Introduction
According to cancer stem cell (CSC) theory, only
some cells within a tumor will initiate tumorigenic growth
(1). It is becoming evident that
cancer treatment that fails to eliminate cancer stem cells may
allow for regrowth of the tumor (2).
CSCs have been identified and isolated from hematopoietic
malignancies and solid tumors, including glioblastoma, breast
cancer, colon cancer, hepatocellular carcinoma and other types of
tumors (3).
Cervical cancer is the fourth most common malignancy
affecting women worldwide, and 250,000 deaths have been estimated
annually (4). Notably, the high
mortality rate of patients is mainly due to poor loco-regional
control, including local tissue invasion by the primary tumor and
regional lymph node involvement, rather than distant metastasis.
Human papillomavirus (HPV) infection is a factor in the development
of most cases of cervical cancer (5).
At the same time, HPV infection alone is not sufficient to generate
a fully malignant phenotype. Thus, the exact molecular and genetic
mechanisms of malignant transformation remain to be explored. The
prospective discrimination and isolation of cervical cancer stem
cells are the most important steps in elucidating cervical
carcinogenesis and establishing new therapeutic approaches for this
cancer type.
It has been suggested that polycomb complex protein
Bmi1 (Bmi1) is associated with cancer initiation and progression in
various types of tumor-initiating cells, and plays important roles
in the development and progression of carcinomas (6). The Bmi1 gene belongs to the polycomb
gene family and is a component of polycomb repressive complex 1,
which is implicated in the stable maintenance of gene repression
(7). Bmi1 was first isolated as an
oncogene that cooperates with c-Myc in the generation of lymphomas
in mice (8). Various types of human
cancer display a similar pattern in terms of overexpression of
Bmi1, such as non-small cell lung cancer, breast cancer, colorectal
cancer, prostate cancer and nasopharyngeal carcinoma (9).
Sox2, a major transcription factor belonging to
group B of the SOX family, is a key transcription factor in
embryonic development and plays a critical role in determining the
fate of stem cells. In our previous study, it was demonstrated that
Sox2 may participate in the carcinogenesis of cervical carcinomas
(10).
Both Bmi1 and Sox2 are specific markers of neural
stem cells (11,12). Meanwhile, Bmi1 and Sox2 are both
overexpressed in some human cancer types, such as non-small cell
lung cancer, breast cancer and cervical cancer (13). Until now, no evidence has supported
the interaction between Bmi1 and Sox2 in cervical carcinogenesis,
to the best of our knowledge. The present study evaluated Bmi1
expression using immunohistochemical staining of tumor tissues from
patients with cervical squamous cell carcinoma, analyzed its role
in cervical carcinogenesis, and explored the relationship between
Bmi1 and Sox2.
Materials and methods
Clinical specimens
A total of 112 cervical tissue specimens, including
high-grade squamous intraepithelial lesions (HSILs; n=41) and
cervical cancer (n=71), were examined, and their
clinicopathological backgrounds are summarized in Table I. The samples were derived from either
surgical resection or biopsy. A total of 47 histologically normal
cervical specimens were obtained from biopsy materials. All the
specimens were obtained from the Department of Gynecology, First
Affiliated Hospital of Xi'an Jiaotong University between January
2016 and December 2017, and were fixed in 10% formalin for 30 min
at room temperature and embedded in paraffin. Serial sections, 4 µm
in thickness, were cut from each paraffin block. A total of two
pathologists, in a blinded fashion, processed several sections for
histopathology studies according to the International Federation of
Gynecology and Obstetrics classification system (14), while the remaining sections were
processed for subsequent immunohistochemistry.
 | Table I.Relationship between Bmi1 or Sox2
expression and the characteristics of the cervical squamous
carcinomas. |
Table I.
Relationship between Bmi1 or Sox2
expression and the characteristics of the cervical squamous
carcinomas.
|
|
| Bmi-1 | SOX-2 |
|
|---|
|
|
|
|
|
|
|---|
|
Characteristics | n | low | high | P-value | Negative | Positive | P-value |
|---|
| Age, years |
|
≤45 | 33 | 18 | 15 | 0.777 | 8 | 25 | 0.655 |
|
>45 | 38 | 22 | 16 |
| 11 | 27 |
|
| SCC
differentiation |
|
Well | 18 | 14 | 4 | 0.034 | 9 | 9 | 0.015 |
|
Moderate-poor | 53 | 26 | 27 |
| 10 | 43 |
|
| Lymph node
metastasis |
|
Absence | 57 | 34 | 23 | 0.256 | 13 | 44 | 0.178 |
|
Present | 14 | 6 | 8 |
| 6 | 8 |
|
| Clinical stage |
|
I–II | 60 | 35 | 25 | 0.516 | 15 | 45 | 0.470 |
|
III–IV | 11 | 5 | 6 |
| 4 | 7 |
|
Immunohistochemistry
The slides were deparaffinized in xylene and then
were rehydrated through an ethanol gradient. Following a rinse in
PBS, the sections were heated at 120°C in 0.01 mol/l sodium citrate
buffer (pH 6.0) for 2 min to for antigen retrieval. After blocking
nonspecific reactions with the 3% H2O2 for 30
min at room temperature (Sigma-Aldrich; Merck KGaA), the sections
were incubated with Bmi1 antibody (1:150; EMD Millipore; cat. no.
05-1322) and Sox2 antibody (1:100; Santa Cruz Biotechnology, Inc.;
cat. no. sc-17320) at 4°C overnight. The sections were then stained
with horseradish peroxidase (HRP)-labeled goat-mouse and
rabbit-goat (1:100; OriGene Technologies, Inc.; cat. nos. SPN-9001
and SPN-9002) at 30°C for 20 min. The expression of proteins was
stained with 3,3′-diaminobenzidine (DAB). A similar dilution of the
control mouse or control goat IgG (1:100; R&D Systems, Inc.;
cat. nos. MAB0031 and AB-108-C) was applied instead of the primary
antibody as a negative control. The degree of immunostaining of
each formalin-fixed, paraffin-embedded section was reviewed and
scored by two independent observers. The immunoreactivity of Bmi1
and Sox2 was semi quantitatively analyzed by light microscope,
according methods from previous studies (15). In brief, the percentage of positive
cells was divided into five categories of score: <10, 0; 10–25,
1; 25–50, 2; 50–75, 3; and >75%, 4. The intensity of staining
was divided into four categories of score: No staining, 0; light
brown, 1; brown, 2; and dark brown, 3. Bmi1 and Sox2 staining was
assessed by the following formula: Immunohistochemistry score ×
intensity score. An overall score <3 was defined as negative;
>3 and <6 as weak positive; and >6 as strong positive. All
scores were evaluated by two different researchers at a
magnification of ×400.
Cell lines and cell culture
The human cervical cancer cell lines SiHa, HeLa and
C33A were purchased from the American Type Culture Collection. All
the cervical cancer cells were grown in DMEM (Sigma Aldrich; Merck
KGaA) supplemented with 10% FBS (Invitrogen; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 µg/m streptomycin at
37°C in an atmosphere of 5% CO2. Tera-1 cells were
cultured in McCoy 5A medium (Sigma-Aldrich; Merck KGaA) with 15%
FBS at 37°C in an atmosphere with 5% CO2.
Western blotting
Protein samples from each lysate from fresh cells
treated with RIPA lysis buffer (cat. no. sc-24948; Santa Cruz
Biotechnology, Inc.) were firstly quantified with a protein
bicinchoninic acid kit (Pierce-23225; Thermo Fisher Scientific,
Inc.), loaded and separated by 10% SDS-PAGE with 30 ng per lane,
and then transferred to PVDF membranes. The PVDF membranes were
blocked with fat-free milk at a concentration of 5% for 1 h at room
temperature. The membranes were probed with anti-Bmi1 (1:1,000; EMD
Millipore; cat. no. 05-1322), anti-Sox2 (1:500; Santa Cruz
Biotechnology, Inc.; cat. no. sc-17320), and anti-actin (1:1,000;
Santa Cruz Biotechnology, Inc.; cat. no. sc-47778) at 4°C
overnight. After reacting with HRP-conjugated anti-mouse or
anti-goat immunoglobulin (1:10,000; cat. nos. G-21040 and 81-1620;
Thermo Fisher Scientific Inc.) for 1 h at room temperature, the
proteins were detected using enhanced chemiluminescence (EMD
Millipore).
Vector construction and
transfection
Full-length Bmi1 cDNA was amplified (16), and then the Bmi1 cDNA fragment was
subsequently cloned into the EcoRI and BamHI sites of
the internal ribosome entry site vector pIRES2-AcGFP (Clontech
Laboratories, Inc.) to generate the pIRES2-AcGFP-Bmi1 recombinant
plasmid. The Bmi1 short hairpin (sh)RNA named GenePharma
SuperSilencing shRNA™, for which the plasmid was
pGPU6/GFP/Neo, was used to specifically silence the expression of
Bmi1 (Shanghai GenePharma Co., Ltd.).
All transfection experiments were performed using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions. In
brief, 105 cells were seeded in 6-well plate and
transfected with 2 µg pIRES2-AcGFP-Bmi1 and shBmi1 or empty vector.
After culturing in medium containing 1 mg/ml geneticin (cat. no.
G418; Genetical; Ameresco, Inc.) for 2–3 weeks, pooled stable
clones were isolated. Clones that expressed the Bmi1 cDNA coding
region, or Bmi1 silenced cells, were maintained in medium
containing 0.8 mg/ml geneticin and used for further
investigation.
Tumor sphere formation assay
For clinical cervical cancer samples, the tissues
were collected immediately after surgical resection and were then
washed, minced and dissociated to single cells using collagenase IV
(Sigma-Aldrich; Merck KGaA) at 37°C. After 16 h of digestion, the
cells were grown in serum-free stem cell medium containing DMEM/F12
(cat. no. 12400-024, Gibco; Thermo Fisher Scientific, Inc.)
supplemented with basic fibroblast growth factor, epidermal growth
factor, N2 supplement and B27 supplement. For in vivo
propagation, the tumor spheres were dissociated to single cells
after 7 days and quantified. Next, 1,000 cells were injected into
the subcutaneous tissue in the dorsum of 4- to 6-week-old female
BALB/c nude mice (Shanghai SLAC Laboratory Animal Co., Ltd.). A
total of 30 mice weighing 12–14 g were housed in SPF conditions
with a temperature of 24±2°C and a humidity of 40–60%. Mice had
free access to regular chow which was replaced every three days,
and were housed on a 12 h light/12 h dark cycle. The
xenotransplanted tumor passage in vivo were performed in 3
number of mice (30 number mice for 10-serial-passage). After 60
days, the mice were sacrificed, and the formed tumors were
dissected and handled as described previously (17).
For cervical cancer cell lines, the cells were
plated at a density of 200 cells/well in 24-well ultralow
attachment plates, or at a density of 1 cell/well in 96-well
plates, and maintained in stem cell medium at 37°C in an atmosphere
of 5% CO2. Tumor spheres that arose within 2 weeks were
recorded. For serial tumor sphere formation assays, the spheres
were harvested, disaggregated with 0.25% trypsin/EDTA, filtered
through a 40-µm mesh and re-plated according to the aforementioned
method. For each cell type, triplicate samples were used, and two
individuals counted the number of spheres in a blind fashion.
Tumor formation assays
Female 6-week-old NOD/SCID mice (Shanghai SLAC
Laboratory Animal Co., Ltd.) weighing 12–14 g were used to assess
the tumor formation properties in vivo; A total number of 9
mice (3 mice/group: SiHa-GFP cell and SiHa-Bmi1 cell group;
HeLa-GFP cell and HeLa-Bmi1 cell group; and C33A-shControl cell and
C33A-shBmi1 cell group) were housed in SPF conditions with a
temperature of 24±2°C and a humidity of 40–60%. Mice had free
access to regular chow which was replaced every three days, and
were housed on a 12 h light/12 h dark cycle. A total of
106 cells were injected into the subcutis on the dorsum.
Engrafted mice were inspected twice per week, until the observation
was terminated at 8–12 weeks, by visual observation and palpation
for the appearance of tumors. The tumor volume (V) was determined
by the length (a) and width (b) as follows: V=ab2/2. The
experimental protocols were evaluated and approved by the Animal
Care and Use Committee of the Medical School of Xi'an Jiaotong
University. A portion of each tumor was fixed for 24 h in 10%
formaldehyde at room temperature and embedded in paraffin for
immunohistochemistry analysis according to the aforementioned
protocol.
Dual luciferase reporter assay
Sox2 promoter regions containing 2 E-Box motifs that
can bind to the Bmi1 protein were predicted using Promoter 2.0
Prediction Server (http://www-bimas.cit.nih.gov/molbio/proscan/).
Promoter luciferase reporters containing the Sox2 promoter regions
of −1,000 bp-+1 bp, −900 bp-+1 bp, −400 bp-+1 bp and −100 bp-+1 bp,
and pTK-RL plasmids (Promega Corporation), were transiently
co-transfected with the Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) into tumor cells
(5×104) plated in 24-well plate dishes, while the
activities of both firefly and Renilla luciferase reporters
were determined 48 h post-transfection using the Dual Luciferase
Assay kit (Promega Corporation), according to the manufacturer's
instructions. The promoter luciferase reporter activity is
presented as the relative ratio of firefly luciferase activity to
Renilla luciferase activity. The specific activity was
displayed as a fold change of the experimental group compared with
the control group. All experiments were performed in
triplicate.
Quantitative chromatin
immunoprecipitation (ChIP)
Quantitative ChIP assays were performed according to
the manufacturer's protocol for the EZ-ChIP™ assay kit
(cat. no. 17-371; EMD Millipore). Each sample was assayed in
triplicate, and the amount of precipitated DNA was calculated as
10% of the input sample. A total of 20 µg Bmi1 antibody was
validated to immunoprecipitate the chromatin DNA cross-linked
complex with 1% formaldehyde. Normal mouse IgG and histone H3
rabbit monoclonal antibodies were used as the negative and positive
controls, respectively. Regions of interest were amplified from
precipitated samples by reverse transcription-quantitative PCR
(RT-qPCR). Each sample was assayed in triplicate, and the amount of
precipitated DNA was calculated as the percentage of the input
sample.
RT-qPCR
Total RNA was extracted using TRIzol reagent,
according to the manufacturer's protocol (Invitrogen; Thermo Fisher
Scientific, Inc.). A total of 2 µg total RNA was reverse
transcribed using Takara reverse transcriptase (Takara
Biotechnology Co., Ltd.). A volume of 2.0 µl of each diluted cDNA
(1:20) was subjected to RT-qPCR in a final volume of 20 µl
containing 100 nM of each specific primer and SYBR Green Mix
(Takara Biotechnology, Co., Ltd.). The sequences of the primers are
supplied in Table SI.
The amplification was carried out as follows:
Initial enzyme activation at 95°C for 30 sec, followed by 40 cycles
of 95°C for 5 sec and 60°C for 30 sec, and then a dissociation
stage using an iQ5 multicolor Real-time PCR Detection System
(Bio-Rad, Laboratories, Inc.). The quantification cycle (Cq) value
was determined as the point at which the fluorescence exceeded a
threshold value preset by the instrument software. Relative
expression in each experiment set (fold-change vs. control) was
calculated according to comparative Cq method using the formula:
RQ=2−ΔΔCq (18).
Database
Expression of Bmi1 and SOX2 in patients with
cervical cancer compared to normal cervix tissue was assessed using
data from the Gene Expression Profiling Interactive Analysis
website (GEPIA; http://gepia.cancer-pku.cn/index.html) based on The
Cancer Genome Atlas database (normal cervix, n=13; cervical cancer,
n=306).
Statistical analysis
All statistical analyses were carried out using the
SPSS 16.0 statistical software package (SPSS, Inc.). A Tukey HSD
post hoc test after two-way ANOVA was used to analyze the
significance of protein expression in the normal cervix, HSIL and
cervical carcinoma samples. The relationship between the expression
of Bmi1 and clinicopathological characteristics was assessed by
χ2 test. Spearman correlation analysis was used to
demonstrate the relationship between Bmi1 and Sox2. All the data
are presented as mean ± SD and P<0.05 was considered to indicate
a statistically significant difference.
Results
Expression of Bmi1 protein in
paraffin-embedded cervical tissue specimens
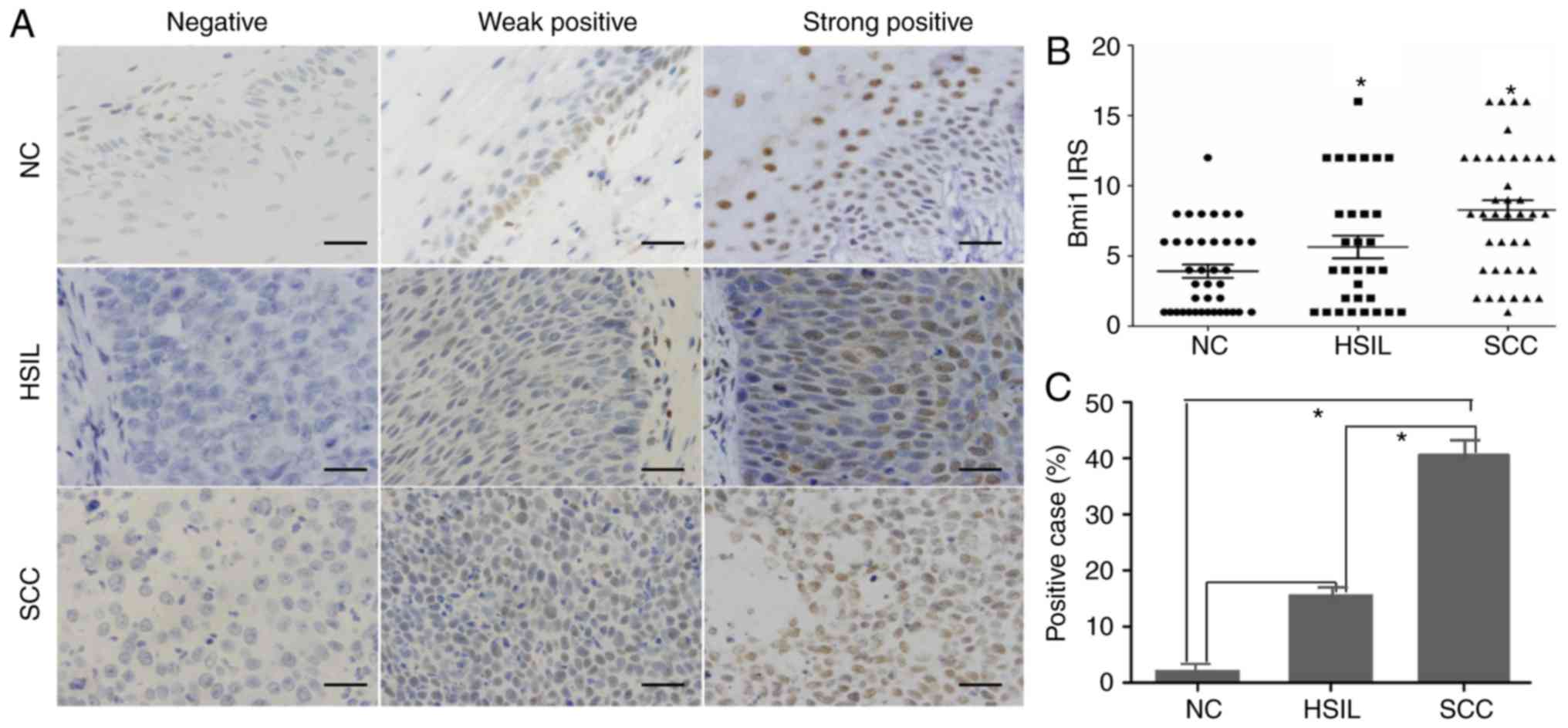
The expression and subcellular localization of Bmi1
protein were determined by immunohistochemistry in 47
paraffin-embedded normal cervix (NC) samples, 41 HSILs, and 71
cervical squamous cell carcinoma (SCC) samples. As shown in
Fig. 1, Bmi1 was localized to the
nucleus. Among the 47 NC samples, 46 cases exhibited low Bmi1
expression (weakly positive or no staining), and high Bmi1
expression (strongly positive) was found only in 1 case (2.12%;
Fig. 1A). Of the low Bmi1 expression
cases, 14 cases showed no staining and 28 cases showed weak
staining. In our study, basal cells in the cervical epithelium were
Bmi1-positive with 32 weakly staining samples and 1 strongly
positive staining sample. A significantly higher frequency of Bmi1
staining was observed in the HSIL (7/41; 16.13%) and SCC samples
(31/71; 43.66%; P<0.05; Fig. 1B and
C).
Statistical evaluation of the immunohistochemistry
was compared with clinical reports and pathological findings
(Table I). The expression level of
Bmi1 was significantly associated with histological grade
(P<0.05) but was not associated with age, tumor size, lymph node
metastasis or clinical stage.
Bmi1 promotes tumorigenicity in
cervical cancer
Bmi1 is associated with the initiation and
progression of various types of tumor-initiating cells and plays
important roles in the development and progression of carcinomas.
Here, to determine whether Bmi1 affects the tumorigenicity of
cervical cancer cell lines, the expression of Bmi1 was evaluated in
cervical cancer cell lines by western blotting (Fig. 2A). Next, exogenous Bmi1 in SiHa and
HeLa cells was overexpressed by stable gene transfection, and the
expression of Bmi1 was knocked down in C33A cells by shRNA
(Fig. 2B and C). To test the tumor
formation ability, cells were subcutaneously injected into nude
mice. When 105 cells were inoculated into nude mice, the
tumors formed by Bmi1-overexpressed SiHa cells grew more quickly
and to a larger size, with an average net weight of 532±6.23 mg,
than those formed by control cells, with an average net weight of
324±4.76 mg, in three mice/group (P<0.05; Fig. 2D-F). Similarly, HeLa-Bmi1 cells formed
tumors of 925±12.32 mg, which were twice as large as those with
HeLa-GFP cells (P<0.05; Fig.
2D-F). Additionally, Bmi1-silenced C33A cells formed tumors
that grew more slowly and to a smaller size than those formed by
C33A-shControl cells (482±6.02 mg vs. 0; P<0.001; Fig. 2G-I), suggesting that Bmi1 contributes
to the tumorigenesis of cervical cancer.
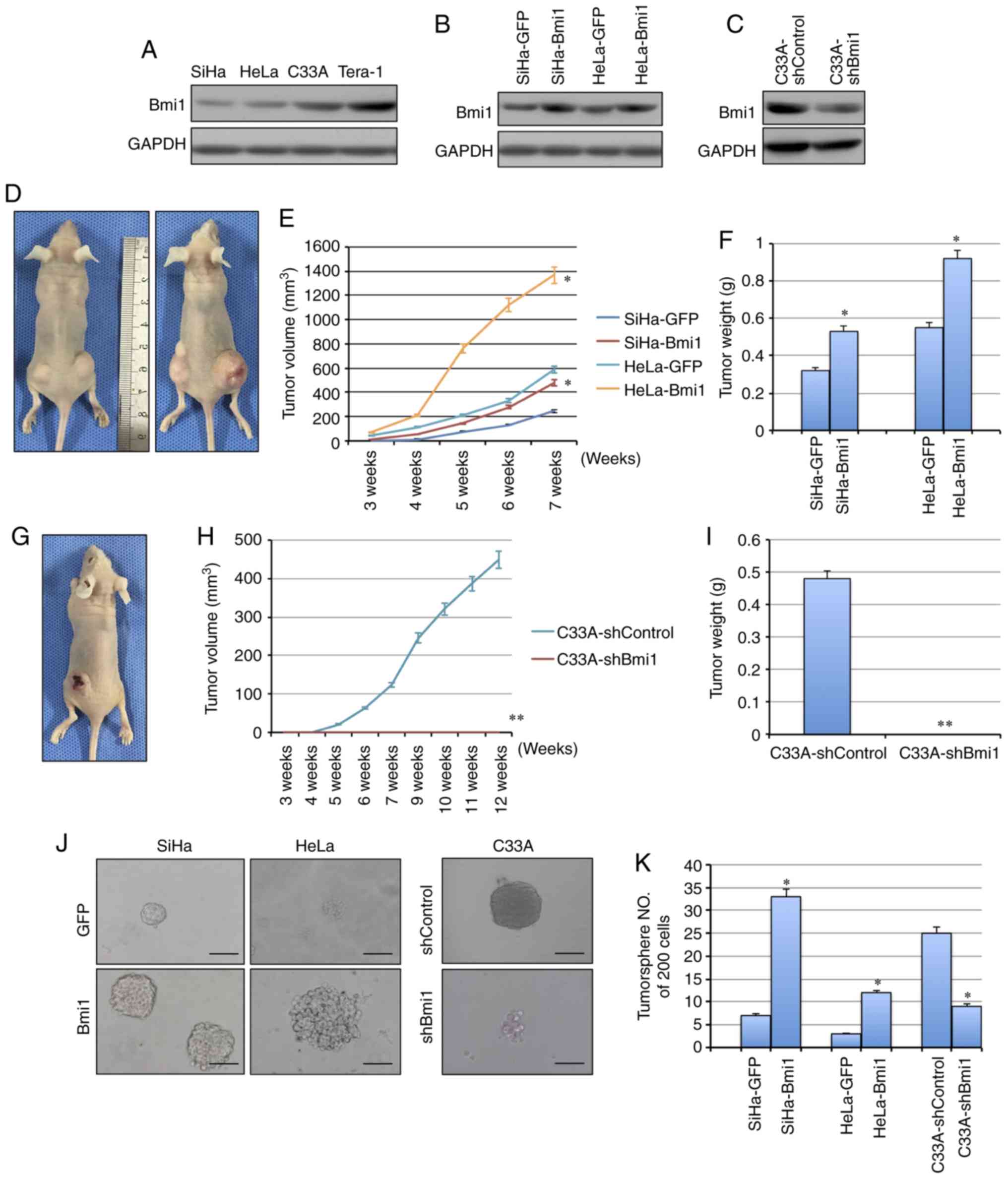 | Figure 2.Bmi1 promotes carcinogenicity and
tumor sphere formation ability in cervical cancer. (A) The
expression of Bmi1 protein in three cervical cancer cell lines was
detected by western blotting; the teratoma cell line Tera-1 was
used as the positive control. (B) Bmi1 protein expression was
evaluated in Bmi1-overexpressing SiHa and HeLa cells by western
blotting. (C) Bmi1 protein expression was evaluated in
Bmi1-silenced C33A cells by western blotting. (D) Tumor growth was
assessed over (E) 7 weeks, as well as the (F) the tumor net weight
of nude mice inoculated with SiHa-Bmi1, SiHa-GFP, HeLa-Bmi1 and
HeLa-GFP cells with three replicates (1×105 cells).
Additionally, (G) tumor growth was measured over (H) 12 weeks, as
well as the (I) tumor weights of nude mice inoculated with
C33A-shBmi1 and C33A-shControl cells with three replicates
(1×106 cells). (J) The tumor sphere formation assay was
performed in FBS-free medium in low-density cultures per 200 cells
with six replicates, and then (K) the number of tumor spheres
formed by Bmi1-overexpressing SiHa and HeLa cells and Bmi1-silenced
C33A cells was calculated. Bars=SE. *P<0.05, **P<0.01 vs.
respective control. Bmi1, polycomb complex protein Bmi1; GFP, green
fluorescent protein; sh, short hairpin. |
Bmi1 promotes tumor sphere formation
ability in cervical cancer
Besides the tumor formation ability, CSCs share
critical properties with stem cells: Self-renewal and
differentiation. The tumor sphere formation assay is recognized as
a classical assay for self-renewal. Cells were cultured in
serum-free medium under conditions that were optimal for growing
tumor spheres. As shown in Fig. 2J,
Bmi1-overexpressing SiHa and HeLa cells were generated by
consecutive passages of tumor sphere cultures, and these cells
formed more tumor spheres (33±4.12/200 cells in SiHa-Bmi1 and
12±1.29/200 cells in HeLa-Bmi1) than the control cells (7±0.87/200
cells in SiHa-GFP and 3±0.41/200 cells in HeLa-GFP) in each culture
passage (Fig. 2K; P<0.05).
Furthermore, the Bmi1-silenced C33A cells showed significantly
decreased tumor sphere formation capacity compared with the control
cells (9±1.23/200 cells in C33A-shBmi1 vs 25±3.14/200 cells in
C33A-shControl; P<0.05; Fig. 2J and
K). Taken together, these findings indicated that
Bmi1-expressing cells possibly contain more cells that have
self-renewal ability.
Bmi1-positive cervical cancer cells
express more stem cell-related proteins
Sox2, Oct4, c-Myc and Nanog are recognized as stem
cell self-renewal-related nuclear transcription factors. The
present study compared the expression of these transcription
factors in Bmi1-overexpressing SiHa and HeLa cells with their
respective controls. As shown in Fig.
3, qPCR and western blot analysis (Fig. 3A and B) revealed that
Bmi1-overexpressing SiHa and HeLa cells expressed higher levels of
Sox2, Oct4 and c-Myc than control cells at both the transcriptional
and translational levels. Kruppel like factor 4 (KLF4), a tumor
suppressor in Bmi1-overexpressing cells, was downregulated.
However, Nanog protein expression was unchanged. Additionally, in
Bmi1-silenced C33A cells, the factors Sox2, Oct4 and c-Myc showed
lower expression than in C33A-shControl cells by both qPCR and
western blotting (Fig. 3C and D).
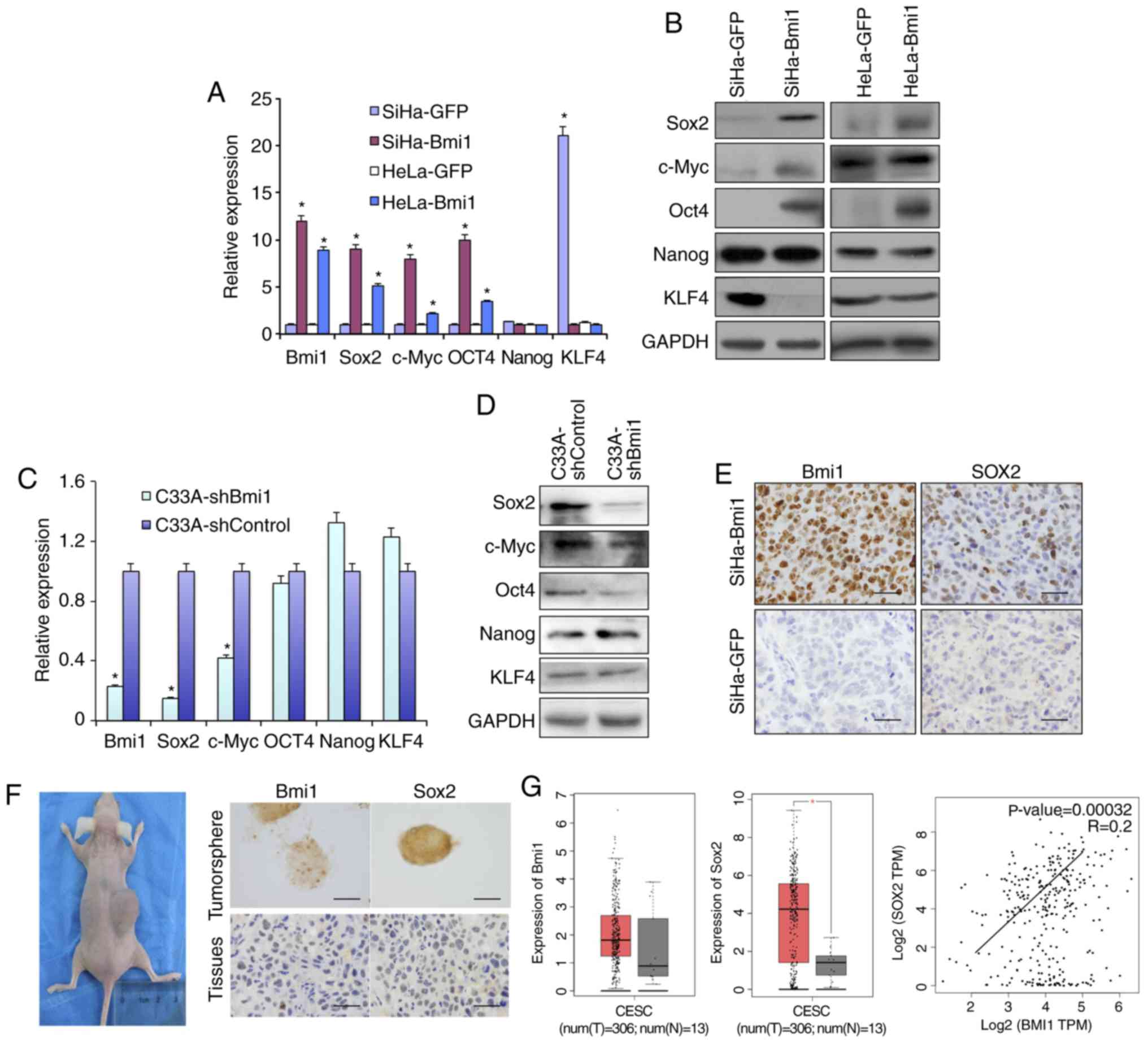 | Figure 3.Bmi1-positive cervical cancer cells
express more stem cell-related genes, including Sox2. The
differential expression of several stem cell-related genes,
including Sox2, c-Myc, Oct4, Nanog and KLF4, in Bmi1-overexpressing
SiHa and HeLa cells and controls was validated by (A) qPCR and (B)
western blotting. Additionally, the stem cell-related factors were
detected in C33A-shBmi1 and C33A-shControl cells by (C) qPCR and
(D) western blotting. (E) Expression of the stem cell-related
factor Sox2 in tumor xenografts formed by SiHa-Bmi1 and SiHa-GFP
cells was detected by immunohistochemistry. (F) Bmi1 and Sox2
immunoreactivity was predominantly detected in primary cervical
tumor spheres and xenografts formed by tumor sphere propagation
in vivo. (G) The dataset from the GEPIA repository showed
the expression and correlation of Bmi1 and Sox2 in cervical cancer
tissues and normal cervical tissues. Error bars represent SD (with
three replicates). *P<0.05 vs. respective control. qPCR,
quantitative PCR; KLF4, Kruppel like factor 4; Bmi1, polycomb
complex protein 1; GFP, green fluorescent protein; sh, short
hairpin. |
In a previous study, immunofluorescence analysis
showed that Bmi1 colocalizes to the nucleus with Sox2 in both
normal cervical and cervical carcinoma tissues (16). However, their mechanism of action
should be further explored. Here, the Sox2 level was increased in
Bmi1-overexpressing cells and was decreased in Bmi1-silenced cells,
as revealed by both qPCR and western blotting (Fig. 3A-D). The expression levels of Sox2
were also positively correlated with those of Bmi1 in the
Sox2-overexpressing xenograft tumor tissues (Fig. 3E). The present study also detected
Sox2 and Bmi1 protein in fresh tissue-derived cervical cancer tumor
sphere cells and 10-serial-passage xenograft tissue formed by
primary cervical cancer cells. Both Sox2 and Bmi1 staining was
found in the nuclei of cervical cancer tumor sphere cells and in
10th xenograft tissue (Fig. 3F).
Additionally, as shown in Fig. 3G, by
analyzing data from GEPIA, the expression levels of Bmi1 and Sox2
were both upregulated and positively correlated in human cervical
tissues (r=+0.2; P<0.05). These results suggest that Bmi1
maintains cervical cancer ‘stemness’, possibly by upregulating the
expression of Sox2.
Bmi1 upregulates Sox2 expression by
binding to the E-Box region of the Sox2 promoter
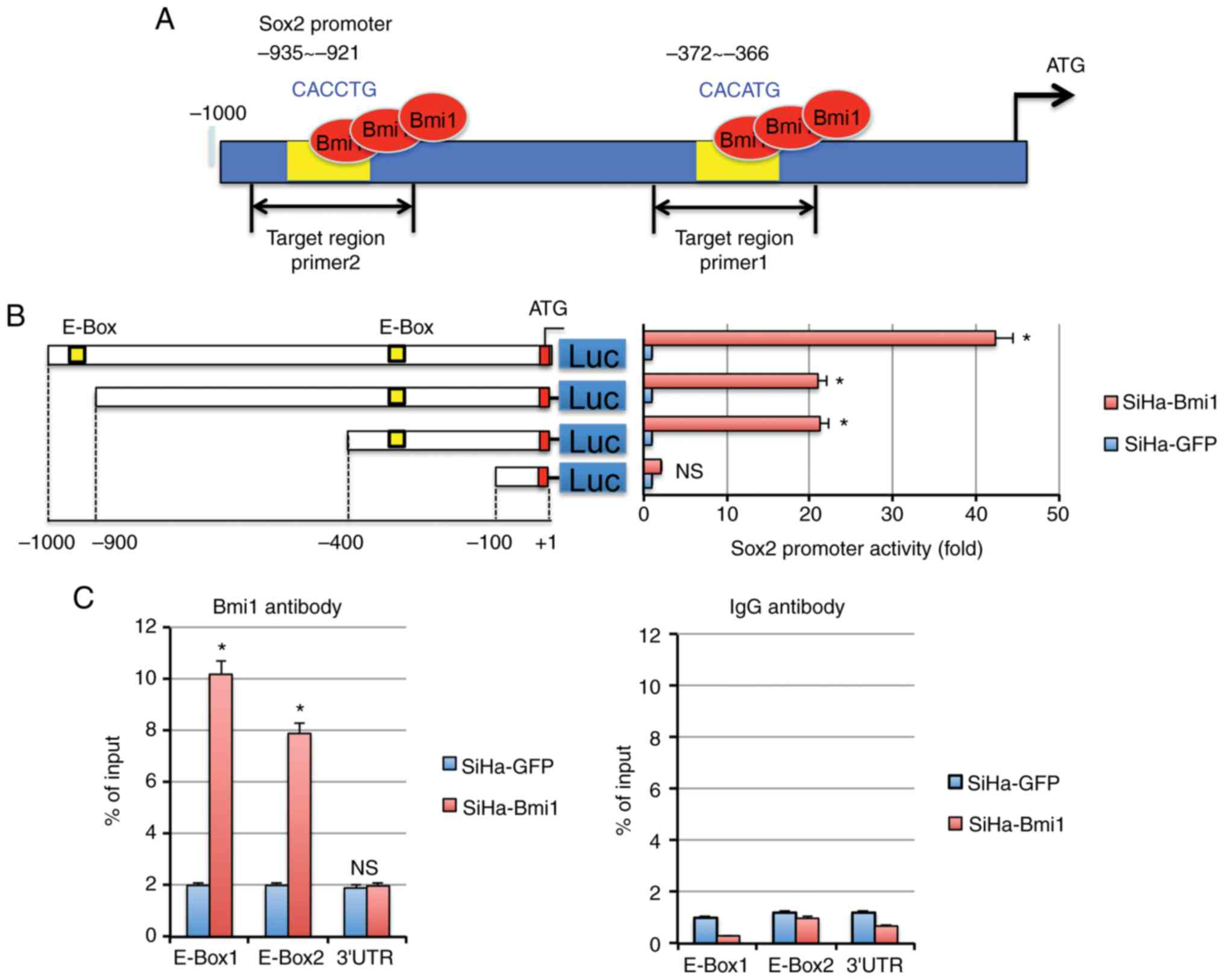
It was found that the Sox2 promoter contains 2 E-Box
motifs using the prediction software Promoter Scan (Fig. 4A) that could be recognized by Bmi1. To
identify the relationship between Bmi1 and Sox2 proteins in
cervical carcinoma, the Sox2 promoter-luciferase constructs were
transfected into SiHa-Bmi1 and SiHa-GFP cells. The construct with
either one or both E-Box motifs in the Sox2 promoter showed the
strongest luciferase signals in SiHa-Bmi1 cells compared with those
in SiHa-GFP cells (Fig. 4B;
P<0.05), suggesting that Bmi1 transactivates Sox2 expression
through the E-Box motifs in the Sox2 promoter in cervical
carcinoma.
Furthermore, quantitative ChIP assays were used to
identify the E-Box of the Sox2 promoter to which Bmi1 binds.
Primers were designed to amplify the two specific regions, and a
primer pair was used to amplify a 150-bp fragment in the Sox2 3′
untranslated region as a control (Fig.
4A). Following immunoprecipitation using the Bmi1 antibody, the
E-Box primer was amplified 8–10-fold more from SiHa-Bmi1 cells than
from SiHa-GFP cells (Fig. 4C).
However, no significant differences were observed in the control
IgG immunoprecipitate for all primers, demonstrating the
specificity of Bmi1 binding to the Sox2 promoter in cervical
carcinoma cells.
Discussion
Presently, the molecular mechanisms of the
initiation and progression of cervical cancer remain unclear,
although many genetic factors have been found to be associated with
cervical cancer.
Previously, it was shown that deregulation of Bmi1
is associated with the pathogenesis of different human cancer
types, including breast cancer (19,20), Ewing
sarcoma (21) and leukemia (22,23).
Furthermore, Bmi1 was shown to be a useful molecular marker to
predict the prognosis of bladder cancer (24) and nasopharyngeal carcinoma (25,26). The
significantly high frequency of Bmi1 expression in invasive SCC
compared with that in HSIL and NC samples is a finding of great
interest. First, Bmi1 staining in the normal cervical epithelium
was found only in basal cells where epithelial ‘reserve’ cells are
located. It has been suggested that reserve cells appear to be the
candidate for cervical stem cells (27), and they play a central role in the
pathogenesis of cervical intraepithelial neoplasia. Because Bmi1 is
necessary to maintain normal stem cells and cancer stem cells
(28), the present experiments
indicated that Bmi1 expression is more upregulated in HSILs and
invasive cervical cancer than in normal cancer. At the same time,
squamous carcinoma stage II and III showed a relatively higher
intensity of Bmi1 staining than squamous carcinoma stage I. These
results were consistent with those of the previous study.
Furthermore, exogenously expressed Bmi1 enhanced tumorigenicity,
and knockdown of Bmi1 suppressed tumorigenicity, indicating that
KLF4 works as a promoter for cervical cancer. These results support
the notion that highly expressed Bmi1 promotes the pathogenesis of
cervical carcinoma.
Furthermore, it was found that Bmi1 upregulated the
expression of stem cell-related factors such as Sox2, Oct4 and
c-Myc. Previous studies have reported that Bmi1 showed
colocalization with Sox2 in cervical tissues (16,29).
Interestingly, Bmi1 positively regulates factors through binding to
the E-Box motif in the proximal region. In this study, promoter
analysis revealed that Bmi1 activated Sox2 expression by directly
binding to the proximal E-Box sequence of the Sox2 promoter in
cervical carcinoma cells. Therefore, the results from our study
revealed that the stemness characteristics induced by Bmi1 in
cervical carcinoma likely occurs through activating the expression
of Sox2.
Additionally, Sox2 belongs to a highly conserved
family of high mobility group transcription factors with the
sex-determining gene Sry as the first-identified member. Sox
proteins are important factors in regulating stem cell biology, and
the determination of cell fate and maintenance. Sox2 has also been
reported to play a critical role in developmental processes
(30), including neural stem cell
specification and maintenance (31,32) and
lung morphogenesis (33,34). A previous study showed that Sox2 may
participate in the carcinogenesis of cervical carcinomas (28). The present study investigated the
expression of Bmi1 in human cervical squamous cell carcinoma and
its relationship to the expression of Sox2. Interestingly, a high
frequency of expression of Bmi1 and Sox2 was observed in invasive
cervical carcinomas compared with that in the normal cervix or
HSIL. The present study characterized the role of Sox2 and Bmi1 in
the maintenance cervical cancer stem cells using tumor sphere
formation assays and propagation in vivo. These factors are
necessary for stem-cell-like cancer cells in cervical cancer
because they are clearly and strongly expressed in stem-cell-like
cells of cervical cancer formed under in vitro culture
conditions and in vivo, facilitating the proliferation of
only CSCs and progenitor cells. The results suggested that the
co-deregulation of Sox2 and Bmi1 is a general phenomenon in
cervical cancer.
In summary, the present study suggested a general
role for Bmi1 and Sox2 in cervical cancer development. The finding
that cervical cancer cells require Bmi1 and Sox2 for their
tumorigenic activity suggests that interference with Bmi1 and Sox2
activity could be a therapeutic strategy for cervical cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by a grant to Dr Rui Xu from
the National Natural Science Foundation of Shaanxi province (grant
no. 2012JM4006) in the design of the study, collection, analysis
and interpretation of data, and manuscript writing.
Availability of data and materials
The datasets used and analyzed during the current
study are available in the GEPIA repository (http://gepia.cancer-pku.cn/detail.php?gene=BMI1;
http://gepia.cancer-pku.cn/detail.php?gene=BMI1 and
http://gepia.cancer-pku.cn/detail.php?gene=BMI1).
Authors' contributions
WTY analyzed and interpreted the patient data
regarding the cervical dissection. RX and LC performed histological
examination of the cervix, cell experiments and animal assays, and
were major contributors in the writing of the manuscript. All the
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The institutional review board, named as ‘Ethics
Committee of Medical School of Xi'an Jiaotong University’, approved
the population study. The Ethics Committee of Medical School of
Xi'an Jiaotong University approved the design of the cervical
cancer study, including tissue sample collection. The experimental
protocols involving animals were evaluated and approved by the
Animal Care and Use Committee of the Medical School of Xi'an
Jiaotong University.
Patient consent for publication
The patients provided written informed consent for
the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dick JE: Breast cancer stem cells
revealed. Proc Natl Acad Sci USA. 100:3547–3549. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Graham SM, Jørgensen HG, Allan E, Pearson
C, Alcorn MJ, Richmond L and Holyoake TL: Primitive, quiescent,
Philadelphia-positive stem cells from patients with chronic myeloid
leukemia are insensitive to STI571 in vitro. Blood. 99:319–325.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ginestier C, Liu S, Diebel ME, Korkaya H,
Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum
D, et al: CXCR1 blockade selectively targets human breast cancer
stem cells in vitro and in xenografts. J Clin Invest. 120:485–497.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uchida N, Buck DW, He D, Reitsma MJ, Masek
M, Phan TV, Tsukamoto AS, Gage FH and Weissman IL: Direct isolation
of human central nervous system stem cells. Proc Natl Acad Sci USA.
97:14720–14725. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rountree CB, Ding W, He L and Stiles B:
Expansion of CD133-expressing liver cancer stem cells in
liver-specific phosphatase and tensin homolog deleted on chromosome
10-deleted mice. Stem Cells. 27:290–299. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paavonen J: Human papillomavirus infection
and the development of cervical cancer and related genital
neoplasias. Int J Infect Dis. 11 (Suppl 2):S3–S9. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Walboomers JM, Jacobs MV, Manos MM, Bosch
FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ and Muñoz N:
Human papillomavirus is a necessary cause of invasive cervical
cancer worldwide. J Pathol. 189:12–19. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Molofsky AV, He S, Bydon M, Morrison SJ
and Pardal R: Bmi-1 promotes neural stem cell self-renewal and
neural development but not mouse growth and survival by repressing
the p16Ink4a and p19Arf senescence pathways. Genes Dev.
19:1432–1437. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bertolini JA, Favaro R, Zhu Y, Pagin M,
Ngan CY, Wong CH, Tjong H, Vermunt MW, Martynoga B, Barone C, et
al: Mapping the global chromatin connectivity network for Sox2
function in neural stem cell maintenance. Cell Stem Cell.
24:462–476.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Douglas D, Hsu JH, Hung L, Cooper A,
Abdueva D, van Doorninck J, Peng G, Shimada H, Triche TJ and Lawlor
ER: BMI-1 promotes ewing sarcoma tumorigenicity independent of
CDKN2A repression. Cancer Res. 68:6507–6515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Spremović-Rađenović S, Stefanović A,
Kadija S, Jeremić K and Sparić R: Classification and the
diagnostics of abnormal uterine bleeding in nongravid women of
reproductive age: The PALM-COEIN classification system adopted by
the international federation of gynecology and obstetrics.
Vojnosanit Pregl. 73:1154–1159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo WJ, Zeng MS, Yadav A, Song LB, Guo BH,
Band V and Dimri GP: Mel-18 acts as a tumor suppressor by
repressing Bmi-1 expression and down-regulating Akt activity in
breast cancer cells. Cancer Res. 67:5083–5089. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu R, Yang WT and Zheng PS: Coexpression
of B-lymphoma Moloney murine leukemia virus insertion region-1 and
sex-determining region of Y chromosome-related high mobility group
box-2 in cervical carcinogenesis. Hum Pathol. 44:208–217. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu XF, Yang WT, Xu R, Liu JT and Zheng
PS: Cervical cancer cells with positive Sox2 expression exhibit the
properties of cancer stem cells. PLoS One. 9:e870922014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wiederschain D, Chen L, Johnson B, Bettano
K, Jackson D, Taraszka J, Wang YK, Jones MD, Morrissey M, Deeds J,
et al: Contribution of polycomb homologues Bmi-1 and Mel-18 to
medulloblastoma pathogenesis. Mol Cell Biol. 27:4968–4979. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim JH, Yoon SY, Jeong SH, Kim SY, Moon
SK, Joo JH, Lee Y, Choe IS and Kim JW: Overexpression of Bmi-1
oncoprotein correlates with axillary lymph node metastases in
invasive ductal breast cancer. Breast. 13:383–388. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang J, Chai L, Liu F, Fink LM, Lin P,
Silberstein LE, Amin HM, Ward DC and Ma Y: Bi-1 is a target gene
for SALL4 in hematopoietic and leukemic cells. Proc Natl Acad Sci
USA. 104:10494–10499. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Valk-Lingbeek ME, Bruggeman SW and van
Lohuizen M: Stem cells and cancer; the polycomb connection. Cell.
118:409–418. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lessard J and Sauvageau G: Bmi-1
determines the proliferative capacity of normal and leukaemic stem
cells. Nature. 423:255–260. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vonlanthen S, Heighway J, Altermatt HJ,
Gugger M, Kappeler A, Borner MM, van Lohuizen M and Betticher DC:
The bmi-1 oncoprotein is differentially expressed in non-small cell
lung cancer and correlates with INK4A-ARF locus expression. Br J
Cancer. 84:1372–1376. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, He SS, Cai XY, Chen HY, Yang XL,
Lu LX and Chen Y: The novel prognostic score combining red blood
cell distribution width and body mass index (COR-BMI) has
prognostic impact for survival outcomes in nasopharyngeal
carcinoma. J Cancer. 9:2295–2301. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu J, Hu D, Yang G, Zhou J, Yang C, Gao Y
and Zhu Z: Down-regulation of BMI-1 cooperates with artemisinin on
growth inhibition of nasopharyngeal carcinoma cells. J Cell
Biochem. 112:1938–1948. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peters WM: Nature of ‘basal’ and ‘reserve’
cells in oviductal and cervical epithelium in man. J Clin Pathol.
39:306–312. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu S, Dontu G, Mantle ID, Patel S, Ahn
NS, Jackson KW, Suri P and Wicha MS: Hedgehog signaling and Bmi-1
regulate self-renewal of normal and malignant human mammary stem
cells. Cancer Res. 66:6063–6071. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ji J and Zheng PS: Expression of Sox2 in
human cervical carcinogenesis. Hum Pathol. 41:1438–1447. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Maurizi G, Verma N, Gadi A, Mansukhani A
and Basilico C: Sox2 is required for tumor development and cancer
cell proliferation in osteosarcoma. Oncogene. 37:4626–4632. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Andreu-Agullo C, Maurin T, Thompson CB and
Lai EC: Ars2 maintains neural stem-cell identity through direct
transcriptional activation of Sox2. Nature. 481:195–198. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miyagi S, Nishimoto M, Saito T, Ninomiya
M, Sawamoto K, Okano H, Muramatsu M, Oguro H, Iwama A and Okuda A:
The Sox2 regulatory region 2 functions as a neural stem
cell-specific enhancer in the telencephalon. J Biol Chem.
281:13374–13381. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Danopoulos S, Alonso I, Thornton ME,
Grubbs BH, Bellusci S, Warburton D and Al Alam D: Human lung
branching morphogenesis is orchestrated by the spatiotemporal
distribution of ACTA2, SOX2, and SOX9. Am J Physiol Lung Cell Mol
Physiol. 314:L144–L149. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gontan C, de Munck A, Vermeij M, Grosveld
F, Tibboel D and Rottier R: Sox2 is important for two crucial
processes in lung development: Branching morphogenesis and
epithelial cell differentiation. Dev Biol. 317:296–309. 2008.
View Article : Google Scholar : PubMed/NCBI
|