Introduction
Patients with breast cancer who undergo surgery are
likely to develop a second cancer in the contralateral breast, with
a 2- to 6-fold increased risk of contralateral breast cancer
(1–5).
Additionally, patients with breast cancer have a generally
increased risk of developing multiple primary cancer (MPC) in other
organs, such as ovarian, pancreatic and skin cancer (6). For these reasons, patients with breast
cancer often consider prophylactic surgery for the contralateral
breast and other organs. The registry of contralateral prophylactic
mastectomy (CPM) has more than doubled over a 6-year period
(7,8),
and the proportion of breast-conserving procedures for the
treatment of early stage breast cancer has declined accompanied by
a compensatory increase in the number of CPMs (9). Although patients with hereditary breast
cancer caused by a germline mutation in the breast cancer
susceptibility gene (BRCA) are at high risk of developing
multiple tumors, the mechanisms underlying the increased risk of
apparently non-hereditary multiple primary breast cancer have not
been elucidated.
Multiple cancers can arise simultaneously in regions
of normal tissue containing certain genetic and epigenetic
alterations (10). This phenomenon is
exemplified by ‘field cancerization’ (11), which makes common embryological
regions susceptible to neoplasms. Our previous study demonstrated
that global DNA demethylation was associated with genomic
instability in gastrointestinal cancer (12). Although genetic alterations are found
in only a minor fraction of cells from normal tissue (11,13),
somatic epigenetic alterations are commonly detected in normal
tissues adjacent to various types of cancers (14–20). Our
previous study evaluating levels of DNA demethylation in common
targets [i.e., repetitive sequences in the whole genome (10), such as long interspersed nucleotide
element-1 (LINE-1)] demonstrated that DNA demethylation of LINE-1
is associated with a predisposition to multiple tumors in patients
with colorectal cancer (10);
however, the general significance of LINE-1 demethylation in
carcinogenesis remains unknown. Additionally, we evaluated
satellite DNA comprising highly repetitive noncoding sequences
located in centromeric regions and implicated in chromosomal
stability (21).
Recent studies have provided insight into the role
of noncoding DNA in cancer susceptibility (10,21),
although these regions are poorly transcribed due to
heterochromatin structure. The appropriate transcription of
satellite regions is essential for accurate chromosomal segregation
(22), and elevated satellite-DNA
expression has been observed in various epithelial tumors (23), with the overexpression of satellite
RNA inducing abnormal segregation of chromosomes in experimental
studies (24). Additionally, we
demonstrated that overexpression of satellite alpha transcripts
(SAT) leads to chromosomal mis-segregation in normal mammary
epithelial cells, thereby enhancing chromosomal instability
(25). Moreover, SAT expression
levels correlate with DNA hypomethylation levels of SAT in both
normal and tumor tissues (25),
whereas demethylation of satellite DNA in normal gastric tissues
increases susceptibility to multiple gastric cancers (21). These results suggest that excessive
satellite RNA plays an important role in carcinogenesis and could
be involved in the mechanism underlying the development of multiple
tumors as a result of field cancerization.
In the present study, we evaluated the role of SAT
in field cancerization and cancer development in the bilateral
breast or MPCs in other organs in patients with breast cancer.
Materials and methods
Patients and specimens
Samples of tumor tissues and normal tissues without
cancerous mammary glands were collected from 167 female patients
who underwent incisional biopsy or a surgical procedure for breast
cancer diagnosis and treatment from July 2015 to July 2017 at
Saitama Medical Center, Jichi Medical University (Saitama, Japan).
The sample sizes were 165 tumor tissues and 109 normal tissues. The
tumor tissue samples were obtained during a surgical operation or
preoperative biopsy when patients were candidates for neoadjuvant
chemotherapy. When tumor tissue samples were obtained,
ultrasonography and 14G biopsy needle (ACECUT; TSK Laboratory,
Tochigi, Japan) were used both in preoperative biopsy and surgical
operations for accuracy. Normal tissues were also collected from
patients with breast cancer during the surgical operation and
defined as tissues at least 3 cm from the tumor and nipple and
microscopically identified as normal mammary glands according to
histologic examination of hematoxylin and eosin-stained sections.
All tissue specimens were immediately soaked in RNAlater (Ambion),
and after 24 to 48 h, removed from RNAlater and stored at
−80°C.
Clinical and pathological findings are presented in
Table IA. Family history was defined
as positive when one or more relatives of first and/or second
degree had a medical history of breast cancer. Human epidermal
growth factor receptor 2 (HER2) testing was performed according to
the American Society of Clinical Oncology/College of American
Pathologists guidelines (26). HER2
status was determined in all patients with invasive breast cancer
on the basis of one or more HER2 test results (negative, equivocal,
or positive). HER2-positive status was defined as an area of the
tumor with >10% contiguous and homogeneous tumor cells. If the
results were equivocal, reflex testing was performed using
fluorescence in situ hybridization to define HER2-positive
or -negative status (26).
Triple-negative breast cancer (TNBC) was characterized as cancer
exhibiting low expression of estrogen receptor, progesterone
receptor, and HER2. Tumor, Nodes, Metastasis (TNM) staging was
performed according to the American Joint Committee on Cancer and
the International Union for Cancer Control staging manual, 8th
edition (UICC.org). BRCA1 or BRCA2 mutation
status was not examined in this study.
 | Table I.Clinicopathological features of the
breast cancer patients and multivariate analysis. |
Table I.
Clinicopathological features of the
breast cancer patients and multivariate analysis.
| A,
Clinicopathological features of the patients with single breast
cancer (SBC), bilateral breast cancer (BBC), and multiple primary
cancer (MPC) |
|---|
|
|---|
| Variables | SBC (n=120) | BBC (n=27) |
P-valuec | MPC (n=27) |
P-valued |
|---|
| Agea (years) (<50/≥50) | 33/87 | 3/24 | 0.0861 |
|
|
|
| Ageb (years) (<63/≥63) | 61/59 |
|
| 11/16 | 0.398 |
| BMI
(kg/m2) (≥25/<25) | 37/83 | 9/18 | 0.821 | 6/21 | 0.485 |
| Family history
(negative/positive) | 104/16 | 23/4 | 0.764 | 25/2 | 0.528 |
| HER2e (negative/positive) | 69/37 | 16/9 | 1.00 | 10/10 | 0.216 |
| ERe (negative/positive) | 29/77 | 5/20 | 0.613 | 6/14 | 0.791 |
| TNBCe (negative/positive) | 88/18 | 21/4 | 1.00 | 18/2 | 0.738 |
| Te (0/1/2/3/4) | 18/55/38/6/9 | 10/15/6/1/0 | 0.113 | 7/16/2/0/0 | 0.0255 |
| Ne (0/1,2) | 87/39 | 25/9 | 0.678 | 21/5 | 0.342 |
| M (0/1) | 113/6 | 25/2 | 0.641 | 27/0 | 0.593 |
| lye (0/1/2/3) | 35/54/2/2 | 8/10/0/1 | 0.679 | 9/7/0/1 | 0.352 |
| ve (0/1/2) | 49/42/2 | 11/8/0 | 0.865 | 11/6/0 | 0.601 |
| rSAT of tumor
tissue [median (range)] | 1.20
(−0.0139–3.44) | 1.89
(0.201–3.28) | 0.0312 | 1.79
(0.690–2.60) | 0.0420 |
| rSAT of normal
tissue [median (range)] | 1.88
(−0.0331–3.38) | 2.50
(1.19–3.87) | 0.119 | 2.32
(1.03–3.87) | 0.407 |
|
| B, Multivariate
analysis to predict MPC |
|
| Multivariate
analysis | Stepwise
multivariate analysis |
|
|
|
|
|
Variables | OR | 95% CI | P-value | OR | 95% CI | P-value |
|
| Tf (0,1 vs. 2,3,4) | 8.07 | 1.79–36.3 | 0.00652 | 8.07 | 1.79–36.3 | 0.00652 |
| rSAT of tumor
tissueg (>1.5 vs.
≤1.5) | 2.96 | 1.15–7.61 | 0.0243 | 2.96 | 1.15–7.61 | 0.0243 |
This study was approved by the Research Ethics
Committee at Jichi Medical University, and written informed consent
was obtained from each study participant.
RNA extraction
Total RNA was extracted from the samples using the
Illustra RNAspin Mini RNA Isolation kit (GE Healthcare UK)
according to the manufacturer's instructions. To assess RNA quality
and yield, A260/A280 and A260/A230 ratios for RNA samples were
analyzed using a NanoDrop ND-1000 spectrophotometer (NanoDrop
Technologies, Inc.). Only RNA with an A260/A280 ratio >1.8 was
used for subsequent experiments.
DNA extraction
Dissected tissues or cultured cells were placed in
buffered proteinase K solution at 56°C for 3 h, and genomic DNA was
isolated and purified using an EZ1 Advanced XL and an EZ1 DNA
tissue kit (Qiagen) according to manufacturer's instructions. DNA
purity was assessed using a NanoDrop ND-1000 spectrophotometer
(NanoDrop Technologies, Inc.) at A260 and A280, with the A260/A280
ratio >1.8 in all instances.
Real-time reverse transcription
(RT)-PCR
RT was performed using a High Capacity RNA-to-cDNA
kit (Applied Biosystems) with thermal cycling of 37°C for 60 min,
followed by 95°C for 5 min and maintenance at 4°C. Real-time RT-PCR
assays were performed using SYBR Green technology, SYBR Premix Ex
Taq reagents (Tli RNaseH Plus; Applied Biosystems) and the
QuantStudio 12K Flex real-time PCR system (Applied Biosystems).
Thermal cycling conditions were as follows: 95°C for 30 sec for
denaturation, followed by 40 cycles of 95°C for 15 sec and 58°C for
1 min as the cycling stage, 95°C for 15 sec, 60°C for 1 min, and
95°C for 15 sec as the melting-curve stage. Gene expression was
determined using fluorescence-intensity measurements obtained using
QuantStudio 12K Flex data analysis (Applied Biosystems). A
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) fragment
was amplified as an internal control. Primers targeting satellite
alpha (forward, AAGGTCAATGGCAGAAAAGAA and reverse,
CAACGAAGGCCACAAGATGTC) and GAPDH (forward,
GAAGGTGAAGGTCGGAGT and reverse, GAAGATGGTGATGGGATTTC) were used.
Log (SAT/GAPDH expression) values were calculated from the
mean measurements and represented relative to SAT (rSAT)
levels.
Statistical analysis
Fisher's exact test comparing single breast cancer
(SBC) to bilateral breast cancer (BBC) used a cut-off value for
patient age at 50 years based on a report indicating that patients
aged <50 years have a higher cumulative 10-year risk of
contralateral breast cancer, despite a lack of BRCA
mutations (27). Fisher's exact test
comparing SBC to MPC used a cut-off value for patient age as the
median value (i.e., 63 years). Other cut-off values were determined
by median values. Cut-off values for the relative SAT-expression
levels were determined by receiver operating characteristic (ROC)
curve analysis. All statistical analyses were performed using EZR
(v.2.4; Saitama Medical Center, Jichi Medical University, Saitama,
Japan), which is a graphical user interface for R (v.3.4.1; The R
Foundation for Statistical Computing, Vienna, Austria) (28). Fisher's exact test was used to examine
associations between two categorical variables. Continuous
variables such as relative SAT-expression (rSAT) levels were
evaluated in Kolmogorov-Sminov's test and Bartlett's test, which
showed they were normally distributed and homoscedastic.
Thereafter, rSAT levels were compared with one-way ANOVA followed
by Dunnett's ‘many-to one’ post hoc test. Medians (ranges) of
continuous variables are presented in each table. Multivariate
analysis was performed by logistic regression. The level of
statistical significance was set at P<0.05 unless otherwise
specified.
Results
Comparisons of clinical and
pathological features in patients with breast cancer
Our analysis included 167 patients divided into
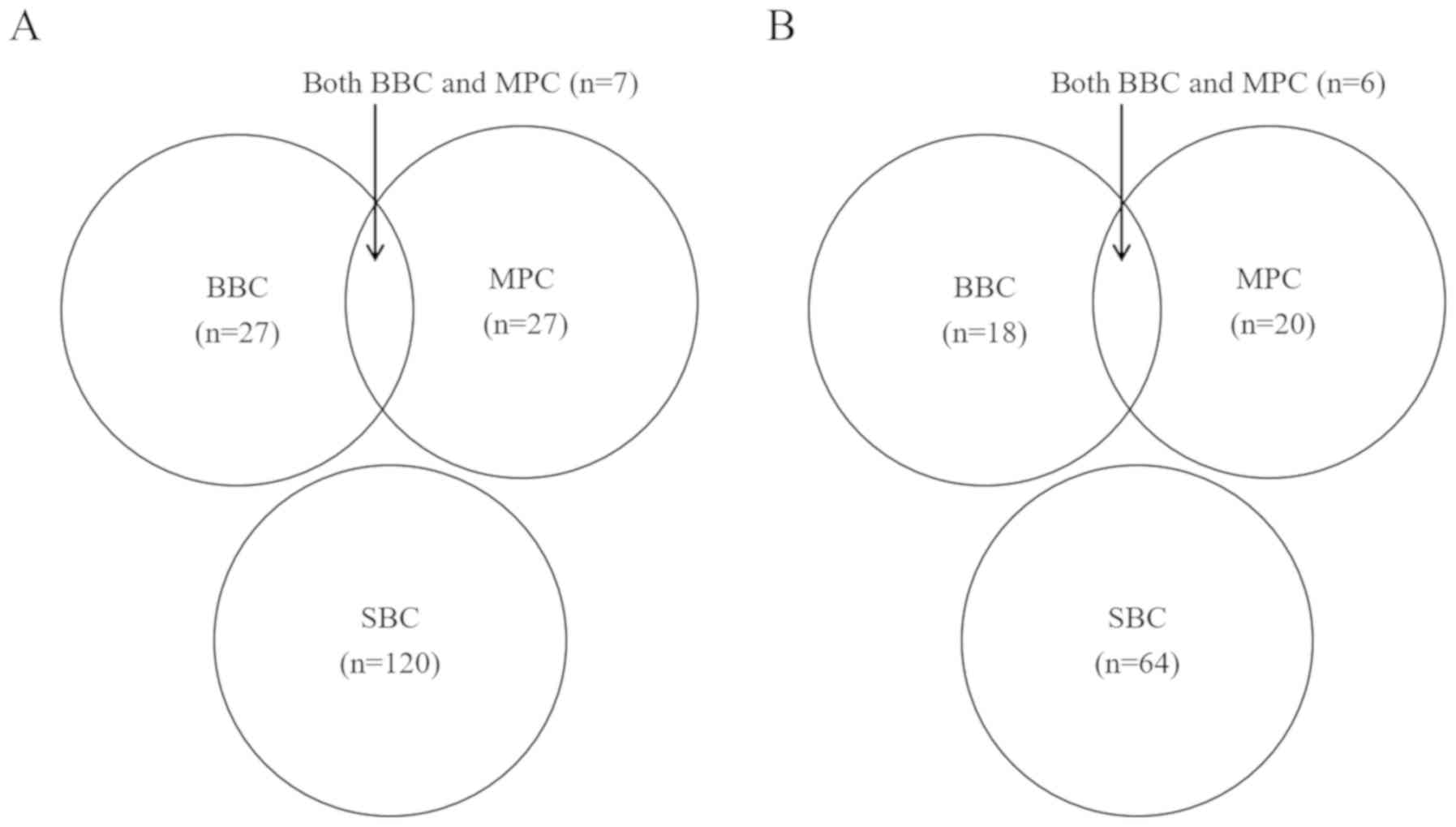
three groups according to their clinical characteristics (Fig. 1A) including 120 patients with SBC
(which excluded those with MPC), 27 patients with BBC (including 13
with synchronous BBC and 14 with metachronous BBC), and 27 patients
with MPC (including 4 with cervical cancer, 4 with gastric cancer,
3 with colorectal cancer, 3 with pancreatic cancer or IPMN, 2 with
uterine cancer, 2 with ovarian cancer, 2 with myelodysplastic
syndromes and lymphoma, and one each with lung cancer, thyroid
cancer, biliary tract cancer, tongue cancer, renal cancer, and
retroperitoneal sarcoma). Seven patients were included in both the
BBC and MPC groups. Clinicopathological factors of SBC patients
were compared to those of the BBC and MPC cases (Table I).
Relative expression level of SAT
(rSAT) in patients with SBC, BBC or MPC
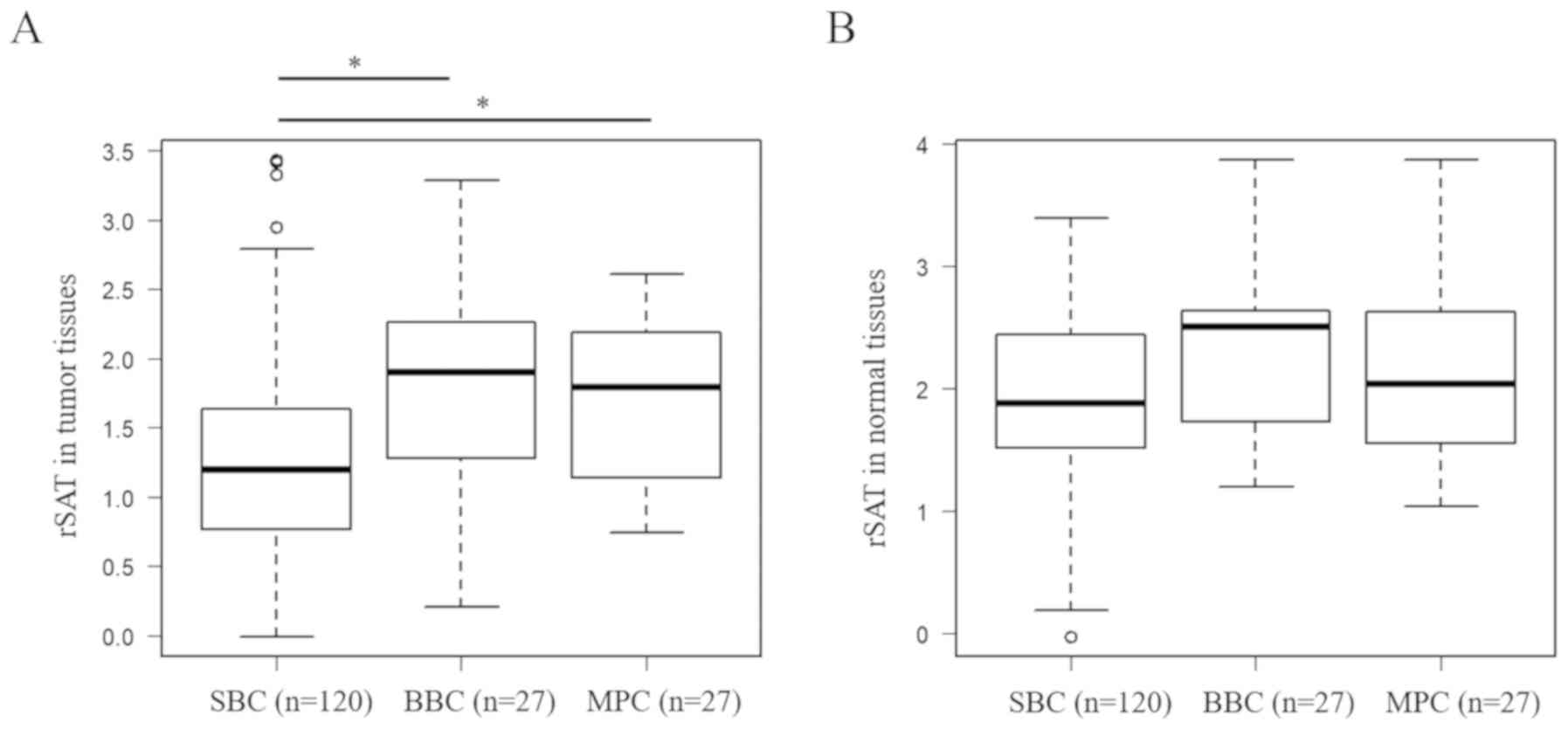
We measured rSAT levels in tumor tissues (Fig. 2A) and normal tissues (Fig. 2B) in each group. First, we performed
univariate analysis to determine the association between several
factors and the probability of developing BBC. Comparing SBC with
BBC, univariate analysis revealed that the rSAT level in tumor
tissues was the only significant factor associated with BBC
development [1.20 (−0.0139–3.44) in SBC vs. 1.89 (0.201–3.28) in
BBC; P=0.0312] (Table IA and Fig. 2A). Comparing SBC with MPC, univariate
analysis revealed that T (P=0.0255) and rSAT level in tumor tissues
[1.20 (−0.0139–3.44) in SBC vs. 1.79 (0.690–2.60) in MPC; P=0.0420]
were significantly associated with MPC development (Table IA and Fig.
2A).
For multivariate analysis, we established cut-off
values for rSAT level (according to ROC curve analysis) of 1.58
[area under the ROC curve (AUC): 0.649, 95% confidence interval
(CI): 0.534–0.764; sensitivity, 0.735; specificity, 0.621) for
tumor tissues in BBC and 1.54 (AUC: 0.679, 95% CI: 0.578–0.780;
sensitivity, 0.709; specificity, 0.615) for tumor tissues in MPC.
Based on these data, we utilized a cut-off value of 1.5 for
multivariate analysis. Similarly, in normal tissues, the cut-off
values were 2.47 (AUC: 0.615, 95% CI: 0.481–0.750; sensitivity,
0.770; specificity, 0.542) for rSAT level in BBC and 2.32 (AUC:
0.559, 95% CI: 0.406–0.713; sensitivity, 0.689; specificity, 0.421)
for rSAT level in MPC. Based on these data, we utilized a cut-off
value of 2.4 for multivariate analysis. Multivariate stepwise
logistic regression analysis showed that high rSAT levels in tumor
tissue (cut-off: 1.5, determined by ROC) and low T (T = 0.1;
cut-off: 1, determined by the median) were retained as significant
factors [odds ratio (OR): 2.96, 95% CI: 1.15–7.61; P=0.0243 for
rSAT level in tumor tissues; and OR: 8.07, 95% CI: 1.79–36.3;
P=0.00652 for T) (Table IB).
However, we failed to confirm the significance of
high rSAT levels in normal tissues. We excluded 71 patients with
BRCA-related clinical features, including a family history
of breast cancer, pathological TNBC (including 18 patients with
SBC, 4 with BBC, and 2 with MPC), history of ovarian cancer, and
younger age (<50 years). We then performed analyses using the
remaining 96 patients (Table II),
who were divided into three groups [i.e., SBC (n=64), BBC (n=18;
including 10 patients with synchronous BBC and 8 with metachronous
BBC), and MPC (n=20; including 3 with cervical cancer, 4 with
gastric cancer, 2 with colorectal cancer, 3 with pancreatic cancer
or IPMN, 1 with uterine cancer, 2 with myelodysplastic syndromes
and lymphoma, and 1 each with lung cancer, thyroid cancer, biliary
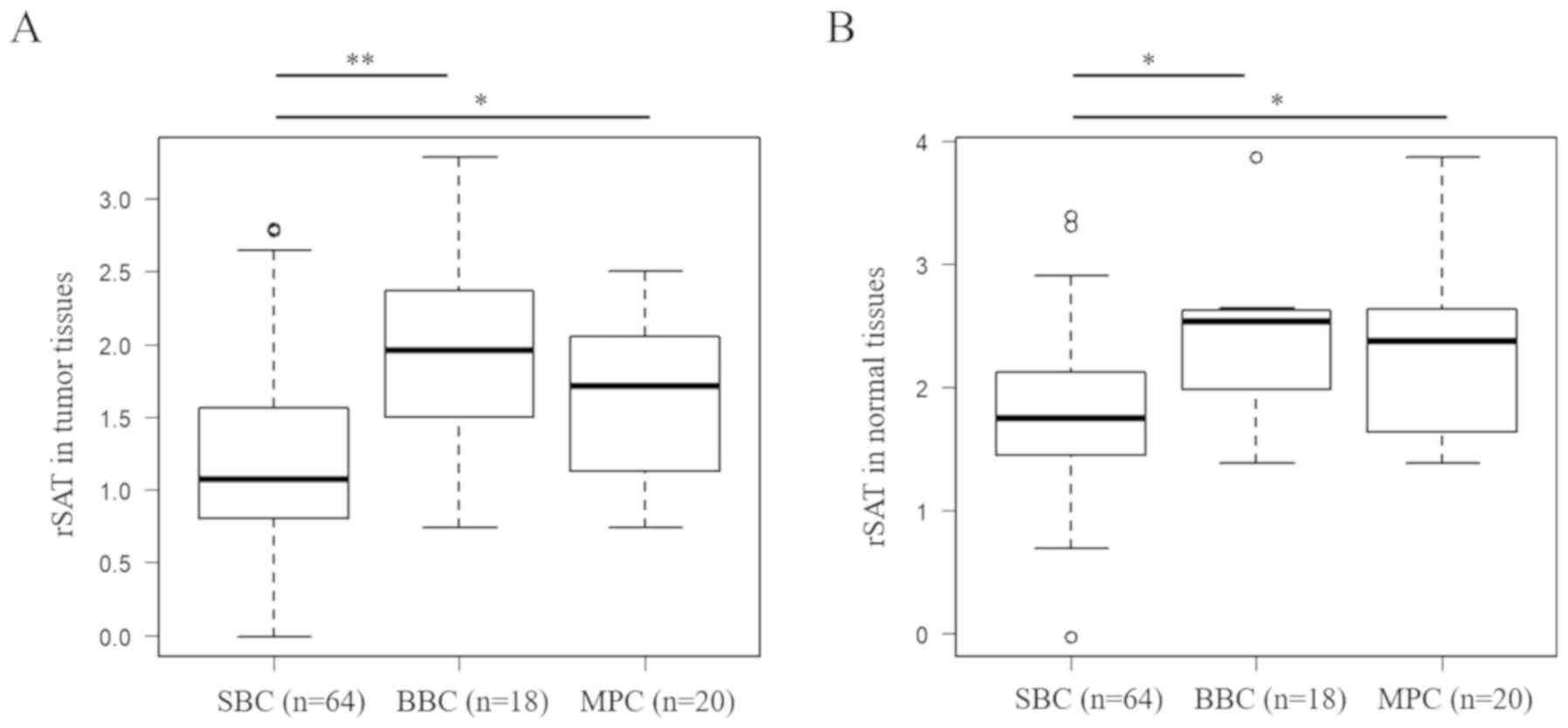
tract cancer, renal cancer, and retroperitoneal sarcoma)] (Table IIIA and Fig. 1B). Univariate analysis revealed that T
(P=0.0328), rSAT level in tumor tissues [1.08 (−0.0139–2.79) in SBC
vs. 1.95 (0.736–3.28) in BBC; P=0.000330] (Fig. 3A), and rSAT level in normal tissues
[1.74 (−0.0331–3.38) in SBC vs. 2.53 (1.38–3.87) in BBC; P=0.0310]
(Fig. 3B) were significant factors
for BBC development (Table IIIA).
Multivariate stepwise logistic regression analysis showed that high
rSAT level in normal tissues (cut-off: 2.4, determined by ROC) was
the only statistically significant factor (OR: 22.7, 95% CI:
3.43–151; P=0.00120) (Table IIIB).
Comparing SBC and MPC, univariate analysis revealed that T
(P=0.00960) and rSAT level in tumor tissues [1.08 (−0.0139–2.79) in
SBC vs. 1.71 (0.693–2.50) in MPC; P=0.0286] (Fig. 3A) and rSAT level in normal tissues
[1.74 (−0.0331–3.38) in SBC vs. 2.51 (1.41–3.87) in MPC; P=0.0267]
(Fig. 3B) were significant factors
for MPC development (Table IIIA).
Additionally, multivariate stepwise logistic regression analysis
showed that high rSAT level in normal tissues (cut-off: 2.4,
determined by ROC) was the only statistically significant factor
(OR: 13.0, 95% CI: 2.09–81.0; P=0.00601) (Table IIIC). In total, 20.0% of normal
tissues showed high rSAT levels (>2.4) among all 167 patients,
and 15.5% of normal tissues showed high rSAT levels (>2.4) among
96 patients harboring no BRCA-related features (Table II). These data suggested that 15 to
20% of patients had a high risk of developing BBC and MPC based on
normal tissue features.
 | Table II.Clinicopathological features of the
96 patients who harbored no BRCA-related clinical
features. |
Table II.
Clinicopathological features of the
96 patients who harbored no BRCA-related clinical
features.
|
| Data values |
|---|
|
|
|
|---|
| Variables | (median or n) | (range or %) |
|---|
| Age (years) | 66 | 50–89 |
| BMI
(kg/m2) | 23 | 14–38 |
| Follow-up period
(months) | 25.5 | 1.16–38.7 |
| BBC
(negative/positive) | 78/18 | 81.2/18.7 |
| HER2a (negative/positive) | 46/37 (NA20) | 44.7/35.9 |
| ERa (negative/positive) | 10/73 (NA20) | 9.71/70.9 |
| MPC
(negative/positive) | 76 / 20 | 79.2/20.8 |
| Ta (0/1/2/3/4) | 19/49/23/6/4
(NA2) |
18.5/47.6/22.3/5.83/3.88 |
| Na (0/1,2) | 76/27 | 73.8/26.2 |
| M (0/1) | 92/4 | 95.8/4.16 |
| lya (0/1/2/3) | 28/40/1/3
(NA31) |
27.2/38.8/0.97/2.91 |
| va (0/1/2) | 39/32/1 (NA31) | 37.9/31.1/0.97 |
 | Table III.Clinicopathological features of
patients who harbor no BRCA-related clinical features and
multivariate analyses to predict BBC and MPC. |
Table III.
Clinicopathological features of
patients who harbor no BRCA-related clinical features and
multivariate analyses to predict BBC and MPC.
| A,
Clinicopathological features of patients who harbored no
BRCA-related clinical features with single breast cancer
(SBC), bilateral breast cancer (BBC) and multiple primary cancer
(MPC) |
|---|
|
|---|
| Variables | SBC (n=64) | BBC (n=18) |
P-valueb | MPC (n=20) |
P-valuec |
|---|
| Agea (years) (<63/≥63) | 22/41 | 11/7 | 0.0593 | 9/14 | 0.801 |
| BMI
(kg/m2) (≥25/<25) | 26/38 | 9/8 | 0.783 | 7/13 | 0.795 |
| HER2d (negative/positive) | 31/25 | 9/7 | 1.00 | 7/8 | 0.574 |
| ERd (negative/positive) | 8/48 | 1/15 | 0.673 | 2/13 | 1.00 |
| Td (0/1/2/3/4) | 6/31/18/6/4 | 8/9/4/0/0 | 0.0328 | 7/12/1/0/0 | 0.00960 |
| Nd (0/1,2) | 44/21 | 19/4 | 0.281 | 4/19 | 0.284 |
| M (0/1) | 61/2 | 17/1 | 0.535 | 19/1 | 0.547 |
| lyd (0/1/2/3) | 18/31/1/2 | 5/4/0/1 | 0.438 | 7/6/0/0 | 0.668 |
| vd (0/1/2) | 27/24/1 | 6/4/0 | 0.783 | 9/4/0 | 0.487 |
| rSAT of tumor
tissue [median (range)] | 1.08
(−0.0139–2.79) | 1.95
(0.736–3.28) | 0.000330 | 1.71
(0.693–2.50) | 0.0286 |
| rSAT of normal
tissue [median (range)] | 1.74
(−0.0331–3.38) | 2.53
(1.38–3.87) | 0.0310 | 2.51
(1.41–3.87) | 0.0267 |
|
| B, Multivariate
analysis to predict BBC in patients who do not have
BRCA-related clinical features |
|
|
| Multivariate
analysis | Stepwise
multivariate analysis |
|
|
|
|
|
Variables | OR | 95% CI | P-value | OR | 95% CI | P-value |
|
| Te (0,1 vs. 2,3,4) | 2.51 | 0.349–18.0 | 0.361 | Excluded |
|
|
| rSAT of tumor
tissuef (>1.5 vs.
≤1.5) | 7.11 | 0.664–76.0 | 0.105 | Excluded |
|
|
| rSAT of normal
tissueg (>2.4 vs.
≤2.4) | 14.2 | 1.74–117 | 0.0133 | 22.7 | 3.43–151 | 0.00120 |
|
| C, Multivariate
analysis to predict MPC in patients who do not have
BRCA-related clinical features |
|
| Multivariate
analysis | Stepwise
multivariate analysis |
|
|
|
|
|
Variables | OR | 95% CI | P-value | OR | 95% CI | P-value |
|
| Th (0,1 vs. 2,3,4) | – | 0.00-inf | 0.994 | Excluded |
|
|
| rSAT of tumor
tissuei (>1.5 vs.
≤1.5) | 1.20 | 0.164–8.80 | 0.858 | Excluded |
|
|
| rSAT of normal
tissuej (>2.4 vs.
≤2.4) | 10.0 | 0.777–129 | 0.0774 | 13.0 | 2.09–81.0 | 0.00601 |
Discussion
We demonstrated that satellite alpha transcripts
(SAT) in tumor tissues were involved in the development of BBC and
MPC in 167 patients with breast cancer. However, in our initial
analysis, SAT in normal tissues did not have predictive value,
which might be explained by selection bias of the SBC patients
harboring BRCA-related clinical features and higher or lower
rSAT levels in normal tissues. We excluded these patients for
sub-analysis, finding higher normal-tissue rSAT levels in patients
with both BBC and MPC relative to those in patients with SBC. In
these selected patients with breast cancer, multivariate analysis
revealed rSAT levels >2.4 in normal tissues as a significant
predictor of development of BBC (OR, 22.7; P=0.00120) and MPC (OR,
13.0; P=0.00601). Our data indicated that patients with breast
cancer and exhibiting high rSAT levels in normal breast tissues had
a 10- to 20-fold increased risk for the development of multiple
cancers when they harbored no BRCA-related clinical
features. These patients had been excluded from the high-risk group
for BBC; however, our subsequent results highlighted unique
findings.
Well-established risk factors for bilateral breast
cancer include young age, a family history of breast cancer, and
TNBC, which are strongly associated with familial cancer due to a
BRCA mutation (1–5,27).
Nevertheless, some noncarriers of BRCA mutations show a high
cumulative risk for BBC and similar to that in women with
BRCA mutations (27). The
prevalence of BRCA mutations was found to be 16.3% (34/209)
for Korean breast cancer patients with BBC, implying that genetic
testing for BRCA is insufficient to determine predisposition
for BBC (29). Moreover, despite the
increased risk for developing BBC in patients harboring these
factors, few clinical risk factors for BBC have been
identified.
Epigenetic alterations are frequently observed in
normal tissues surrounding cancer tissues and can contribute to
epigenetic field cancerization. Epigenetic alterations have been
identified in many types of human cancers in connection with
carcinogenesis in the absence of genetic sequence abnormalities
(22–24,30) and
associated with chromosomal instability. We previously evaluated
DNA demethylation and its targets, including repetitive sequences,
LINE-1, and SAT, demonstrating that increased SAT-demethylation
levels in normal tissues of the stomach are associated with
susceptibility to multiple gastric cancers (21), and that LINE-1 demethylation levels in
normal tissues of patients with colon cancer are associated with
multiple primary colon cancer (10,21). These
results indicate that demethylation of satellite DNAs plays an
important role in field cancerization, resulting in the development
of multiple primary cancers. Moreover, our previous study revealed
that rSAT levels were correlated significantly with levels of SAT
hypomethylation (25), indicating
that the rSAT level is involved in the development of multiple
cancers. Additionally, a study of mitotic errors in SAT-transfected
cells indicated that SAT overexpression induces chromosomal
alterations (25). Our data combined
with those of previous studies indicate a potential role of
epigenetic alterations in field cancerization and its contribution
to the development of multiple cancers of the stomach, colon and
breast. The possibility that demethylation-associated
overexpression of satellite sequences and its correlation with
chromosomal instability results in field cancerization underlying
the development of multiple cancers is highly speculative.
Furthermore, it is possible that field cancerization may occur
prior to the presence of chromosomal instability.
Regarding prophylactic surgery, of 496,488 women
diagnosed with unilateral invasive breast cancer, 59.6% underwent
breast-conserving surgery, 33.4% underwent unilateral mastectomy,
and 7.0% underwent CPM; however, the survival benefits remain
controversial. Mortality does not differ between bilateral
mastectomy and breast-conserving surgery plus radiation (16), and studies report that prophylactic
mastectomy can decrease the risk of future breast cancer by 90 to
97% (31). The National Comprehensive
Cancer Network Guidelines and Preventive Service Task Force
Recommendations suggest that prophylactic mastectomy should be part
of the discussion among patients who test positive for BRCA
mutations or have a strong family history of breast cancer
(32). Howard-McNattesal reported
that 37% of BRCA-negative patients at their institution
chose contralateral prophylactic mastectomy, and another study
reported that 83.4% of patients who underwent contralateral
prophylactic mastectomy did not have a known BRCA mutation
(33). Contralateral prophylactic
mastectomy is acceptable to reduce fear or to circumvent the risk
of developing a second primary breast cancer, but insufficient
surgery should be avoided. In our study, patients with rSAT levels
>2.4 in normal tissues had a 20-fold increased risk of BBC when
lacking BRCA-related clinical features. Our data provide
important information for treatment decisions.
MPC in other organs is also likely to develop in
patients with breast cancer. Massive autopsy data demonstrate that
patients with breast cancer have a generally increased risk of
developing MPC in other organs, such as ovarian, pancreatic, and
skin cancer, and a decreased incidence of colorectal and cervical
cancer (6). Patients in our study
developed six gynecologic (22.2% in patients with MPC), seven
gastrointestinal (25.9% in patients with MPC), and three pancreatic
cancers (11.1% in patients with MPC), as well as other types of
cancer. Kato et al (34)
reported that 7.58% of patients develop primary breast cancer after
the resection of colorectal cancer. In the present study, our data
revealed a high frequency of gynecologic and gastrointestinal MPCs,
suggesting the need for wide surveillance, from gynecologic to
gastrointestinal areas, for patients who harbor higher rSAT levels
in normal tissues.
This study has limitations. The normal breast tissue
was donated during surgical operations, and SAT-expression levels
in normal tissues could only be assessed after surgery. It is
difficult to accurately obtain normal breast tissues around
malignant tumors by preoperative biopsy; however, it is possible to
resolve the difficulty of obtaining normal tissue samples
preoperatively by measuring SAT preoperatively using plasma
samples. Kondratova et al (35) measured two subtypes of satellite DNA
(HSATII and GSATII) in blood plasma by RT-PCR, finding that
transcript levels differed between healthy donors and patients with
colon cancer. Similarly, plasma SAT levels in patients with breast
cancer may be higher than those in healthy donors, and these levels
in patients with BBC or MPC may also be higher than those in
patients with SBC. These levels might provide a basis for detecting
the risk of developing BBC and MPC preoperatively. Moreover, the
continual measurement of plasma SAT levels during follow-up might
provide insight into the need for surveillance testing.
In conclusion, we demonstrated for the first time a
strong association between SAT-expression levels in normal breast
tissues and the development of BBC, as well as MPC, in other organs
when patients with breast cancer lack BRCA-related features.
Additionally, these patients exhibited a 22- and 13-fold increased
risk for the development of BBC and MPC, respectively. Importantly,
these patients lacking BRCA-related features had previously
been excluded from high-risk BBC categorization; therefore, our
study improves the understanding of the risk for the development of
BBC and MPC and the need for prophylactic surgery.
Acknowledgements
Not applicable.
Funding
This study was supported by a grant-in-aid for
Scientific Research (no. JP 17K10562) from the Ministry of
Education, Culture, Sports, Science and Technology and the JKA
Foundation through its promotion funds from the Keirin Race [no.
27-1-068 (2)].
Availability of data and materials
The datasets generated during this study are
available from the corresponding author upon reasonable
request.
Authors' contributions
NK designed this study and wrote the initial draft
of the manuscript. KS contributed to the analysis and
interpretation of data and assisted with the preparation of the
manuscript. IA, YE, ST, HI, FW, KI, MS, KF, FK and TR conducted the
data collection, analysis and interpretation of the data and
critically reviewed the manuscript. All authors read and approved
the final version of the manuscript and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
This study was approved by the Research Ethics
Committee at Jichi Medical University. Written informed consent was
obtained from each study participant.
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SAT
|
satellite alpha transcripts
|
|
BBC
|
bilateral breast cancer
|
|
SBC
|
single breast cancer
|
|
MPC
|
multiple primary cancer
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
TNBC
|
triple-negative breast cancer
|
|
CPM
|
contralateral prophylactic
mastectomy
|
|
BRCA
|
breast cancer susceptibility gene
|
|
rSAT
|
relative expression of satellite alpha
transcripts
|
|
RDL
|
relative demethylation level
|
|
ROC
|
receiver operating characteristic
|
|
LINE-1
|
long interspersed nucleotide
element-1
|
|
IPMN
|
intraductal papillary mucinous
neoplasm
|
|
CI
|
confidence interval
|
|
OR
|
odds ratio
|
|
HR
|
hazard ratio
|
References
|
1
|
Beinart G, Gonzalez-Angulo AM, Broglio K,
Mejia J, Ruggeri A, Mininberg E, Hortobagyi GN and Valero V:
Clinical course of 771 patients with bilateral breast cancer:
Characteristics associated with overall and recurrence-free
survival. Clin Breast Cancer. 7:867–874. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen Y, Thompson W, Semenciw R and Mao Y:
Epidemiology of contralateral breast cancer. Cancer Epidemiol
Biomarkers Prev. 8:855–861. 1999.PubMed/NCBI
|
|
3
|
Kheirelseid EA, Jumustafa H, Miller N,
Curran C, Sweeney K, Malone C, McLaughlin R, Newell J and Kerin MJ:
Bilateral breast cancer: Analysis of incidence, outcome, survival
and disease characteristics. Breast Cancer Res Treat. 126:131–140.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hartmann LC, Schaid DJ, Woods JE, Crotty
TP, Myers JL, Arnold PG, Petty PM, Sellers TA, Johnson JL,
McDonnell SK, et al: Efficacy of bilateral prophylactic mastectomy
in women with a family history of breast cancer. N Engl J Med.
340:77–84. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li CI, Malone KE, Porter PL and Daling JR:
Epidemiologic and molecular risk factors for contralateral breast
cancer among young women. Br J Cancer. 89:513–518. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shibahara Y, Sugawara Y, Miki Y, Hata S,
Takahashi H, Nakamura Y, Suzuki T, Ohuchi N, Tsuji I and Sasano H:
Analysis of multiple primary cancer autopsy cases associated with
breast cancer: 2002-2010. Pathol Int. 66:695–700. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Momoh AO, Cohen WA, Kidwell KM, Hamill JB,
Qi J, Pusic AL, Wilkins EG and Matros E: Tradeoffs associated with
contralateral prophylactic mastectomy in women choosing breast
reconstruction: Results of a prospective multicenter cohort. Ann
Surg. 266:158–164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tuttle TM, Habermann EB, Grund EH, Morris
TJ and Virnig BA: Increasing use of contralateral prophylactic
mastectomy for breast cancer patients: A trend toward more
aggressive surgical treatment. J Clin Oncol. 25:5203–5209. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Albornoz CR, Matros E, Lee CN, Hudis CA,
Pusic AL, Elkin E, Bach PB, Cordeiro PG and Morrow M: Bilateral
mastectomy versus breast-conserving surgery for early-stage breast
cancer: The role of breast reconstruction. Plast Reconstr Surg.
135:1518–1526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kamiyama H, Suzuki K, Maeda T, Koizumi K,
Miyaki Y, Okada S, Kawamura YJ, Samuelsson JK, Alonso S, Konishi F
and Perucho M: DNA demethylation in normal colon tissue predicts
predisposition to multiple cancers. Oncogene. 31:5029–5037. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Braakhuis BJ, Tabor MP, Kummer JA, Leemans
CR and Brakenhoff RH: A genetic explanation of Slaughter's concept
of field cancerization: Evidence and clinical implications. Cancer
Res. 63:1727–1730. 2003.PubMed/NCBI
|
|
12
|
Suzuki K, Suzuki I, Leodolter A, Alonso S,
Horiuchi S, Yamashita K and Perucho M: Global DNA demethylation in
gastrointestinal cancer is age dependent and precedes genomic
damage. Cancer Cell. 9:199–207. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deng G, Lu Y, Zlotnikov G, Thor AD and
Smith HS: Loss of heterozygosity in normal tissue adjacent to
breast carcinomas. Science. 274:2057–2059. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ahlquist T, Lind GE, Costa VL, Meling GI,
Vatn M, Hoff GS, Rognum TO, Skotheim RI, Thiis-Evensen E and Lothe
RA: Gene methylation profiles of normal mucosa, and benign and
malignant colorectal tumors identify early onset markers. Mol
Cancer. 7:942008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Belshaw NJ, Pal N, Tapp HS, Dainty JR,
Lewis MP, Williams MR, Lund EK and Johnson IT: Patterns of DNA
methylation in individual colonic crypts reveal aging and
cancer-related field defects in the morphologically normal mucosa.
Carcinogenesis. 31:1158–1163. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Konishi K, Shen L, Jelinek J, Watanabe Y,
Ahmed S, Kaneko K, Kogo M, Takano T, Imawari M, Hamilton SR and
Issa JP: Concordant DNA methylation in synchronous colorectal
carcinomas. Cancer Prev Res (Phila). 2:814–822. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Menigatti M, Truninger K, Gebbers JO,
Marbet U, Marra G and Schär P: Normal colorectal mucosa exhibits
sex- and segment-specific susceptibility to DNA methylation at the
hMLH1 and MGMT promoters. Oncogene. 28:899–909. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paun BC, Kukuruga D, Jin Z, Mori Y, Cheng
Y, Duncan M, Stass SA, Montgomery E, Hutcheon D and Meltzer SJ:
Relation between normal rectal methylation, smoking status, and the
presence or absence of colorectal adenomas. Cancer. 116:4495–4501.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ushijima T: Epigenetic field for
cancerization. J Biochem Mol Biol. 40:142–150. 2007.PubMed/NCBI
|
|
20
|
Worthley DL, Whitehall VL, Buttenshaw RL,
Irahara N, Greco SA, Ramsnes I, Mallitt KA, Le Leu RK, Winter J, Hu
Y, et al: DNA methylation within the normal colorectal mucosa is
associated with pathway-specific predisposition to cancer.
Oncogene. 29:1653–1662. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saito M, Suzuki K, Maeda T, Kato T,
Kamiyama H, Koizumi K, Miyaki Y, Okada S, Kiyozaki H and Konishi F:
The accumulation of DNA demethylation in Sat α in normal
gastric tissues with Helicobacter pylori infection renders
susceptibility to gastric cancer in some individuals. Oncol Rep.
27:1717–1725. 2012.PubMed/NCBI
|
|
22
|
Herrera LA, Prada D, Andonegui MA and
Dueñas-González A: The epigenetic origin of aneuploidy. Curr
Genomics. 9:43–50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kawano H, Saeki H, Kitao H, Tsuda Y, Otsu
H, Ando K, Ito S, Egashira A, Oki E, Morita M, et al: Chromosomal
instability associated with global DNA hypomethylation is
associated with the initiation and progression of esophageal
squamous cell carcinoma. Ann Surg Oncol. 21 (Suppl 4):S696–S702.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rodriguez J, Frigola J, Vendrell E,
Risques RA, Fraga MF, Morales C, Moreno V, Esteller M, Capellà G,
Ribas M and Peinado MA: Chromosomal instability correlates with
genome-wide DNA demethylation in human primary colorectal cancers.
Cancer Res. 66:8462–9468. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ichida K, Suzuki K, Fukui T, Takayama Y,
Kakizawa N, Watanabe F, Ishikawa H, Muto Y, Kato T, Saito M, et al:
Overexpression of satellite alpha transcripts leads to chromosomal
instability via segregation errors at specific chromosomes. Int J
Oncol. Mar 16–2018.(Epub ahead of print). doi:
10.3892/ijo.2018.4321 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wolff AC, Hammond ME, Hicks DG, Dowsett M,
McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M,
Fitzgibbons P, et al: Recommendations for human epidermal growth
factor receptor 2 testing in breast cancer: American Society of
Clinical Oncology/College of American Pathologists clinical
practice guideline update. J Clin Oncol. 31:3997–4013. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reiner AS, John EM, Brooks JD, Lynch CF,
Bernstein L, Mellemkjaer L, Malone KE, Knight JA, Capanu M, Teraoka
SN, et al: Risk of asynchronous contralateral breast cancer in
noncarriers of BRCA1 and BRCA2 mutations with a family history of
breast cancer: A report from the Women's Environmental Cancer and
Radiation Epidemiology Study. J Clin Oncol. 31:433–439. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kang E, Seong MW, Park SK, Lee JW, Lee J,
Kim LS, Lee JE, Kim SY, Jeong J, Han SA, et al: The prevalence and
spectrum of BRCA1 and BRCA2 mutations in Korean population: Recent
update of the Korean Hereditary Breast Cancer (KOHBRA) study.
Breast Cancer Res Treat. 151:157–168. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feinberg AP and Vogelstein B:
Hypomethylation distinguishes genes of some human cancers from
their normal counterparts. Nature. 301:89–92. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rebbeck TR, Friebel T, Lynch HT, Neuhausen
SL, vant Veer L, Garber JE, Evans GR, Narod SA, Isaacs C, Matloff
E, et al: Bilateral prophylactic mastectomy reduces breast cancer
risk in BRCA1 and BRCA2 mutation carriers: The PROSE Study Group. J
Clin Oncol. 22:1055–1062. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bevers TB, Armstrong DK, Arun B, Carlson
RW, Cowan KH, Daly MB, Fleming I, Garber JE, Gemignani M, Gradishar
WJ, et al: Breast cancer risk reduction. J Natl Compr Canc Netw.
5:676–701. 2007.PubMed/NCBI
|
|
33
|
Fu Y, Zhuang Z, Dewing M, Apple S and
Chang H: Predictors for contralateral prophylactic mastectomy in
breast cancer patients. Int J Clin Exp Pathol. 8:3748–3764.
2015.PubMed/NCBI
|
|
34
|
Kato T, Suzuki K, Muto Y, Sasaki J,
Tsujinaka S, Kawamura YJ, Noda H, Horie H, Konishi F and Rikiyama
T: Multiple primary malignancies involving primary sporadic
colorectal cancer in Japan: Incidence of gastric cancer with
colorectal cancer patients may be higher than previously
recognized. World J Surg Oncol. 13:232015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kondratova VN, Botezatu IV, Shelepov VP
and Likhtenshtein AV: Transcripts of satellite DNA in blood plasma:
Probable markers of tumor growth. Mol Biol (Mosk). 48:999–1007.
2014.(In Russian). View Article : Google Scholar : PubMed/NCBI
|