|
Tolcher AW, Gerson SL, Denis L, Geyer C,
Hammond LA, Patnaik A, Goetz AD, Schwartz G, Edwards T, Reyderman
L, et al: Marked inactivation of O6-alkylguanine-DNA
alkyltransferase activity with protracted temozolomide schedules.
Br J Cancer. 88:1004–1011. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Amakye D, Jagani Z and Dorsch M:
Unraveling the therapeutic potential of the Hedgehog pathway in
cancer. Nat Med. 19:1410–1422. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang K, Chen D, Qian Z, Cui D, Gao L and
Lou M: Hedgehog/Gli1 signaling pathway regulates MGMT expression
and chemoresistance to temozolomide in human glioblastoma. Cancer
Cell Int. 17:1172017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Santoni M, Burattini L, Nabissi M, Morelli
MB, Berardi R, Santoni G and Cascinu S: Essential role of Gli
proteins in glioblastoma multiforme. Curr Protein Pept Sci.
14:133–140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beauchamp EM, Ringer L, Bulut G, Sajwan
KP, Hall MD, Lee YC, Peaceman D, Özdemirli M, Rodriguez O,
Macdonald TJ, et al: Arsenic trioxide inhibits human cancer cell
growth and tumor development in mice by blocking Hedgehog/GLI
pathway. J Clin Invest. 121:148–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang D, Cao F, Ye X, Zhao H, Liu X, Li Y,
Shi C, Wang H and Zhou J: Arsenic trioxide inhibits the hedgehog
pathway which is aberrantly activated in acute promyelocytic
leukemia. Acta Haematol. 130:260–267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Breccia M and Lo-Coco F: Arsenic trioxide
for management of acute promyelocytic leukemia: Current evidence on
its role in front-line therapy and recurrent disease. Expert Opin
Pharmacother. 13:1031–1043. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang J, Li H, Chen YY, Wang XJ, Shi GY, Hu
QS, Kang XL, Lu Y, Tang XM, Guo QS and Yi J: Anthraquinones
sensitize tumor cells to arsenic cytotoxicity in vitro and in vivo
via reactive oxygen species-mediated dual regulation of apoptosis.
Free Radic Biol Med. 37:2027–2041. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pettersson HM, Pietras A, Munksgaard
Persson M, Karlsson J, Johansson L, Shoshan MC and Påhlman S:
Arsenic trioxide is highly cytotoxic to small cell lung carcinoma
cells. Mol Cancer Ther. 8:160–170. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma Y, Wang J, Liu L, Zhu H, Chen X, Pan S,
Sun X and Jiang H: Genistein potentiates the effect of arsenic
trioxide against human hepatocellular carcinoma: Role of Akt and
nuclear factor-κB. Cancer Lett. 301:75–84. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu J, Qian H, Li Y, Wang Y, Zhang X, Liang
X, Fu M and Lin C: Arsenic trioxide (As2O3)
reduces the invasive and metastatic properties of cervical cancer
cells in vitro and in vivo. Gynecol Oncol. 106:400–406. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maeda H, Hori S, Nishitoh H, Ichijo H,
Ogawa O, Kakehi Y and Kakizuka A: Tumor growth inhibition by
Arsenic Trioxide (As2O3) in the orthotopic
metastasis model of Androgen-independent prostate cancer. Cancer
Res. 61:5432–5440. 2001.PubMed/NCBI
|
|
18
|
Nakamura S, Nagano S, Nagao H, Ishidou Y,
Yokouchi M, Abematsu M, Yamamoto T, Komiya S and Setoguchi T:
Arsenic trioxide prevents osteosarcoma growth by inhibition of GLI
transcription via DNA damage accumulation. PLoS One. 8:e694662013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Subbarayan PR and Ardalan B: In the war
against solid tumors arsenic trioxide needs partners. J
Gastrointest Cancer. 45:363–371. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Murgo AJ: Clinical trials of arsenic
trioxide in hematologic and solid tumors: Overview of the national
cancer institute cooperative research and development studies.
Oncologist. 6 (Suppl 2):S22–S28. 2001. View Article : Google Scholar
|
|
21
|
Meiss F and Zeiser R: Vismodegib. Recent
Results Cancer Res. 201:405–417. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nagao-Kitamoto H, Nagata M, Nagano S,
Kitamoto S, Ishidou Y, Yamamoto T, Nakamura S, Tsuru A, Abematsu M,
Fujimoto Y, et al: GLI2 is a novel therapeutic target for
metastasis of osteosarcoma. Int J Cancer. 136:1276–1284. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mazzucchelli S, Truffi M, Baccarini F,
Beretta M, Sorrentino L, Bellini M, Rizzuto MA, Ottria R, Ravelli
A, Ciuffreda P, et al: H-Ferritin-nanocaged olaparib: A promising
choice for both BRCA-mutated and sporadic triple negative breast
cancer. Sci Rep. 7:75052017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kumar S, Eroglu E, Stokes JA III,
Scissum-Gunn K, Saldanha SN, Singh UP, Manne U, Ponnazhagan S and
Mishra MK: Resveratrol induces mitochondria-mediated,
caspase-independent apoptosis in murine prostate cancer cells.
Oncotarget. 8:20895–20908. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wen W, Zhu F, Zhang J, Keum YS, Zykova T,
Yao K, Peng C, Zheng D, Cho YY, Ma WY, et al: MST1 promotes
apoptosis through phosphorylation of histone H2AX. J Biol Chem.
285:39108–39116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu J, Cai Y, Xu K, Ren X, Sun J, Lu S,
Chen J and Xu P: Beclin1 overexpression suppresses tumor cell
proliferation and survival via an autophagydependent pathway in
human synovial sarcoma cells. Oncol Rep. 40:1927–1936.
2018.PubMed/NCBI
|
|
27
|
Saitoh Y, Setoguchi T, Nagata M, Tsuru A,
Nakamura S, Nagano S, Ishidou Y, Nagao-Kitamoto H, Yokouchi M,
Maeda S, et al: Combination of Hedgehog inhibitors and standard
anticancer agents synergistically prevent osteosarcoma growth. Int
J Oncol. 48:235–242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin CJ, Lee CC, Shih YL, Lin TY, Wang SH,
Lin YF and Shih CM: Resveratrol enhances the therapeutic effect of
temozolomide against malignant glioma in vitro and in vivo by
inhibiting autophagy. Free Radic Biol Med. 52:377–391. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hegi ME, Diserens AC, Gorlia T, Hamou MF,
de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani
L, et al: MGMT gene silencing and benefit from temozolomide in
glioblastoma. N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dosch J, Christmann M and Kaina B:
Mismatch G-T binding activity and MSH2 expression is quantitatively
related to sensitivity of cells to methylating agents.
Carcinogenesis. 19:567–573. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yip S, Miao J, Cahill DP, Iafrate AJ,
Aldape K, Nutt CL and Louis DN: MSH6 mutations arise in
glioblastomas during temozolomide therapy and mediate temozolomide
resistance. Clin Cancer Res. 15:4622–4629. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yoon JW, Gilbertson R, Iannaccone S,
Iannaccone P and Walterhouse D: Defining a role for Sonic hedgehog
pathway activation in desmoplastic medulloblastoma by identifying
GLI1 target genes. Int J Cancer. 124:109–119. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ulasov IV, Nandi S, Dey M, Sonabend AM and
Lesniak MS: Inhibition of sonic hedgehog and notch pathways
enhances sensitivity of CD133+ glioma stem cells to
temozolomide therapy. Mol Med. 17:103–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nasr R, Guillemin MC, Ferhi O, Soilihi H,
Peres L, Berthier C, Rousselot P, Robledo-Sarmiento M,
Lallemand-Breitenbach V, Gourmel B, et al: Eradication of acute
promyelocytic leukemia-initiating cells through PML-RARA
degradation. Nat Med. 14:1333–1342. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim J, Lee JJ, Kim J, Gardner D and Beachy
PA: Arsenic antagonizes the Hedgehog pathway by preventing ciliary
accumulation and reducing stability of the Gli2 transcriptional
effector. Proc Natl Acad Sci USA. 107:13432–13437. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sekulic A, Migden MR, Oro AE, Dirix L,
Lewis KD, Hainsworth JD, Solomon JA, Yoo S, Arron ST, Friedlander
PA, et al: Efficacy and safety of vismodegib in advanced basal-cell
carcinoma. N Engl J Med. 366:2171–2179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gould SE, Low JA, Marsters JC Jr, Robarge
K, Rubin LL, de Sauvage FJ, Sutherlin DP, Wong H and Yauch RL:
Discovery and preclinical development of vismodegib. Expert Opin
Drug Discov. 9:969–984. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chang AL, Solomon JA, Hainsworth JD,
Goldberg L, McKenna E, Day BM, Chen DM and Weiss GJ: Expanded
access study of patients with advanced basal cell carcinoma treated
with the Hedgehog pathway inhibitor, vismodegib. J Am Acad
Dermatol. 70:60–69. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tomita A, Kiyoi H and Naoe T: Mechanisms
of action and resistance to all-trans retinoic acid (ATRA)
and arsenic trioxide (As2O3) in acute
promyelocytic leukemia. Int J Hematol. 97:717–725. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pricl S, Cortelazzi B, Dal Col V, Marson
D, Laurini E, Fermeglia M, Licitra L, Pilotti S, Bossi P and
Perrone F: Smoothened (SMO) receptor mutations dictate resistance
to vismodegib in basal cell carcinoma. Mol Oncol. 9:389–397. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee PC, Kakadiya R, Su TL and Lee TC:
Combination of bifunctional alkylating agent and arsenic trioxide
synergistically suppresses the growth of drug-resistant tumor
cells. Neoplasia. 12:376–387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Melamed JR, Morgan JT, Ioele SA, Gleghorn
JP, Sims-Mourtada J and Day ES: Investigating the role of
Hedgehog/GLI1 signaling in glioblastoma cell response to
temozolomide. Oncotarget. 9:27000–27015. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chou TC: Drug Combination studies and
their synergy quantification using the chou-talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: The combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Alves TR, Lima FR, Kahn SA, Lobo D, Dubois
LG, Soletti R, Borges H and Neto VM: Glioblastoma cells: A
heterogeneous and fatal tumor interacting with the parenchyma. Life
Sci. 89:532–539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
McDonald K, Joshi S, Jue TR, Yin J and
Khasraw M: ATPS-54genomically Unstable Glioblastoma (U-GBM) show
exquisite sensitivity to parp inhibition. Neuro Oncol. 17:v302015.
View Article : Google Scholar :
|
|
47
|
Shou Y, Robinson DM, Amakye DD, Rose KL,
Cho YJ, Ligon KL, Sharp T, Haider AS, Bandaru R, Ando Y, et al: A
five-gene hedgehog signature developed as a patient preselection
tool for hedgehog inhibitor therapy in medulloblastoma. Clin Cancer
Res. 21:585–593. 2015. View Article : Google Scholar : PubMed/NCBI
|