Introduction
Oesophageal squamous cell carcinoma (ESCC) is one of
the most highly malignant neoplasms of the digestive system. In
China, the dominant histological subtype of oesophageal cancer (EC)
is ESCC, and it has a low 5-year survival rate after surgery,
chemotherapy, radiotherapy and target therapy (1,2). Cancer
stem cells (CSCs) have been defined as a small population of tumour
cells that may contribute to tumour self-renewal, tumour
maintenance, differentiation into heterogeneous lineages of cancer
cells, tumour progression and resistance to therapies (3,4). Previous
research on ESCC stem-like cells were related to the detection of
side population (SP) cells in ESCC cell lines. Further studies
identified that p75NTR, CD133, CD44, KLF4, ALDH1A1, OCT4, SOX2 and
NANOG were oesophageal stem-like cancer cell markers that can
maintain the self-renewal and progression ability of cancer cells.
Concurrently, spheroid body cells could be enriched and maintain
the stem-like cell characteristics of ESCC (5–10). In a
previous study, our group demonstrated that ESCC KYSE450 spheroid
body cells overexpressed the stemness genes SOX2, ALDH1A1 and
NANOG, and were highly tumorigenic in xenograft models. In
addition, it was revealed that Lgr5-positive spheroid body cells
may represent a type of ESCC cancer stem cell, and Lgr5 may be an
essential regulatory factor modulating stemness in ESCC (11).
Numerous studies have discovered that hypoxia
promoted the self-renewal of embryonic stem cells, haematopoietic
stem cells, neurospheres and maintained the proliferation of CSCs
(12). Hypoxia-inducible factor 1α
(HIF-1α) is an important transcription factor that regulates cell
responses to hypoxia. Increased expression of HIF-1α is associated
with cancer cell metabolism, proliferation, invasiveness,
angiogenesis and metastasis, and HIF-1α is a marker for poor
prognosis in the majority of cancer types (13,14). In
addition, accumulating evidence supports the hypothesis that HIF-1α
is essential for maintaining CSCs and that the ability of CSCs and
tumour-initiating cells exists in a hypoxic niche microenvironment
(12,15). Mazumdar et al reported that
HIF-1α regulates the Wnt/β-catenin signalling pathway in hypoxic
embryonic stem cells by promoting β-catenin activation and the
expression of downstream effectors (16). Furthermore, certain researchers
revealed that the depletion of HIF-1α decreased the expression of
colon cancer stem cell markers and the Wnt/β-catenin signalling
transcriptional activity (17). The
Wnt/β-catenin signalling pathway has also been identified to play a
critical role in the regulation, formation and maintenance of
stemness in cancer stem cells (18).
Previously, we revealed that Lgr5 activated the Wnt/β-catenin
signalling pathway, leading to the progression of ESCC, and the
related stem cell gene Lgr5 also played a key role in maintaining
the functions of ESCC stem-like cells through the Wnt/β-catenin
signalling pathway (11).
The present study examined the effects of stable
HIF-1α knockdown on the proliferation, migration and tumour growth
in vivo of ESCC. Moreover, the role of stable HIF-1α
knockdown in the expression of CSC-related genes and spheroid body
formation was investigated in serum-free medium at low adherence in
ESCC cells. Subsequently, the activity of the Wnt/β-catenin
signalling pathway and the expression of Wnt/β-catenin
pathway-related target genes were detected in ESCC cells. The
present results indicated that HIF-1α promotes the stemness of ESCC
by activating the Wnt/β-catenin pathway.
Materials and methods
Cell lines and culture
The human ESCC cell lines KYSE450, KYSE70, Eca9706
and Eca109 were obtained from the Institute of the Chinese Academy
of Medical Sciences. For normoxic conditions, all of the cell lines
were cultured in RPMI-1640 supplemented with 10% foetal bovine
serum (both from HyClone; GE Healthcare Life Sciences) at 37°C in a
5% CO2 humidified incubator (Thermo Fisher Scientific,
Inc.). For hypoxic conditions, the chemical anoxic agent
CoCl2 (Sigma-Aldrich; Merck KGaA) was used to induce
hypoxia in ESCC using standard methods.
Lentivirus transfection assay
Lentivirus vectors encoding shRNA targeting HIF-1α
were constructed using the sequence 5′-TTCTCCGAACGTGTCACGT-3′, and
designed by GenePharma. Lentiviruses were packaged in ESCC by
co-transfection with the plasmid pTOPFlash and the void plasmid
pSuper. The supernatant was collected and concentrated 48 h after
co-transfection. The lentivirus was transfected using an enhanced
solution with 8 µg/ml polybrene (Sigma-Aldrich; Merck KGaA), and
then the packed lentivirus was added to cells with a multiplicity
of infection (MOI) of 10. Following incubation for 12 h, the
lentivirus solution was replaced with normal culture medium
supplemented with 10% FBS. The infected cells were subcultured
every 5–7 days.
Quantitative real-time polymerase
chain reaction (qRT-PCR)
Total RNA was isolated with TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), and cDNA was
synthesized with the PrimeScript RT Reagent kit (Takara Bio, Inc.).
qRT-PCR was performed using a SYBR Green I kit (Roche Diagnostics)
and was assessed in triplicate using Agilent Mx3005P. The following
primers were used for HIF-1α: forward,
5′-GAACGTCGAAAAGAAAAGTCTCG-3′ and reverse,
5′-CCTTATCAAGATGCGAACTCACA-3′; β-actin: Forward,
5′-AGGCACCAGGGCGTGAT-3′ and reverse, 5′-GCCCACATAGGAATCCTTCTGAC-3′.
Other primer sequences and additional PCR conditions are available
upon request. PCR was conducted at 95°C for 15 min, followed by 40
cycles at 94°C for 15 sec, 55°C for 30 sec, and 64°C for 30 sec.
Gene expression values were normalized to the housekeeping gene,
and relative expression values were calculated based on the
2−ΔΔCq method (19).
Tissue patient sample collection
The ESCC tissues and normal esophageal squamous
epithelial tissues were collected from patients who underwent
surgical resection with curative intent at the First Affiliated
Hospital of Henan University of Traditional Chinese Medicine. The
study included 20 human tissues (9 females and 11 males, aged 46–75
years) collected from February 2018 to November 2018. The
Institutional Ethics Review Board of The First Affiliated Hospital
of Henan University of Traditional Chinese Medicine (Zhengzhou,
China) approved the use of human samples and patients provided
written informed consent.
Western blotting
ESCC cells, ESCC tissues and normal esophageal
squamous epithelial tissues were lysed, and the total protein was
extracted using lysis buffer (Beyotime Institute of Biotechnology,
Haimen, China). A Nuclear and Cytoplasmic Protein Extraction kit
(Sigma-Aldrich; Merck KGaA) was used to separate nuclear and
cytoplasmic proteins. Total protein (20–40 mg) and nuclear protein
extracts were separated by 10% SDS-PAGE and transferred onto PVDF
membranes. After blocking with 5% skim milk at room temperature for
1 h, the primary antibodies were incubated overnight at 4°C. Then,
the membranes were washed three times in TBST and incubated with a
secondary antibody at room temperature for 1 h. Protein expression
was quantified by densitometry (Quantity One software; Bio-Rad
Laboratories, Hercules, CA, USA). The following primary antibodies
were used: Anti-HIF-1α (cat. no. 66730; dilution 1:10,000),
anti-Lgr5 (cat. no. 29025; dilution 1:1,000), anti-NANOG (cat. no.
14295; dilution 1:1,000), anti-p75NTR (cat. no. 55014; dilution
1:1,000), anti-ALDH1A1 (cat. no. 15910; dilution 1:1,000),
anti-SOX2 (cat. no. 11064; dilution 1:1,000), anti-β-catenin (cat.
no. 17565; dilution 1:2,000), anti-c-Myc (cat. no. 10828; dilution
1:2,000), anti-cyclin D1 (cat. no. 60186; dilution 1:2,000) and
anti-β-actin (cat. no. 20536; dilution 1:2,000) (all antibodies
from Proteintech Group Inc., Wuhan, China). Subsequently, the
membranes were incubated with specific horseradish
peroxidase-conjugated antibodies (cat. no. bs-2188R; dilution
1:5,000; from Beijing Biosynthesis Biotechnology Co., Ltd.,
Beijing, China) The immunoreactive proteins were detected using an
enhanced chemiluminescence (ECL) detection system (Tanon Science
and Technology Co., Ltd., Shanghai, China).
Culture of spheroid body cells
The ESCC cells were plated in 6-well ultra-low
attachment plates (Corning, Inc.), and were incubated in serum-free
DMEM/F12 medium (Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 20 ng/ml EGF (PeproTech, Inc.), B27 (1:50; Gibco;
Thermo Fisher Scientific, Inc.), and 20 ng/ml bFGF (PeproTech,
Inc.). Then the number and the formation rate of spheroid body
cells were examined under light microscopy in ESCC cells after 7
days.
Cell proliferation and migration
assay
Isolated cells were seeded into 96-well culture
plates at 2,000 cells/well in three replicates for cell
proliferation assays and then were incubated for 24 h. Viable cells
were counted by Cell Counting Kit-8 (CCK-8) (Dojindo Molecular
Technologies, Inc.) at 24, 36, 48, 60 and 72 h. Subsequently, the
cells were incubated with CCK-8 reagent for 1 h at 37°C. Colour
intensity was assessed using a microplate reader set at 450 nm to
obtain cell growth curves. For the migration assay, cells were
seeded in the upper chamber of Transwell plates (BD Biosciences)
with serum-free DMEM/F12. The lower chambers were filled with
chemoattractant medium (DMEM/F12 plus 10% FBS). After 20 h of
incubation at 37°C, the cells that did not migrate to the lower
chamber were removed with a cotton swab, while the migrating cells
were stained with 0.1% crystal violet after 36–48 h at room
temperature and counted under a light microscope in five different
fields.
Luciferase reporter assay
The 3′untranslated regions (UTRs) of HIF-1α were
amplified using the following primers: HIF-1α-F,
5′-GATCTCGAGGCTTTTTCTTAATTTCATTCCT-3′ and HIF-1α-R,
5′-GATGCGGCCGCGCCTGGTCCACAGAAGATGTTTA-3′. The ESCC cells
(2×105)/well were cultured in 24-well plates and
co-transfected with 0.5 µg of the TOP-flash or FOP-flash plasmid
(Promega, Madison, WI, USA) using 2 µl Lipofectamine 2000
transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
in serum-free DMEM/F12 medium. After 6 h of transfection, the
DMEM/F12 medium was replaced with DMEM/F12 medium containing 10%
serum. Subsequently, the cells were lysed and luciferase activity
was detected using the Dual-Luciferase reporter assay (Promega)
system kit after 24 h of incubation. For each experiment, the assay
was performed three times. Renilla luciferase values were
then divided by the firefly luciferase activity values to normalize
the difference in transfection efficiency.
In vivo xenograft tumourigenicity
assay
Sixty 5-week-old male BALB/c nude mice with a body
weight of ~15.5 g were housed under specific pathogen-free
conditions and were cared at a temperature of 25°C with a humidity
level of 40–60% with food and water provided ad libitum at
the Laboratory Animal Centre of The First Affiliated Hospital of
Henan Medical University of Traditional Chinese Medicine. The
protocol of raising the animals was approved by the Institutional
Ethics Review Board of the First Affiliated Hospital of Henan
University of Traditional Chinese Medicine. A total of
1×106 cancer cells were injected subcutaneously into
5-week-old female BALB/c nude mice (5 mice per group), and tumour
size was determined with callipers by measuring the long and short
diameters every 3 days. Animals were sacrificed by cervical
dislocation after 4 weeks, then tumours were photographed,
collected and measured by bodyweight.
Statistical analysis
The data represent the mean ± SD from three
independent experiments. The statistical analysis was performed
using GraphPad Prism (GraphPad Software, Inc., La Jola, CA, USA).
The data were then analyzed using Student's t-test when only two
groups were present. In addition, one-way analysis of variance
(ANOVA) was used to assess comparison in three groups, and multiple
comparison between the groups was performed by analyzing data with
the Student-Newman-Keuls (SNK) method. All comparisons were
performed relative to untreated groups, and significant differences
are indicated as P<0.05 and P<0.01.
Results
HIF-1α and CSC-related genes are
upregulated in ESCC in hypoxic conditions
To detect the levels of HIF-1α expression in ESCC
cells, western blotting and qRT-PCR techniques were used to detect
HIF-1α expression in four ESCC cell lines (KYSE70, KYSE450, Eca109
and Eca9706). The results demonstrated that HIF-1α expression was
markedly higher in KYSE450 cells than in the other ESCC cell lines
(Fig. 1A). Furthermore, it was
revealed that the protein expression of HIF-1α in hypoxic
conditions (CoCl2 treatment) was higher than in normoxic
conditions in ESCC cell line KYSE450 (Fig. 1B). In addition, to assess the clinical
importance of the expression of HIF-1α, the protein level of HIF-1α
in ESCC tissues and normal esophageal squamous epithelial tissues
was examined by western blotting. The results revealed that the
protein level of HIF-1α was increased in ESCC tissues, compared
with normal esophageal squamous epithelial tissues (Fig. 1C). Subsequently, the expression levels
of the CSC-related genes Lgr5, ALDH1A1, NANOG, SOX2 and p75NTR were
evaluated in KYSE450 cells under normoxic and hypoxic conditions.
Compared with expression levels in the normoxic environment, the
mRNA and protein expression of CSC-related genes in hypoxic
conditions were significantly enhanced in the ESCC cell line
KYSE450 (Fig. 1D).
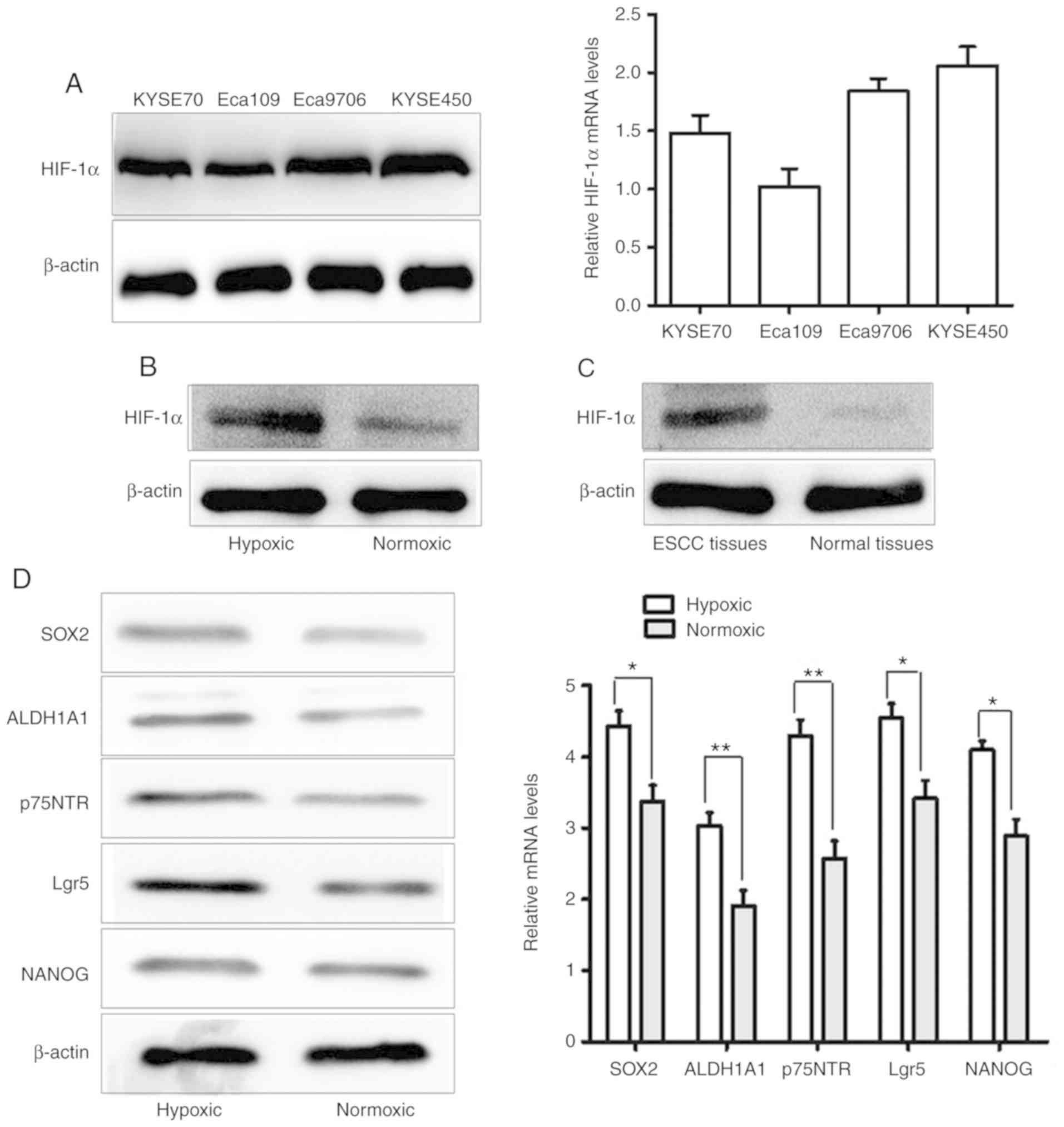 | Figure 1.HIF-1α and CSC-related genes are
upregulated in ESCC in hypoxic conditions. (A) mRNA and protein
levels of HIF-1α in four ESCC cell lines (KYSE70, KYSE450, Eca109
and Eca9706). (B) The protein expression of HIF-1α in hypoxic
conditions (CoCl2 treatment) was higher than in normoxic conditions
in ESCC cell line KYSE450. (C) The protein level of HIF-1α was
elevated in ESCC tissues, compared with normal esophageal squamous
epithelial tissues. (D) qRT-PCR and western blot analysis revealed
that the mRNA and protein expression levels of the CSC-related
genes Lgr5, ALDH1A1, NANOG, SOX2 and p75NTR in KYSE450 cells was
increased under hypoxic conditions, compared with in normoxic
conditions (*P<0.05 and **P<0.01). HIF-1α, hypoxia-inducible
factor 1; CSC, cancer stem cell; ESCC, oesophageal squamous cell
carcinoma. |
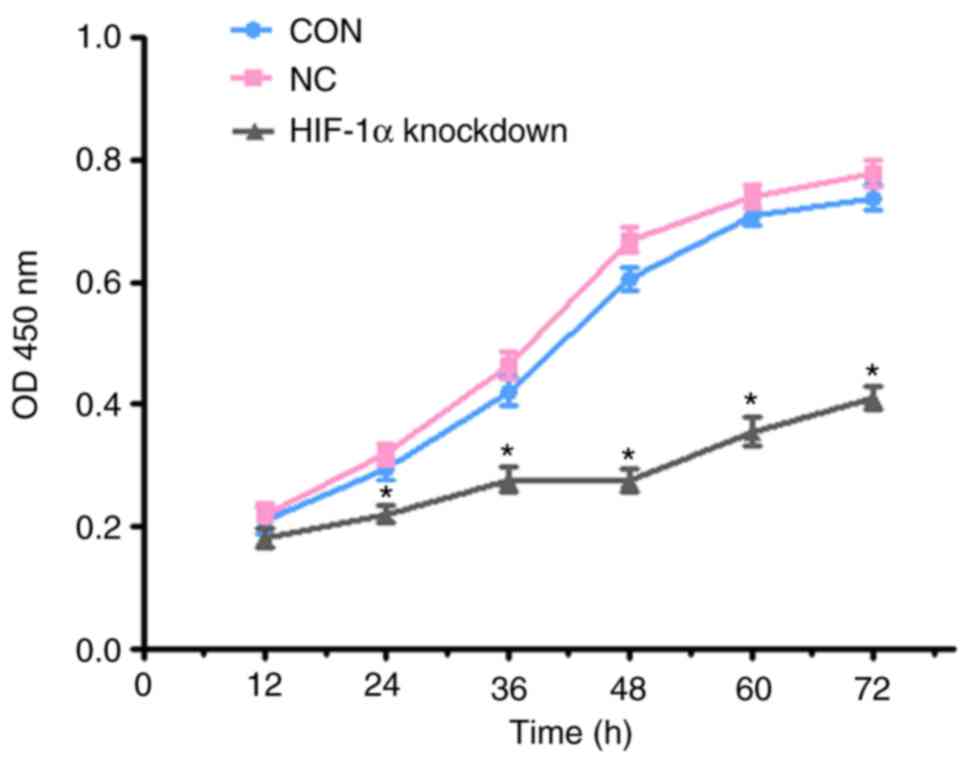
Stable knockdown of HIF-1α decreases
ESCC cell proliferation
Among the four ESCC cell lines, KYSE450 cells were
used for further experiments. The proliferation of ESCC cells was
detected by the CCK-8 assay. After transfection with the
lentivirus, the results from the CCK-8 assay demonstrated that the
proliferation rate of the HIF-1α-knockdown cell group was inhibited
compared to the control cell (CON) group and the negative control
cell (NC) group on the third day (P<0.05) (Fig. 2).
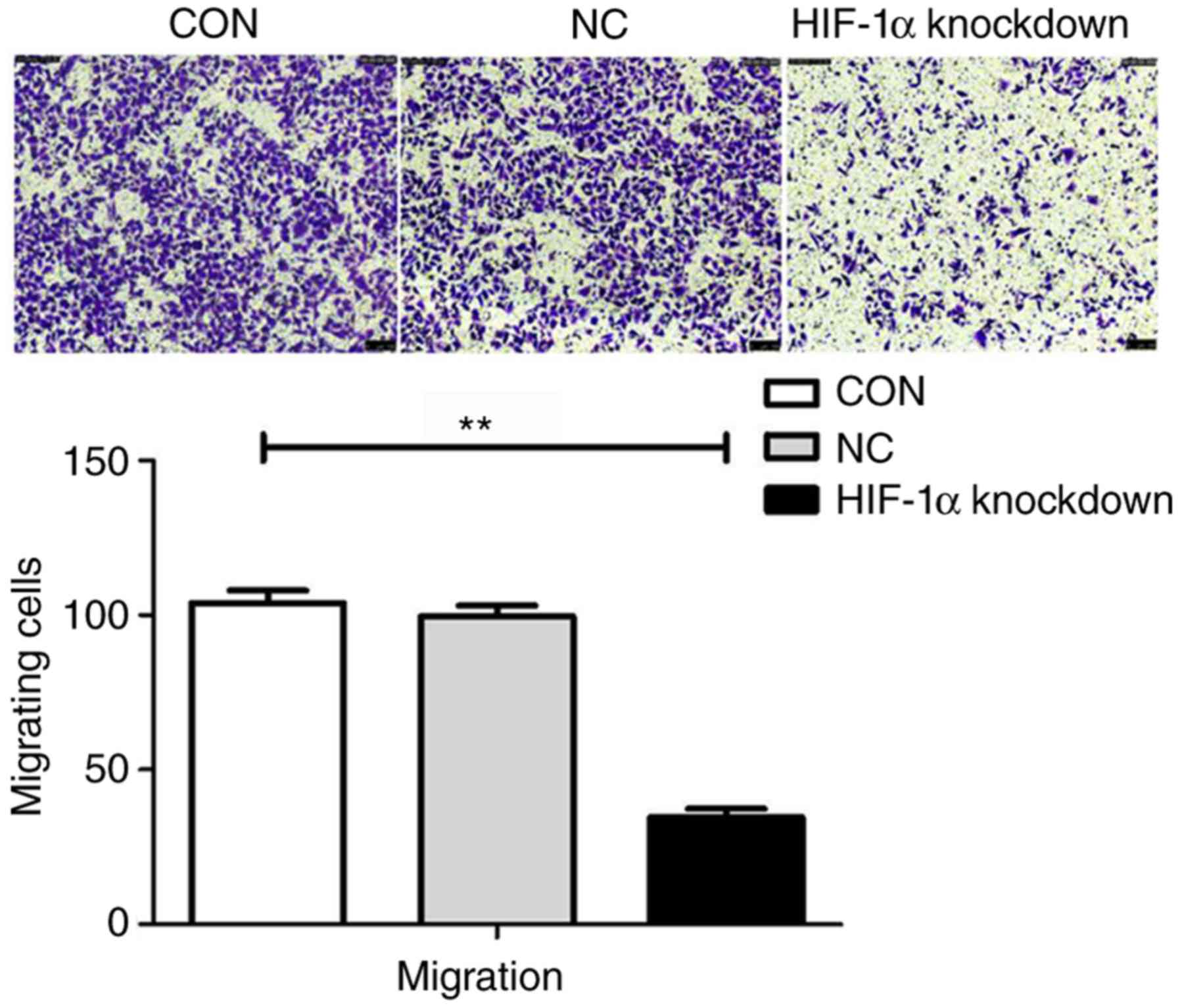
Stable knockdown of HIF-1α diminishes
ESCC cell migration
To study whether HIF-1α knockdown affects tumour
migration, Transwell assays were performed to investigate the
effects of HIF-1α in ESCC cells. Cells from the HIF-1α knockdown
group, CON group and NC group were seeded in Transwell chambers and
cultured for 24 h. A significant decrease in migration was observed
in the HIF-1α-knockdown cells compared to the migration of the
cells in the CON group and the NC group (P<0.05). The migration
rate of HIF-1α knockdown cells was 65% lower than that of the cells
in the CON group. The data indicated that HIF-1α was involved in
ESCC progression (Fig. 3).
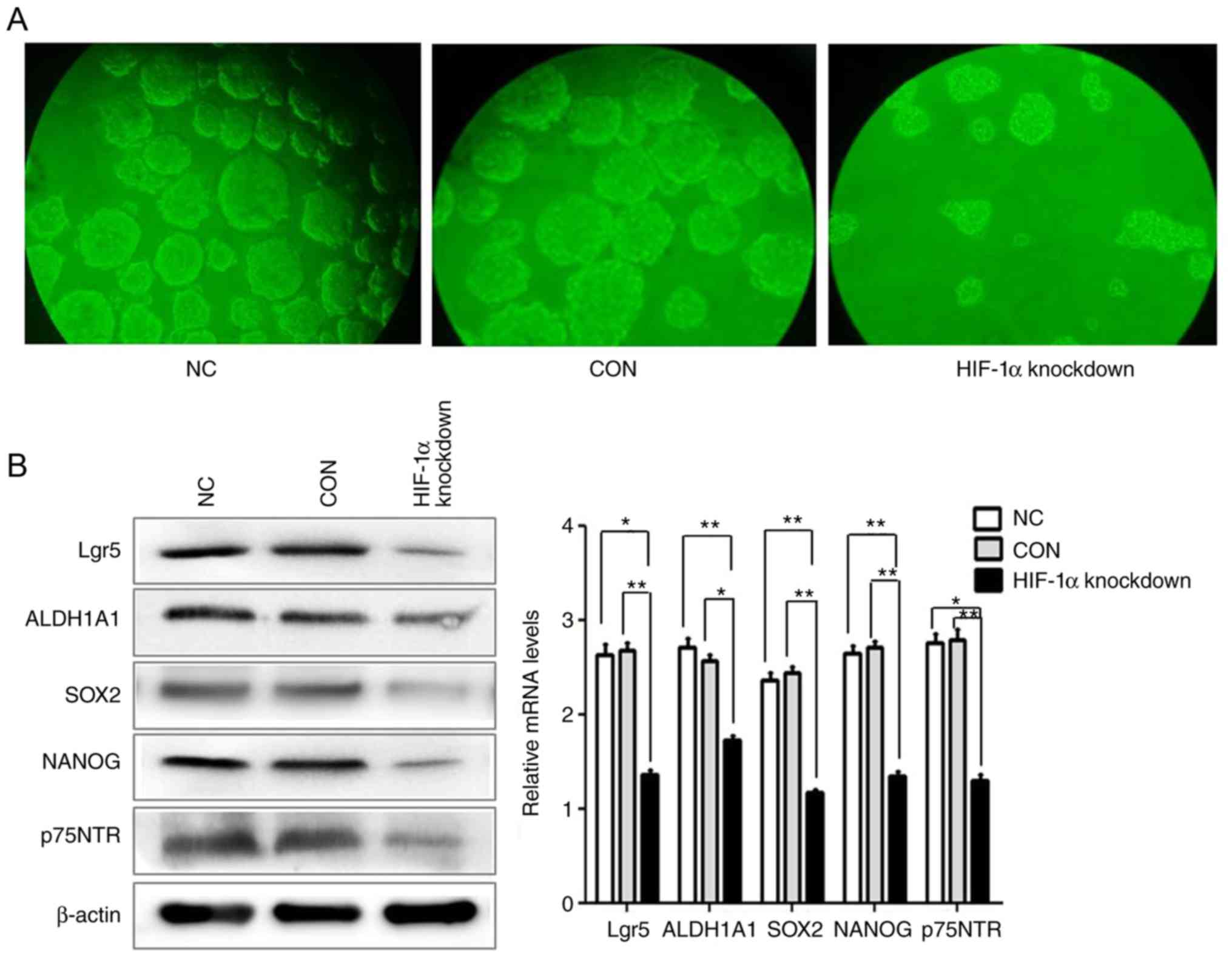
Stable knockdown of HIF-1α
significantly reduces the stemness of ESCC cells
It has been revealed that stem cell properties in
ESCC cells contribute to ESCC recurrence and metastasis. To
investigate whether HIF-1α plays a major role in ESCC stem cells,
it was first determined that hypoxia facilitates the maintenance of
ESCC stem cells. Mounting evidence demonstrates that CSCs can be
enriched and maintained using spheroid body formation assay. Thus,
ultra-low attachment surface plates and serum-free culture
conditions were used to culture the spheroid bodies in the
HIF-1α-knockdown group, CON group and NC group. The results
revealed that the number of the spheroid body cells in the HIF-1α
knockdown group was markedly reduced, compared with CON group and
NC group (Fig. 4A). Subsequently, the
effect of HIF-1α knockdown on the expression of CSC-related markers
was examined by western blotting and qRT-PCR. The results revealed
that knockdown of HIF-1α decreased the protein and mRNA expression
of the CSC-related genes Lgr5, ALDH1A1, NANOG, SOX2 and p75NTR
compared with the CON group and the NC group. These data revealed
that HIF-1α may have an important function in promoting the
stemness of ESCC (Fig. 4B).
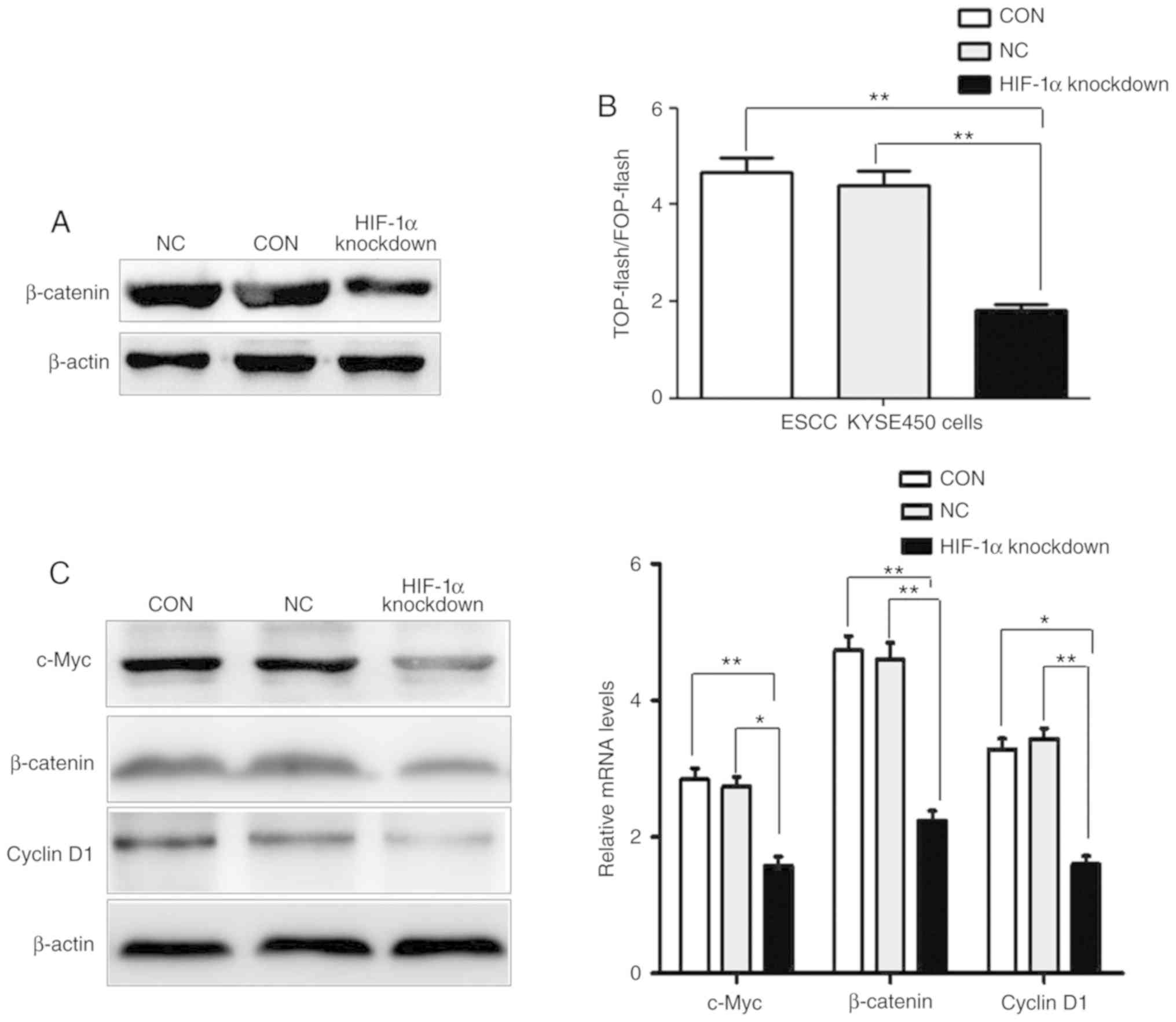
Downregulated HIF-1α directly
suppresses the activity of the Wnt/β-catenin pathway in ESCC
cells
Accumulating evidence supports that the
Wnt/β-catenin pathway plays a major role in cancer cell
proliferation, activation, and progression and the renewal of
cancer stem cells. The Wnt/β-catenin pathway could enter into the
nucleus through β-catenin and combine with TCF/LEF transcription
factors, thereby activating transcription of important target genes
cyclin D1 and c-Myc. Thus, subcellular fractionation assays were
used to confirm that the knockdown of HIF-1α in ESCC cells
decreased the protein expression of nuclear accumulation of
β-catenin (Fig. 5A). Furthermore, the
TOP-flash and FOP-flash dual luciferase reporter assay revealed
that transcriptional activity of TCF/LEF transcription factors in
the HIF-1α-knockdown group of cells was significantly reduced,
compared with the CON group and NC group (Fig. 5B). Moreover, the total protein and
mRNA expression of β-catenin and important target genes cyclin D1
and c-Myc in the Wnt/β-catenin pathway were decreased in the
HIF-1α-knockdown group of cells compared with the expression in the
CON group and the NC group (Fig. 5C).
Collectively, our data indicated that knockdown of HIF-1α could
suppress the activity of the Wnt/β-catenin pathway in ESCC
cells.
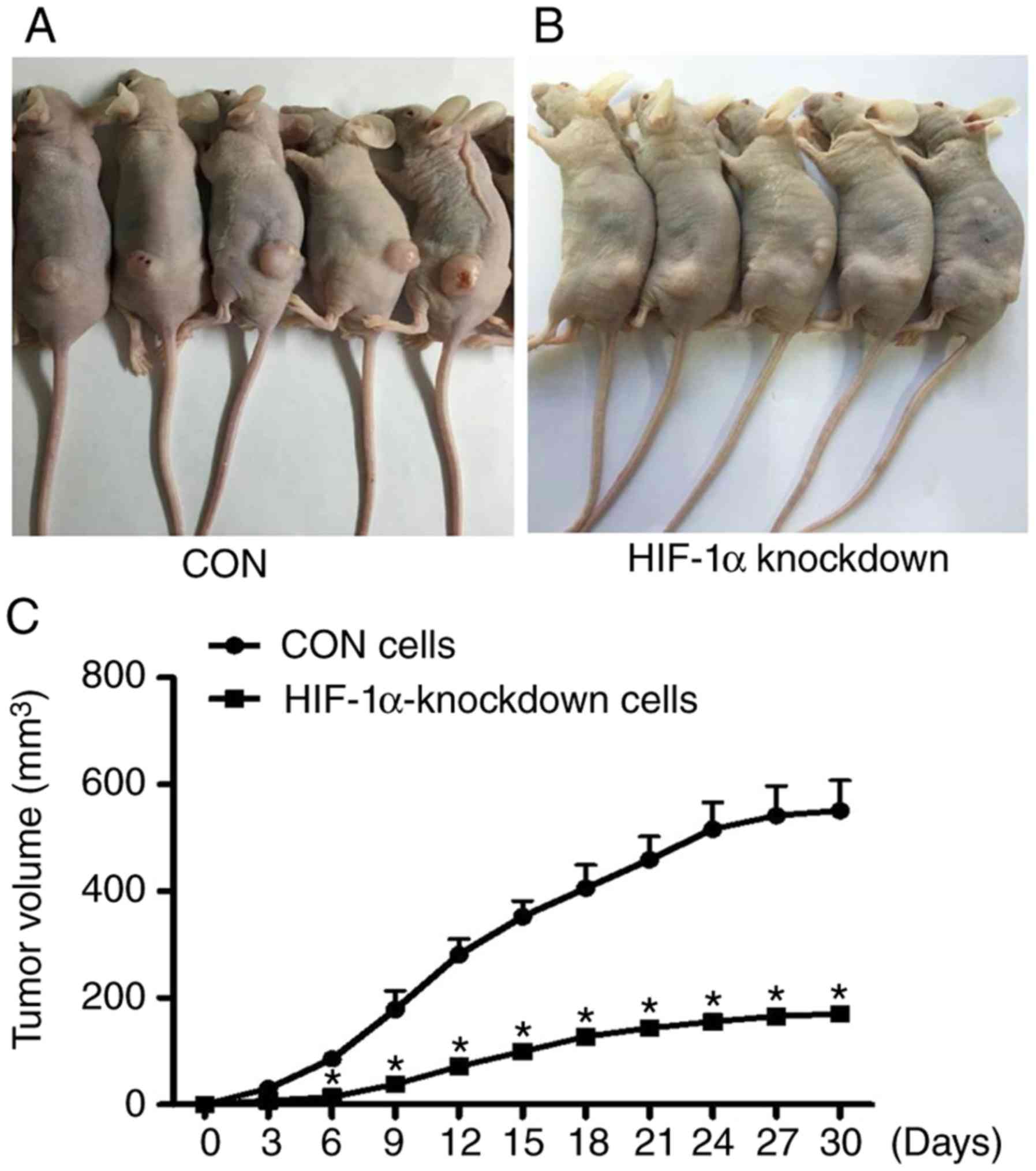
Stable knockdown of HIF-1α inhibits
tumour growth in vivo
To further evaluate the role of HIF-1α in the
progression of ESCC, tumour models were established. The HIF-1α
knockdown cells and CON cells were injected subcutaneously into the
dorsum of the BALB/c male nude mice. Tumour growth in the mice was
measured weekly after implantation. After four weeks, the mice were
euthanized, and the tumours were removed and weighed. As revealed
in Fig. 6, compared with the CON
cells, the mean volumes and the growth rate of the tumour treated
with HIF-1α-knockdown cells exhibited a decrease in the BALB/c male
nude mice (Fig. 6).
Discussion
Hypoxia-inducible factors (HIFs) are a crucial
pathological feature of all solid tumours and play a key role in
cancer proliferation, migration, progression, metabolic
reprogramming, angiogenesis, poor clinical prognosis and CSC
maintenance (20–22). HIF-1α is a key transcriptional factor
that responds to hypoxia, and accumulating data indicate that HIF
activation is stimulated by increased transcriptional and
translational activity of HIF-1α (23). Consistent with these functions,
increased HIF-1α expression has been observed in various human
cancer cell types and has been associated with cancer
proliferation, migration, progression and poor prognosis in many
cases. Conley et al revealed that the expression of HIF-1α
increased under hypoxic conditions in breast cancer (24). In this respect, it was determined in
the present study that HIF-1α expression was markedly higher in
KYSE450 cells than in other ESCC cell lines, and compared with a
normoxic environment, the protein expression level of HIF-1α under
hypoxic conditions was upregulated significantly in the ESCC cell
line KYSE450. In addition, it was demonstrated that the protein
expression of HIF-1α was markedly higher in ESCC tissues than
normal esophageal squamous epithelial tissues.
Furthermore, previous studies revealed that
knockdown of HIF-1α resulted in lower rates of proliferation,
migration, tumour metabolism and tumourigenic activity in colon
cancer cell lines. Researchers have revealed that HIF-1α plays an
important role in the progression of colon cancer (17,25). In
the present study, it was demonstrated that the proliferation rate
of the HIF-1α-knockdown group of ESCC cells was inhibited compared
with the CON group cells and NC group cells. It was also revealed
that the migration rate of HIF-1α-knockdown ESCC cells was 65%
lower than that of the CON group and NC group cells. These data
indicated that HIF-1α was involved in the modulation of ESCC
proliferation and progression.
Numerous studies have indicated that hypoxia may
result in poor clinical outcomes in cancers through enhanced
survival, self-renewal, and tumourigenesis abilities of the CSC
subpopulation. Previous studies have revealed that hypoxia could
induce the CSC phenotype in breast and colon cancer, as well as
glioma and pancreatic cancer through the activity of HIFs (12,26–28). Xiang
et al demonstrated that HIF-1α could stimulate both the
expression and activity of TAZ, a transcriptional co-activator that
is required for maintenance of breast cancer stem cells. They also
observed that suppression of HIF-1α decreased the expression of
CSC-related genes, tumour angiogenesis, tumour proliferation and
tumourigenicity (29). Qiang et
al revealed that HIF-1α enhanced the self-renewal activity of
CD133-positive glioblastoma cells and inhibited the induction of
glioblastoma stem cell differentiation. They determined that HIF-1α
may play an important role in the hypoxia-mediated maintenance of
glioblastoma stem cells partly due to its interaction with NICD
(30). A previous study revealed that
SOX2, ALDH1A1, NANOG, p75NTR and Lgr5 may be CSC-related genes in
ESCC, and spheroid body cell culture is used to enrich and identify
potential CSCs (8–11). Emerging evidence has indicated that
CSCs contribute to tumour maintenance, tumour progression,
migration and tumourigenicity (4,31,32). To investigate the biological role of
HIF-1α in ESCC CSCs, it was observed that knockdown of HIF-1α
decreased the number of spheroid body cells, and supressed the
protein and mRNA expression of the CSCs-related genes Lgr5,
ALDH1A1, NANOG, p75NTR and SOX2, compared with the CON group and NC
group cells. In addition, it was also observed that
HIF-1α-knockdown cells exhibited a decreased growth rate of tumours
in vivo. Collectively, these data revealed that HIF-1α plays
an important role in regulating the stemness of ESCC.
Accumulating evidence supports the hypothesis that
the Wnt/β-catenin signalling pathway plays a critical role in the
regulation, formation, renewal and maintenance of stem cells and
cancer stem cells (18,33). Similar to the results from our
previous study, the Wnt/β-catenin signalling pathway may play a key
role in the maintenance and progression of ESCC stem cells. Certain
researchers have revealed that HIF-1α depletion not only decreases
β-catenin protein levels and nuclear β-catenin levels, but also
decreases β-catenin transcriptional activity in colon cancer cells.
Finally, researchers have revealed that HIF-1α is essential for the
maintenance of the stemness and malignancy of colon cancer cells by
activating the Wnt/β-catenin signalling pathway (17). β-catenin is a crucial signalling
molecule in the Wnt/β-catenin signalling pathway that functions as
a transcriptional factor to activate the expression of cell
proliferation, migration, and important target genes, such as
cyclin D1 and c-Myc (34–36). In the present study, it was observed
that the knockdown of HIF-1α in ESCC cells decreased the expression
of nuclear accumulation of β-catenin. In addition, the TOP-flash
and FOP-flash dual luciferase reporter assay revealed that
transcriptional activity of TCF/LEF transcription factors in
HIF-1α-knockdown group of cells was significantly reduced, compared
with the CON group and NC group. Furthermore, the total protein and
mRNA expression of β-catenin and the Wnt/β-catenin pathway-related
target genes cyclinD1 and c-Myc were decreased in the
HIF-1α-knockdown group of cells compared with the CON group and NC
group of cells. This indicated that knockdown of HIF-1α could
reduce the activity of Wnt/β-catenin pathway in ESCC cells.
Collectively, the present results demonstrated that
HIF-1α-induced activation of the Wnt/β-catenin pathway is essential
for self-renewal, tumourigenesis and progression of ESCC stem
cells. These results could serve as a foundation for the study of
new targets for the development of potential targeted therapeutic
strategies by inhibiting the expression of HIF-1α in ESCC. In
addition, the further exploration of the impact of HIF-1α in ESCC
stem cells, and the relationship of ESCC stem cell-related genes
and HIF-1α is required.
Acknowledgements
We would like to thank the researchers and
participants for contributing samples to this study.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81804063) and the
Henan Province Traditional Chinese Medicine Research Special
Subject (grant no. 2018ZY2032).
Availability of data and materials
The datasets used during the present study are
available from the corresponding authors upon reasonable
request.
Authors' contributions
Collection, analysis and drafting the manuscript was
performed by ZL, XQC and RDL. Analysis and interpretation of
figures and data was performed by XQR, BW and LFL. Statistical
analysis was conducted by XQC, YSG and XJC. Revision of manuscript
for important intellectual content was performed by XQR and XQC.
All authors read and approved the final manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The human and animal protocols were approved by the
Institutional Ethics Review Board of The First Affiliated Hospital
of Henan University of Chinese Medicine, Zhengzhou, Henan, China.
Written informed consent was obtained from all patients and consent
for the publication of the human tissues from all patients who were
involved in the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kanamoto A, Ninomiya I, Harada S, Tsukada
T, Okamoto K, Nakanuma S, Sakai S, Makino I, Kinoshita J, Hayashi
H, et al: Valproic acid inhibits irradiation-induced
epithelial-mesenchymal transition and stem cell-like
characteristics in esophageal squamous cell carcinoma. Int J Oncol.
49:1859–1869. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y,
Ueda J, Wei W, Inoue M and Tanaka H: Epidemiology of esophageal
cancer in Japan and China. J Epidemiol. 23:233–242. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alferez DG, Simões BM, Howell SJ and
Clarke RB: The role of steroid hormones in breast and effects on
cancer stem cells. Curr Stem Cell Rep. 4:81–94. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dalerba P and Clarke MF: Cancer stem cells
and tumor metastasis: First steps into uncharted territory. Cell
Stem Cell. 1:241–242. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang SD, Yuan Y, Liu XH, Gong DJ, Bai CG,
Wang F, Luo JH and Xu ZY: Self-renewal and chemotherapy resistance
of p75NTR positive cells in esophageal squamous cell carcinomas.
BMC Cancer. 9:92009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rassouli FB, Matin MM, BahramiA R,
Ghaffarzadegan K, Cheshomi H, Lari S, Memar B and Kan MS:
Evaluating stem and cancerous biomarkers in CD15+CD44+ KYSE30
cells. Tumour Biol. 34:2909–2920. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Le Bras GF, Allison GL, Richards NF,
Ansari SS, Washington MK and Andl CD: CD44 upregulation in
E-cadherin-negative esophageal cancers results in cell invasion.
PLoS One. 6:e270632011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ajani JA, Wang X, Song S, Suzuki A, Taketa
T, Sudo K, Wadhwa R, Hofstetter WL, Komaki R, Maru DM, et al:
ALDH-1 expression levels predict response or resistance to
preoperative chemoradiation in resectable esophageal cancer
patients. Mol Oncol. 8:142–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Najafi M, Abbaszadegan MR, Rad A, Dastpak
M, Boroumand-Noughabi S and Forghanifard MM: Crosstalk between SHH
and stemness state signaling pathways in esophageal squamous cell
carcinoma. J Cell Commun Signal. 11:147–153. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Izadpanah MH, Abbaszadegan MR, Fahim Y and
Forghanifard MM: Ectopic expression of TWIST1 upregulates the
stemness marker OCT4 in the esophageal squamous cell carcinoma cell
line KYSE30. Cell Mol Biol Lett. 22:332017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lv Z, Yu JJ, Zhang WJ, Xiong L, Wang F, Li
LF, Zhou XL, Gao XY, Ding XF, Han L, et al: Expression and
functional regulation of stemness gene Lgr5 in esophageal squamous
cell carcinoma. Oncotarget. 8:26492–26504. 2017.PubMed/NCBI
|
|
12
|
Mohyeldin A, Garzón-Muvdi T and
Quiñones-Hinojosa A: Oxygen in stem cell biology: A critical
component of the stem cell niche. Cell Stem Cell. 7:150–161. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shin S, Im HJ, Kwon YJ, Ye DJ, Baek HS,
Kim D, Choi HK and Chun YJ: Human steroid sulfatase induces
Wnt/β-catenin signaling and epithelial-mesenchymal transition by
upregulating Twist1 and HIF-1α in human prostate and cervical
cancer cells. Oncotarget. 8:61604–61617. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Semenza GL: The hypoxic tumor
microenvironment: A driving force for breast cancer metastasis.
Biochim Biophys Acta. 1863:382–391. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schwab LP, Peacock DL, Majumdar D, Ingels
JF, Jensen LC, Smith KD, Cushing RC and Seagroves TN:
Hypoxia-inducible factor 1α promotes primary tumor growth and
tumor-initiating cell activity in breast cancer. Breast Cancer Res.
14:R62012. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mazumdar J, O'Brien WT, Johnson RS,
LaManna JC, Chavez JC, Klein PS and Simon MC: O2 regulates stem
cells through Wnt/β-catenin signalling. Nature Cell Biol.
12:1007–1013. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Santoyo-Ramos P, Likhatcheva M,
García-Zepeda EA, Castañeda-Patlán MC and Robles-Flores M:
Hypoxia-inducible factors modulate the stemness and malignancy of
colon cancer cells by playing opposite roles in canonical Wnt
signaling. PLoS One. 9:e1125802014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wend P, Holland JD, Ziebold U and
Birchmeier W: Wnt signaling in stem and cancer stem cells. Semin
Cell Dev Biol. 21:855–863. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Semenza GL: Hypoxia-inducible factors:
Mediators of cancer progression and targets for cancer therapy.
Trends Pharmacol Sci. 33:207–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wenger RH: Cellular adaptation to hypoxia:
O2-sensing protein hydroxylases, hypoxia-inducible transcription
factors, and O2-regulated gene expression. FASEB J. 16:1151–1162.
2012. View Article : Google Scholar
|
|
22
|
Jubb AM, Buffa FM and Harris AL:
Assessment of tumour hypoxia for prediction of response to therapy
and cancer prognosis. J Cell Mol Med. 14:18–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang GL and Semenza GL: Purification and
characterization of hypoxia-inducible factor 1. J Biol Chem.
270:1230–1237. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Conley SJ, Gheordunescu E, Kakarala P,
Newman B, Korkaya H, Heath AN, Clouthier SG and Wicha MS:
Antiangiogenic agents increase breast cancer stem cells via the
generation of tumor hypoxia. Proc Natl Acad Sci USA. 109:2784–2789.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Imamura T, Kikuchi H, Herraiz MT, Park DY,
Mizukami Y, Mino-Kenduson M, Lynch MP, Rueda BR, Benita Y, Xavier
RJ and Chung DC: HIF-1alpha and HIF-2alpha have divergent roles in
colon cancer. Int J Cancer. 124:763–771. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ezashi T, Das P and Roberts RM: Low O2
tensions and the prevention of differentiation of hES cells. Proc
Natl Acad Sci USA. 102:4783–4788. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoshida Y, Takahashi K, Okita K, Ichisaka
T and Yamanaka S: Hypoxia enhances the generation of induced
pluripotent stem cells. Cell Stem Cell. 5:237–241. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Louie E, Nik S, Chen JS, Schmidt M, Song
B, Pacson C, Chen XF, Park S, Ju J and Chen EI: Identification of a
stem-like cell population by exposing metastatic breast cancer cell
lines to repetitive cycles of hypoxia and reoxygenation. Breast
Cancer Res. 12:R942010. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xiang L, Gilkes DM, Hu H, Takano N, Luo W,
Lu H, Bullen JW, Samanta D, Liang H and Semenza GL:
Hypoxia-inducible factor 1 mediates TAZ expression and nuclear
localization to induce the breast cancer stem cell phenotype.
Oncotarget. 5:12509–12527. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qiang L, Wu T, Zhang HW, Lu N, Hu R, Wang
YJ, Zhao L, Chen FH, Wang XT, You QD and Guo QL: HIF-1α is critical
for hypoxia-mediated maintenance of glioblastoma stem cells by
activating Notch signaling pathway. Cell Death Differ. 19:284–294.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sato T and Clevers H: Primary mouse small
intestinal epithelial cell cultures. Methods Mol Biol. 945:319–328.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yue D, Zhang Z, Li J, Chen X, Ping Y, Liu
S, Shi X, Li L, Wang L, Huang L, et al: Transforming growth
factor-beta1 promotes the migration and invasion of sphere-forming
stem-like cell subpopulations in esophageal cancer. Exp Cell Res.
336:141–149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Clevers H, Loh KM and Nusse R: Stem cell
signaling. An integral program for tissue renewal and regeneration:
Wnt signaling and stem cell control. Science. 346:12480122014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
He TC, Sparks AB, Rago C, Hermeking H,
Zawel L, da Costa LT, Morin PJ, Vogelstein B and Kinzler KW:
Identification of c-MYC as a target of the APC pathway. Science.
281:1509–1512. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tetsu O and McCormick F: Beta-catenin
regulates expression of cyclin D1 in colon carcinoma cells. Nature.
398:422–426. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Klaus A; Birchmeier, : Wnt signalling and
its impact on development and cancer. Nat Rev Cancer. 8:387–398.
2008. View
Article : Google Scholar : PubMed/NCBI
|