Introduction
Ovarian cancer has the highest mortality rate among
gynecological malignancies worldwide (1,2). Tumor
metastasis is a complex biological process involving cell
signaling, regulation of cell proliferation, motility and invasion;
in addition, metastasis is the primary cause of death in patients
with advanced ovarian cancer and is closely associated with
unfavorable outcomes and poor prognosis (3–7). Recently,
the rat sarcoma-mitogen-activated protein kinase, phosphoinositide
3-kinase-protein kinase B and janus kinase-signal transducer and
activator of transcription signaling pathways have been reported to
be associated with tumor metastasis and invasion in ovarian cancer
(8). Furthermore, the tumor
microenvironment, which includes stromal cells, extracellular
matrix components and exosomes, can establish a communication
circuit that enhances cancer cell invasion and metastasis via
reciprocal signaling (9). However,
the molecular mechanisms underlying ovarian cancer metastasis are
currently not well elucidated (10).
Myelin protein zero like 1 (MPZL1), also known as
protein zero-related, is a hyperphosphorylated transmembrane
glycoprotein involved in extracellular matrix-induced signal
transduction (11–15). Previous studies have demonstrated that
MPZL1 promotes hepatocellular carcinoma cell migration through the
Src tyrosine kinase, and may be involved in adhesion-dependent
signaling (11,14,16).
Furthermore, MPZL1 forms a complex with the growth factor
receptor-bound protein 2 adaptor and tyrosine-protein phosphatase
non-receptor type 11 phosphatase, and is involved in cell adhesion
in human epidermal growth factor receptor 2-positive breast cancer
cells (12). Additionally, as a major
receptor of concanavalin A, MPZL1 has an important role in cell
signaling via c-Src (13). However,
the functional role and clinical implications of MPZL1 in ovarian
cancer are largely unknown.
The present study demonstrated that amplification of
MPZL1 was associated with malignant features of ovarian cancer, and
promoted tumor cell proliferation, migration and invasion.
Furthermore, overexpression of MPZL1 significantly promoted
cell growth and metastasis of ovarian cancer. Conversely, knockdown
of MPZL1 via short hairpin RNA (shRNA) attenuated
proliferation and migration of ovarian cancer cells. This study
also demonstrated that MPZL1 overexpression-induced
activation of Src kinase mediated the phosphorylation and
activation of cortactin and p130 in ovarian cancer. Taken together,
these findings suggested that MPZL1 may be considered a
novel pro-metastatic gene that promotes tumor cell proliferation
and migration via activation of Src kinase in ovarian cancer.
Materials and methods
Collection of ovarian cancer
specimens
The present study was approved in January 2017 by
the Ethics Committee of the Tongren Hospital, Shanghai Jiao Tong
University School of Medicine. The collection of ovarian cancer
specimens was performed in conformity to ethical standards. All
participants provided written informed consent. The tissues were
obtained during surgery and were fixed with 4% paraformaldehyde
(Beijing Solarbio Science & Technology Co., Ltd.) for 24 h at
room temperature and embedded in paraffin. Specimens were obtained
from 78 patients (age, 29–77 years, including 16 benign, 16
borderline and 46 malignant patients) with epithelial ovarian
cancer who had not previously undergone treatment; the benign group
consisted of patients with a benign ovarian tumor. No distant
metastasis was detected in the selected patients prior to surgery.
Detailed pathological data, including histological type, tumor
size, tumor stage and lymph node metastasis, were obtained and
summarized. The tumor stage was defined using the 8th edition of
the Union for International Cancer Control-tumor node metastasis
classification system (17).
Reagents and cell lines
The human ovarian cancer cell lines 293T, HO8910,
SKOV3, HEY and TOV-21G were purchased from American Type Culture
Collection and were cultured in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Gibco; Thermo Fisher Scientific,
Inc.).
Virus production and infection
293T cells (60% confluent) in a 10 cm dish were
co-transfected with 5 µg lentiviral constructs (pLenti 7.3;
Invitrogen; Thermo Fisher Scientific, Inc.), 5 µg plasmid Δ8.9
(Invitrogen; Thermo Fisher Scientific, Inc.) and 3 µg plasmid
vesicular stomatitis virus G (Invitrogen; Thermo Fisher Scientific,
Inc.) using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). Cells were incubated at 37°C and the
medium was replaced after 12 h. Virus-containing medium was
collected 48 h post-transfection and supplemented with 8 µg/ml
polybrene to infect target cells in 6-well dishes (60% confluent)
at 37°C. Infected cells were selected with 3 µg/ml puromycin at
37°C for ≥1 week post-infection. The lentiviral shRNA vectors
targeting MPZL1 and scrambled control shRNA were purchased
from Open Biosystems; Dharmacon Inc. For MPZL1 knockdown,
the shRNA sequences were: MPZL1-shRNA-1,
5′-TGACATCACAGATATAGGT-3′; MPZL1-shRNA-2,
5′-TCAAGTGGCATAGCCAATG-3′; and shRNA-negative control (NC),
5′-ACCTCCACCCTCACTCTGCCAT-3′. For MPZL1 overexpression, the
coding sequence of MPZL1 was inserted into the lentiviral
vector pLenti 7.3.
For Src knockdown, Src-small
interfering RNA (siRNA) was used at a final concentration of 25 nM
and was transfected into cells (60% confluent) using
Lipofectamine® RNAiMAX reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) for 72 h at 37°C, according to the
manufacturer's protocol. For Src knockdown, the siRNA sequences
(Shanghai GenePharma Co., Ltd.) were: Src-siRNA,
5′-CAGGCUGAGGAGUGGUAUUTT-3′; and siRNA-NC,
5′-TTCTCCGAACGTGTCACGT-3′.
Western blotting
Cells were lysed in RIPA buffer [Tris (pH 7.4), 50
mM; NaCl, 150 mM; 1% NP-40; 0.1% SDS; EDTA, 2 µM] containing
proteinase inhibitors (Roche Molecular Diagnostics) and phosphatase
inhibitors (Roche Molecular Diagnostics), and the protein
concentration was determined using the bicinchoninic acid assay.
The cell lysates (20 µg total protein) were subjected to 8–10%
SDS-PAGE and immunoblotting. Subsequently, proteins were
transferred to nitrocellulose membranes, which were blocked for 1 h
with 5% nonfat milk at room temperature, and were then incubated
with primary antibodies (1:1,000) at 4°C overnight. The membranes
were then incubated for 1 h with horseradish peroxidase-linked
anti-rabbit IgG antibody (1:1,000; cat. no. 7074; Cell Signaling
Technology, Inc) at room temperature. Blots were visualized with
ECL western blotting reagents (Thermo Fisher Scientific, Inc.)
using ChemiDoc XRS+ (Bio-Rad Laboratories, Inc.).
Semi-quantification was conducted using ImageLab 2.0 software
Bio-Rad Laboratories, Inc.). Antibodies against the following
proteins were used: p130 (cat. no. 13846), phosphorylated (p)-p130
(cat. no. 4015), cortactin (cat. no. 3502), p-cortactin (cat. no.
4569), Src (cat. no. 2108), p-Src (cat. no. 12432), MPZL1 (cat. no.
9893) and GAPDH (cat. no. 5174) (all Cell Signaling Technology,
Inc). Notably, since MPZL1 has three alternatively spliced
isoforms, at least three bands can be seen in MPZL1 blots, and all
bands were measured when semi-quantifying the blots.
Cell proliferation assay
Cell proliferation was assessed using the Cell
Counting Κit-8 (CCK-8; Dojindo Molecular Technologies, Inc.). Cells
(5,000 cells/well) were seeded in triplicate in 96-well plates.
After 24, 48, 72, 96 or 120 h at 37°C, CCK-8 reagent (1/20,
volume/volume) was added to the cells for 2 h at 37°C. The
absorbance of each well was measured at 450 nm, according to the
manufacturer's protocol.
Cell migration and invasion
assays
Transwell chambers with 8 µm pore membranes
(Corning, Inc.) were placed in 24-well culture plates and were
incubated with serum-free RPMI-1640 medium at 37°C for 1 h. A 200
µl suspension of 0.5–1×105 cells was seeded into the
upper compartment of the Transwell chambers in FBS-free medium,
whereas 600–800 µl RPMI-1640 medium supplemented with 10% FBS was
added into the bottom wells; cells were incubated at 37°C for 12–24
h. The migrating cells that were attached to the lower membranes of
the Transwell chambers were stained with crystal violet (0.1%) at
room temperature for 2 h and images were captured at ×200
magnification under a light microscope. Images of three random
fields from three replicate wells were obtained, and the migrated
cells were counted. The cell invasion assay was the same as the
migration assay; however, the chambers were coated with
Matrigel.
Colony formation assay
Cells were seeded in triplicate in 6-well plates.
After 12 days, the cells were washed with PBS, fixed with methanol
for 1 h and stained with 0.1% crystal violet for 1 h at room
temperature. Subsequently, images of the colonies were captured and
counted.
Immunohistochemistry
Ovarian cancer specimens were collected from the 78
patients during surgery at Tongren Hospital, Shanghai Jiao Tong
University. The tissue specimens were fixed in 4% paraformaldehyde
(Beijing Solarbio Science & Technology Co., Ltd.) for 24 h at
room temperature and embedded in paraffin, before being cut into 5
µm sections. The tissue sections were deparaffinized, treated with
3% H2O2 for 10 min at room temperature,
autoclaved in 10 mM citric sodium (pH 6.0) for 30 min to unmask
antigens and rinsed in PBS. Subsequently, sections were incubated
with primary antibodies against MPZL1 (1:200; cat. no. GTX46451;
GeneTex, Inc.) at 4°C overnight, followed by incubation with a
biotinylated secondary antibody (1:1,000; cat. no. ab6844; Abcam)
for 1 h at room temperature. Signal amplification and detection was
performed using the DAB system (Dako) according to the
manufacturer's instructions.
Immunohistochemistry scoring
All cases were analyzed independently with the help
of two expert pathologists and were assigned a score according to
the following criteria: Strongly positive, 3+; positive, 2+; weakly
positive, 1+; and negative, 0.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 6 software (GraphPad Software, Inc.). Genomic analysis of
MPZL1 in ovarian cancer (489 high-grade serous ovarian
cancer specimens were surgically resected prior to systemic
treatment; all patients received a platinum agent and 94% received
a taxane) was performed with The Cancer Genome Atlas (TCGA) copy
number portal (www.broadinstitute.org/tcga). In all experiments,
comparisons between two groups were conducted using two-sided
Student's t-test, and one-way analysis of variance followed by
Tukey's multiple comparisons test was used to test for differences
among more groups. Fisher's exact test was used to determine
differences between stages, as presented in Table I. P<0.05 was considered to indicate
a statistically significant difference.
 | Table I.Association between
clinicopathological characteristics and MPZL-1 expression. |
Table I.
Association between
clinicopathological characteristics and MPZL-1 expression.
|
|
| MPZL-1 expression
score |
|
|---|
|
|
|
|
|
|---|
|
|
| Low level | High level |
|
|---|
|
|
|
|
|
|
|---|
| Variable | Cases (n) | − | + | ++ | +++ | P-value |
|---|
| Age (years) |
|
<55 | 28 | 1 | 8 | 9 | 10 |
|
|
≥55 | 18 | 0 | 4 | 4 | 10 | 0.69 |
| Clinical stage |
|
I–II | 9 | 1 | 5 | 2 | 1 |
|
|
III | 25 | 0 | 5 | 8 | 12 | 0.01a |
| IV | 12 | 0 | 2 | 3 | 7 | 0.02b |
| CA125 (U/ml) |
|
≤200 | 33 | 1 | 12 | 10 | 10 |
|
|
>200 | 13 | 0 | 0 | 3 | 10 | 0.01 |
Results
Overexpression of MPZL1 is associated
with malignant features of ovarian cancer
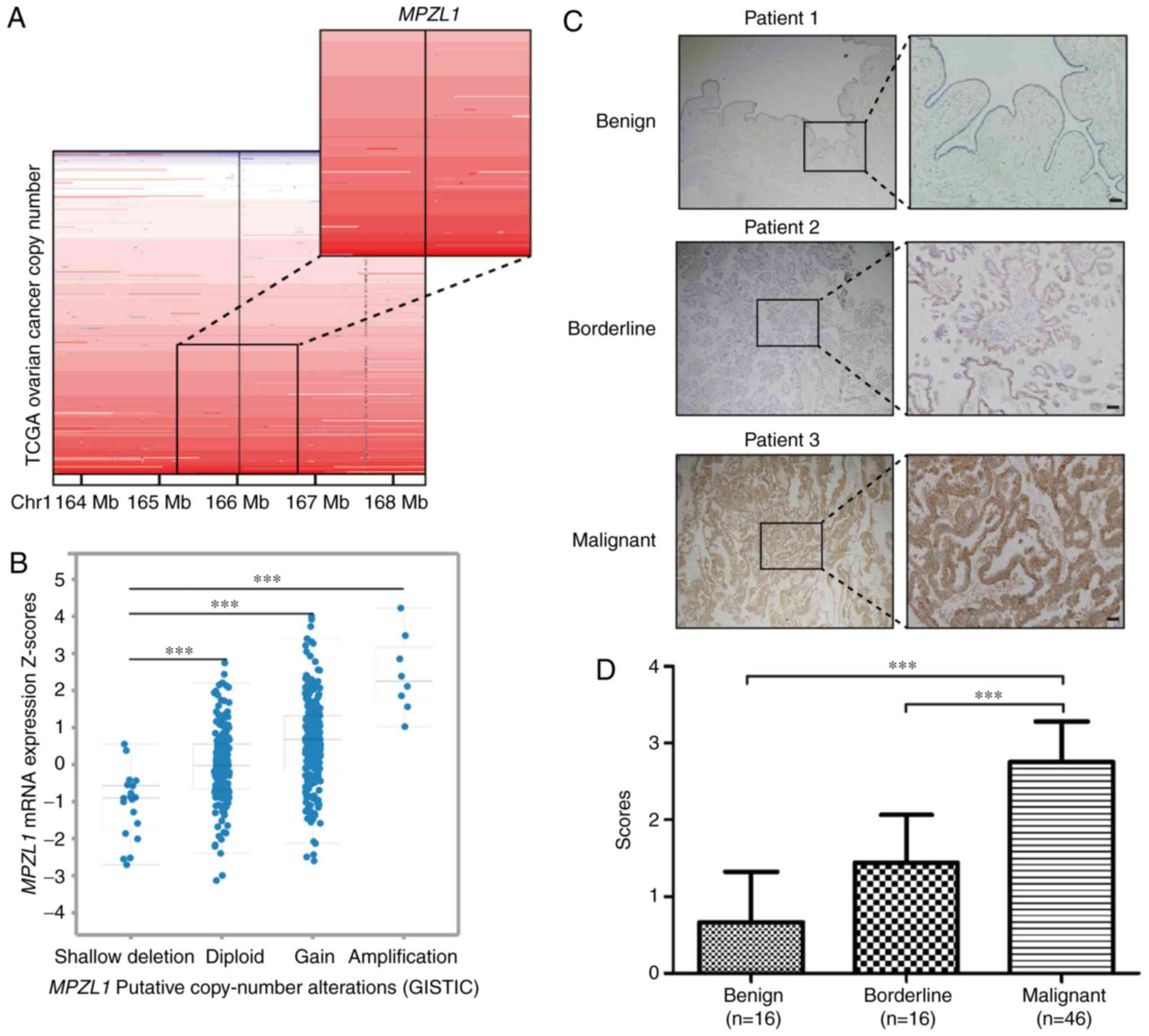
Copy number analysis of TCGA ovarian serous
adenocarcinoma samples in the cBioportal database revealed that
MPZL1, located at chromosome 1q24 (magnified section in
Fig. 1A), was genomically amplified
in a considerable proportion of cases (Fig. 1A). Notably, the expression levels of
MPZL1 were associated with its amplification status
(Fig. 1B), thus indicating that the
high expression of MPZL1 in tumor tissues may be regulated
by copy number amplification of the MPZL1 gene. To further
determine the association of MPZL1 expression with the
malignant features of ovarian cancer, MPZL1 protein expression was
assessed by immunohistochemistry among benign, borderline and
malignant epithelial ovarian cancer patient tissues (Fig. 1C; Table
I). The results revealed that MPZL1 expression was almost
undetectable in patients with benign ovarian cancer, whereas it was
expressed at significantly higher levels in malignant cancer
patient tissues compared with in benign/borderline cancer patient
tissues (Fig. 1D). In addition, the
expression profile of the MPZL1 protein was determined using The
Human Protein Atlas (18). MPZL1 was
ubiquitously expressed in different human tissues as well as
various cancer types. In normal ovarian tissues, MPZL1 was lowly
expressed, whereas in some ovarian cancer tissues, MPZL1 was
overexpressed (data not shown). Taken together, these data
indicated that MPZL1 was overexpressed in a subset of patients with
ovarian cancer, suggesting that MPZL1 may serve a pivotal role in
ovarian cancer.
Overexpression of MPZL1 promotes
ovarian cancer cell proliferation and migration
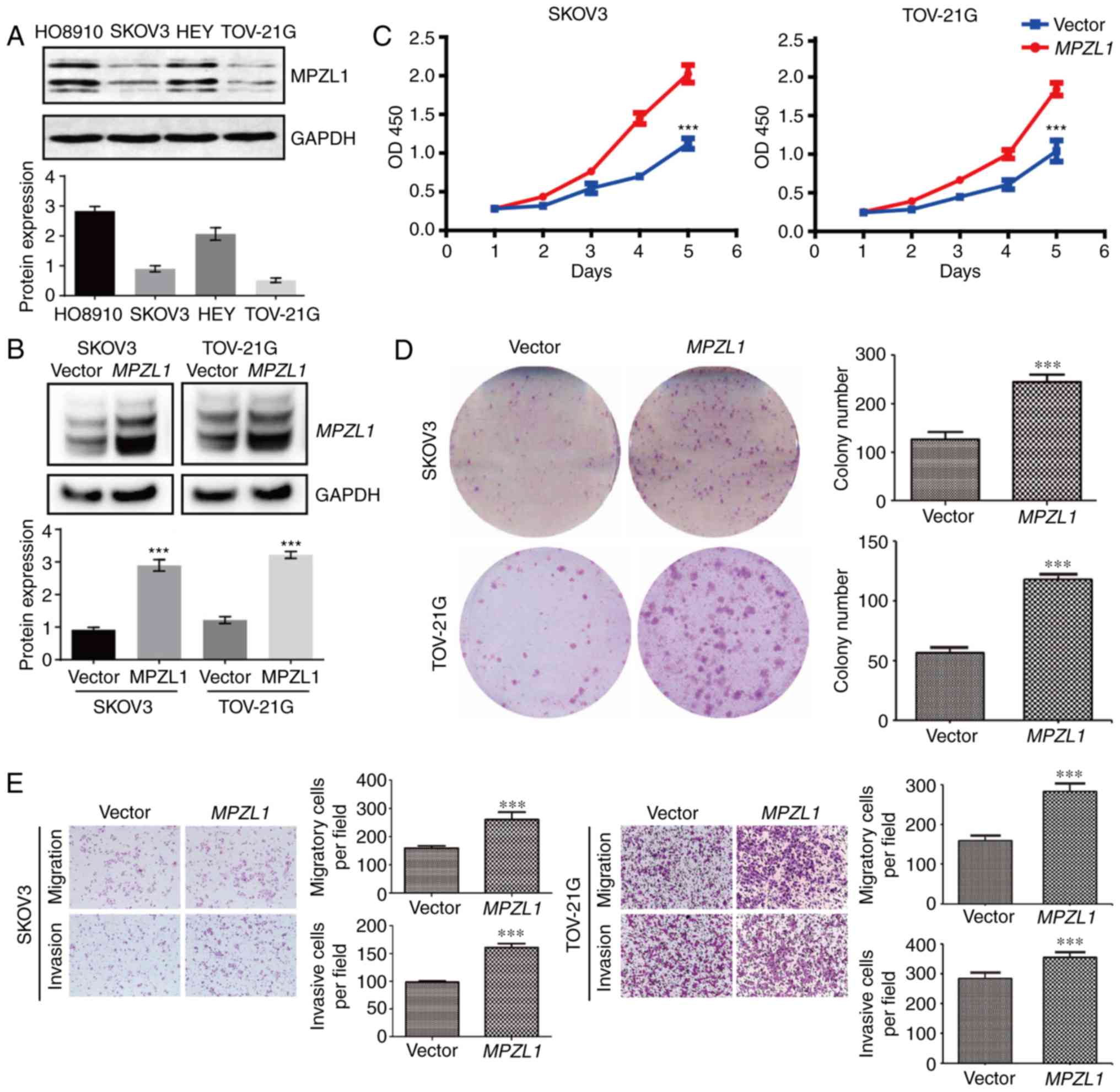
To study the biological function of MPZL1, the
protein expression levels of MPZL1 were detected in four ovarian
cancer cell lines (Fig. 2A).
Subsequently, the MPZL1 gene was overexpressed via
lentiviral infection in SKOV3 and TOV-21G cells (Fig. 2B); these two cell lines were selected
as they exhibited relatively lower endogenous MPZL1 expression.
Subsequently, the effects of MPZL1 overexpression on the
proliferation of these ovarian cancer cells were determined using
the CCK-8 assay. Notably, the results demonstrated that exogenous
overexpression of the MPZL1 gene promoted ovarian cancer
cell proliferation (Fig. 2C).
Similarly, the results of the colony formation assay demonstrated
that the number of colonies was significantly increased in
MPZL1-overexpressed SKOV3 and TOV-21G cells (Fig. 2D). Furthermore, the effects of
MPZL1 overexpression were examined on the migratory and
invasive abilities of ovarian cancer cells by Transwell assays;
ectopic expression of MPZL1 significantly enhanced the in
vitro migration and invasion of ovarian cancer cells (Fig. 2E). These results indicated that
MPZL1 may serve an important role in promoting cell
migration and metastasis of ovarian cancer.
Targeted downregulation of MPZL1
attenuates ovarian cancer cell proliferation and migration
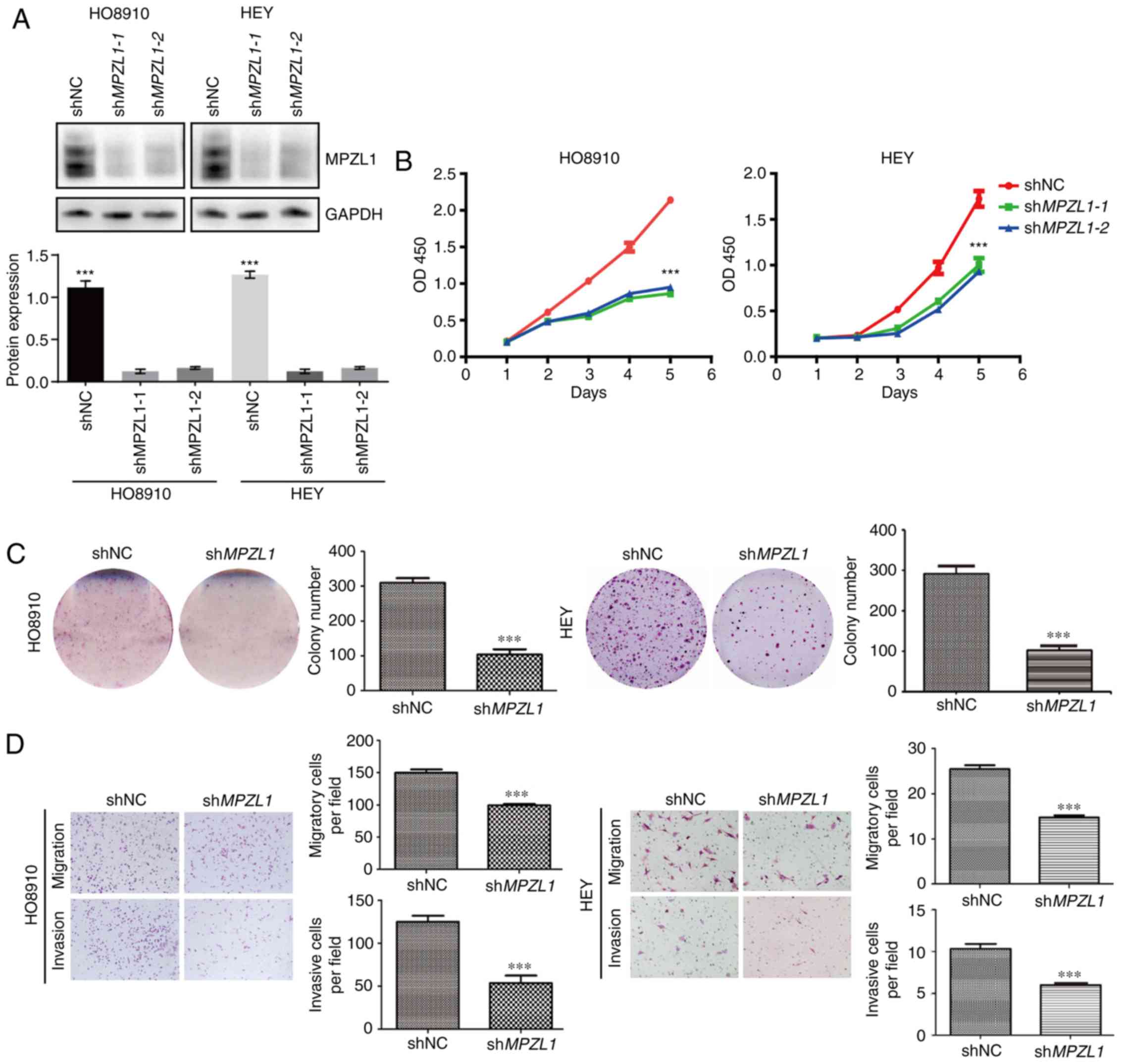
To further verify the role of MPZL1 in ovarian
cancer cell migration, HO8910 and HEY cells were selected as
cellular models for loss-of-function studies. Firstly, the
MPZL1 gene was stably knocked down in HO8910 and HEY cells
using a lentiviral shRNA specifically targeting MPZL1
(Fig. 3A). Consequently, cell
proliferation was significantly inhibited under normal growth
conditions (Fig. 3B). Consistently,
the colony formation assay revealed that the number of colonies was
evidently decreased following knockdown of MPZL1 in HO8910
and HEY cells (Fig. 3C). Furthermore,
Transwell assays demonstrated that the in vitro migration
and invasion of ovarian cancer cells were inhibited by MPZL1
depletion (Fig. 3D). These findings
indicated that MPZL1 knockdown attenuated proliferation,
migration and invasion of ovarian cancer cells.
MPZL1 regulates phosphorylation levels
of numerous pro-metastatic proteins
Recently, many studies have reported that
pro-metastatic proteins, including p130, Src and cortactin, are
important for cell migration and tumor metastasis (19–21).
Furthermore, p130 and cortactin were originally identified as
substrate proteins of the Src family kinases, and a previous report
demonstrated that the MPZL1/Src/cortactin signaling cascade
functions in the process of hepatocellular carcinoma cell migration
(22). However, to the best of our
knowledge, the potential role of MPZL1 in the phosphorylation of
pro-metastatic proteins has not been determined in ovarian cancer.
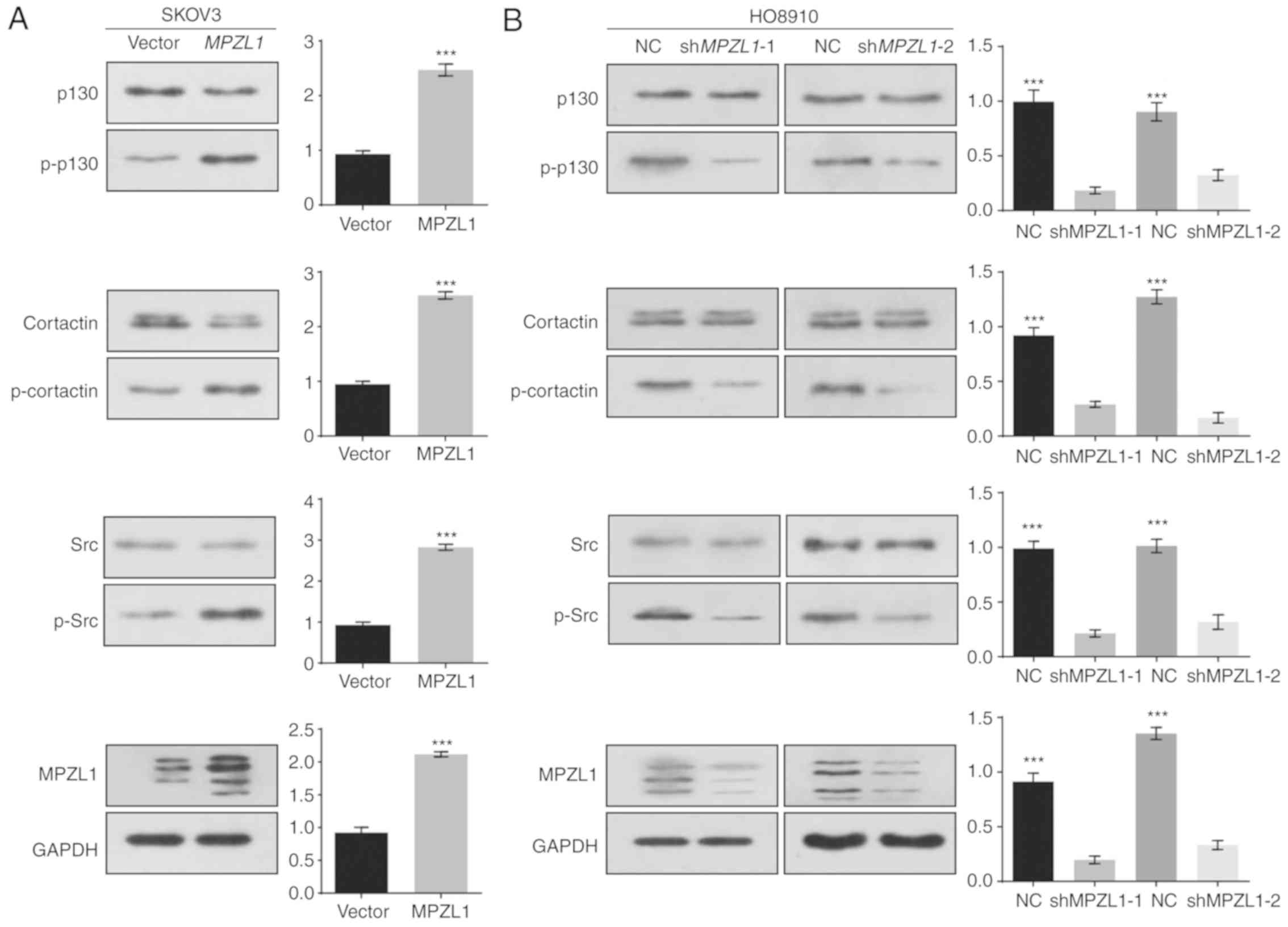
To investigate whether the MPZL1/Src/cortactin signaling cascade
exists in ovarian cancer, the expression levels of pro-metastatic
proteins were detected by western blotting. The results indicated
that stable overexpression of MPZL1 led to increased
phosphorylation of p130, Src and cortactin in SKOV3 cells (Fig. 4A). Furthermore, targeted knockdown of
the MPZL1 gene by shRNA reduced phosphorylation of these
three proteins in HO8910 cells (Fig.
4B). These findings indicated that MPZL1 overexpression
reprogrammed the pro-metastatic signaling network in ovarian
cancer.
Transient transfection of Src siRNA
suppresses migration and invasion in SKOV3-MPZL1 cells
According to previous studies, Src tyrosine kinases
serve critical roles in several cellular signal transduction
pathways that regulate cell proliferation, adhesion, migration and
invasion (22–25). Additionally, as substrate proteins of
Src kinase, p-p130 and p-cortactin participate in cell migration
(26). Furthermore, amplification of
MPZL1 promotes tumor cell migration via Src-mediated
cortactin phosphorylation in hepatocellular carcinoma cells
(27). To ascertain whether Src
kinase activity is essential for MPZL1-induced phosphorylation of
p130 and cortactin in ovarian cancer cells, MPZL1 was
overexpressed in SKOV3 cells, and the Src gene was then
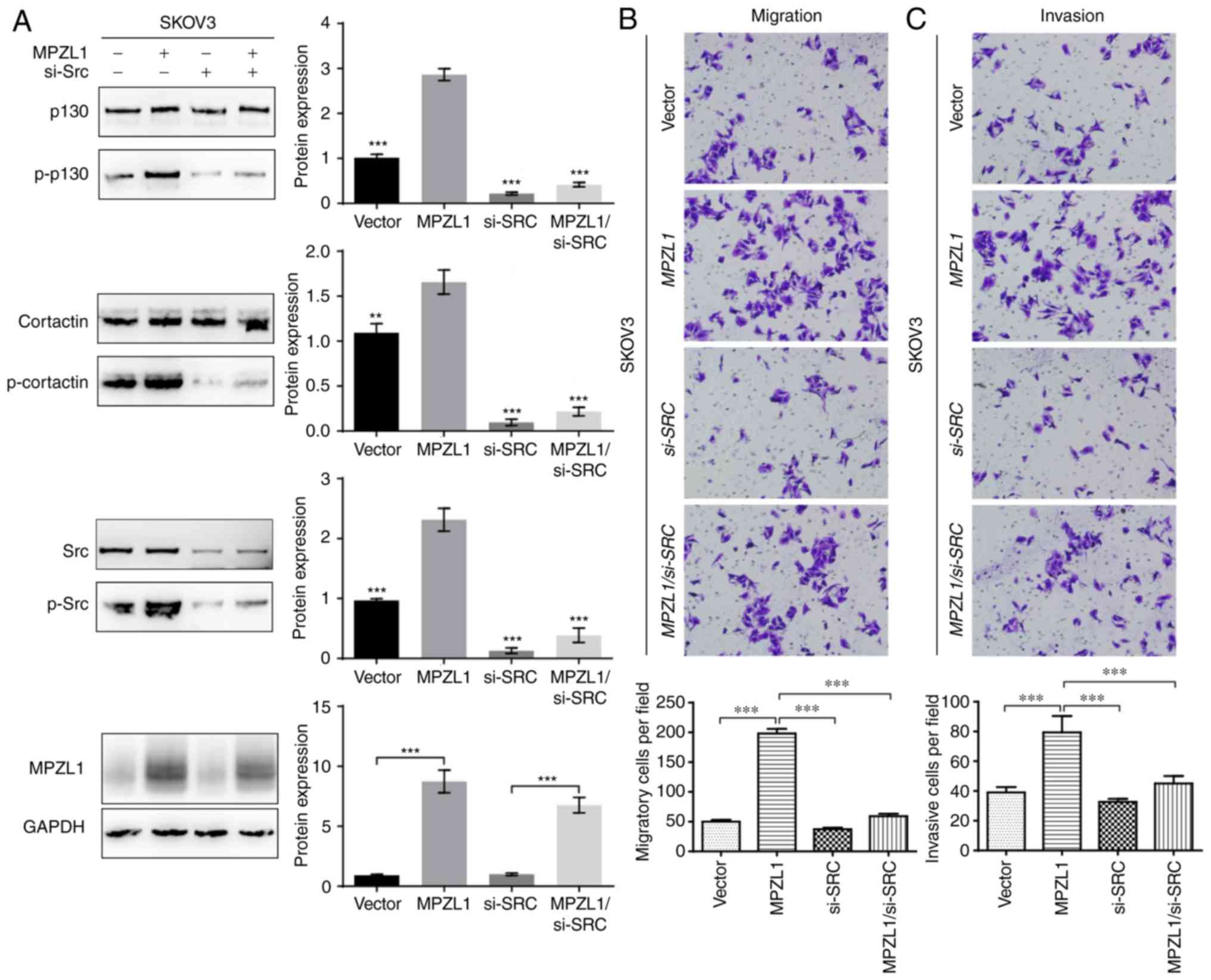
knocked down by siRNA. Notably, stable overexpression of
MPZL1 significantly increased phosphorylation of p130,
cortactin and Src in SKOV3-MPZL1 cells, and enhanced the
migratory and invasive abilities of SKOV3-MPZL1 cells
(Fig. 5A-C). Conversely, targeted
knockdown of Src via siRNA resulted in reduced
phosphorylation of p130 and cortactin, and suppressed the migration
and invasion of SKOV3-MPZL1 cells (Fig. 5A-C). Taken together, these findings
indicated that Src kinase activity may be essential for
MPZL1-mediated migration and invasion of ovarian cancer cells.
Discussion
In the present study, amplified MPZL1 was
associated with the malignant features of ovarian cancer.
Subsequently, overexpression and knockdown of MPZL1
indicated that MPZL1 served a prominent role in the promotion of
ovarian cancer cell proliferation, migration and invasion. In
addition, amplification of MPZL1 promoted tumor cell
migration through Src-mediated phosphorylation of p130 and
cortactin. Therefore, these data suggested that MPZL1 may operate
as a novel oncoprotein in ovarian cancer.
Previous studies have reported that the MPZL1
protein is involved in cell signaling, proliferation,
differentiation and transformation (15,28).
Furthermore, amplification of MPZL1 promotes tumor cell
migration through Src-mediated phosphorylation of cortactin in
hepatocellular carcinoma (27,29).
However, the biological functions and clinical implications of
MPZL1 in other types of human cancer remain unclear. This study
identified a positive association between the protein expression
levels of MPZL1 and cell proliferation, migration and invasion of
ovarian cancer cells.
Src family protein tyrosine kinases participate in
numerous signaling pathways that control cellular responses,
including proliferation, survival, adhesion and migration in normal
and cancer cells (30–33). As substrate proteins of Src kinase,
p130 and cortactin become tyrosine-phosphorylated during
integrin-mediated cell adhesion to the extracellular matrix, cell
attachment and cell invasion (24,34–36).
Furthermore, it has been demonstrated that tyrosine-phosphorylated
cortactin via Src kinase, which is activated upon MPZL1
overexpression, increases the migratory potential of hepatocellular
carcinoma cells (14,27). In this study, it was demonstrated that
overexpression of MPZL1 resulted in increased
phosphorylation of Src, p130 and cortactin. Additionally, the
migration and proliferation of ovarian cancer cells were promoted.
These findings indicated that MPZL1 may serve an important role in
ovarian cancer cell metastasis. However, the physiological
relevance of these data requires further mechanistic investigations
and in vivo studies.
In conclusion, the present results suggested that
amplification of MPZL1 in ovarian cancer may be associated
with the malignant features of ovarian cancer. MPZL1 was involved
in Src-mediated phosphorylation of p130 and cortactin, thereby
promoting ovarian cancer cell proliferation, migration and
invasion. Furthermore, these data identifies MPZL1 as a
novel pro-metastatic gene in ovarian cancer. Together with a report
implicating MPZL1 in other cancer types (27), the present study expands our
understanding of human ovarian cancer metastasis and indicates
potential therapeutic avenues for the treatment of ovarian
cancer.
Acknowledgements
The authors would like to thank Dr Shengzhe Zhang
and Ms. Zhenfeng Zhang (Shanghai Jiao Tong University School of
Medicine) for their technical assistance.
Funding
This work was supported by a grant from the Science
and Technology Commission of Changning District, Shanghai (grant
no. CNKW2017Y08 to X.W.).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW and DC designed and conceived the experiments. LC
analyzed the data. XW, DC and LC drafted the manuscript and wrote
the manuscript. XW supervised the study. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Ethics Committee of the Tongren Hospital, Shanghai Jiao Tong
University School of Medicine. All patients provided written
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Marcus CS, Maxwell GL, Darcy KM, Hamilton
CA and McGuire WP: Current approaches and challenges in managing
and monitoring treatment response in ovarian cancer. J Cancer.
5:25–30. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oronsky B, Ray CM, Spira AI, Trepel JB,
Carter CA and Cottrill HM: A brief review of the management of
platinum- resistant-platinum-refractory ovarian cancer. Med Oncol.
34:1032017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Walker JL, Powell CB, Chen Lm, Carter J,
Bae Jump VL, Parker LP, Borowsky ME and Gibb RK: Society of
gynecologic oncology recommendations for the prevention of ovarian
cancer. Cancer. 121:2108–2120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matz M, Coleman MP, Carreira H, Salmerón
D, Chirlaque MD and Allemani C; CONCORD Working Group, : Worldwide
comparison of ovarian cancer survival: Histological group and stage
at diagnosis (CONCORD-2). Gynecol Oncol. 144:396–404. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jiménez-Sánchez A, Memon D, Pourpe S,
Veeraraghavan H, Li Y, Vargas HA, Gill MB, Park KJ, Zivanovic O,
Konner J, et al: Heterogeneous tumor-immune microenvironments among
differentially growing metastases in an ovarian cancer patient.
Cell. 170:927–938. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hillman RT, Lu KH and Futreal PA: A novel
genomic rearrangement signature to predict poor survival among
women with high grade serous ovarian cancer. J Clin Oncol. 35
(Suppl 15):S55092017. View Article : Google Scholar
|
|
7
|
Lengyel E: Ovarian cancer development and
metastasis. Am J Pathol. 177:1053–1064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bast RC, Hennessy B and Mills GB: The
biology of ovarian cancer: New opportunities for translation. Nat
Rev Cancer. 9:415–428. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Naora H and Montell DJ: Ovarian cancer
metastasis: Integrating insights from disparate model organisms.
Nat Rev Cancer. 5:355–366. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo Z, Wang Q, Lau WB, Lau B, Xu L, Zhao
L, Yang H, Feng M, Xuan Y, Yang Y, et al: Tumor microenvironment:
The culprit for ovarian cancer metastasis? Cancer Lett.
377:174–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He G, Liu X, Qin W, Chen Q, Wang X, Yang
Y, Zhou J, Xu Y, Gu N, Feng G, et al: MPZL1/PZR, a novel candidate
predisposing schizophrenia in Han Chinese. Mol Psychiatry.
11:748–751. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Beigbeder A, Chartier FJ and Bisson N:
MPZL1 forms a signalling complex with GRB2 adaptor and PTPN11
phosphatase in HER2-positive breast cancer cells. Sci Rep.
7:115142017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao R, Guerrah A, Tang H and Zhao ZJ:
Cell surface glycoprotein PZR is a major mediator of concanavalin
A-induced cell signaling. J Biol Chem. 277:7882–7888. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fonseca NA, He Y, Greger L, Brazma A and
Zhang Z: Comprehensive genome and transcriptome analysis reveals
genetic basis for gene fusions in cancer. bioRxiv. 1486842017.
|
|
15
|
Zhao ZJ and Zhao R: Purification and
cloning of PZR, a binding protein and putative physiological
substrate of tyrosine phosphatase SHP-2. J Biol Chem.
273:29367–29372. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Playford MP and Schaller MD: The interplay
between Src and integrins in normal and tumor biology. Oncogene.
23:7928–7946. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang L, Dong P, Zhang Y, Yang M, Chen Y
and Tian BL: Prognostic validation of the updated 8th edition
Tumor-Node- Metastasis classification by the Union for
international cancer control: Survival analyses of 307 patients
with surgically treated gallbladder carcinoma. Oncol Lett.
16:4427–4433. 2018.PubMed/NCBI
|
|
18
|
Uhlen M, Oksvold P, Fagerberg L, Lundberg
E, Jonasson K, Forsberg M, Zwahlen M, Kampf C, Wester K, Hober S,
et al: Towards a knowledge-based human protein atlas. Nature
Biotechnol. 28:1248–1250. 2010. View Article : Google Scholar
|
|
19
|
Dai Y, Qi L, Zhang X, Li Y, Chen M and Zu
X: CrkI and p130(Cas) complex regulates the migration and invasion
of prostate cancer cells. Cell Biochem Funct. 29:625–629. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Padhye A, Ungewiss C, Fradette JJ,
Rodriguez BL, Albritton JL, Miller JS and Gibbons DL: A novel ex
vivo tumor system identifies Src-mediated invasion and metastasis
in mesenchymal tumor cells in non-small cell lung cancer. Sci Rep.
9:48192019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu H, Jing X, Cheng X, He Y, Hu L, Wu H,
Ye F and Zhao R: Asporin enhances colorectal cancer metastasis
through activating the EGFR/src/cortactin signaling pathway.
Oncotarget. 7:73402–73413. 2016.PubMed/NCBI
|
|
22
|
Zhang XH, Wang Q, Gerald W, Hudis CA,
Norton L, Smid M, Foekens JA and Massagué J: Latent bone metastasis
in breast cancer tied to Src-dependent survival signals. Cancer
cell. 16:67–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Summy JM and Gallick GE: Src family
kinases in tumor progression and metastasis. Cancer Metastasis Rev.
22:337–358. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang J, Asawa T, Takato T and Sakai R:
Cooperative roles of fyn and cortactin in cell migration of
metastatic murine melanoma. J Biol Chem. 278:48367–48376. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brábek J, Constancio SS, Siesser PF, Shin
NY, Pozzi A and Hanks SK: Crk-associated substrate tyrosine
phosphorylation sites are critical for invasion and metastasis of
SRC-transformed cells. Mol Cancer Res. 3:307–315. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Parsons SJ and Parsons JT: Src family
kinases, key regulators of signal transduction. Oncogene.
23:7906–7909. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jia D, Jing Y, Zhang Z, Liu L, Ding J,
Zhao F, Ge C, Wang Q, Chen T, Yao M, et al: Amplification of
MPZL1/PZR promotes tumor cell migration through src-mediated
phosphorylation of cortactin in hepatocellular carcinoma. Cell Res.
24:204–217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao R and Zhao ZJ: Dissecting the
interaction of SHP-2 with PZR, an immunoglobulin family protein
containing immunoreceptor tyrosine-based inhibitory motifs. J Biol
Chem. 275:5453–5459. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yeh YT, Dai HY and Chien CY: Amplification
of MPZL1/PZR gene in hepatocellular carcinoma. Hepatobiliary surg
Nutr. 3:87–90. 2014.PubMed/NCBI
|
|
30
|
Giannoni E, Buricchi F, Raugei G, Ramponi
G and Chiarugi P: Intracellular reactive oxygen species activate
Src tyrosine kinase during cell adhesion and anchorage-dependent
cell growth. Mol Cell Biol. 25:6391–6403. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shor AC, Keschman EA, Lee FY, Muro-Cacho
C, Letson GD, Trent JC, Pledger WJ and Jove R: Dasatinib inhibits
migration and invasion in diverse human sarcoma cell lines and
induces apoptosis in bone sarcoma cells dependent on SRC kinase for
survival. Cancer Res. 67:2800–2808. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu H and Jove R: The STATs of cancer-new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mitra SK and Schlaepfer DD:
Integrin-regulated FAK-src signaling in normal and cancer cells.
Curr Opin Cell Biol. 18:516–523. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vuori K and Ruoslahti E: Tyrosine
phosphorylation of p130Cas and cortactin accompanies
integrin-mediated cell adhesion to extracellular matrix. J Biol
Chem. 270:22259–22262. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ammer AG, Kelley LC, Hayes KE, Evans JV,
Lopez-Skinner LA, Martin KH, Frederick B, Rothschild BL, Raben D,
Elvin P, et al: Saracatinib impairs head and neck squamous cell
carcinoma invasion by disrupting invadopodia function. J Cancer Sci
Ther. 1:52–61. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Agerer F, Lux S, Michel A, Rohde M, Ohlsen
K and Hauck CR: Cellular invasion by Staphylococcus aureus
reveals a functional link between focal adhesion kinase and
cortactin in integrin-mediated internalisation. J Cell Sci.
118:2189–2200. 2005. View Article : Google Scholar : PubMed/NCBI
|