Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide (1). Clinical
studies have established cisplatin-based chemotherapy as a standard
chemotherapeutic regimen for patients with non-small cell lung
cancer (NSCLC) (2). However,
cisplatin can give rise to refractory tumors. Recent research has
shown that tumor progression might be caused by tumor cell escape
from immunosurveillance (3). Natural
killer (NK) cells play an important role in immunosurveillance
against cancer cells (4), and are
recognized as a promising tool in cancer immunotherapy (5). NK cell-mediated tumor cytotoxicity is
mainly promoted by NK group 2 member D (NKG2D) receptors on NK
cells (6–8). The major histocompatibility complex
(MHC) class I-related chains A and B (MICA and MICB, respectively)
(6), as well as UL16-binding proteins
(ULBPs) (9), are ligands of NKG2D and
they are expressed at low levels in non-malignant cells and at high
levels in transformed cells (10).
NKG2D ligands render transformed cells susceptible to NK
cell-mediated death (6,11); however, tumor cells can evade immune
recognition by downregulating the expression of NKG2D ligands.
NKG2D ligands have been described as stress-related proteins and
their expression can be increased through activation of the DNA
damage pathway (12). Although
cytotoxic anticancer drugs can induce apoptosis of NSCLC cells by
inhibiting DNA replication and mitosis (13), tumors develop resistance to
chemotherapy in many cases. There is no study reporting the
expression of NKG2D ligands in NSCLC tissues before and after
chemotherapy. However, we recently reported that a single exposure
to cisplatin upregulated the expression of MICA and MICB (MICA/B),
resulting in enhanced NK cell-mediated cytotoxicity via NKG2D-NKG2D
ligand interaction in NSCLC cell lines (14). Therefore, it is of particular interest
to understand whether cisplatin-based chemotherapy affects the
expression of ligands for NK cell-activated or inhibitory receptors
in tumor tissues collected from patients with NSCLC.
Furthermore, immune checkpoint inhibitors targeting
the programmed cell death-1 (PD-1)/PD-1 ligand 1 (PD-L1) axis have
shown promising results in recent clinical studies of patients with
NSCLC. It is also of particular interest to investigate the effect
of chemotherapy on the expression of PD-L1 since PD-L1 is one major
mechanism of the immunoevasion of tumor cells (15–17). It
has been reported that chemotherapy increases PD-L1 expression in
thymic epithelial tumors and ovarian cancer (18,19), but
another study showed that platinum-based chemotherapy tends to
decrease the expression of PD-L1 in lung cancer (20). Thus, the effect of chemotherapy on the
expression of PD-L1 in tumor cells has not been fully elucidated.
However, the addition of the anti-PD-1 antibody pembrolizumab to
standard chemotherapy was found to improve overall survival and
progression-free survival to a greater extent than chemotherapy
alone (21), suggesting that this
combination may be a promising strategy for patients with
NSCLC.
In the present study, we showed that repeated
exposure to cisplatin in vitro enhanced the expression of
both NKG2D ligands and PD-L1 in NSCLC cell lines, whereas
platinum-based chemotherapy attenuated the expression of NKG2D
ligands and enhanced the expression of PD-L1 in patients with
NSCLC, which mimicked the effects of interferon γ (IFNγ) stimuli on
NSCLC cells in vitro. Our findings suggested that residual
tumors are the result of immunoselection by host immunity, and that
possible mechanisms of tumor escape from host immunity during
platinum-based chemotherapy include both the induction of PD-L1
expression and reduction of NKG2D ligand expression, which inhibit
both T cell- and NK cell-mediated cytotoxicity. Notably, IFNγ
stimuli enhanced PD-L1 expression and attenuated NKG2D ligand
expression in NSCLC cell lines, which could explain the benefit of
chemotherapy combined with a PD-1/PD-L1 inhibitor. Moreover, in
NSCLC cell lines, the JAK-STAT inhibitor tofacitinib blocked
IFNγ-induced increase and decrease in the expression of PD-L1 and
NKG2D ligands, respectively, suggesting that triplet therapy with
chemotherapy, a PD-1/PD-L1 targeting immunocheckpoint inhibitor,
and a JAK-STAT inhibitor is a promising strategy to enhance the
host antitumor immunity for patients with NSCLC.
Materials and methods
Patients and specimens
The present study was approved by the Kawasaki
Medical School Ethics Committee (no. 1673-3), and written informed
consent was obtained from all patients for the use of specimens.
This study included patients with NSCLC who received platinum-based
chemotherapy followed by surgery at the Kawasaki Medical School
Hospital between January 2006 and October 2013. Histologic
diagnosis was confirmed using hematoxylin and eosin (H&E)
staining according to the WHO 2004 criteria (22) and the IASLC/ATS/ERS classification of
lung adenocarcinoma (23).
Pathological stages were defined according to the 7th edition of
the TNM classification (24).
Immunohistochemical staining
NSCLC tissue sections were stained according to a
previously described protocol (14).
The expression levels of MICA/B, ULBP-2/5/6, PD-L1, and HLA class I
were determined by immunohistochemical staining (IHC) using a mouse
anti-MICA/B antibody (1:50 dilution; clone no. D-8; Santa Cruz
Biotechnology, Dallas, TX, USA), goat anti-ULBP-2/5/6 polyclonal
antibody (polyclonal, 1:20 dilution, cat. no. AF1298; R&D
Systems, Minneapolis, MN, USA), mouse monoclonal anti-PD-L1
antibody (1:100 dilution; clone no. SP142; Spring Bioscience Corp.,
Pleasanton, CA, USA), or mouse monoclonal anti-HLA class I (HLA-A,
B and C) antibody (1:100 dilution; clone no. EMR8-5; Medical
Biological Laboratories Co., Ltd., Nagoya, Japan) followed by
poly-HRP-conjugated goat anti-mouse/rabbit secondary antibody (cat.
no. K5007; Dako, Santa Clara, CA, USA) or anti-goat HRP-DAB Cell
& Tissue Staining kit (cat. no. CTS008; R&D Systems). To
assess the expression level of each marker, the slides were
evaluated by two investigators (RO and AM) who were blinded to the
corresponding clinicopathological data. The intensity scoring for
staining was defined as follows: 0, no staining; 1+, weak staining
that was visible only with high magnification; 2+, moderate
staining (between 1+ and 3+), and 3+, strong staining that was
visible with low magnification.
NSCLC cell lines
The human NSCLC cell lines A549 and PC-9 were
obtained from Riken BRC through the National Bio-Resource Project
of MEXT (Tsukuba, Japan) and the IBL Cell Bank (Gunma, Japan),
respectively. The genotypes of the cell lines were identified with
the PowerPlex 16 STR System (Promega, Fitchburg, WI, USA). Both
cell lines were maintained as previously described (25). For cell culture experiments, cisplatin
(Wako Pure Chemicals Industries Ltd., Osaka, Japan) and tofacitinib
(Selleck Chemicals, Houston, TX, USA) stock solutions were prepared
in dimethyl sulfoxide (DMSO) (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany), whereas IFNγ (PBL Assay Science, Piscataway, NJ, USA)
stock solution was prepared in phosphate-buffered saline (PBS)
(−).
Repeated exposure to cisplatin does
not induce a cisplatin- resistant phenotype in NSCLC cell
lines
Previously, the pharmacokinetics study showed that
the platinum concentration in plasma ranged from 1.7 to 9.6 µg/ml
in patients treated with 50–100 mg/m2 of cisplatin
(26), which was equivalent to
cisplatin concentration of 8.7–49.2 µM. Based on this report, both
A549 and PC-9 cells were treated with 10 µM cisplatin thrice every
1–2 weeks to establish the NSCLC cell models by repeated exposure
to cisplatin. Second or third treatment with cisplatin was
undergone after the cells reached 50% confluency in a cell culture
dish.
WST-1 cell proliferation assay
The WST-1 assay was performed using the Cell
Proliferation Reagent WST-1 (Roche Diagnostics, Basel, Switzerland)
as previously described (25). In
brief, NSCLC cells were cultured in triplicate wells of 96-well
flat-bottomed plates with 1–100 µM of cisplatin in culture medium
for 48 h, then WST reagent were added to the wells according to the
manufacturer's protocol. The colorimetric reaction was measured
with the spectral scanning multimode reader Varioskan Flash (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Cisplatin-mediated
inhibition of cell proliferation was calculated as described
previously with the following formula: 100× (absorbance of the
wells for the cells treated with cisplatin/absorbance of the wells
for the cells treated with DMSO) (25).
Flow cytometry
Extracellular staining was performed with
fluorochrome-conjugated antibodies as previously described
(27). The following antibodies were
used for staining: PE-labeled MICA (clone no. 159227; R&D
Systems), allophycocyanin-labeled MICB (clone no. 236511; R&D
Systems), PE-labeled MICA/B (clone no. 6D4; BioLegend, San Diego,
CA, USA), Alexa Fluor 488-labeled ULBP-1 (clone no. 170818; R&D
Systems), PE- and allophycocyanin-labeled ULBP-2/5/6 (clone no.
165903; R&D Systems), PE-labeled ULBP-3 (clone no. 1166510;
R&D Systems), Alexa Fluor 488-labeled ULBP-4 (clone no. 709116;
R&D Systems), PE-labeled PD-L1 (clone no. 29E.2A3; BioLegend),
allophycocyanin-labeled HLA-A, B and C (clone no. G46-2.6;
BioLegend), as well as PE-, allophycocyanin- and Alexa Fluor
488-labeled anti-mouse IgG1κ (clone no. MOPC-21; BioLegend) and
IgG2bκ (clone no. MOPC-173; BioLegend) as isotype controls. The
cells were assayed using a FACSCanto II flow cytometer (BD
Biosciences, San Diego, CA, USA) and analyzed using FlowJo software
6.4.7 (Tree Star, Inc., Ashland, OR, USA). The increase in mean
fluorescence intensity (ΔMFI) was calculated as: (MFI with specific
mAb-MFI with isotype control)/MFI with isotype control). The
relative MFI (rMFI) values were calculated to compare the
differences between ΔMFI of a specific treatment and control as:
100× (ΔMFI of a specific treatment/ΔMFI of the control
treatment).
NK cell-mediated cytotoxicity
assay
Peripheral blood mononuclear cells (PBMCs) were
collected from healthy donors by gradient centrifugation with
Vacutainer CPT mononuclear cell preparation tubes (BD Biosciences,
San Jose, CA, USA). NK cells were purified using a NK cell
isolation kit (Stem Cell Technologies, Vancouver, BC, Canada) and
incubated with 100 IU/ml IL-2 (Teceleukin; Shionogi & Co.,
Ltd., Osaka, Japan) for 24 h as previously described (25). The NSCLC cells, with or without
repeated exposure to 10 µM cisplatin, were tested for sensitivity
to NK cell-mediated cytotoxicity using an LDH release assay kit
(CytoTox 96 Non-Radioactive Cytotoxicity assay; Promega) and a
Varioskan Flash spectral scanning multimode reader (Thermo Fisher
Scientific, Inc.) as previously described (25). To analyze the involvement of NKG2D in
the cytotoxicity of NK cells, NK cells were co-incubated with 20
µg/ml of anti-NKG2D blocking antibody (clone no. 1D11; BioLegend)
or an isotype-matched control antibody (clone no. 11711; R&D
Systems). For the LDH release assay, blood samples were collected
only from researchers who were involved with this study; therefore,
written informed consent was not required. The Ethics Research
Committee of the Kawasaki Medical School approved both the study
and this consent procedure (no. 1217-5).
Statistical analysis
Differences in means were evaluated with the
Student's t-test. All analyses were performed at a significance
level of 5% (P<0.05) using GraphPad Prism 5 (GraphPad Software
Inc., La Jolla, CA, USA).
Results
Tumor expression of NKG2D ligands
MICA/B and ULBP-2/5/6 is attenuated by platinum-based chemotherapy
in patients with NSCLC
We collected tissue samples from 10 patients who
received more than two courses of platinum-based chemotherapy
followed by surgery, and the expression levels of MICA/B and
ULBP-2/5/6 were assessed by IHC. The patient characteristics are
shown in Table I. Following
chemotherapy, MICA/B expression was downregulated in 2 of 10
patients (Patient #1 and #3) (Fig.
S1), whereas ULBP-2/5/6 expression was downregulated in 4 of 10
patients (Patient #1, #8, #9 and #10) (Fig. S2 and Table
II), which could lead to tumor immunoescape from NK cells.
Surprisingly, these findings contrasted the results of our previous
in vitro study, which showed that cisplatin enhances NKG2D
ligand expression in NSCLC cell lines (14). One important difference between our
present and previous studies (14) is
the number of exposures to chemotherapeutic reagents: Repeated vs.
single, respectively. For this reason, in the subsequent in
vitro experiments, we further investigated the effect of
repeated exposure to cisplatin on NKG2D ligand expression.
 | Table I.Clinicopathological characteristics
of the NSCLC patients who were treated with platinum-based
chemotherapy followed by surgery. |
Table I.
Clinicopathological characteristics
of the NSCLC patients who were treated with platinum-based
chemotherapy followed by surgery.
| Patient no. | Age (years) | Sex | T (c/yp) | N (c/yp) | M (c/yp) | yp Stage | Histology | Regimen | Cycles | OR | pR |
|---|
| #1 | 69 | Male | 2/3 | 2/X | 0 | IIIA | pleo | CDDP+GEM | 2 | SD | Ef1a |
| #2 | 66 | Male | 2/2 | 2/2 | 0 | IIIA | Sq | CDDP+GEM | 2 | SD | Ef1a |
| #3 | 70 | Male | 3/2b | 0/1 | 0 | IIB | Sq | CDDP+GEM | 2 | PR# | Ef1a |
| #4 | 54 | Male | 2/2b | 2/2 | 1 (BRA) | IV | La | CDDP+DTX | 2 | SD | Ef1b |
| #5 | 68 | Male | 4/4 | 0/1 | 0 | IIIA | Sq | CDDP+DTX | 2 | PR | Ef1a |
| #6 | 72 | Male | 4/3 | 1/2 | 0 | IIIA | Sq | CDDP+DTX | 2 | PR | Ef2 |
| #7 | 47 | Male | 4/4 | 0/0 | 0 | IIIB | Ad | CDDP+PTX | 4 | SD | Ef1a |
| #8 | 61 | Male | 2/2 | 2/2 | 0 | IIIA | Ad | CDDP+PTX | 2 | PR | Ef1b |
| #9 | 57 | Male | 1b/1b | 0/2 | 1 (BRA) | IV | Ad | CBDCA+PTX | 4 | SD | Ef1a |
| #10 | 70 | Female | 2a/2a | 2/2 | 0 | IIIA | AdSq | CDDP+VNR | 2 | SD | Ef1a |
 | Table II.Immunohistochemical staining scores
in NSCLC tissues before and after induction chemotherapy. |
Table II.
Immunohistochemical staining scores
in NSCLC tissues before and after induction chemotherapy.
|
| MICA/B | ULBP-2/5/6 | PD-L1 | HLA-A, B, C |
|---|
|
|
|
|
|
|
|---|
| Patient no. | pre-CTx | post-CTx | pre-CTx | post-CTx | pre-CTx | post-CTx | pre-CTx | post-CTx |
|---|
| #1 | 1 | 0 | 3 | 2 | 0 | 0 | 3 | 1 |
| #2 | 1 | 1 | 2 | 2 | 0 | 0 | 1 | 1 |
| #3 | 3 | 0 | 1 | 2 | 0 | 0 | 0 | 2 |
| #4 | 0 | 0 | 1 | 2 | 1 | 2 | 2 | 0 |
| #5 | 0 | 0 | 1 | 2 | 0 | 3 | 2 | 2 |
| #6 | 0 | 0 | 2 | 2 | 0 | 0 | 2 | 2 |
| #7 | 0 | 0 | 1 | 2 | 1 | 1 | 3 | 2 |
| #8 | 0 | 0 | 3 | 2 | 1 | 2 | 3 | 2 |
| #9 | 0 | 0 | 2 | 1 | 0 | 0 | 1 | 1 |
| #10 | 0 | 0 | 3 | 2 | 1 | 0 | 3 | 1 |
Tumor expression of PD-L1 is enhanced
while that of HLA-class I is attenuated by platinum-based
chemotherapy in NSCLC patients
Through IHC, we also evaluated the expression of
both PD-L1 and HLA class I, which are important for tumor
recognition by T cells. Notably, the expression of PD-L1 was
upregulated in 3 of the 10 patients (Patient #4, #5 and #8)
(Fig. S3) and that of HLA class I
was downregulated in 5 of the 10 patients (Patient #1, #4, #7, #8
and #10) (Fig. S4 and Table II), supporting tumor immunoescape
from T cells.
Repeated exposure to cisplatin does
not induce cisplatin- resistant phenotype in NSCLC cell lines
We previously reported that MICA/B was overexpressed
in ~30% of patients with NSCLC (14)
and all the tested NSCLC cell lines (25). Although no study has evaluated ULBP-2
expression in tumor tissues from patients with NSCLC, soluble
ULBP-2 was detected in the serum of patients with lung cancer
(28), and this ligand was expressed
in all the tested NSCLC cell lines (25). To investigate whether repeated
exposure to cisplatin alters the expression of NKG2D ligands in
NSCLC cell lines, NSCLC cells were treated with 10 µM cisplatin
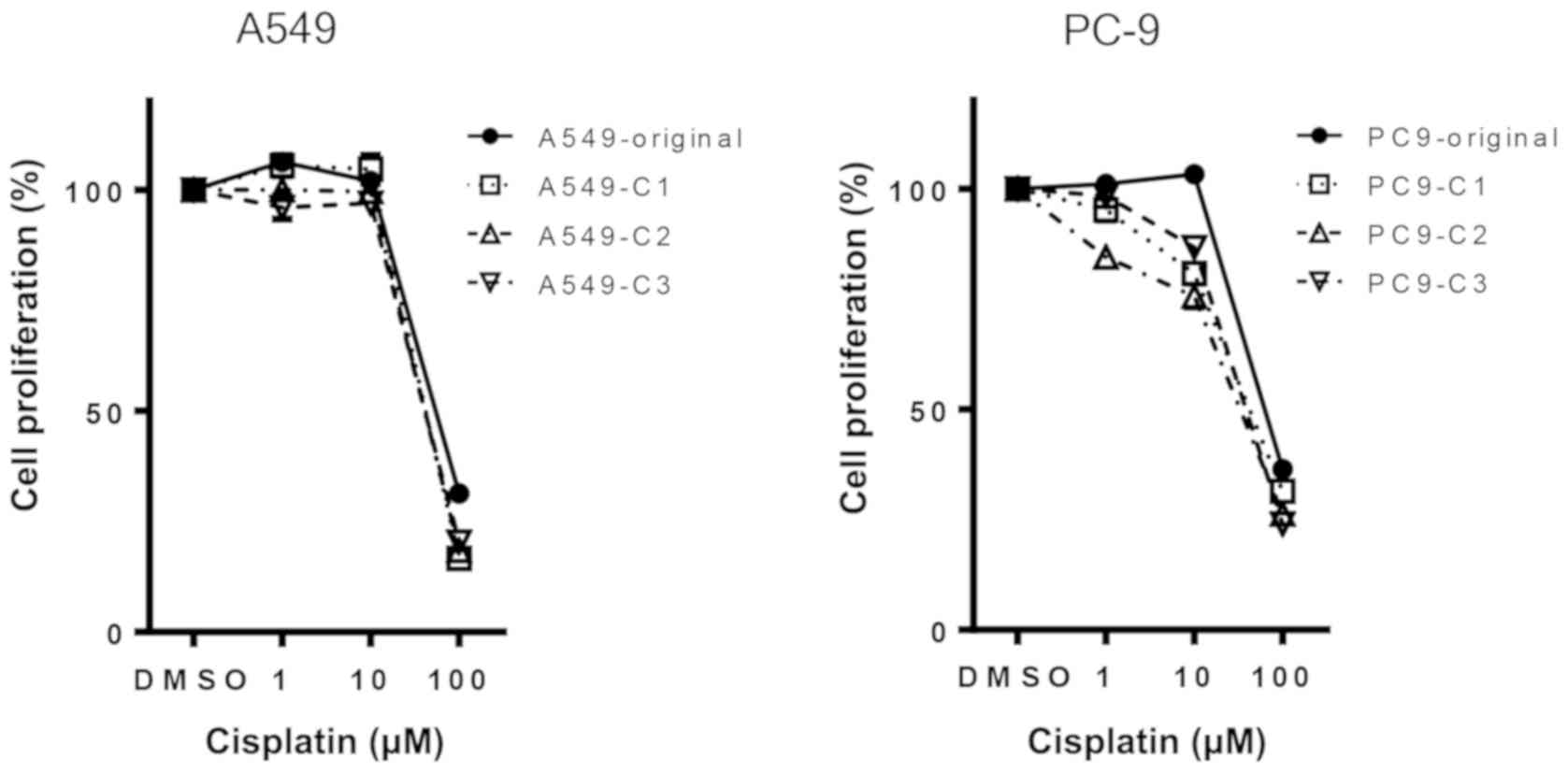
thrice, once every 1–2 weeks. First of all, cisplatin resistance
was assessed by a WST-1 cell proliferation assay in both the
original cells (-original) and cells repeatedly exposed to
cisplatin (-C1, -C2, -C3). A549-C cells showed marginal resistance,
whereas PC-9-C cells showed marginal sensitivity to cisplatin
(Fig. 1).
Repeated exposure to cisplatin
enhances NK cell-mediated cytotoxicity via the upregulation of
NKG2D ligands in NSCLC cell lines
Next, the expression levels of NKG2D ligands (MICA,
MICB and ULBP-1, ULBP-2/5/6, ULBP-3, ULBP-4), PD-L1 and HLA class I
in NSCLC cell lines were evaluated by flow cytometry. After
exposure to 10 µM cisplatin thrice, the expression of MICA in A549
cells and one of ULBP-2/5/6 in PC-9 cells was significantly
upregulated. Additionally, the expression of PD-L1 was
significantly upregulated in both the cell lines, and the
expression of one of the HLA class I was significantly enhanced in
the PC-9 cells. The basal expression of MICA in the PC-9 cell line
and the expression levels of MICB, ULBP-1, ULB-3 and ULB-4 in both
the cell lines were too weak (ΔMFI <0.5) to analyze (Fig. 2).
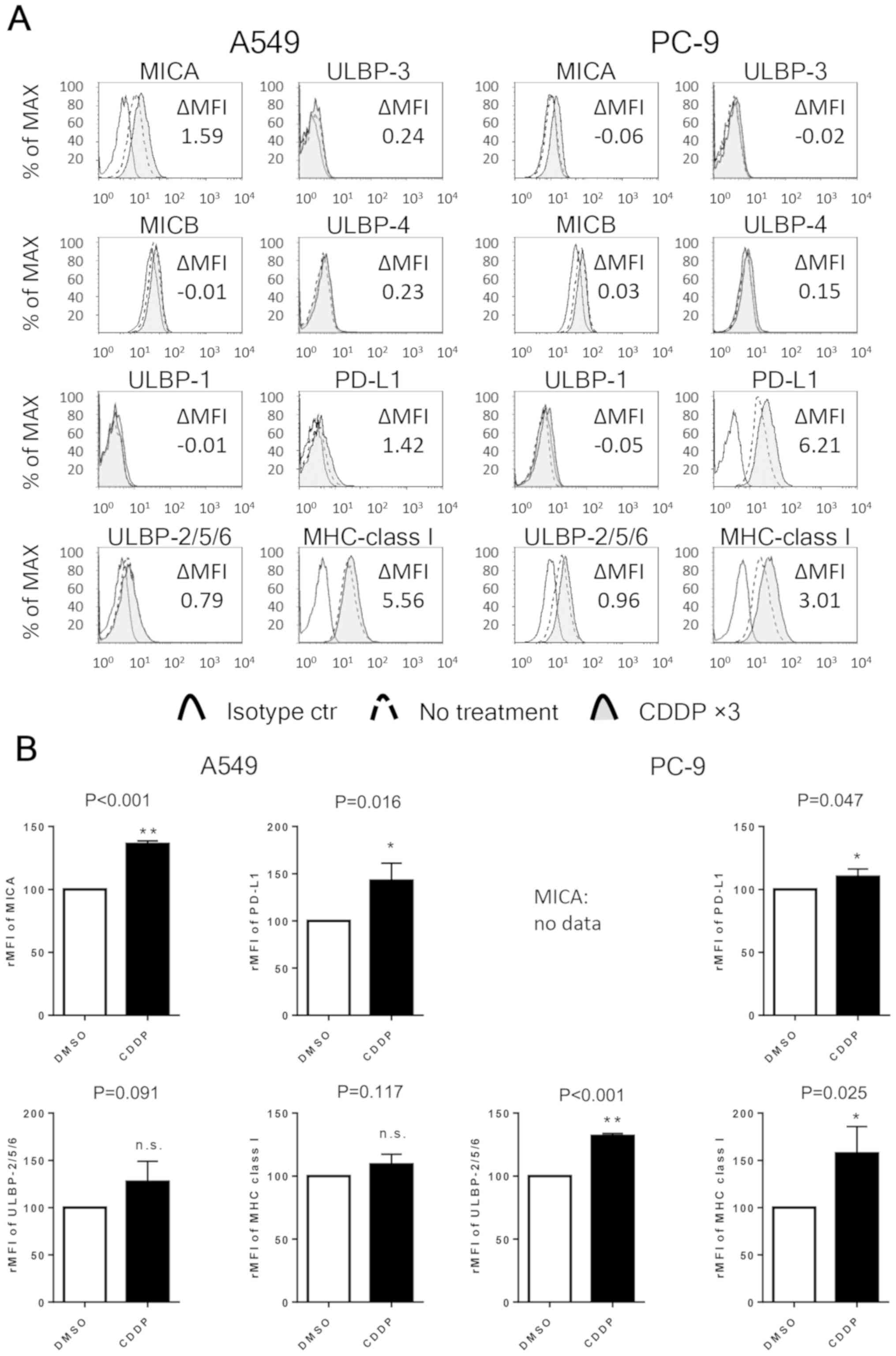 | Figure 2.Repeated exposure to cisplatin
enhances the expression of NKG2D ligands, PD-L1 and HLA class I in
NSCLC cell lines. (A) The expression of MICA, MICB, ULBP-1,
ULBP-2/5/6, ULBP-3, ULBP-4, PD-L1 and HLA class I in NSCLC cell
lines. The expression of each cell surface molecule was assessed by
flow cytometry in both untreated cells and cells thrice exposed to
10 µM cisplatin (CDDP ×3). The cells were stained with an isotype
control antibody or antibody specific for the indicated molecule.
The results represent three independent experiments. (B) The
relative MFI (rMFI) of MICA, ULBP-2/5/6, PD-L1 and MHC class I
molecules were calculated based on three independent experiments
and evaluated with the Student's t-test. Bars indicate SEM.
*P<0.05 and **P<0.01. NKG2D, natural killer group 2 member D;
NSCLC, non-small cell lung cancer; PD-L1, programmed cell death-1
ligand 1; HLA-class I, human leucocyte antigen; MICA, MHC class
I-related chain molecule A; MICB, MHC class I-related chain
molecule B; ULBP-1, UL16 binding protein 1; rMFI, relative mean
fluorescence intensity; MHC, major histocompatibility complex; SEM,
standard error of the mean; n.s., not significant. |
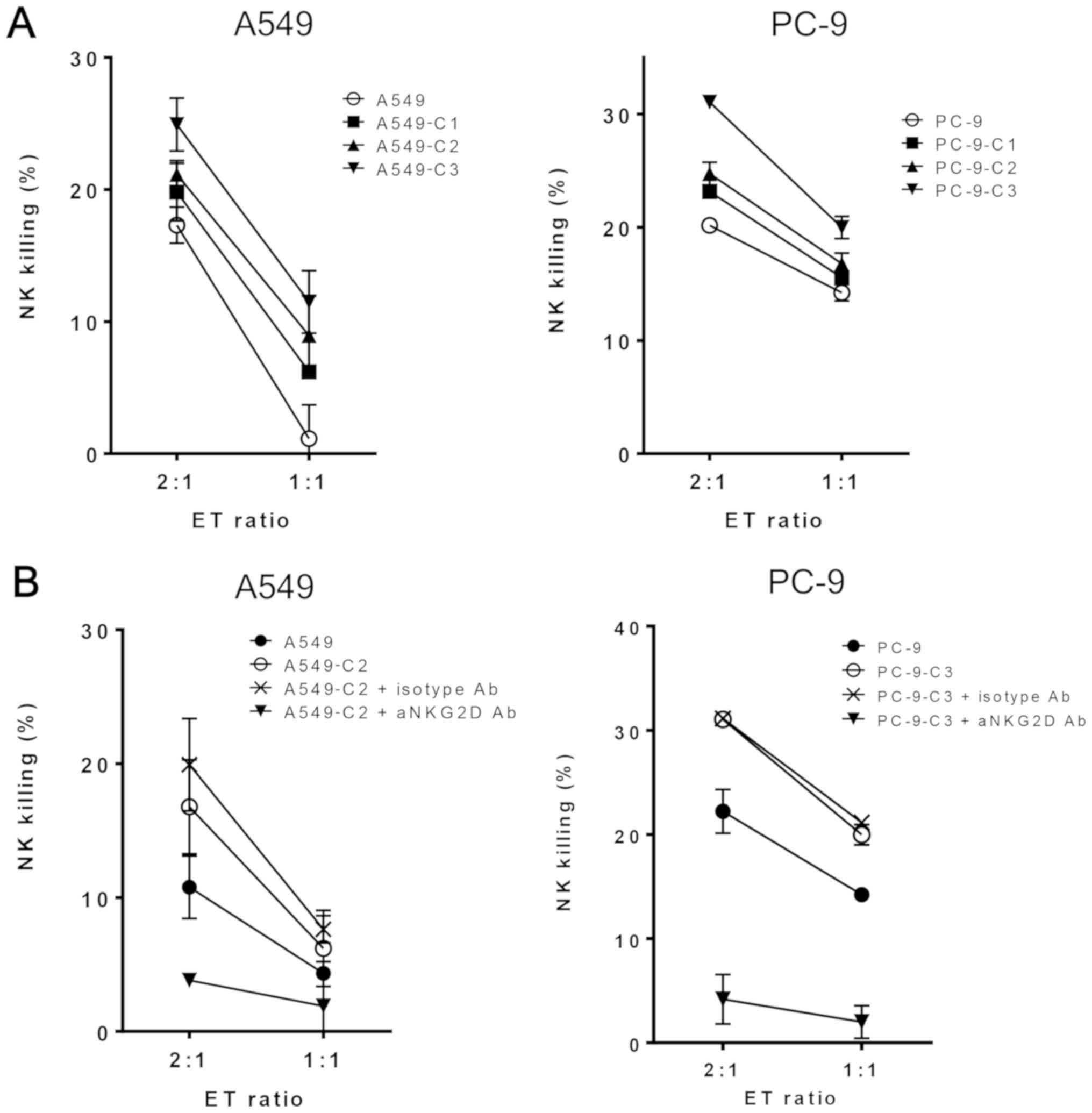
To analyze the effect of repeated exposure to
cisplatin on NK cell-mediated cytotoxicity, the sensitivity of A549
and PC-9 cells to NK cells was evaluated by an LDH release assay.
As expected, NK cell-mediated death increased in the A549 and PC-9
cells after exposure to cisplatin thrice (Fig. 3A). To verify that an NKG2D receptor
was involved in the repeated exposure to cisplatin-induced
sensitivity to NK cell-mediated death, purified NK cells were
pretreated with an anti-NKG2D blocking antibody prior to the assay.
In this assay, we selected A549-C2 and PC-9-C3 cells since these
cells showed the highest cisplatin-resistance phenotype among the
three established phenotypes. The anti-NKG2D blocking antibody
inhibited NK cell-mediated lysis of the cisplatin-treated tumor
cells, whereas an isotype control antibody had no effect on both
cell lines (Fig. 3B). These findings
indicated that the NK cell-mediated cytotoxicity of repeated
cisplatin exposure was dependent on NKG2D-NKG2D ligand
interaction.
IFNγ downregulates NKG2D ligands but
upregulates PD-L1 in NSCLC cell lines
To determine the reason for the difference in NKG2D
ligand expression in vivo and in vitro, we focused on
the immune response of NSCLC cells as another major difference
between the two environments is the presence of host immunity. IFNγ
is an important cytokine secreted by NK cells to attack tumor cells
(29). Previous reports have shown
that IFNγ increases PD-L1 expression (15) and decreases NKG2D ligand expression
(30) in cancer cells. Thus, we
evaluated the effect of IFNγ stimuli on the expression of NKG2D
ligands, PD-L1, and HLA class I in NSCLC cell lines. We found that
IFNγ significantly downregulated MICA/B in A549 and ULBP-2/5/6 in
PC-9 cells, respectively. We previously reported that IFNγ enhanced
PD-L1 expression in A549 cells, which was blocked by the JAK
inhibitor tofacitinib (31). In line
with our previous report (31), the
present study demonstrated that IFNγ significantly upregulated
PD-L1 in A549 cells and marginally but significantly enhanced PD-L1
expression in PC-9 cells. On the other hand, IFNγ upregulated HLA
class I in both cell lines. Moreover, the JAK-STAT inhibitor
tofacitinib significantly blocked the IFNγ-induced decrease in
MICA/B and blocked the IFNγ-induced increase in PD-L1 in both cell
lines (Fig. 4). These findings
suggest that both IFNγ-induced decrease in MICA/B and IFNγ-induced
increase in PD-L1 are mainly regulated by the JAK/STAT pathway in
NSCLC cells. Notably, the IFNγ-treated NSCLC cell lines showed a
similar trend for the expression of NKG2D ligands and PD-L1 to that
of patients with NSCLC who received platinum-based chemotherapy
(Figs. S1–S3), suggesting that the expression levels
of NKG2D ligands and PD-L1 after platinum-based chemotherapy are
determined not by the chemotherapeutic drug but by IFNγ. This
process is one reasonable mechanism that may explain the effect of
chemotherapy combined with a PD-1/PD-L1 axis-targeting drug on the
improvement of clinical outcome of patients with NSCLC (21).
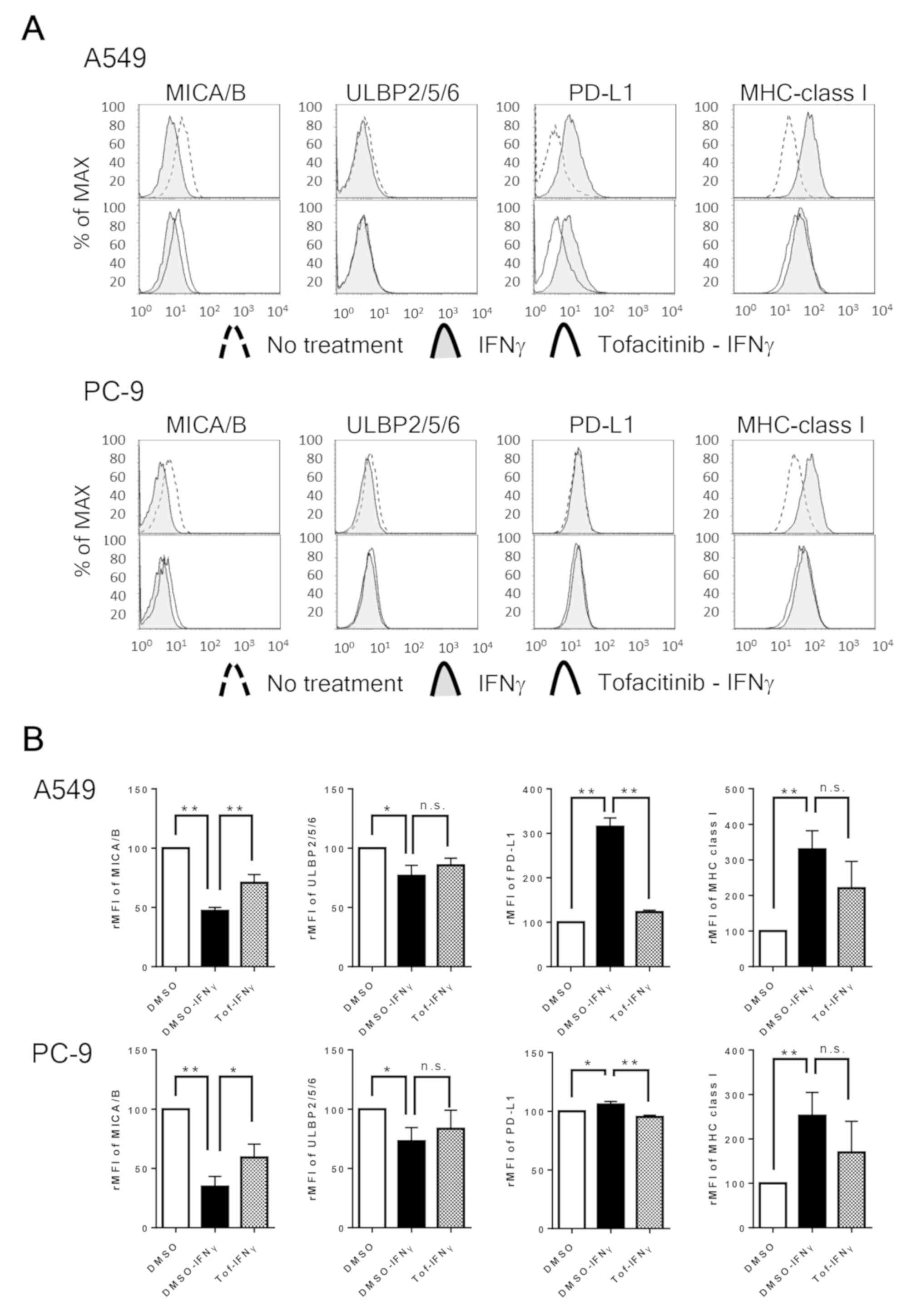 | Figure 4.IFNγ downregulates NKG2D ligands and
upregulates PD-L1 and HLA class I in NSCLC cells, and is blocked by
tofacitinib. (A) The panels show representative images of the
expression of MICA/B, ULBP-2/5/6, PD-L1 and HLA class I in NSCLC
cell lines pretreated with or without 1 µM of tofacitinib (Tof)
followed by 0.5 ng/ml of IFNγ. (B) The relative MFI (rMFI) of
MICA/B, ULBP-2/5/6, PD-L1 and HLA class I were calculated based on
three independent experiments and evaluated with the Student's
t-test. The bars represent the standard deviations. *P<0.05 and
**P<0.01. Bars indicate SEM. IFNγ, interferon γ; NKG2D, natural
killer group 2 member D; PD-L1, programmed cell death-1 ligand 1;
HLA class I, human leucocyte antigen class I; NSCLC, non-small cell
lung cancer; rMFI, relative mean fluorescence intensity; MICA/B,
major histocompatibility complex (MHC) class I-related chains A and
B; ULBP-2/5/6, UL16-binding proteins 2/5/6; SEM, standard error of
the mean; DMSO, dimethyl sulfoxide; n.s., not significant. |
Discussion
The expression of NKG2D ligands is regulated by DNA
stress-induced ataxia-telangiectasia mutated (ATM) and
ataxia-telangiectasia and Rad3-related (ATR) protein kinase
signaling. The ATM-ATR pathway is frequently activated in tumor
cells and is found to regulate NKG2D ligands via transcriptional
regulation (12). An increase in
NKG2D ligand expression by chemotherapeutic reagents could
represent an important antitumor mechanism whereby tumor cells are
eradicated by the innate immune system (5). We previously reported that a single
exposure to cisplatin enhanced NKG2D ligand expression and NK
cell-mediated cytotoxicity in NSCLC cells in vitro (14). However, the present study showed that
the expression of both MICA/B and ULBP-2/5/6 in the tissue samples
was attenuated by cisplatin-based chemotherapy, which were not in
line with the results of our previous in vitro study
(14).
A primary difference between our previous and
present studies concerns the numbers of exposure to cisplatin:
Single vs. repeated, respectively. In the present study, the effect
of repeated exposure to cisplatin on NKG2D ligand expression was
first evaluated. In agreement with our previous study, repeated
exposure to cisplatin enhanced the expression of MICA in A549 and
the expression of one of ULBP-2/5/6 in PC-9 cells resulting in
enhanced NK cell-mediated cytotoxicity via NKG2D-NKG2D ligand
interaction in both cell lines.
Another important difference between our previous
and present study is the study setting: In vitro vs. in
patients, respectively. The in vitro study showed that the
direct response of cancer cells to cisplatin can be assessed
without the influence of host immune cells. However, the residual
tumors in patients were caused by both chemotherapy and antitumor
immunity resistance. Our conflicting findings between the in
vitro and in patient studyies support the hypothesis that
repeated exposure to cisplatin upregulates the expression of NKG2D
ligands in cancer cells and then NK cells eradicate tumor cells via
NKG2D-NKG2D ligand interaction. Tumor heterogeneity is expected in
the expression levels of both basal and chemotherapeutic
reagent-induced increase in NKG2D ligand expression. Tumor cells
expressing low levels of NKG2D ligands may escape from NK
cell-mediated immunosurveillance and be immunoselected as residual
tumor. Recently, chemotherapy was recognized as a PD-L1 inducer in
cancer cells (18). Our findings
suggested that repeated exposure to cisplatin and host
immunoreaction with IFNγ enhanced PD-L1 expression, leading to the
inhibition of T cell- or NK cell-mediated cytotoxicity in patients
and resulting in tumor escape from host immunity. It is well known
that NSCLC cells have different genetic backgrounds, such as EGFR
driver mutation and EML4-ALK fusion gene (32). Both EGFR and ALK signaling regulate
PD-L1 in NSCLC cells (33,34), and we have also reported that EGFR
signaling regulates the expression of NKG2D ligand in PC-9 cells
having EGFR driver mutation (25).
These findings suggest genetic effects on the escape from host
immunity via regulation of immune-related molecules.
One major limitation of the present study was that
we could evaluate only the effect of NK cell-mediated
immunoselection on NSCLC cells in vitro. Antitumor immunity
involves not only NK cells but also several phenotypes of T cells,
antibody-producing B cells, antigen-presenting cells and cytokines.
Since host immunity against tumor cells is a complicated system, it
is difficult to be reproduced in an in vitro assay with
several types of immune cells. Another limitation was the very low
number of patients who were evaluated using IHC for NKG2D ligand
and PD-L1 expression as induction chemotherapy is not a standard
therapy for locally advanced NSCLC.
In summary, the present clinical study suggests that
residual tumor following chemotherapy is the result of tumor
immunoescape via the downregulation of NKG2D ligands and/or
upregulation of PD-L1 in tumor cells as tumor cells with this
phenotype can easily escape from host immunity, even though
cisplatin-based chemotherapy works in concert with NK cell-mediated
cytotoxicity via the upregulation of NKG2D ligands in vitro.
Although a recent clinical study showed that cisplatin-based
chemotherapy combined with a PD-1/PD-L1 inhibitor is the optimal
therapeutic approach for patients with advanced NSCLC (21), our current findings suggest that
addition of the JAK-STAT inhibitor tofacitinib to
chemo-immunocheckpoint therapy is a promising strategy as it may
block IFNγ-induced downregulation of NKG2D ligands and upregulation
of PD-L1 in NSCLC cells.
Supplementary Material
Supporting Data
Acknowledgements
We thank the JCRB Cell Bank for the cell line
authentication. We also thank Ms. Maitani and the staff of the
Tissue Culture & Immunology Center and Tissue Biology &
Electron Microscopy Research Center (Kawasaki Medical School) for
the technical assistance.
Funding
The present study was supported by the Japanese
Society for the Promotion of Science (JSPS) Kakenhi Grants (grant
nos. 25462189 and 16K10696 to RO) and the Strategic Research
Foundation Grant-Aided Project for Private Universities from the
Ministry of Education, Culture, Sport, Science, and Technology
(grant no. S1291010 to MN).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RO substantially contributed to the conception and
design of the study. RO and AM substantially contributed to the
acquisition of data, the analysis and interpretation of the data.
KS, SS, YN and MN contributed to the acquisition of the data, the
analysis and interpretation of the data. RO and MN drafted the
article and all authors revised it critically for important
intellectual content, and agreed to be accountable for all aspects
of the work in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
The present study was approved by the Kawasaki
Medical School Ethics Committee (nos. 1217-5 and 1673-3). Written
informed consent was obtained from all patients for the use of
specimens.
Patient consent for publication
Not applicable.
Competing interests
Dr Masao Nakata received research funding from Kyowa
Hakko Kirin, Taiho Pharma, Ono Pharma, and Nihon Medi-Physics Co.
The sponsors had no control over the interpretation, writing, and
publication of this study. All other authors declare that they have
no competing interests.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baggstrom MQ, Stinchcombe TE, Fried DB,
Poole C, Hensing TA and Socinski MA: Third-generation chemotherapy
agents in the treatment of advanced non-small cell lung cancer: A
meta-analysis. J Thorac Oncol. 2:845–853. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lanier LL: A renaissance for the tumor
immunosurveillance hypothesis. Nat Med. 7:1178–1180. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ljunggren HG and Malmberg KJ: Prospects
for the use of NK cells in immunotherapy of human cancer. Nat Rev
Immunol. 7:329–339. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bauer S, Groh V, Wu J, Steinle A, Phillips
JH, Lanier LL and Spies T: Activation of NK cells and T cells by
NKG2D, a receptor for stress-inducible MICA. Science. 285:727–729.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu J, Song Y, Bakker AB, Bauer S, Spies T,
Lanier LL and Phillips JH: An activating immunoreceptor complex
formed by NKG2D and DAP10. Science. 285:730–732. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ogasawara K and Lanier LL: NKG2D in NK and
T cell-mediated immunity. J Clin Immunol. 25:534–540. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cosman D, Mullberg J, Sutherland CL, Chin
W, Armitage R, Fanslow W, Kubin M and Chalupny NJ: ULBPs, novel MHC
class I-related molecules, bind to CMV glycoprotein UL16 and
stimulate NK cytotoxicity through the NKG2D receptor. Immunity.
14:123–133. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Groh V, Bahram S, Bauer S, Herman A,
Beauchamp M and Spies T: Cell stress-regulated human major
histocompatibility complex class I gene expressed in
gastrointestinal epithelium. Proc Natl Acad Sci USA.
93:12445–12450. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bryceson YT, March ME, Ljunggren HG and
Long EO: Activation, coactivation, and costimulation of resting
human natural killer cells. Immunol Rev. 214:73–91. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gasser S, Orsulic S, Brown EJ and Raulet
DH: The DNA damage pathway regulates innate immune system ligands
of the NKG2D receptor. Nature. 436:1186–1190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tolis C, Peters GJ, Ferreira CG, Pinedo HM
and Giaccone G: Cell cycle disturbances and apoptosis induced by
topotecan and gemcitabine on human lung cancer cell lines. Eur J
Cancer. 35:796–807. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Okita R, Yukawa T, Nojima Y, Maeda A,
Saisho S, Shimizu K and Nakata M: MHC class I chain-related
molecule A and B expression is upregulated by cisplatin and
associated with good prognosis in patients with non-small cell lung
cancer. Cancer Immunol Immunother. 65:499–509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Blank C, Gajewski TF and Mackensen A:
Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T
cells as a mechanism of immune evasion: Implications for tumor
immunotherapy. Cancer Immunol Immunother. 54:307–314. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peng J, Hamanishi J, Matsumura N, Abiko K,
Murat K, Baba T, Yamaguchi K, Horikawa N, Hosoe Y, Murphy SK, et
al: Chemotherapy induces programmed cell death-ligand 1
overexpression via the nuclear factor-κB to Foster an
Immunosuppressive tumor microenvironment in ovarian cancer. Cancer
Res. 75:5034–5045. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Katsuya Y, Horinouchi H, Asao T, Kitahara
S, Goto Y, Kanda S, Fujiwara Y, Nokihara H, Yamamoto N, Watanabe S,
et al: Expression of programmed death 1 (PD-1) and its ligand
(PD-L1) in thymic epithelial tumors: Impact on treatment efficacy
and alteration in expression after chemotherapy. Lung Cancer.
99:4–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rojkó L, Reiniger L, Téglási V, Fábián K,
Pipek O, Vágvölgyi A, Agócs L, Fillinger J, Kajdácsi Z, Tímár J, et
al: Chemotherapy treatment is associated with altered PD-L1
expression in lung cancer patients. J Cancer Res Clin Oncol.
144:1219–1226. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gandhi L, Rodríguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al: Pembrolizumab plus chemotherapy in metastatic
non-small-cell lung cancer. N Engl J Med. 378:2078–2092. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Travis WD, Brambilla E, Müller-Hermelink
HK and Harris CC: Pathology and Genetics of Tumours of the Lung,
Pleura, Thymus and Heart. IARCPress; Lyon: 2004
|
|
23
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ,
Van Schil PE, et al: International association for the study of
lung cancer/american thoracic society/european respiratory society
international multidisciplinary classification of lung
adenocarcinoma. J Thorac Oncol. 6:244–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L;
International Association for the Study of Lung Cancer
International Staging Committ, : The IASLC lung cancer staging
project: Proposals for the revision of the TNM stage groupings in
the forthcoming (seventh) edition of the TNM Classification of
malignant tumours. J Thorac Oncol. 2:706–714. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Okita R, Wolf D, Yasuda K, Maeda A, Yukawa
T, Saisho S, Shimizu K, Yamaguchi Y, Oka M, Nakayama E, et al:
Contrasting effects of the cytotoxic anticancer drug gemcitabine
and the EGFR tyrosine kinase inhibitor gefitinib on NK
cell-mediated cytotoxicity via regulation of NKG2D ligand in
non-small-cell lung cancer cells. PLoS One. 10:e01398092015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vermorken JB, van der Vijgh WJ, Klein I,
Gall HE, van Groeningen CJ, Hart GA and Pinedo HM: Pharmacokinetics
of free and total platinum species after rapid and prolonged
infusions of cisplatin. Clin Pharmacol Ther. 39:136–144. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Okita R, Mougiakakos D, Ando T, Mao Y,
Sarhan D, Wennerberg E, Seliger B, Lundqvist A, Mimura K and
Kiessling R: HER2/HER3 signaling regulates NK cell-mediated
cytotoxicity via MHC class I chain-related molecule A and B
expression in human breast cancer cell lines. J Immunol.
188:2136–2145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamaguchi K, Chikumi H, Shimizu A, Takata
M, Kinoshita N, Hashimoto K, Nakamoto M, Matsunaga S, Kurai J,
Miyake N, et al: Diagnostic and prognostic impact of serum-soluble
UL16-binding protein 2 in lung cancer patients. Cancer Sci.
103:1405–1413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zitvogel L, Galluzzi L, Kepp O, Smyth MJ
and Kroemer G: Type I interferons in anticancer immunity. Nat Rev
Immunol. 15:405–414. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schwinn N, Vokhminova D, Sucker A, Textor
S, Striegel S, Moll I, Nausch N, Tuettenberg J, Steinle A, Cerwenka
A, et al: Interferon-gamma down-regulates NKG2D ligand expression
and impairs the NKG2D-mediated cytolysis of MHC class I-deficient
melanoma by natural killer cells. Int J Cancer. 124:1594–1604.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shimizu K, Okita R, Saisho S, Maeda AI,
Nojima Y and Nakata M: Impact of COX2 inhibitor for regulation of
PD-L1 expression in non-small cell lung cancer. Anticancer Res.
38:4637–4644. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular profiling of lung adenocarcinoma. Nature.
511:543–550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Akbay EA, Koyama S, Carretero J, Altabef
A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp
EM, Pugh TJ, et al: Activation of the PD-1 pathway contributes to
immune escape in EGFR-driven lung tumors. Cancer Discov.
3:1355–1363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ota K, Azuma K, Kawahara A, Hattori S,
Iwama E, Tanizaki J, Harada T, Matsumoto K, Takayama K, Takamori S,
et al: Induction of PD-L1 expression by the EML4-ALK oncoprotein
and downstream signaling pathways in non-small cell lung cancer.
Clin Cancer Res. 21:4014–4021. 2015. View Article : Google Scholar : PubMed/NCBI
|