Introduction
Bladder cancer is a common urinary cancer worldwide
and its high rate of recurrence results in poor clinical outcomes
(1). Patients with high-risk
bladder tumors have approximately a 50% chance of recurrence if
treated with surgery alone (2). The
American Cancer Society estimates approximately 81,190 new cases
and 17,240 deaths from bladder cancer in 2018 (3). There are two major phenotypes in
bladder cancer: Muscle-invasive bladder cancer and
non-muscle-invasive bladder cancer (4). After surgical resection, the current
standard treatment is cisplatin-based chemotherapy (5).
Cancer stem cells (CSCs), which are found in various
solid cancers, including bladder cancer, comprise a small
population of tumor cells, which play indispensable roles in cancer
development and metastasis (6,7). CSCs
are characterized by a slow growth rate and high expression levels
of drug efflux proteins including ATP-binding cassette transporters
(8). CSCs have significant clinical
implications because they have been correlated with chemoresistance
(9). Bladder CSCs were first
identified in 2009 and the existence of bladder CSCs contributes to
the resistance to anticancer therapeutics (10). The sonic hedgehog protein (Shh)
signaling pathway plays an important role in the maintenance of
CSCs and mediates tumorigenesis in many cancers (11). Shh proteins bind to and inactivate
the 12-pass transmembrane protein protein patched homolog 1
(Ptch1), which inhibits the seven-pass transmembrane protein
smoothened homolog (Smo) (12). The
release of Smo leads to the activation of zinc finger protein GLI
(Gli) family transcription factors, which induce the transcription
of target genes (12). The Shh
signaling pathway is critical for the development, initiation and
progression of bladder cancer, and promotes bladder tumorigenicity
and stemness by activating epithelial-to-mesenchymal transition
(13). Shh signaling pathway
activity shows distinct spatial and temporal distribution during
bladder development (13).
(−)-Epigallocatechin-3-gallate (EGCG) is an abundant
polyphenol from green tea and plays antitumor roles in various
cancers including bladder cancer (14). EGCG downregulates nuclear factor-κB
and matrix metalloproteinase-9 to inhibit bladder cancer SW780 cell
proliferation and migration both in vitro and in vivo
(15). In addition, EGCG also
suppresses bladder cancer cell metastasis via the PI3K/AKT
signaling pathway (16).
Furthermore, EGCG was shown to inhibit lung and colorectal CSCs by
affecting the Wnt/β-catenin signaling pathway, and EGCG
downregulates STAT3 to suppress the stem-cell-like characteristics
of nasopharyngeal cancer (17–19).
Moreover, EGCG significantly reduces recurrence in patients with
colorectal adenoma, and acts synergistically with cisplatin and
oxaliplatin via the autophagy pathway in human colorectal cancer
cells (20). However, the molecular
mechanisms underlying the effect of EGCG on bladder CSCs remain
unknown. In the present study, the authors investigated the effect
of EGCG on bladder CSCs.
Materials and methods
Cell culture and reagents
Human bladder cancer cell lines EJ and UM-UC-3 were
obtained from American Type Culture Collection and maintained at
37°C in incubator containing 5% CO2. Both cell lines
were grown in RPMI-1640 medium supplemented with 10% fetal bovine
serum (FBS) and 1% penicillin/streptomycin (all from Gibco; Thermo
Fisher Scientific, Inc.). EGCG was obtained from Sigma-Aldrich
(Merck KGaA). Epidermal growth factor (EGF), basic fibroblast
growth factor (bFGF) and insulin were obtained from Peprotech,
Inc., and B27 was obtained from Gibco (Thermo Fisher Scientific,
Inc.). MTT and dimethyl sulfoxide (DMSO) were obtained from
Sigma-Aldrich (Merck KGaA). Purmorphamine was obtained from
Selleckchem; it was dissolved in DMSO to make a stock solution at a
concentration of 10 mM. Antibodies against CD44 (cat. no. 15675),
CD133 (cat. no. 18470), Oct4 (cat. no. 60242), ALDH1A1 (cat. no.
15910), Nanog (cat. no. 14295), Bcl2 (cat. no. 12789), Bax (cat.
no. 50599), cleaved-caspase-3 (cat. no. 25546), caspase-8 (cat. no.
13423), Cyclin D1 (cat. no. 60186), proliferating cell nuclear
antigen (PCNA; cat. no. 24036), Shh (cat. no. 20697), Smo (cat. no.
20787) and GAPDH (cat. no. 10494) were obtained from ProteinTech
Group, Inc. Antibodies against cleaved-caspase-9 (cat. no. 9509)
were obtained from Cell Signaling Technology, Inc. Antibodies
against Gli1 (cat. no. DF7523) and Gli2 (cat. no. DF7541) were
obtained from Affinity Biosciences, Inc. The primers of CD44,
CD133, Oct4, ALDH1A1, Nanog and GAPDH were acquired from BGI
Genomics Co., Ltd. The EJ cell line has been authenticated by short
tandem repeat profiling.
Tumorsphere formation assay
The bladder EJ and UM-UC-3 cells were cultured in
serum-supplemented medium (SSM), which consisted of RPMI 1640
medium with 10% FBS and 1% penicillin/streptomycin. Cells were
maintained in the incubator with 5% CO2 at 37°C for 48
h. In order to achieve tumorsphere formation, bladder cancer cells
were seeded into 24-well plates at a density of 5,000 cells per
well and were cultured in serum-free medium (SFM) consisting of
Dulbecco's modified Eagle's medium: Nutrient Mixture F-12
(DMEM/F12; Gibco; Thermo Fisher Scientific, Inc.) with 20 ng/ml
bFGF, 20 ng/ml EGF, 5 µg/ml insulin and 2% B27 for 3 days, when the
tumorspheres began to form. Tumorspheres were maintained in the
incubator with 5% CO2 at 37°C for 7 days. Tumorspheres
were imaged by an inverted microscope at a magnification of ×100.
For investigating the effect of EGCG and purmorphamine on the
tumorsphere formation capacity, various concentrations of EGCG (0,
30, 60 or 90 µM) or purmorphamine (1 µM) were added to each well.
After 7 days of treatment in the incubator with 5% CO2
at 37°C, the number of EJ and UM-UC-3 tumorspheres >50 µm in
diameter that had formed was photographed and counted with the
inverted microscope.
Western blot analysis
UM-UC-3 and EJ cells were incubated in SSM, and
tumorspheres were incubated in SFM in the incubator with 5%
CO2 at 37°C. EGCG- and purmorphamine-treated
tumorspheres, and cells were washed with PBS, followed by lysing in
Radio Immunoprecipitation assay buffer (Beyotime Institute of
Biotechnology). Bicinchoninic Acid Protein assay (Pierce; Thermo
Fisher Scientific, Inc.) were utilized to detect the concentration
of each protein. Proteins (50 µg per lane) were then separated by
SDS-PAGE on a 10% gel and transferred to nitrocellulose membranes
(EMD Millipore). After blocking in 5% non-fat dry milk at room
temperature for 1 h, the membranes were incubated with primary
antibodies against Cyclin D1, Shh, Nanog (all 1:500 dilution),
CD44, CD133, Oct4, ALDH1A1, Bcl2, Bax, cleaved-caspase-3,
cleaved-caspase-9, caspase-8, PCNA, Smo, Gli1, Gli2 and GAPDH (all
1:1,000 dilution) at 4°C overnight. Then they were incubated with
horseradish peroxidase-conjugated goat anti-rabbit (cat. no. S0001)
or anti-mouse (cat. no. S0002; both 1:10,000 dilution; Affinity
Biosciences) immunoglobin G secondary antibodies for 1 h at room
temperature. GAPDH was used as the loading control. The signals
were detected with enhanced chemiluminescence detection system
(cat. no. WBKLS0500; EMD Millipore). The target bands were
quantified using ImageJ version 1.46 software (National Institutes
of Health).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Bladder cancer adherent cells were cultured in SSM,
which consisted of RPMI-1640 medium with 10% FBS and 1%
penicillin/streptomycin, and tumorspheres were cultured in SFM.
Bladder cancer adherent cells and tumorspheres were maintained at
37°C in an incubator containing 5% CO2 for 7 days. Total
RNA from the cells was isolated with TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) and transcribed into cDNA using 5X
All-In-One RT MasterMix (Applied Biological Materials, Inc.)
according to the manufacturers' protocol with 1 µg total RNA. The
RT-qPCR analysis was performed using the EvaGreen 2X qPCR MasterMix
(Applied Biological Materials, Inc.) and primers of CD44, CD133,
Oct4, ALDH1A1, Nanog and GAPDH on the LightCycler® 96
real-time PCR detection system (Roche Molecular Systems, Inc.). The
mRNA expression for each gene was normalized by GAPDH. The
amplification reactions were as follows: An initial hold step (95°C
for 5 min) and 45 cycles of a two-step PCR (95°C for 15 sec, 54°C
for 30 sec and 72°C for 30 sec). Fold changes in the expression of
each gene were calculated by the 2−ΔΔCq method (21). The primer sequences are shown in
Table I.
 | Table I.Reverse transcription-quantitative
polymerase chain reaction sequences. |
Table I.
Reverse transcription-quantitative
polymerase chain reaction sequences.
| Gene | Direction | Sequence
(5′-3′) |
|---|
| CD44 | F |
GACACATATTGTTTCAATGCTTCAGC |
|
| R |
GATGCCAAGATGATCAGCCATTCTGGAAT |
| CD133 | F |
TACAACGCCAAACCACGACTGT |
|
| R |
TCTGAACCAATGGAATTCAAGACCCTTT |
| Oct4 | F |
TGGGATATACACAGGCCGATG |
|
| R |
TCCTCCACCCACTTCTGAG |
| ALDH1A1 | F |
GCACGCCAGACTTACCTGTC |
|
| R |
CCTCCTCAGTTGCAGGATTAAAG |
| Nanog | F |
TTTGTGGGCCTGAAGAAAACT |
|
| R |
AGGGCTGTCCTGAATAAGCAG |
| GAPDH | F |
CAAGGTCACCATGACAACTTTG |
|
| R |
GTCCACCACCCTGTTGCTGTAG |
Cell viability analysis
Both bladder cancer cells (1×104/well)
were seeded into 96-well plates (Corning Incorporated), which were
grown in SSM and incubated with various concentrations of EGCG (0,
30, 60, 90, 120, 150 and 180 µM) in an incubator with 5%
CO2 at 37°C for 48 h. After incubation, 20 µl MTT
solution (5 mg/ml in PBS) was added to each well, and the plates
were further incubated for 4 h at 37°C. Then, the medium containing
MTT was discarded and 150 µl DMSO was added to dissolve the
formazan crystals. Absorbance was measured at 490 nm using a
microplate reader. All measurements were performed in
triplicate.
Cell cycle analysis
Bladder cancer adherent cells, which cultured in
SSM, and tumorspheres, which cultured in SFM, were maintained at
37°C in an incubator containing 5% CO2 for 7 days. The
cells were collected and then washed twice with ice-cold PBS. Then
the cells were fixed in 70% cold ethanol at 4°C overnight. After
that, cells were treated with 200 µg/ml PureLinkRNase A, 20 µg/ml
propidium iodide (PI) and 0.1% Triton X-100 (all from Invitrogen;
Thermo Fisher Scientific, Inc.) at 37°C for 15 min. Next, the cell
cycle was analyzed in FACSCalibur™ (BD Biosciences).
Detection of apoptotic cells by flow
cytometry
The apoptosis assay was performed using a
fluorescein isothiocyanate (FITC)-Annexin V Apoptosis Detection kit
purchased from BD Biosciences. EJ and UM-UC-3 tumorspheres, which
cultured in SFM, were seeded into 6-well plates at a density of
1×106/well and treated with various concentrations of
EGCG (0, 30, 60 and 90 µM) for 7 days. Cells were centrifuged at
1,000 × g for 5 min at 4°C and washed twice with PBS. Then the
cells were resuspended in 100 µl cold binding buffer, and incubated
with 5 µl FITC-Annexin-V and 5 µl PI. After 1 min at room
temperature in dark, the apoptotic cells were detected by flow
cytometry within 1 h. The data were analyzed using FlowJo version
10.0.7 software (FlowJo LLC).
Immunofluorescence staining
EJ and UM-UC-3 tumorspheres, which cultured in SFM,
were seeded into 6-well plates at a density of
1×106/well and treated with various concentrations of
EGCG (0, 30, 60 and 90 µM) for 7 days. The bladder tumorspheres
were washed in PBS Tween-20 (PBST), fixed with 4% paraformaldehyde
for 15 min at room temperature, and then were washed three times
with PBST. After that, plasma membranes were ruptured with 0.1%
Triton X-100 (Sigma-Aldrich; Merck KGaA) for 30 min. The
tumorspheres were then blocked with 5% bovine serum albumin
(Sigma-Aldrich; Merck KGaA) for 1 h at room temperature and stained
with rabbit anti-CD133 antibodies (1:100 dilution) at 4°C
overnight. They were then stained with FITC-conjugated goat
anti-rabbit antibodies (cat. no. S0008; 1:200 dilution; Affinity
Biosciences, Inc.) for 2 h at room temperature. Next,
4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich; Merck KGaA) was
used to stain the nuclei for 15 min at room temperature and mounted
in aqueous mounting medium (cat. no. ab128982; Abcam). The
fluorescent images were imaged using a fluorescence microscope at a
magnification of ×100.
Cell transfection
EJ and UM-UC-3 cells were seeded into 6-well plates
at a density of 2×105 cells in RPMI-1640 medium
containing 10% FBS without antibiotics. After 12 h of incubation at
37°C, cells were then transiently transfected with
Gli1-overexpressing vectors (EX-NEG-M29-Gli1) or control vectors
(EX-NEG-M29; both 2 µg; GeneCopoeia, Inc.) with Lipofectamine
Reagent 3000 (Invitrogen; Thermo Fisher Scientific, Inc.) according
to the manufacturer's protocols. After 12 h of transfection, cells
were trypsinized and seeded into 24-well plates at a density of
5,000 cells per well containing SFM. Another 12 h later, cells were
treated with or without 60 µM EGCG for 4 days. Finally, the western
blot analysis was used to detect the protein expression levels.
Statistical analysis
Data are presented as mean ± standard deviation.
Student's t-test was used for comparison between two groups.
One-way analysis of variance with Dunnett post hoc test and Tukey
test was used for comparison between multiple groups. Statistical
tests were performed using Graph-Pad Prism 7.0 software (GraphPad
Software, Inc.). P<0.05 indicated that the difference between
groups was statistically significant.
Results
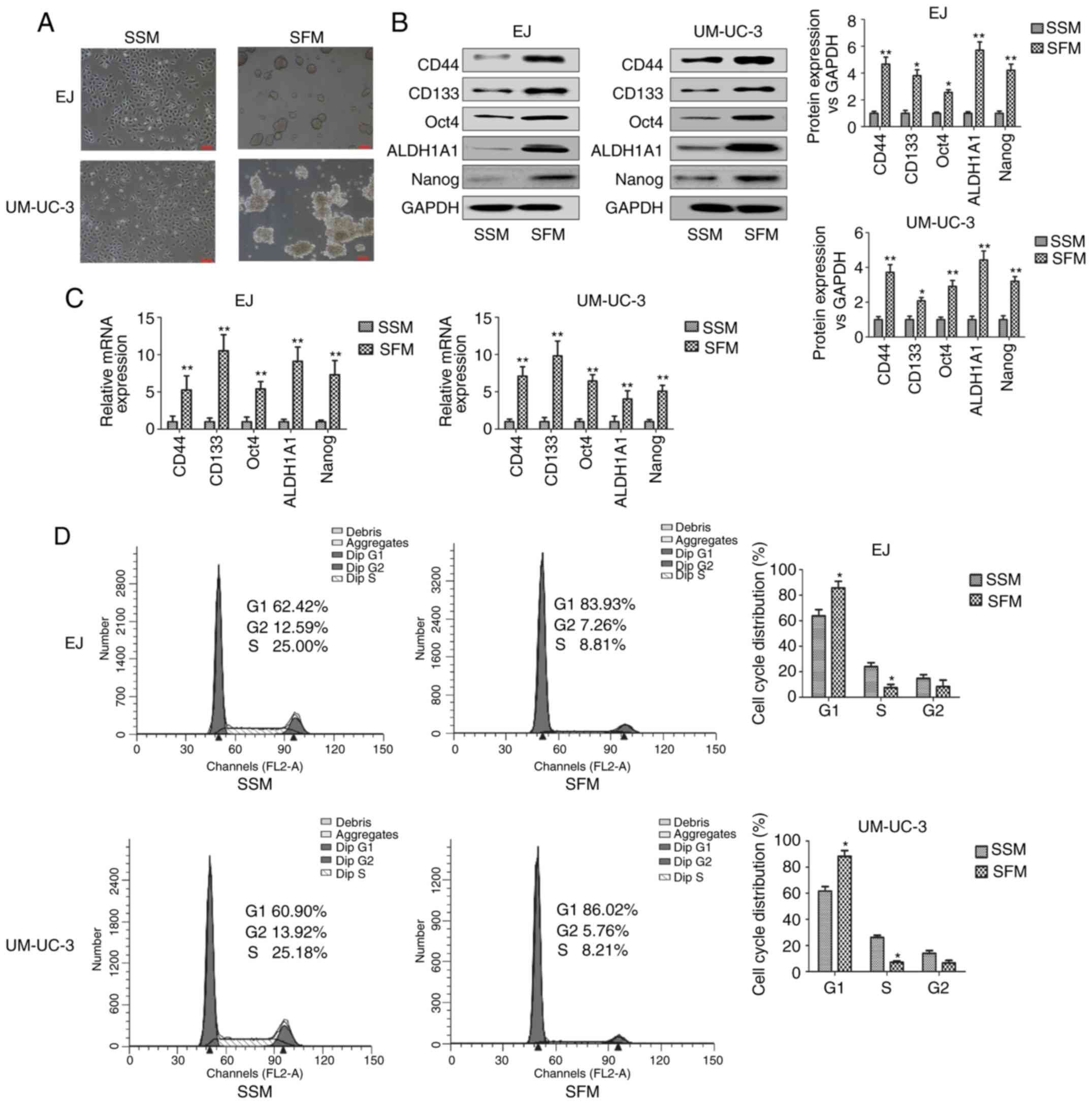
Enrichment of bladder CSCs by SFM
culture in vitro
SFM culture is commonly performed to enrich CSCs
properties, such as the capability of forming tumorspheres in
vitro (22,23). The expression of bladder CSCs
markers were examined including CD44, CD133, Oct4, ALDH1A1 and
Nanog. As shown in Fig. 1A, bladder
adherent cancer cells were grown in SSM and tumorspheres were
formed in EJ and UM-UC-3 bladder cancer cells cultured for 7 days
in SFM. The protein and mRNA expression levels of bladder CSCs
markers were significantly higher in cells grown in SFM compared
with those cultured in SSM (Fig. 1B and
C). Increasingly, reports have indicated that CSCs were more
likely to be quiescent than cancer cells (24,25).
Cell cycle analysis showed that the percentage of bladder CSCs in
the G1 phase of the cell cycle was greater than that of cells in
the S phase (Fig. 1D). It also
showed that the percentage of tumorspheres, which were cultured in
SFM, in the G1 phase was significantly greater than that of bladder
adherent cells, which were cultured in SSM. Compared with adherent
cells, the percentage of tumorspheres in the G1 phase greater by
~20%, and the percentage of tumorspheres in the S phase was
significantly lower by 17%. This result is consistent with the
theory of CSCs. CSCs have been shown to always have a slower growth
rate compared with cancer cells as they are resistant to anticancer
drugs (26). These results
suggested that EJ and UM-UC-3 tumorsphere-forming cells cultured in
SFM showed the characteristics of bladder CSCs.
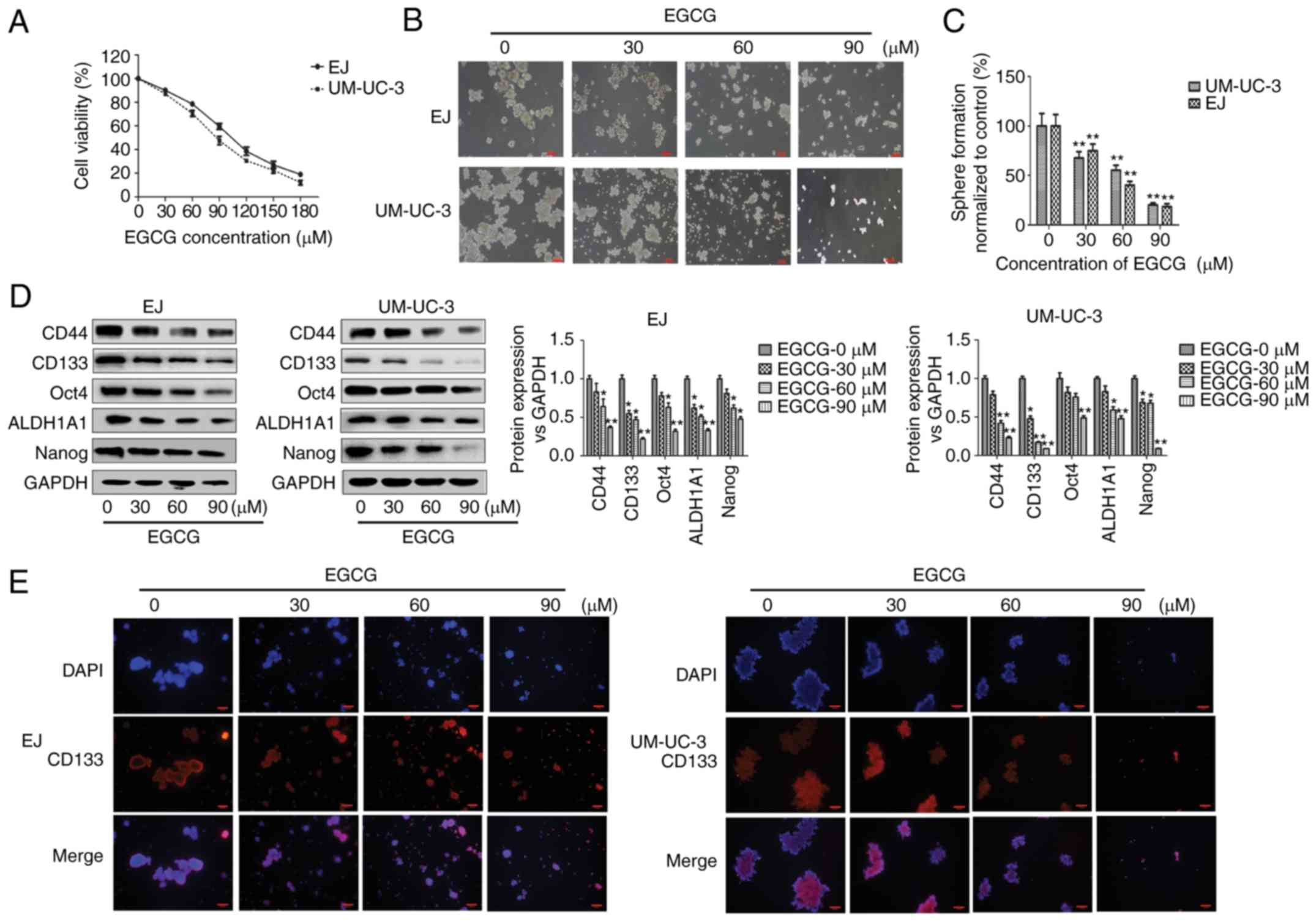
EGCG inhibits bladder CSCs
properties
To investigate the effect of EGCG on bladder cancer,
cells were treated with EGCG for 48 h, which inhibited cell
proliferation in what appeared to be a dose-dependent manner
(Fig. 2A). EGCG inhibited the
growth of EJ and UM-UC-3 cells with an IC50 of 100.9 and
84.5 µM at 48 h, respectively. Treatment of EJ and UM-UC-3
tumorspheres with increasing concentrations of EGCG (0, 30, 60 and
90 µM) decreased the size and number of bladder tumorspheres in
what appeared to be a dose-dependent manner (Fig. 2B and C). The number of tumorspheres
in cells treated with 30, 60 and 90 µM EGCG was significantly lower
compared with untreated cells. Western blot analysis showed that
the protein expression of bladder CSCs markers was significantly
downregulated in the tumorspheres of both cell lines compared with
untreated cells (Fig. 2D). In EJ
cells, CD44, Oct4 and Nanog were significantly downregulated at 60
and 90 µM EGCG, while CD133 and ALDH1A1 were significantly
downregulated at 30, 60 and 90 µM EGCG compared with untreated
cells. In UM-UC-3 cells, CD44 and ALDH1A1 were significantly
downregulated at 60 and 90 µM EGCG, CD133 and Nanog at 30, 60 and
90 µM EGCG, and Oct4 at 90 µM EGCG compared with untreated cells.
Immunofluorescent staining showed the seemingly dose-dependent
decrease of CD133-positive sphere-forming cells (Fig. 2E). These results indicateed that
EGCG inhibited bladder CSCs properties in EJ and UM-UC-3
tumorspheres.
EGCG induces apoptosis and inhibits
the expression of proliferation-related proteins in bladder
CSCs
Next, the authors examined the effects of EGCG on
cell apoptosis and protein expression in bladder CSCs. As shown in
Fig. 3A, EGCG treatment
significantly downregulated Bcl-2, and significantly upregulated
Bax, caspase-8, cleaved caspase-9 and cleaved caspase-3 compared
with untreated cells. In EJ cells, Bcl-2 was significantly
downregulated at 30, 60 and 90 µM EGCG. In these cells, Bax and
cleaved caspase-3 were significantly upregulated at 30, 60 and 90
µM EGCG, while caspase-8 was significantly upregulated at 90 µM
EGCG and cleaved caspase-9 was significantly upregulated at 60 and
90 µM EGCG. In UM-UC-3 cells, Bcl-2 was significantly downregulated
at 90 µM EGCG, while Bax, caspase-8, cleaved caspase-9 and cleaved
caspase-3 were significantly upregulated at 30, 60 and 90 µM
EGCG.
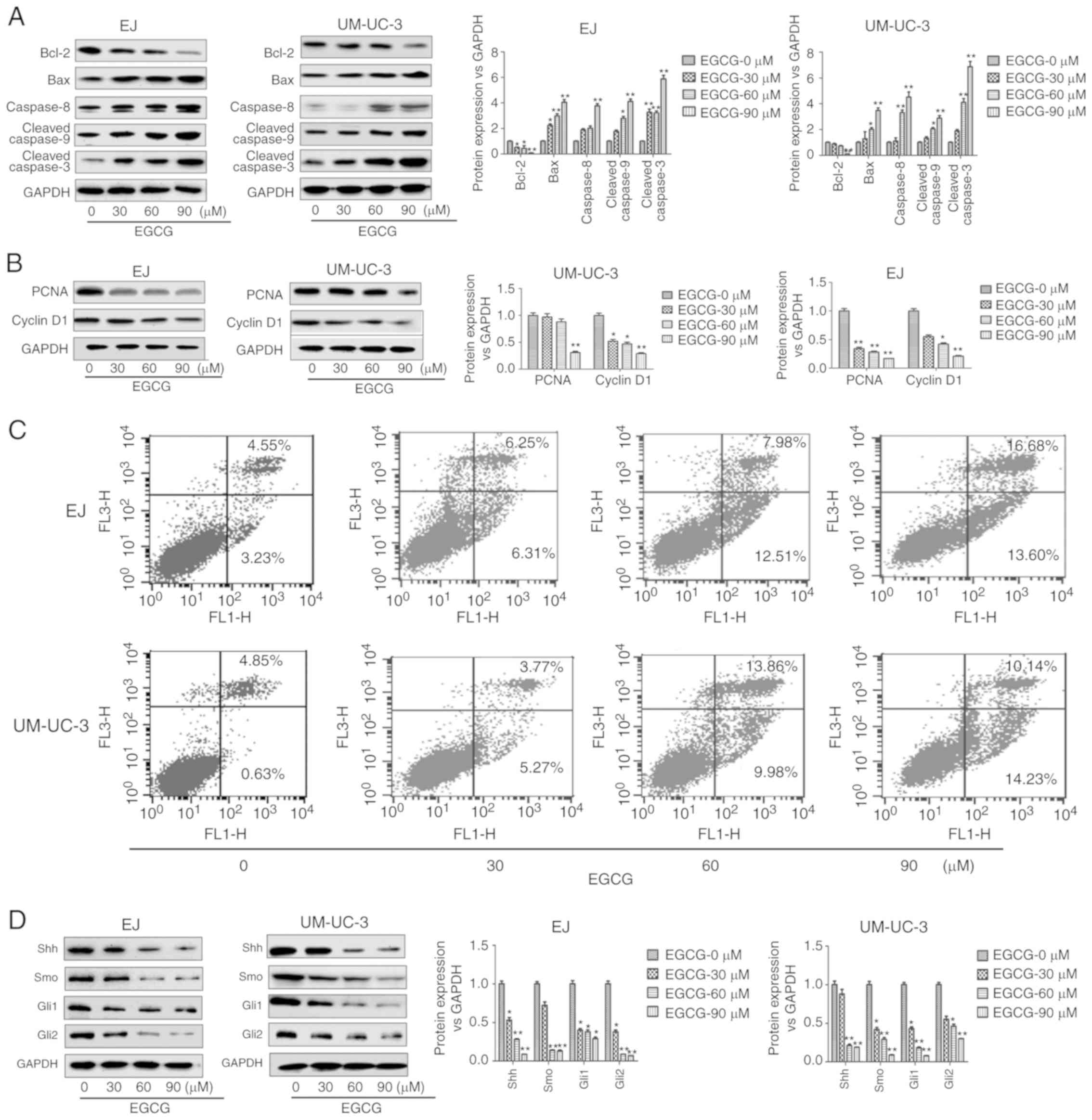 | Figure 3.EGCG induces apoptosis and reduces
the expression of cell proliferation-related proteins on bladder
CSCs. Bladder CSCs were treated with different concentrations of
EGCG for 7 days. (A) Expression levels of apoptosis-related
proteins, including Bcl-2, Bax, caspase-8, cleaved caspase-9 and
cleaved caspase-3 were measured by western blot analysis. (B)
Expression levels of cell proliferation-related proteins, including
PCNA and Cyclin D1 were measured by western blot analysis. (C) Cell
apoptosis was analyzed by flow cytometry. (D) The expression levels
of Shh signaling pathway-related proteins including Shh, Smo, Gli1
and Gli2 were detected by western blot analysis. Data are expressed
as mean ± standard deviation. *P<0.05, **P<0.01 vs. the
EGCG-0 µm group. EGCG, (−)-epigallocatechin-3-gallate; CSCs, cancer
stem cells; PCNA, proliferating cell nuclear antigen; Shh, sonic
hedgehog protein; Smo, smoothened homolog; Gli, zinc finger protein
GLI. |
EGCG treatment also downregulated the expression of
cell proliferation-related proteins PCNA and Cyclin D1 (17) (Fig.
3B). In UM-UC-3 cells, PCNA was significantly downregulated at
90 µM EGCG, while Cyclin D1 was significantly downregulated at 30,
60 and 90 µM EGCG compared with untreated cells. In EJ cells, PCNA
and Cyclin D1 were significantly downregulated at 60 and 90 µM
EGCG, while only PCNA was significantly downregulated at 30 µM EGCG
compared with untreated cells. Meanwhile, flow cytometry analysis
also showed that EGCG induced apoptosis of sphere-forming cells in
both EJ and UM-UC-3 cell lines as the rates of cell apoptosis
increased with increasing EGCG doses in what appeared to be a
dose-dependent manner (Fig. 3C).
These results suggested that EGCG decreased cell proliferation and
induced apoptosis in bladder CSCs.
Downregulation of Shh pathway
components mediates the inhibitory effect of EGCG on bladder
CSCs
To investigate whether EGCG could modulate the sonic
hedgehog signaling pathway on bladder CSCs, the expression of
Shh-related markers after the treatment of EGCG was analysed.
Western blot analysis showed that 30, 60 and 90 µM EGCG
significantly decreased the expression levels of Smo, Shh, Gli1 and
Gli2 compared with untreated cells (Fig. 3D). However, no significant
differences were observed in Smo expression in EJ cells and Shh
expression in UM-UC-3 cells treated with 30 µm EGCG.
To further investigate the involvement of the Shh
pathway in the effect of EGCG, cells were co-treated with
purmorphamine, a specific activator of the Shh pathway (27), and EGCG. As shown in Fig. 4A and 4B, purmorphamine treatment promoted
tumorsphere formation and abolished the effect of EGCG on bladder
CSCs. EGCG significantly decreased and purmorphamine significantly
increased tumorsphere formation compared with untreated cells
(Fig. 4B). Tumorsphere formation in
EGCG and purmorphamine co-treated cells was similar to untreated
cells, and significantly increased when compared with EGCG-treated
cells. These results were confirmed by western blot analysis, which
showed that the effects of EGCG on the expression of bladder CSCs
protein markers (Fig. 4C), cell
proliferation-related proteins (Fig.
4D) and apoptosis-related proteins (Fig. 4E) were significantly reversed by
purmorphamine-induced Shh signaling pathway activation compared
with EGCG-treated cells. In addition, purmorphamine significantly
promoted the activation of Shh signaling pathway (Fig. 4F).
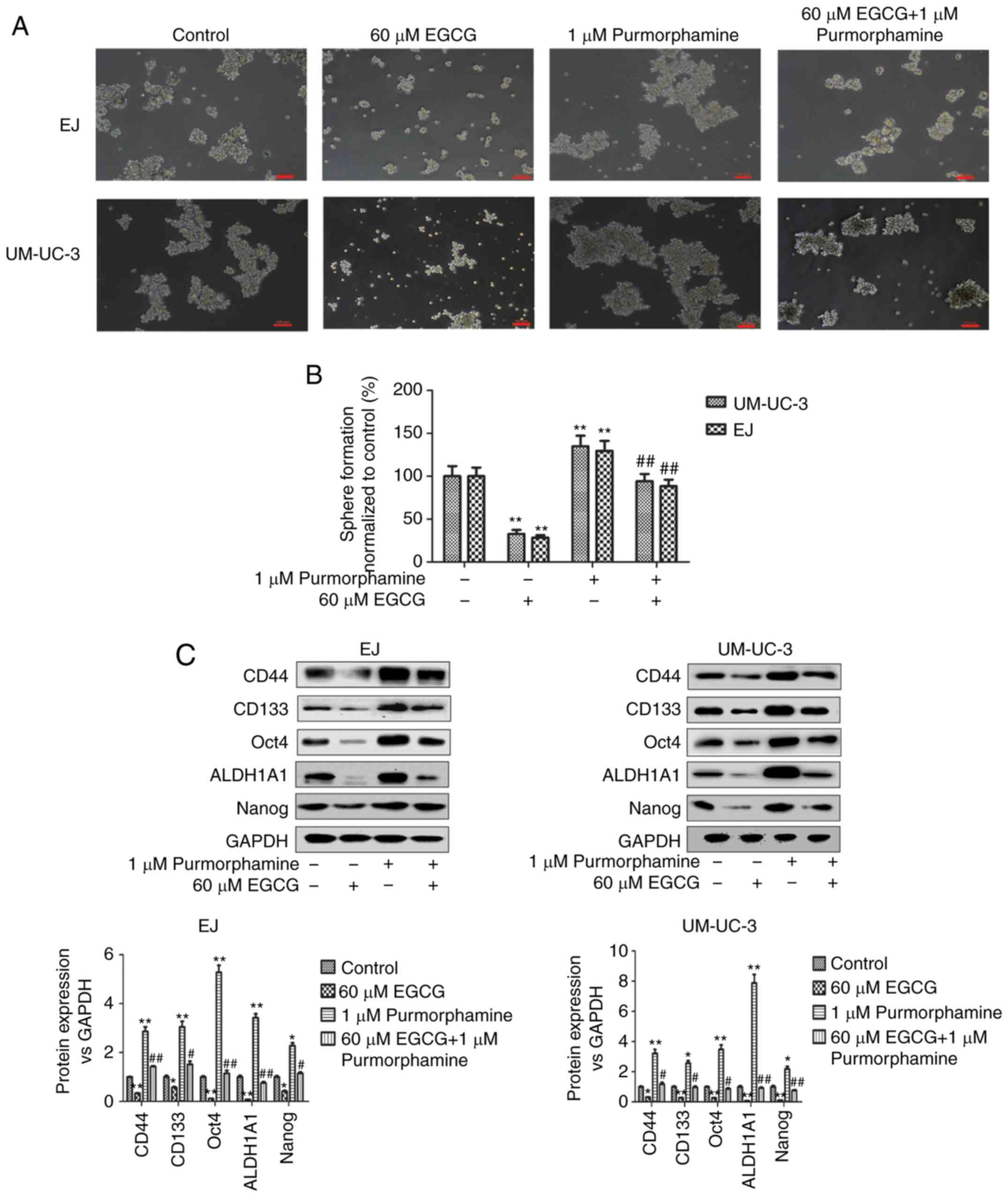 | Figure 4.Downregulation of the Shh signaling
pathway mediates the inhibitory effects of EGCG on bladder CSCs.
Bladder CSCs were treated with EGCG and purmorphamine for 7 days.
(A) Representative images of tumorspheres after co-treatment. Scale
bar, 100 µm. (B) Tumorspheres quantitation (mean ± standard
deviation, n=3). Western blot analysis was used to measure the
protein levels of (C) bladder CSCs markers. Bladder CSCs were
transfected with control vector or Gli1-overexpressing plasmids,
and then treated with 60 µM EGCG for 4 days. Western blot analysis
was used to measure the protein levels of (D) cell
proliferation-related proteins and (E) cell apoptosis-related
proteins. Bladder CSCs were transfected with control vector or
Gli1-overexpressing plasmids, and then treated with 60 µM EGCG for
4 days. Western blot analysis was used to measure the protein
levels of (F) Shh signaling pathway-related proteins. Bladder CSCs
were transfected with control vector or Gli1-overexpressing
plasmids, and then treated with 60 µM EGCG for 4 days. (G) Western
blot analysis was used to measure the protein levels of bladder
CSCs markers. Data are expressed as mean ± standard deviation.
*P<0.05, **P<0.01 vs. the control group.
#P<0.05, ##P<0.01 vs. the 60 µM EGCG
group. EGCG, (−)-epigallocatechin-3-gallate; CSCs, cancer stem
cells; Shh, sonic hedgehog protein; Smo, smoothened homolog; Gli,
zinc finger protein GLI; CD, cluster of differentiation; ALDH1A1,
retinal dehydrogenase 1; PCNA, proliferating cell nuclear
antigen. |
To confirm the involvement of the Shh signaling
pathway, cells were transfected with Gli1-overexpressing plasmids.
Overexpression of Gli1 significantly reversed the EGCG-induced
downregulation of bladder CSCs markers compared with EGCG-treated
cells (Fig. 4G). However, Gli1
expression in EJ cells treated with EGCG and EX-NEG-M29-Gli1 was
not significantly different from EJ cells treated with EGCG alone.
These results suggested that EGCG inhibited bladder CSCs by
suppressing the activity of the Shh signaling pathway.
Discussion
Previous studies have indicated that the
intervention effect of EGCG is common among CSC cell lines
(28–30). CSCs play an important role in the
recurrence and chemoresistance of bladder cancer; therefore,
targeting bladder CSCs is considered to be a critical strategy
(9). The Shh signaling pathway
plays a significant role in cancer and stem cells, and is involved
in maintaining the stemness of CSCs (11). Green tea has been demonstrated to
act as cancer preventive for primary cancer prevention and
catechins combine with anticancer drugs in tertiary cancer
prevention (31). EGCG is the most
abundant catechin in green tea and has shown anticancer activity
(14). EGCG inhibits breast CSCs by
decreasing the expression levels of the proliferation markers
Cyclin D1 (32). EGCG also
upregulates Bax, caspase-8, and cleaved caspases-3 and −9 in lung
CSCs (17).
Here, the authors of the present study demonstrated
that EGCG inhibited bladder CSCs by targeting the Shh signaling
pathway. In the present study, bladder CSCs were isolated from
adherent bladder cancer EJ and UM-UC-3 cells using the SFM culture
system. SFM is a commonly used method for the enrichment of CSCs
according to the characteristics of stem cells (33). In in vitro experiments, cells
cultured in SFM formed three-dimensional tumorspheres, and the
expression of bladder CSCs markers (CD44, CD133, Oct4, ALDH1A and,
Nanog) was upregulated. Evidence showed that CD133+
bladder cancer cells exhibited higher tumorigenic potential and
chemoresistance than CD133− cells (34). CD44+ bladder cancer was
first isolated from tumor specimens and associated with poor
clinical outcomes in bladder cancer (10). ALDH1A1 has been associated with poor
prognosis in urothelial carcinoma (35). Nanog and Oct4 have been shown to be
expressed at high levels in bladder cancer, and play a significant
role in tumor carcinogenesis (36,37). A
cell cycle analysis detected a greater percentage of EJ and UM-UC-3
tumorspheres contained cells in the G1 phase than cells in the S
phase (38). These results were
consistent with the theory of stem cells, as CSCs are mostly
quiescent cells; they may survive treatment, rebuild tumors and
subsequently become the cause of resistance to cancer treatment
(39). The present data suggest
that cells acquired stem cells characteristics when cultured in SFM
in vitro.
Increasing evidence supports the role of natural
products in inhibiting tumor cells, including CSCs. The effect of
common natural products, such as curcumin, sulforaphane and diallyl
trisulfide, on inhibiting CSCs has been shown, and studies have
indicated that they contribute to the chemotherapy for cancers
(40–42). Tessmann et al (43) found that
1-thiocarbamoyl-3,5-diaryl-4,5-dihydro-1H-pyrazole, as one of the
derivatives of pyrazoline, has an antitumor potential towards
bladder cancer. Liu et al (44) showed that metformin repressed
bladder CSCs through the prostaglandin G/H synthase 2/prostaglandin
E2/STAT3 signaling pathway. Natural products have shown potential
in the prevention and treatment of different tumors (45). EGCG is an important polyphenol
antioxidant from green tea, and its antitumor effect on bladder
tumors involves different pathways (31). In the present study, the authors
showed that EGCG suppressed the bladder CSCs properties by
decreasing tumorsphere formation activity and reducing the
expression of bladder CSCs markers. Moreover, EGCG inhibited the
expression of cell proliferation-related proteins and stimulated
the apoptosis of bladder CSCs. These data indicated that EGCG
exhibits a suppressive effect on bladder CSCs.
Increasingly, studies are demonstrating the role of
the Shh signaling pathway in the maintenance of CSCs (11,33).
During early development, Shh is expressed in the notochord and
promotes the development of the bladder (46). The secreted Shh molecule represses
Ptch1, thereby activating the Gli transcription factor in target
cells during urothelial mesenchymal differentiation (47). In bladder cancer, Shh signaling
pathway activation is related to tumor development and metastasis
(13). Additionally, Gli1 and Gli2
overexpression promotes mammosphere formation and increases
mammosphere size (48). However,
the involvement of the Shh signaling pathway in the effect of EGCG
on bladder CSCs remains poorly understood. Therefore, the authors
of the current study aimed to determine whether the Shh signaling
pathway mediates the effect of EGCG on bladder CSCs.
In the present study, it was showed that the Shh
signaling pathway was significantly downregulated after EGCG
treatment of bladder CSCs, as evidenced by the decreased expression
of Smo, Shh, Gli1 and Gli2. Purmorphamine is a Shh signaling
pathway agonist that targets the Smo transmembrane protein
(39,49,50).
Wu et al (49) showed that
purmorphamine treatment upregulated the expression of Gli1 and
Gli2. The current study showed that activation of the Shh signaling
pathway by purmorphamine abolished the effects of EGCG on
tumorsphere formation activity, the expression of stem cell markers
and cell proliferation-related proteins, and apoptosis in bladder
CSCs. Overexpression of Gli1 has been previously shown to
upregulate CD44, ALDH1A1 and Oct4 (51–53).
Taken together, these results indicated that the
suppressive effect of EGCG on bladder CSCs is mediated by Shh
signaling pathway inhibition. However, the current study is based
on in vitro findings involving a SFM culture system.
Additional in vivo studies are warranted to validate the
findings of the current study and other CSCs model in bladder
cancer, such as side population and cell isolation using specific
markers.
In conclusion, the present demonstrated that EGCG
suppressed bladder CSCs by inhibiting the Shh signaling pathway.
These findings provide new insight into the potential mechanism
underlying the effect of EGCG on bladder cancer.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National
Natural Science Foundation of China (grant no. 81373005), Anhui
Medical University scientific research funds (Hefei, China; grant
no. H0514) and the Natural Science Foundation of Anhui Province
(grant no. 1608085QH173).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
DY and CZ designed the experiment. XS, JS, EL, HG
and YL performed the experiment. XS analyzed the data and wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interest
The authors declare that they have no conflict of
interest.
References
|
1
|
Witjes JA, Compérat E, Cowan NC, De Santis
M, Gakis G, Lebret T, Ribal MJ, Van der Heijden AG and Sherif A;
European Association of Urology, : EAU guidelines on
muscle-invasive and metastatic bladder cancer: Summary of the 2013
guidelines. Eur Urol. 65:778–792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DeGeorge KC, Holt HR and Hodges SC:
Bladder cancer: Diagnosis and treatment. Am Fam Physician.
96:507–514. 2017.PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dinney CP, McConkey DJ, Millikan RE, Wu X,
Bar-Eli M, Adam L, Kamat AM, Siefker-Radtke AO, Tuziak T, Sabichi
AL, et al: Focus on bladder cancer. Cancer Cell. 6:111–116. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y, Wang Z, Yu J, Shi JZ, Wang C, Fu
WH, Chen ZW and Yang J: Cancer stem-like cells contribute to
cisplatin resistance and progression in bladder cancer. Cancer
Lett. 322:70–77. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Y, Lin K, Yang Z, Han N, Quan X, Guo X
and Li C: Bladder cancer stem cells: Clonal origin and therapeutic
perspectives. Oncotarget. 8:66668–66679. 2017.PubMed/NCBI
|
|
7
|
Shiozawa Y, Nie B, Pienta KJ, Morgan TM
and Taichman RS: Cancer stem cells and their role in metastasis.
Pharmacol Ther. 138:285–293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shervington A and Lu C: Expression of
multidrug resistance genes in normal and cancer stem cells. Cancer
Invest. 26:535–542. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hatina J, Parmar HS, Kripnerova M, Hepburn
A and Heer R: Urothelial carcinoma stem cells: Current concepts,
controversies, and methods. Methods Mol Biol. 1655:121–136. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chan KS, Espinosa I, Chao M, Wong D,
Ailles L, Diehn M, Gill H, Presti J Jr, Chang HY, van de Rijn M, et
al: Identification, molecular characterization, clinical prognosis,
and therapeutic targeting of human bladder tumor-initiating cells.
Proc Natl Acad Sci USA. 106:14016–14021. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Syed IS, Pedram A and Farhat WA: Role of
sonic hedgehog (Shh) signaling in bladder cancer stemness and
tumorigenesis. Curr Urol Rep. 17:112016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qian W, Kong X, Zhang T, Wang D, Song J,
Li Y, Li X, Geng H, Min J, Kong Q, et al: Cigarette smoke
stimulates the stemness of renal cancer stem cells via sonic
hedgehog pathway. Oncogenesis. 7:242018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Islam SS, Mokhtari RB, Noman AS, Uddin M,
Rahman MZ, Azadi MA, Zlotta A, van der Kwast T, Yeger H and Farhat
WA: Sonic hedgehog (Shh) signaling promotes tumorigenicity and
stemness via activation of epithelial-to-mesenchymal transition
(EMT) in bladder cancer. Mol Carcinog. 55:537–551. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fujiki H, Watanabe T, Sueoka E, Rawangkan
A and Suganuma M: Cancer prevention with green tea and its
principal constituent, EGCG: From early investigations to current
focus on human cancer stem cells. Mol Cells. 41:73–82.
2018.PubMed/NCBI
|
|
15
|
Luo KW, Chen W, Lung WY, Wei XY, Cheng BH,
Cai ZM and Huang WR: EGCG inhibited bladder cancer SW780 cell
proliferation and migration both in vitro and in vivo via
downregulation of NF-κB and MMP-9. J Nutr Biochem. 41:56–64. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo KW, Lung WY, Chun-Xie, Luo XL and
Huang WR: EGCG inhibited bladder cancer T24 and 5637 cell
proliferation and migration via PI3K/AKT pathway. Oncotarget.
9:12261–12272. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu J, Jiang Y, Yang X, Wang S, Xie C, Li
X, Li Y, Chen Y, Wang X, Meng Y, et al: Wnt/β-catenin pathway
mediates (−)-Epigallocatechin-3-gallate (EGCG) inhibition of lung
cancer stem cells. Biochem Biophys Res Commun. 482:15–21. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Y, Wang XQ, Zhang Q, Zhu JY, Li Y,
Xie CF, Li XT, Wu JS, Geng SS, Zhong CY and Han HY:
(−)-Epigallocatechin-3-Gallate inhibits colorectal cancer stem
cells by suppressing Wnt/β-catenin pathway. Nutrients. 9:E5722017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin CH, Chao LK, Hung PH and Chen YJ: EGCG
inhibits the growth and tumorigenicity of nasopharyngeal
tumor-initiating cells through attenuation of STAT3 activation. Int
J Clin Exp Pathol. 7:2372–2381. 2014.PubMed/NCBI
|
|
20
|
Fujiki H, Sueoka E, Watanabe T and
Suganuma M: Synergistic enhancement of anticancer effects on
numerous human cancer cell lines treated with the combination of
EGCG, other green tea catechins, and anticancer compounds. J Cancer
Res Clin Oncol. 141:1511–1522. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun FF, Hu YH, Xiong LP, Tu XY, Zhao JH,
Chen SS, Song J and Ye XQ: Enhanced expression of stem cell markers
and drug resistance in sphere-forming non-small cell lung cancer
cells. Int J Clin Exp Pathol. 8:6287–6300. 2015.PubMed/NCBI
|
|
23
|
Pozzi V, Sartini D, Rocchetti R,
Santarelli A, Rubini C, Morganti S, Giuliante R, Calabrese S, Di
Ruscio G, Orlando F, et al: Identification and characterization of
cancer stem cells from head and neck squamous cell carcinoma cell
lines. Cell Physiol Biochem. 36:784–798. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells-perspectives on current status and future directions:
AACR Workshop on cancer stem cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Williams SA, Anderson WC, Santaguida MT
and Dylla SJ: Patient-derived xenografts, the cancer stem cell
paradigm, and cancer pathobiology in the 21st century. Lab Invest.
93:970–982. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ohishi T, Koga F and Migita T: Bladder
cancer stem-like cells: Their origin and therapeutic perspectives.
Int J Mol Sci. 17:E432015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sinha S and Chen JK: Purmorphamine
activates the hedgehog pathway by targeting Smoothened. Nat Chem
Biol. 2:29–30. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wubetu GY, Shimada M, Morine Y, Ikemoto T,
Ishikawa D, Iwahashi S, Yamada S, Saito Y, Arakawa Y and Imura S:
Epigallocatechin gallate hinders human hepatoma and colon cancer
sphere formation. J Gastroenterol Hepatol. 31:256–264. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang P, Xu C, Chen L, Chen A, Wu X, Zhou
M, Haq IU, Mariyam Z and Feng Q: Epigallocatechin-3-gallate
inhibited cancer stem cell-like properties by targeting
hsa-mir-485-5p/RXRα in lung cancer. J Cell Biochem. 119:8623–8635.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Toden S, Tran HM, Tovar-Camargo OA,
Okugawa Y and Goel A: Epigallocatechin-3-gallate targets cancer
stem-like cells and enhances 5-fluorouracil chemosensitivity in
colorectal cancer. Oncotarget. 7:16158–16171. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fujiki H, Sueoka E, Rawangkan A and
Suganuma M: Human cancer stem cells are a target for cancer
prevention using (−)-epigallocatechin gallate. J Cancer Res Clin
Oncol. 143:2401–2412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mineva ND, Paulson KE, Naber SP, Yee AS
and Sonenshein GE: Epigallocatechin-3-gallate inhibits stem-like
inflammatory breast cancer cells. PLoS One. 8:e734642013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang D, Kong X, Li Y, Qian W, Ma J, Wang
D, Yu D and Zhong C: Curcumin inhibits bladder cancer stem cells by
suppressing sonic hedgehog pathway. Biochem Biophys Res Commun.
493:521–527. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang P, Watanabe M, Kaku H, Ueki H,
Noguchi H, Sugimoto M, Hirata T, Yamada H, Takei K, Zheng S, et al:
Cancer stem cell-like characteristics of a CD133+
subpopulation in the J82 human bladder cancer cell line. Mol Clin
Oncol. 1:180–184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Keymoosi H, Gheytanchi E, Asgari M,
Shariftabrizi A and Madjd Z: ALDH1 in combination with CD44 as
putative cancer stem cell markers are correlated with poor
prognosis in urothelial carcinoma of the urinary bladder. Asian Pac
J Cancer Prev. 15:2013–2020. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sedaghat S, Gheytanchi E, Asgari M, Roudi
R, Keymoosi H and Madjd Z: Expression of cancer stem cell markers
OCT4 and CD133 in transitional cell carcinomas. Appl
Immunohistochem Mol Morphol. 25:196–202. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Amini S, Fathi F, Mobalegi J,
Sofimajidpour H and Ghadimi T: The expressions of stem cell
markers: Oct4, Nanog, Sox2, nucleostemin, Bmi, Zfx, Tcl1, Tbx3,
Dppa4, and Esrrb in bladder, colon, and prostate cancer, and
certain cancer cell lines. Anat Cell Biol. 47:1–11. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Morata-Tarifa C, Jiménez G, García MA,
Entrena JM, Griñán-Lisón C, Aguilera M, Picon-Ruiz M and Marchal
JA: Low adherent cancer cell subpopulations are enriched in
tumorigenic and metastatic epithelial-to-mesenchymal
transition-induced cancer stem-like cells. Sci Rep. 6:187722016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu J, Yang X, Chen Y, Jiang Y, Wang SJ,
Li Y, Wang XQ, Meng Y, Zhu MM, Ma X, et al: Curcumin suppresses
lung cancer stem cells via inhibiting Wnt/β-catenin and sonic
hedgehog pathways. Phytother Res. 31:680–688. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li X, Meng Y, Xie C, Zhu J, Wang X, Li Y,
Geng S, Wu J, Zhong C and Li M: Diallyl Trisulfide inhibits breast
cancer stem cells via suppression of Wnt/β-catenin pathway. J Cell
Biochem. 119:4134–4141. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang F, Wang W, Li J, Zhang J, Wang X and
Wang M: Sulforaphane reverses gefitinib tolerance in human lung
cancer cells via modulation of sonic hedgehog signaling. Oncol
Lett. 15:109–114. 2018.PubMed/NCBI
|
|
42
|
Li X, Wang X, Xie C, Zhu J, Meng Y, Chen
Y, Li Y, Jiang Y, Yang X, Wang S, et al: Sonic hedgehog and
Wnt/β-catenin pathways mediate curcumin inhibition of breast cancer
stem cells. Anticancer Drugs. 29:208–215. 2018.PubMed/NCBI
|
|
43
|
Tessmann JW, Buss J, Begnini KR, Berneira
LM, Paula FR, de Pereira CM, Collares T and Seixas FK: Antitumor
potential of 1-thiocarbamoyl-3,5-diaryl-4,5-dihydro-1H-pyrazoles in
human bladder cancer cells. Biomed Pharmacother. 94:37–46. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu Q, Yuan W, Tong D, Liu G, Lan W, Zhang
D, Xiao H, Zhang Y, Huang Z, Yang J, et al: Metformin represses
bladder cancer progression by inhibiting stem cell repopulation via
COX2/PGE2/STAT3 axis. Oncotarget. 7:28235–28246. 2016.PubMed/NCBI
|
|
45
|
Dutta S, Mahalanobish S, Saha S, Ghosh S
and Sil PC: Natural products: An upcoming therapeutic approach to
cancer. Food Chem Toxicol. 128:240–255. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Villavicencio EH, Walterhouse DO and
Iannaccone PM: The sonic hedgehog-patched-gli pathway in human
development and disease. Am J Hum Genet. 67:1047–1054. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
DeSouza KR, Saha M, Carpenter AR, Scott M
and McHugh KM: Analysis of the sonic hedgehog signaling pathway in
normal and abnormal bladder development. PLoS One. 8:e536752013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu S, Dontu G, Mantle ID, Patel S, Ahn
NS, Jackson KW, Suri P and Wicha MS: Hedgehog signaling and Bmi-1
regulate self-renewal of normal and malignant human mammary stem
cells. Cancer Res. 66:6063–6071. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu X, Walker J, Zhang J, Ding S and
Schultz PG: Purmorphamine induces osteogenesis by activation of the
hedgehog signaling pathway. Chem Biol. 11:1229–1238. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Oliveira FS, Bellesini LS, Defino HL, da
Silva Herrero CF, Beloti MM and Rosa AL: Hedgehog signaling and
osteoblast gene expression are regulated by purmorphamine in human
mesenchymal stem cells. J Cell Biochem. 113:204–208. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xu M, Gong A, Yang H, George SK, Jiao Z,
Huang H, Jiang X and Zhang Y: Sonic hedgehog-glioma associated
oncogene homolog 1 signaling enhances drug resistance in
CD44(+)/Musashi-1(+) gastric cancer stem cells. Cancer Lett.
369:124–133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang L, Li L, Jiao M, Wu D, Wu K, Li X,
Zhu G, Yang L, Wang X, Hsieh JT and He D: Genistein inhibits the
stemness properties of prostate cancer cells through targeting
hedgehog-Gli1 pathway. Cancer Lett. 323:48–57. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ma D, Yu H, Xu S, Wang H, Zhang X, Ning T
and Wu B: Stathmin inhibits proliferation and differentiation of
dental pulp stem cells via sonic hedgehog/Gli. J Cell Mol Med.
22:3442–3451. 2018. View Article : Google Scholar : PubMed/NCBI
|