Introduction
Gastric cancer (GC) is the fourth most common type
of cancer globally, with high frequency and mortality rates
(1). GC remains one of the most
severe public health problems worldwide, and particularly in China
(2). Therefore, it is necessary to
explore potential novel therapeutic methods for treating GC.
Classical adjuvant treatment methods for patients
with GC are based on MacDonald's protocol, combining 5-fluoruracil
(5-FU) and radiation in patients with stage IB-IVA, which is
associated with increased progression free survival (PFS) and
overall survival (OS) of patients with GC (3,4).
Radiotherapy is the major loco-regional control
method for unresectable GC. Unfortunately, intrinsic
radio-resistance of cells results in failure of radiotherapy in
numerous patients (5). The guidelines
of the National Comprehensive Cancer Network recommend radiotherapy
as a standard therapy for patients with GC. There are two major
limitations associated with treating GC via radiation: Intrinsic or
acquired resistance to radiotherapy, and nonspecific toxicity to
gastric mucosa and the surrounding normal tissues (6,7). For
instance, radiotherapy is routinely used for treating cancer and
generates various DNA lesions, which in turn activates the DNA
damage response (8).
DNA double strand breaks (DSBs) are generated by
ionizing radiation (IR), and can be repaired by non-homologous
end-joining (NHEJ) and homologous recombination (9,10).
DNA-dependent protein kinase catalytic subunit (DNA-PKcs) is a
crucial factor involved in NHEJ, and the DNA-PK complex contributes
to early-stage damage-induced DNA repair (11). DNA-PKcs expression predicts response
to radiotherapy in patients with prostate cancer (12). Silencing of DNA-PKcs leads to
increased radiosensitivity and DSBs (13,14).
Overexpression of DNA-PKcs in patients with nasopharyngeal
carcinoma has been reported to be associated with a relatively poor
clinical outcome (15). Silence and
loss-of-function mutations of DNA-PKcs were demonstrated to promote
apoptosis resistance in a number of types of cancer cells,
including head and neck cancer, leukemia and skin cells (16–18).
Thus, DNA-PK may be a radiotherapeutic target for
cancer. In the current study, the function of a DNA-PKcs inhibitor
in GC cell lines, and the corresponding molecular mechanisms were
investigated, aiming to identify a potential novel treatment method
for GC.
Materials and methods
Cell culture
Human BGC823, SGC7901, MGC803, HGC-27, MKN45 and
MKN74 GC cell lines were obtained from Shanghai Institute of Cell
Biology (Shanghai, China) and cultured in RPMI-1640 medium,
supplemented with 10% calf bovine serum, 50 U/ml penicillin and 50
U/ml streptomycin in an incubator at 37°C in 5% CO2.
Ionizing radiation
The DNA-PK inhibitor NU7441 (Tocris Bioscience,
Bristol, UK) was dissolved in dimethyl sulfoxide (DMSO) as a 5
mmol/l stock solution and stored at −20°C. A casein kinase 2 (CK2)
inhibitor, CX4945, was purchased from Selleck Chemicals (Houston,
TX, USA). Cells were exposed to X-rays generated by a Rad Source
RS2000 irradiator (Rad Source Technologies, Inc., Buford, GA, USA)
operating at 25 mA with a 0.3 mm Al filter and effective photon
energy of 160 kV. The dose rate at an irradiation distance of 48.6
cm was 1.31 Gy/min.
Clonogenic survival assay
Cells were seeded into 6-well plates
(2×106) and treated with or without drug (0.1 µM NU7441
or 0.5 µM CX4945) following attachment for 4–6 h. The next day, the
cells were exposed to different doses of IR. Cells were incubated
for 10–14 days to form colonies, then fixed with 100% methyl
alcohol for 5 min and stained with 1% crystal violet for 10 min at
room temperature. Colonies containing >50 cells were counted.
Survival fractions were normalized according to the non-irradiated
subgroup to eliminate cytotoxicity generated by drug pretreatment.
Cell survival curves were obtained using GraphPad Prism 7 software
(GraphPad Software, Inc., La Jolla, CA, USA) according to the
multitarget single hit model. The radiation-associated parameters,
mean lethal dose (D0) and quasi-threshold dose (Dq), were
calculated to evaluate the effect of drug pretreatment on the
radiosensitivity of GC cells.
Determination of half maximal
inhibitory concentration (IC50)
Determination of the IC50 of NU7441
treatment of GC cells was performed using Cell Counting Kit-8
(CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan).
Briefly, following treatment with increasing concentrations of
NU7441 (5, 10, 15 and 20 µM) for 48 h, 10 µl CCK-8 solution was
added to each well and incubated for 2 h. Subsequently, the cells
were transferred into another 96-well plate and the absorbance was
detected at 450 nm using a microplate reader (Bio-Rad Laboratories,
Inc.) to reflect cell viability. The IC50 was calculated
using CompuSyn software version 1.0 (ComboSyn, Inc., Paramus, NJ,
USA).
Western blot analysis
Cell extracts were prepared in
radioimmunoprecipitation assay lysis buffer (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) and the concentration was measured using
a Pierce BCA Protein Assay kit (Sigma-Aldrich; Merck KGaA)
according to the manufacturer's protocol. Briefly, each sample (20
µg protein), prepared with loading buffer, was separated by
SDS-PAGE on 8% gels and electrotransferred onto polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). Membranes
were blocked in 5% non-fat milk for 1 h at room temperature and
incubated with primary antibody at 4°C overnight. GAPDH primary
antibody (cat. no. G8795; 1:5,000) was purchased from Sigma-Aldrich
(Sigma-Aldrich; Merck KGaA), γ H2A histone family member X (γH2AX;
cat. no. 33686; 1:1,000), poly [ADP-ribose] polymerase 1 (PARP1;
cat. no. 31288; 1:1,000) and caspase3 (cat. no. 29034; 1:1,000)
primary antibodies were from Signalway Antibody LLC (College Park,
MD, USA). The membranes were then washed with three times with
Tris-buffered saline and incubated with a horseradish
peroxidase-conjugated secondary antibody (cat. no. 7074, 1:10,000;
Cell Signaling Technology, Inc., Danvers, MA, USA) for 2 h.
Finally, the blots were visualized using enhanced chemiluminescence
(EMD Millipore) and the semi-quantification of bands was performed
using ImageJ (version 1.51; National Institutes of Health,
Bethesda, MD, USA).
Immunostaining analysis
Cells were incubated with 3%
H2O2 for 10 min, permeabilized with 0.3%
Triton X-100 in PBS for 15 min and blocked with 10% goat serum for
1 h at room temperature. The sections were then incubated with
primary antibody (γH2AX; cat. no. 33686; 1:100) at 4°C overnight.
The next day, sections were washed, incubated with biotinylated
goat anti-rabbit IgG (cat. no. BA-1000; 1:10,000; Vector
Laboratories, Inc., Burlingame, CA, USA) in PBS for 1 h at room
temperature and with VECTASTAIN (PK-6200; Vector Laboratories,
Inc.) for 1 h. A horseradish peroxidase reaction product was
visualized using an enhanced DAB peroxidase substrate kit (cat. no.
SK-4100; Vector Laboratories, Inc.). Immunostaining signals were
analyzed using the optical fractionator method with
Microbrightfield Stereo-Investigator (MBF Bioscience, Williston,
VT, USA). The total number of cells in four or five fields of view
was counted per sample.
Statistical analysis
Data are presented as the mean ± standard deviation.
Comparisons between two groups were evaluated by the Student's
t-test, and comparisons among multiple groups were analyzed by
analysis of variance followed by Newman-Keuls post-hoc analysis
using GraphPad Prism 7 software (GraphPad Software, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
BGC823 and MGC803 cell lines are
relatively resistant to IR
In the present study, six human GC cell lines,
SGC7901, HGC-27, MKN45, MKN74, BGC823 and MGC803, were used, and
the relative sensitivities to IR were determined by clonogenic
survival assay. BGC823 and MGC803 cells were the most resistant to
IR, with higher D0 and Dq values (Fig.
1A and Table I). In addition, the
expression levels of key regulators involved in cell apoptosis and
cell survival were detected in all cell lines treated with IR. The
expression levels of cleaved caspase3 and γH2AX were relatively low
in BGC823 and MGC803 cells compared with other cell lines following
IR. PARP1, the substrate of active caspase3, was expressed at a
relatively high level in BGC823 and MGC803 cells (Fig. 1B and C).
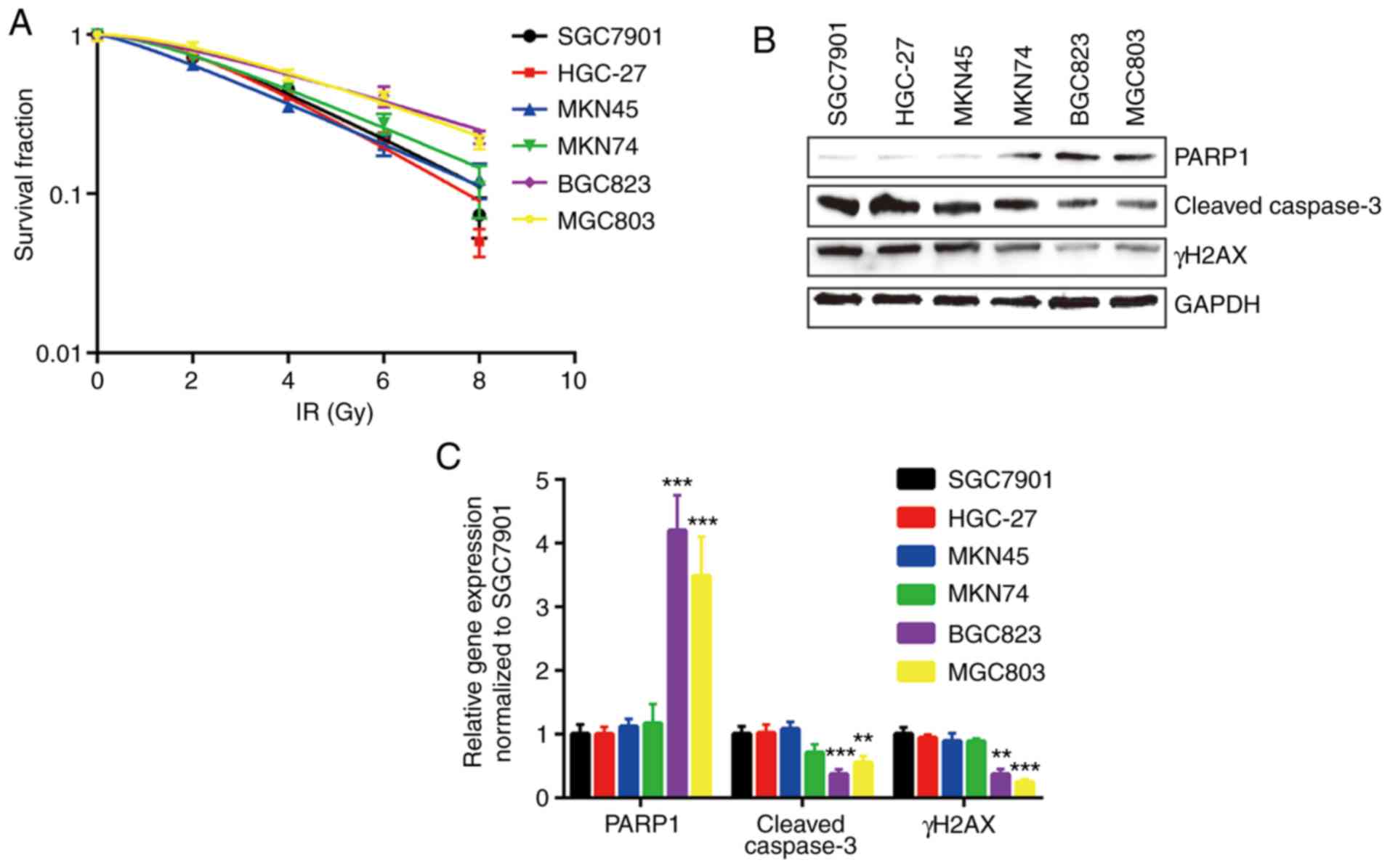 | Figure 1.BGC823 and MGC803 cells are relatively
insensitive to IR. (A) Six gastric cancer cell lines (SGC7901,
HGC-27, MKN45, MKN74, BGC823 and MGC803) were exposed to increasing
doses of IR (0, 2, 4, 6 and 8 Gy). (B) Protein expression levels of
γH2AX and cleaved-caspase3 were decreased, and PARP1 protein
expression was increased, in BGC823 and MGC803 cells following IR
(4 Gy). (C) Quantification of protein expression levels.
**P<0.01 and ***P<0.0001 vs. SGC7901. IR, ionizing radiation;
PARP1, poly [ADP-ribose] polymerase 1; γH2AX, γ H2A histone family
member X. |
 | Table I.D0 and Dq of six gastric cancer cell
lines towards ionizing radiation. |
Table I.
D0 and Dq of six gastric cancer cell
lines towards ionizing radiation.
| Parameter | SGC7901 | HGC-27 | MKN-45 | MKN-28 | BGC-823 | MGC-803 |
|---|
| lnN | 0.689641 | 0.822859 | 0.266203 | 0.524137 | 0.431782 | 0.66114 |
| D0 | 2.795639 | 2.497502 | 3.273322 | 3.303601 | 4.539265 | 3.863988 |
| Dq | 1.927987 | 2.055092 | 0.871368 | 1.731538 | 1.959975 | 2.554638 |
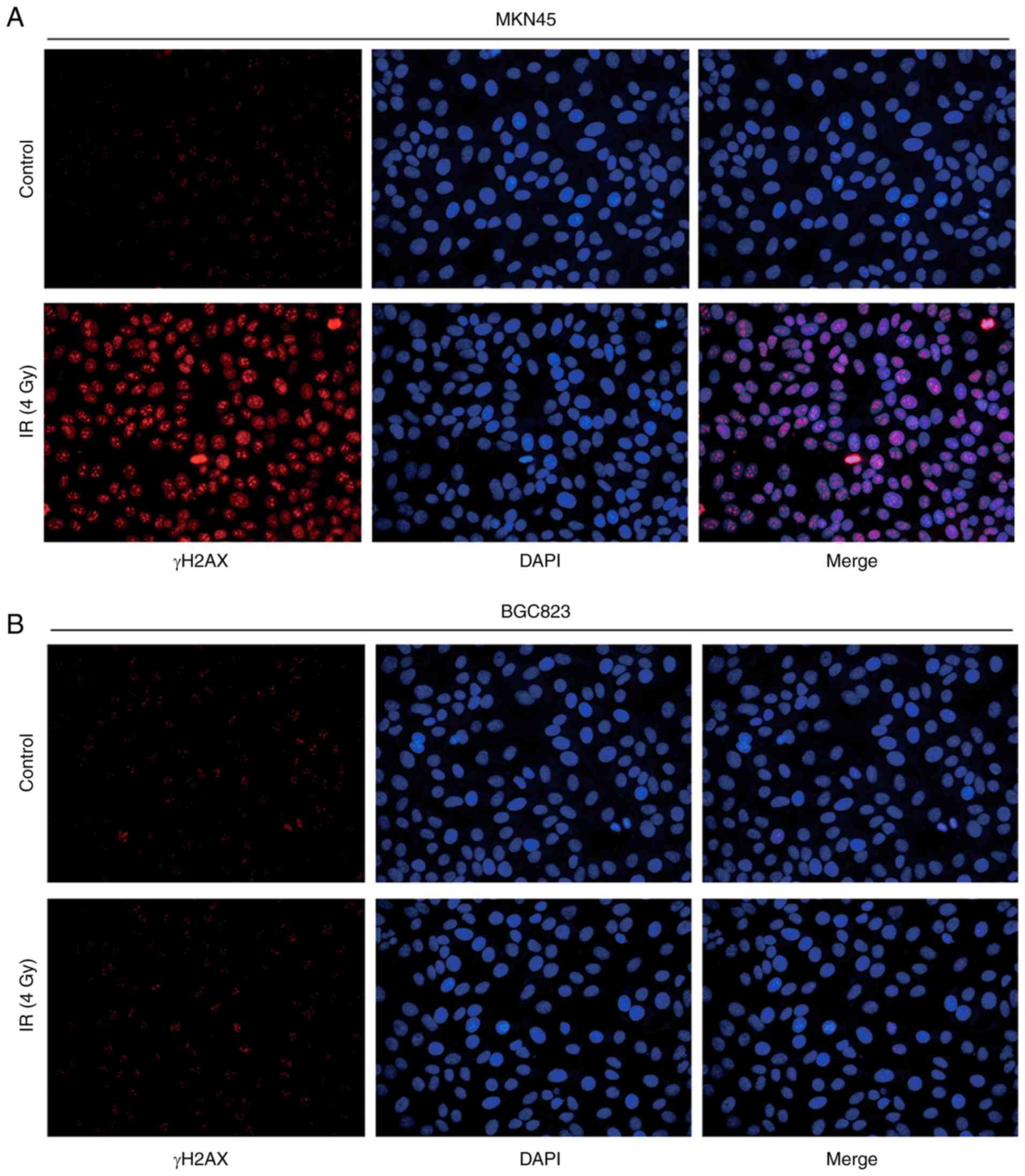
To further evaluate their response to IR,
immunofluorescence was used to observe γH2AX expression following
IR treatment. BGC823 cells exhibited weak fluorescence signals
compared with MKN45 cells, indicating a mild double strand break
induced by IR (Fig. 2A and B).
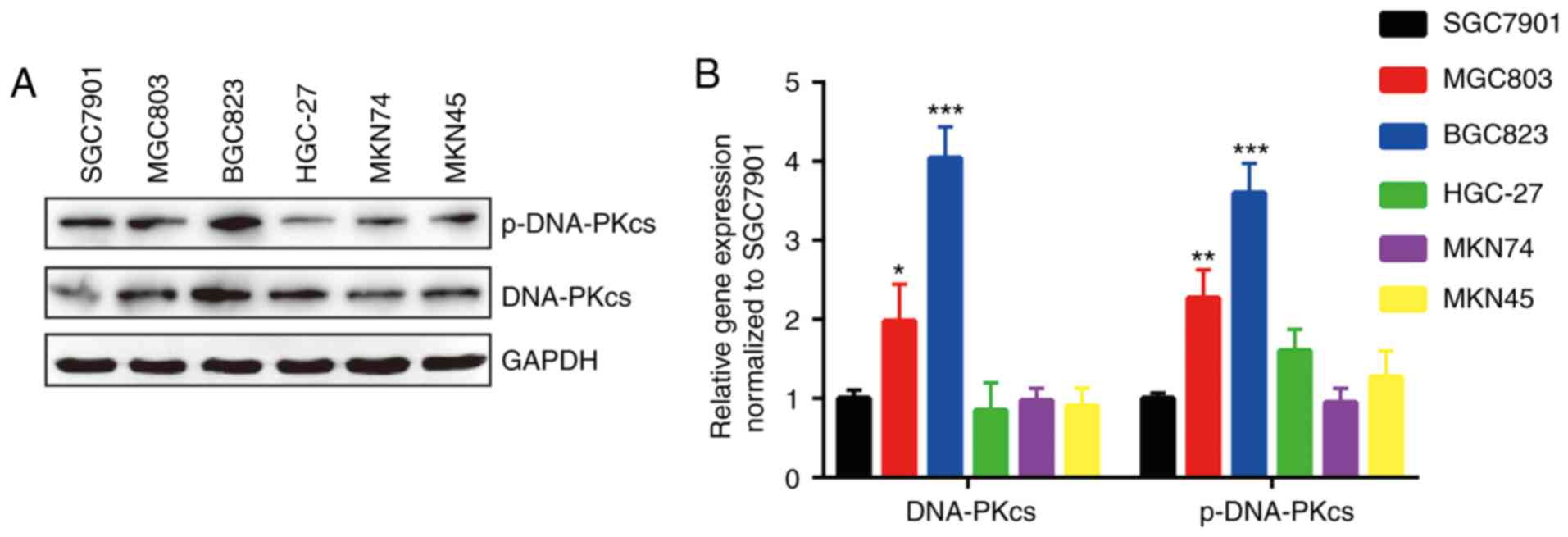
Overexpression of DNA-PKcs and its
phosphorylated form in BGC823 and MGC803 cells
The expression of DNA-PKcs, whose expression
predicts response to radiotherapy in various types of cancers, is
responsible for DNA repair (12,19).
Western blotting detection of DNA-PKcs and p-DNA-PKcs proteins
indicated that DNA-PKcs was expressed more highly in BGC823 and
MGC803 cells compared with other GC cell lines (Fig. 3A and B).
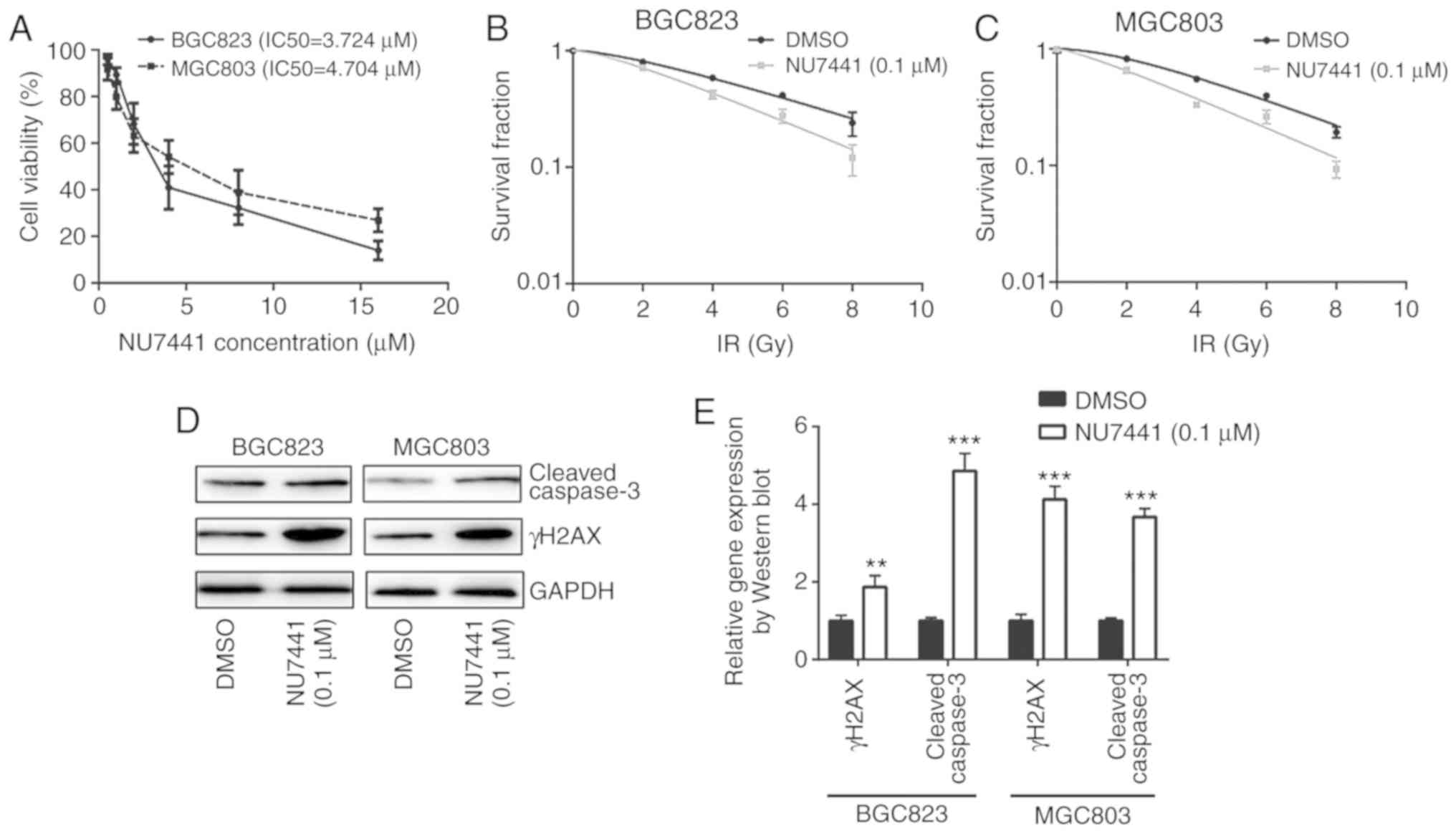
Inhibition of DNA-PKcs sensitizes
BGC823 and MGC803 cells towards IR
To determine whether DNA-PKcs were responsible for
different sensitivities towards IR, NU7441 (a DNA-PKcs inhibitor)
was used to inhibit DNA-PKcs activity. Cell viability assays
revealed that the IC50 was 3.724 µM and 4.704 µM for
BGC823 and MGC803, respectively (Fig.
4A). At a very low concentration without significant growth
inhibitory effect, 0.1 µM NU7441 induced a decreased survival
fraction in response to IR compared with cells treated with DMSO in
both BGC823 and MGC803 cells (Fig. 4B and
C; Table II). In addition,
NU7441 treatment increased γH2AX and cleaved-caspase3 protein
expression levels in BGC823 and MGC803 cells treated with IR
(Fig. 4D and E); 0.1 µM NU7441
significantly elevated IR induced cell apoptosis of BGC823 and
MGC803 cells (Fig. 4F and G).
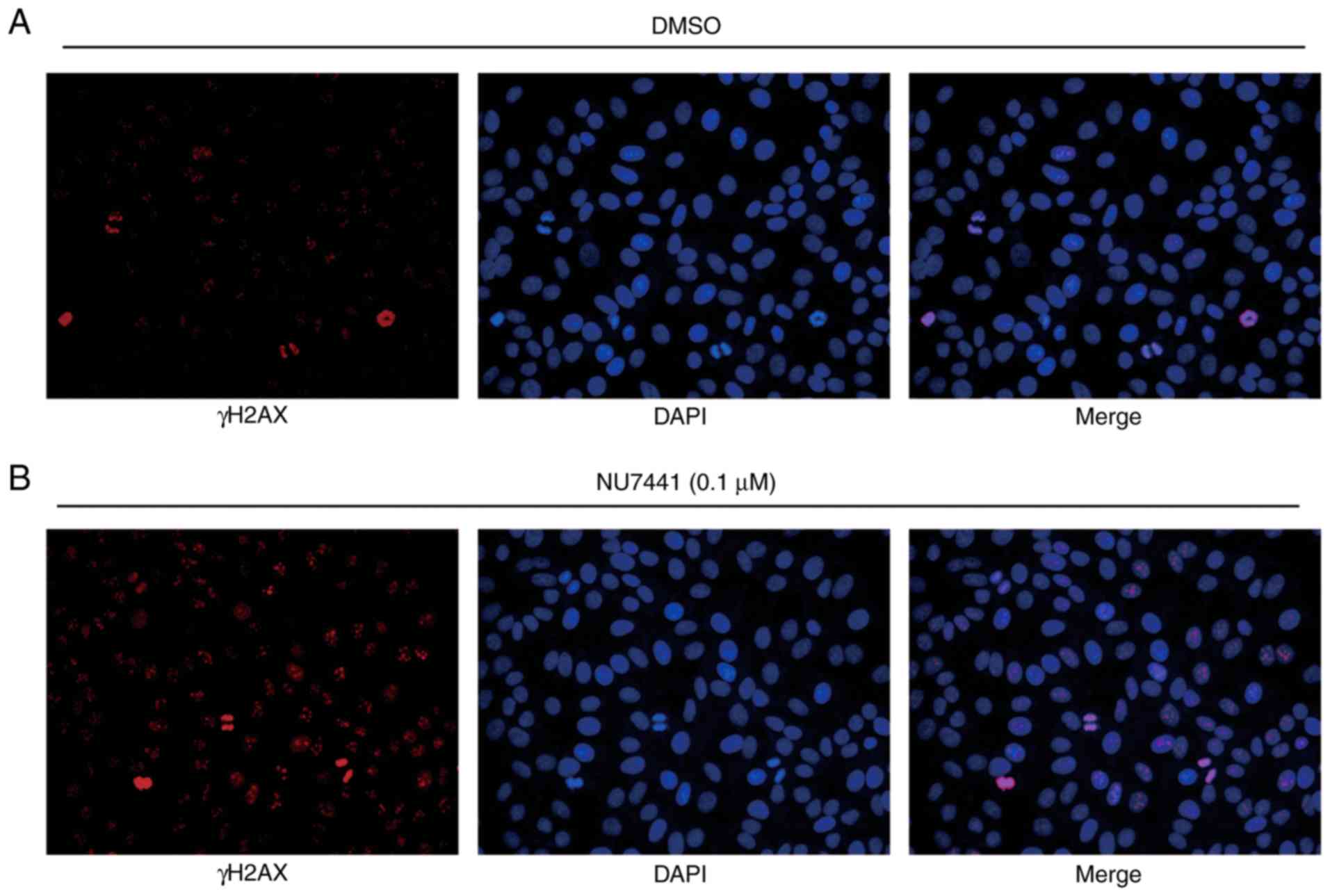
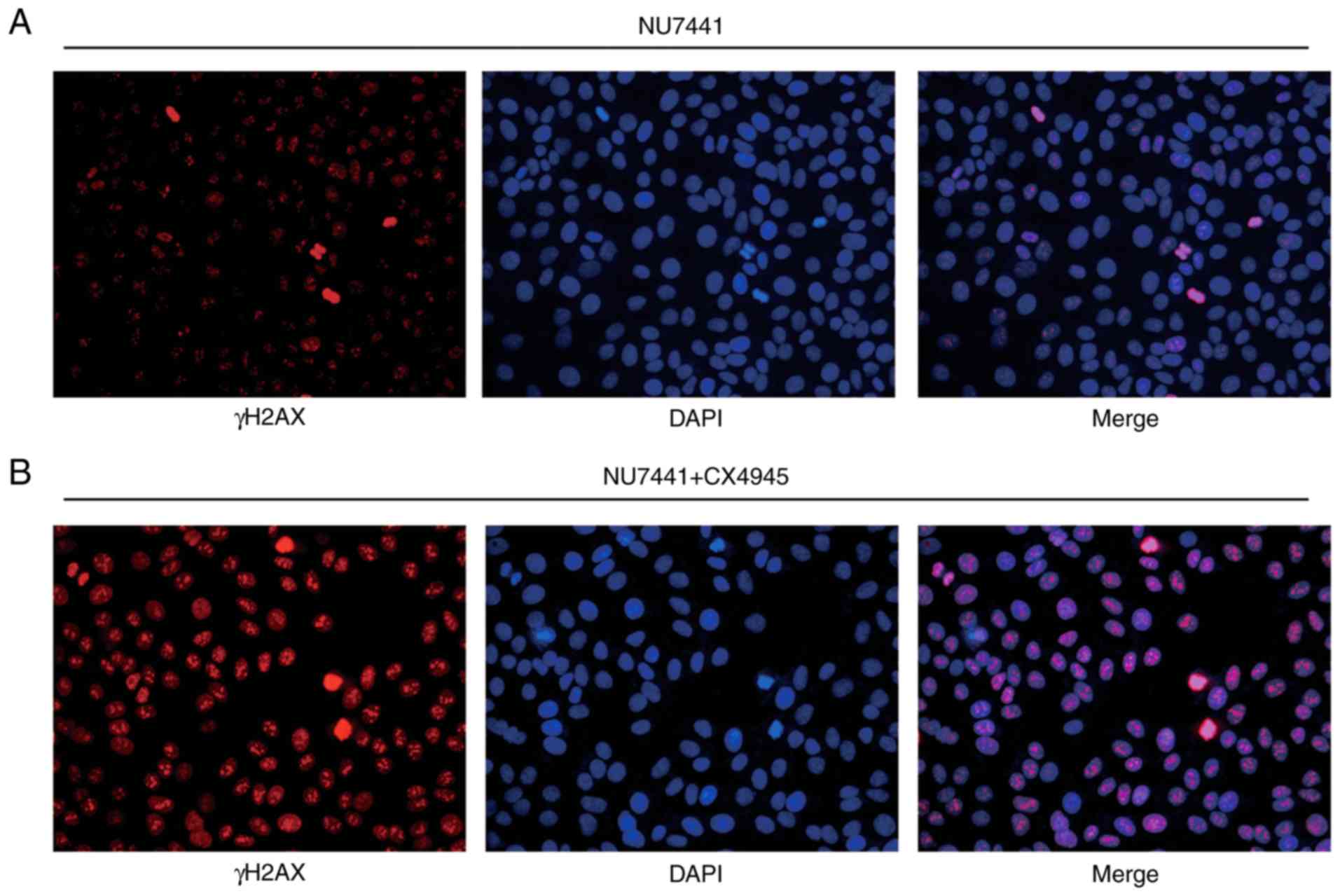
Furthermore, immunofluorescence analysis confirmed an elevation of
γH2AX expression in the nucleus in cells treated with NU7441 after
IR in BGC823 (Fig. 5A and B). These
data collectively demonstrated that DNA-PKcs was involved in
radiotherapy sensitivity in GC.
 | Table II.D0 and Dq in BGC823 and MGC803
treated with or without NU7441 (0.1 µM) towards ionizing
radiation. |
Table II.
D0 and Dq in BGC823 and MGC803
treated with or without NU7441 (0.1 µM) towards ionizing
radiation.
|
| BGC823 | MGC803 |
|---|
|
|
|
|
|---|
| Parameter | DMSO | NU7441 | DMSO | NU7441 |
|---|
| lnN | 0.480573 | 0.421338 | 0.677018 | 0.285931 |
| D0 | 4.526935 | 3.402518 | 3.762227 | 3.30142 |
| Dq | 2.175521 | 1.433612 | 2.547095 | 0.943977 |
Combination of CK2 inhibitor and
DNA-PKcs inhibitor strongly enhances response to radiotherapy in GC
cell lines
A previous study indicated that CK2 inhibition could
increase radiotherapy sensitivity via inhibition of X-ray repair
cross complementing 1 (XRCC1) (20).
In BGC823 and MGC803 cells, the combination of CK2 inhibitor
(CX-4945) and DNA-PKcs inhibitor (NU7441), demonstrated a stronger
enhancing effect in radiotherapy sensitivity compared with NU7441
alone (Fig. 6A and B; Table III). Furthermore, the combination of
CX-4945 and NU7441 further increased γH2AX and cleaved-caspase3
protein expression levels in both cell lines (Fig. 6C and D); additionally, CX4945 further
significantly elevated NU7441 induced cell apoptosis of GC823 cells
and MGC803 cells (Fig. 6E and F). The
elevation of γH2AX expression was validated in an
immunofluorescence assay (Fig. 7A and
B).
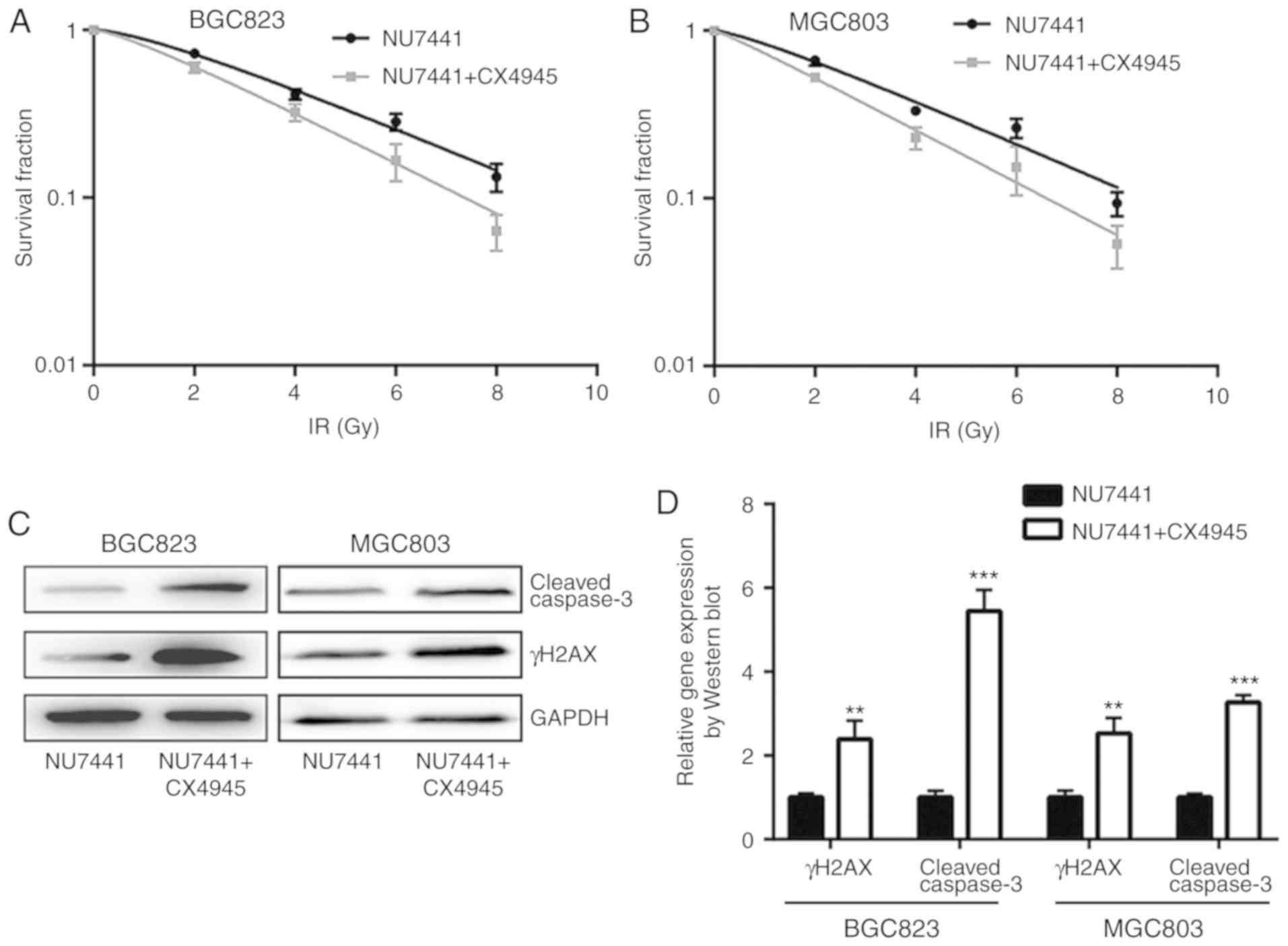 | Figure 6.Combination of DNA-dependent protein
kinase catalytic subunit inhibition and CK2 inhibition further
increases sensitivity of gastric cancer cells to IR. (A) BGC823 and
(B) MGC803 cells treated with NU7441 (0.1 µM), or a combination of
NU7441 (0.1 µM) and CX4945 (0.5 µM; a CK2 inhibitor), were exposed
to increasing doses of IR (0, 2, 4, 6 and 8 Gy). (C) Western
blotting showed that the combination of NU7441 (0.1 µM) and CX4945
(0.5 µM) induced a significant elevation of cleaved-caspase3 and
γH2AX protein expression levels compared with treatment with NU7441
(0.1 µM) alone in BGC823 and MGC803 cells. (D) Quantification of
protein expression levels. **P<0.01 and ***P<0.0001 vs.
NU7441. (E) CX4945 further significantly elevated NU7441 induced
cell apoptosis of GC823 cells and MGC803 cells. (F) Quantification
of cell apoptosis rates. **P<0.01 and ***P<0.0001. CK2,
casein kinase 2; IR, ionizing radiation; γH2AX, γ H2A histone
family member X; PI, propidium iodide; FITC, fluorescein
isothiocyanate. |
 | Table III.Comparison of D0 and Dq of BGC823 and
MGC803 treated with NU7441 (0.1 µM) or NU7441 (0.1 µM) and CX4945
(0.5 µM) towards ionizing radiation. |
Table III.
Comparison of D0 and Dq of BGC823 and
MGC803 treated with NU7441 (0.1 µM) or NU7441 (0.1 µM) and CX4945
(0.5 µM) towards ionizing radiation.
|
| BGC823 | MGC803 |
|---|
|
|
|
|
|---|
| Parameter | NU7441 | NU7441 +
CX4945 | NU7441 | NU7441 +
CX4945 |
|---|
| lnN | 0.42853 | 0.295650242 | 0.285931 | 0.104360015 |
| D0 | 3.434066 | 2.849814762 | 3.30142 | 2.748763057 |
| Dq | 1.471602 | 0.842548424 | 0.943977 | 0.286860955 |
Discussion
Radiotherapy is one of the standard treatment
approaches for GC, and can significantly improve the 5 year
survival rate of patients with GC (21). It is important to maximize the
therapeutic effect of radiotherapy towards tumor tissues and
minimize damage to normal tissues (22). Thus, increasing sensitivity of GC
towards radiotherapy may reduce the side effects of radiation. In
the current study, inhibition of DNA-PKcs enhanced radiotherapy
sensitivity of GC cells. Combination of DNA-PKcs and CK2 inhibition
could further augment sensitivity towards radiotherapy.
Activation of DNA-PKcs has been demonstrated to be
crucial for DNA double strand break repair (23). As DNA-PKcs is indispensable for DNA
damage repair, its expression is an important indicator for
response to DNA-damaging agents in various types of cancer
(12,24). DNA-PKcs also promotes cancer cell
metastasis via transcriptional regulation (25). In GC, the role of DNA-PKcs is
controversial. A report demonstrated that the expression of
DNA-PKcs was frequently elevated compared with normal gastric
tissues and was associated with relatively lower differentiation
(26). In another study, loss of
DNA-PKcs in GC was associated with advanced cancer, lymphatic
invasion, lymph node metastasis and advanced pTNM stage (27). The activity of DNA-PKcs is largely
reliant on Ku70/80 and DNA (28).
Mechanistically, Ku-DNA complex induces conformation change of
phosphoinositide 3-kinase domain to activate DNA-PKcs (29). In human tumor cells, a study revealed
that DNA-PKcs activation was also controlled by the Akt
serine/threonine kinase pathway. Activated Akt directly interacts
with DNA-PKcs to promote DSB accumulation of DNA-PKcs, which would
lead to radiotherapy resistance (30). A recent report discovered that
microRNA-101 negatively regulates DNA-PKcs and downregulation of
microRNA-101 was responsible for overexpression of DNA-PKcs in
renal cell carcinoma (31). The
function of DNA-PKcs in mediating radiotherapy sensitivity of GC
remains unknown. Expression of DNA-PKcs and its active form were
increased in GC cell lines that were relatively insensitive towards
IR. In addition, inhibition of DNA-PKcs sensitized GC cells exposed
to IR, alongside significantly increased expression of γH2AX and
cleaved caspase3.
CK2 is a highly conserved kinase that controls the
DNA damage repair regulation process through phosphorylation of its
substrates (32). Several reports
have indicated that CK2 enhances radiosensitivity of non-small cell
lung cancer cells via regulation of XRCC4 (33,34).
However, although it modulated DNA damage response, CK2 inhibition
failed to increase radiosensitivity of malignant glioma cells
(35). In GC, CK2 expression promoted
cancer progression and chemoresistance, and predicted poor
prognosis (36,37). Inhibition of CK2 activity further
increased radiosensitivity of GC cells treated with DNA-PKcs
inhibitor. This indicated that CK2 and DNA-PKcs are both involved
in the radiosensitivity of GC cells. A double inhibition strategy
using CK2 and DNA-PKcs may be effective in improving the effect of
radiotherapy in patients with GC.
In conclusion, the findings of the study revealed
that DNA-PKcs expression is important for the sensitivity of GC to
radiotherapy. Combination of CK2 and DNA-PKcs inhibition further
sensitized GC cells to IR. These results suggest that DNA-PKcs and
CK2 may be novel therapeutic targets for enhancing the effect of
radiotherapy for patients with GC.
Acknowledgements
Not applicable.
Funding
The current study was funded by the Nanjing Military
Area Medical Science and Technology Innovation Key Project Funding
Project (grant no. 15ZD011) and the Science and Technology Project
of Huaian (grant no. HAB201822).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WG, HS, DT, QW and SS performed the experiments and
acquired the data. HS, JZ and WX analyzed the data. HS and WG wrote
the paper. WG and HS supervised the current study. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yeh JM, Kuntz KM, Ezzati M, Hur C, Kong CY
and Goldie SJ: Development of an empirically calibrated model of
gastric cancer in two high-risk countries. Cancer Epidemiol
Biomarkers Prev. 17:1179–1187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Macdonald JS, Smalley SR, Benedetti J,
Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA,
Gunderson LL, Jessup JM and Martenson JA: Chemoradiotherapy after
surgery compared with surgery alone for adenocarcinoma of the
stomach or gastroesophageal junction. N Engl J Med. 345:725–730.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Toiyama Y, Tanaka K, Konishi N, Mohri Y,
Tonouchi H, Miki C and Kusunoki M: Administration
sequence-dependent antitumor effects of paclitaxel and
5-fluorouracil in the human gastric cancer cell line MKN45. Cancer
Chemother Pharmacol. 57:368–375. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Valentini V and Cellini F: Radiotherapy in
gastric cancer: A systematic review of literature and new
perspectives. Expert Rev Anticancer Ther. 7:1379–1393. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garg AK, Buchholz TA and Aggarwal BB:
Chemosensitization and radiosensitization of tumors by plant
polyphenols. Antioxid Redox Signal. 7:1630–1647. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dai Y and Grant S: New insights into
checkpoint kinase 1 in the DNA damage response signaling network.
Clin Cancer Res. 16:376–383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu P, Gan W, Guo C, Xie A, Gao D, Guo J,
Zhang J, Willis N, Su A, Asara JM, et al: Akt-mediated
phosphorylation of XLF impairs non-homologous end-joining DNA
repair. Mol Cell. 57:648–661. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang S, Matsunaga S, Lin YF, Sishc B,
Shang Z, Sui J, Shih HY, Zhao Y, Foreman O, Story M, et al:
Spontaneous tumor development in bone marrow-rescued
DNA-PKcs(3A/3A) mice due to dysfunction of telomere leading strand
deprotection. Oncogene. 35:3909–3918. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee SH and Kim CH: DNA-dependent protein
kinase complex: A multifunctional protein in DNA repair and damage
checkpoint. Mol Cells. 13:159–166. 2002.PubMed/NCBI
|
|
12
|
Bouchaert P, Guerif S, Debiais C, Irani J
and Fromont G: DNA-PKcs expression predicts response to
radiotherapy in prostate cancer. Int J Radiat Oncol Biol Phys.
84:1179–1185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng Y, Zhang Q, Nagasawa H, Okayasu R,
Liber HL and Bedford JS: Silencing expression of the catalytic
subunit of DNA-dependent protein kinase by small interfering RNA
sensitizes human cells for radiation-induced chromosome damage,
cell killing, and mutation. Cancer Res. 62:6400–6404.
2002.PubMed/NCBI
|
|
14
|
Toulany M, Kehlbach R, Florczak U, Sak A,
Wang S, Chen J, Lobrich M and Rodemann HP: Targeting of AKT1
enhances radiation toxicity of human tumor cells by inhibiting
DNA-PKcs-dependent DNA double-strand break repair. Mol Cancer Ther.
7:1772–1781. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee SW, Cho KJ, Park JH, Kim SY, Nam SY,
Lee BJ, Kim SB, Choi SH, Kim JH, Ahn SD, et al: Expressions of Ku70
and DNA-PKcs as prognostic indicators of local control in
nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys.
62:1451–1457. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bjork-Eriksson T, West C, Nilsson A,
Magnusson B, Svensson M, Karlsson E, Slevin N, Lewensohn R and
Mercke C: The immunohistochemical expression of DNA-PKCS and Ku
(p70/p80) in head and neck cancers: Relationships with
radiosensitivity. Int J Radiat Oncol Biol Phys. 45:1005–1010. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eriksson A, Lewensoh R, Larsson R and
Nilsson A: DNA-dependent protein kinase in leukaemia cells and
correlation with drug sensitivity. Anticancer Res. 22:1787–1793.
2002.PubMed/NCBI
|
|
18
|
Tu Y, Ji C, Yang B, Yang Z, Gu H, Lu CC,
Wang R, Su ZL, Chen B, Sun WL, et al: DNA-dependent protein kinase
catalytic subunit (DNA-PKcs)-SIN1 association mediates ultraviolet
B (UVB)-induced Akt Ser-473 phosphorylation and skin cell survival.
Mol Cancer. 12:1722013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu JY, Lu S, Xu XY, Hu SL, Li B, Qi RX,
Chen L and Chang JY: Knocking down nucleolin expression enhances
the radiosensitivity of non-small cell lung cancer by influencing
DNA-PKcs activity. Asian Pac J Cancer Prev. 16:3301–3306. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Strom CE, Mortusewicz O, Finch D, Parsons
JL, Lagerqvist A, Johansson F, Schultz N, Erixon K, Dianov GL and
Helleday T: CK2 phosphorylation of XRCC1 facilitates dissociation
from DNA and single-strand break formation during base excision
repair. DNA Repair (Amst). 10:961–969. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang ZX, Gu XZ, Yin WB, Huang GJ, Zhang
DW and Zhang RG: Randomized clinical trial on the combination of
preoperative irradiation and surgery in the treatment of
adenocarcinoma of gastric cardia (AGC)-report on 370 patients. Int
J Radiat Oncol Biol Phy. 42:929–934. 1998. View Article : Google Scholar
|
|
22
|
Moran JM, Elshaikh MA and Lawrence TS:
Radiotherapy: What can be achieved by technical improvements in
dose delivery? Lancet Oncol. 6:51–58. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kurimasa A, Kumano S, Boubnov NV, Story
MD, Tung CS, Peterson SR and Chen DJ: Requirement for the kinase
activity of human DNA-dependent protein kinase catalytic subunit in
DNA strand break rejoining. Mol Cell Biol. 19:3877–3884. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Beskow C, Skikuniene J, Holgersson A,
Nilsson B, Lewensohn R, Kanter L and Viktorsson K: Radioresistant
cervical cancer shows upregulation of the NHEJ proteins DNA-PKcs,
Ku70 and Ku86. Br J Cancer. 101:816–821. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goodwin JF, Kothari V, Drake JM, Zhao S,
Dylgjeri E, Dean JL, Schiewer MJ, McNair C, Jones JK, Aytes A, et
al: DNA-PKcs-mediated transcriptional regulation drives prostate
cancer progression and metastasis. Cancer Cell. 28:97–113. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li W, Xie C, Yang Z, Chen J and Lu NH:
Abnormal DNA-PKcs and Ku 70/80 expression may promote malignant
pathological processes in gastric carcinoma. World J Gastroenterol.
19:6894–6901. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee HS, Yang HK, Kim WH and Choe G: Loss
of DNA-dependent protein kinase catalytic subunit (DNA-PKcs)
expression in gastric cancers. Cancer Res Treat. 37:98–102. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hammarsten O and Chu G: DNA-dependent
protein kinase: DNA binding and activation in the absence of Ku.
Proc Natl Acad Sci USA. 95:525–530. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rivera-Calzada A, Maman JD, Spagnolo L,
Pearl LH and Llorca O: Three-dimensional structure and regulation
of the DNA-dependent protein kinase catalytic subunit (DNA-PKcs).
Structure. 13:243–255. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Toulany M, Lee KJ, Fattah KR, Lin YF,
Fehrenbacher B, Schaller M, Chen BP, Chen DJ and Rodemann HP: Akt
promotes post-irradiation survival of human tumor cells through
initiation, progression, and termination of DNA-PKcs-dependent DNA
double-strand break repair. Mol Cancer Res. 10:945–957. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zheng B, Mao JH, Li XQ, Qian L, Zhu H, Gu
DH and Pan XD: Over-expression of DNA-PKcs in renal cell carcinoma
regulates mTORC2 activation, HIF-2α expression and cell
proliferation. Sci Rep. 6:294152016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meggio F and Pinna LA:
One-thousand-and-one substrates of protein kinase CK2? FASEB J.
17:349–368. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Q, Li K, Yang T, Zhang S, Zhou Y, Li Z,
Xiong J, Zhou F, Zhou X, Liu L, et al: Association of protein
kinase CK2 inhibition with cellular radiosensitivity of non-small
cell lung cancer. Sci Rep. 7:161342017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin YC, Hung MS, Lin CK, Li JM, Lee KD, Li
YC, Chen MF, Chen JK and Yang CT: CK2 inhibitors enhance the
radiosensitivity of human non-small cell lung cancer cells through
inhibition of stat3 activation. Cancer Biother Radiopharm.
26:381–388. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kroonen J, Artesi M, Capraro V,
Nguyen-Khac MT, Willems M, Chakravarti A, Bours V and Robe PA:
Casein kinase 2 inhibition modulates the DNA damage response but
fails to radiosensitize malignant glioma cells. Int J Oncol.
41:776–782. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu W, Chen Q, Wang Q, Sun Y, Wang S, Li A,
Xu S, Røe OD, Wang M, Zhang R, et al: JWA reverses cisplatin
resistance via the CK2-XRCC1 pathway in human gastric cancer cells.
Cell Death Dis. 5:e15512014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bae JS, Park SH, Kim KM, Kwon KS, Kim CY,
Lee HK, Park BH, Park HS, Lee H, Moon WS, et al: CK2alpha
phosphorylates DBC1 and is involved in the progression of gastric
carcinoma and predicts poor survival of gastric carcinoma patients.
Int J Cancer. 136:797–809. 2015. View Article : Google Scholar : PubMed/NCBI
|