Introduction
Gallbladder cancer (GBC), a lethal aggressive
malignant neoplasm, is the most common malignancy of the biliary
tract and the leading cause of cancer-related mortalities in
western countries and China (1–5). The
majority of patients with GBC are diagnosed at an advanced stage
due to early occult symptoms, and treating GBC is associated with
many problems, not only due to the poor results of surgical
resection for the disease, but also due to its insensitivity to
chemo-radiotherapy; thus, the prognosis of the patients is still
very poor (5–7). Therefore, it is necessary to explore the
prognostic indicators and drug targets of GBC, and provide more
effective and individualized treatments for patients with GBC.
Cancer-associated fibroblasts (CAFs) are key
cellular components in tumor stroma and the most primary stromal
cells of the tumor microenvironment (TME). Recent studies have
demonstrated the critical role of CAFs in cancer stroma for
tumorigenesis, development and the targeted therapy of cancers
(8–10). In addition, tumor treatment
effectiveness is complicated by the presence of reactive stroma in
the TME, which is associated with tumor invasiveness and drug
resistance (10–13). CAFs have their own gene expression
profiles that are different from normal fibroblasts (NFs), and they
interact with cancer cells via a variety of signals in a paracrine
or autocrine manner to affect the TME, determine cancer cell
growth, invasion, metastasis, angiogenesis and therapeutic
tolerance (14–17), and predict the poor prognosis of
patients (18,19). In addition, anti-CAFs can effectively
prevent tumor progression before tumor invasion. In pancreatic
cancer, as well as in other cancer treatments, inhibiting CAFs can
prolong the survival of patients compared with chemotherapy alone
(20–23). However, little is known about the
relationship between the complex components of CAFs and the
prognosis of patients with tumors, and there is a lack of studies
on related molecular mechanisms. In order to predict the poor
prognosis of patients and to tailor treatments more effectively to
the individual patient, it is important to clearly define the tumor
stroma, particularly CAFs, at a molecular level, which will enable
researchers to identify biomarkers that will more accurately
predict patient prognosis and responsiveness to treatments.
Nicotinamide adenine dinucleotide phosphate (NADPH)
oxidase 1 (NOX1) is a member of the NADPH oxidase family. The main
biological function of NOX1 and other NOX family proteins is to
produce reactive oxygen species (ROS) (24). ROS are oxygen-derived small molecules
that are oxidizing factors and can be easily converted into free
radicals (25,26). NOX-dependent ROS regulation
abnormalities are thought to be closely associated with tumor
development (27,28). NOX1 has been reported to be highly
expressed in a variety of tumor types including gastric (29–31) and
liver cancer (32), and is associated
with poor prognosis. However, tumor stromal NOX1 expression and its
relationship with the prognosis of tumor patients have not been
reported. In the present study, the relationship between NOX1
expression in GCAFs and the prognosis of GBC patients was
investigated. The results revealed that NOX1 expression was
significantly upregulated in the stroma of GBCs, and this
observation was confirmed by GBC specimens expressing the
interstitial marker α-smooth muscle actin (SMA) and fibroblast
secreted protein (FSP)-1 positive in vivo and by GCAF and
GBC-SD+GCAF co-culture at the mRNA and protein levels in
vitro. GBC patients with upregulated stromal NOX1 expression
had a lower survival rate. Thus, it was concluded that upregulated
NOX1 expression in GCAFs may predict an unfavorable prognosis. This
may contribute to the discovery of novel tumor markers and
potential targeted therapeutics for human GBCs.
Materials and methods
Patients and clinical specimens
The present study was conducted in accordance with
ethical standards, the Declaration of Helsinki and the official
recommendations of the Chinese Community Guidelines, and was
approved by the Ethics Committee and the Institutional Review Board
of Tongji Hospital. Written informed consent was obtained from all
patients. A total of 81 cases of paraffin-embedded gallbladder
tissue specimens, including 65 GBC specimens, 8 gallbladder
precancerous lesion (GBPL; adenoma and severe dysplasia) specimens
and 8 gallbladder benign lesion (GBBL; cholecystitis) specimens
were collected. All GBC patients underwent surgery from January
2007 to September 2012 at Tongji Hospital, Tongji University School
of Medicine, were histopathologically diagnosed and did not receive
chemotherapy or radiotherapy before surgery. To avoid the direct
impact of surgery, patients who died within a month after surgical
resection were excluded. Two independent pathologists who were
unaware of the clinical status of patients validated the diagnosis
of these GBC samples. Detailed clinicopathological and follow-up
data were collected from the medical records of patients at Tongji
Hospital, and completed by telephone survey. The median follow-up
time was 19.9 (range, 1–62) months for all GBC patients. The 5-year
overall survival (OS) rate was 12.3% (8/65). The demographic and
clinicopathological data for a total of 65 GBC patients are
summarized in Table I.
 | Table I.Demographic and clinicopathological
parameters of GBC patients included in the present study. |
Table I.
Demographic and clinicopathological
parameters of GBC patients included in the present study.
| Demographic and
clinicopathological parameters | Patients with GBC
[n (%)] |
|---|
| No. of
patients | 65 |
| Sex |
|
Male | 25 (38.5) |
|
Female | 40 (61.5) |
| Age (years) |
|
>65 | 34 (52.3) |
|
≤65 | 31 (47.7) |
| Tumor size
(cm) |
|
>3 | 27 (41.5) |
| ≤3 | 38 (58.5) |
| Tumor location |
|
Bottom | 26 (40) |
|
Corporis and others | 39 (60) |
| Pathological
diagnosis |
|
Adenocarcinoma | 61 (93.8) |
|
Othersa | 4 (6.2) |
| Differentiation
degree |
| G1
(high) | 11 (16.9) |
| G2
(moderate) | 29 (44.6) |
| G3
(poor) | 25 (38.5) |
| Nevin stage |
|
S1-S2 | 8 (12.3) |
|
S3-S5 | 57 (87.7) |
| Lymph node
metastasis |
|
(−) | 21 (32.3) |
|
(+) | 44 (67.7) |
| Liver
infiltration |
|
(−) | 34 (52.3) |
|
(+) | 31 (47.7) |
| Venous
invasion |
|
(−) | 36 (55.4) |
|
(+) | 29 (44.6) |
| Curability |
| R0 | 32 (49.2) |
| R1,
R2 | 33 (50.8) |
Immunohistochemistry (IHC) in
vivo
IHC staining was used to detect the expression of
NOX1 protein in the stroma of different gallbladder tissue
specimens. After deparaffinization and inactivating endogenous
peroxide, sections (4 µm) were pretreated using bovine serum
albumin V working solution (cat. no. A8020; Beijing Solarbio
Science & Technology Co., Ltd.), and then incubated with the
primary anti-rabbit antibody against NOX1 (1:500; cat. no. GTX
103888; GeneTech), followed by the secondary antibody
immunoglobulin (Ig; H+L; 1:200; cat. no. 074-1506; KPL) and DAB
(cat. no. K346711; Dako; Agilent Technologies, Inc.) solution, and
were counterstained with hematoxylin according to the
manufacturer's instructions. A negative control was conducted by
replacing the primary antibody with PBS (Gibco; Thermo Fisher
Scientific, Inc.) in all samples. Known immunoassay-positive colon
cancer sections were used as the positive controls.
The positive expression of NOX1 protein was
localized in the cytoplasm, nucleus and stroma. In the present
study, only the expression of NOX1 was observed in the stroma of
gallbladder tissues. To analyze biomarker expression in stromal
tissues, the present study modified the methods described in
previous studies (33–35). The staining of NOX1 in cancer tissues
was evaluated in five randomly selected high-power fields, which
were considered to represent the average value of a tumor at ×200
magnification. Images were first imported into Adobe Photoshop. Hue
and saturation of the images were normalized using Auto-Contrast.
Tumor epithelium was distinguished from the stroma by differences
in nuclear and cellular morphology, and tissue architecture. Using
the lasso tool, epithelial tissues were selected and cropped out
from the image, leaving the stromal tissues behind. These stromal
tissues were labeled as ‘total stromal area’. DAB chromogen
staining (brown) was selected using the Magic Wand Tool in the
Color Range Window. The selected pixels were copied and pasted into
a new window and saved as a separate file. Then, NOX1 positive
staining in ‘total stromal area’ was scored using mean optical
density (MOD). The MOD formula was as follows: Integrated optical
density/(960×1,280, pixel value). Based on the results of stroma
positive staining, a MOD value of 0.138 was used to distinguish low
(MOD <0.138) and high (MOD ≥0.138) NOX1 expression. Two
independent researchers blinded to the clinicopathological
parameters and patient outcome scored all gallbladder tissue
samples. Experienced pathologists assessed any inconsistencies.
Co-immunofluorescence (CIF) in
vivo
CIF staining was used to verify the expression of
NOX1 protein in the stroma of GBC specimens using stromal markers
such as α-SMA and FSP-1. After deparaffinizing and pretreating
specimens as aforementioned for IHC, sections (4 µm) from GBC
tissues were permeabilized in PBS containing 10% methanol for 30
min, washed in PBS and blocked for 1 h with PBS containing 3% FBS
(Hangzhou Sijiqing Biological Engineering Materials Co., Ltd.).
Mouse IgG was blocked using the M.O.M kit (cat. no. BMK-2202;
Vector Laboratories, Inc.) according to the manufacturer's
protocol. For CIF staining of NOX1 and α-SMA, or NOX1 and FSP-1,
sections were respectively incubated with rabbit anti-NOX1 (1:500;
cat. no. gtx103888; GeneTex) and mouse anti-α-SMA (1:200; cat. no.
ab7817; Abcam), or rabbit anti-NOX1 (1:500) and mouse anti-FSP-1
(1:100; cat. no. ab93283; Abcam) at 4°C overnight; then incubated
with the corresponding secondary antibody, goat anti-rabbit IgG
(1:1,000; cat. no. ab6717; Abcam) to detect NOX1 expression, goat
anti-mouse IgG (1:200; cat. no. A32727; Thermo Fisher Scientific,
Inc.) to detect α-SMA or FSP-1 expression. Finally, sections were
stained with DAPI for 5 min, and observed at ×200 magnification
using an immunofluorescence microscopy.
Cell lines and cultures
The human gallbladder cancer cell line GBC-SD
(Shanghai Cell Biology Research Institute of Chinese Academy of
Sciences) was maintained and propagated in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS and
105 U·ml−1 penicillin and streptomycin
(Shanghai Pharmaceutical Works) in an incubator (Forma series II
HEPA Class 100; Thermo Fisher Scientific, Inc.) at 37°C with a 5%
CO2 atmosphere. Human GCAFs and NFs were isolated from
the clinical specimens of human GBC tissues and adjacent normal
tissues, and identified by the detection of the stromal markers
α-SMA and fibroblast activation protein (FAP) using IHC,
immunofluorescence and western blotting. Established GCAFs and NFs
were incubated in DMEM/F-12 medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS in an incubator (Thermo
Fisher Scientific, Inc.) at 37°C with a 5% CO2
atmosphere. The cells used in the experiment were between the 4th
and 9th generations.
Co-cultures of GBC-SD cells and GCAFs or NFs were
performed by coating 96-well U-bottom plates (cat. no. 3799;
Corning Incorporated) with poly-2-hydroxyethyl methacrylate
(poly-Hema; Polysciences Europe GmbH). In poly-Hema coated 96-well
plates, 2.5×105 GBC-SD cells were seeded per well as the
mono-culture, and 1×105 GBC-SD cells and
1.5×105 GCAFs or NFs per well for co-cultures.
Monocultures and co-cultures were incubated for 7 days in an
incubator at 37°C with a 5% CO2 atmosphere until
spheroid formation.
Affymetrix chip analysis of gene
expression profile and reverse transcription-quantitative (RT-q)PCR
in GCAFs/NFs in vitro
To further verify the expression of NOX1 protein in
the stroma of GBCs, especially in CAFs, the present study analyzed
the gene expression profile in GCAFs/NFs using Affymetrix chip
analysis and detected the different expression levels of NOX1 at
the mRNA level in GCAFs/NFs using RT-qPCR in vitro.
Affymetrix chip analysis in vitro was
performed using Affymetrix GeneChip Human 1.0ST array (Affymetrix;
Thermo Fisher Scientific, Inc.). Briefly, total RNA was extracted
in triplicate from GCAFs/NFs. After total RNA quality detection,
RNA RT and in vitro transcription (IVT) of cRNA were
performed by adding 130 µl of the IVT Master Mix using a GeneChip
3′IVT PLUS Kit (Affymetrix; Thermo Fisher Scientific, Inc.) to 130
µl of double-stranded cRNA. The generated cRNA was then
synthesized, purified and labeled. Finally, after being hybridized
and washed using a GeneChip Hybridization Wash and Stain kit
(Affymetrix; Thermo Fisher Scientific, Inc.), arrays were scanned
for differentially expressed genes between GCAFs/NFs using a
Genechip Array scanner 3000 (Affymetrix; Thermo Fisher Scientific,
Inc.). Array data were normalized using log scale robust
multi-array analysis and were analyzed by R-Project software. Gene
expression was deemed significant if the fold change (FC) value was
>1.5 or <0.67, and P<0.05. Gene Ontology (GO) analysis was
used to perform functional enrichment analysis. For statistical
analysis of GO, gene set enrichment analysis and Fisher exact
analysis were performed. For the study of gene expression profile
variance between GCAFs and NFs, potentially related up- or
downregulated genes involved in biological processes were selected
for verification.
For RT-qPCR, total RNA from GCAFs or NFs was
prepared using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). The concentration of RNA was determined by
absorption at 260–280 nm. PCR amplifications were performed with
gene-specific primers with annealing temperatures and the number of
amplification cycles optimized using cDNA in each group. The
primers for NOX1 and GAPDH were as follows: NOX1,
5′-ACCTCTTGACAATGGGAAAC-3′ (sense) and 5′-CTCCACTGTCGTGTTTCG-3′
(antisense); and GAPDH, 5′-CTCCTCCTGTTCGACAGTCA-3′ (sense) and
5′-GCTCCGCCCAGATACCATT-3′ (antisense). PCR amplification reactions
were performed as follows: 94°C for 3 min, followed by 40 cycles of
95°C for 15 sec, 60°C for 30 sec, 72°C for 30 sec, and 82–86°C
(fluorescence collection) for 5–10 sec, and finally 72–99°C for 5
min. GAPDH primers were used as the control for PCR amplification.
PCR products (10 µl) were placed onto 15 g·l-1 agarose gels and
observed by ethidium bromide (Huamei Bioengineering Co., Ltd.)
staining using ABI Prism 7300 SDS software (Bio-Rad Laboratories,
Hercules, CA, USA). Data were analyzed using the ΔΔcq method
(36).
IHC and western blotting in
co-cultures of GBC-SD cells/GCAFs or NFs in vitro
To verify the elevated NOX1 expression in GCAFs, the
present study further detected the altered expression of NOX1 at
the protein level in co-cultures of GBC-SD cells/GCAFs or NFs using
IHC and western blotting in vitro.
Co-culture spheroids of one 96-well plate were
harvested, washed once in PBS and fixed in 4% paraformaldehyde in
PBS for 1 h at room temperature. After spheroid embedding in 1%
agarose with PBS and a dehydration series, spheroids were embedded
in paraffin. Sections (1.5 µm) were incubated with primary
anti-rabbit antibody against NOX1, then the secondary antibody Ig
(H+L) and finally with DAB solution according to the aforementioned
steps used for IHC, and observed under an optical microscope
(Olympus CH-2; Olympus Corporation). Negative controls were
established by replacing the primary antibody with PBS in all
samples. Ten sample slides (10 visual fields per slide) in each
group were selected for analysis.
For western blotting, co-culture spheroids of one
96-well plate were harvested and washed once in PBS. Cells were
lysed, the supernatant was recovered and BCA protein was determined
with a protein quantitative kit (KangChen KC-430; Kangchen BioTech,
Co., Ltd.). Then, an aliquot of 20 µg of proteins was subjected to
SDS-PAGE (10%) under reducing conditions, and proteins were
transferred to a PVDF membrane. The membrane was incubated with the
NOX1 primary anti-rabbit antibody (1:3,000; cat. no. GTX 103888;
GeneTech) and mouse anti-human GAPDH antibody (1:10,000; cat. no.
KC-5G4; KangChen Biotech), followed by an appropriate anti-mouse
horseradish peroxidase-labeled secondary antibody (1:5,000; cat.
no. KC-MM-035; Kangchen BioTech Co., Ltd.). The target proteins
were visualized using an enhanced chemiluminescent reagent (KC™
Chemiluminescent kit; Kangchen BioTech Co., Ltd.), and imaged on a
Bio-Rad chemiluminescence imager. The gray value and gray
coefficient ratio of every protein were analyzed and calculated
with ImageJ 1.37v analysis software (National Institutes of
Health).
Statistical analysis
All statistical analyses were conducted using SPSS
22.0 software (IBM Corp.). Data are expressed as the mean ±
standard deviation (SD). Student's t-test was used to analyze
independent samples. The χ2 test was used to analyze the
relationship between NOX1 expression and clinicopathological
characteristics. The Kaplan-Meier method and the log-rank test were
used for survival analysis. Single variables and multivariate
analysis of prognostic factors were performed using the Cox's
regression model. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of NOX1 protein is
upregulated in GBC stroma with positive α-SMA and FSP-1 expression,
and elevated NOX1 expression in the stroma of GBC predicts poor
prognosis
To determine the significance of NOX1 expression in
the stroma of GBCs, the present study analyzed the expression of
NOX1 at the protein level in the stroma of GBCs. Using IHC, the
present study first analyzed the expression of NOX1 protein in
different types of gallbladder tissues from GBBLs, GBPLs and GBCs.
As revealed in Fig. 1, NOX1 protein
was positively expressed (brown staining) in the tumor epithelium
and in the stroma of different gallbladder tissues (Fig. 1A). The MOD value of NOX1 in the stroma
of GBCs was significantly higher than that observed in the stroma
of GBPLs (0.138±0.030 vs. 0.110±0.025, P=0.028) or GBBLs
(0.138±0.030 vs. 0.109±0.019, P=0.021); the MOD value of NOX1 in
the GBPL stroma was higher than in the GBBL stroma, but the
difference was not significant (0.110±0.025 vs. 0.109±0.019,
P=0.997; Fig. 1B). Overall, these
results indicated that NOX1 expression was upregulated in the
stroma of GBCs compared with the stroma of GBPLs and GBBLs.
Furthermore, to determine whether NOX1 protein was expressed in the
stroma of GBCs, the present study performed CIF staining for the
expression of NOX1 in GBC stroma tissues with α-SMA and FSP-1. It
was observed that the expression of NOX1 protein was positive not
only in the tumor epithelium but also in the stroma of GBCs,
consistent with DAB expression patterns. In addition, NOX1
overlapped with both α-SMA and FSP-1-expressing cells, and
co-localized with α-SMA and FSP-1 positive stroma (Fig. 2), presenting NOX1 expression in both
α-SMA and FSP-1-positive fibroblasts in the stroma of GBCs. Thus,
it is considered that the expression of NOX1 protein is upregulated
in the stroma of GBCs.
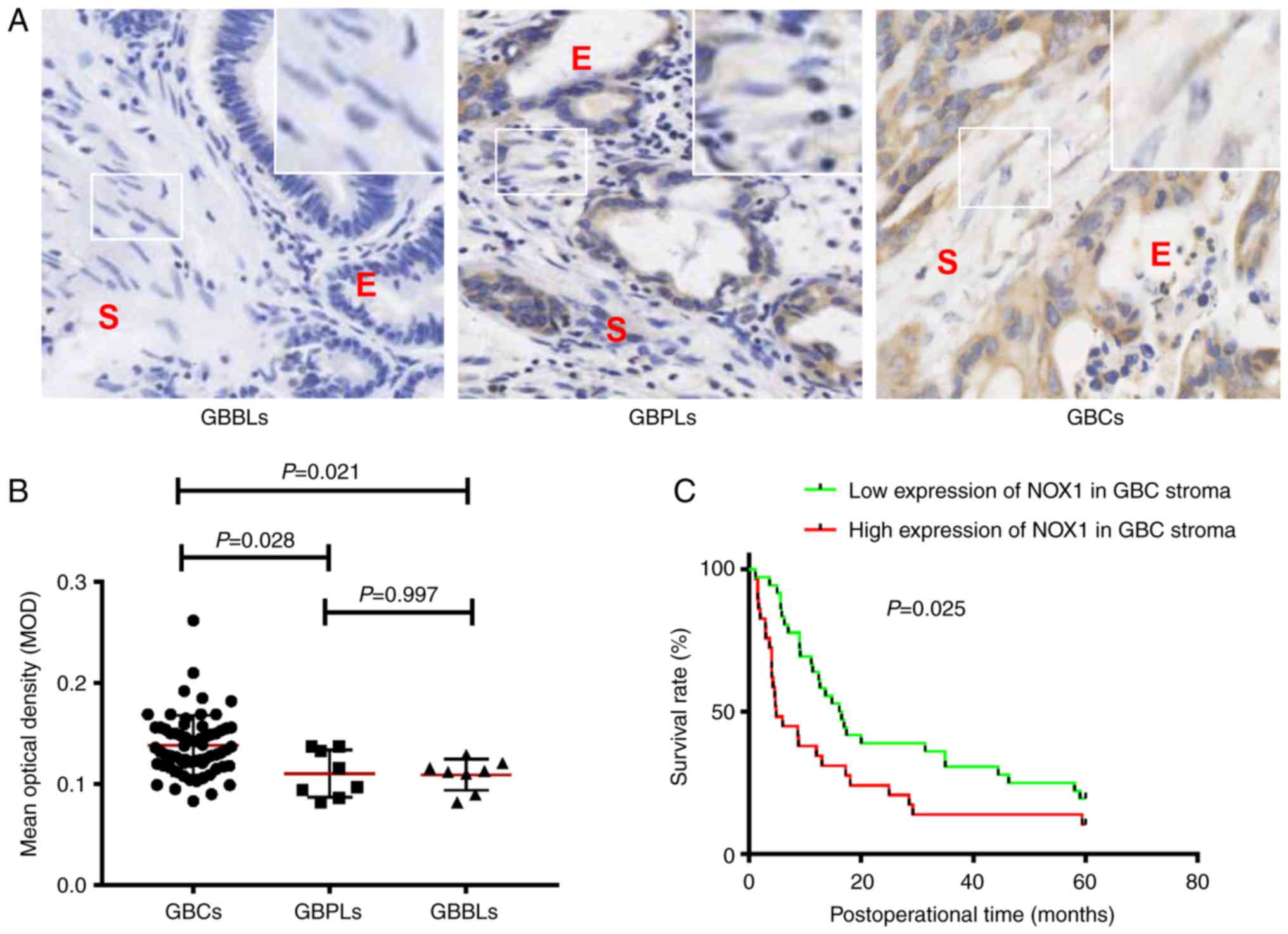 | Figure 1.NOX1 expression is upregulated in the
stroma of GBCs and Kaplan-Meier survival curves for the GBC
patients with high and low stromal NOX1 expression. (A) Magnified
images show representative NOX1 staining in the stroma
(immunohistochemistry; magnification, ×200). (B) Staining in the
stroma was scored using MOD. Values are expressed as the mean ±
standard deviation. The expression of NOX1 protein in the stroma of
GBCs (n=65) was significantly upregulated compared with the stroma
of GBBLs (n=8; 0.138±0.030 vs. 0.109±0.019, P=0.021) or GBPLs (n=8;
0.138±0.030 vs. 0.110±0.025, P=0.028); however, the difference of
NOX1 expression in the stroma of both GBPLs and GBBLs was not
significant (0.110±0.025 vs. 0.109±0.019, P=0.997). (C) GBC
patients with upregulated stromal NOX1 expression had a lower
survival rate than downregulated stromal NOX1 expression patients
(P=0.025, log-rank test). E, epithelium; S, stroma; MOD, mean
optical density; NOX1, nicotinamide adenine dinucleotide phosphate
oxidase 1; GBPL, gallbladder precancerous lesions; GBBLs,
gallbladder benign lesions; GBC, gallbladder cancers. |
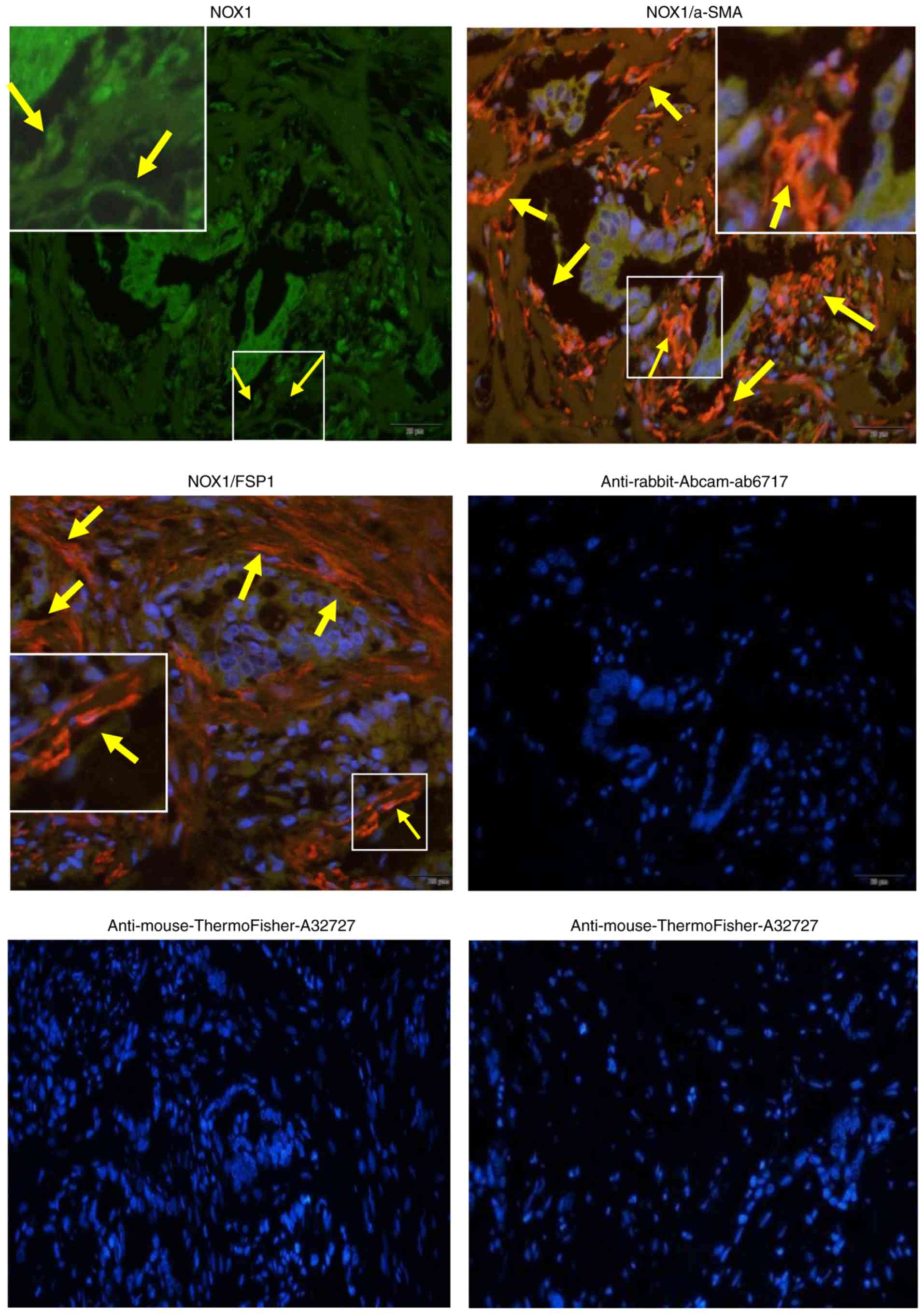 | Figure 2.NOX1 is positively expressed in the
stroma of GBCs with α-SMA and FSP-1 positive expression. The
expression of NOX1 (green) and α-SMA or FSP-1 (red) in GBC tissues
stained using co-immunofluorescence staining (immunofluorescence
microscopy; magnification, ×200). Representative samples of NOX1,
α-SMA and FSP-1 are presented. Sections were counterstained with
DAPI. Secondary antibody only controls are presented: Anti-rabbit
(cat. no. ab6717; Abcam) for NOX1, anti-mouse (cat. no. A32727;
Thermo Fisher Scientific, Inc.) for α-SMA or FSP-1. Arrows and
insets indicate positive staining in fibroblastic cells. NOX1
overlapped with both α-SMA and FSP-1-expressing cells, and
co-localized with the positive stroma of α-SMA and FSP-1, thereby
indicating that NOX1 was positively expressed in both α-SMA and
FSP-1 positive fibroblasts in the stroma of GBCs. NOX1,
nicotinamide adenine dinucleotide phosphate oxidase 1; GBCs,
gallbladder cancers; α-SMA, α-smooth muscle actin; FSP-1,
fibroblast secreted protein-1. |
To determine the significance of NOX1 expression in
the stroma of GBCs, the present study used a MOD value of 0.138 to
distinguish low (MOD <0.138) and high (MOD ≥0.138) NOX1
expression, based on the results of the positive staining of the
stroma. It was observed that stromal NOX1 was highly expressed in
29 cases (44.6%) and poorly expressed in 36 cases (55.4%) of GBCs.
Upregulated NOX1 expression in the stroma of GBCs was significantly
correlated with differentiation degree (P=0.042), venous invasion
(P=0.041) and resection methods (P=0.002); but no significant
correlations were observed between stromal NOX1 expression and the
clinicopathological variables such as sex, age, tumor size, tumor
location, histological type, Nevin stage, lymph node metastasis and
liver infiltration (all P>0.05; Table
II). In addition, the present study used the Cox proportional
hazards model to identify prognostic factors involved in GBC
patients (Table III). Univariate
analysis indicated that tumor histological type (P=0.011),
differentiation degree (P=0.002), Nevin staging (P=0.012), lymph
node metastasis (P=0.001), liver infiltration (P=0.002), vascular
invasion (P<0.00001), curability (P<0.00001) and stromal NOX1
expression (P=0.027) were significantly associated with the OS of
GBC patients. Multivariate analysis validated that histological
type [hazard ratio (HR), 0.308; 95% confidence interval (CI),
0.105–0.902; P=0.032], differentiation degree (HR, 0.038; 95% CI,
0.151–0.974; P=0.044), vascular invasion (HR, 2.375; 95% CI,
1.363–4.139; P=0.002), curability (HR, 1.833; 95% CI, 1.010–3.325;
P=0.046) and stromal NOX1 expression (HR, 1.745; 95% CI,
1.001–2.658; P=0.047) were the independent prognostic factors for
the OS rate of GBC patients. Furthermore, the log-rank test was
used to evaluate the effect of stromal NOX1 expression on the
survival of GBC patients. For 65 GBC patients enrolled in the
present study, the mean and median survival time of the
high-stromal NOX1 expression group (29/65, 44.6%) were 15.7 and 4.8
months, respectively, with a 5-year survival rate of 10.3% (3/29),
compared with 26.6 and 16.1 months, respectively, and a 5-year
survival rate of 19.4% (7/36) for the low-stromal NOX1 expression
group (36/65, 55.4%). The survival rate of GBC patients with
upregulated stromal NOX1 expression was significantly lower than
that of those with downregulated stromal NOX1 expression (Fig. 1C; P=0.025, log-rank test). These
results revealed that GBC patients with high stromal NOX1
expression have poorer prognoses.
 | Table II.Relationship between the expression
of NOX1 in the stroma of GBC and clinicopathological parameters in
patients with GBCs. |
Table II.
Relationship between the expression
of NOX1 in the stroma of GBC and clinicopathological parameters in
patients with GBCs.
|
|
| NOX-1 expression [n
(%)] |
|
|
|---|
|
|
|
|
|
|
|---|
| Variable | n | Low | High | χ2
value | P-value |
|---|
| Sex |
|
Male | 25 | 14 (56) | 11 (44) | 0.006 | 0.937 |
|
Female | 40 | 22 (55) | 18 (45) |
|
|
| Age (years) |
|
>65 | 34 | 21 (61.8) | 13 (38.2) | 1.174 | 0.279 |
|
≤65 | 31 | 15 (48.4) | 16 (51.6) |
|
|
| Tumor size
(cm) |
|
>3 | 27 | 14 (51.9) | 13 (48.1) | 0.233 | 0.629 |
| ≤3 | 38 | 22 (57.9) | 16 (42.1) |
|
|
| Tumor location |
|
Bottom | 26 | 18 (69.2) | 8 (30.8) | 3.362 | 0.067 |
|
Corporis and others | 39 | 18 (46.2) | 21 (53.8) |
|
|
| Histological
type |
|
Adenocarcinoma | 61 | 34 (55.7) | 27 (44.3) | 0.000 | 1.000 |
|
Othersa | 4 | 2 (50) | 2 (50) |
|
|
| Differentiation
degree |
| G1
(high) | 11 | 8 (72.7) | 3 (27.3) | 6.346 | 0.042b |
| G2
(moderate) | 29 | 19 (65.5) | 10 (34.5) |
|
|
| G3
(poor) | 25 | 9 (36) | 16 (64) |
|
|
| Nevin stage |
|
S1-S2 | 8 | 7 (87.5) | 1 (12.5) | 2.470 | 0.116 |
|
S3-S5 | 57 | 29 (50.9) | 28 (49.1) |
|
|
| Lymph node
metastasis |
|
(−) | 44 | 21 (47.7) | 23 (52.3) | 3.232 | 0.072 |
|
(+) | 21 | 15 (71.4) | 6 (28.6) |
|
|
| Liver
infiltration |
|
(+) | 31 | 15 (48.4) | 16 (51.6) | 1.174 | 0.279 |
|
(−) | 34 | 21 (61.8) | 13 (38.2) |
|
|
| Venous
invasion |
|
(+) | 29 | 12 (41.4) | 17 (58.6) | 4.156 | 0.041b |
|
(−) | 36 | 24 (66.7) | 12 (33.3) |
|
|
| Curability |
| R0 | 32 | 24 (75) | 8 (25) | 9.815 | 0.002b |
| R1,
R2 | 33 | 12 (36.4) | 21 (63.6) |
|
|
 | Table III.Univariate and multivariate analysis
of overall survival rate of GBC patients with Cox proportional
hazards model. |
Table III.
Univariate and multivariate analysis
of overall survival rate of GBC patients with Cox proportional
hazards model.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Sex |
| Male
vs. female | 1.283 | 0.748–2.203 | 0.365 |
|
|
|
| Age (years) |
| >65
vs. ≤65 | 1.109 | 0.652–1.888 | 0.703 |
|
|
|
| Tumor size
(cm) |
| >3.0
vs. ≤3.0 | 1.425 | 0.833–2.436 | 0.196 |
|
|
|
| Tumor location |
| Bottom
vs. corporis and other | 0.771 | 0.447–1.331 | 0.351 |
|
|
|
| Histological
type |
|
Adenocarcinoma vs.
Othera | 0.250 | 0.086–0.727 | 0.011b | 0.308 | 0.105–0.902 | 0.032b |
| Differentiation
degree |
| G1 vs.
G2 and G3 | 0.263 | 0.111–0.622 | 0.002b | 0.038 | 0.151–0.974 | 0.044b |
| Nevin staging |
| S3-S5
vs. S1-S2 | 3.732 | 1.339–10.401 | 0.012b |
|
|
|
| Lymph node
metastasis |
| (+) vs.
(−) | 3.024 | 1.607–5.690 | 0.001b |
|
|
|
| Liver
infiltration |
| (+) vs.
(−) | 2.335 | 1.360–4.007 | 0.002b |
|
|
|
| Venous
invasion |
| (+) vs.
(−) | 2.771 | 1.615–4.756 |
P<0.001b | 2.375 | 1.363–4.139 | 0.002b |
| Curability |
| R1, R2
vs. R0 | 2.903 | 1.672–5.041 |
P<0.001b | 1.833 | 1.010–3.325 | 0.046b |
| NOX1 expression in
GBC stroma |
| High
vs. low | 1.822 | 1.069–3.104 | 0.027b | 1.745 | 1.001–2.658 | 0.047b |
NOX1 expression is upregulated in
GCAFs
To verify the upregulated NOX1 expression in the
stroma of GBCs, especially GCAFs, the present study performed
Affymetrix chip analysis on the gene expression profile for GCAFs
and NFs using the Affymetrix GeneChip Human 1.0ST array, and
detected the expression of NOX1 at the mRNA level in GCAFs and NFs
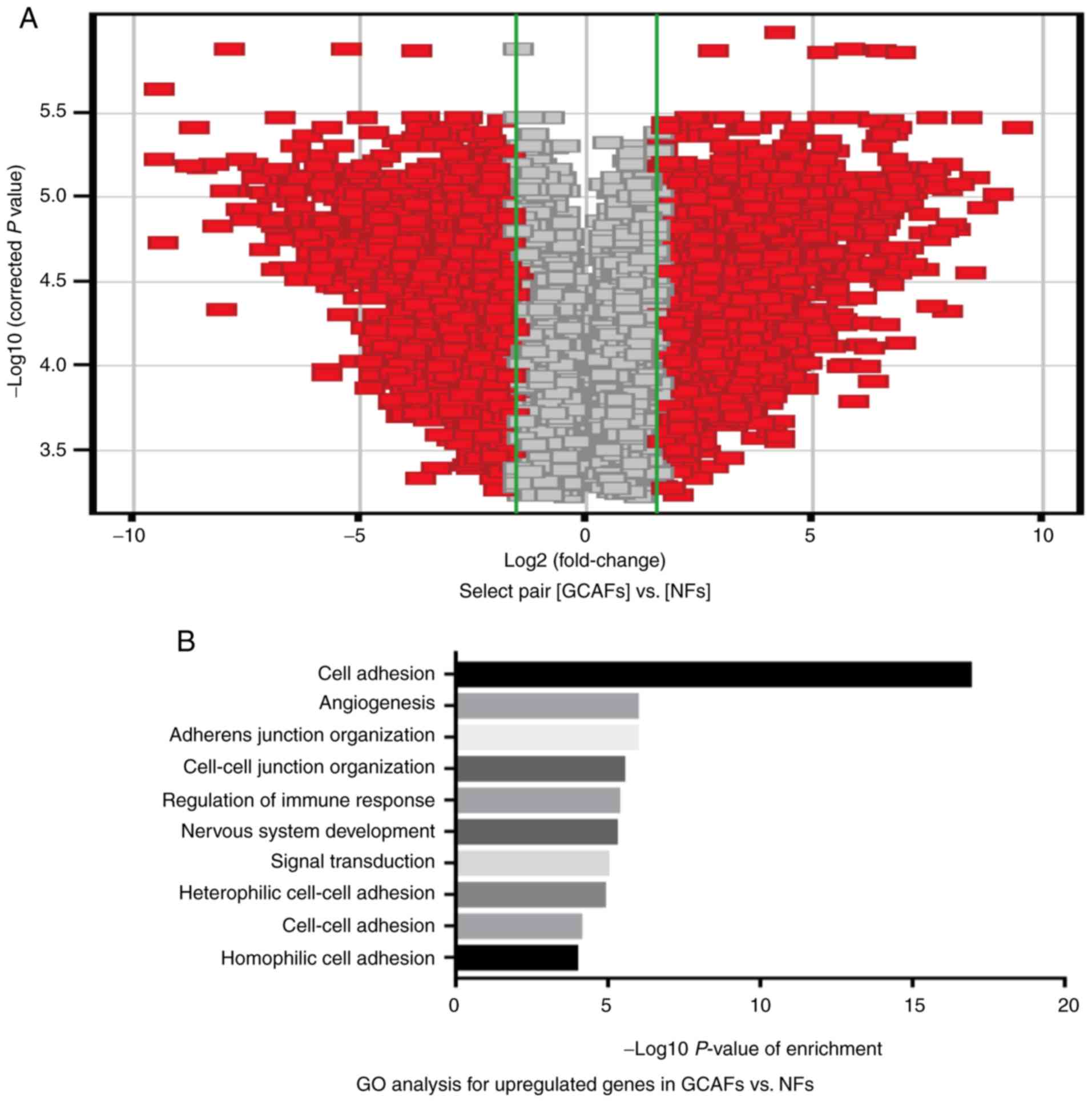
using RT-qPCR in vitro. As presented in Fig. 3, the associated volcano-map provided
an overview of the significantly affected genes (Fig. 3A); GO analysis indicated the
upregulated expression genes, based on the classification of gene
numbers such as biological processes (Fig. 3B). A total of 466 upregulated genes
(FC >1.5) and 596 downregulated genes (FC <0.67) were
identified in GCAFs/NFs according to the inclusion criteria, and of
the total 466 upregulated genes, the NOX1 gene was significantly
upregulated (FC=2.49) in GCAFs (Fig.
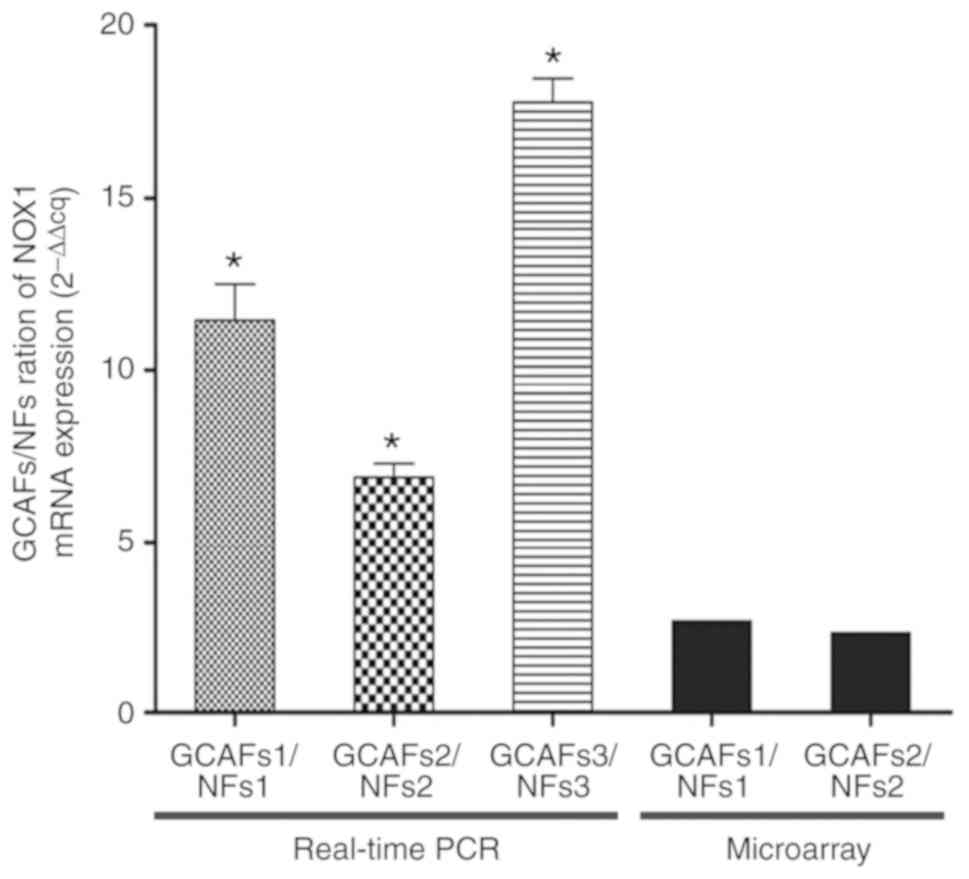
3C). Furthermore, the expression of NOX1 mRNA was significantly
increased in all of the GCAFs compared with adjacent gallbladder
NFs (mean ± SD, 11.45±1.03; 7.04±0.32; 17.58±0.74, all P<0.05;
Fig. 4). The results were consistent
with the results of the Affymetrix chip analysis. Thus, the results
revealed that NOX1 expression was upregulated in GCAFs.
NOX1 expression is upregulated in
co-cultures of GBC-SD cells/GCAFs
To further verify the upregulated NOX1 expression in
GCAFs, the present study detected the expression of NOX1 at the
protein level in the simulated structures (spheroid formation) of
human GBC and adjacent tissues using the co-cultures of GBC-SD
cells/GCAFs or NFs by IHC and western blotting in vitro.
Co-culture of GBC-SD cells with GCAFs strongly enhanced the ability
to form spheroids with higher viability; while GBC-SD cells
co-cultured with NFs formed loose cell aggregates on day 7.
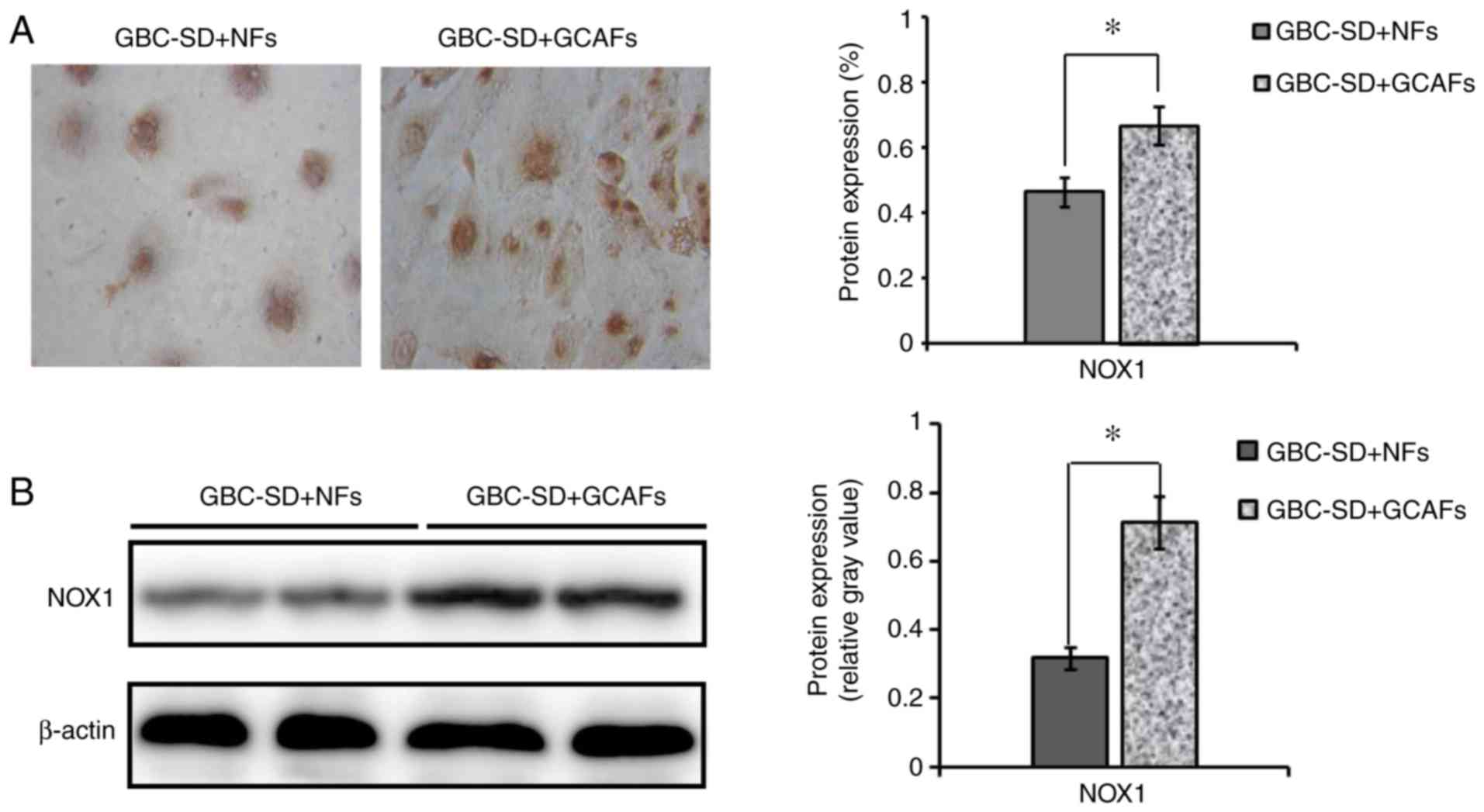
Notably, as determined by IHC, the expression of NOX1 protein was
significantly upregulated in the GBC-SD+GCAFs co-culture when
compared with GBC-SD+NFs co-culture in vitro (0.668±0.058
vs. 0.465±0.045%; P<0.01; Fig.
5A); using western blotting, the expression of NOX1 protein in
the GBC-SD+GCAFs co-culture was also significantly upregulated
compared with the GBC-SD+NFs co-culture in vitro
(0.715±0.077 vs. 0.318±0.031, relative gray value; P<0.001;
Fig. 5B). Thus, the present study
further verified that NOX1 expression was upregulated in GCAFs.
Discussion
NOX1, as one of the members of the NADPH oxidase
family, is thought to play a vital role in tumorigenesis and tumor
development through the generation of ROS and important
intracellular signaling molecules (27,28), has
been reported to be highly expressed in a variety of tumor types
such as gastric (29–31) and liver cancers (32), and is associated with the poor
prognosis of these patients. However, these studies mainly focused
on the expression of NOX1 in the tumor cells themselves. Recently,
some studies have investigated the effect of NOX1 expression in
fibroblasts on tumorigenesis and the development of the tumor.
Increased NOX1 expression in fibroblasts has the ability to induce
malignant transformation and can produce tumors in athymic mice
(37). NOX1 converted tumors from
dormant to aggressive growth, by rendering them capable of forming
well vascularized tumors and inducing molecular markers of
angiogenesis, and revealed that NOX1 is a potent trigger of the
angiogenic switch (38). Fibroblasts
regulated ROS production via NOX1 and NOX4 to mediate
cytokine-triggered DNA damage, which may contribute to malignant
transformation (39). However, the
expression of NOX1 in tumor stroma, particularly GCAFs, and its
role in tumor prognosis have not been well clarified. In the
present study, it was firstly observed that NOX1 expression was
upregulated in the stroma of GBCs compared with GBBLs and GBPLs,
and the expression of NOX1 in the stroma of GBCs was localized to
stromal fibroblasts expressing α-SMA and FSP-1 using CIF staining.
Secondly, the results revealed that NOX1 expression was upregulated
in GCAFs in vitro using Affymetrix chip analysis of the gene
expression profile and RT-qPCR analysis for GCAFs/NFs. Finally, it
was identified that NOX1 was highly expressed in the simulated
spheroid formation of the GBC-SD+GCAFs co-culture, compared with
the GBC-SD+NFs co-culture, using IHC and western blotting. Thus, it
is believed that NOX1 expression was upregulated in GCAFs.
An increasing body of evidence has revealed that the
TME serves a critical role in the growth and development of the
tumor. The TME, the ‘soil’ of tumor growth, is composed of cancer
cells and various interstitial cells, including the extracellular
matrix, proteolytic enzymes, cytokines and chemokines, and
interacts with tumors by altering the proteome and degradome
(8–10). As a prominent member of the stroma and
as the most stromal or interstitial cells in the TME, CAFs have
their own gene expression profiles that are different from NFs, and
interact with cancer cells via a variety of signals to affect the
TME, tumorigenesis, development and therapeutic tolerance of cancer
cells (11–13), which are also associated with the
prognosis of various tumors by expressing FAP, FGF-2, IL-1β-IRAK4
and Podoplanin (15,16,40–44).
Notably, the presence of reactive stroma in the TME, particularly
in CAFs, was revealed to affect the tumor treatment effectiveness
and was associated with tumor drug resistance (17–20).
Anti-CAFs can effectively prevent tumor progression before tumor
invasion, and prolong the survival of patients compared with
chemotherapy alone in pancreatic and other cancer treatments
(20–23). In the present study, NOX1 expression
was significantly upregulated in the stroma of GBC tissues with
positive expression of α-SMA and FSP-1 in vivo, and NOX1 was
highly expressed in GCAFs and the simulated spheroid formation of
the GBC-SD+GCAFs co-culture with positive expression of α-SMA and
FAP, compared with NFs with negative expression of α-SMA and FAP
in vitro. It is recognized that α-SMA, FSP-1 and FAP are the
most important stromal or interstitial markers, their positive
expression localized to stromal GCAFs. Thus, the present study
verified that NOX1 expression was upregulated in GCAFs. To
determine the significance of upregulated NOX1 expression in GCAF,
the present study further analyzed the relationship between NOX1
expression and the clinicopathological characteristics and
prognostic factors of GBC patients. The results revealed the
upregulated NOX1 expression in the stroma of GBCs; GCAFs were
correlated with aggressive characteristics such as differentiation
degree, venous invasion, resection methods (all P<0.05), and the
lower survival rate (P=0.025, log-rank test) of GBC patients.
Stromal NOX1 expression (P=0.047) was an independent prognostic
factor for the OS rate of GBC patients. Thus, it was concluded that
GBC patients with upregulated NOX1 expression in GCAFs have a
poorer prognosis.
GBC, as a highly malignant tumor, is insensitive to
chemotherapy and radiotherapy. It was recently reported that NOX1
overexpression mediated the chemical resistance of cisplatin
through elevated intracellular ROS levels by activating the
HIF1α/MDR1 signaling pathway in GBC cells, and that NOX1 was a
novel accelerant of chemoresistance in GBC (45). It is possible that high stromal NOX1
expression in GBC after chemotherapy maintains the viability of
tumor cells by secreting some anti-drug molecules. NOX1-targeted
therapeutics may be exploited as a strategy for increasing the
efficacy of cisplatin treatment (45). Collectively, NOX1 expression was
upregulated in GCAFs and was associated with the unfavorable
prognosis of GBC patients, which will enable the establishment of
novel biomarkers and potential therapeutic targets that will more
accurately predict patient prognosis and responsiveness to
treatments for human GBCs.
In conclusion, the present study revealed that NOX1
expression was upregulated in the stroma of GBCs, and that GCAFs
with positive α-SMA and FSP-1 expression were sources of NOX1
expression. Upregulated NOX1 expression was related to the tumor
differentiation degree, venous invasion and survival rate of
patients, and appeared to be an independent prognostic factor,
thereby suggesting a poor prognosis in patients with GBC. An
in-depth study of NOX1 expression in GCAFs and its molecular
mechanisms will contribute to the development of novel prognostic
markers and therapeutic targets for human GBCs.
Acknowledgements
Not applicable.
Funding
The present study was supported by funds from the
National Nature Science Foundation of China (grant nos. 30672073
and 81372614) and the Shanghai Science and Technology Commission
Research Project (grant no. 19411966300).
Availability of data and materials
Not applicable.
Authors' contributions
FTW, MH, KHA and YZF conceived and designed the
experiments. FTW, MH, KHA and YZF performed the experiments. FTW,
GLX and XPL analyzed the data. GLX and XPL contributed
reagents/materials/analysis tools. FTW and YZF wrote the paper. All
authors have read and approved the final manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee and the Institutional Review Board of Tongji Hospital.
Written informed consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GBC
|
gallbladder cancer
|
|
CAFs
|
cancer-associated fibroblasts
|
|
GCAFs
|
gallbladder cancer-associated
fibroblasts
|
|
NFs
|
normal fibroblasts
|
|
NOX
|
nicotinamide adenine dinucleotide
phosphate oxidase
|
|
TME
|
tumor microenvironment
|
|
ROS
|
reactive oxygen species
|
|
GBPL
|
gallbladder precancerous lesion
|
|
GBBL
|
gallbladder benign lesion
|
|
OS
|
overall survival
|
|
IHC
|
immunohistochemistry
|
|
DAB
|
3,3-diaminobenzidine
|
|
PBS
|
phosphate-buffered saline
|
|
MOD
|
mean optical density
|
|
IOD
|
integrated optical density
|
|
α-SMA
|
α-smooth muscle actin
|
|
FSP-1
|
fibroblast secreted protein-1
|
|
FAP
|
fibroblast activation protein
|
|
CIF
|
co-immunofluorescence
|
|
FBS
|
fetal bovine serum
|
|
DAPI
|
diamidine phenylindole
|
|
DMEM
|
Dulbecco's modified Eagle's media
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
FC
|
fold change
|
|
GO
|
gene ontology
|
|
IVT
|
in vitro transcription
|
References
|
1
|
Valle JW, Lamarca A, Goyal L, Barriuso J
and Zhu AX: New horizons for precision medicine in biliary tract
cancers. Cancer Discov. 7:943–962. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer Statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu ZY, Cao J, Zhang JT, Xu GL, Li XP,
Wang FT, Ansari KH, Mohamed H and Fan YZ: Ring finger protein 125,
as a potential highly aggressive and unfavorable prognostic
biomarker, promotes the invasion and metastasis of human
gallbladder cancers via activating the TGF-β1-SMAD3-ID1 signaling
pathway. Oncotarget. 8:49897–49914. 2017.PubMed/NCBI
|
|
5
|
Hundal R and Shaffer EA: Gallbladder
cancer: Epidemiology and outcome. Clin Epidemiol. 6:99–109.
2014.PubMed/NCBI
|
|
6
|
Horgan AM, Amir E, Walter T and Knox JJ:
Adjuvant therapy in the treatment of biliary tract cancer: A
systematic review and meta-analysis. J Clin Oncol. 30:1934–1940.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Malka D, Cervera P, Foulon S, Trarbach T,
de la Fouchardière C, Boucher E, Fartoux L, Faivre S, Blanc JF,
Viret F, et al: Gemcitabine and oxaliplatin with or without
cetuximab in advanced biliary-tract cancer (BINGO): A randomised,
open-label, non-comparative phase 2 trial. Lancet Oncol.
15:819–828. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liotta L and Kohn E: The microenvironment
of the tumour-host interface. Nature. 411:375–379. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim JB, Stein R and O'Hare MJ:
Tumour-stromal interactions in breast cancer: The role of stroma in
tumourigenesis. Tumour Biol. 26:173–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reisfeld R: The tumor microenvironment: A
target for combination therapy of breast cancer. Crit Rev Oncog.
18:115–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Finak G, Bertos N, Pepin F, Sadekova S,
Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu
A, et al: Stromal gene expression predicts clinical outcome in
breast cancer. Nat Med. 14:518–527. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ahn S, Cho J, Sung J, Lee JE, Nam SJ, Kim
KM and Cho EY: The prognostic significance of tumor-associated
stroma in invasive breast carcinoma. Tumor Biol. 33:1573–1580.
2012. View Article : Google Scholar
|
|
13
|
Shi S, Liang C, Xu J, Meng Q, Hua J, Yang
X, Ni Q and Yu X: The strain ratio as obtained by endoscopic
ultrasonography elastograhy correlates with the stroma proportion
and the prognosis of local pancreatic cancer. Ann Surg. 2018.
View Article : Google Scholar
|
|
14
|
Orimo A, Gupta PB, Sgroi DC,
Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL
and Weinberg RA: Stromal fibroblasts present in invasive human
breast carcinomas promote tumor growth and angiogenesis through
elevated SDF-1/CXCL12 secretion. Cell. 121:335–348. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao Q, Wang XY, Qiu SJ, Zhou J, Shi YH,
Zhang BH and Fan J: Tumor stroma reaction-related gene signature
predicts clinical outcome in human hepatocellular carcinoma. Cancer
Sci. 102:1522–1531. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Herrera M, Herrera A, Domínguez G, Silva
J, García V, García JM, Gómez I, Soldevilla B, Muñoz C, Provencio
M, et al: Cancer-associated fibroblast and M2 macrophage markers
together predict outcome in colorectal cancer patients. Cancer Sci.
104:437–444. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Micke P and Ostman A: Tumour-stroma
interaction: Cancer-associated fibroblasts as novel targets in
anti-cancer therapy? Lung Cancer. 45 (Suppl 2):S163–S175. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Johansson AC, Ansell A, Jerhammar F, Lindh
MB, Grénman R, Munck-Wikland E, Östman A and Roberg K:
Cancer-associated fibroblasts induce matrix
metalloproteinase-mediated cetuximab resistance in head and neck
squamous cell carcinoma cells. Mol Cancer Res. 10:1158–1168. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Affolter A, Schmidtmann I, Mann WJ and
Brieger J: Cancer-associated fibroblasts do not respond to combined
irradiation and kinase inhibitor treatment. Oncol Rep. 29:785–790.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gonda TA, Varro A, Wang TC and Tycko B:
Molecular biology of cancer-associated fibroblasts: Can these cells
be targeted in anti-cancer therapy? Semin Cell Dev Biol. 21:2–10.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Al-Ansari MM, Hendrayani SF, Tulbah A,
Al-Tweigeri T, Shehata AI and Aboussekhra A: p16INK4A represses
breast stromal fibroblasts migration/invasion and their
VEGF-A-dependent promotion of angiogenesis through Akt inhibition.
Neoplasia. 14:1269–1277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mertens JC, Fingas CD, Christensen JD,
Smoot RL, Bronk SF, Werneburg NW, Gustafson MP, Dietz AB, Roberts
LR, Sirica AE, et al: Therapeutic effects of deleting
cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res.
73:897–907. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Olive KP, Jacobetz MA, Davidson CJ,
Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA,
Caldwell ME, Allard D, et al: Inhibition of Hedgehog signaling
enhances delivery of chemotherapy in a mouse model of pancreatic
cancer. Science. 324:1457–1461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Altenhöfer S, Kleikers PW, Radermacher KA,
Scheurer P, Rob Hermans JJ, Schiffers P, Ho H, Wingler K and
Schmidt HH: The NOX toolbox: Validating the role of NADPH oxidases
in physiology and disease. Cell Mol Life Sci. 69:2327–2343. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bartosz G: Reactive oxygen species:
Destroyers or messengers? Biochem Pharmacol. 77:1303–1315. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schröder K: Isoform specific functions of
Nox protein-derived reactive oxygen species in the vasculature.
Curr Opin Pharmacol. 10:122–126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kamata T: Roles of Nox1 and other Nox
isoforms in cancer development. Cancer Sci. 100:1382–1388. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Roy K, Wu Y, Meitzler JL, Juhasz A, Liu H,
Jiang G, Lu J, Antony S and Doroshow JH: NADPH oxidases and cancer.
Clin Sci (Lond). 128:863–875. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
You X, Ma M, Hou G, Hu Y and Shi X: Gene
expression and prognosis of NOX family members in gastric cancer.
Onco Targets Ther. 11:3065–3074. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Augusto AC, Miguel F, Mendonça S,
Pedrazzoli J Jr and Gurgueira SA: Oxidative stress expression
status associated to Helicobacter pylori virulence in
gastric diseases. Clin Biochem. 40:615–622. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Montalvo-Javé EE, Olguín-Martínez M,
Hernández-Espinosa DR, Sánchez-Sevilla L, Mendieta-Condado E,
Contreras-Zentella ML, Oñate-Ocaña LF, Escalante-Tatersfield T,
Echegaray-Donde A, Ruiz-Molina JM, et al: Role of NADPH oxidases in
inducing a selective increase of oxidant stress and cyclin D1 and
checkpoint 1 over-expression during progression to human gastric
adenocarcinoma. Eur. J Cancer. 57:50–57. 2016.
|
|
32
|
Eun HS, Cho SY, Joo JS, Kang SH, Moon HS,
Lee ES, Kim SH and Lee BS: Gene expression of NOX family members
and their clinical significance in hepatocellular carcinoma. Sci
Rep. 7:110602017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Linder N, Konsti J, Turkki R, Rahtu E,
Lundin M, Nordling S, Haglund C, Ahonen T, Pietikäinen M and Lundin
J: Identification of tumor epithelium and stroma in tissue
microarrays using texture analysis. Diagn Pathol. 7:222012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lehr HA, van der Loos CM, Teeling P and
Gown AM: Complete chromogen separation and analysis in double
immunohistochemical stains using Photoshop-based image analysis. J
Histochem Cytochem. 47:119–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zou A, Lambert D, Yeh H, Yasukawa K,
Behbod F, Fan F and Cheng N: Elevated CXCL1 expression in breast
cancer stroma predicts poor prognosis and is inversely associated
with expression of TGF-β signaling proteins. BMC Cancer.
14:7812014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Suh YA, Arnold RS, Lassegue B, Shi J, Xu
X, Sorescu D, Chung AB, Griendling KK and Lambeth JD: Cell
transformation by the superoxide-generating oxidase Mox1. Nature.
401:79–82. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arbiser JL, Petros J, Klafter R,
Govindajaran B, McLaughlin ER, Brown LF, Cohen C, Moses M, Kilroy
S, Arnold RS and Lambeth JD: Reactive oxygen generated by Nox1
triggers the angiogenic switch. Proc Natl Acad Sci USA. 99:715–720.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Illeperuma RP, Kim DK, Park YJ, Son HK,
Kim JY and Kim J, Lee DY, Kim KY, Jung DW, Tilakaratne WM and Kim
J: Areca nut exposure increases secretion of tumor-promoting
cytokines in gingival fibroblasts that trigger DNA damage in oral
keratinocytes. Int J Cancer. 137:2545–2557. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Franco OE, Shaw AK, Strand DW and Hayward
SW: Cancer associated fibroblasts in cancer pathogenesis. Semin
Cell Dev Biol. 21:33–39. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pecqueux C, Arslan A, Heller M,
Falkenstein M, Kaczorowski A, Tolstov Y, Sültmann H, Grüllich C,
Herpel E, Duensing A, et al: FGF-2 is a driving force for
chromosomal instability and a stromal factor associated with
adverse clinico-pathological features in prostate cancer. Urol
Oncol. 36:365.e15–365.e26. 2018. View Article : Google Scholar
|
|
42
|
Zhang D, Li L, Jiang H, Li Q, Wang-Gillam
A, Yu J, Head R, Liu J, Ruzinova MB and Lim KH: Tumor-stroma
IL1β-IRAK4 feedforward circuitry drives tumor fibrosis,
chemoresistance, and poor prognosis in pancreatic cancer. Cancer
Res. 78:1700–1712. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wikberg ML, Edin S, Lundberg IV, Van
Guelpen B, Dahlin AM, Rutegård J, Stenling R, Oberg A and Palmqvist
R: High intratumoral expression of fibroblast activation protein
(FAP) in colon cancer is associated with poorer patient prognosis.
Tumour Biol. 34:1013–1020. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Schoppmann SF, Berghoff A, Dinhof C,
Jakesz R, Gnant M, Dubsky P, Jesch B, Heinzl H and Birner P:
Podoplanin-expressing cancer-associated fibroblasts are associated
with poor prognosis in invasive breast cancer. Breast Cancer Res
Treat. 134:237–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhan M, Wang H, Chen T, Chen W, Yang L, He
M, Xu S and Wang J: NOX1 mediates chemoresistance via HIF1α/MDR1
pathway in gallbladder cancer. Biochem Biophys Res Commun.
468:79–85. 2015. View Article : Google Scholar : PubMed/NCBI
|