Introduction
Acute pancreatitis (AP) is associated with an annual
incidence ranging from 13 to 45 per 100,000 individuals in the USA
(1–3).
Thus, the attenuation of the increasing prevalence of AP is a major
challenge in the medical field (4).
In clinical situations of pancreatitis, including endoscopic
retrograde pancreatography and gallstone passing, the pancreatic
ductal epithelium would appear to be the most exposed to noxious
stimuli (5). Pancreatic ductal
epithelial cells and their secreted mucus constitute a pancreatic
duct mucosal barrier, which prevents bile and trypsin from flowing
back into the pancreas in a physiological state and has the barrier
function of protecting pancreatic tissue from damage by internal
and external substances (6). It has
been reported that transient hypertension in the pancreatic duct
triggers a pancreatic inflammatory cascade and destroys tight
junction integrity in a mouse model (7,8). The
inappropriate activation of pancreatic zymogen in the pancreas
results in exocrine gland parenchymal cell damage and necrosis,
which eventually causes AP (9–11).
Furthermore, injured pancreatic ductal cells can produce and
release inflammatory factors, including interleukin (IL)-6 and IL-8
(5,12). Thus, pancreatic ductal cells play
critical roles in injury in AP. However, previous studies on AP
have focused on pancreatic acinar cells (13–15), while
studies on pancreatic ductal cells are limited (7).
Several recent studies have indicated that adenosine
triphosphate (ATP), mainly released by acinar cells in the
pancreas, can regulate pancreatic duct function via P2Y and P2X
purinoceptor, and ATP-activated purinergic receptors may play
important roles in pancreas pathophysiology (16,17). The
study by Hoque et al also demonstrated that ATP released
from upstream pancreatic acinar cells induced the activation of
inflammatory responses (18). As
regards the underlying molecular mechanisms, purinergic receptor
P2X, ligand-gated ion channel, 7 (P2X7), is a member of the P2X
family of ATP-gated cation channels, and it can trigger diverse
downstream signaling cascades, mainly including mitogen-activated
protein kinases (MAPKs), reactive oxygen species (ROS) and nuclear
factor-κB (NF-κB) transcription through coupling the ATP channels
(19–23). Previous studies have demonstrated that
P2X7 respectively cleaves pro-IL-1β and pro-IL-18 into mature IL-1β
and IL-18 via the recruitment of the NOD-like receptor protein 3
(NLRP3) inflammasome, which is composed of NLRP3, the adaptor
protein apoptosis-associated speck-like protein containing a CARD
(ASC) and the effector protein caspase-1. IL-1β and IL-18-dependent
pathways further cause pancreatic injury (19,24). Thus,
the ATP activated-P2X7/NLRP3 signaling pathway may be a potent
therapeutic target for AP insult.
In China, Rheum palmatum L. has been utilized
in the treatment of AP for a number of years as a separate or
principal component in traditional formulas (e.g., Da-Cheng-Qi
decoction). Emodin, also known as
1,3,8-trihydroxy-6-methylanthraquinone, is major component of
Rheum palmatum L. (25). Over
the past decades, significant progress has been made to evaluate
the pharmacological activities of emodin in vivo and in
vitro. Our previous study demonstrated that emodin exerts
significant protective effects on sodium taurocholate-treated
pancreatic acinar cells, although its effects on the injury of
pancreatic ductal cells have not been reported to date, at least to
the best of our knowledge (26). The
present study evaluated the effects and potential mechanisms of
action of emodin on the injury of pancreatic ductal cells in AP
using an ATP-induced pancreatic ductal cell injury model.
Materials and methods
Cells and cell culture
The pancreatic human duct cell line, HPDE6-C7, was
purchased from the American Type Culture Collection (ATCC). The
cells were cultured in Dulbecco's modified Eagle's medium (DMEM;
HyClone) supplemented with high glucose and 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific) at 37°C in a 5%
CO2 incubator.
ATP-induced cell injury
Cell viability assay using the MTT method was
carried out to determine the most suitable damaging concentration
of ATP (Solarbio) in vitro. In brief, HPDE6-C7 cells
(1×105 cells/ml) were treated with various
concentrations of ATP (40, 20, 10, 5, 2.5 and 1.25 mM) for 16 h
after 24 h of culture in 96-well plates. Subsequently, 10 µl MTT
solution (5 mg/ml) were added into each well followed by 4 h of
incubation at 37°C. Finally, the formazan crystals were dissolved
with 100 µl dimethyl sulfoxide (DMSO) and measured with a
microplate reader (BioTek) at a wavelength of 490 nm.
Emodin cytotoxicity
The HPDE6-C7 cells (1×105 cells/ml) were
treated with gradient concentrations of emodin (180, 90, 45, 22.5,
11.25 and 5.625 µM) (Solarbio) for 24 h in a humidified atmosphere
of 5% CO2 at 37°C. Emodin was dissolved in DMSO to a
final concentration of <0.1%. Finally, the MTT method was used
to evaluate cell viability as described above.
Cell viability and morphology
To evaluate the effects of emodin on ATP-induced
HPDE6-C7 cell injury, the cells were divided 5 five groups as
follows: i) The control group; ii) ATP group; and iii) low-, iv)
medium- and v) high-dose emodin groups. The cells in the control
group were cultured under normal conditions without being subjected
to any other treatment. The cells in the ATP group were pre-treated
with 40 mM ATP for 16 h, while the cells in the emodin groups were
respectively first treated with 11.25, 22.5 and 45 µM emodin for 24
h prior to a 16 h-incubation with ATP (40 mM). Cell viability was
then assessed using the aforementioned MTT method. To observe the
effects of emodin on the morphology of the HPDE6-C7 cells, the
cells were grown in 6-well plates and divided into 3 groups as
follows: i) The control group; ii) ATP group; and iii) high-dose
emodin (45 µM) group. The cells were pre-treated as described
above, and then imaged using a phase contrast microscope (Olympus
Corp.).
Immunofluorescence detection of
P2X7
Following culture in 6-well plates for 24 h at 37°C,
the HPDE6-C7 cells were subjected to different treatments. The
cells were then fixed in 4% paraformaldehyde and incubated with
diluted anti-P2X7 antibody (1:100; cat. no. ab48871; Abcam) at 4°C
overnight. The cells were then incubated with FITC-conjugated goat
anti-rabbit IgG (1:50; cat. no. SA00003-2; ProteinTech Group) for 1
h and re-stained with DAPI (1 µg/ml; ProteinTech Group) for 5 min
in the dark at 37°C. Immunohistochemical images (×100
magnification) were obtained using an Olympus BX63 fluorescence
microscope (Olympus Corp.).
Overexpression of the P2X7 gene
The HPDE6-C7 cells (1×105 cells/ml) were
seeded into 6-well plates for 24 h, and then transfected with a
P2X7 overexpression plasmid using Lipofectamine 2000 according to
the manufacturer's instructions (Thermo Fisher Scientific). The
P2X7 overexpression plasmid, also termed pEX-3-P2X7 cDNA (cat. no.
Y5251), was purchased from GenePharma Co., Ltd. Briefly, pEX-3-P2X7
cDNA (2 µg) was diluted in 200 µl of DMEM without serum, and then
Lipofectamine 2000 (4 µl) was diluted in 200 µl of DMEM. Following
a 5-min incubation at room temperature, the diluted DNA was
combined with the diluted Lipofectamine 2000. This was followed by
gentle mixing and incubation for 20 min at room temperature to
allow the DNA-Lipofectamine 2000 complexes to form. Finally, 400 µl
of the complexes were added to each well, and the wells were
replenished with 1.6 ml of DMEM. Following 24 h of transfection,
the cells were treated with emodin (45 µM) and ATP (40 mM).
Finally, the P2X7, NLRP3, ASC and caspase-1 protein expression
levels, and the contents of IL-1β and IL-18 in the cell supernatant
were measured by western blot analysis and ELISA, respectively.
ELISA
According to the manufacturer's instructions, the
IL-1β and IL-18 contents in the cell supernatant were evaluated
using commercially available human ELISA kits (Boster Bio). The OD
values in each well were measured using a multifunctional
microplate reader (BioTek) at a wavelength of 450 nm.
Western blot assay
Total proteins from the HPDE6-C7 cells were
extracted using a protein extraction kit (cat. no. KGP2100; KeyGen
Biotech), and the protein concentrations were determined using a
BCA protein assay kit (cat. no. KGP902; KeyGen Biotech). Protein
samples were separated by SDS-PAGE (10–12%) and then transferred
onto PVDF membranes (EMD Millipore). The membranes were then
blocked and incubated with primary antibodies against P2X7 (cat.
no. ab48871), NLRP3 (cat. no. ab214185), ASC (cat. no. ab155970)
and caspase-1 (cat. no. ab1872) (all from Abcam and 1:800 dilution
in TBST) overnight at 4°C. Following incubation with the secondary
antibody [cat. no. SA00001-2; HRP-conjugated Affinipure goat
anti-rabbit IgG (H+L) ProteinTech Group] (1:2,000 dilution in TBST)
at room temperature for 2 h, the protein expression in the
membranes was visualized by enhanced ECL using Tanon-5200 Multi Gel
Imaging System (Tanon Science and Technology). β-actin (cat. no.
ab179467; Abcam) was used as the internal control. Quantitative
analysis was performed using Gel-Pro analyzer 4.0 software (Media
Cybernetics).
Statistical analysis
Data are expressed as the means ± standard deviation
(SD) and analyzed using SPSS 17.0 software (SPSS, Inc.).
Differences between multiple groups were analyzed using one-way
analysis of variance with Tukey's post-hoc test. Comparisons
between 2 groups were made using an unpaired Student's t-test. A
value of P<0.05 was considered to indicate a statistically
significant difference.
Results
Emodin attenuates ATP-induced HPDE6-C7
cell injury
In order to establish a model of ATP-induced injury
using normal human pancreatic ductal epithelial cells, we adopted
various concentrations of ATP to stimulate the HPDE6-C7 cells. As
shown in Fig. 1A, ATP at a
concentration of 40 mM significantly decreased the viability of the
HPDE6-C7 cells. Thus, 40 mM was selected as the stimulus dose for
the in vitro model in the subsequent experiments.
Furthermore, to identify the safe concentration of emodin, the
cytotoxicity of emodin against the HPDE6-C7 cells was evaluated by
MTT assay. As shown in Fig. 1B, the
results indicated that treatment with 90 µM emodin exerted marked
cellular cytotoxicity (P<0.001), which demonstrated that 45 µM
was the maximum safe concentration of emodin. In addition, as shown
in Fig. 1C, emodin (45 µM) notably
increased cell viability compared with the ATP model group.
Furthermore, the cell morphology images presented in Fig. 1D indicated that ATP significantly
induced HPDE6-C7 cell death, and that this was attenuated by emodin
(45 µM). Therefore, these results suggested that emodin exerted a
potent protective effect against ATP-induced HPDE6-C7 cell
injury.
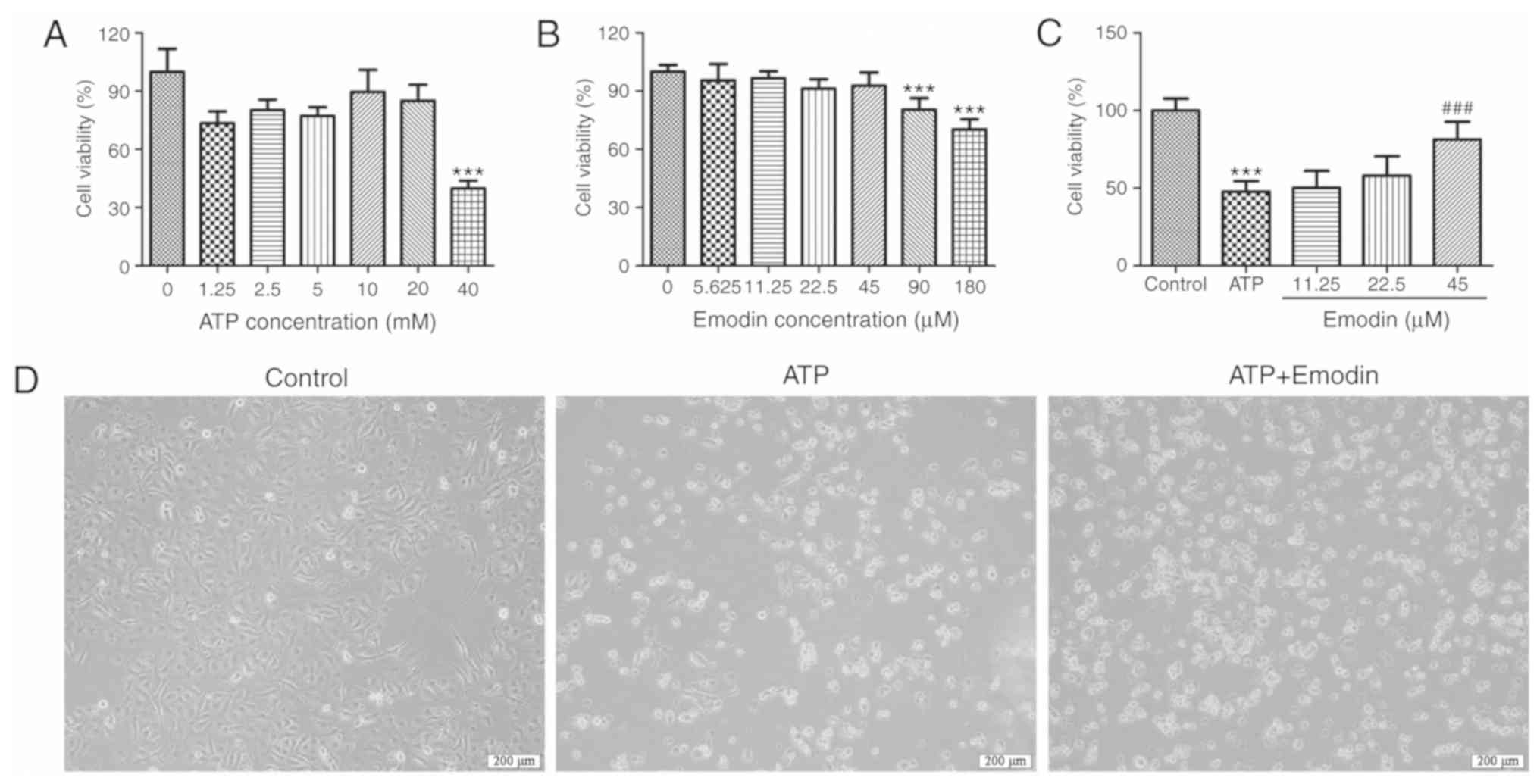 | Figure 1.Emodin attenuates ATP-induced HPDE6-C7
cell injury. (A) Effects of various concentrations doses of ATP
(1.25, 2.5, 5, 10, 20 and 40 mM) on HPDE6-C7 cell viability
following 16 h of pre treatment. (B) Cytotoxicity of emodin (5.625,
11.25, 22.5, 45 and 90 µM) on HPDE6-C7 cells following 24 h of
pre-treatment. (C) Effects of emodin (11.25, 22.5 and 45 µM) on the
viability of ATP (40 mM)-treated HPDE6-C7 cells. (D) Representative
images of HPDE6-C7 cell morphology in the different groups
(magnification, ×100); ATP, 40 mM; emodin, 45 µM (n=6; ‘n’ refers
to the number of samples). ***P<0.001 vs. control group,
###P<0.001 vs. ATP group. ATP, adenosine
triphosphate. |
Emodin suppresses the expression of
proteins associated with the P2X7/NLRP3 signaling pathway in
ATP-stimulated HPDE6-C7 cells
In order to examine the effects of emodin on P2X7
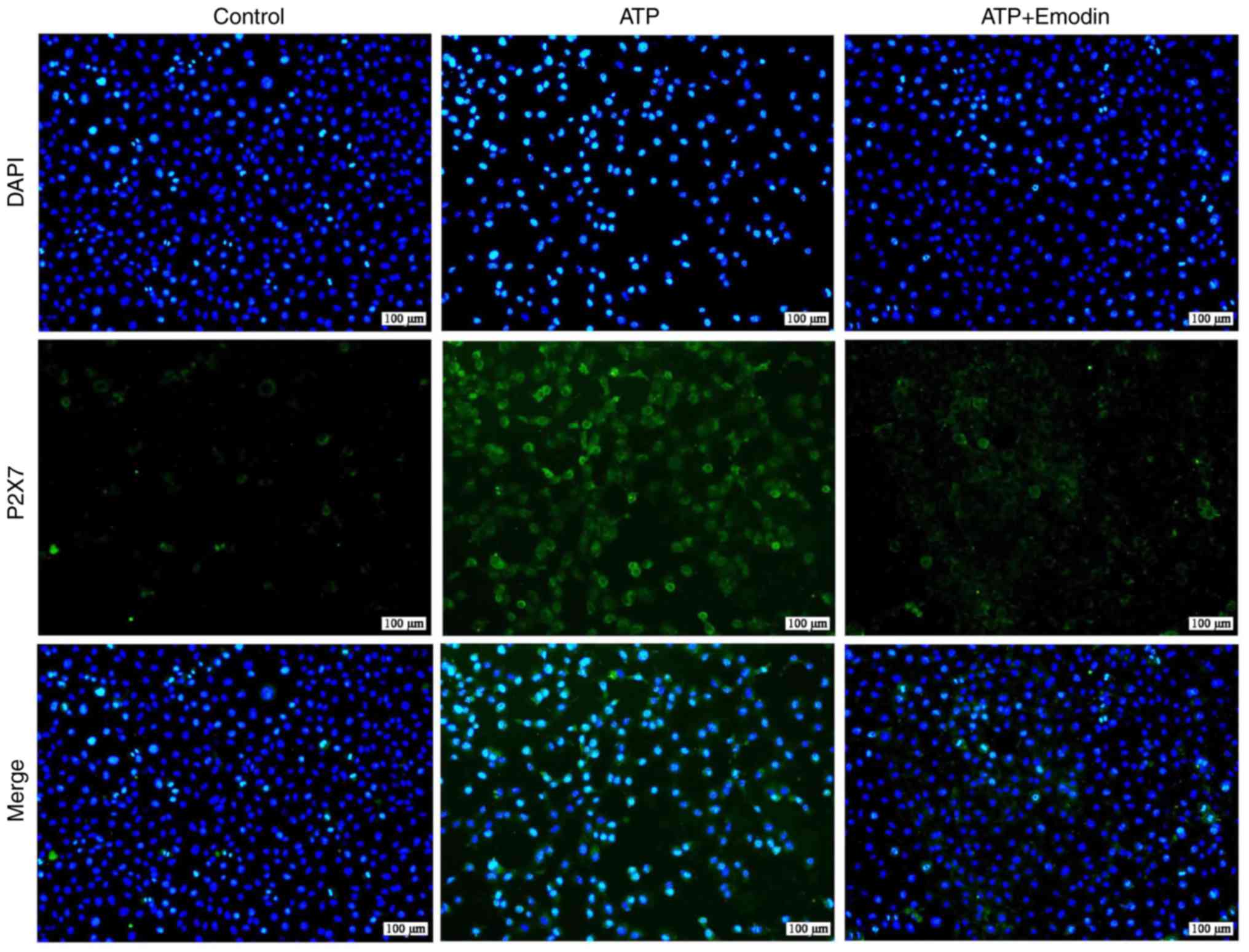
protein expression, we performed a P2X7 immunofluorescence assay.
As shown in Figs. 2 and S1, the number of P2X7-positive cells
exhibiting green fluorescence was significantly increased in the
ATP group; however, treatment with emodin (45 µM) markedly
decreased this green fluorescence. Subsequently, we evaluated
whether the P2X7/NLRP3 signaling pathway is involved in the
protective effects of emodin against ATP-induced HPDE6-C7 cell
injury. The results shown in Fig. 3
revealed that the expression of proteins associated with the
P2X7/NLRP3 signaling pathway, including P2X7, NLRP3, ASC and
caspase-1 in the ATP group was significantly increased compared
with the control group. Treatment with emodin (45 µM) markedly
inhibited the levels of these proteins. Therefore, emodin can
attenuate HPDE6-C7 cell injury induced by ATP through the
inhibition of the P2X7/NLRP3 signaling pathway.
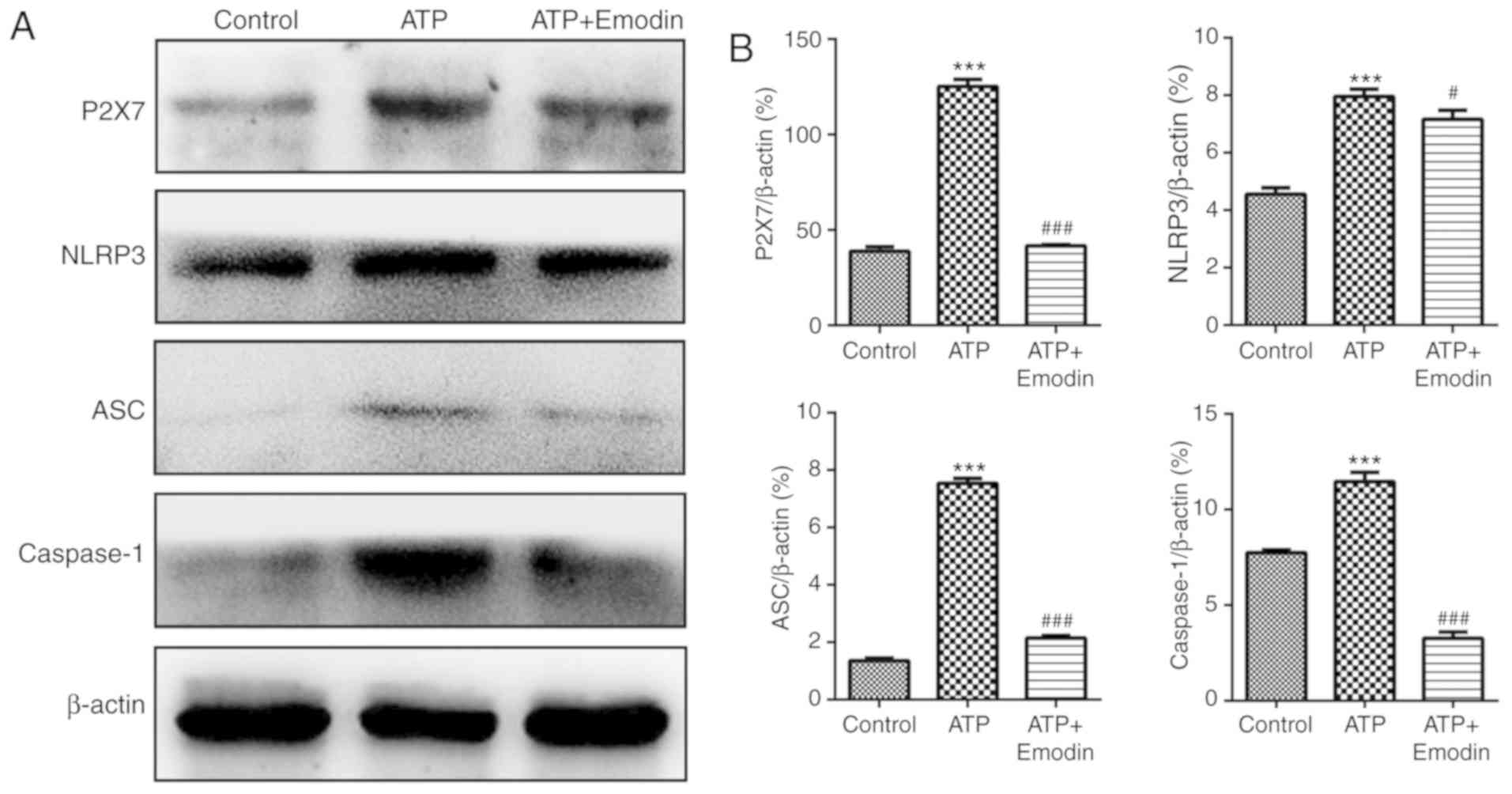 | Figure 3.Emodin inhibits the expression of
proteins associated with the P2X7/NLRP3 signaling pathway in
ATP-stimulated HPDE6-C7 cells. (A) Effects of emodin (45 µM) on the
protein expression of P2X7, NLRP3, ASC and caspase-1 in
ATP-stimulated HPDE6-C7 cells. (B) Statistical analysis of the
effects of emodin on protein expression (n=3; ‘n’ refers to the
number of repeats). ***P<0.001 vs. control group,
#P<0.05 vs. ATP group, ###P<0.001 vs.
ATP group. ATP, adenosine triphosphate; P2X7, purinergic receptor
P2X, ligand-gated ion channel, 7; NLRP3, NOD-like receptor protein
3; ASC, apoptosis-associated speck-like protein containing a
CARD. |
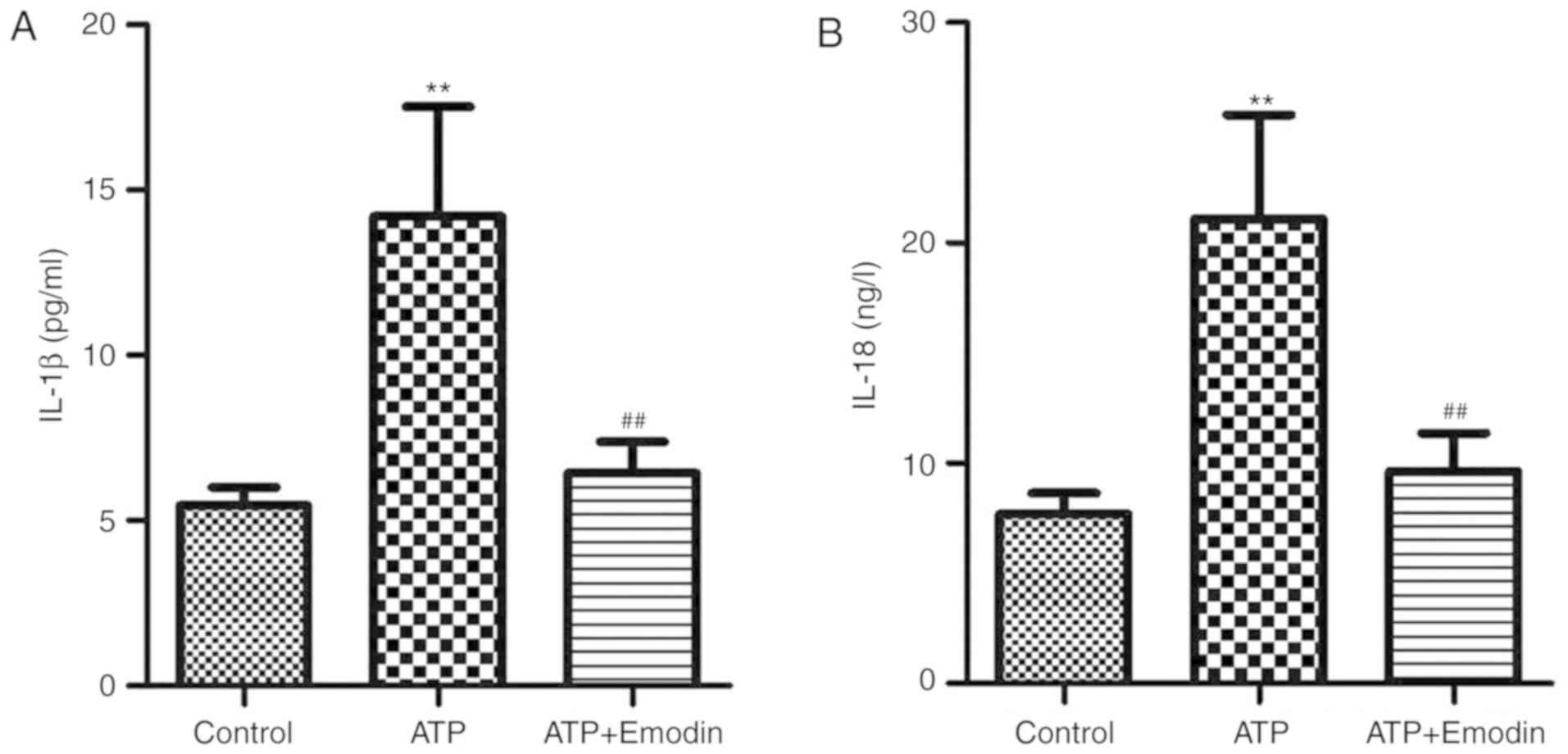
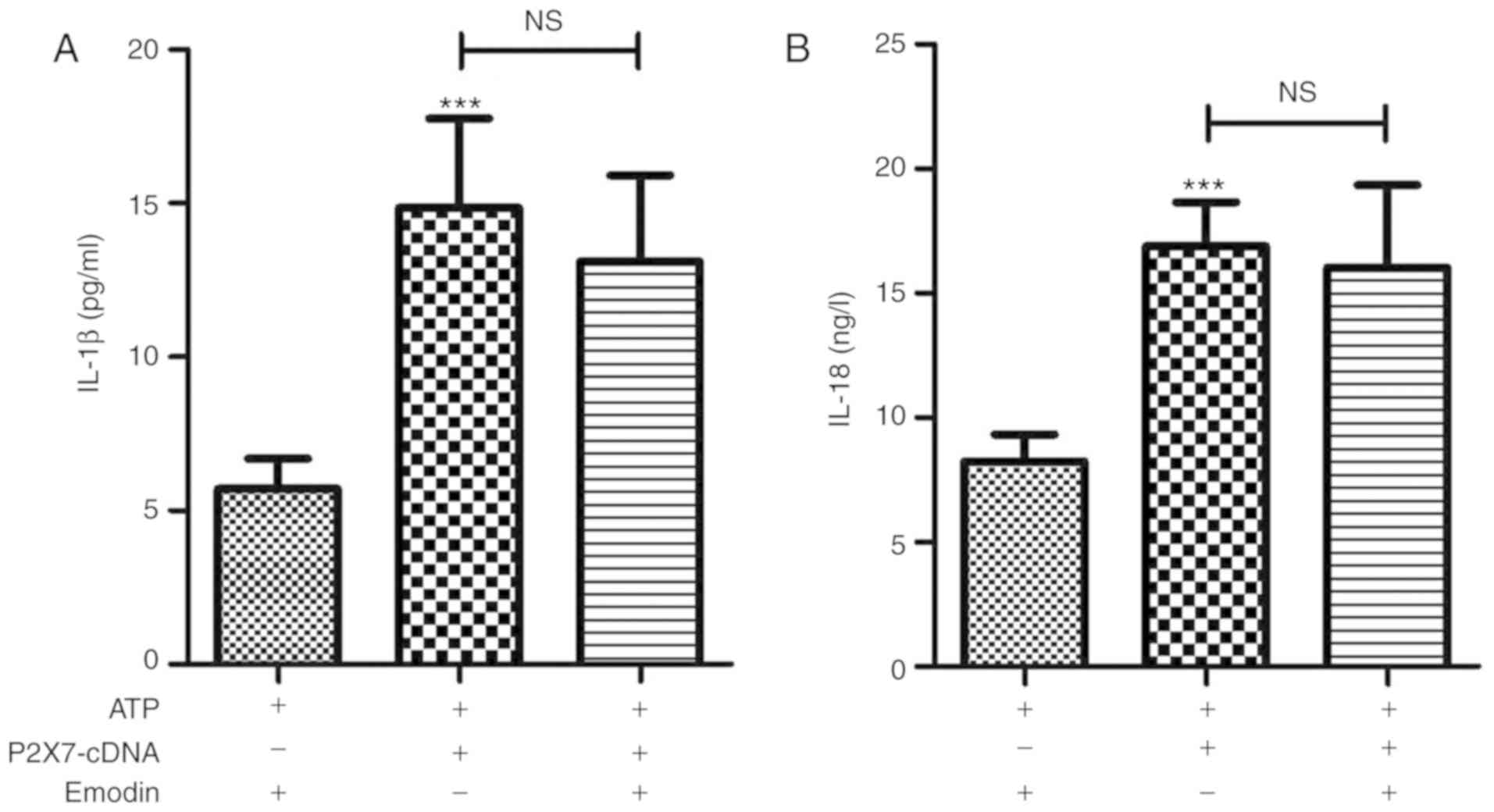
Emodin reduces the contents of IL-1β
and IL-18 in the supernatant of ATP-stimulated HPDE6-C7 cells
The present study examined the effects of emodin on
the conents of IL-1β and IL-18 in ATP-stimulated HPDE6-C7 cells.
The results shown in Fig. 4 indicated
that the contents of IL-1β and IL-18 in the cell supernatant were
significantly increased in the model group, and that these effects
were reversed by treatment with emodin (45 µM).
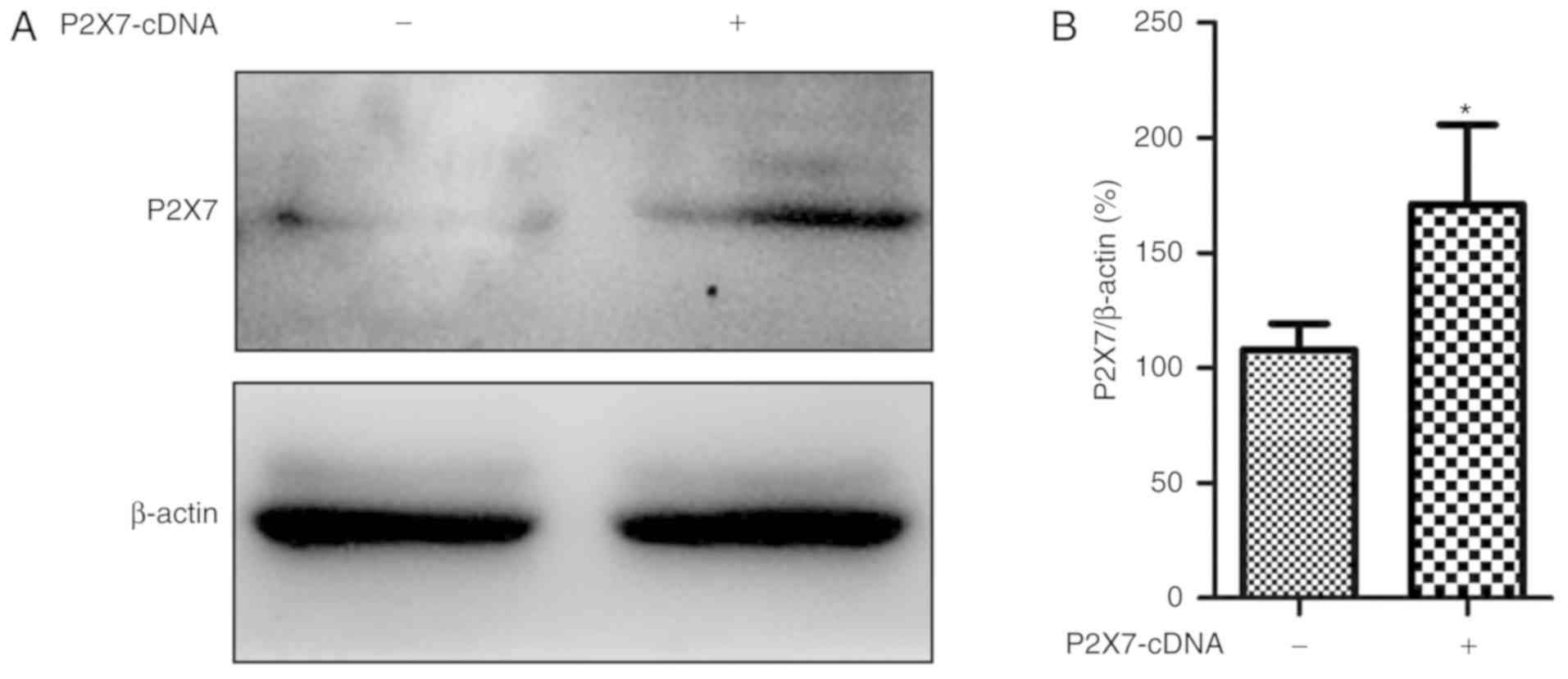
Overexpression of P2X7 abrogates the
protective effects of emodin on ATP-stimulated HPDE6-C7 cells
In order to explore the role of P2X7 on the
protective effects of emodin against ATP-induced HPDE6-C7 cell
injury, a P2X7-cDNA plasmid was used to overexpress the P2X7 gene.
As shown in Fig. 5, the protein level
of P2X7 was notably increased following transfection with the P2X7
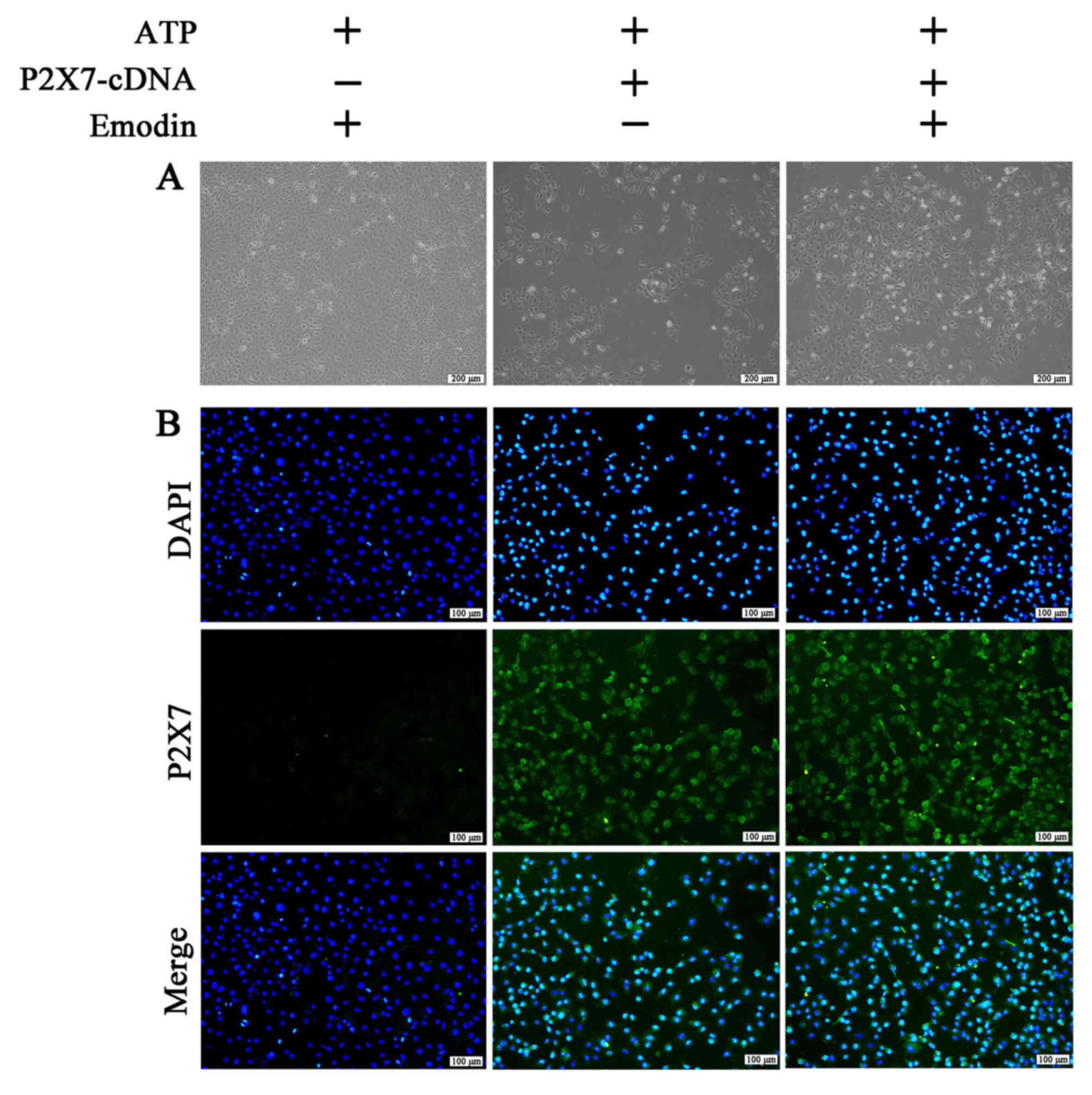
plasmid compared with the negative control group. Furthermore, as
shown in Figs. 6 and S2, the morphological damage and the number
of P2X7-positive cells in the P2X7 overexpression group were
significantly increased compared with the negative control group.
However, these effects were not attenuated by treatment with emodin
(45 µM). Therefore, P2X7 overexpression reversed the protective
effects of emodin against ATP-induced HPDE6-C7 cell injury.
In addition, as shown in Fig. 7, following transfection with the P2X7
overexpression plasmid, regardless of the presence of emodin, the
expression levels of P2X7, NLRP3, ASC and caspase-1 were all
notably upregulated compared with the control group. In addition,
as shown in Fig. 8, similar results
were also observed regarding the release of IL-1β and IL-18 in the
cell supernatant. Overall, these results suggested that P2X7
overexpression abrogated the regulation of the P2X7/NLRP3 signaling
pathway mediated by emodin, which further demonstrated that the
protective actions of emodin on ATP-induced pancreatic ductal cell
injury were mainly due to the inhibition of the P2X7/NLRP3
signaling pathway.
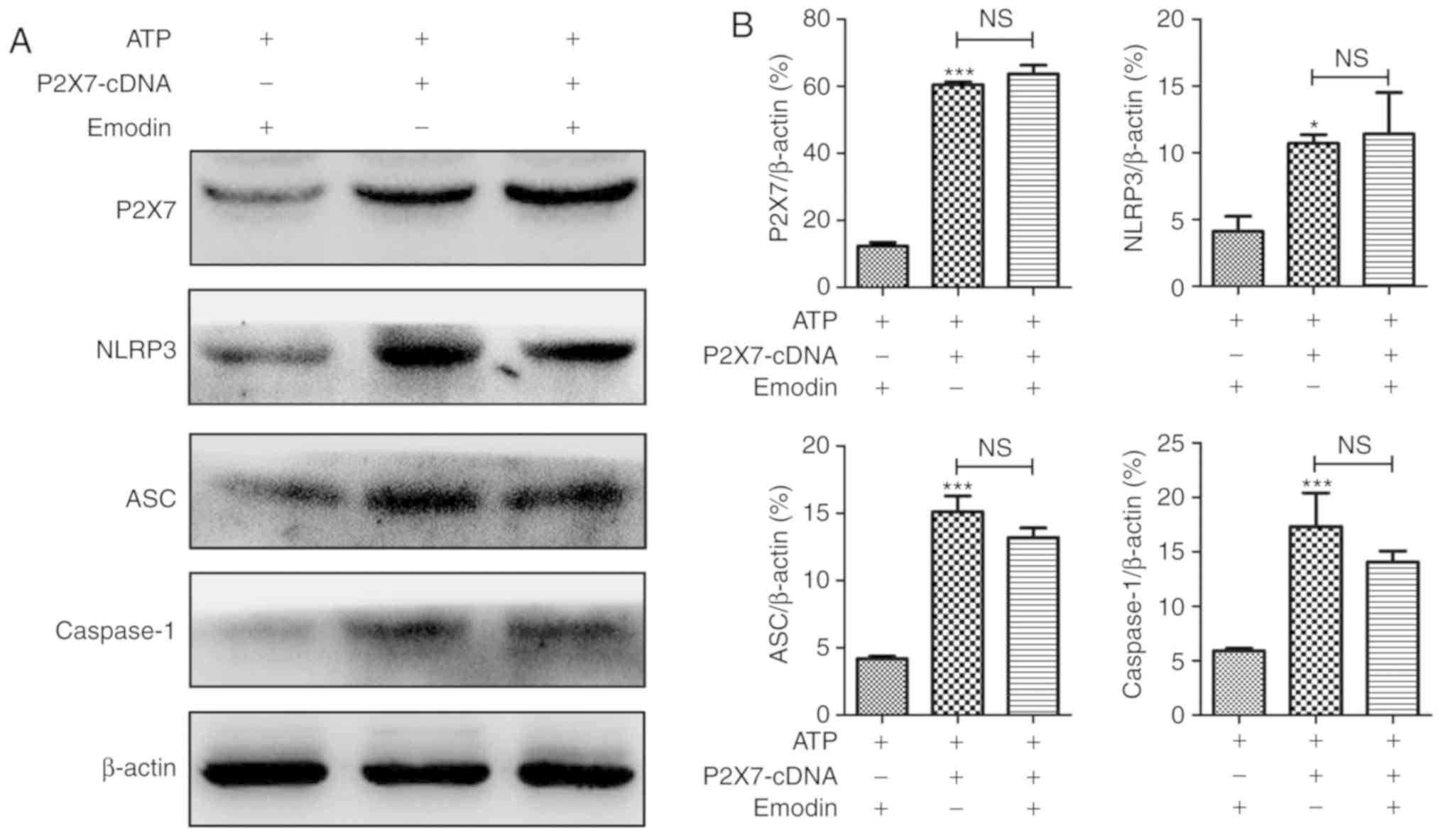 | Figure 7.The inhibitory effects of emodin on
the P2X7/NLRP3 pathway are abrogated by P2X7 overexpression
performed by transfection with pEX-3-P2X7 cDNA. (A) Effects of
emodin and P2X7 overexpression on P2X7, NLRP3, ASC and caspase-1
expression levels in adenosine triphosphate-treated HPDE6-C7 cells
based on the results of western blot analysis. (B) Statistical
analysis of the effects of emodin and P2X7 overexpression on
protein expressions levels (n=3; ‘n’ refers to the number of
repeats). *P<0.05 vs. control group, ***P<0.001 vs. control
group, NS, not significant; ATP, adenosine triphosphate; P2X7,
purinergic receptor P2X, ligand-gated ion channel, 7; NLRP3,
NOD-like receptor protein 3; ASC, apoptosis-associated speck-like
protein containing a CARD. |
Discussion
The pancreatic duct is mainly formed by epithelial
cells, since it is linked by centro-acinar cells interfaced with
pancreatic acini at the terminal end (27). A previous study reported that
pancreatic acini can release ATP into the ductal lumen in response
to various stimuli, thus activating P2Y and P2X receptors to
regulate the epithelial secretion of the pancreatic duct (28). Ductal occlusion and injury have been
postulated to lead to focal pancreatic inflammation, and the time
of ductal occlusion determines the severity of AP. Thus, the
present study established a model of ATP-induced injury using
normal human pancreatic ductal epithelial cells (HPDE6-C7) and
revealed that ATP at a concentration of 40 mM markedly decreased
cell viability. Furthermore, to examine the protective effects of
emodin against ATP-induced HPDE6-C7 cell injury, the cytotoxicity
of emodin on HPDE6-C7 cells was evaluated, and it was observed that
45 µM was the maximum safe concentration for emodin. Emodin at this
safe concentration markedly increased cell viability, and
attenuated the adverse morphological changes in the HPDE6-C7 cells
stimulated with ATP. Therefore, the results of the present study
indicated that emodin exerted a potent effect against ATP-induced
pancreatic ductal epithelial cell injury.
P2X7 is primarily expressed in rodent duct cells in
the pancreas and is a member of the P2X family of ATP-gated cation
channels (29,30). The activation of P2X7 can effectively
stimulate NLRP3, which is a member of the NOD-like receptor family,
and can respond to a series of intrinsic stimuli and external
stress signals (31). Activated NLRP3
protein can recruit ASC and caspase-1 proteins to form the NLRP3
inflammasome. The activated NLRP3 inflammasome promotes the
caspase-1-dependent cleavage of pro-IL-1β and pro-IL-18 into active
IL-1β and IL-18, respectively, and increases their subsequent
release from the cell (32). The
study by Hoque et al reported that the genetic deletion of
Nlrp3 reduced pro-IL-1β expression, inflammatory responses
and pancreatic edema in caerulein-induced pancreatitis (18). Therefore, the ATP-dependent P2X7/NLRP3
signaling pathway may be a potent therapeutic target for pancreatic
ductal epithelial cell injury in AP. The present study detected the
expression levels of the proteins involved in the P2X7/NLRP3
signaling pathway, and observed that emodin markedly downregulated
the protein expression of P2X7, NLRP3, ASC and caspase-1.
Mature IL-1β can modify and activate
pro-inflammatory cytokines, including IL-8, IL-17 and tumor
necrosis factor-α by binding to IL-1β receptors. Thus, IL-1β is an
early and potent pro-inflammatory factor in response to infections
and tissue damage (33,34). IL-18 has a similar structure and
function to IL-1β, and plays an important role in regulating innate
immunity and inflammation in multiple inflammatory diseases, thus
acting as a novel pro-inflammatory cytokine (24). The findings of this study demonstrated
that emodin notably decreased the levels of IL-1β and IL-18.
In addition, in this study, P2X7 overexpression by
the P2X7 plasmid abrogated the protective effects of emodin on
pancreatic ductal cells and its inhibition of the P2X7/NLRP3
signaling pathway. Therefore, P2X7 may be a drug target of emodin
against ATP-induced pancreatic ductal cell injury. These results
further confirmed that emodin exerts potent effects against
ATP-induced pancreatic ductal cell injury by inhibiting the
P2X7/NLRP3 signaling pathway and the subsequent inflammatory
reaction.
In conclusion, P2X7 acts as a potent drug target of
emodin for preventing the spontaneous activation of ATP-dependent
P2X7/NLRP3 signaling, which has important implications in
pancreatic ductal cell injury in AP pathologies. These findings may
contribute to further advancements in the development of emodin for
medical interventions in patients with AP.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Key Project
Supported by Clinical Ability Construction of Liaoning Province
(No. LNCCC-A03-2015) and the National Natural Science Foundation of
China (No. 81873156).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HX and DS conceived and designed the experiments,
and drafted the manuscript. QZhang, FH, and FG performed the cell
culture experiments and gene transfection experiments. QZhang and
FH performed the western blot analyses, and QZhou performed the
ELISA. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lankisch PG, Apte M and Banks PA: Acute
pancreatitis. Lancet. 386:85–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Manohar M, Verma AK, Venkateshaiah SU,
Sanders NL and Mishra A: Pathogenic mechanisms of pancreatitis.
World J Gastrointest Pharmacol Ther. 8:10–25. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
O'Reilly DA and Kingsnorth AN: A brief
history of pancreatitis. J R Soc Med. 94:130–132. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou Q, Xia S, Guo F, Hu F, Wang Z, Ni Y,
Wei T, Xiang H and Shang D: Transforming growth factor-β in
pancreatic diseases: Mechanisms and therapeutic potential.
Pharmacol Res. 142:58–69. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blanchard JN II, Barve S, Joshi-Barve S,
Talwalker R and Gates LK Jr: Cytokine production by CAPAN-1 and
CAPAN-2 cell lines. Dig Dis Sci. 45:927–932. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Neoptolemos JP, London NJ and Carr-Locke
DL: Assessment of main pancreatic duct integrity by endoscopic
retrograde pancreatography in patients with acute pancreatitis. Br
J Surg. 80:94–99. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wen L, Javed TA, Yimlamai D, Mukherjee A,
Xiao X and Husain SZ: Transient high pressure in pancreatic ducts
promotes inflammation and alters tight junctions via calcineurin
signaling in mice. Gastroenterology. 155:1250–1263.e5. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Venglovecz V, Rakonczay Z Jr, Gray MA and
Hegyi P: Potassium channels in pancreatic duct epithelial cells:
Their role, function and pathophysiological relevance. Pflugers
Arch. 467:625–640. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiang H, Zhang QK, Qi B, Tao XF, Xia SL,
Song HY, Qu JL and Shang D: Chinese herbal medicines attenuate
acute pancreatitis: Pharmacological activities and mechanisms.
Front Pharmacol. 8:2162017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hegyi P and Petersen OH: The exocrine
pancreas: The acinar-ductal tango in physiology and
pathophysiology. Rev Physiol Biochem Pharmacol. 165:1–30. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Novak I, Haanes KA and Wang J: Acid-base
transport in pancreas-new challenges. Front Physiol. 4:3802013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blanchard JN II, Blanchard JA, Barve S,
Joshi-Barve S, Talwalker R and Gates LK Jr: Antioxidants inhibit
cytokine production and suppress NF-kappaB activation in CAPAN-1
and CAPAN-2 cell lines. Dig Dis Sci. 46:2768–2772. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wen L, Voronina S, Javed MA, Awais M,
Szatmary P, Latawiec D, Chvanov M, Collier D, Huang W, Barrett J,
et al: Inhibitors of ORAI1 prevent cytosolic calcium-associated
injury of human pancreatic acinar cells and acute pancreatitis in 3
mouse models. Gastroenterology. 149:481–492.e7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mrazek AA, Bhatia V, Falzon M, Spratt H,
Chao C and Hellmich MR: Apigenin decreases acinar cell damage in
pancreatitis. Pancreas. 48:711–718. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kong L, Wu Q, Zhao L, Ye J, Li N and Yang
H: Effect of microRNA-27a-5p on apoptosis and inflammatory response
of pancreatic acinar cells in acute pancreatitis by targeting PTEN.
J Cell Biochem. 120:15844–15850. 2019.PubMed/NCBI
|
|
16
|
Novak I, Jans IM and Wohlfahrt L: Effect
of P2X(7) receptor knockout on exocrine secretion of pancreas,
salivary glands and lacrimal glands. J Physiol. 588:3615–3627.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Henriksen KL and Novak I: Effect of ATP on
intracellular pH in pancreatic ducts involves P2X7 receptors. Cell
Physiol Biochem. 13:93–102. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hoque R, Sohail M, Malik A, Sarwar S, Luo
Y, Shah A, Barrat F, Flavell R, Gorelick F, Husain S and Mehal W:
TLR9 and the NLRP3 inflammasome link acinar cell death with
inflammation in acute pancreatitis. Gastroenterology. 141:358–369.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Giuliani AL, Sarti AC, Falzoni S and Di
Virgilio F: The P2X7 receptor-interleukin-1 liaison. Front
Pharmacol. 8:1232017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sanz JM, Chiozzi P, Ferrari D, Colaianna
M, Idzko M, Falzoni S, Fellin R, Trabace L and Di Virgilio F:
Activation of microglia by amyloid {beta} requires P2X7 receptor
expression. J Immunol. 182:4378–4385. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu S, Wang Y, Wang X, Li J and Hu F:
Emodin inhibits ATP-induced IL-1β secretion, ROS production and
phagocytosis attenuation in rat peritoneal macrophages via
antagonizing P2X7 receptor. Pharm Biol. 52:51–57. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu H, Xiong C, He L, Wu B, Peng L, Cheng
Y, Jiang F, Tan L, Tang L, Tu Y, et al: Trans-resveratrol
attenuates high fatty acid-induced P2X7 receptor expression and
IL-6 release in PC12 cells: Possible role of P38 MAPK pathway.
Inflammation. 38:327–337. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Q, Wu H, Qin S, Liu C, Chen Y, Yang Y
and Xu C: The P2X7 receptor involved in gp120-induced cell injury
in BV2 microglia. Inflammation. 39:1814–1826. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yuan BS, Zhu RM, Braddock M, Zhang XH, Shi
W and Zheng MH: Interleukin-18: A pro-inflammatory cytokine that
plays an important role in acute pancreatitis. Expert Opin Ther
Targets. 11:1261–1271. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dong X, Fu J, Yin X, Cao S, Li X, Lin L
and Huyiligeqi Ni J: Emodin: A review of its pharmacology, toxicity
and pharmacokinetics. Phytother Res. 30:1207–1218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiang H, Tao X, Xia S, Qu J, Song H, Liu J
and Shang D: Emodin alleviates sodium taurocholate-induced
pancreatic acinar cell injury via MicroRNA-30a-5p-mediated
inhibition of high-temperature requirement a/transforming growth
factor beta 1 inflammatory signaling. Front Immunol. 8:14882017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou Q and Melton DA: Pancreas
regeneration. Nature. 557:351–358. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kowal JM, Yegutkin GG and Novak I: ATP
release, generation and hydrolysis in exocrine pancreatic duct
cells. Purinergic Signal. 11:533–550. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Novak I, Nitschke R and Amstrup J:
Purinergic receptors have different effects in rat exocrine
pancreas. Calcium signals monitored by fura-2 using confocal
microscopy. Cell Physiol Biochem. 12:83–92. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Burnstock G and Novak I: Purinergic
signalling in the pancreas in health and disease. J Endocrinol.
213:123–141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yan Y, Jiang W, Liu L, Wang X, Ding C,
Tian Z and Zhou R: Dopamine controls systemic inflammation through
inhibition of NLRP3 inflammasome. Cell. 160:62–73. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li X and Zhong F: Nickel induces
interleukin-1β secretion via the NLRP3-ASC-caspase-1 pathway.
Inflammation. 37:457–466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de Torre-Minguela C, Mesa Del Castillo P
and Pelegrin P: The NLRP3 and pyrin inflammasomes: Implications in
the pathophysiology of autoinflammatory diseases. Front Immunol.
8:432017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wen H, Miao EA and Ting JP: Mechanisms of
NOD-like receptor-associated inflammasome activation. Immunity.
39:432–441. 2013. View Article : Google Scholar : PubMed/NCBI
|