Introduction
Liver cancer is the fifth most frequently diagnosed
cancer and the second most common cause of cancer mortality in
males worldwide (1). In females, the
numbers are as high as ninth and sixth worldwide for diagnosed
cancer and cause of mortality, respectively (1). Liver cancer constitutes a major global
health problem, with the highest incidence rates in East Asia
(1). The major causes of liver cancer
include chronic hepatitis B and C virus infection, aflatoxin
exposure, alcohol abuse, smoking and obesity (2). Most liver cancer patients are diagnosed
at advanced stages with a poor prognosis; surgical resection and
chemotherapy may prolong the overall survival time of liver cancer
patients (3). Therefore, there is an
urgent need to identify effective biomarkers and therapeutic
targets for the treatment of liver cancer.
MicroRNAs (miRNAs) are small noncoding RNAs composed
of ~22 nt. They usually bind to the 3′-untranslated region (UTR) of
mRNAs and suppress gene expression by either degrading the mRNA or
suppressing mRNA translation into protein, depending on pairing
complementarity (4,5). Growing evidence indicates that miRNAs
play important roles in many biological processes, including
development, proliferation, differentiation, apoptosis and
oncogenesis (5). It has been shown
that miRNAs are abnormally expressed in hepatocellular carcinoma
(HCC), one of the most common primary liver cancers representing
75–85% of cases (1), and act as tumor
oncogenes or suppressors by affecting various targets to regulate
tumorigenesis, invasion and metastasis (6). Furthermore, many miRNAs show promise as
prognostic markers or therapeutic targets for the treatment of
different cancer types (7,8).
miRNA-124 (miR-124) was first identified to be
highly expressed in the central nervous system, and it regulates
many neuronal activities (9–11). Subsequent studies revealed that
miR-124 is significantly downregulated in several types of human
cancer and functions as a tumor suppressor (9,12,13). miR-124 is able to recognize cell
proliferation-related genes such as EPH receptor A2 (14), Smad4 (15), and AKT-solute carrier family 2 member
1/HKII (16). Several studies have
shown aberrant expression of miR-124 in liver cancer, and decreased
expression of miR-124 is correlated with shorter overall survival
and poor prognosis in patients with liver cancer, suggesting that
miR-124 might be a potential biomarker for the early diagnosis of
liver cancer (17,18).
Chloride channels are membrane proteins with
Cl− permeation pores. The mammalian chloride
intracellular ion channel (CLIC) family consists of 6 subfamilies:
CLIC1, CLIC-2, CLIC-3, CLIC-4, CLIC-5 and CLIC-6 (19). CLIC1 was initially identified in the
human myelomonocytic cell line U937 in 1997 (20). Studies have shown that the CLIC1
protein level is increased in different types of cancer, including
epithelial ovarian cancer (21),
gastric cancer (22), pancreatic
cancer (23) and HCC (24). All of these findings suggest that
CLIC1 is an oncogene or a tumor marker in the progression of
cancer. The present study identified CLIC1 as a miR-124 target gene
by mass-spectrometry, luciferase assay and western blot analysis.
Using further knockdown and functional assays, it was confirmed
that miR-124 functions as a tumor suppressor through the
downregulation of CLIC1. Thus, miR-124 and CLIC1 may serve as a
biomarkers or have therapeutic potential for the treatment of human
liver cancer.
Materials and methods
Hepatic cell culture and
transfection
Human liver cancer HepG2 cells and human fetal
hepatic HL-7702 cells were cultured in DMEM (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C and 5% CO2. The cell lines
were characterized by Shanghai Yihe Applied Biotechnology Co., Ltd.
using short tandem repeat markers. miR-124 precursor
oligonucleotide (miR-124) and mismatched sequence as a negative
control (cat. no. AM17111; pre-scrambled miRNA control; NC) were
synthesized by Ambion; Thermo Fisher Scientific, Inc. The sequence
of miR-124 was as follows: Sense, 5′-GGCAUUCACCGCGUGCCUUATT-3′ and
antisense, 5′-UAAGGCACGCGGUGAAUGCCAA-3′. HepG2 cells were seeded in
12-well plates (1×105 cells/well) and incubated for 20 h
before transfection. The transfection of miRNA (100 nm) or plasmid
(2 µg) was carried out with Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. For rescue experiments, the miRNA (100
nm) and plasmids (2 µg) were transfected into HepG2 cells using
Lipofectamine® 2000.
Plasmid construction
Total RNA was extracted from HepG2 cells using
TRIzol® reagent (Thermo Fisher Scientific, Inc.) and was
reverse-transcribed using a murine Moloney Leukemia Virus (M-MLV)
kit (Takara Biotechnology Co., Ltd.). The PCR was performed using
Ex Taq (Takara Biotechnology Co., Ltd.). The 3′-UTR of CLIC1 was
amplified by PCR and constructed into the
SpeI/HindIII sites downstream of the luciferase gene
in the pMIR-REPORT Luciferase miRNA Expression Reporter Vector
(Ambion; Thermo Fisher Scientific, Inc.). The primers for the
3′-UTR of CLIC1 were as follows: Forward,
5′-gactagtGCCCCTCCTGGGACTCCCT-3′ and reverse primer,
5′-atgcaagcttTTTTGCGTAAAAACACTTG-3′. A CLIC1 expression vector,
pEGFP-N1-CLIC1, was constructed in our laboratory. Primers for
CLIC1 mRNA were as follows: Forward,
5′-atcgctcgagATGGCTGAAGAACAACCGCAGG-3′ and reverse,
5′-agtcgacTTATTTGAGGGCCTTTGCCAC-3′. The PCR reaction was carried
out under the following conditions: 95°C for 5 min; 30 cycles of
95°C for 30 sec, 56°C for 30 sec and 72°C for 1 min; and 72°C for 5
min. All constructs were confirmed by SpeI/HindIII or
XhoI/BamHI restriction digestion and DNA sequencing.
The restriction digestion was performed at 37°C for 1 h and the DNA
sequencing was performed by BGI.
CLIC1 knockdown
CLIC1 small interfering RNA (siR-CLIC1) and scramble
siRNA control (siR-NC) was purchased from Shanghai GeneChem Co.,
Ltd. The siR-CLIC1 sequence was 5′-GGACCGAGACAGTGCAGAA-3′ and the
siR-NC sequence was 5′-TTCTCCGAACGTGTCACGT-3′. HepG2 cells were
seeded 80% confluent and transfected with 50 nm siRNA using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The knockdown effect was evaluated by reverse
transcription-quantitative PCR (RT-qPCR) and western blotting at
24, 48 and 72 h post-transfection.
RT-qPCR
Total RNA from HepG2 and HL-7702 cells was extracted
with TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). The expression of miR-124 was measured using a
TaqMan MicroRNA assay kit (Ambion; Thermo Fisher Scientific, Inc.),
according to the manufacturer's instructions (25). Relative expression was calculated
using the 2−ΔΔCq method and normalized to the expression
of RNU6B (Ambion; Thermo Fisher Scientific, Inc.) (26). For the CLIC1 mRNA analysis, the total
RNA was extracted from cells transfected with miR-124 or control
with TRIzol® reagent. cDNA was synthesized using a M-MLV
(Takara Biotechnology, Co., Ltd.) under the following program: 70°C
for 10 min, then cooling on ice, 42°C for 1 h, 70°C for 15 min and
held at 4°C. qPCR was performed in triplicate in a 20 µl reaction
volume with SYBR-Green (Bio-Rad Laboratories, Inc.) as follows:
95°C for 3 min; and 40 cycles of 95°C for 20 sec, 58°C for 30 sec
and 72°C for 20 sec. The primers used for RT-qPCR were as follows:
CLIC1 forward, 5′-AATTCAAACCCAGCACTCAATG-3′ and reverse primer,
5′-CAGCACTGGTTTCATCCACTT-3′; and GAPDH forward,
5′-CCACTCCTCCACCTTTGAC-3′ and reverse primer,
5′-ACCCTGTTGCTGTAGCCA-3′. The expression level of GAPDH was used as
a control. Relative expression was calculated via the comparative
Cq method. The specificity of the PCR products was confirmed by
melting curve analysis.
Immunoblotting
Total protein was isolated from cell lines
transfected with RNA oligonucleotide and/or plasmid DNA in cell
lysis buffer [50 mM Tris-HCl (pH 7.5), 150 mm NaCl, 1% Triton
X-100, 1 mM EDTA, 1 mM PMSF and 1% sodium deoxycholate]. Protein
concentration was measured using a Bio-Rad protein assay kit (cat.
no. 5000002; Bio-Rad Laboratories, Inc.). Protein samples were
separated by 10% SDS-PAGE and transferred to a polyvinylidene
difluoride membrane (Amersham; GE Healthcare). Primary antibody
against CLIC1 (1:1,000; Santa Cruz Biotechnology, Inc.; cat. no.
sc-81873) and tubulin (1:5,000; Santa Cruz Biotechnology, Inc.;
cat. no. sc-365791) was applied after blocking the membrane with 5%
nonfat milk in PBS + 0.1% Tween-20, at room temperature for 1 h,
followed by the appropriate horseradish peroxidase-conjugated
secondary antibody (1:5,000; Santa Cruz Biotechnology, Inc.; cat.
no. sc-516102). The membranes were detected using an ECL western
blotting detection system (EMD Millipore). Signals were calculated
using ImageJ 1.50 (National Institutes of Health).
Proteomic analysis
HepG2 cells were transfected with miR-124 or
pre-scrambled miRNA control (NC). After 48 h of transfection,
cellular proteins were extracted using the ProteoPrep Total
Extraction Sample kit (Sigma-Aldrich; Merck KGaA) and the protein
concentration was determined by Bio-Rad Protein Assay (Bio-Rad
Laboratories, Inc.). Proteins were used for two-dimensional gel
electrophoresis (2-DGE) as described in the manufacturer's
instructions (Bio-Rad Laboratories, Inc.). The first-dimension
isoelectric-focusing of 2-DGE was carried out using pH 3–10
immobilized pH gradient ReadyStrips, and in the second dimension,
the protein was separated by 8–14% gradient SDS-PAGE. The gels were
stained with Coomassie brilliant blue and scanned with a flatbed
scanner. The analysis included picture merge and protein spot
detection performed by PDQuest 2-DE analysis software (BioRad
Laboratories, Inc., version 8.0). Gel comparison was performed
using the ‘Automated Detection and Matching’ function in PDQuest
software, combined with manual pair correction. The yellow-labeled
spots showed at least a 2-fold decrease between the miR-124 sample
and control, with statistical significance (P<0.05). Selected
protein spots were excised from the gels and identified by mass
spectrometry. Mass spectrometry analysis was performed at the
Teaching Center of Biology Experiment, School of Life Sciences, Sun
Yat-Sen University.
Luciferase assay
HepG2 cells with 80% confluence were transfected
with luciferase constructs containing the 3′-UTR of CLIC1 (2 µg)
and/or miR-124 or pre-scrambled miRNA control (NC) (100 nm) using
Lipofectamine® 2000. After 48 h of transfection, cells
were harvested for luciferase assays using the Luciferase Assay
System (Promega Corporation), according to the manufacturer's
protocol. pMIR-REPORT-β-gal was used for normalization. TargetScan
(targetscan.org) was used to search for
complementary sites of miR-124 in the 3′-UTR of CLIC1 mRNA.
Cell growth and viability
Cells were counted and plated at a density of
3×103 cells/well in 96-well plates in triplicate. Cell
viability was determined at 24, 48 and 72 h post-transfection.
Spectrophotometry was performed at λ=450 nm and λref
=630 nm after incubation with 10 µl water-soluble tetrazolium salt
(WST)-1 in 100 µl medium (Roche Molecular Diagnostics) for 2 h. For
the colony formation assays, cells were seeded in a 6-well plate
(0.8×103 cells/well) and cultured for 2 weeks. Colonies
were fixed with cooled methanol for 10 min at 4°C and stained using
0.1% crystal violet for 10 min at room temperature. The images were
taken using a camera. Visible colonies were manually counted. Each
group was measured in triplicate.
Scratch wound-healing motility
assays
A total of 1.0×106 cells were seeded and
grown to confluence in 60-mm dishes with 10% FBS DMEM media. A
scratch was made on the cell monolayer using a pipette tip at 48 h
after miR-124 or pre-scrambled miRNA control transfection. The
cells was washed in PBS twice and cultured in DMEM without FBS. The
wound areas were imaged at 24 and 48 h after the scratch was made.
The distance between the two edges of the scratch was measured
using the ImageJ 1.50 (National Institutes of Health). The images
were taken with a Leica Microsystems, Inc. light microscope at a
magnification of ×100.
Transwell assay
Cell migration was detected using a 6-well plate
Transwell system without Matrigel coating (BD Biosciences). Cell
invasion assays were performed using Matrigel Invasion Chambers (BD
Biosciences) precoated with ECM gel (Sigma-Aldrich; Merck KGaA). A
total of 1.0×106 cells at 48 h post-transfection were
seeded into the upper chamber with serum-free medium. The lower
chamber was prepared with medium containing 10% FBS, which served
as a chemoattractant. After incubating for 24 h, the non-invasive
cells were mechanically removed. The invasive cells on the lower
surface of the membrane were then washed, fixed with methanol for
20 min at 4°C and stained with 0.1% crystal violet for 10 min at
room temperature. Cells were counted in 10 random optical fields
under a Leica Miscrosystems, Inc. light microscope at ×400
magnification. The images were taken by a Leica Miscrosystems, Inc.
light microscope at a magnification of ×100.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 5.0 (GraphPad Software, Inc.). All numerical data are
displayed as the mean ± SEM. Statistical comparisons among two or
more groups were conducted using one-way ANOVA test followed by the
Newman-Keuls multiple comparison test, or Student's t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
miR-124 functions as a tumor
suppressor in liver cancer cells
miR-124 levels were compared between a human liver
cancer cell line and a normal liver epithelial cell line using
RT-qPCR. In HepG2 liver cancer cells, the expression of miR-124 was
significantly decreased compared with that in the normal liver
epithelial cells (Fig. 1A),
suggesting that miR-124 may function as a tumor suppressor in liver
cancer cells.
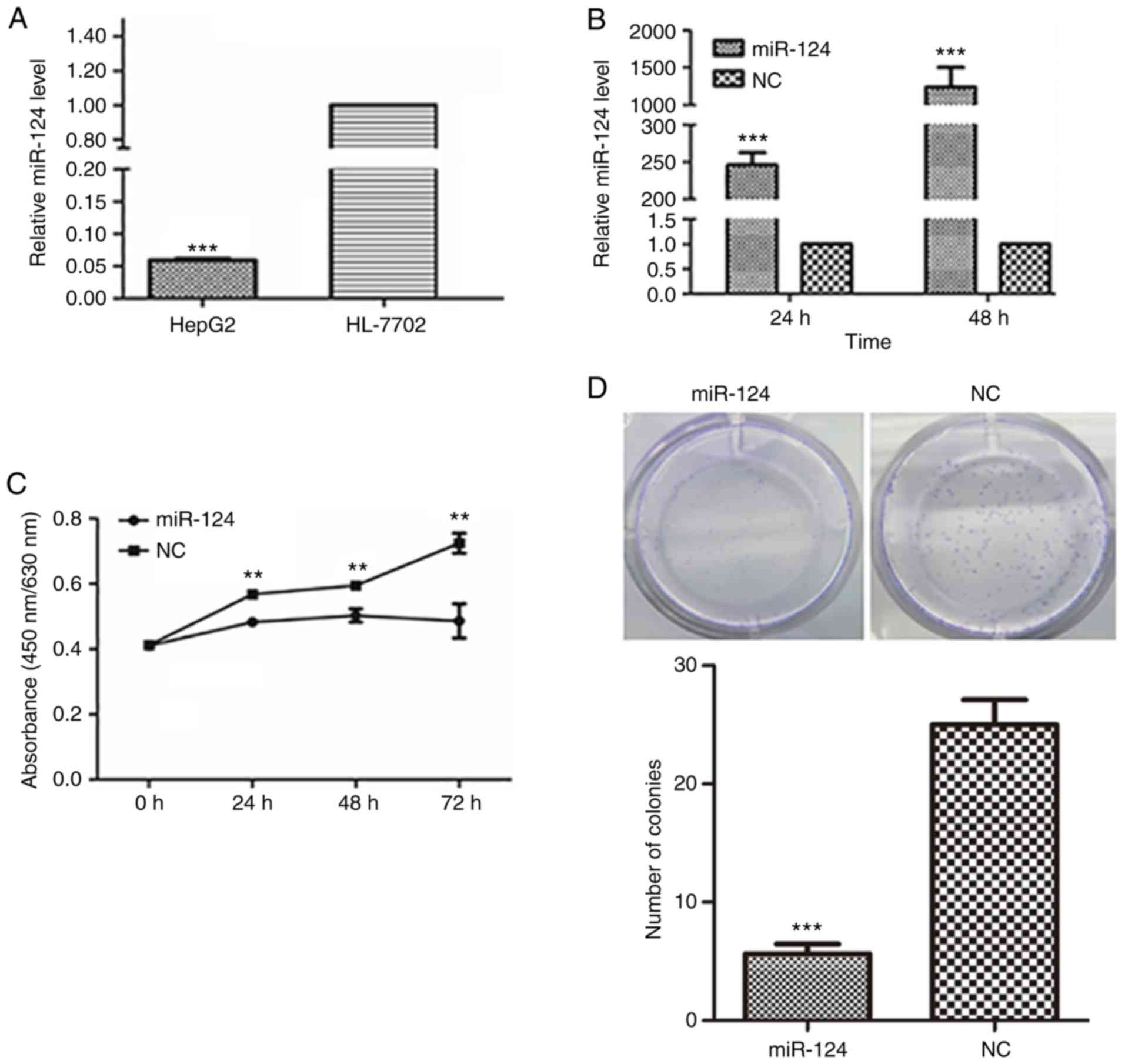 | Figure 1.Ectopic expression of miR-124
suppresses liver cancer cell proliferation, migration and invasion.
(A) Detection of the miR-124 expression level in human liver cancer
cells and a normal liver epithelial cell line by RT-qPCR with
normalization to U6 ***P<0.001 vs. HL-7702. (B) Evaluation of
the efficiency of the miR-124 precursor at 24 and 48 h after
transfection using RT-qPCR. (C) Summary of the effect of miR-124 on
the viability of liver cancer cells, according to the water soluble
tetrazolium salt-1 assay. (D) Colony formation assay showing the
effect of miR-124 on the cell growth of liver cancer cells. Scale
bar, 200 µm. **P<0.01, ***P<0.001 vs. respective NC. RT-qPCR,
reverse transcription-quantitative PCR; miR, microRNA; NC, negative
control. (E) Analysis of the function of miR-124 on the migration
of liver cancer cells by scratch wound-healing motility assay.
Scale bar, 200 µm. (F) The effect of miR-124 on the migration of
liver cancer cells in the Transwell assay without Matrigel coating.
Scale bar, 200 µm. (G) A Matrigel-coated Transwell assay was used
to analyze the effect of miR-124 on cell invasion in liver cancer
cells. Scale bar, 200 µm. ***P<0.001 vs. respective NC. RT-qPCR,
reverse transcription-quantitative PCR; miR, microRNA; NC, negative
control. |
To further confirm the function of miR-124 in liver
cancer cells, HepG2 cells with low miR-124 expression were used as
a cell model, and miR-124 was overexpressed in HepG2 cells by
transfecting miR-124 precursor; scrambled miRNA precursor was used
as the NC. The increased level of miR-124 was confirmed by RT-qPCR
(Fig. 1B). Overexpression of miR-124
significantly inhibited the viability and proliferation of HepG2
cells (Fig. 1C and D). In the scratch
wound-healing motility assay, transfecting miR-124 into HepG2 cells
resulted in slower closure of the wound compared with that in
control cells (Fig. 1E). In the
Transwell assay without Matrigel coating, HepG2 cells expressing
miR-124 migrated to a lesser extent to the lower chamber, compared
with the control cells (Fig. 1F).
Similarly, cell invasion was also largely inhibited by transfecting
miR-124, as demonstrated by the Transwell assay with Matrigel
(Fig. 1G). All these observations
suggested that miR-124 functions as a tumor suppressor in liver
cancer.
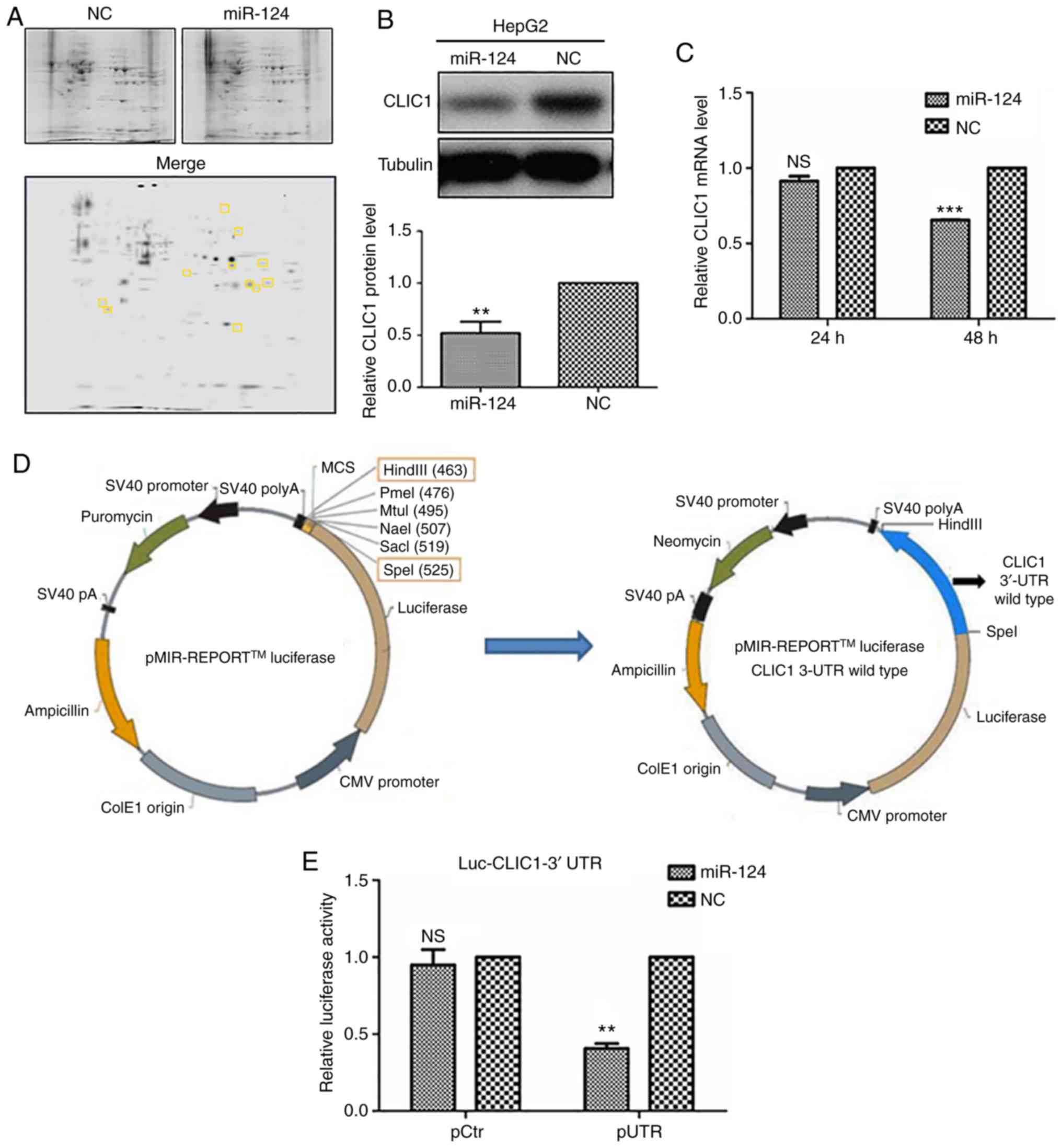
miR-124 targets CLIC1 and negatively
regulates its expression
To explore the targets of miR-124 in liver cancer
cells, mass spectrometry was used to analyze the differential
expression of proteins between miR-124-overexpressing HepG2 cells
and control cells (Fig. 2A). Among
the proteins detected by two-dimensional gel electrophoresis, CLIC1
expression was decreased in miR-124-overexpressing cells, as
compared with control cells (Fig.
2A), suggesting that CLIC1 is one of the potential targets of
miR-124. To confirm this finding, HepG2 cells were further
transfected with either miR-124 or NC, and the endogenous
expression of CLIC1 was detected at both the protein and mRNA
levels. Overexpression of miR-124 caused a significant decrease in
CLIC1 protein expression (Fig. 2B).
miRNAs are able to regulate gene expression by either degrading the
mRNA of a target gene or preventing its translation. To better
understand the mechanism underlying the downregulation of CLIC1 by
miR-124, the mRNA level of CLIC1 was examined in
miR-124-overexpressing cells. The RT-qPCR results revealed that the
mRNA level of CLIC1 was reduced at 48 h after transfection with
miR-124, compared with the NC (Fig.
2C). These data indicated that CLIC1 is a target gene of
miR-124, which is likely to downregulate CLIC1 through mRNA
degradation.
miR-124 targets the 3′-UTR of CLIC1
mRNA
To further identify the target region of CLIC1 for
miR-124, the 3′-UTR of CLIC1 mRNA was cloned into a luciferase
vector (Fig. 2D). Co-transfection of
miR-124 significantly inhibited the luciferase activity of the
CLIC1 3′-UTR construct (Fig. 2E),
suggesting that miR-124 targets the 3′-UTR of CLIC1 mRNA and
prevents subsequent translation. Usually, the regulatory effect of
miRNAs on target genes depends on the seed region of the miRNA.
Bioinformatics analysis using TargetScan was used to search for
complementary sites in the 3′-UTR of CLIC1 mRNA for the seed region
of miR-124 (data not shown). Unexpectedly, there is no
complementary site in the 3′-UTR of CLIC1 for the seed region of
miR-124.
Inhibition of cancer progression in
liver cancer cells by CLIC1 knockdown
To explore the function of CLIC1 in liver cancer,
CLIC1 protein and mRNA levels were measured in a liver cancer cell
line (HepG2) and in the normal human hepatocyte cell line HL-7702.
Both the protein and mRNA of CLIC1 were highly expressed in HepG2
liver cancer cells, but not in the normal hepatocyte cells
(Fig. 3A and B). To confirm that
miR-124 functions as a tumor suppressor by downregulating CLIC1,
the effect of silencing CLIC1 was assessed in liver cancer cells.
As shown in Fig. 3C and D,
transfecting CLIC1 siRNA caused significant downregulation of CLIC1
protein and mRNA levels in HepG2 cells compared with control (NC).
The CLIC1-deficient cells exhibited a growth rate similar to that
of control cells in WST-1 and colony formation assays (Fig. 3E and F), suggesting that CLIC1 had no
effects on the viability and proliferation of liver cancer cells.
In the scratch wound-healing motility assay, CLIC1-deficient cells
showed significantly slower closure of the wound than the control
cells (Fig. 3G). In the Transwell
assays without Matrigel coating, fewer CLIC1-deficient cells were
able to migrate through pores in the membrane to the lower chamber
compared with control cells transfected with scramble siRNA
(Fig. 3H). In the Matrigel-coated
Transwell invasion assay, silencing of CLIC1 inhibited the invasion
of liver cancer cells (Fig. 3I).
These results demonstrated that suppression of CLIC1 functions as a
metastasis inhibitor in liver cancer cells and has an effect
similar to that of miR-124 in liver cancer cells.
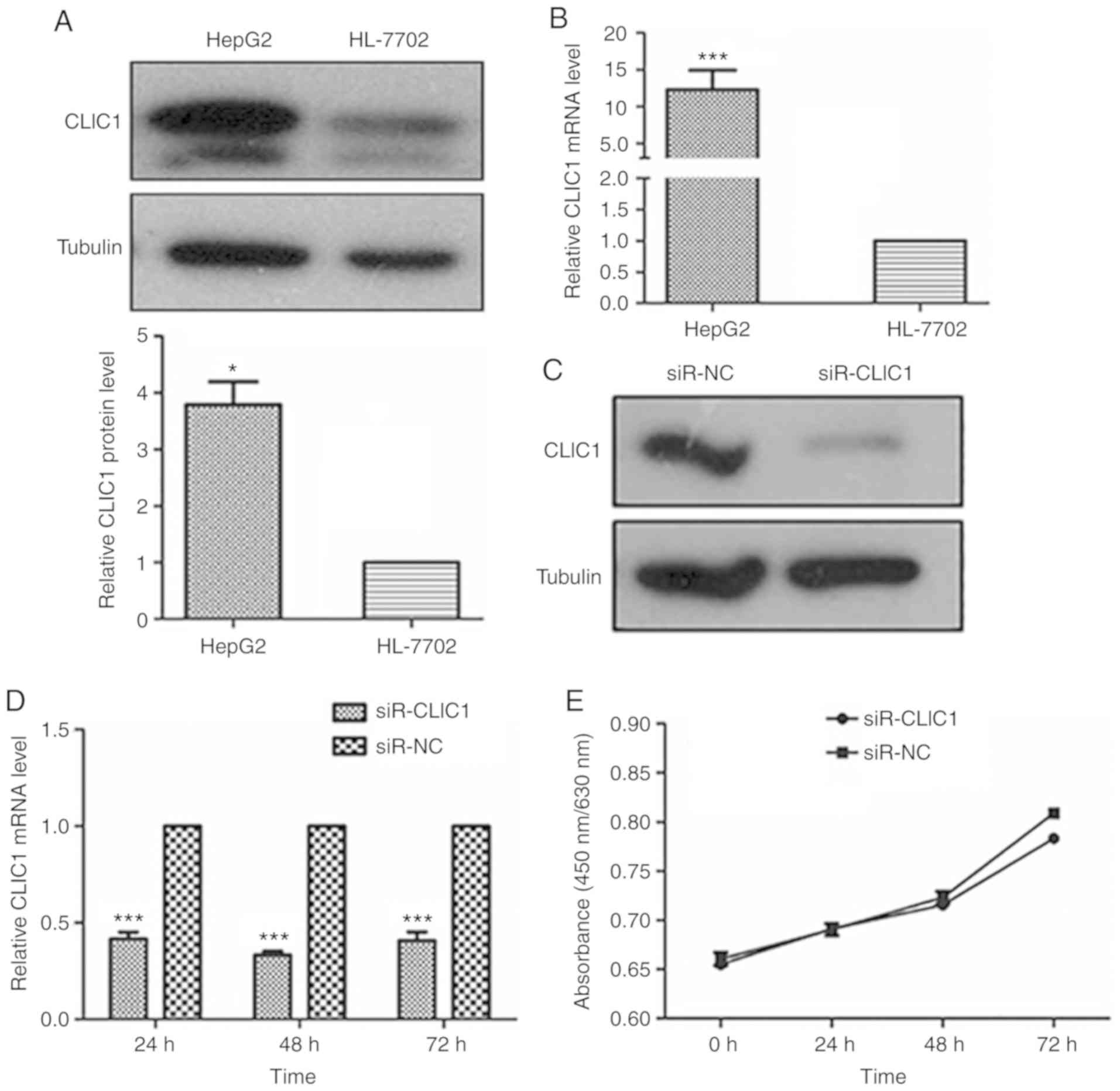 | Figure 3.CLIC1 is essential for liver cancer
cell migration and invasion, but not proliferation. (A) CLIC1
protein is overexpressed in liver cancer cells compared with normal
liver epithelial cells. (B) Analysis of the mRNA expression level
of CLIC1 by reverse transcription-quantitative PCR. *P<0.05,
***P<0.001 vs. HL-7702. (C) CLIC1 protein and (D) mRNA were
reduced by siR-CLIC1 in liver cancer cells. (E) WST1 showed that
CLIC1 had no effect on cell viability and cell growth. siR, small
interfering RNA; CLIC1, chloride intracellular protein 1; NC,
negative control; n.s., not significant. (F) Colony formation
assays showed that CLIC1 had no effect on cell viability and cell
growth. The cell migration of liver cancer cells after knockdown of
CLIC1 was evaluated by (G) scratch wound-healing motility assay and
(H) Transwell assay without Matrigel coating. Scale bar, 200 µm.
(I) The cell invasion ability of liver cancer cells was evaluated
by Matrigel-coated Transwell assay. Scale bar, 200 µm.
***P<0.001 vs. respective siR-NC. siR, small interfering RNA;
CLIC1, chloride intracellular protein 1; NC, negative control;
n.s., not significant. |
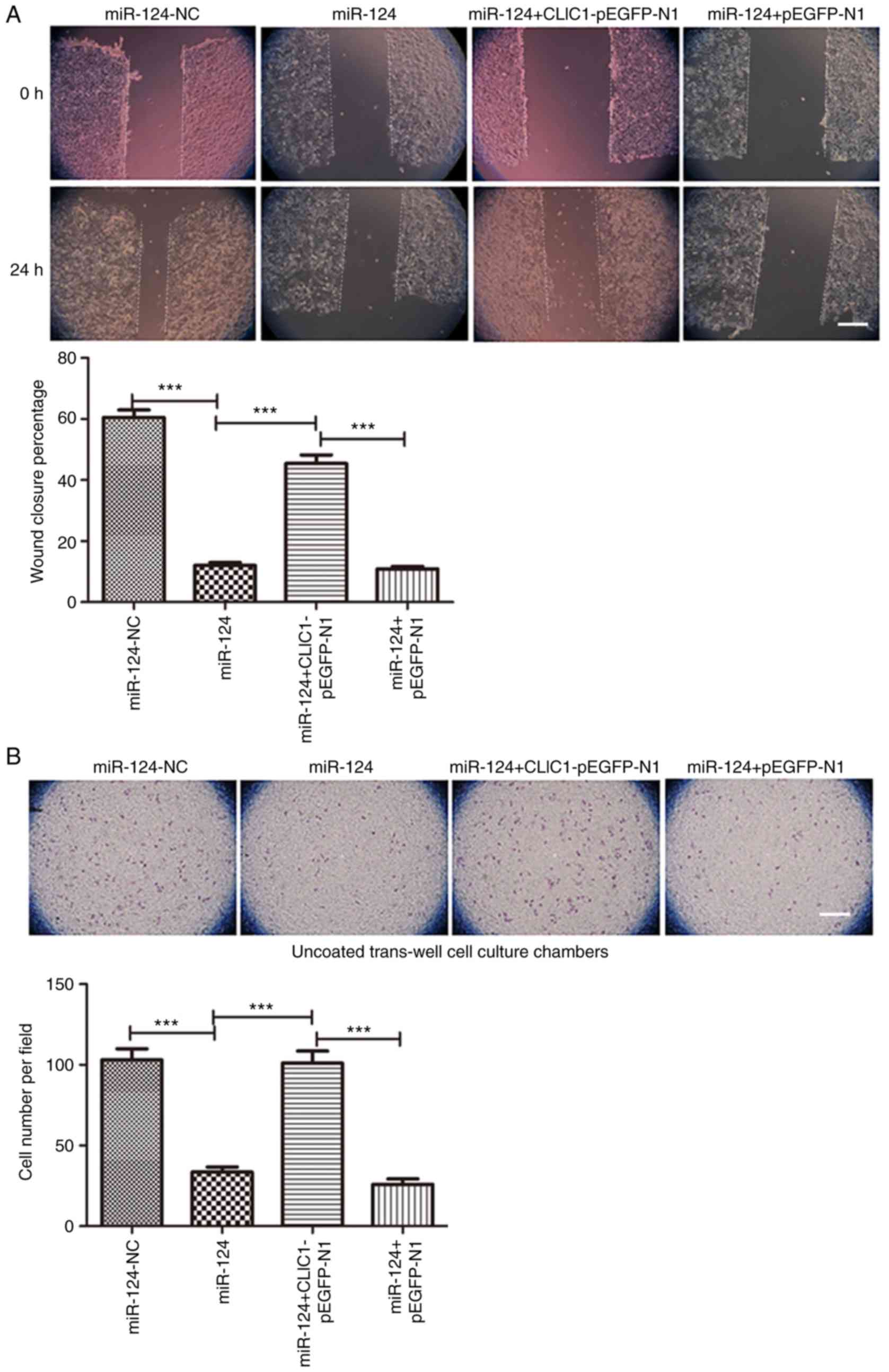
CLIC1 overexpression reverses the
miR-124-mediated inhibition of cancer cell migration and
invasion
Since CLIC1 is one of the targets of miR-124, CLIC1
was overexpressed in HepG2 cells transfected with an expression
plasmid together with miR-124. As expected, miR-124 itself and
miR-124 with the pEGFP-N1 vector both inhibited cell migration in
the scratch wound-healing motility assay (Fig. 4A). Similar to the miR-124 negative
control, cells expressing CLIC1 and miR-124 migrated faster and had
faster closure of wound healing than those transfected with miR-124
alone or miR-124 with the vehicle vector pEGFP-N1. In the Transwell
assay without Matrigel coating, there were fewer cells able to
migrate through the membrane in both the miR-124 and miR-124 +
pEGFP-N1 vector groups, but cells expressing CLIC1 and miR-124
migrated faster than cells transfected with miR-124 or miR-124 +
pEGFP-N1 vector (Fig. 4B). Similar to
cell migration, CLIC1 also reversed the miR-124-induced inhibition
of liver cancer cell invasion in the Matrigel-coated Transwell
invasion assay (Fig. 4C). These
results further support the hypothesis that CLIC1 is target of
miR-124 and is involved in the inhibition of liver cancer cell
migration and invasion.
Discussion
The goal of the present study was to investigate the
role of miR-124 in hepatocarcinogenesis. miR-124 is highly
expressed in the brain and was previously demonstrated to be a
‘brain-enriched’ miRNA (10,11). In recent years, researchers have
demonstrated that the expression of miR-124 is dysregulated in
almost all tumors, such as colorectal cancer (27), gastric cancer (28), lung cancer (16) and hepatocellular carcinoma (29). In the progression of cancer, miR-124
not only acts as a suppressor of tumor growth but also decreases
cell invasion and metastasis. These previous results suggest that
miR-124 plays an important role in tumorigenesis and tumor
progression. The present study found that miR-124 was downregulated
in liver cancer cells and that miR-124 could suppress liver cancer
cell proliferation, migration and invasion, indicating its role as
a tumor suppressor in liver cancer.
In an earlier study, it was shown that
overexpression of miR-124 suppressed tumor growth by targeting
STAT3 (30), but the mechanism of
action of miR-124 in liver cancer metastasis remained elusive.
Invasion, as one of the hallmarks of malignant tumors, renders
liver cancer difficult to cure. To elucidate the mechanism of
metastasis, miR-124 targets were investigated using 2-DGE and mass
spectrometry to analyze the differentially expressed proteins
between miR-124-overexpressing and control cells. The results of
the mass spectrometry led to a focus on CLIC1 as a potential
target. Furthermore, the results revealed that miR-124 directly
targeted CLIC1 to inhibit cell migration and invasion in liver
cancer cells. Moreover, CLIC1 rescued the miR-124-mediated
repression of cell migration and invasion. For the first time, to
the best of our knowledge, CLIC1 was identified as a functional
target of miR-124 in the inhibition of cell migration and invasion.
These findings suggested that miR-124 and CLIC1 play critical roles
in liver cancer metastasis.
From the 2-DGE and mass spectrometry results,
several proteins with decreased expression were identified. Based
on literature research, CLIC1 is highly expressed in several human
malignant tumors, such as nasopharyngeal carcinoma, ovarian cancer
and glioma, and was considered a promising diagnostic biomarker
(31–33). According to functional research, CLIC1
plays an important role in neoplastic transformation (34,35), and
promotes the cell motility and invasion of gallbladder carcinoma
(36) and prostate cancer (37). However, the function of CLIC1 in liver
cancer is still elusive. The present results identified CLIC1 to be
a functional target of miR-124; thus, this was a focus of further
investigation.
A 3′-UTR luciferase assay was performed, and it was
observed that co-transfection of miR-124 and the 3′-UTR of CLIC1 in
the pMIR-REPORT vector resulted in decreased luciferase activity.
Notably, in the 3′-UTR of CLIC1 mRNA, there was no complementary
sequence of miR-124 found using the target prediction software
(TargetScan). The best-known mechanism of miRNA regulation is
direct binding with the seed sequence located in the 3′-UTR of the
mRNA. Studies have demonstrated that miRNAs have some other
regulatory methods, such as interactions with regulatory proteins,
miRNAs and long noncoding RNAs to regulate protein expression.
Eiring et al (38) showed that
miR-328 interacts with poly(rC) binding protein 2 to modulate mRNA
translation, and the interaction is independent of the miRNA seed
sequence. Tang et al (39)
reported that miRNA-709 regulates cell apoptosis through the
miRNA-15a/16-1 pathway. miRNAs also regulate gene expression by
targeting long noncoding RNAs. Du et al (40) reported that miR-124 regulates ERK/MAPK
by targeting MALAT1. Altogether, these data suggest that miRNAs
function in cells both through base pairing with seed sequences and
though interference with other regulatory proteins, miRNAs, and
long non coding RNAs. However, many miRNA regulatory mechanisms
remain unknown. There may exist some unknown regulatory interaction
between miR-124 and CLIC1. A future study look into the mechanism
of how miR-124 regulates CLIC1 expression. CLIC1 protein expression
was also markedly decreased in HepG2 cells transfected with
miR-124. Subsequently, it was demonstrated that CLIC1 is a
functional target of miR-124. The knockdown of CLIC1 affected
cancer cell migration and invasion, but not cell proliferation.
Similarly, overexpressed CLIC1 reversed the tumor suppressor
function of miR-124 in terms of cell migration and invasion, but
not cell viability. This suggested that the oncogenic function of
CLIC1 in liver cancer cells correlates with cell motility and
related pathways, but not pathways related to the cell cycle. It
has been reported that CLIC1 regulates the migration and invasion
of colon cancer cells by decreasing the regulatory volume decrease
capacity (41). A study by Wang et
al (42) reported that the
inhibition of CLIC1 channel activity leads to decreased cell
migration through the ROS/ERK pathway. Further studies may be
undertaken to examine the regulatory function of CLIC1 in cell
motility-related signaling pathways.
In conclusion, the present study demonstrated that
miR-124 is downregulated in liver cancer cells, and identified a
new functional target gene of miR-124. Further in vitro
studies showed that miR-124 repressed the migration and invasion of
liver cancer cells by reducing the expression of CLIC1. Moreover,
the knockdown of CLIC1 in miR-124 ectopically-expressing liver
cancer cells reversed the effects of miR-124. Combined with all
aforementioned studies, the present data contribute to the
understanding of the biological function of miR-124 in tumor
metastasis.
Acknowledgements
The authors would like to thank Mr. Yang Yang and
Ms. Manhui Li for their insight and technical support. The authors
would also like to thank Ms. Li Li for the correction of the
English in the manuscript.
Funding
The present study was supported by research grants
from the Scientific Cooperation Planning Project of Guizhou
Province [grant. no. Qian Ke He LH Zi (2015)7530]; the National
Natural Scientific Foundation of China (grant. no. 81171447);
Program of Science and Technology Department of Guizhou Province
(grant. no. QKHPTRC-201905612); and the ZMU Startup Fund for
Doctors (grant. no. F-696).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article. The datasets used
and/or analyzed during the current study are available from the
corresponding author on reasonable request.
Authors' contributions
JZ and YL designed the research. XY and YL performed
the research and prepared all the figures. YC and QY assisted with
the data analysis. YL, JZ and YC wrote the main manuscript text.
All authors reviewed the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marengo A, Rosso C and Bugianesi E: Liver
cancer: Connections with obesity, fatty liver, and cirrhosis. Annu
Rev Med. 67:103–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruix J, Qin S, Merle P, Granito A, Huang
YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al:
Regorafenib for patients with hepatocellular carcinoma who
progressed on sorafenib treatment (RESORCE): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet. 389:56–66.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu Y, Yang C, Yang S, Cheng F, Rao J and
Wang X: MiR-665 promotes hepatocellular carcinoma cell migration,
invasion, and proliferation by decreasing Hippo signaling through
targeting PTPRB. Cell Death Dis. 9:9542018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sarvizadeh M, Malekshahi ZV, Razi E,
Sharifi H, Moussavi N and Taghizadeh M: MicroRNA: A new player in
response to therapy for colorectal cancer. J Cell Physiol.
234:8533–8540. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Imani S, Wu RC and Fu J: MicroRNA-34
family in breast cancer: From research to therapeutic potential. J
Cancer. 9:3765–3775. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Makeyev EV, Zhang J, Carrasco MA and
Maniatis T: The MicroRNA miR-124 promotes neuronal differentiation
by triggering brain-specific alternative pre-mRNA splicing. Mol
Cell. 27:435–448. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chandrasekar V and Dreyer JL: microRNAs
miR-124, let-7d and miR-181a regulate cocaine-induced plasticity.
Mol Cell Neurosci. 42:350–362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng LC, Pastrana E, Tavazoie M and
Doetsch F: MiR-124 regulates adult neurogenesis in the
subventricular zone stem cell niche. Nat Neurosci. 12:399–408.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dal Moro F, Valotto C, Guttilla A and
Zattoni F: Urinary markers in the everyday diagnosis of bladder
cancer. Urologia. 80:265–275. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi XB, Xue L, Ma AH, Tepper CG,
Gandour-Edwards R, Kung HJ and deVere White RW: Tumor suppressive
miR-124 targets androgen receptor and inhibits proliferation of
prostate cancer cells. Oncogene. 32:4130–4138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Q, Xu L, Wang C, Fan W, Yan H and Li Q:
MicroRNA-124-3p represses cell growth and cell motility by
targeting EphA2 in glioma. Biochem Biophys Res Commun.
503:2436–2442. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Z, Gong Q, Li M, Xu J, Zheng Y, Ge P
and Chi G: MicroRNA-124 inhibits the proliferation of C6 glioma
cells by targeting Smad4. Int J Mol Med. 40:1226–1234. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao X, Lu C, Chu W, Zhang B, Zhen Q, Wang
R, Zhang Y, Li Z, Lv B, Li H and Liu J: MicroRNA-124 suppresses
proliferation and glycolysis in non-small cell lung cancer cells by
targeting AKT-GLUT1/HKII. Tumour Biol. 39:10104283177062152017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Long HD, Ma YS, Yang HQ, Xue SB, Liu JB,
Yu F, Lv ZW, Li JY, Xie RT, Chang ZY, et al: Reduced hsa-miR-124-3p
levels are associated with the poor survival of patients with
hepatocellular carcinoma. Mol Biol Rep. 45:2615–2623. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu LP, Wu J, Shang A, Yang M, Li LL, Yu J,
Xu LR, Wang CB, Wang WW, Zhu JJ and Lu WY: MiR-124 inhibits
progression of hepatocarcinoma by targeting KLF4 and promises a
novel diagnostic marker. Artif Cells Nanomed Biotechnol. 46 (Suppl
1):S159–S167. 2018. View Article : Google Scholar
|
|
19
|
Ulmasov B, Bruno J, Woost PG and Edwards
JC: Tissue and subcellular distribution of CLIC1. BMC Cell Biol.
8:82007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Valenzuela SM, Martin DK, Por SB, Robbins
JM, Warton K, Bootcov MR, Schofield PR, Campbell TJ and Breit SN:
Molecular cloning and expression of a chloride ion channel of cell
nuclei. J Biol Chem. 272:12575–12582. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu W, Cui R, Qu H, Liu C, Deng H and Zhang
Z: Expression and prognostic value of CLIC1 in epithelial ovarian
cancer. Exp Ther Med. 15:4943–4949. 2018.PubMed/NCBI
|
|
22
|
Li BP, Mao YT, Wang Z, Chen YY, Wang Y,
Zhai CY, Shi B, Liu SY, Liu JL and Chen JQ: CLIC1 Promotes the
progression of gastric cancer by regulating the MAPK/AKT pathways.
Cell Physiol Biochem. 46:907–924. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu J, Dong Q, Zhang B, Wang X, Ye B, Zhang
F, Song X, Gao G, Mu J, Wang Z, et al: Chloride intracellular
channel 1 (CLIC1) is activated and functions as an oncogene in
pancreatic cancer. Med Oncol. 32:6162015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wei X, Li J, Xie H, Wang H, Wang J, Zhang
X, Zhuang R, Lu D, Ling Q, Zhou L, et al: Chloride intracellular
channel 1 participates in migration and invasion of hepatocellular
carcinoma by targeting maspin. J Gastroenterol Hepatol. 30:208–216.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Lu Y, Yue X, Li H, Luo X, Wang Y,
Wang K and Wan J: MiR-124 suppresses growth of human colorectal
cancer by inhibiting STAT3. PLoS One. 8:e703002013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Taniguchi K, Sugito N, Kumazaki M,
Shinohara H, Yamada N, Nakagawa Y, Ito Y, Otsuki Y, Uno B, Uchiyama
K and Akao Y: MicroRNA-124 inhibits cancer cell growth through
PTB1/PKM1/PKM2 feedback cascade in colorectal cancer. Cancer Lett.
363:17–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xiao HJ, Ji Q, Yang L, Li RT, Zhang C and
Hou JM: In vivo and in vitro effects of microRNA-124 on human
gastric cancer by targeting JAG1 through the Notch signaling
pathway. J Cell Biochem. 119:2520–2534. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cao J, Qiu J, Wang X, Lu Z, Wang D, Feng
H, Li X, Liu Q, Pan H, Han X, et al: Identification of microRNA-124
in regulation of hepatocellular carcinoma through BIRC3 and the
NF-kB pathway. J Cancer. 9:3006–3015. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu Y, Yue X, Cui Y, Zhang J and Wang K:
MicroRNA-124 suppresses growth of human hepatocellular carcinoma by
targeting STAT3. Biochem Biophys Res Commun. 441:873–879. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang YH, Wu CC, Chang KP, Yu JS, Chang YC
and Liao PC: Cell secretome analysis using hollow fiber culture
system leads to the discovery of CLIC1 protein as a novel plasma
marker for nasopharyngeal carcinoma. J Proteome Res. 8:5465–5474.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Singha B, Harper SL, Goldman AR, Bitler
BG, Aird KM, Borowsky ME, Cadungog MG, Liu Q, Zhang R, Jean S, et
al: CLIC1 and CLIC4 complement CA125 as a diagnostic biomarker
panel for all subtypes of epithelial ovarian cancer. Sci Rep.
8:147252018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang L, He S, Tu Y, Ji P, Zong J, Zhang J,
Feng F, Zhao J, Zhang Y and Gao G: Elevated expression of chloride
intracellular channel 1 is correlated with poor prognosis in human
gliomas. J Exp Clin Cancer Res. 31:442012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
He YM, Zhang ZL, Liu QY, Xiao YS, Wei L,
Xi C and Nan X: Effect of CLIC1 gene silencing on proliferation,
migration, invasion and apoptosis of human gallbladder cancer
cells. J Cell Mol Med. 22:2569–2579. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peretti M, Angelini M, Savalli N, Florio
T, Yuspa SH and Mazzanti M: Chloride channels in cancer: Focus on
chloride intracellular channel 1 and 4 (CLIC1 AND CLIC4) proteins
in tumor development and as novel therapeutic targets. Biochim
Biophys Acta. 1848:2523–2531. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang JW, Peng SY, Li JT, Wang Y, Zhang ZP,
Cheng Y, Cheng DQ, Weng WH, Wu XS, Fei XZ, et al: Identification of
metastasis-associated proteins involved in gallbladder carcinoma
metastasis by proteomic analysis and functional exploration of
chloride intracellular channel 1. Cancer Lett. 281:71–81. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tian Y, Guan Y, Jia Y, Meng Q and Yang J:
Chloride intracellular channel 1 regulates prostate cancer cell
proliferation and migration through the MAPK/ERK pathway. Cancer
Biother Radiopharm. 29:339–344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Eiring AM, Harb JG, Neviani P, Garton C,
Oaks JJ, Spizzo R, Liu S, Schwind S, Santhanam R, Hickey CJ, et al:
miR-328 functions as an RNA decoy to modulate hnRNP E2 regulation
of mRNA translation in leukemic blasts. Cell. 140:652–665. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tang R, Li L, Zhu D, Hou D, Cao T, Gu H,
Zhang J, Chen J, Zhang CY and Zen K: Mouse miRNA-709 directly
regulates miRNA-15a/16-1 biogenesis at the posttranscriptional
level in the nucleus: Evidence for a microRNA hierarchy system.
Cell Res. 22:504–515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Du M, Chen W, Zhang W, Tian XK, Wang T, Wu
J, Gu J, Zhang N, Lu ZW, Qian LX, et al: TGF-? regulates the
ERK/MAPK pathway independent of the SMAD pathway by repressing
miRNA-124 to increase MALAT1 expression in nasopharyngeal
carcinoma. Biomed Pharmacother. 99:688–696. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang P, Zhang C, Yu P, Tang B, Liu T, Cui
H and Xu J: Regulation of colon cancer cell migration and invasion
by CLIC1-mediated RVD. Mol Cell Biochem. 365:313–321. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang P, Zeng Y, Liu T, Zhang C, Yu PW, Hao
YX, Luo HX and Liu G: Chloride intracellular channel 1 regulates
colon cancer cell migration and invasion through ROS/ERK pathway.
World J Gastroenterol. 20:2071–2078. 2014. View Article : Google Scholar : PubMed/NCBI
|