Introduction
Despite many advancements in treatments and annual
screenings, breast cancer remains as one of the leading causes of
cancer-related deaths in women, second only to lung cancer. In
2017, there was a projected number of 252,710 new cases of invasive
breast cancer occurrence, with increases in breast cancer incidence
observed from 2005 to 2014 in Asian/Pacific Islanders, non-Hispanic
Black, and Hispanic ethnic groups (1). One factor that may complicate
treatment for a patient is the specific subtype of breast cancer
diagnosed. Cancers of the breast are diverse and distinct from one
another; each subtype possessing its own prognosis and treatment
implications. The diversity among subtypes makes treatment and
prevention plans differ based on the molecular and receptor status
of the cancer, complicating research endeavors for new and
effective treatments (2). Subtypes
resulting from estrogen receptor-positive (ER-positive) cancer
cells, representing ~70% of diagnosed cases, are characterized by
expressing both the estrogen receptor (ER) and progesterone (PR)
that serve as targetable receptor sites for chemotherapeutic
treatments (2,3). Owing to previous study on
hormone-signaling pathways as a therapeutic target, the majority of
ER-positive breast cancer cases are treated with advanced endocrine
therapies that function to block estrogen activity and downregulate
tumor growth. Drugs that resemble the molecular structure of
estrogens are a common means of therapy due to similarities in
chemical composition that allow binding at these receptors to
either decrease endogenous estrogen activity or act
antagonistically with receptors for estrogen signaling (4,5). As
the need for more effective treatment methods arises, the potential
use of plant-derived compounds, such as resveratrol, has gained
popularity in the treatment of breast cancer due to their phenolic
structure and role in endocrine receptor binding (Fig. 1) (5,6).
Plant-derived compounds known as stilbenes have been associated
with many health benefits including the treatment of metabolic
disorders such as diabetes and obesity, as well as increasing
cardiovascular health.
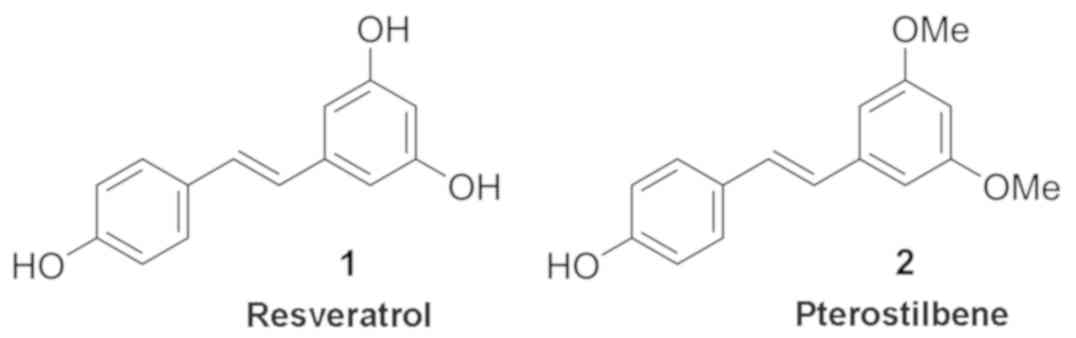
Resveratrol (Fig.
1A) has received much attention as a chemotherapeutic agent in
recent years for its anticancerous properties exhibited in many
types of cancer, including lung, pancreatic, ovarian, and breast.
Found naturally in many plants such as peanuts, cocoa, grapes,
berries, and red wine, the antioxidant properties of resveratrol
are attributed to its polyphenolic stilbene structure (7). Resveratrol and its analogues have also
been classified as phytoestrogens since their structural
resemblance allows them to bind estrogen receptors, enabling them
to signal through the associated pathways, inducing alterations of
kinase activity, transcription of mRNA, or other key cellular
functions (8). In the ER-positive
cell line MCF-7, several resveratrol phytoestrogenic analogues have
demonstrated the ability to: induce pro-apoptotic events leading to
significant inhibitory effects on proliferation through
ER-dependent signaling (9,10); induce epigenetic modifications in
the regulation of cancer (11); and
promote reversal of the epithelial-mesenchymal transition (EMT)
(12). While resveratrol has shown
great promise as a chemotherapeutic agent, low bioavailability
limits its effectiveness in clinical settings as it is metabolized
rapidly and has a very low solubility, with an oral bioavailability
reported in a study by Walle of <1% (13,14).
More resent investigations have unveiled novel analogues of
resveratrol that exhibit increased effects in cytotoxicity in
addition to increased potency in the treatment of breast cancer
cells when compared to resveratrol (13,15).
Tumors resulting from the triple-negative breast
cancer (TNBC) subtype lack both ER and PR receptors and are
negative for the human epidermal growth factor receptor 2 (HER2)
gene. While the TNBC subtype accounts for only 10–17% of breast
cancer cases, these are among the most difficult to treat since
they lack receptors predominantly targeted and exploited by
contemporary therapies, leaving traditional chemotherapy as the
primary treatment (2,16). In addition, patients diagnosed with
the TNBC subtype often experience early and increased rates of
metastasis resulting from induction of the epithelial-mesenchymal
transition (EMT) in cells (17).
This change in morphology decreases cell adherence to primary tumor
location, allowing cells, devoid of contact restrictions, to
metastasize (16).
Traditionally, resveratrol analogues were thought to
affect breast cancer cells through ER-dependent signaling pathways
and reversal of the EMT. However, we have found similar effects of
resveratrol and 28 analogues on both ER-positive and TNBC cell
lines. The lack of ER and other significant modalities often
targeted in breast cancer treatments should make resveratrol
analogue treatments of TNBC cells ineffective, according to the
previously proposed mode of action; yet we demonstrate significant
effects of 8 resveratrol analogues in decreasing cell viability in
a dose-dependent manner, indicating the existence of an
alternative, ER-independent mechanism. To better understand this
activity and the potential for future chemotherapeutic use in the
treatment of breast cancer, we investigated modulation of intra-
and inter-cellular pathways within TNBCs by these resveratrol
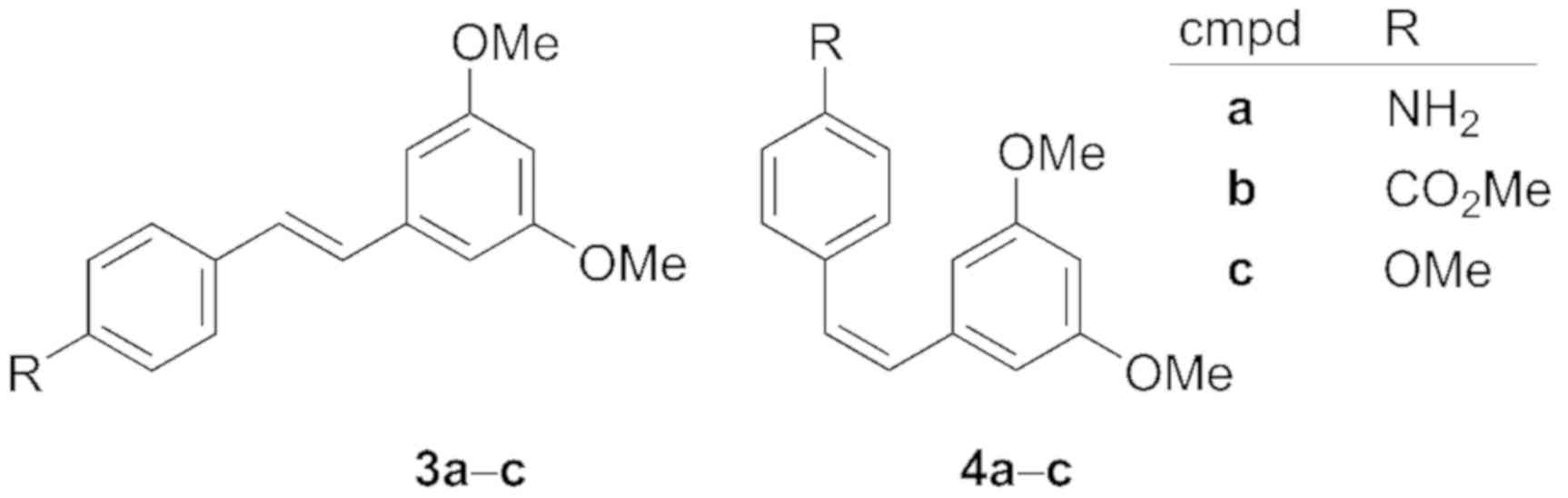
analogues. Our results suggest that stilbene compounds 3c and 4a-c
(Fig. 2) impede TNBC cell viability
by inhibiting cell survival pathways and upregulating cell death
pathways, including apoptosis signaling.
Materials and methods
Chemical methods
Our resveratrol analogue screening library was
generously donated by Agnes Rimando at the United States Department
of Agriculture (USDA). Larger quantities of select compounds were
obtained thus: resveratrol and pterostilbene (Fig. 1) were purchased from Thermo Fisher
Scientific Inc. (Waltham, MA, USA), and compounds 3a, 3c and 4a-c
were synthesized by known methods (18,19);
Spectra agreed with the aggregate of previously reported
characterizations (18–21). All compounds were analyzed by
1H NMR and GCMS to confirm identity and purity prior to
biological analysis. E/Z isomers were determined by
1H NMR, by observing J=16-16.5 Hz (E) and
J=12-12.5 Hz (Z) for the vinyl protons, and isomers
were confirmed pure (>99:1 isomeric ratio) by GC (FID).
1H NMR analysis was performed on a JEOL 400 MHz
spectrometer in solutions of CDCl3. GC separations were
performed on a Restek RTX-5MS column (30 m, 0.25 mm, 0.25 µm); 2
runs, 60°C/min, 2 ml/min, and 20°C/min, 3 ml/min. Compound weights
were obtained on a Mettler Toledo MS105 micro balance (0.01 mg
precision). 4-Hydroxy-tamoxifen and 17β-estradiol were obtained
from (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Breast cancer cell culture
ER-positive cell lines ZR-75-1 and MCF-7, and TNBC
cell lines MDA-MB-231, MDA-MB-157, and BT-549 were generously
provided by Dr Matthew E. Burow of Tulane University [all
originally obtained from the American Type Culture Collection
(ATCC; Manassas, VA, USA)]. Liquid nitrogen stocks were conducted
upon receipt and maintained until the start of each study. Cells
were used for no >6 months after being thawed with periodic
recording of morphology and doubling times to ensure maintenance of
phenotype (22). Cell lines were
maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc.), supplemented with 10% fetal bovine
serum (FBS; Atlanta Biologicals, Flowery Branch, GA, USA), insulin
(24 µg) and 1% each of antibiotic-antimycotic, essential and
non-essential amino acids, and sodium pyruvate (Thermo Fisher
Scientific, Inc.). Cells were cultured at 37°C in 5% CO2
and 95% relative humidity, re-fed every three to four days, and
passaged as needed.
Proliferation and viability
assays
Cell culture plates (96-well) were seeded at a cell
density of 1.5×103 cells/well for proliferation and
7.5×103 cells/well for viability at 100 µl/well of 10%
DMEM and incubated at 37°C in 5% humidified CO2 for
attachment overnight. Following incubation, cells were treated with
the corresponding compound suspended in 100 µl 10% DMEM at
designated concentrations in internal triplicates. Cells were
incubated at 37°C and harvested after 7 days. For Fig. S1, cells were harvested and fixed
after 24 h. Each plate was fixed with 10 µl 25% glutaraldehyde/well
for 20 min at room temperature and stained with 50 µl 0.4% crystal
violet in 20% methanol solution/well for 20 min at room
temperature. Excess crystal violet was removed, and plates were
rinsed with water, inverted, and allowed to dry overnight. Cells
were lysed with 50 µl 33% acetic acid and rocked for 15 min at room
temperature to ensure complete lysing. Lysates were read on plate
reader at 490 nm absorbance. Readings were normalized to dimethyl
sulfoxide (DMSO) control set to 100%.
RNA isolation, cDNA synthesis and
RT2-PCR assays
To identify effects on gene expression induced by
compounds, five compounds were chosen to screen in 88 breast cancer
gene arrays through RT-PCR. ER-negative cell line BT-549 were
chosen as representative line and treated as outlined above with
compounds 1, 3c, and 4a-c and DMSO control at 10 µM for 24 h before
harvesting. Total RNA was extracted using RNeasy Mini Kit (Qiagen,
Inc., Valencia, CA, USA) and cDNA was prepared using RT2
First Strand Kit (Qiagen, Inc.). RT2 Breast Cancer PCR
Array panels were run according to the manufacturers' instructions
(Qiagen, Inc.) on the CFX96 Bio-Rad Real-Time PCR thermocycler
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). CT values were
exported to an Excel file to create a table of Cq values. This
table was then uploaded onto the data analysis web portal at
http://www.qiagen.com/geneglobe. Samples
were assigned to controls and test groups. Cq values were
normalized based on an automatic selection from HKG panel of
reference genes. The data analysis web portal calculated fold
change/regulation using the ∆∆Cq method, where ∆Cq is calculated
between the gene of interest (GOI) and an average of reference
genes (HKG), followed by ∆∆Cq calculations [∆Cq (Test Group) ∆Cq
(Control Group)]. Fold change was then calculated using
2−∆∆Cq formula (23).
The data analysis web portal also created the clustergram heat map.
This data analysis report was exported from the Qiagen web portal
at GeneGlobe (https://www.qiagen.com/us/shop/genes-and-pathways).
Caspase-3/-7 apoptosis assays
Induction of apoptosis by resveratrol analogues was
detected using CellEvent Caspase-3/7 Green Detection reagent as per
the manufacturer's protocol (Thermo Fisher Scientific, Inc.). Cells
were plated at 5×103 cells/well in 96-well cell culture
plates and incubated overnight under standard culture conditions.
Cells were treated with corresponding analogues at 10 µM final
concentration, with 125 µM hydrogen peroxide and DMSO included for
positive and negative controls, respectively. Caspase-3/-7
detection reagent was diluted in phosphate-buffered saline (PBS)
with 5% FBS to 5 µM stock concentration immediately preceding
usage. At 48 h, media was removed from cells and 100 µl of diluted
reagent was added to each well. Cells were incubated for 30 min at
37°C in 5% humidified CO2. Cells were imaged at the
corresponding time-points using an Olympus CKX41 inverted
microscope (Olympus Corp., Tokyo, Japan) with fluorescence (X-Cite
120Q; EXFO Inc., Quebec, QC, Canada) absorption/emission at 502/530
nm and a CMS camera (Olympus DP21; Olympus Corp.). All cell images
were captured at ×40 original magnification. For each condition,
the number of caspase-positive (green) was divided by the total
number of cells per image (bright field) and multiplied by 100 to
determine the percent of positive apoptotic cells/condition.
Statistical analysis
All statistical analysis was conducted using
GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA, USA). All
treatment groups were compared to control (DMSO) using one-way
ANOVA testing with multiple comparisons post hoc tests
(Bonferroni). Each trial was plated as internal triplicates with a
minimum of three biological replicates for statistical testing.
Results
Analogues of resveratrol reduce cell
viability and proliferation in ER-positive cells
To confirm the anticancerous effects of resveratrol
reported in previously published studies (12), and to identify additional active
analogues and their effects on cell viability, resveratrol and 28
of its analogues, synthesized and obtained from the USDA (18), were screened in two ER-positive,
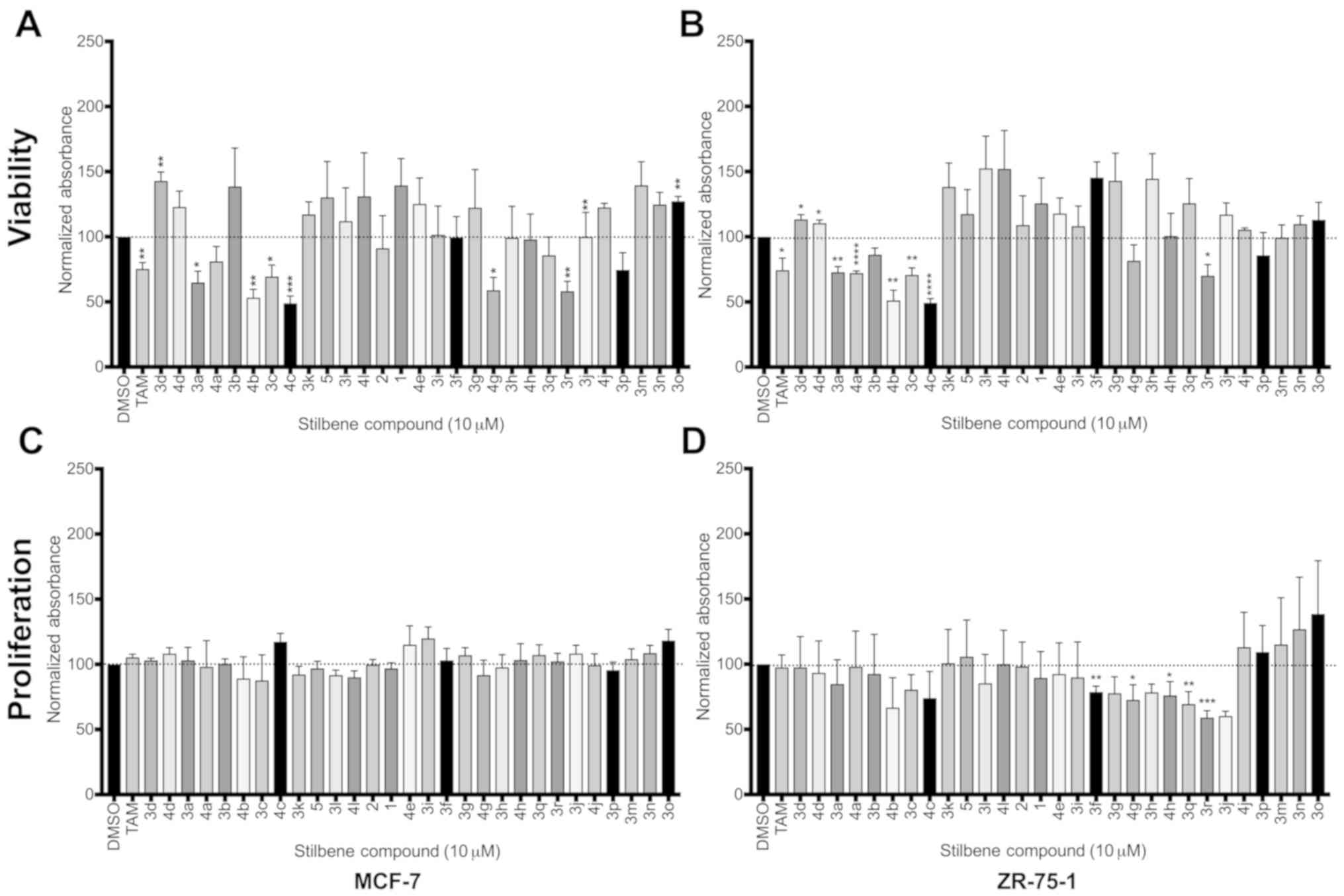
luminal cell lines: MCF-7 and ZR-75-1 (Fig. 3). Effects of our compounds on cell
viability were tested against those of tamoxifen, a known
anti-estrogen chemotherapeutic agent, as a positive control
(24). Of the 29 compounds screened
(Figs. 3A and B), we observed
significant activity of several compounds on cell viability in both
cell lines tested, with minimal variation between active compounds
in the two lines. Five of these analogues (3a, 3c, 3r, 4b and 4c,
Table I) significantly reduced cell
viability in both MCF-7 and ZR-75-1 lines, and an additional two
compounds were implicated in the reduction of viability in one of
the two cell lines (4a in ZR-75-1 and 4g in MCF-7).
 | Table I.Resveratrol analogue library
(Fig. 4). |
Table I.
Resveratrol analogue library
(Fig. 4).
| Compound | R1 | R2 | R3 | R4 | R5 |
|---|
| 1 | OH | H | OH | H | OH |
| 2 | OH | H | OMe | H | OMe |
| Dihydro-2 | OH | H | OMe | H | OMe |
| a | NH2 | H | OMe | H | OMe |
| b |
CO2Me | H | OMe | H | OMe |
| c | OMe | H | OMe | H | OMe |
| d | NO2 | H | OMe | H | OMe |
| eb |
CO2H | H | OMe | H | OMe |
| fa | F | H | OMe | H | OMe |
| g | Br | H | OMe | H | OMe |
| h | CF3 | H | OMe | H | OMe |
| ia |
Glucosec | H | OMe | H | OMe |
| j | H | H | OMe | H | OMe |
| ka | OH | H | OH | H | OMe |
| l | OMe | H | OH | H | OH |
| ma | OH | H | OH | H | OAc |
| n | OH | H | OAc | H | OAc |
| o | OAc | H | OAc | H | OAc |
| p | OH | H | OMe | Me | OMe |
| q | OH | OMe | OMe | H | OMe |
| r | OH | OH | OMe | H | OMe |
When comparing the effects of resveratrol analogues
to resveratrol itself, surprisingly, we observed that resveratrol
exhibited no significant decreases on cell viability for either
line tested at 10 µM concentration. However, this could be due in
part to the inhibitory dose-dependent effects of resveratrol
(25), where our screen identified
several analogues that may possess a greater potency than
resveratrol, having the capacity to induce these inhibitory effects
with greater efficiency than the well-studied parent compound. Of
particular interest were the effects of two compounds, 4b and 4c,
that induced marked decreases at or below 50% cell viability.
Structural comparison of these two compounds reveal various
chemical differences between the two analogues and resveratrol,
suggesting functional group substitutions alter compound
activity.
Notably, when tested under proliferation conditions
with the MCF-7 line, no significant effects on cell proliferation
were observed for any of the compounds tested. Several compounds
were found to possess significant proliferation inhibition, but
only in the ZR-75-1 cell line (Fig. 3C
and D). The compounds found to be active in suppression of
proliferation were unique from those effective at decreasing
viability, suggesting differences in mechanism between inhibition
of these cell effects. It also indicated that the absolute
configuration as well as functional group substitution of the
compounds play key roles in determining the mode of inhibition.
Several resveratrol analogues
significantly decrease viability in TNBC cells in a dose-dependent
manner
A common assumption is that phytoestrogens,
including stilbenes, elicit their effects by binding to and
regulating estrogen receptor function. As a preliminary probe into
the mechanism of action of our resveratrol analogues on the
ER-positive cell lines, we screened the 29-compound library in
three triple-negative breast cancer (TNBC) cell lines (MDA-MB-157,
MDA-MB-231 and BT-549). Our results demonstrated unprecedentedly
similar activity of these phytoestrogenic compounds in both
ER-positive and ER-negative cell lines; six compounds exhibited
significant inhibitory effects in at least one of the three TNBC
lines, with 4c being common among all three lines (Fig. 5A-C). In the BT-549 cells, 4c induced
a marked decrease in viability of ~70%, whereas in the MDA-MB-157
and MDA-MB-231 lines, the decrease was closer to 25 and 10%,
respectively, suggesting even slight genetic variation may
significantly affect the effectiveness of a compound. As with our
ER-positive cell line screen, neither resveratrol (Fig. 1A) nor pterostilbene (Fig. 1B) exhibited significant inhibition
of cell viability. Notably, the resveratrol analogues that
exhibited significant effects in the ER-positive lines also
exhibited marked decreases on cell viability in the ER-negative
lines, with compounds 3a, 3c, 3r and 4a-c significantly decreasing
viability in at least one of the three TNBC lines screened
(Fig. 5A-C). Although we did not
anticipate observing significant effects in the ER-negative cells,
these results indicated that select stilbenes may utilize an
ER-independent mechanism to induce effects on cell viability.
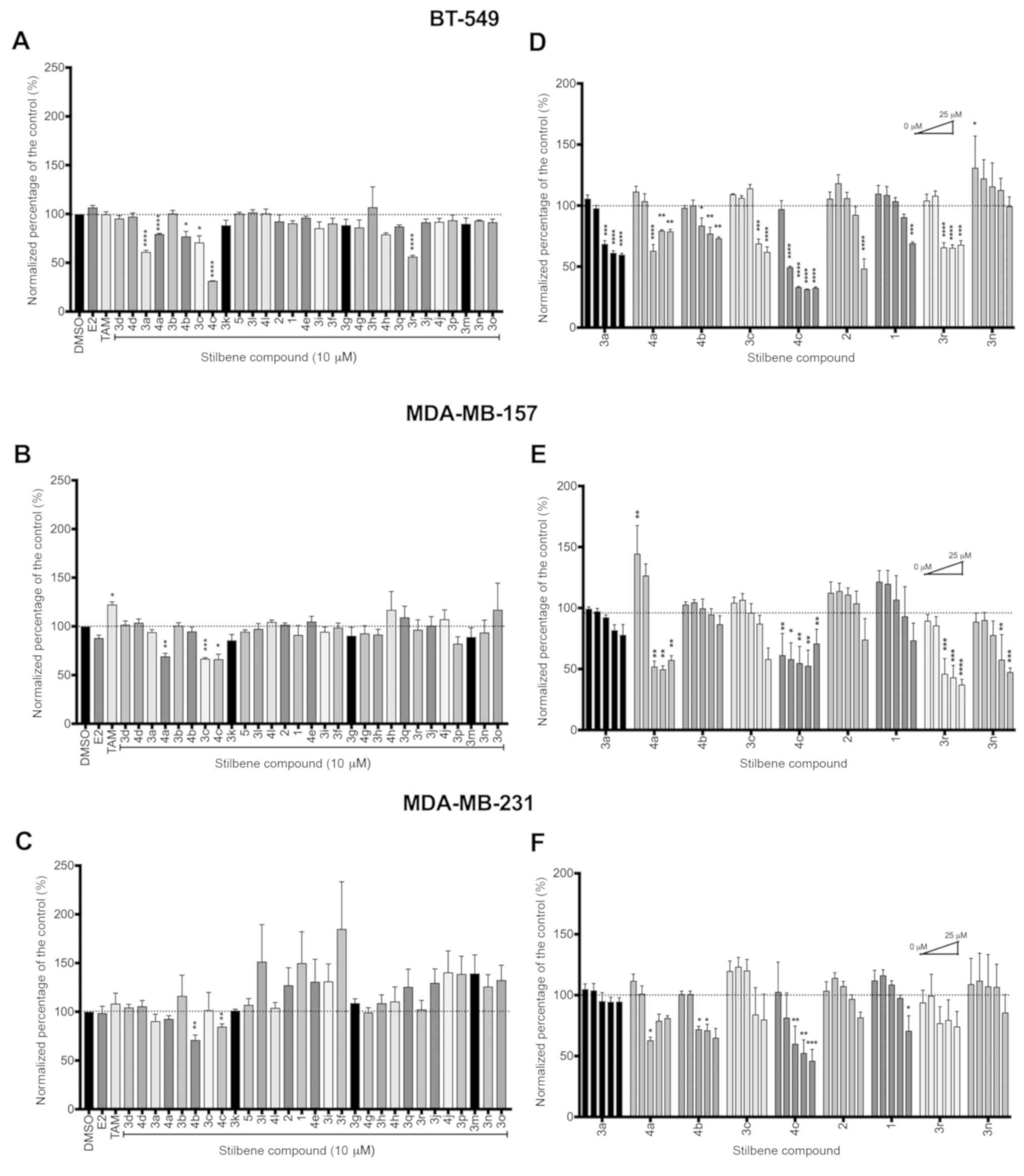 | Figure 5.Analogues of resveratrol exhibit
significant decreases on cell viability of triple-negative breast
cancers (TNBCs) with dose-dependent effects and high potency. Three
TNBC cells lines, (A and D) BT-549, (B and E) MDA-MB-157 and (C and
F) MDA-MB-231, were treated with stilbene compounds (10 µM final
concentration) and examined for viability at a 7-day time-point.
Dimethyl sulfoxide (DMSO) served as a vehicle control;
17β-estradiol (1 nM) and 4-hydroxy-tamoxifen (100 nM) were used as
additional controls. (D-F) Compounds 1, 2, 3a, 3c, 3n, 3r, and 4a-c
were assessed in a dose-response assay. Cells were treated with
corresponding compounds at 0.1, 1, 5, 10 or 25 µM and cultured for
seven days. Bars represent normalized mean (DMSO control set to
100) ± SEM for biological triplicate experiments. Statistical
significance was denoted by *P<0.05, **P<0.01, ***P<0.001
or ****P<0.0001). |
Further investigation into the six most effective
compounds led us to examine the potency of these compounds through
a dose-response assay. TNBC cells were treated with increasing
doses of each compound as follows: 0.1, 1, 5, 10 and 25 µM final
concentration. Resveratrol (Fig.
1A) and pterostilbene (Fig. 1B)
were included as our representative parent compounds as proven
anticancerous effects in breast cancer cells. Compounds 4a, 4c and
3r exhibited the highest potency with significant inhibition of
viability evident at 1 µM and increasing effectiveness as
concentration increased in at least 2 out of the 3 cell lines
tested (Fig. 5D-F). Consistent with
the 10 µM screen, resveratrol (Fig.
1A) and pterostilbene (Fig. 1B)
behaved with little effect on cancer cell viability in any of the
TNBC lines, with significant decreases observed at only the highest
doses tested.
Resveratrol analogues alter gene
expression of cell death and survival signaling pathways
Due to the lack of classical estrogen signaling via
ER in TNBC cells, we set out to understand the mechanism of action
of stilbenes in the inhibition of cell viability in this system.
Investigation into the intracellular pathways was carried out
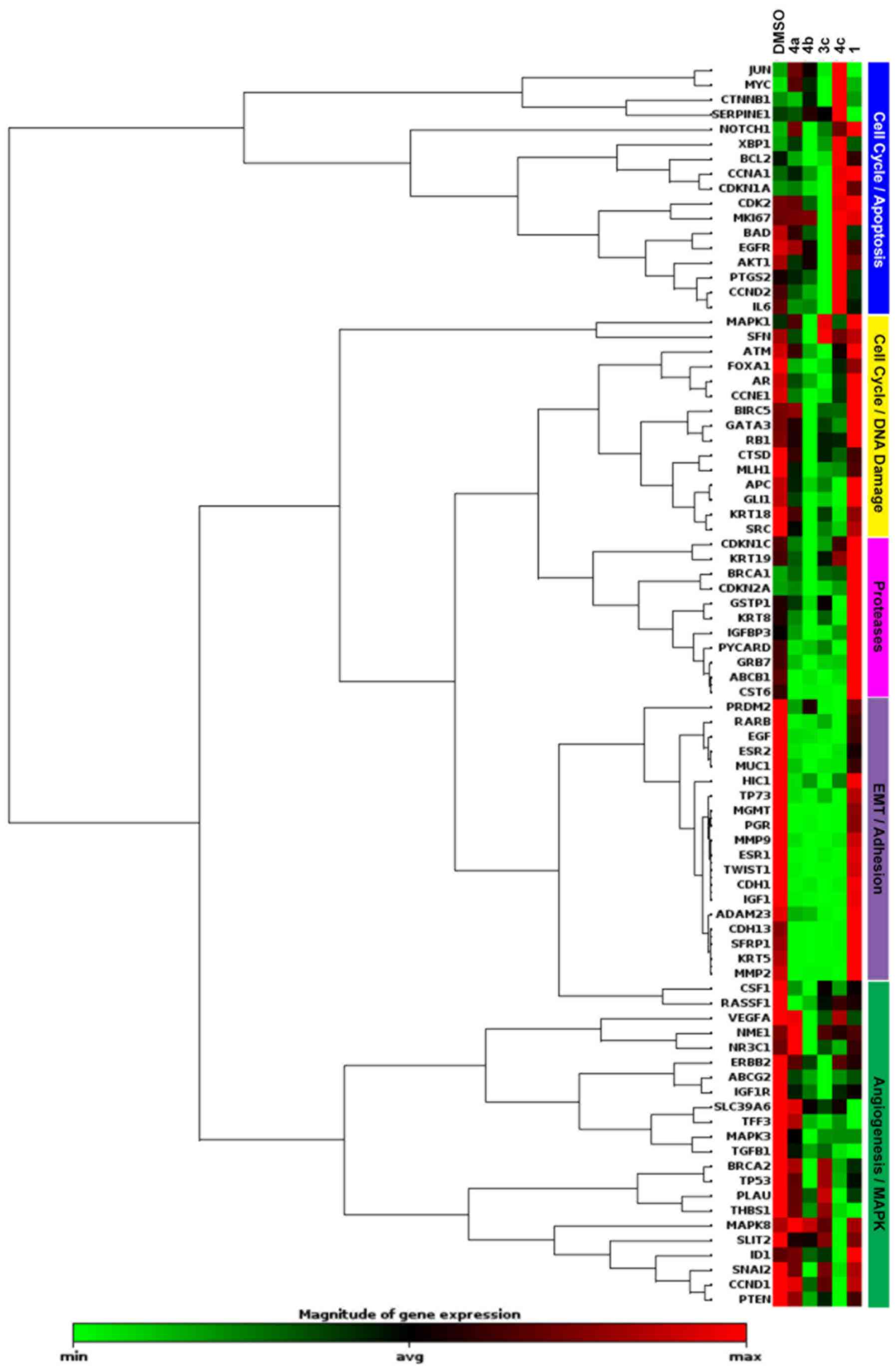
through RT2-PCR profiling. The effects of four of the
most potent representative compounds (3c, 4a-c) and the parent
compound resveratrol (Fig. 1A) on
breast cancer-associated gene expression were assessed in the
BT-549 cell line. The RT2 Breast Cancer Profiler PCR
assay containing specific primers for 88 known breast
cancer-associated genes was run for each sample and compared to
DMSO-treated control cells. Resveratrol exhibited little change in
the BT-549 cell line, only 4 of the 84 genes tested responded
>2-fold compared to the control (Fig. 6). Overall, the greatest differences
in gene expression for the stilbene compounds tested were observed
in genes associated with the cell cycle, apoptosis, and DNA repair.
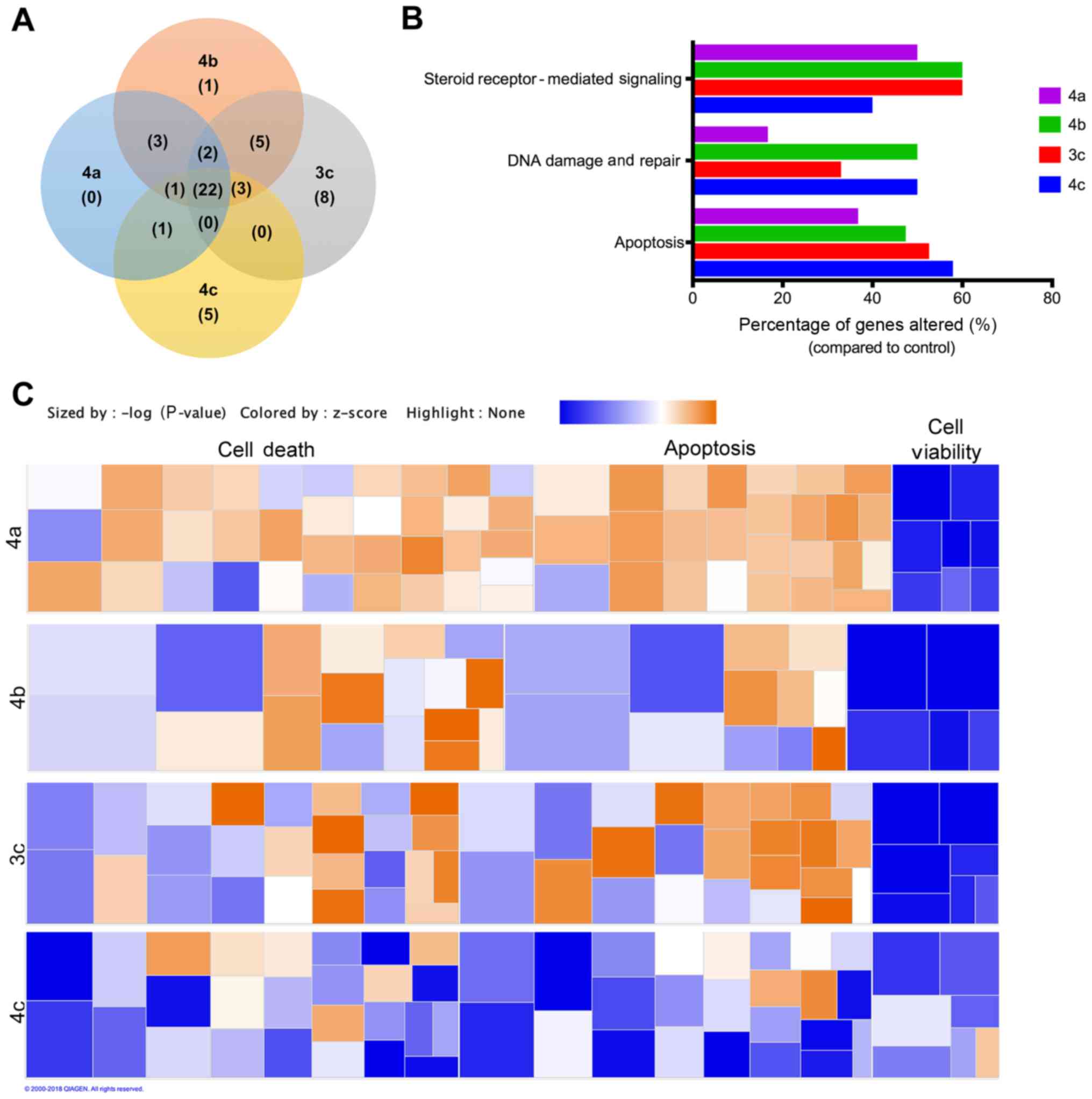
Fig. 7A summarizes the similarities
and differences in gene expression changes between compounds 3c,
4a-c for genes exhibiting >2-fold regulation. Of the 22 genes
that were commonly regulated by the compounds tested,
downregulation of steroid hormone signaling, particularly estrogen
(ESR1, ESR2, PgR and RARB), inhibition of growth factors and
oncogenes associated with cancer (EGF, IGF1, MUC1, ADAM23, GLI1 and
MGMT), and inhibition of EMT and metastasis-related genes (CDH1,
CDH13, TWIST1, MMP2, MMP9 and GRB7) were among the top altered. Top
pathways commonly regulated by these stilbenes included apoptosis,
DNA damage and repair, and, surprisingly, steroid receptor-mediated
signaling (Fig. 7B).
Pathway analysis for each compound was conducted
using Ingenuity Pathway Analysis. Of all the Disease and Function
pathways tested, cell death and survival were the top predicted
pathways altered by all stilbenes tested. Upon further
investigation, cell death pathways, including apoptosis, necrosis,
and anoikis, were the most upregulated pathways predicted for 3c,
4a and 4b, while cell viability and survival were among the most
downregulated pathways predicted for 3c and 4a-c (Fig. 7C). Compound 4c presents an
interesting case. As the most effective compound on the viability
of the cell lines tested, we expected to see broad effects
throughout the cell death and survival spectrum. However, the cell
death and apoptotic pathways were not upregulated in the 4c-treated
cells. Although cell death pathways appear to be activated in cells
treated with 3c, 4a and 4b, but not 4c, we did not observe any
significant cell death at 24 h via crystal violet staining
(Fig. S1). Yet we observe that,
collectively, the overall effect from the aforementioned results
presented is clearly broad-spectrum downregulation of cancer cell
viability. While there are obvious differences in the specific gene
regulation induced by each stilbene, these results indicated that
select stilbenes induce cell death via apoptosis with simultaneous
inhibition of cell survival signaling pathways.
cis-Resveratrol analogues with methoxy
functional groups induce apoptosis in TNBC cell lines
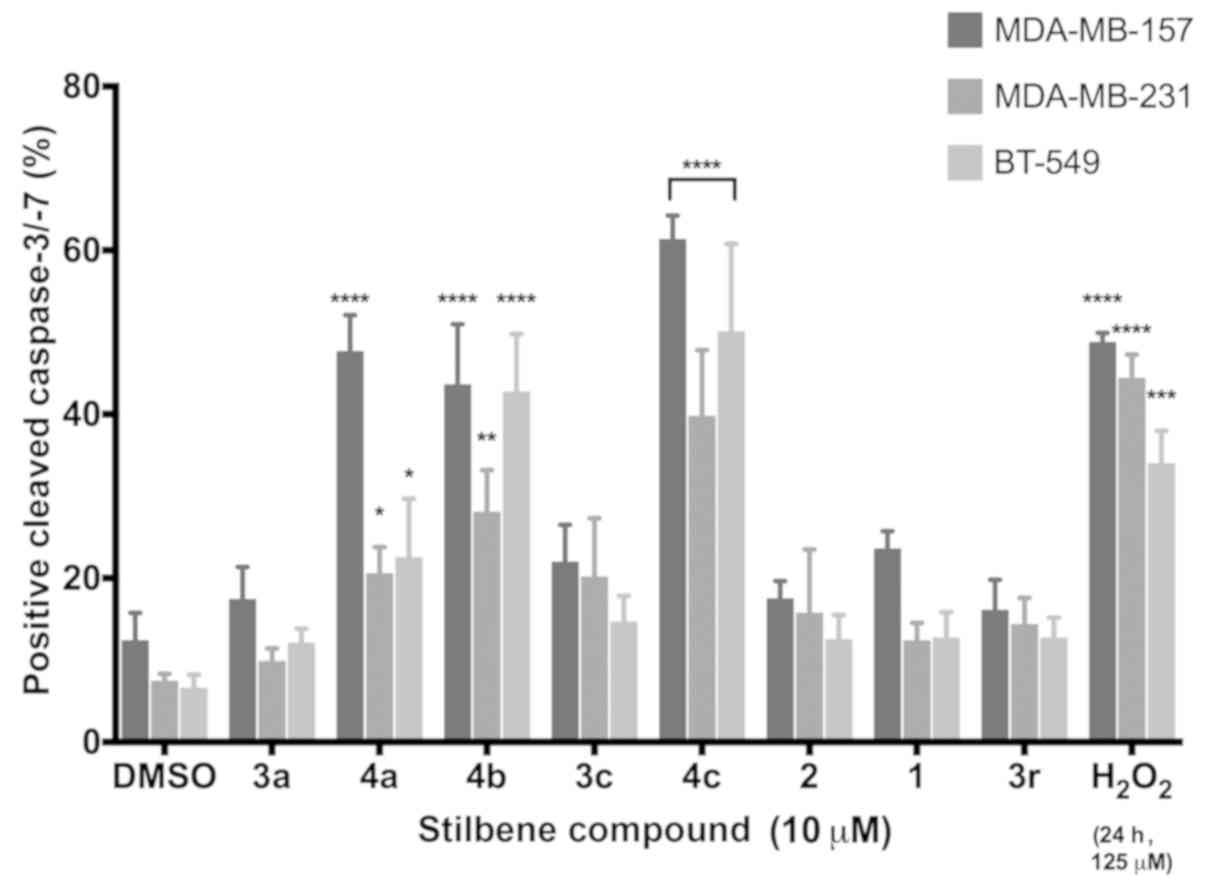
To confirm that the effects on cell viability were
due to activation of apoptosis as suggested by gene expression
array, caspase activation assays were conducted with stilbenes 3a,
3c, 3r and 4a-c, with resveratrol (Fig.
1A) and pterostilbene (Fig. 1B)
serving as negative controls and hydrogen peroxide (125 µM) serving
as a positive control. Significant induction of apoptosis was
observed in 3 of the 8 compounds dosed at 10 µM 48 h
post-treatment. While differences in the magnitude of caspase
activation were observed across all three TNBC cell lines tested, a
clear and consistent induction of apoptosis was revealed. These
results confirmed that the decrease in cell viability caused by
these compounds was due, at least in part, to activation of
programmed cell death pathways. These findings also supported gene
expression pathway analysis that indicated increased cell death
including apoptotic pathways.
Discussion
With the occurrence of breast cancer on the rise,
quickly becoming the most commonly diagnosed cancer in the world,
the need for reliable and effective treatments has never been more
necessary. With a majority of breast cancer cases being derived
from a subtype with targetable modalities, significant advancements
in modern endocrine therapies have been made, leaving these
patients with hopeful prognosis as they journey to remission
(3). In the search of alternative
means for the utilization of hormone therapy, such as
phytoestrogenic compounds, resveratrol is one that has long been
studied in the treatment of ER-positive breast cancer cases.
Investigations into the mechanism of stilbenes in ER-positive
subtypes has revealed great promise for their use in hormone
therapy (3), and more recently, in
overcoming therapy-resistant breast cancer cells.
A recent investigation into cisplatin-resistant
MCF-7 cells, a widely used chemotherapeutic agent, demonstrated the
ability of resveratrol to enhance cisplatin antiproliferation
effects, lowering the IC50 in both normal and resistant MCF-7
lines, while causing downregulation of Rad51 protein levels and
homologous recombination (HR) complex initiators (26). Increased levels of both Rad51 and HR
have been revealed to be associated with genetically predisposed
risks for breast cancer contraction and increased development of
therapy-resistant cells (27). De
Amicis et al evaluated the use of resveratrol in cases of
tamoxifen-resistant MCF-7 cells, reporting its ability to increase
cell-sensitivity by increasing p53 protein expression. They
reported that increased p53 resulted in decreased ER expression,
leading to downregulation of growth factor pathways and resulting
in cross-talk known to arise between pro-survival and hormonal
pathways (28). Various studies
have highlighted the ability of resveratrol, through downregulation
of ERα mRNA transcription, to inhibit expression of major cell
cycle and cell proliferation-dependent genes, all initiated by
downstream upregulation of p53 and p21 (28–30).
While there exists a general consensus on the ability of
resveratrol to modulate both ERα and p53 expression in ER-positive
breast cancer, a recent study investigated the relationship between
this tumor-suppressor protein and hormonal signaling receptor,
suggesting an alternate pathway in which resveratrol induces p53
expression to promote anti-survival effects, due to the variable,
dose-dependent actions of resveratrol reported (25).
One alternative mechanism recently proposed is of
particular interest as it has been observed in both ER-positive and
ER-negative breast cancers. A membrane receptor site for
resveratrol on an integrin has been uncovered in both subtypes of
breast cancer, where p53-dependent apoptosis induction has been
confirmed upon resveratrol binding (25,31).
Further investigation of binding site affinity for resveratrol and
analogues has uncovered an interesting interaction with the
integrin. Hsieh et al evaluated the binding of resveratrol
and trans−3,4′,5-triacetylstilbene (compound 3o from our
library) in MDA-MB-231 cells, revealing significant increases in
both p53 and p21 expression and resultant induction of
p53-dependent apoptosis at treatment concentrations ranging from 10
to 50 µM (9). While we did not
observe these reductions in TNBC viability at 10 µM doses of either
resveratrol (1) or 3o, some
significant inhibition was observed for resveratrol and 3n, a
diacetyl-substituted analogue, at 25 µM (Fig. 5D-F) in select TNBC lines. Our
results again support that dose-dependent proliferation verses
growth-inhibitory effects that resveratrol and related compounds
may exhibit, with doses >20 µM more commonly be associated with
antiproliferation effects (25).
Where H-donating functional groups have been confirmed to interact
with high affinity for the integrin receptor site, analogues
substituted with methoxy groups exhibit little interaction with the
protein (9). Of greater
significance were our results of several resveratrol analogues
which increased the amount of apoptosis under the same conditions
(Fig. 8). Notably our results
elucidate the anticancerous effects of these methoxy resveratrol
analogues at low-dose treatment concentrations (0.1–25 µM),
supporting various alternative mechanisms that these unique
stilbenes may function through in TNBC (9).
Caspase-3/-7 quantification revealed three compounds
identified in our screen (4a-c) to significantly decrease TNBC
viability, suggesting differential, pro-apoptotic signaling
pathways not yet explored in ER-negative cells. When comparing the
molecular structures of compounds 4a-c, we have identified several
common elements that we believe may play a major role in their
apoptotic activity. First, these compounds are all in the
cis-confirmation that has been a well-established factor in
increasing both the cytotoxic and apoptotic functionality of
various stilbene compounds (20,32).
Additionally, these three compounds all have the
3,5-dimethoxystyryl structure and are thus closer analogues of
pterostilbene, a well-studied trans-analogue of resveratrol.
Studies into the substitution of the hydroxyl groups for methoxy
groups on resveratrol have revealed marked increases in the
anticancerous effects (33,34). In one investigation, Hong et
al compared the activity of resveratrol and
trimethoxy-substituted resveratrol analogue (3c and 4c) in
pancreatic and breast cancer cells, revealing significantly
increased potency of the Z-analogue, as demonstrated though
increased inhibition of proliferation, increased cell cycle arrest,
and overall increased induction of apoptosis (35). These pro-apoptotic events resulting
in increased cellular toxicity in both MCF-7 and MDA-MB-231 cell
lines was attributed to the blocking of tubulin formation by
stilbenes, resulting in cell cycle arrest (35). Since the blocking of α- and
β-tubulin interaction is not dependent upon estrogen signaling,
this further supports our hypothesis that some of our compounds are
functioning in an ER-independent manner. Compound 4c contains an
additional methoxy group on the second benzene ring that may be
responsible for its consistently high levels of cellular toxicity
observed across our studies and supports future investigation into
the intracellular pathways being regulated.
While TNBC cells lack ERα expression, the primary
estrogen receptor, they many retain expression of other
non-dominant estrogen receptors such as ERβ and GPR30. Our results
clearly indicated an increase in apoptosis, and pathway analyses
also suggested inhibition of ER-dependent signaling. Even though
several of our stilbene compounds elicited anti-viability and/or
anti-proliferative effects in ER-positive breast cancer cell lines,
in agreement with recent similarly conducted studies, more
significantly they elicit greater anti-viability and pro-apoptotic
effects in ER-negative TNBC cell lines, a phenomenon that has not
been fully explored to understand these compounds potential for
therapeutic use. While our gene expression studies raise the
possibility of suppression of potentially metastatic pathways
(decreased expression of TWIST, MMP2, MMP9 and TGFB), the goal of
the present study was to determine general activity of compounds.
Our current and future studies will explore the mechanisms
regulating their anticancer activity as well as any potential
anti-migration or -invasion activity.
Although there has been much success with
resveratrol as an anticancer compound in laboratory studies, when
taken by patients, its bioavailability continues to limit its
effectiveness in clinical settings (14). Thus, the investigation into the
activity of analogues of this ‘miracle’ compound may be a pivotal
step in overcoming this biological barrier in the use of
resveratrol use as a therapeutic agent. Through our investigation,
we have uncovered previously unknown actions of multiple
synthetically derived analogues of resveratrol that not only
possess increased effects on inhibition of TNBC growth compared to
the parent compound, but also increased potency.
Supplementary Material
Supporting Data
Acknowledgements
The authors thank Dr Agnes Rimando at the USDA for
providing the initial stilbene compound library, and Dr Matthew E.
Burow for providing all the breast cancer cell lines used in this
study.
Funding
The authors would like to thank the Seidler Family
Foundation and FGCU's Office of Research and Graduate Studies
(ORSP-15066-1), the FGCU Whitaker Center for STEM Education, the
FGCU Office of Undergraduate Scholarships, and the FGCU Honors
College for their financial support. DHP thanks the NSF for MRI
funding (CHE-1530959) and the Sheffield Foundation for financial
support. These funds supported this research with materials,
supplies, and travel to conferences, except the NSF MRI which
supported chemical structure determination by NMR. A portion of the
Seidler Foundation funding was also allocated specifically for
summer research stipend for DHP and LVR.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
XJH planned and conducted the experiments, analyzed
the data and drafted the manuscript. HT and EB conducted the
experiments and analyzed the data. DHP synthesized the analogues,
conducted the characterization/purity studies, and contributed to
the drafting and editing of the manuscript. LVR conceptualized the
study, planned the experiments, conducted data analysis and
statistics, prepared the figures, and assisted in drafting and
editing of the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no financial or conflicts of
interests of any kind.
Glossary
Abbreviations
Abbreviations:
|
ER
|
estrogen receptor
|
|
TNBC
|
triple-negative breast cancer
|
References
|
1
|
DeSantis CE, Ma J, Goding Sauer A, Newman
LA and Jemal A: Breast cancer statistics, 2017, racial disparity in
mortality by state. CA Cancer J Clin. 67:439–448. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chavez KJ, Garimella SV and Lipkowitz S:
Triple negative breast cancer cell lines: One tool in the search
for better treatment of triple negative breast cancer. Breast Dis.
32:35–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rajappa S, Bajpai J, Basade M, Ganvir M,
Goswami C, Murali A, Rathi AK, Kaushal V, Jain S, Parikh PM, et al:
Practical consensus recommendations regarding the use of hormonal
therapy in metastatic breast cancer. South Asian J Cancer.
7:137–141. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alfarsi L, Johnston S, Liu DX, Rakha E and
Green AR: Current issues with luminal subtype classification in
terms of prediction of benefit from endocrine therapy in early
breast cancer. Histopathology. 73:545–558. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bowers JL, Tyulmenkov VV, Jernigan SC and
Klinge CM: Resveratrol acts as a mixed agonist/antagonist for
estrogen receptors α and β. Endocrinology. 141:3657–3667. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu R and Serrero G: Resveratrol, a natural
product derived from grape, exhibits antiestrogenic activity and
inhibits the growth of human breast cancer cells. J Cell Physiol.
179:297–304. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sirerol JA, Rodríguez ML, Mena S, Asensi
MA, Estrela JM and Ortega AL: Role of natural stilbenes in the
prevention of cancer. Oxid Med Cell Longev. 2016:31289512016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ko JH, Sethi G, Um JY, Shanmugam MK,
Arfuso F, Kumar AP, Bishayee A and Ahn KS: The role of resveratrol
in cancer therapy. Int J Mol Sci. 18:25892017. View Article : Google Scholar :
|
|
9
|
Hsieh TC, Wong C, John Bennett D and Wu
JM: Regulation of p53 and cell proliferation by resveratrol and its
derivatives in breast cancer cells: An in silico and biochemical
approach targeting integrin αvβ3. Int J Cancer. 129:2732–2743.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yenugonda VM, Kong Y, Deb TB, Yang Y,
Riggins RB and Brown ML: Trans-resveratrol boronic acid
exhibits enhanced anti-proliferative activity on estrogen-dependent
MCF-7 breast cancer cells. Cancer Biol Ther. 13:925–934. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee PS, Chiou YS, Ho CT and Pan MH:
Chemoprevention by resveratrol and pterostilbene: Targeting on
epigenetic regulation. Biofactors. 44:26–35. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chin YT, Hsieh MT, Yang SH, Tsai PW, Wang
SH, Wang CC, Lee YS, Cheng GY, HuangFu WC, London D, et al:
Anti-proliferative and gene expression actions of resveratrol in
breast cancer cells in vitro. Oncotarget. 5:12891–12907. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Liu Y, Chen H, Yao X, Xiao Y, Zeng
X, Zheng Q, Wei Y, Song C, Zhang Y, et al: Synthetic resveratrol
derivatives and their biological activities: A review. Open J Med
Chem. 05:97–105. 2015. View Article : Google Scholar
|
|
14
|
Walle T: Bioavailability of resveratrol.
Ann NY Acad Sci. 1215:9–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Licznerska B, Szaefer H, Wierzchowski M,
Mikstacka R, Papierska K and Baer-Dubowska W: Evaluation of the
effect of the new methoxy-stilbenes on expression of receptors and
enzymes involved in estrogen synthesis in cancer breast cells. Mol
Cell Biochem. 444:53–62. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jo SJ, Park PG, Cha HR, Ahn SG, Kim MJ,
Kim H, Koo JS, Jeong J, Park JH, Dong SM, et al: Cellular inhibitor
of apoptosis protein 2 promotes the epithelial-mesenchymal
transition in triple-negative breast cancer cells through
activation of the AKT signaling pathway. Oncotarget. 8:78781–78795.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paul S, Mizuno CS, Lee HJ, Zheng X,
Chajkowisk S, Rimoldi JM, Conney A, Suh N and Rimando AM: In vitro
and in vivo studies on stilbene analogs as potential treatment
agents for colon cancer. Eur J Med Chem. 45:3702–3708. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mizuno CS, Ma G, Khan S, Patny A, Avery MA
and Rimando AM: Design, synthesis, biological evaluation and
docking studies of pterostilbene analogs inside PPARalpha. Bioorg
Med Chem. 16:3800–3808. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roberti M, Pizzirani D, Simoni D, Rondanin
R, Baruchello R, Bonora C, Buscemi F, Grimaudo S and Tolomeo M:
Synthesis and biological evaluation of resveratrol and analogues as
apoptosis-inducing agents. J Med Chem. 46:3546–3554. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pettit GR, Grealish MP, Jung MK, Hamel E,
Pettit RK, Chapuis JC and Schmidt JM: Antineoplastic agents. 465.
Structural modification of resveratrol: Sodium resverastatin
phosphate. J Med Chem. 45:2534–2542. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tate CR, Rhodes LV, Segar HC, Driver JL,
Pounder FN, Burow ME and Collins-Burow BM: Targeting
triple-negative breast cancer cells with the histone deacetylase
inhibitor panobinostat. Breast Cancer Res. 14:R792012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sauter ER: Breast cancer prevention:
Current approaches and future directions. Eur J Breast Health.
14:64–71. 2018.PubMed/NCBI
|
|
25
|
Saluzzo J, Hallman KM, Aleck K, Dwyer B,
Quigley M, Mladenovik V, Siebert AE and Dinda S: The regulation of
tumor suppressor protein, p53, and estrogen receptor (ERα) by
resveratrol in breast cancer cells. Genes Cancer. 7:414–425.
2016.PubMed/NCBI
|
|
26
|
Leon-Galicia I, Diaz-Chavez J,
Albino-Sanchez ME, Garcia-Villa E, Bermudez-Cruz R, Garcia-Mena J,
Herrera LA, García-Carrancá A and Gariglio P: Resveratrol decreases
Rad51 expression and sensitizes cisplatin-resistant MCF-7 breast
cancer cells. Oncol Rep. 39:3025–3033. 2018.PubMed/NCBI
|
|
27
|
Bhattacharyya A, Ear US, Koller BH,
Weichselbaum RR and Bishop DK: The breast cancer susceptibility
gene BRCA1 is required for subnuclear assembly of Rad51 and
survival following treatment with the DNA cross-linking agent
cisplatin. J Biol Chem. 275:23899–23903. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
De Amicis F, Giordano F, Vivacqua A,
Pellegrino M, Panno ML, Tramontano D, Fuqua SA and Andò S:
Resveratrol, through NF-Y/p53/Sin3/HDAC1 complex phosphorylation,
inhibits estrogen receptor alpha gene expression via
p38MAPK/CK2 signaling in human breast cancer cells.
FASEB J. 25:3695–3707. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ronghe A, Chatterjee A, Bhat NK, Padhye S
and Bhat HK: Tamoxifen synergizes with
4-(E)-{(4-hydroxyphenylimino)-methylbenzene, 1,2-diol} and
4-(E)-{(p-tolylimino)-methylbenzene-1,2-diol}, novel azaresveratrol
analogs, in inhibiting the proliferation of breast cancer cells.
Oncotarget. 7:51747–51762. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ronghe A, Chatterjee A, Singh B, Dandawate
P, Murphy L, Bhat NK, Padhye S and Bhat HK: Differential regulation
of estrogen receptors α and β by
4-(E)-{(4-hydroxyphenylimino)-methylbenzene,1,2-diol}, a novel
resveratrol analog. J Steroid Biochem Mol Biol. 144:500–512. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chin YT, Yang SH, Chang TC, Changou CA,
Lai HY, Fu E, HuangFu WC, Davis PJ, Lin HY and Liu LF: Mechanisms
of dihydrotestosterone action on resveratrol-induced
antiproliferation in breast cancer cells with different ERα status.
Oncotarget. 6:35866–35879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Simoni D, Roberti M, Invidiata FP,
Rondanin R, Baruchello R, Malagutti C, Mazzali A, Rossi M, Grimaudo
S, Capone F, et al: Heterocycle-containing retinoids. Discovery of
a novel isoxazole arotinoid possessing potent apoptotic activity in
multidrug and drug-induced apoptosis-resistant cells. J Med Chem.
44:2308–2318. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kumar A, Rimando AM and Levenson AS:
Resveratrol and pterostilbene as a microRNA-mediated
chemopreventive and therapeutic strategy in prostate cancer. Ann NY
Acad Sci. 1403:15–26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Moon D, McCormack D, McDonald D and
McFadden D: Pterostilbene induces mitochondrially derived apoptosis
in breast cancer cells in vitro. J Surg Res. 180:208–215. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hong YB, Kang HJ, Kim HJ, Rosen EM,
Dakshanamurthy S, Rondanin R, Baruchello R, Grisolia G, Daniele S
and Bae I: Inhibition of cell proliferation by a resveratrol analog
in human pancreatic and breast cancer cells. Exp Mol Med.
41:151–160. 2009. View Article : Google Scholar : PubMed/NCBI
|