Introduction
Circular RNAs (circRNAs) are a novel class of
endogenous noncoding RNAs. circRNAs were long thought to be
artifacts of aberrant RNA splicing, however, the recent development
of next-generation sequencing has led to the discovery of numerous
endogenous circRNAs with unique functions. In fact, circRNAs are
functionally involved in normal processes as well as pathogenic
status (1–3).
Colorectal cancer is a leading cause of
tumor-associated mortality worldwide, and its incidence continues
to gradually increase (4). Recently,
it was reported that a representative circRNA, cerebellar
degeneration-related protein 1 transcript (CDR1-AS), is frequently
upregulated in colorectal cancer (5).
Since CDR1-AS can bind >70 copies of microRNA-7 (miR7) due to
their sequence similarities, CDR1-AS functions as a competing
endogenous RNA (ceRNA) against miR7 (6,7). miR7 can
function as either an oncogenic or tumor-suppressive microRNA
depending on the cellular context, and may exert tumor-suppressive
effects in colorectal cancer (5). In
fact, colon cancer cases with CDR1-AS expression exhibited a poorer
prognosis, and overexpression of CDR1-AS in colon cancer cells
resulted in biological consequences due to functional impairment of
miR7 when it was overexpressed (5).
However, because miR7, which is principally expressed in
neuroendocrine cells (8), is not
always abundant in colon tissues, it was hypothesized that CDR1-AS
may have functions other than the impairment of miR7 that lead to
worse prognosis in colorectal carcinoma.
In the present study, to examine CDR1-AS function in
colorectal cancer cells, the factors that potentially influence
colorectal cancer prognosis due to the overexpression of CDR1-AS
were determined. The present results may provide insights into
novel mechanisms underlying the link between CDR1-AS expression and
poor prognosis in colorectal cancer.
Materials and methods
Cells
The cell line 293T and the human colon carcinoma
cell lines SW620 and Caco2 were purchased from the American Type
Culture Collection and cultured in Dulbecco's Modified Eagle's
medium (DMEM) or L-15 medium (Leibovitz), respectively,
supplemented with 10% fetal bovine serum. Human umbilical vein
endothelial cells (HUVECs) were purchased from PromoCell and
cultured in endothelial cell Growth Medium 2 (PromoCell).
Plasmid production and lentivirus
infection
The CDR1-AS-expressing plasmid,
pcDNA3.1-laccase2-MCS-ciRS7, whose transcript naturally
circularizes due to DNAREP1_DM cassettes, and its control
plasmid were obtained from Addgene, Inc. The lentiviral vector
pCDH-CDR1-AS was constructed by subcloning the corresponding
sequences from pcDNA3.1 into pCDH vector, using the EcoRI
and NotI sites. Lentiviruses produced from the pCDH control
vector were used as a negative control.
To generate stably expressed polyclonal cells, the
Lentivirus Packaging System (System Biosciences) was used according
to the manufacturer's protocol. The viruses were transduced into
SW620 cells and Caco2 cells using polybrene (Santa Cruz
Biotechnology, Inc.), followed by selection with 6 µg/ml puromycin
to obtain polyclonal cells stably expressing CDR1-AS RNA.
RNA extraction and RNase R
treatment
RNA was extracted using ISOGEN II (Nippon Gene Co.,
Ltd.) according to the manufacturer's protocol. After RNA
extraction, 6 µg RNA was treated with RNase R (AR Brown Co., Ltd.)
at 37°C for 15 min, followed by purification using the RNeasy
MinElute Cleanup kit, according to the manufacturer's protocol
(Qiagen).
Northern blotting
Northern blotting was performed as described
previously (9). Briefly, 5 µg of RNAs
were separated in 1% formaldehyde-denatured agarose gel and
hydrostatically transferred to a Hybond N+ membrane (GE
Healthcare Life Sciences). Membranes were UV-crosslinked and
hybridization was performed overnight at 42°C in ULTRAhyb Buffer
(Ambion; Thermo Fisher Scientific, Inc.) containing 10 ng/ml
biotin-labeled RNA probe. The membranes were stringently washed and
bound probe was visualized using a BrightStar BioDetect kit
(Ambion) according to the manufacturer's protocol. The probes for
detecting CDR1-AS were generated using in vitro
transcription MEGAscript T7 kit, and the pre-made probe for β-actin
was obtained using a DIG Northern Starter kit (Roche
Diagnostics).
Cell growth, hanging-drop method, and
cell invasion
The Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.) was used to evaluate 2D cell growth according
to the manufacturer's instructions. Cells were seeded at
1×103 cells/well in 96-well plates. CCK-8 solution was
added at 24-, 48-, 72-, and 96-h time-points. The absorbance at 450
nm was measured using a microplate reader (Thermo Fisher
Scientific, Inc.).
For the 3D cell culture, SW620 cells
(5×104 cells/well) were seeded in hanging droplets in a
volume of 40 µl medium/droplet using the GravityPlus system
(InSphero AG). After 8 days, the spheroids were transferred to a
96-well microtissue receiver plate with a non-adhesive surface
(GravityTRAP; InSphero) in a volume of 70 µl medium/well.
CellTiter-Glo 3D cell reagent (Promega Corp.) was added at 24-,
48-, 72-, and 96-h time-points. Following 30 min of incubation at
room temperature, luminescence was quantified on a GloMax 96
Microplate Luminometer (Promega Corp.).
An invasion assay was performed using a CytoSelect
96-well cell invasion assay kit (Cell Biolabs, Inc.) with
1×105 cells placed in the upper chamber of the assay
plate. Chemoattractant was added to the feeder tray. The assay
plate was cultured for 24 h, and cells that migrated were collected
and quantified using CyQUANT GR dye solution (Cell Biolabs, Inc.).
Invasion values were reported as the mean relative fluorescence
units (RFUs) measured at 480 nm/520 nm using a GloMax Discover
Multimode Microplate Reader (Promega Corp.).
Angiogenesis assay
An Angiogenesis Assay Kit (PromoCell) was used to
evaluate endothelial tube formation according to the manufacturer's
instructions. HUVECs were seeded onto 96-well plates coated with
basement membrane extract at 1.0×104 cells/well. The
culture supernatant of the negative control and the
CDR1-AS-expressing SW620 cells were added to each well, and the
plate was incubated for 6.5 h. The tubular networks were analyzed
using ImageJ software 1.51J8 (National Institutes of Health).
cDNA microarray analyses
cDNA microarray analysis was performed using cDNA
oligo chips (Toray Industries, Inc.). The data and the protocols
were deposited in a public database (GEO; accession no.
GSE125687).
RT-qPCR
Quantitative RT-PCR (qRT-PCR) was performed as
described previously (10). All
values were normalized to the mRNA levels of the β-actin. Relative
expression was calculated according to the ΔΔCq method
as follows: ΔΔCq=ΔCqsample
-ΔCqβ-actin (11). The
primers used were as follows: CDR1-AS forward,
5′-GCTGATCTTCTGACATTCAGG-3′ and reverse,
5′-GAGTTGTTGGAAGACCTTGAC-3′; PD-L1 forward,
5′-GGTGCCGACTACAAGCGAAT-3′ and reverse, 5′-AGCCCTCAGCCTGACATGTC-3′;
CMTM4 forward, 5′-CTGGCGTCTTGCTGATTATG-3′ and reverse,
5′-ATTTCTGCTCCGGCTCTATG-3′; CMTM6 forward,
5′-ATGAAGGCCAGCAGAGACAG-3′ and reverse, 5′-GTGTACAGCCCCACTACGGA-3′;
β-actin forward, 5′-TCCCTGGAGAAGAGCTACGA-3′ and reverse,
5′-AGCACTGTGTTGGCGTACAG-3′.
Transfection, reporter assay, and miR
mimic
pGL4-miR7RE, a luciferase-based reporter construct
to monitor miR7 function, was generated by inserting two tandem
sequences of the miR7 responsive elements
(ACAACAAAATCACTAGTCTTCCAACAACAAAATCACTAGTCTTCCA) into the
FseI site at the 3′UTR of the pGL4.5 luciferase vector
(Promega Corp.). To construct the control mutated miR7 reporter,
the following insertion sequence was used:
ACAACAAAATCACTAGTCAAGGTACAACAAAATCACTAGTCAAGGT, which has mutations
in the seed sequences of the miR7-recognizing sites.
To determine the functional activities of miR7, the
pGL4-miR7RE plasmid, along with a pGL4-TK plasmid-expressing sea
pansy luciferase (Promega Corp.) as an internal control, were
transiently transfected into the cells using FuGENE6 (Promega
Corp.). When adding the miR7 mimic, locked nucleic acid (LNA)-based
miR7 oligonucleotides (Qiagen) were transfected using
oligofectamine (Invitrogen; Thermo Fisher Scientific, Inc.) at the
same time as the reporter transfection. At 48 h after transfection,
dual luciferase assays were performed using a Dual-Luciferase
Reporter Assay System (Promega Corp.) as previously described
(10).
Flow cytometry
Flow cytometric analyses were performed as
previously described (12). Cells
were hybridized with anti-PDL1 (1:1,000; cat. no. PA5-20343; Thermo
Fisher Scientific, Inc.) or the isotype control IgG (1:1,000; cat.
no. 2729; Cell Signaling Technology, Inc.) for 40 min at 4°C. After
washing, the cells were incubated with goat anti-mouse Alexa Fluor
488 (1:1,000; Molecular Probes; Thermo Fisher Scientific, Inc.) for
20 min. Flow cytometry was performed, and the data were analyzed
using Guava Easy Cyte Plus (GE Healthcare Life Sciences). To
quantify the PD-L1-positive cells, cells with fluorescence
intensity exceeding the negative control cells were counted, and
the ratio was calculated.
Mice and xenograft
Experimental protocols were approved by the Ethics
Committee for Animal Experimentation at the University of Tokyo
(approval no. P18-017) and conducted in accordance with the
Guidelines for the Care and Use of Laboratory Animals of the
Department of Medicine, University of Tokyo.
Four 8-week old male BALB/c (nu/nu) nude mice
(weight ~30 g) were purchased from CREA Japan and maintained in
14/10-h light/dark cycle at 25°C. Briefly, 2×106 control
or CDR1-AS cells were suspended in 30 µl of PBS containing 1%
Matrigel (BD Biosciences) and injected subcutaneously into two mice
to establish xenograft models. Mice were placed in standard
conditions (4/cage) under specific pathogen-free conditions in
laminar flow cabinets. All animals received food and water ad
libitum. At 4 weeks post-transplantation, xenograft tissues
were collected for immunohistochemistry analyses.
Immunohistochemistry
Immunohistochemistry was performed as previously
described (13). Tissues were
incubated overnight at 4°C with anti-PDL1 antibody (cat. no.
PA5-20343; Thermo Fisher Scientific, Inc.) diluted with Can Get
Signal Immunostain Immunoreaction Enhancer Solution (Toyobo Life
Science). Signals were enhanced using a VECTASTAIN ABC kit (Vector
Laboratories, Inc.) according to the manufacturer's protocol and
visualized with 3,3′-diaminobenzidine in a buffered substrate
(Nichirei Biosciences, Inc.). To determine the relative intensity
on the cell surface with PD-L1 staining, the average pixel
intensity was determined from at least 20 randomly selected cells
using ImageJ 1.51J8.
miR target site prediction
Putative miRs targeting the 3′untranslated region
(UTR) sequences of CMTM4 and CMTM6 transcripts were determined
using TargetScan 7.2 software (14).
Statistical analyses
Statistically significant differences between two
groups were identified using Student's t-test when the
variances were equal and Welch's t-test when the variances
were unequal. When comparing multiple groups, ANOVA and
Bonferroni post hoc test were used to determine the
statistical significances. P-values <0.05 were considered to
indicate statistical significance.
Results
Establishment of CDR1-AS-expressing
SW620 colon cancer cells
To establish stably-CDR1-AS-expressing colon cancer
cells, laccase cassettes were used to construct lentiviruses
containing CDR1-AS sequences, which efficiently produced circular
RNAs because a pair of inverted DNAREP1_DM family
transposons were located very close to the circularizing exon and
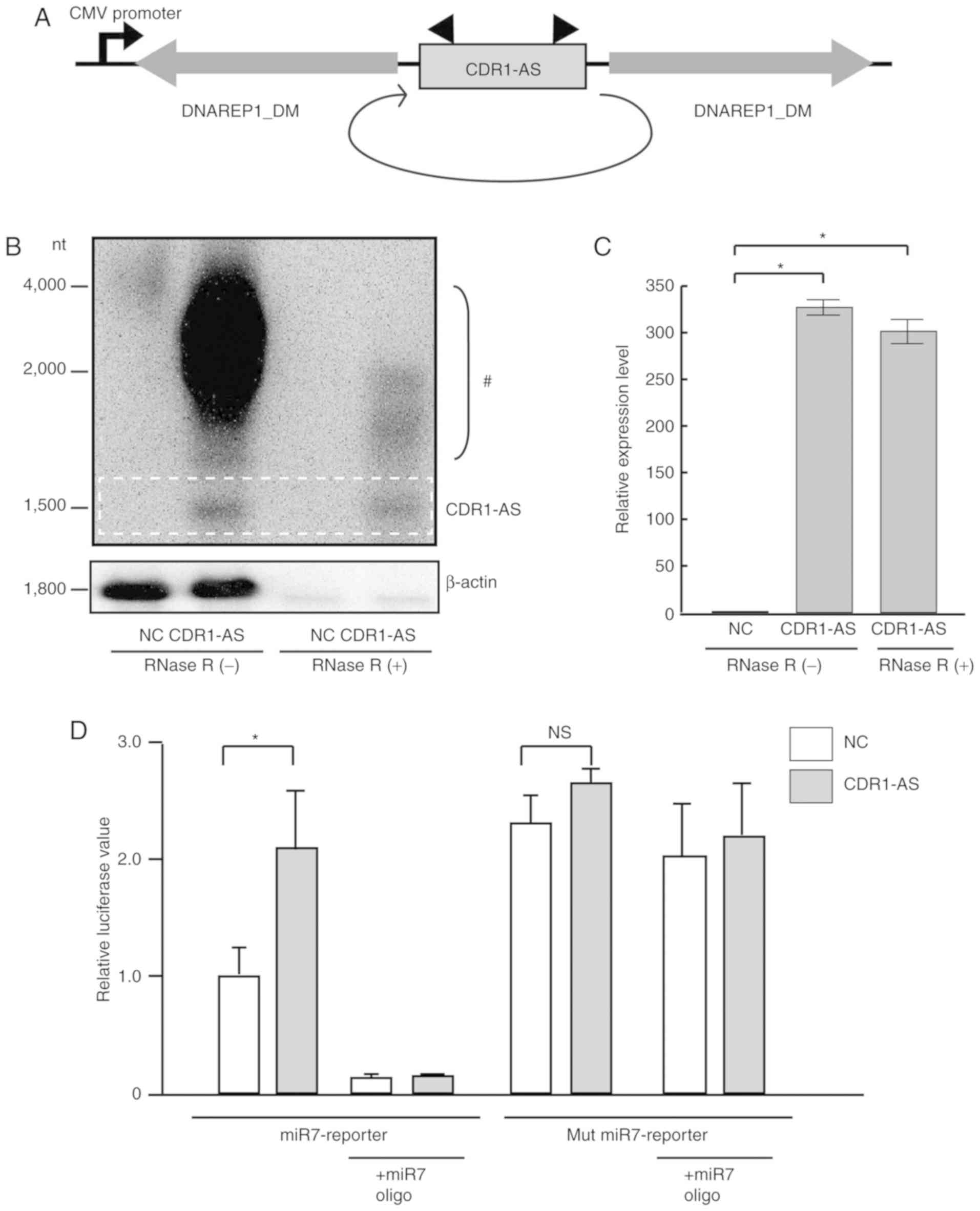
these repeats are highly complementary to each other (15) (Fig. 1A).
The viruses were infected into SW620 cells, a colon cancer cell
line with relatively high miR7 expression (16). To confirm the circularity of the
expressed CDR1-AS RNA, the expression after RNase R treatment,
which digests all linear RNAs but does not digest lariat or
circular RNA structures, was examined by Northern blotting with a
probe against the back-splice junction of the CDR1-AS RNA (Fig. 1B). CDR1-AS was almost the expected
length (1,480 nucleotides) in agarose gel electrophoresis, and the
bands were visible even after RNase R treatment, whereas the bands
corresponding to β-actin completely disappeared, indicating that
CDR1-AS forms a circular RNA resistant to RNase R treatment
(Fig. 1B). The high intensity levels
of bands from the CDR1-AS-expressing constructs (from 2,000 to
4,000 nucleotides) disappeared after RNase R treatment. It was
speculated that these bands corresponded to incompletely
circularized transcripts and/or nicked circular RNAs. As RNase R is
a 3′-5′ exoribonuclease that digests RNAs with 3′ends, completely
circularized CDR1-AS transcripts were unaffected and remained even
after RNase R treatment. RT-PCR determined that CDR1-AS RNA was
expressed at levels ~300 times higher than those in the
non-expressing control cells, and did not significantly diminish
even after RNase R treatment (Fig.
1C). Consistent with a previous study (6), CDR1-AS expression restored miR7 function
as determined by a reporter assay, which was reversed by the forced
expression of miR7-mimic LNA oligonucleotides (Fig. 1D). Although these results confirmed
that CDR1-AS acted as an inhibitor of miR7 function in our system,
endogenous miR7 levels were not prominent in colon cancer cells
(8).
CDR1-AS does not affect the growth or
invasiveness of colon cancer cells
Since CDR1-AS expression is closely linked with poor
prognosis in various cancers, including colon carcinoma (5,17), the
effects of CDR1-AS on the growth and invasiveness of SW620 cells
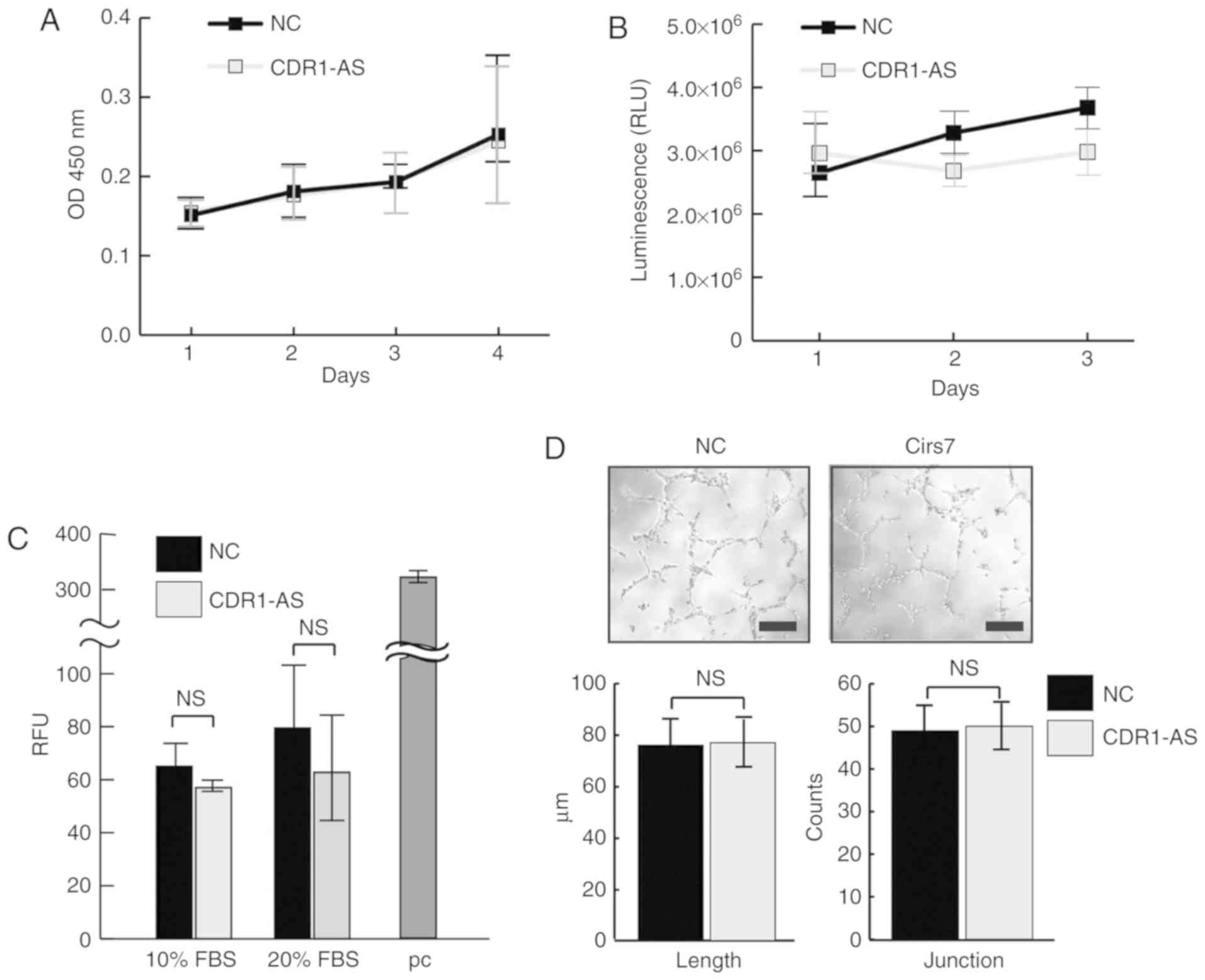
were examined. The growth of SW620 cells in both 2D and 3D cultures
was examined since a 3D culture may render different results from
conventional 2D cultures; however, there was no significant growth
advantage observed in CDR1-AS-expressing SW620 cells (Fig. 2A and B). Furthermore, a Transwell
invasion assay did not reveal any increased invasiveness of
CDR1-AS-expressing SW620 cells (Fig.
2C). Angiogenesis was also examined using supernatant from
CDR1-AS-expressing SW620 cells to determine whether they promote
angiogenesis. However, there were no significant differences in the
length and branch counts in in vitro angiogenesis between
the supernatants from the control and the CDR1-AS-expressing cells
(Fig. 2D). These results appear to
exclude the possibility that angiogenetic factors are produced by
CDR1-AS expression and suggest that, although CDR1-AS expression in
colon cancer cells is clinically linked with poor prognosis, cell
growth, invasiveness, and angiogenesis are unaffected.
CMTM4 and CMTM6 mRNA levels are
increased in CDR1-AS-expressing SW620 cells
To gain insight into the biological causes of the
poor prognosis of colon cancers with CDR1-AS expression, changes in
transcript expression levels in CDR1-AS-expressing SW620 cells (the
cDNA microarray results were deposited in GEO; accession no.
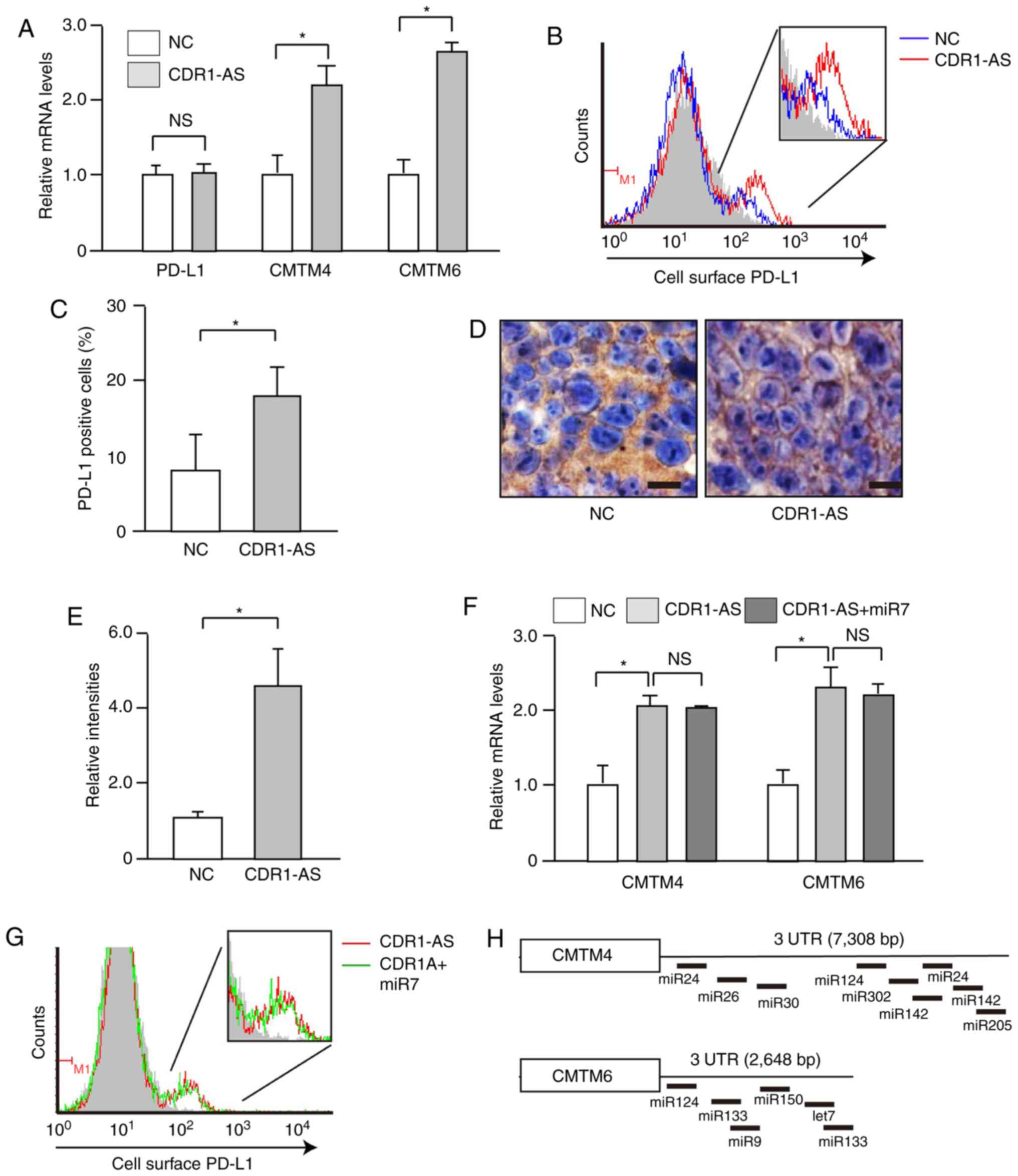
GSE125687) were assessed. The simultaneous increases in CMTM4 and
CMTM6 transcript levels were noteworthy since these genes were
recently reported as stabilizers of PD-L1 protein at the cell
surface (18,19). The PD-L1 ligand plays a crucial role
in suppressing tumor-specific T-cell responses, and its
upregulation on the surface of cancer cells is linked to enhanced
inhibition of T cells and thus, poor prognosis (20). The upregulation of CMTM4 and CMTM6
transcript levels using RT-PCR was confirmed (Fig. 3A). Accordingly, cell surface PD-L1
levels, which were expressed only in a subpopulation, were
upregulated in CDR1-AS-expressing SW620 cells (Fig. 3B and C), although PD-L1 mRNA levels
were unchanged (Fig. 3A). The
increased expression of PD-L1 on the surface of CDR1-AS-expressing
cells was also detected in xenograft models established using SW620
cells (Fig. 3D and E), although
tumour growth/size was not analysed in the present study in the
presence/absence of CDR1AS expression due to inconsistencies in the
growth rates rendering the appropriate analysis difficult.
Similarly, in Caco2 cells, another colon cancer cell line, forced
expression of CDR1-AS RNA (Fig. S1A)
induced significantly increased expression of CMTM transcripts
(Fig. S1B) and PD-L1 protein on the
cell surface (Fig. S1C and D). These
results inidcated that CDR1-AS expression leads to an increase in
CMTM4 and CMTM6 expression levels, resulting in an increase in cell
surface PD-L1 levels.
Increased cell surface PD-L1
expression in CDR1-AS-expressing cells is not dependent on miR7
function
Since CDR1-AS functionally antagonizes miR7 due to
sequence similarities (Fig. 1D)
(6,7),
miR7 mimic LNA oligonucleotides were ectopically expressed in
CDR1-AS-expressing cells to examine the involvement of deregulated
miR7 function in the upregulated cell surface PD-L1 expression
levels. However, the increased CMTM4 and CMTM6 transcript levels
(Fig. 3F) and the cell surface PD-L1
protein levels (Fig. 3G), which were
expressed in a subpopulation of the cells, were not reduced by
forced expression of miR7 mimic LNA oligonucleotides. These results
were consistent with the fact that the 3′UTRs of CMTM4 and CMTM6 do
not share sequence similarities with miR7 (Fig. 3H). Thus, the upregulated expression
levels of CMTM4 and CMTM6 in CDR1-AS-expressing cells do not appear
to be dependent on impaired miR7 function, but likely depend on
other undefined factors induced by CDR1-AS expression.
Discussion
In the present study, it was revealed that the
expression of circular RNA CDR1-AS in colon cancer cells induces
the upregulation of CMTM6 expression levels, which may be linked
with increased PD-L1 protein expression on the cell surface. This
may underlie the poor prognosis in colon cancer cases with CDR1-AS
expression.
CDR1-AS is frequently overexpressed in colon cancer
tissues compared with normal tissues. High CDR1-AS expression
levels have been revealed to be correlated with poor survival in
patients (5,21). In the present study, unexpectedly,
cell growth, invasion ability, and vascular growth did not increase
according to CDR1-AS expression, although these are often reported
as results of impaired miR7 function by CDR1-AS (22). This may be due to differences in the
balance between miR7 expression levels and the expression levels of
CDR1-AS in the cells. Instead, it was revealed that cell surface
expression levels of PD-L1 protein were upregulated in
CDR1-AS-expressing colon cancer cells. Sicne PD-L1 expression on
tumor cells is associated with poor prognosis in patients with
colorectal cancer, possibly through T-cell inhibition by cell
surface PD-L1 expression in cancer cells (20), this may account for the poor prognosis
of colon cancer patients with high CDR1-AS expression levels.
CMTM6/4 were recently identified as regulators of
PD-L1 stability at the cell surface (18,19).
Although CDR1-AS is recognized to antagonize miR7 function
(6), the 3′UTR sequences of CMTM6 and
CMTM4 do not contain any similar sequences complementary to miR7.
Additionally, overexpression of miR7 in CDR1-AS-expressing cells
did not decrease the expression levels of CMTM6 or CMTM4 mRNA.
Although the mechanisms underlying the regulation of the expression
of CMTM6 or CMTM4 remain unknown, the present results indicated
that the upregulation of CMTM6 and CMTM4 by CDR1-AS expression may
indirectly affect expression by modulating the function or
expression of transcription factors required for CMTM6 and CMTM4
expression.
To further confirm the involvement of CMTM6 and
CMTM4 in the changes of PD-L1 expression levels by CDR1-AS,
blocking experiments of CMTM6 and CMTM4 were attempted in
CDR1-AS-expressing cells, however, solid data could not be obtained
because it was difficult to control the knockdown levels of CMTM4
and CMTM6. Thus, these data were omitted from this study. Several
studies have reported that CMTM4 and CMTM6 are involved in the cell
surface expression of PD-LI (18,19). The
decreased PD-L1 expression in CMTM4 and CMTM6-knockdown cells was
also observed, which suggested the involvement of these proteins in
PD-L1 expression.
Since CDR1-AS expression is closely linked with poor
prognosis in various cancers (5,22), our
findings may be generally applied to other cancers in addition to
colon cancer. Although the mechanism by which CDR1-AS expression is
upregulated in some cancers is unknown, interventional methods
against CDR1-AS as a non-coding RNA may enhance the effectiveness
of current PD-L1-PD1 blocking therapies. In summary, a link between
the expression of a circular RNA and its onco-immunological
function was identified, which explains why some cancer patients
with high CDR1-AS expression have a poor prognosis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Grants-in-Aid
from the Ministry of Education, Culture, Sports, Science and
Technology, Japan (nos. 16H05149, 16KT0109 and 17K15923) (to MO and
TK), by the Grant-in-Aid for Scientific Research on Innovative
Areas (no. 18H05024 to MO), by The Yasuda Medical Foundation (to
MO), and by the Project for Cancer Research And Therapeutic
Evolution (P-CREATE) from Japan Agency for Medical Research and
Development (AMED) (to MO, no. JP18cm0106602).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
ET and MO planned the research and wrote the
manuscript. ET, YM, TK, TSe, TI, KF, NO, KS, MY, and TSu performed
majority of the experiments. RI and MO analyzed the data. KK
supervised the entire project and wrote the manuscript. All authors
read and approved the final version to be published and agree to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Experimental protocols were approved by the Ethics
Committee for Animal Experimentation at the University of Tokyo
(Approval no. P18-017) and conducted in accordance with the
Guidelines for the Care and Use of Laboratory Animals of the
Department of Medicine, University of Tokyo.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
circRNAs
|
circular RNAs
|
|
CDR1-AS
|
cerebellar degeneration-related
protein 1 transcript
|
|
miR
|
microRNA
|
References
|
1
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ebbesen KK, Hansen TB and Kjems J:
Insights into circular RNA biology. RNA Biol. 14:1035–1045. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kristensen LS, Hansen TB, Venø MT and
Kjems J: Circular RNAs in cancer: Opportunities and challenges in
the field. Oncogene. 37:555–565. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weng W, Wei Q, Toden S, Yoshida K,
Nagasaka T, Fujiwara T, Cai S, Qin H, Ma Y and Goel A: Circular RNA
ciRS-7-A promising prognostic biomarker and a potential therapeutic
target in colorectal cancer. Clin Cancer Res. 23:3918–3928. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Memczak S, Jens M, Elefsinioti A, Torti F,
Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer
M, et al: Circular RNAs are a large class of animal RNAs with
regulatory potency. Nature. 495:333–338. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Horsham JL, Ganda C, Kalinowski FC, Brown
RA, Epis MR and Leedman PJ: MicroRNA-7: A miRNA with expanding
roles in development and disease. Int J Biochem Cell Biol.
69:215–224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kishikawa T, Otsuka M, Yoshikawa T, Ohno
M, Yamamoto K, Yamamoto N, Kotani A and Koike K: Quantitation of
circulating satellite RNAs in pancreatic cancer patients. JCI
Insight. 1:e866462016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamagami M, Otsuka M, Kishikawa T, Sekiba
K, Seimiya T, Tanaka E, Suzuki T, Ishibashi R, Ohno M and Koike K:
ISGF3 with reduced phosphorylation is associated with constitutive
expression of interferon-induced genes in aging cells. NPJ Aging
Mech Dis. 4:112018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sekiba K, Yamagami M, Otsuka M, Suzuki T,
Kishikawa T, Ishibashi R, Ohno M, Sato M and Koike K:
Transcriptional activation of the MICA gene with an engineered
CRISPR-Cas9 system. Biochem Biophys Res Commun. 486:521–525. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshikawa T, Wu J, Otsuka M, Kishikawa T,
Suzuki N, Takata A, Ohno M, Ishibashi R, Yamagami M, Nakagawa R, et
al: Repression of MicroRNA function mediates
inflammation-associated colon tumorigenesis. Gastroenterology.
152:631–643. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:2015. View Article : Google Scholar
|
|
15
|
Kramer MC, Liang D, Tatomer DC, Gold B,
March ZM, Cherry S and Wilusz JE: Combinatorial control of
Drosophila circular RNA expression by intronic repeats, hnRNPs, and
SR proteins. Genes Dev. 29:2168–2182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang N, Li X, Wu CW, Cai M, Mok MT, Wang
H, Chen J, Ng SS, Chen M, Sung JJ and Yu J: microRNA-7 is a novel
inhibitor of YY1 contributing to colorectal tumorigenesis.
Oncogene. 32:5078–5088. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng L, Yuan XQ and Li GC: The emerging
landscape of circular RNA ciRS-7 in cancer (Review). Oncol Rep.
33:2669–2674. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mezzadra R, Sun C, Jae LT, Gomez-Eerland
R, de Vries E, Wu W, Logtenberg MEW, Slagter M, Rozeman EA, Hofland
I, et al: Identification of CMTM6 and CMTM4 as PD-L1 protein
regulators. Nature. 549:106–110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Burr ML, Sparbier CE, Chan YC, Williamson
JC, Woods K, Beavis PA, Lam EYN, Henderson MA, Bell CC, Stolzenburg
S, et al: CMTM6 maintains the expression of PD-L1 and regulates
anti-tumour immunity. Nature. 549:101–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen DS, Irving BA and Hodi FS: Molecular
pathways: Next-generation immunotherapy-inhibiting programmed
death-ligand 1 and programmed death-1. Clin Cancer Res.
18:6580–6587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tang W, Ji M, He G, Yang L, Niu Z, Jian M,
Wei Y, Ren L and Xu J: Silencing CDR1as inhibits colorectal cancer
progression through regulating microRNA-7. Onco Targets Ther.
10:2045–2056. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang J, Hu H and Zhao Y and Zhao Y:
CDR1as is overexpressed in laryngeal squamous cell carcinoma to
promote the tumour's progression via miR-7 signals. Cell Prolif.
51:e125212018. View Article : Google Scholar : PubMed/NCBI
|