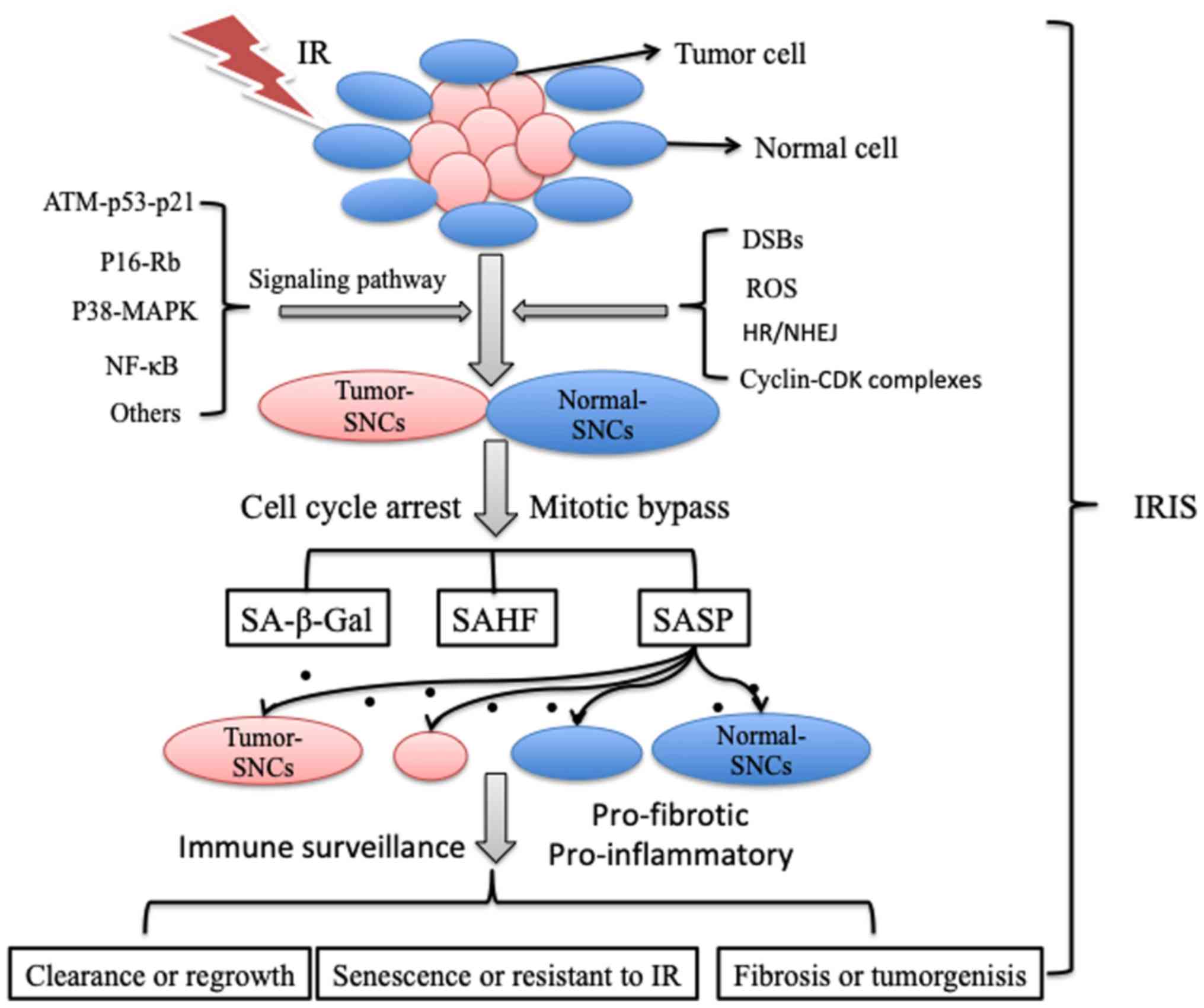
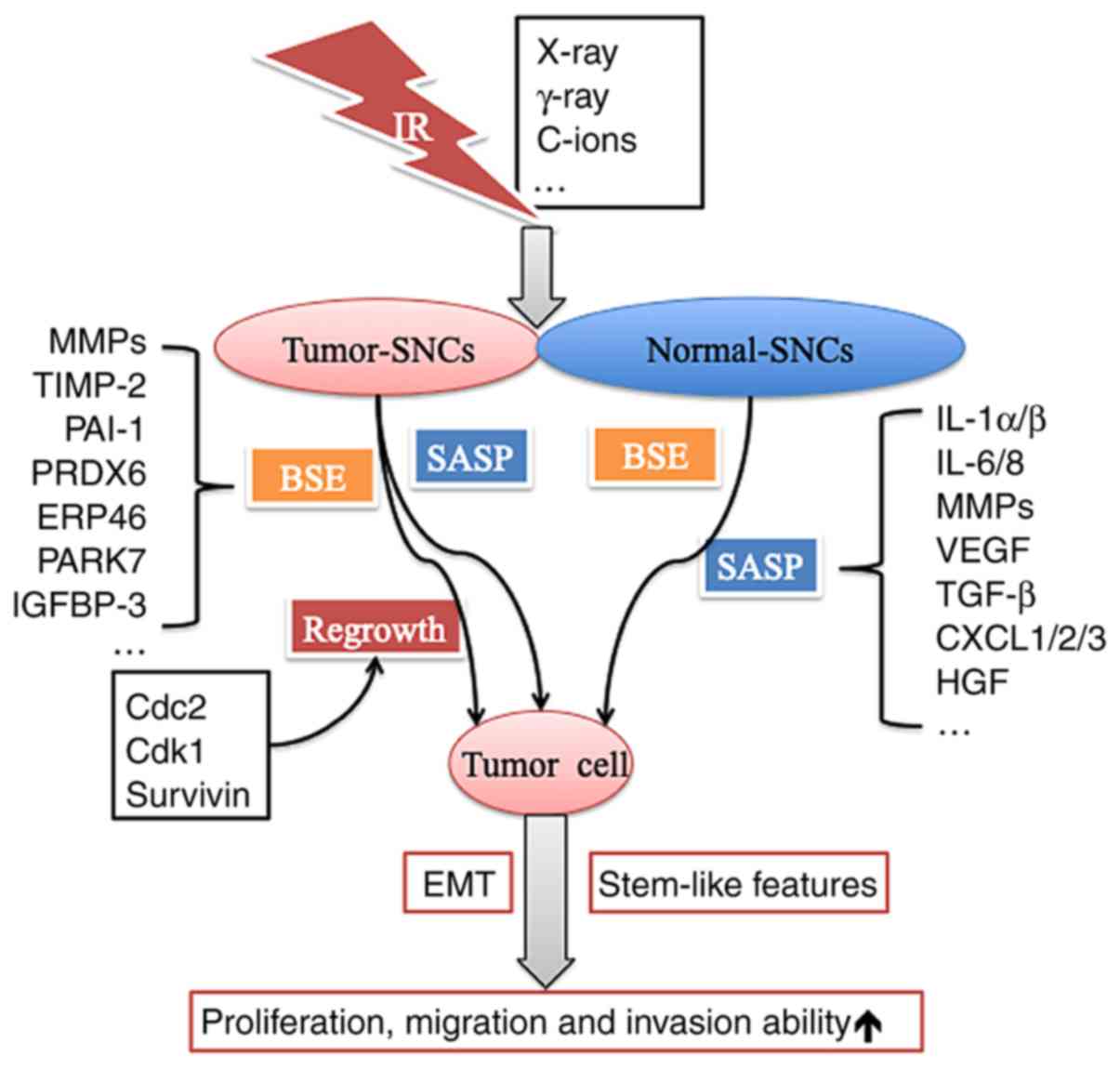
|
1
|
Hayflick L and Moorhead PS: The serial
cultivation of human diploid cell strains. Exp Cell Res.
25:585–621. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharpless NE and Sherr CJ: Forging a
signature of in vivo senescence. Nat Rev Cancer. 15:397–408. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sulli G, Rommel A, Wang X, Kolar MJ, Puca
F, Saghatelian A, Plikus MV, Verma IM and Panda S: Pharmacological
activation of REV-ERBs is lethal in cancer and oncogene-induced
senescence. Nature. 553:351–355. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McRobb LS, McKay MJ, Gamble JR, Grace M,
Moutrie V, Santos ED, Lee VS, Zhao Z, Molloy MP and Stoodley MA:
Ionizing radiation reduces ADAM10 expression in brain microvascular
endothelial cells undergoing stress-induced senescence. Aging
(Albany NY). 9:1248–1262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Davalos AR, Coppe JP, Campisi J and
Desprez PY: Senescent cells as a source of inflammatory factors for
tumor progression. Cancer Metastasis Rev. 29:273–283. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Childs BG, Gluscevic M, Baker DJ, Laberge
RM, Marquess D, Dananberg J and van Deursen JM: Senescent cells: An
emerging target for diseases of ageing. Nat Rev Drug Discov.
16:718–735. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eriksson D and Stigbrand T:
Radiation-induced cell death mechanisms. Tumour Biol. 31:363–372.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Wang Y, Liu S, Liu Y, Xu H, Liang
J, Zhu J, Zhang G, Su W, Dong W and Guo Q: Upregulation of EID3
sensitizes breast cancer cells to ionizing radiation-induced
cellular senescence. Biomed Pharmacother. 107:606–614. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nguyen HQ, To NH, Zadigue P, Kerbrat S, De
La Taille A, Le Gouvello S and Belkacemi Y: Ionizing
radiation-induced cellular senescence promotes tissue fibrosis
after radiotherapy. A review. Crit Rev Oncol Hematol. 129:13–26.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sun Y, Campisi J, Higano C, Beer TM,
Porter P, Coleman I, True L and Nelson PS: Treatment-induced damage
to the tumor microenvironment promotes prostate cancer therapy
resistance through WNT16B. Nat Med. 18:1359–1368. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hess J, Unger K, Orth M, Schötz U,
Schüttrumpf L, Zangen V, Gimenez-Aznar I, Michna A, Schneider L,
Stamp R, et al: Genomic amplification of Fanconi anemia
complementation group A (FancA) in head and neck squamous cell
carcinoma (HNSCC): Cellular mechanisms of radioresistance and
clinical relevance. Cancer Lett. 386:87–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Noda A, Hirai Y, Hamasaki K, Mitani H,
Nakamura N and Kodama Y: Unrepairable DNA double-strand breaks that
are generated by ionising radiation determine the fate of normal
human cells. J Cell Sci. 125:5280–5287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rossiello F, Herbig U, Longhese MP,
Fumagalli M and d'Adda di Fagagna F: Irreparable telomeric DNA
damage and persistent DDR signalling as a shared causative
mechanism of cellular senescence and ageing. Curr Opin Genet Dev.
26:89–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Suzuki M and Boothman DA: Stress-induced
premature senescence (SIPS)-influence of SIPS on radiotherapy. J
Radiat Res. 49:105–112. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
He S and Sharpless NE: Senescence in
health and disease. Cell. 169:1000–1011. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li M, You L, Xue J and Lu Y: Ionizing
radiation-induced cellular senescence in normal, non-transformed
cells and the involved DNA damage response: A mini review. Front
Pharmacol. 9:5222018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagane M, Kuppusamy ML, An J, Mast JM,
Gogna R, Yasui H, Yamamori T, Inanami O and Kuppusamy P:
Ataxia-telangiectasia mutated (ATM) kinase regulates eNOS
expression and modulates radiosensitivity in endothelial cells
exposed to ionizing radiation. Radiat Res. 189:519–528. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gire V and Dulic V: Senescence from G2
arrest, revisited. Cell Cycle. 14:297–304. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Krenning L, Feringa FM, Shaltiel IA, Van
Den Berg J and Medema RH: Transient activation of p53 in G2 phase
is sufficient to induce senescence. Mol Cell. 55:59–72. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Müllers E, Silva Cascales H, Jaiswal H,
Saurin AT and Lindqvist A: Nuclear translocation of Cyclin B1 marks
the restriction point for terminal cell cycle exit in G2 phase.
Cell Cycle. 13:2733–2743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ye C, Zhang X, Wan J, Chang L, Hu W, Bing
Z, Zhang S, Li J, He J, Wang J and Zhou G: Radiation-induced
cellular senescence results from a slippage of long-term G2
arrested cells into G1 phase. Cell Cycle. 12:1424–1432. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuilman T, Michaloglou C, Mooi WJ and
Peeper DS: The essence of senescence. Genes Dev. 24:2463–2479.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Scheiber MN, Neumann C, Calin GA
and Zhou D: MicroRNA regulation of ionizing radiation-induced
premature senescence. Int J Radiat Oncol Biol Phys. 81:839–848.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suzuki M, Yamauchi M, Oka Y, Suzuki K and
Yamashita S: Live-cell imaging visualizes frequent mitotic skipping
during senescence-like growth arrest in mammary carcinoma cells
exposed to ionizing radiation. Int J Radiat Oncol Biol Phys.
83:e241–e250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hudson D, Kovalchuk I, Koturbash I, Kolb
B, Martin OA and Kovalchuk O: Induction and persistence of
radiation-induced DNA damage is more pronounced in young animals
than in old animals. Aging (Albany NY). 3:609–620. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim BC, Han NK, Byun HO, Kim SS, Ahn EK,
Chu IS, Leem SH, Lee CK and Lee JS: Time-dependently expressed
markers and the characterization for premature senescence induced
by ionizing radiation in MCF7. Oncol Rep. 24:395–403.
2010.PubMed/NCBI
|
|
27
|
Studencka M and Schaber J: Senoptosis:
Non-lethal DNA cleavage as a route to deep senescence. Oncotarget.
8:30656–30671. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Suzuki K, Mori I, Nakayama Y, Miyakoda M,
Kodama S and Watanabe M: Radiation-induced senescence-like growth
arrest requires TP53 function but not telomere shortening. Radiat
Res. 155:248–253. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liang X, Gu J, Yu D, Wang G, Zhou L, Zhang
X, Zhao Y, Chen X, Zheng S, Liu Q, et al: Low-dose radiation
induces cell proliferation in human embryonic lung fibroblasts but
not in lung cancer cells: Importance of ERK1/2 and AKT signaling
pathways. Dose-Response. 14:15593258156221742016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Boerma M and Zhou D: Ionizing
radiation-induced endothelial cell senescence and cardiovascular
diseases. Radiat Res. 186:153–161. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rezáčová M, Rudolfová G, Tichý A, Bačíková
A, Mutná D, Havelek R, Vávrová J, Odrážka K, Lukášová E and Kozubek
S: Accumulation of DNA damage and cell death after fractionated
irradiation. Radiat Res. 175:708–718. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim BC, Yoo HJ, Lee HC, Kang KA, Jung SH,
Lee HJ, Lee M, Park S, Ji YH, Lee YS, et al: Evaluation of
premature senescence and senescence biomarkers in carcinoma cells
and xenograft mice exposed to single or fractionated irradiation.
Oncol Rep. 31:2229–2235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lagadec C, Vlashi E, Della Donna L, Meng
Y, Dekmezian C, Kim K and Pajonk F: Survival and self-renewing
capacity of breast cancer initiating cells during fractionated
radiation treatment. Breast Cancer Res. 12:R132010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liakou E, Mavrogonatou E, Pratsinis H,
Rizou S, Evangelou K, Panagiotou PN, Karamanos NK, Gorgoulis VG and
Kletsas D: Ionizing radiation-mediated premature senescence and
paracrine interactions with cancer cells enhance the expression of
syndecan 1 in human breast stromal fibroblasts: The role of TGF-β.
Aging (Albany NY). 8:1650–1668. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hernandez-Segura A, de Jong TV, Melov S,
Guryev V, Campisi J and Demaria M: Unmasking transcriptional
heterogeneity in senescent cells. Curr Biol. 27:2652–2660.e4. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ortiz-Montero P, Londoño-Vallejo A and
Vernot JP: Senescence-associated IL-6 and IL-8 cytokines induce a
self- and cross-reinforced senescence/inflammatory milieu
strengthening tumorigenic capabilities in the MCF-7 breast cancer
cell line. Cell Commun Signal. 15:172017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Johnston CJ, Hernady E, Reed C, Thurston
SW, Finkelstein JN and Williams JP: Early alterations in cytokine
expression in adult compared to developing lung in mice after
radiation exposure. Radiat Res. 173:522–535. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Papadopoulou A and Kletsas D: Human lung
fibroblasts prematurely senescent after exposure to ionizing
radiation enhance the growth of malignant lung epithelial cells in
vitro and in vivo. Int J Oncol. 39:989–999. 2011.PubMed/NCBI
|
|
39
|
Liao EC, Hsu YT, Chuah QY, Lee YJ, Hu JY,
Huang TC, Yang PM and Chiu SJ: Radiation induces senescence and a
bystander effect through metabolic alterations. Cell Death Dis.
5:e12552014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sugrue MM, Shin DY, Lee SW and Aaronson
SA: Wild-type p53 triggers a rapid senescence program in human
tumor cells lacking functional p53. Proc Natl Acad Sci USA.
94:9648–9653. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Beauséjour CM, Krtolica AF, Galimi F,
Narita M, Lowe SW, Yaswen P and Campisi J: Reversal of human
cellular senescence: Roles of the p53 and p16 pathways. EMBO J.
22:4212–4222. 2014. View Article : Google Scholar
|
|
42
|
Cheng Z, Zheng YZ, Li YQ and Wong CS:
Cellular senescence in mouse hippocampus after irradiation and the
role of p53 and p21. J Neuropathol Exp Neurol. 76:260–269. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Widel M, Lalik A, Krzywon A, Poleszczuk J,
Fujarewicz K and Rzeszowska-Wolny J: The different radiation
response and radiation-induced bystander effects in colorectal
carcinoma cells differing in p53 status. Mutat Res. 778:61–70.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lindgren T, Stigbrand T, Råberg A, Riklund
K, Johansson L and Eriksson D: Genome wide expression analysis of
radiation-induced DNA damage responses in isogenic HCT116 p53+/+
and HCT116 p53-/- colorectal carcinoma cell lines. Int J Radiat
Biol. 91:99–111. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gong L, Gong H, Pan X, Chang C, Ou Z, Ye
S, Yin L, Yang L, Tao T, Zhang Z, et al: p53 isoform
Δ113p53/Δ133p53 promotes DNA double-strand break repair to protect
cell from death and senescence in response to DNA damage. Cell Res.
25:351–369. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sanada F, Taniyama Y, Muratsu J, Otsu R,
Iwabayashi M, Carracedo M, Rakugi H and Morishita R: Activated
factor X induces endothelial cell senescence through IGFBP-5. Sci
Rep. 6:355802016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sanada F, Taniyama Y, Muratsu J, Otsu R,
Shimizu H, Rakugi H and Morishita R: IGF binding protein-5 induces
cell senescence. Front Endocrinol (Lausanne). 9:532018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kim K, Seu Y, Baek S, Kim MJ, Kim KJ, Kim
JH and Kim JR: Induction of cellular senescence by insulin-like
growth factor binding protein-5 through a p53-dependent mechanism.
Mol Biol Cell. 18:4543–4552. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rombouts C, Aerts A, Quintens R, Baselet
B, El-Saghire H, Harms-Ringdahl M, Haghdoost S, Janssen A, Michaux
A, Yentrapalli R, et al: Transcriptomic profiling suggests a role
for IGFBP5 in premature senescence of endothelial cells after
chronic low dose rate irradiation. Int J Radiat Biol. 90:560–574.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shi W, Tang MK, Yao Y, Tang C, Chui YL and
Lee KK: BRE plays an essential role in preventing replicative and
DNA damage-induced premature senescence. Sci Rep. 6:235062016.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lukášová E, Kovarˇík A, Bacˇíková A, Falk
M and Kozubek S: Loss of lamin B receptor is necessary to induce
cellular senescence. Biochem J. 474:281–300. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Freund A, Laberge RM, Demaria M and
Campisi J: Lamin B1 loss is a senescence-associated biomarker. Mol
Biol Cell. 23:2066–2075. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Toillon RA, Magné N, Laïos I, Castadot P,
Kinnaert E, Van Houtte P, Desmedt C, Leclercq G and Lacroix M:
Estrogens decrease gamma-ray-induced senescence and maintain cell
cycle progression in breast cancer cells independently of p53. Int
J Radiat Oncol Biol Phys. 67:1187–1200. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Abdelmohsen K, Panda A, Kang MJ, Xu J,
Selimyan R, Yoon JH, Martindale JL, De S, Wood WH III, Becker KG
and Gorospe M: Senescence-associated lncRNAs: Senescence-associated
long noncoding RNAs. Aging Cell. 12:890–900. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sohn D, Peters D, Piekorz RP, Budach W and
Jänicke RU: miR-30e controls DNA damage-induced stress responses by
modulating expression of the CDK inhibitor p21WAF1/CIP1 and
caspase-3. Oncotarget. 7:15915–15929. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Velegzhaninov IO, Ermakova AV and Klokov
DY: Low dose ionizing irradiation suppresses cellular senescence in
normal human fibroblasts. Int J Radiat Biol. 94:825–828. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dolan DW, Zupanic A, Nelson G, Hall P,
Miwa S, Kirkwood TB and Shanley DP: Integrated stochastic model of
DNA damage repair by non-homologous end joining and
p53/p21-mediated early senescence signalling. PLoS Comput Biol.
11:e10042462015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Celià-Terrassa T, Liu DD, Choudhury A,
Hang X, Wei Y, Zamalloa J, Alfaro-Aco R, Chakrabarti R, Jiang YZ,
Koh BI, et al: Normal and cancerous mammary stem cells evade
interferon-induced constraint through the miR-199a-LCOR axis. Nat
Cell Biol. 19:711–723. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bai X, Fisher DE and Flaherty KT:
Cell-state dynamics and therapeutic resistance in melanoma from the
perspective of MITF and IFNγ pathways. Nat Rev Clin Oncol. Apr
9–2019.(Epub ahead of print) Doi: 10.1038/s41571-019-0204-6.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Braumüller H, Wieder T, Brenner E, Aßmann
S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M,
Griessinger C, et al: T-helper-1-cell cytokines drive cancer into
senescence. Nature. 494:361–365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sato K, Kato A, Sekai M, Hamazaki Y and
Minato N: Physiologic thymic involution underlies age-dependent
accumulation of senescence-associated CD4(+) T cells. J Immunol.
199:138–148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lord CJ and Ashworth A: PARP inhibitors:
Synthetic lethality in the clinic. Science. 355:1152–1158. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Efimova EV, Mauceri HJ, Golden DW, Labay
E, Bindokas VP, Darga TE, Chakraborty C, Barreto-Andrade JC,
Crawley C, Sutton HG, et al: Poly(ADP-ribose) polymerase inhibitor
induces accelerated senescence in irradiated breast cancer cells
and tumors. Cancer Res. 70:6277–6282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Barreto-Andrade JC, Efimova EV, Mauceri
HJ, Beckett MA, Sutton HG, Darga TE, Vokes EE, Posner MC, Kron SJ
and Weichselbaum RR: Response of human prostate cancer cells and
tumors to combining PARP inhibition with ionizing radiati on. Mol
Cancer Ther. 10:1185–1193. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chatterjee P, Choudhary GS, Sharma A,
Singh K, Heston WD, Ciezki J, Klein EA and Almasan A: PARP
inhibition sensitizes to low dose-rate radiation TMPRSS2-ERG fusion
gene-expressing and PTEN-def icient prostate cancer cells. PLoS
One. 8:e604082013. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Azad A, Bukczynska P, Jackson S, Haupt Y,
Cullinane C, McArthur GA and Solomon B: Co-targeting
deoxyribonucleic acid-dependent protein kinase and poly(adenosine
diphosphate-ribose) polymerase-1 promotes accelerated senescence of
irradiated cancer cells. Int J Radiat Oncol Biol Phys. 88:385–394.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Cao F, Ju X, Chen D, Jiang L, Zhu X, Qing
S, Fang F, Shen Y, Jia Z and Zhang H: Phosphorothioatemodified
antisense oligonucleotides against human telomerase reverse
transcriptase sensitize cancer cells to radiotherapy. Mol Med Rep.
16:2089–2094. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Orun O, Tiber PM and Serakinci N: Partial
knockdown of TRF2 increase radiosensitivity of human mesenchymal
stem cells. Int J Biol Macromol. 90:53–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Nam HY, Han MW, Chang HW, Kim SY and Kim
SW: Prolonged autophagy by MTOR inhibitor leads radioresistant
cancer cells into senescence. Autophagy. 9:1631–1632. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Marampon F, Megiorni F, Camero S,
Crescioli C, McDowell HP, Sferra R, Vetuschi A, Pompili S, Ventura
L, De Felice F, et al: HDAC4 and HDAC6 sustain DNA double strand
break repair and stem-like phenotype by promoting radioresistance
in glioblastoma cells. Cancer Lett. 397:1–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Pérès EA, Gérault AN, Samuel V, Roussel S,
Toutain J, Divoux D, Guillamo JS, Sanson M, Bernaudin M and Petit
E: Silencing erythropoietin receptor on glioma cells reinforces
efficacy of temozolomide and X-rays through senescence and mitotic
catastrophe. Oncotarget. 6:2101–2119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ham SW, Jeon HY and Kim H: Verapamil
augments carmustine- and irradiation-induced senescence in glioma
cells by reducing intracellular reactive oxygen species and calcium
ion levels. Tumour Biol. 39:10104283176922442017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang M, Morsbach F, Sander D, Gheorghiu L,
Nanda A, Benes C, Kriegs M, Krause M, Dikomey E, Baumann M, et al:
EGF receptor inhibition radiosensitizes NSCLC cells by inducing
senescence in cells sustaining DNA double-strand breaks. Cancer
Res. 71:6261–6269. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Mirzayans R, Andrais B and Murray D:
Impact of premature senescence on radiosensitivity measured by high
throughput cell-based assays. Int J Mol Sci. 18:E14602017.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Francica P, Aebersold DM and Medova M:
Senescence as biologic endpoint following pharmacological targeting
of receptor tyrosine kinases in cancer. Biochem Pharmacol.
126:1–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Miao GY, Zhou X, Zhang X, Xie Y, Sun C,
Liu Y, Gan L and Zhang H: Telomere-mitochondrion links contribute
to induction of senescence in MCF-7 cells after carbon-ion
irradiation. Asian Pac J Cancer Prev. 17:1993–1998. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ernst A, Anders H, Kapfhammer H, Orth M,
Hennel R, Seidl K, Winssinger N, Belka C, Unkel S and Lauber K:
HSP90 inhibition as a means of radiosensitizing resistant,
aggressive soft tissue sarcomas. Cancer Lett. 365:211–222. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Li P, Hou M, Lou F, Björkholm M and Xu D:
Telomere dysfunction induced by chemotherapeutic agents and
radiation in normal human cells. Int J Biochem Cell Biol.
44:1531–1540. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Maeda T, Nakamura K, Atsumi K, Hirakawa M,
Ueda Y and Makino N: Radiation-associated changes in the length of
telomeres in peripheral leukocytes from inpatients with cancer. Int
J Radiat Biol. 89:106–109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Jinno-Oue A, Shimizu N, Hamada N, Wada S,
Tanaka A, Shinagawa M, Ohtsuki T, Mori T, Saha MN, Hoque AS, et al:
Irradiation with carbon ion beams induces apoptosis, autophagy, and
cellular senescence in a human glioma-derived cell line. Int J
Radiat Oncol Biol Phys. 76:229–241. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Pan J, Li D, Xu Y, Zhang J, Wang Y, Chen
M, Lin S, Huang L, Chung EJ, Citrin DE, et al: Inhibition of
Bcl-2/xl with ABT-263 selectively kills senescent type II
pneumocytes and reverses persistent pulmonary fibrosis induced by
ionizing radiation in mice. Int J Radiat Oncol Biol Phys.
99:353–361. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chung EJ, McKay-Corkum G, Chung S, White
A, Scroggins BT, Mitchell JB, Mulligan-Kehoe MJ and Citrin D:
Truncated plasminogen activator inhibitor-1 protein protects from
pulmonary fibrosis mediated by irradiation in a murine model. Int J
Radiat Oncol Biol Phys. 94:1163–1172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chung EJ, Sowers A, Thetford A,
McKay-Corkum G, Chung SI, Mitchell JB and Citrin DE: Mammalian
target of rapamycin inhibition with rapamycin mitigates
radiation-induced pulmonary fibrosis in a murine model. Int J
Radiat Oncol Biol Phys. 96:857–866. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Shao L, Feng W, Li H, Gardner D, Luo Y,
Wang Y, Liu L, Meng A, Sharpless NE and Zhou D: Total body
irradiation causes long-term mouse BM injury via induction of HSC
premature senescence in an Ink4a- and Arf-independent manner.
Blood. 123:3105–3115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Chang J, Wang Y, Shao L, Laberge RM,
Demaria M, Campisi J, Janakiraman K, Sharpless NE, Ding S, Feng W,
et al: Clearance of senescent cells by ABT263 rejuvenates aged
hematopoietic stem cells in mice. Nat Med. 22:78–83. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lu L, Wang YY, Zhang JL, Li DG and Meng
AM: p38 MAPK inhibitor insufficiently attenuates HSC senescence
administered long-term after 6 Gy total body irradiation in mice.
Int J Mol Sci. 17:E9052016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ness KK, Armstrong GT, Kundu M, Wilson CL,
Tchkonia T and Kirkland JL: Frailty in childhood cancer survivors.
Cancer. 121:1540–1547. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Ariffin H, Azanan MS, Abd Ghafar SS, Oh L,
Lau KH, Thirunavakarasu T, Sedan A, Ibrahim K, Chan A, Chin TF, et
al: Young adult survivors of childhood acute lymphoblastic leukemia
show evidence of chronic inflammation and cellular aging. Cancer.
123:4207–4214. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Artoni F, Kreipke RE, Palmeira O, Dixon C,
Goldberg Z and Ruohola-Baker H: Loss of foxo rescues stem cell
aging in Drosophila germ line. eLife. 6:e278422017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Alessio N, Capasso S, Di Bernardo G,
Cappabianca S, Casale F, Calarco A, Cipollaro M, Peluso G and
Galderisi U: Mesenchymal stromal cells having inactivated RB1
survive following low irradiation and accumulate damaged DNA: Hints
for side effects following radiotherapy. Cell Cycle. 16:251–258.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Xing Y, Zhang J, Lu L, Li D, Wang Y, Huang
S, Li C, Zhang Z, Li J and Meng A: Identification of hub genes of
pneumocyte senescence induced by thoracic irradiation using
weighted gene coexpression network analysis. Mol Med Rep.
13:107–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Alessio N, Esposito G, Galano G, De Rosa
R, Anello P, Peluso G, Tabocchini MA and Galderisi U: Irradiation
of mesenchymal stromal cells with low and high doses of alpha
particles induces senescence and/or apoptosis. J Cell Biochem.
118:2993–3002. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Ramos Silva C, Cabral FV, de Camargo CF,
Núñez SC, Mateus Yoshimura T, de Lima Luna AC, Maria DA and Ribeiro
MS: Exploring the effects of low-level laser therapy on fibroblasts
and tumor cells following gamma radiation exposure. J Biophotonics.
9:1157–1166. 2016. View Article : Google Scholar
|
|
94
|
Hamdi DH, Chevalier F, Groetz JE, Durantel
F, Thuret JY, Mann C and Saintigny Y: Comparable senescence
induction in three-dimensional human cartilage model by exposure to
therapeutic doses of x-rays or C-ions. Int J Radiat Oncol Biol
Phys. 95:139–146. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Baker DJ, Wijshake T, Tchkonia T,
LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL and van
Deursen JM: Clearance of p16Ink4a-positive senescent cells delays
ageing-associated disorders. Nature. 479:232–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Kosmacek EA, Chatterjee A, Tong Q, Lin C
and Oberley-Deegan RE: MnTnBuOE-2-PyP protects normal colorectal
fibroblasts from radiation damage and simultaneously enhances
radio/chemotherapeutic killing of colorectal cancer cells.
Oncotarget. 7:34532–34545. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Beach TA, Johnston CJ, Groves AM, Williams
JP and Finkelstein JN: Radiation induced pulmonary fibrosis as a
model of progressive fibrosis: Contributions of DNA damage,
inflammatory response and cellular senescence genes. Exp Lung Res.
43:134–149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Philipp J, Azimzadeh O, Subramanian V,
Merl-Pham J, Lowe D, Hladik D, Erbeldinger N, Ktitareva S, Fournier
C, Atkinson MJ, et al: Radiation-induced endothelial inflammation
is transferred via the secretome to recipient cells in a
STAT-mediated process. J Proteome Res. 16:3903–3916. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chatterjee A, Kosmacek EA and
Oberley-Deegan RE: MnTE-2-PyP treatment, or NOX4 inhibition,
protects against radiation-induced damage in mouse primary prostate
fibroblasts by inhibiting the TGF-Beta 1 signaling pathway. Radiat
Res. 187:367–381. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Li H, Wang Y, Pazhanisamy SK, Shao L,
Batinic-Haberle I, Meng A and Zhou D: Mn(III)
meso-tetrakis-(N-ethylpyridinium-2-yl) porphyrin mitigates total
body irradiation-induced long-term bone marrow suppression. Free
Radic Biol Med. 51:30–37. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Xu G, Wu H, Zhang J, Li D, Wang Y, Wang Y,
Zhang H, Lu L, Li C, Huang S, et al: Metformin ameliorates ionizing
irradiation-induced long-term hematopoietic stem cell injury in
mice. Free Radic Biol Med. 87:15–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Kaur E, Rajendra J, Jadhav S, Shridhar E,
Goda JS, Moiyadi A and Dutt S: Radiation-induced homotypic cell
fusions of innately resistant glioblastoma cells mediate their
sustained survival and recurrence. Carcinogenesis. 36:685–695.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Roberson RS, Kussick SJ, Vallieres E, Chen
SY and Wu DY: Escape from therapy-induced accelerated cellular
senescence in p53-null lung cancer cells and in huma n lung
cancers. Cancer Res. 65:2795–2803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Chakradeo S, Elmore LW and Gewirtz DA: Is
senescence reversible? Curr Drug Targets. 17:460–466. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Tato-Costa J, Casimiro S, Pacheco T, Pires
R, Fernandes A, Alho I, Pereira P, Costa P, Castelo HB, Ferreira J
and Costa L: Therapy-induced cellular senescence induces
epithelial-to-mesenchymal transition and increases invasiveness in
rectal cancer. Clin Colorectal Cancer. 15:170–178.e3. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Gewirtz DA, Alotaibi M, Yakovlev VA and
Povirk LF: Tumor cell recovery from senescence induced by radiation
with PARP inhibition. Radiat Res. 186:327–332. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Alotaibi M, Sharma K, Saleh T, Povirk LF,
Hendrickson EA and Gewirtz DA: Radiosensitization by PARP
inhibition in DNA repair proficient and deficient tumor cells:
Proliferative recovery in senescent cells. Radiat Res. 185:229–245.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Hellevik T, Pettersen I, Berg V, Winberg
JO, Moe BT, Bartnes K, Paulssen RH, Busund LT, Bremnes R, Chalmers
A and Martinez-Zubiaurre I: Cancer-associated fibroblasts from
human NSCLC survive ablative doses of radiation but their invasive
capacity is reduced. Radiat Oncol. 7:592012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Frame FM, Savoie H, Bryden F, Giuntini F,
Mann VM, Simms MS, Boyle RW and Maitland NJ: Mechanisms of growth
inhibition of primary prostate epithelial cells following gamma
irradiation or photodynamic therapy include senescence, necrosis,
and autophagy, but not apoptosis. Cancer Med. 5:61–73. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Malaquin N, Martinez A and Rodier F:
Keeping the senescence secretome under control: Molecular reins on
the senescence-associated secretory phenotype. Exp Gerontol.
82:39–49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Wiley CD and Campisi J: From ancient
pathways to aging cells-connecting metabolism and cellular
senescence. Cell Metab. 23:1013–1021. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Rodier F and Campisi J: Four faces of
cellular senescence. J Cell Biol. 192:547–556. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Rzeszowska-Wolnyab J and Widel M: Ionizing
radiation-induced bystander effects, potential targets for
modulation of radiotherapy. Eur J Pharmacol. 625:156–164. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Jalal N, Haq S, Anwar N, Nazeer S and
Saeed U: Radiation induced bystander effect and DNA damage. J
Cancer Res Ther. 10:819–833. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Özcan S, Alessio N, Acar MB, Mert E,
Omerli F, Peluso G and Galderisi U: Unbiased analysis of senescence
associated secretory phenotype (SASP) to identify common components
following different genotoxic stresses. Aging (Albany NY).
8:1316–1329. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Mowla SN, Lam EW and Jat PS: Cellular
senescence and aging: The role of B-MYB. Aging Cell. 13:773–779.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Jin X, Li F, Liu B, Zheng X, Li H, Ye F,
Chen W and Li Q: Different mitochondrial fragmentation after
irradiation with X-rays and carbon ions in HeLa cells and its
influence on cellular apoptosis. Biochem Biophys Res Commun.
500:958–965. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Capasso S, Alessio N, Squillaro T, Di
Bernardo G, Melone MA, Cipollaro M, Peluso G and Galderisi U:
Changes in autophagy, proteasome activity and metabolism to
determine a specific signature for acute and chronic senescent
mesenchymal stromal cells. Oncotarget. 6:39457–39468. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Ziegler DV, Wiley CD and Velarde MC:
Mitochondrial effectors of cellular senescence: Beyond the free
radical theory of aging. Aging Cell. 14:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Lafargue A, Degorre C, Corre I,
Alves-Guerra MC, Gaugler MH, Vallette F, Pecqueur C and Paris F:
Ionizing radiation induces long-term senescence in endothelial
cells through mitochondrial respiratory complex II dysfunction and
superoxide generation. Free Radic Biol Med. 108:750–759. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Masaldan S, Clatworthy SAS, Gamell C,
Meggyesy PM, Rigopoulos AT, Haupt S, Haupt Y, Denoyer D, Adlard PA,
Bush AI and Cater MA: Iron accumulation in senescent cells is
coupled with impaired ferritinophagy and inhibition of ferroptosis.
Redox Biol. 14:100–115. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Doll S, Proneth B, Tyurina YY, Panzilius
E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A,
et al: ACSL4 dictates ferroptosis sensitivity by shaping cellular
lipid composition. Nat Chem Biol. 13:91–98. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Li T and Chen ZJ: The cGAS-cGAMP-STING
pathway connects DNA damage to inflammation, senescence, and
cancer. J Exp Med. 215:1287–1299. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Yang H, Wang H, Ren J, Chen Q and Chen ZJ:
cGAS is essential for cellular senescence. Proc Natl Acad Sci USA.
114:E4612–E4620. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Glück S, Guey B, Gulen MF, Wolter K, Kang
TW, Schmacke NA, Bridgeman A, Rehwinkel J, Zender L and Ablasser A:
Innate immune sensing of cytosolic chromatin fragments through cGAS
promotes senescence. Nat Cell Biol. 19:1061–1070. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Sun Y, Coppé JP and Lam EW: Cellular
senescence: The sought or the unwanted? Trends Mol Med. 24:871–885.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Leite de Oliveira R and Bernards R:
Anti-cancer therapy: Senescence is the new black. EMBO J.
37:e993862018. View Article : Google Scholar : PubMed/NCBI
|