Introduction
Colorectal carcinoma (CRC) arises as a result of
genetic and epigenetic changes in epithelial cells (1–3). Upon its
development, a spectrum of lesions are formed, from colorectal
adenoma (CRA) to CRC, with different malignant potential and
behavior (3). Since the introduction
of colon cancer screening programs, a large number of early lesions
have been discovered and removed, including adenomas with
epithelial misplacement (pseudoinvasion) and malignant polyps
(early carcinomas) (4–6). Despite well-defined morphological
features of adenomas with epithelial misplacement and malignant
polyps (7), there is a significant
number of problematic cases of precancerous lesions with ambiguous
features, leading to different diagnostic opinions among
pathologists (5). Biomarkers
distinguishing among various lesions in colorectal carcinogenesis
could therefore help to make the correct diagnosis in ambiguous
cases, thus enabling the optimal treatment to be selected (8,9).
In a previous study from our group, the most
prominent gene candidates were examined in order to distinguish CRA
from CRC using bioinformatics analysis (9). Using a public genomics data repository
(Gene Expression Omnibus), several projects were identified with
gene expression profiles of normal, adenoma and carcinoma colonic
samples, obtained from microarray experiments. Applying in
silico methods, 16 differentially expressed genes were detected
between CRA and CRC and 10 differentially expressed genes were
detected among healthy colon mucosa, CRA and CRC. Functional
analysis of these differentially expressed genes indicated that at
least 9 of them were associated with the extracellular matrix
(ECM).
The ECM is assembled from diverse biochemical
molecules ranging from proteins to polysaccharides (10,11),
including proteoglycans and glycoproteins, with unique physical and
biochemical properties. The ECM offers structural support to organs
(12) and participates in crucial
cell processes, including proliferation, differentiation, adhesion,
migration and apoptosis (10).
Notably, the ECM also participates in many cellular functions
representing the hallmarks of cancer. This overlap suggests that
the biochemical and biophysical properties of ECM should be
considered when examining tumour behaviour and therapeutic
interventions (13).
Only 3 out of the 9 genes that were differentially
expressed between healthy colon mucosa, CRA and CRC, encode for
collagens, namely collagen type XII α1 chain, collagen type II α1
chain and collagen type III α1 chain. The remaining 6 genes
[decorin (DCN), erythropoietin-producing hepatoma receptor A4
(EPHA4), fibronectin 1 (FN1), secreted protein acidic and cysteine
rich (SPARC), spondin 2 (SPON2) and secreted phosphoprotein 1
(SPP1)], have enzymatic activities. Structural proteins, such as
these three collagens, are known to participate in biomechanical
signaling, but do not have an enzymatic role in tumour progression
(14). Changes in collagen expression
and ratio enable tumour progression, possible due to alterations in
the enzymatic activity of ECM proteins, such as DCN, EPH4, FN1,
SPARC, SPON2 and SPP1. The aim of the present study was to examine
the diagnostic and prognostic potential of these 6 ECM-related
components with enzymatic activity in colorectal carcinogenesis.
All 6 genes were identified as being differentially expressed in
our previous bioinformatics analysis comparing CRA and CRC
(9). All of these 6 components of ECM
have been already associated with CRC (15–38).
However, there are limited data about how the expression levels of
mRNAs of these genes and their related proteins change from healthy
mucosa and CRA to malignant polyps and CRC, including the
development of metastases. The few published studies have mainly
focused on either protein and/or mRNAs levels (18–22). On
the basis of the literature and our previous bioinformatics
analysis (9), it was hypothesized
that the expression of these 6 ECM-related genes may be different,
on both mRNA and protein levels, in healthy mucosa and CRA in
comparison to malignant polyps and CRC (diagnostic potential), as
well as between CRC with and without lymph node metastases
(prognostic potential).
Materials and methods
Patients and tissue selection
The present study included biopsy samples from 40
patients divided into four groups on the basis of the biopsy
diagnosis: CRA, malignant polyp, CRC without lymph node metastases
(CRC N0), or CRC with lymph node metastases (CRC N+). The gender
and age of the patients, and the location of the lesions for each
group, are listed in Table I. Only
cases with clear biopsy diagnoses were included. In the CRA group,
there were 4 tubular adenomas with high grade dysplasia, 4
tubulovillous adenomas with high grade dysplasia and 2
tubulovillous adenomas with low grade dysplasia. The malignant
polyps group consisted of 5 tubulovillous adenomas, 3 tubular
adenomas, 1 villous adenoma and 1 tubular adenoma, all with high
grade dysplasia and with malignant transformation, evidenced by
invasion of the dysplastic glands in the submucosa (pT1 stage). The
TNM stages of the CRC N0 group were T2N0M0, T3N0M0 and T4aN0M0 in
2, 6 and 2 patients, respectively. The TNM stages of the CRC N+
group were T3N1Mx, T4aN1Mx and T4aN2Mx in 5, 2 and 3 patients,
respectively.
 | Table I.Summary of clinical data including
location of the lesions. |
Table I.
Summary of clinical data including
location of the lesions.
|
| Colorectal
adenoma | Malignant
polyp | CRC N0 | CRC N+ |
|---|
| Number of
patients | 10 | 10 | 10 | 10 |
| Male : female | 10:0 | 6:4 | 3:7 | 7:4 |
| Age | 50–83 | 56–72 | 48–84 | 58–90 |
| (mean ± SD) | (65±11) | (65±5) | (73±12) | (77±11) |
| Tumour
location |
|
|
|
|
|
Ascending colon | 1 | 0 | 1 | 1 |
|
Descending colon | 0 | 1 | 0 | 1 |
| Right
hemicolon | 0 | 0 | 2 | 2 |
| Rectum
or rectosigmoid colon | 2 | 3 | 2 | 4 |
| Sigmoid
colon | 6 | 6 | 4 | 1 |
| Splenic
flexure | 1 | 0 | 1 | 1 |
As a control group, healthy colon mucosa from
surgical margins from 20 patients with CRC N0 and CRC N+ was
included.
All tissue samples were fixed for 24 h in 10%
buffered formalin prior to the paraffin embedding. After fixation
and embedding, tissues were cut into 3–4 µm sections and stained
with hematoxylin and eosin for routine histopathological
examination. For the purposes of the present retrospective study,
representative paraffin blocks were retrieved from the archives of
the Institute of Pathology, Faculty of Medicine, University of
Ljubljana (tissues originally collected from December 2013 to
October 2018).
Isolation of RNA from formalin-fixed
paraffin-embedded (FFPE) tissue
Isolation from tissue sections
Four 10 µm-thick FFPE sections were cut from the
archival paraffin blocks for the isolation of total RNA. Total RNA
extraction was performed using the AllPrep DNA/RNA FFPE kit (Qiagen
GmbH), according to the manufacturer's instructions. Apart from the
deparaffinization solution (hexadecane; Sigma-Aldrich; Merck KGaA)
and the ethanol (Merck KGaA), all the reagents were from Qiagen
GmbH. The RNA concentration and quality was evaluated using a
NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Inc.).
The A260/A230 nm intensity ratio was >1.0 and the A260/A280
ratio was >1.8. Reverse transcription and amplification using
SYBR Green technology of the housekeeping gene GAPDH (95 bp) was
performed as quality control. Reverse transcription was performed
as described below. Each qPCR reaction contained 1 µl 10X GAPDH
primers (QuantiTect primer assay Hs_GAPDH_1_S; cat. no. QT00079247;
Qiagen GmbH), 5 µl iTaq Universal SYBR Green (Bio-Rad Laboratories,
Inc.) and 4 µl of cDNA. Thermocycling conditions were as follows: 2
min at 50°C, 2 min at 95°C, and 45 cycles of 15 sec at 94°C, 30 sec
at 55°C and 1 min at 72°C. This approach is similar to the approach
that we use for FusionPlex Sarcoma, a next-generation sequencing
panel, which includes the PreSeq RNA QC assay (39) after reverse transcription. These
quality control assays basically use primers that amplify a
universally expressed transcript (in the present study, GAPDH) to
quantify the amount of amplifiable RNA of sufficient length in a
sample. The presence of lower Cqs in the results is strongly
indicative of successful cDNA preparation and predicts successful
sequencing. All of the samples that were included in the present
study passed amplification of GAPDH using SYBR Green (initial
quality control) and those that did not amplify were not included
in the study (the number of samples initially isolated was
>2-fold the number of samples that passed quality control and
were subsequently included in the final manuscript). Finally, for
the selected genes, TaqMan probes (Thermo Fisher Scientific, Inc.)
were designed to amplify and detect PCR products <84 bp long
(Table SI).
Isolation from tissue cores
Tissue cores from the tumor center and the invasive
front were punched from FFPE tissue blocks using a 600 µm needle.
Three punches were used for each isolation. Total RNA extraction
was performed using the MagMax FFPE DNA/RNA Ultra kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions, with one modification: Protease
digestion was performed overnight with mixing for 15 sec at 300 rpm
every 4 min instead of 1 h. Apart from the deparaffinization
solution (xylene; Sigma-Aldrich; Merck KGaA) and the ethanol (Merck
KGaA), all the reagents were from Applied Biosystems (Thermo Fisher
Scientific, Inc). RNA quantity was assessed on a NanoDrop 1000
(Thermo Fisher Scientific, Inc.) by measuring the absorbance at
A260.
Reverse transcription-quantitative PCR
(RT-qPCR)
Reverse transcription, preamplification and qPCR
were performed to measure the expression levels of genes. The
extracted total RNA was reverse transcribed using the OneTaq-PCR
kit (New England Biolabs, Inc.) according to manufacturer's
instructions. Each reverse transcription reaction contained 60 ng
of extracted total RNA, 6 µM random primers mix, 5 µl 2X reaction
mix and 1 µl 10X enzyme mix. First, RNA and random primers were
mixed and incubated for 5 min at 70°C, then the reaction and enzyme
mix was added and incubated for 5 min at 25°C, 60 min at 42°C and 4
min at 80°C.
Following cDNA synthesis, a preamplification
procedure was performed using TaqMan PreAmp mastermix (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Each reaction
contained 5 µl cDNA, 5 µl of 0.2X TaqMan Gene Expression Assays
(Table SI) and 10 µl 2X TaqMan
PreAmp Mastermix. TaqMan Gene Expression Assays were pooled,
followed by dilution to 0.2X using Tris-EDTA buffer solution, pH
8.0 (Sigma-Aldrich; Merck KGaA). The thermocycling conditions were
as follows: 10 min at 95°C and 10 cycles of 15 sec at 95°C and 4
min at 60°C.
Prior to qPCR, to calculate the efficiency of qPCR
reactions, pools of each group were created by mixing RNA samples.
RNA pools were reverse transcribed and preamplified, as
aforementioned. Then, the preamplified cDNA was diluted in four
steps, ranging from 5-fold dilution to 5,000-fold or 625-fold and
qPCR was performed as described below. All qPCR reactions were
conducted on a Rotor-Gene Q system (Qiagen GmbH), and each sample
was run in triplicate. The thermocycling conditions were as
follows: 2 min at 50°C, 10 min at 95°C, and 40 cycles of 15 sec at
95°C and 1 min at 60°C.
qPCR was performed using TaqMan technology with
FastStart Essential DNA Probe Master (Roche Diagnostics). Each
preamplification reaction (20 µl) was diluted with ddH2O
to a final volume 100 µl (5X). Each qPCR reaction contained 4.5 µl
of diluted preamplification mixture, 5 µl of 2X FastStart Essential
DNA Probe Master (Roche Diagnostics) and 0.5 µl of TaqMan Gene
Expression Assay. The TaqMan Gene Expression assays used in the
present study are listed in Table
SI. Importin 8 (IPO8) and β2 microglobulin (B2M) were used as
reference genes. All qPCR reactions were conducted on a Rotor-Gene
Q system (Qiagen GmbH), and each sample was run in duplicate. The
thermocycling conditions were as follows: 2 min at 50°C, 10 min at
95°C and 40 cycles of 15 sec at 95°C and 1 min at 60°C.
Immunohistochemistry
For immunohistochemistry analysis, commercially
available antibodies against SPP1, SPARC, SPON2 and DCN were used.
Detailed information about the antibodies is presented in Table II. Sections of 4 µm thickness were
cut from paraffin blocks, deparaffinized, and processed for
automated antigen retrieval and staining with the antibodies in an
automatic immunostainer (Ventana BenchMark; Roche Diagnostics).
Sections were treated with biotinylated secondary antibody (8 min
at 37°C), followed by incubation with peroxidase-conjugated
streptavidin (both part of the UltraVIEW DAB Detection kit; Roche
Diagnostics). Visualization of the immunoreaction was established
with 3,3′-diaminobenzidine. The sections were then counterstained
with haematoxylin.
 | Table II.Detailed information for the
antibodies used in the present study. |
Table II.
Detailed information for the
antibodies used in the present study.
| Targeting
protein | Type | Host species | Target species | Dilution | Manufacturer | Catalogue
number | Antigen
retrieval | Temperature and
duration of incubation with primary antibody |
|---|
| SPP1 | Monoclonal | Mouse | Human | 1:300 | Invitrogen;
Thermo | MA5-17180 | Cell conditioning
solution 1 | 24 min at 37°C |
|
|
|
|
|
| Fisher Scientific,
Inc. |
| (Ventana
BenchMark) |
|
| SPON2 | Polyclonal | Rabbit | Human | 1:20 | Invitrogen;
Thermo | PA5-59087 | Cell conditioning
solution 1 | 32 min at 37°C |
|
|
|
|
|
| Fisher Scientific,
Inc. |
| (Ventana
BenchMark) |
|
| SPARC | Monoclonal | Mouse | Human | 1:400 | Invitrogen;
Thermo | 33-5500 | Cell conditioning
solution 1 | 24 min at 37°C |
|
|
|
|
|
| Fisher Scientific,
Inc. |
| (Ventana
BenchMark) |
|
| DCN | Monoclonal | Mouse | Human | 1:1,000 | LifeSpan
BioSciences, Inc. | LS-B4312-50 | No
pretreatment | 12 min at 37°C |
Statistical analysis
To calculate relative gene expression, Cq values
were corrected according to Latham (40). To obtain ∆Cq, the geometric mean of Cq
values of the reference genes (IPO8 and B2M) was deducted from the
gene of interest (DCN, EPH4, FN1, SPARC, SPON2 and SPP1).
Differences in expression in the paired tissue samples of tumour
and adjacent healthy mucosa from the CRC N0 group were analyzed for
significance with the Wilcoxon signed rank test. For all other
comparisons of ΔCqs between different groups of samples (healthy
mucosa, CRA, malignant polyp, CRC N0 and CRC N+), ANOVA was used
with two different post-hoc tests, Bonferroni and Hochberg's GT2
(41). Additionally, the Spearman's
Rank correlation coefficient (ρ) was used to calculate the
statistical significance of association between the expression of
analyzed genes (ΔCq values) and various lesions (CRA, malignant
polyp CRC N0 and CRC N+). All the statistical analyses were
performed using SPSS analytical software version 24 (IBM Corp.),
with a cut-off of P≤0.05.
Results
Expression of DCN, EPHA4, FN1, SPARC,
SPON2 and SPP1 in CRA and malignant polyp groups compared with
healthy mucosa control
The majority of genes tested in the present study
were downregulated in CRA compared with healthy mucosa: DCN
(16.1-fold, P<0.001), FN1 (8.1-fold, P=0.022), SPARC (2.6-fold,
not significant), SPON2 (2.7-fold, not significant) and SPP1
(21.1-fold, P=0.006), whereas EPHA4 was slightly upregulated but
not statistically significant. Results are summarized in Fig. 1 and Table
III.
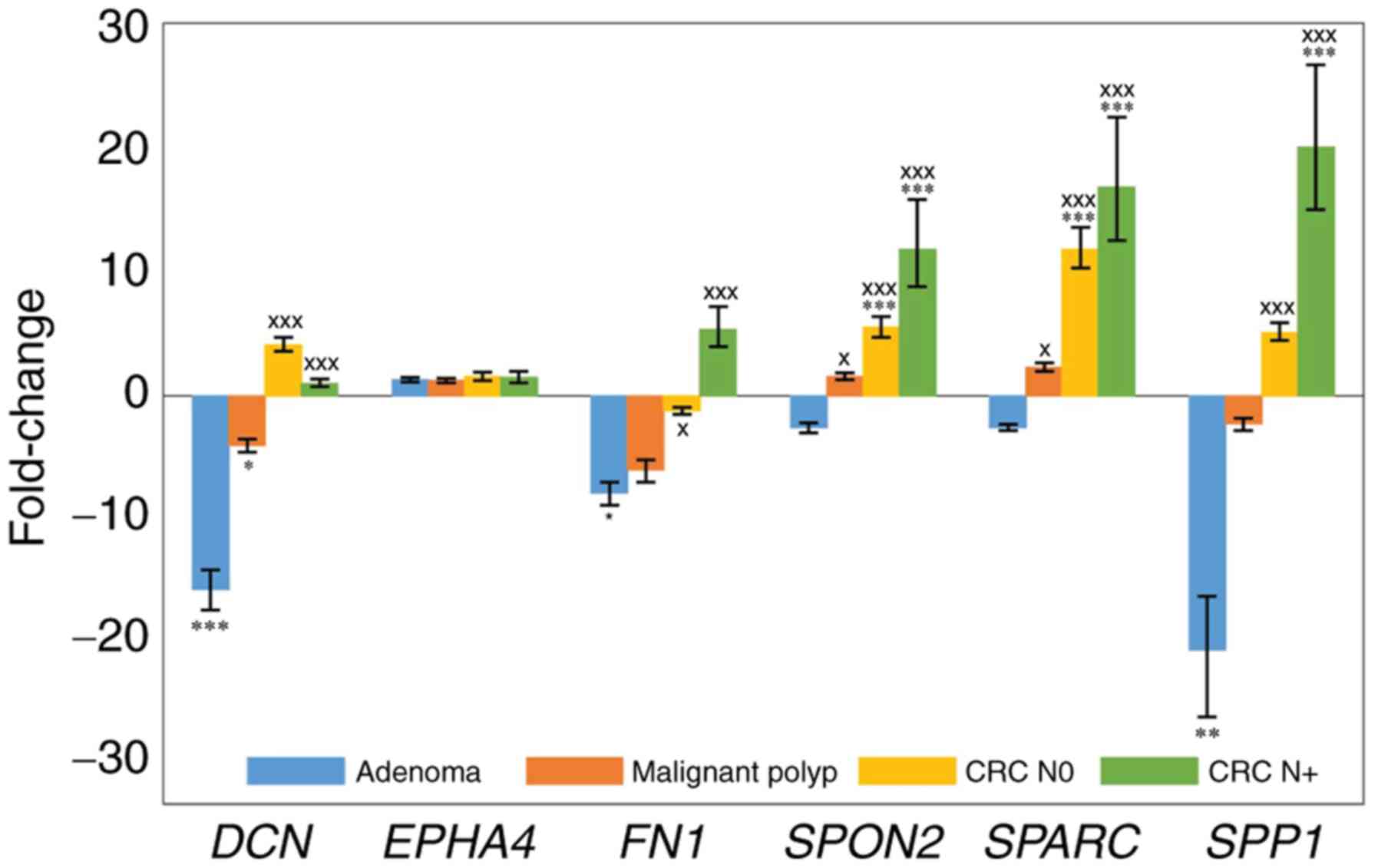 | Figure 1.Expression of DCN, EPHA4, FN1, SPARC,
SPON2 and SPP1 in adenoma, malignant polyp, CRC N0 and CRC N+
groups. Expression is plotted as fold change relative to healthy
colon mucosa tissue control. *P≤0.05, **P<0.01 and ***P<0.001
vs. healthy mucosa; xP≤0.05 and xxxP<0.001
vs. adenoma. DCN, decorin; EPHA4, erythropoietin-producing hepatoma
receptor A4; FN1, fibronectin 1; SPARC, secreted protein acidic and
cysteine rich; SPON2, spondin 2; SPP1, secreted phosphoprotein 1;
CRC, colorectal carcinoma; N0, no lymph node metastases; N+, with
lymph node metastases. |
 | Table III.Genes with statistically significant
differential expression between two groups. |
Table III.
Genes with statistically significant
differential expression between two groups.
| Groups | CRA (n=10) | Malignant polyp
(n=10) | CRC N0 (n=10) | CRC N+ (n=10) |
|---|
| Healthy mucosa
(n=20) | DCN
(P<0.001) | DCN (P=0.031) | SPON2
(P<0.001) | SPON2
(P<0.001) |
|
| FN1 (P=0.022) |
| SPARC
(P<0.001) | SPARC
(P<0.001) |
|
| SPP1 (P=0.006) |
|
| SPP1
(P<0.001) |
| CRA (n=10) | – | SPON2
(P=0.028) | DCN
(P<0.001) | DCN
(P<0.001) |
|
|
| SPARC
(P=0.018) | FN1 (P=0.041) | FN1
(P<0.001) |
|
|
|
| SPON2
(P<0.001) | SPON2
(P<0.001) |
|
|
|
| SPARC
(P<0.001) | SPARC
(P<0.001) |
|
|
|
| SPP1
(P<0.001) | SPP1
(P<0.001) |
DCN was significantly downregulated in the malignant
polyp group compared with healthy mucosa (4.1-fold, P=0.031;
Fig. 1). FN1 and SPP1 also appeared
downregulated in malignant polyps compared with healthy mucosa,
however these changes were not significant. The other three genes
exhibited a trend towards upregulation in malignant polyps, albeit
not significant. Results are summarized in Fig. 1 and Table
III.
Expression of DCN, EPHA4, FN1, SPARC,
SPON2 and SPP1 in CRC N+ and CRC N0 groups compared with healthy
colon mucosa control
Expression of genes in CRC N0 compared with healthy
colon mucosa exhibited a significant upregulation of the levels of
SPARC (12.17-fold, P<0.001) and SPON2 (5.65-fold, P<0.001).
DCN, EPHA4 and SPP1 expression levels were slightly upregulated and
FN1 was downregulated, however these changes were not significant.
Results are summarized in Fig. 1 and
Table III.
Expression of DCN, EPHA4, SPARC, SPON2 and SPP1
showed similar patterns in the CRC N+ and CRC N0 groups, both
compared to their corresponding healthy mucosa. Statistical
significance was reached for SPARC (17.3-fold, P<0.001), SPON2
(12.17-fold, P<0.0017) and SPP1 (20.65-fold, P<0.001)
expression levels. In contrast to CRC N0, FN1 was upregulated
(5.5-fold) in CRC N+ when compared with healthy mucosa, although
this change was not statistically significant. Results are
summarized in Fig. 1 and Table III.
Expression of DCN, EPHA4, FN1, SPARC,
SPON2 and SPP1 in CRA compared with the malignant polyp, CRC N+ or
CRC N0 groups
Statistically significant difference between the CRA
and malignant polyp groups was observed for the SPON2 and SPARC
genes. In addition, statistically significant difference between
the CRA and the CRC N0 and CRC N+ groups was observed for all genes
tested, except for EPHA4. Results are summarized in Table III.
Expression of most of the genes gradually increased
with the level of malignancy, from CRA to malignant polyps and to
CRC. Accordingly, the results demonstrated a statistically
significant association between the various lesions (CRA, malignant
polyp, CRC N0, CRC N+) and the expression levels of DCN, EPHA4,
FN1, SPARC, SPON2 and SPP1 (ρ=0.660, p<0.001; ρ=−0.259, p=0.039;
ρ=0.495, p<0.001; ρ=0.531, p<0.001; ρ=0.505, p<0.001; and
ρ=0.677, p<0.001, respectively).
Expression of DCN, EPHA4, FN1, SPARC,
SPON2 and SPP1 in CRC N+ compared with CRC N0 group
Expression of DCN was slightly upregulated in CRC N0
compared with CRC N+. The difference in DCN expression between CRC
N0 and CRC N+ was statistically significant (P=0.025). There was no
significant change in the expression levels of EPHA4, FN1, SPARC,
SPON2 and SPP1 between the CRC N0 and CRC N+ groups. The expression
levels of EPHA4 were upregulated compared with healthy mucosa, but
the same in CRC N0 and CRC N+. FN1 expression was slightly
downregulated in CRC N0 and upregulated in CRC N+, compared with
healthy mucosa. The expression of SPP1, SPON2, and SPARC was
upregulated both in CRC N0 and CRC N+ compared with healthy mucosa,
and expression of these genes was slightly but not significantly
higher in CRC N+ than in CRC N0. The P-value of the difference of
the SPP1 levels between the CRC N0 and CRC N+ groups was just above
the cut-off (P=0.083).
Expression of DCN, EPHA4, FN1, SPARC,
SPON2 and SPP1 in the central parts of carcinoma and at the
invasive front
Expression of DCN, FN1 and SPARC was upregulated at
the invasive front of the tumour compared with the central parts in
the CRC N+ group, whereas in the CRC N0 group, expression at the
invasive front was similar to the central parts (Fig. 2). The expression levels of SPON2 and
SPP1 were downregulated at the invasive front compared with the
central parts and similar in both CRC N0 and CRC N+ (Fig. 2). The highest difference in expression
levels was observed in the case of the EPHA4 gene; however, there
was no statistically significant difference, even though its
expression was downregulated in CRC N0 and upregulated in CRC N+ at
the invasive front compared with the central parts of the tumour
(Fig. 2).
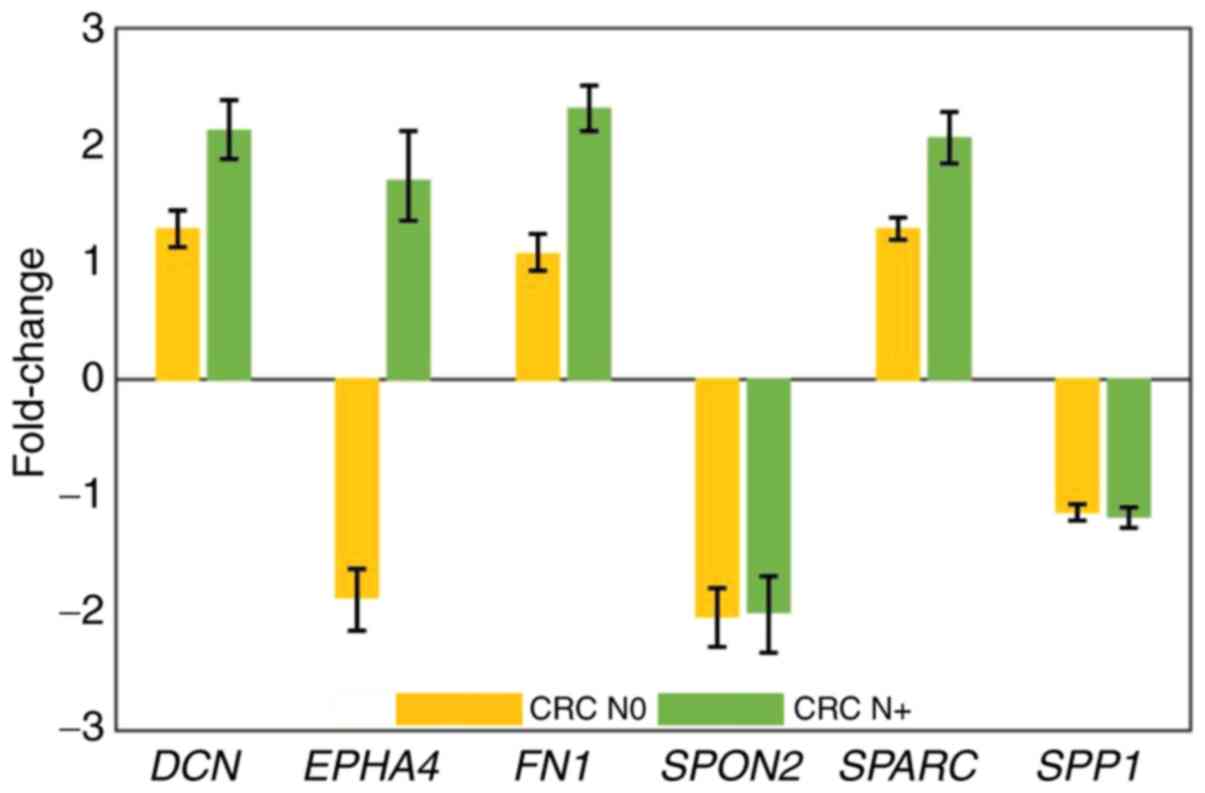 | Figure 2.Expression of DCN, EPH4, FN1, SPARC,
SPON2 and SPP1 at the invasive front compared with the tumour
centre in the CRC N0 and CRC N+ groups. Expression is plotted as
fold change relative to the tumor tissue center. DCN, decorin;
EPHA4, erythropoietin-producing hepatoma receptor A4; FN1,
fibronectin 1; SPARC, secreted protein acidic and cysteine rich;
SPON2, spondin 2; SPP1, secreted phosphoprotein 1; CRC, colorectal
carcinoma; N0, no lymph node metastases; N+, with lymph node
metastases. |
Immunohistochemistry
Expression of most of the genes tested in the
present study appeared increased with the level of malignancy, from
CRA to malignant polyps and to CRC. Therefore, immunohistochemistry
was performed for the DCN, SPARC, SPON2 and SPP1 proteins, since
these four genes exhibited statistically significant differences in
expression using RT-qPCR between CRC N0 and its corresponding
healthy mucosa (DCN, 4.22-fold, P=0.028; SPARC, 12.17-fold,
P=0.007; SPON2, 5.65-fold, P=0.017; and SPP1, 5.24-fold, P=0.036;
Fig. 1).
Immunohistochemistry analysis for the investigated
proteins revealed two patterns. One pattern was observed for the
SPON2 and SPP1 protein expression: Positive staining was evident in
the epithelial cells of the healthy colon mucosa and in dysplastic
glands, both in CRA and CRC tissues, and the intensity of staining
progressively increased with the severity of the lesions, being the
most intense in CRC (Fig. 3).
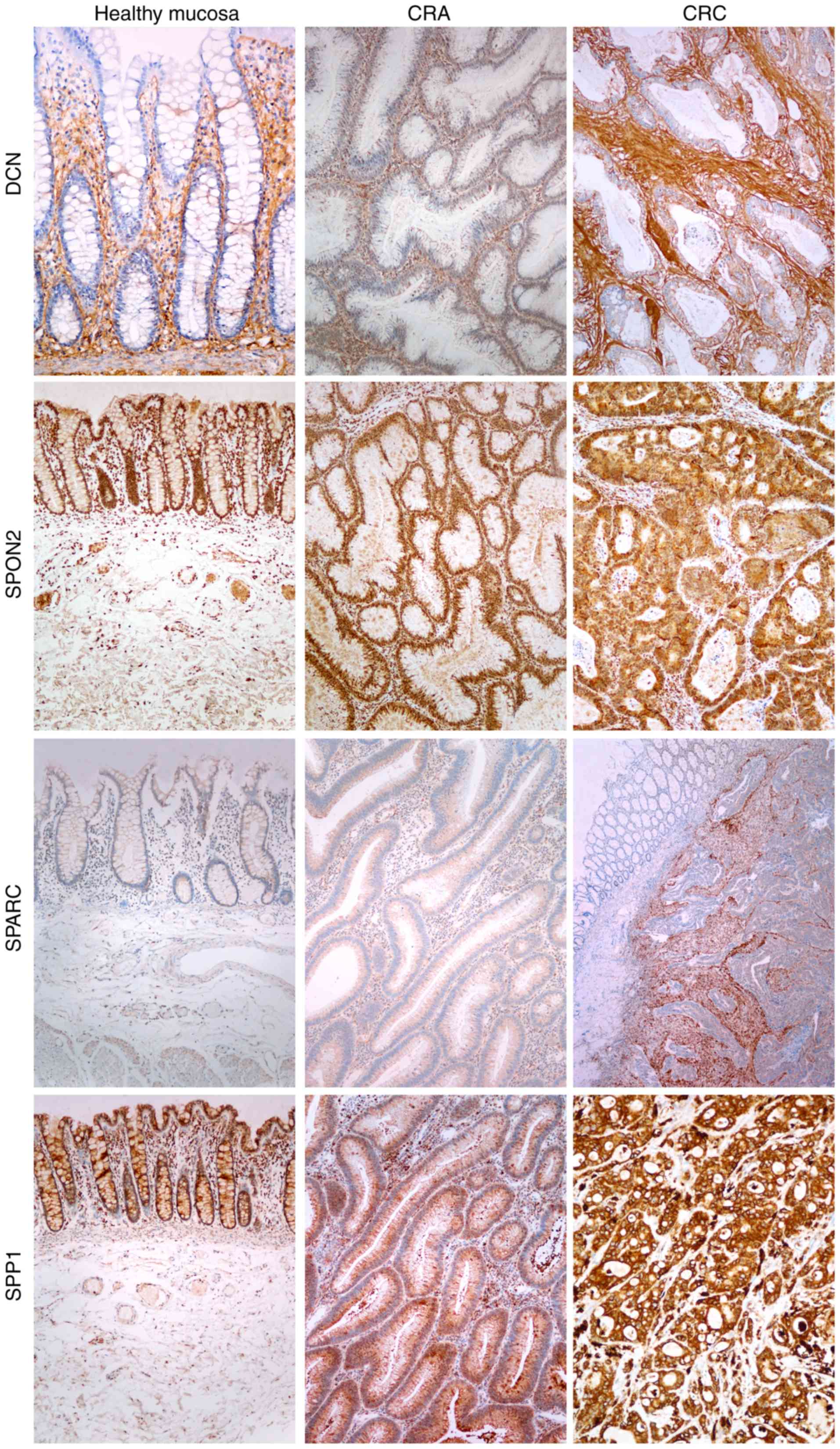 | Figure 3.Immunohistochemical analysis of DCN,
SPARC, SPP1 and SPON2 expression in healthy colon mucosa, CRA and
CRC. SPP1 and SPON2 exhibited positive staining in epithelial cells
in healthy mucosa, in dysplastic glands, in CRA and CRC, with the
most intense staining being in CRC. DCN staining was positive in
the lamina propria in healthy mucosa and in the stroma in CRA and
CRC, with the most intense staining being in CRC. SPARC staining
was negative in healthy mucosa, very faint in CRA and strong in the
stroma in CRC with submucosal invasion. DCN, decorin; SPARC,
secreted protein acidic and cysteine rich; SPP1, secreted
phosphoprotein 1; SPON2, spondin 2; CRA, colorectal adenoma; CRC,
colorectal carcinoma. |
The other pattern was observed for the DCN and SPARC
protein expression: Positive staining was evident in the lamina
propria in healthy mucosa and in the stroma of CRA and CRC
(Fig. 3). In this pattern, too, the
intensity of staining increased with the severity of the lesions,
being most intense in CRC (Fig.
3).
Discussion
Based on a previous bioinformatics analysis, six
ECM-related genes were identified to be differentially expressed
among healthy colon mucosa, CRA and CRC. The present study
performed a validation analysis of these six genes and confirmed
significant differences in the expression of three genes, namely
SPON2, SPARC and SPP1. These were downregulated in healthy mucosa
and CRA, and upregulated in CRC. In addition, immunohistochemistry
analysis of the related proteins showed patterns that were
consistent with the gene expression analysis.
The highest differences in expression among healthy
mucosa, CRA and CRC were observed for SPP1: it was downregulated in
CRA, but upregulated in CRC, with a gradual increase in its
expression from malignant polyp to CRC; the differences among the 3
groups were statistically significant. However, the significance of
the difference in expression between CRC with and CRC without lymph
node metastases was just above the cut-off P-value, and no
significant difference was observed between central parts of the
tumour and the invasive front. These results suggested an important
role of SPP1 in the development of CRC, but not in the progression
of CRC. SPP1 is an extracellular protein involved in
biomineralization, tissue and bone remodelling, cell-mediated
immune response and wound healing (42,43).
Previous studies have also suggested that SPP1 has an important
role in the development of multiple types of cancer, including CRC,
breast, prostate, lung, ovarian and liver cancer (42). For CRC, few studies have been reported
on analysis of SPP1 expression using qPCR and immunohistochemistry.
However, in contrast to the present findings, upregulation of SPP1
has been reported in CRA and CRC in previous studies (17,18). The
present immunohistochemical analysis revealed cytoplasmic
expression of SPP1 in epithelial cells in healthy colon mucosa, CRA
and CRC, and also in some mesenchymal stromal cells and immune
cells. The extent and intensity of staining tended to increase from
healthy colon mucosa to CRA and CRC, similarly to previous studies
(15–19).
Significant differences were also observed for
SPARC: it was slightly downregulated in CRA but upregulated in CRC,
with a gradual increase in its expression from malignant polyp to
CRC, with statistically significant differences among all three
groups. However, there was no significant difference between CRC
with and CRC without lymph node metastases. These results indicate
a specific role of SPARC in reorganization of ECM in the
progression of CRA to CRC. The present results are similar to the
results showed by Galamb et al (18), but not quite consistent with another
study (35), which showed a slight
upregulation in CRA in comparison to healthy tissue, whereas the
present results showed a slight downregulation. A previous
immunohistochemical analysis (37)
reported that SPARC expression was higher in CRC of all stages;
however, a statistically significant difference was observed
between CRC stage I compared with stages II, III or IV. Liu et
al (21) showed that the staining
of SPARC was greater in CRA and CRC without lymph node metastases,
whereas the expression of SPARC was still high but to a lesser
extent in CRC with lymph node metastases compared to CRC without
lymph node metastases. Previously published studies indicate that
expression of SPARC in CRC might also be regulated by methylation
(44,45), leading to various expression patterns.
The SPARC protein, also referred to as osteonectin, is also known
to have different expression patterns in various types of cancer,
with expression being high in breast cancer, melanoma and
glioblastoma, but low in acute myeloid leukaemia, ovarian and
pancreatic cancer (46). It also
participates in tissue remodelling, wound repair, cell migration
and angiogenesis (46,47).
SPON2 exhibited similar expression patterns to those
of SPARC. The SPON2 gene encodes for the protein spondin 2, a
secretory extracellular protein, that participates in the immune
response as a microbial pattern-recognition protein (26). It has been reported that SPON2 is
upregulated in several types of cancer, such as lung adenocarcinoma
(48), hepatocellular carcinoma
(49) and gastric carcinoma (50). A previous study showed that the SPON2
mRNA expression levels are higher in CRC compared with the adjacent
healthy mucosa (26). Another study
also demonstrated positive SPON2 staining in epithelial cells in
CRC (26). The present results are
consistent with these previous studies. Furthermore, the present
results demonstrated that the expression levels of SPON2 increased
with the level of malignancy. Similarly, the differences in
expression of SPON2 between the CRC stages examined in the present
study were all statistically significant, except between CRC with
and CRC without lymph node metastases, indicating that SPON2 may
participate in the reorganization of ECM from CRA to CRC.
The fourth gene, DCN, encodes for the protein
decorin, a member of the small leucine-rich proteoglycans. DCN is a
structural component of the ECM, but it also participates in
cellular processes, such as proliferation, migration and
differentiation (51). The present
study demonstrated downregulation of DCN in CRA and malignant
polyps but upregulation in CRC, with statistical differences among
all groups except between CRA and malignant polyp. Furthermore,
among the investigated genes only DCN showed significantly
different expression between CRC without lymph node metastases and
CRC with lymph node metastases, being higher in CRC without lymph
node metastases than in CRC with lymph node metastases.
Consistently, DCN has been reported to be negatively correlated
with the progression of CRC (20,51,52). With
its diverse functions, DCN may have an important role in the
development of CRA, progression of CRA to CRC and migration of
tumour cells to the regional lymph nodes, forming nodal
metastases.
The next gene, FN1, is a widely expressed
glycoprotein of the ECM that participates in cell adhesion,
migration and differentiation (23,53). It
has been demonstrated that FN1 expression is upregulated in CRC
(53) and that its
immunohistochemical staining is stronger in CRC than in healthy
colon mucosa (23); however, no data
has been currently available regarding FN1 expression in CRA. In
the present study, the results demonstrated downregulation of FN1
in CRA and malignant polyp compared with CRC, regardless of the
presence of lymph node metastases, suggesting its role in the
development of CRC. There was, however, no difference between CRC
without lymph node metastases and CRC with lymph node
metastases.
Analysis of EPHA4 expression showed no significant
differences among the four tissue groups; its expression remained
fairly constant during the different stages of CRC development.
EPHA4 is a transmembrane receptor tyrosine kinase that is attached
to the membrane either by glycosylphosphatidylinositol linkage or a
transmembrane sequence (54). Its
activation affects pathways involved in the regulation of adhesion,
migration and invasiveness (55).
Previous studies of EPHA4 in CRC are consistent with the present
results. Expression levels of EPHA4 were similar in CRC compared
with healthy colon mucosa. There were no significant differences
between the expression of EPHA4 in CRC without lymph node
metastases and CRC with lymph node metastases (29).
Comparison between the central parts of CRC tissues
and the invasive front revealed an upregulation for four out of the
six genes at the invasive front, namely for the genes DCN, EPHA4,
FN1 and SPARC, particularly in CRC with lymph node metastases. This
observation indicates that ECM-related genes may have a role in the
progression of CRC.
The present study has several limitations. The first
one is related to the fact that the current study was performed on
FFPE tissue. Although nucleic acids from FFPE tissues are difficult
to analyse, a great advantage of FFPE tissue is that all samples
have been first evaluated by pathologists, enabling appropriate
diagnosis. RNA fragmentation in FFPE tissue samples has a size
endpoint of ~80 nt and formalin modifications are partially
reversed during isolation (56,57).
Recent publications described that analysis of mRNAs from FFPE is
reliable when performed appropriately (58,59). In
the present study, only the samples that successfully passed
initial quality control were further analysed, thus limiting the
number of included samples. The second limitation is related to the
different stages of CRC. The number of samples was too small to
draw any definite conclusions regarding the correlations between
stage and the expression of the investigated genes and proteins.
Furthermore, the current study did not investigate the causes of
deregulation for these six ECM genes which may be based on DNA,
such as methylation, mutation and copy number alteration.
In conclusion, the present study suggested that at
least three out of six ECM-related genes, identified by a previous
bioinformatics analysis, exhibited significant differences in
expression among healthy colon mucosa, CRA and CRC. These results
provided further evidence that ECM-related genes and proteins may
have an important role in the development of CRC, and identified a
possible diagnostic use for these genes in differentiating among
various lesions, such as between CRA and malignant polyp. However,
the results showed limited potential of these genes to predict
lymph node metastases in CRC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was funded by the Slovenian Research
Agency (research core funding no. P3-0054).
Availability of data and materials
The datasets generated and/or analysed during the
current study are not publicly available due to protecting
confidentiality of personal data but are available from the
corresponding author.
Authors' contributions
MZ contributed to acquisition, analysis and
interpretation of data and writing the manuscript. EB contributed
to analysis and interpretation of data and in drafting and revising
the manuscript. NH contributed to the design of study and
critically revised the manuscript. NZ contributed to analysis and
interpretation of data, in drafting the manuscript and gave final
approval of the version to be published. All authors read and
approved the final manuscript.
Ethical approval and consent to
participate
The research project confirmed to the principles
outlined in The Helsinki Declaration and was approved by the
National Medical Ethics Committee of the Republic of Slovenia,
approval no. 0120-88/2018/4. As stated in the approval document,
the study is retrospective and observational, performed on tissue
samples that were obtained during routine diagnostic/therapeutic
procedures, consisted of either excision or resection. Therefore,
enough tissue was available for routine analysis and research.
Moreover, tissue is still available for any additional analysis in
the future. Our State Ethical Committee does not require informed
consent from patients in such studies.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Simon K: Colorectal cancer development and
advances in screening. Clin Interv Aging. 11:967–976. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mathonnet M, Perraud A, Christou N, Akil
H, Melin C, Battu S, Jauberteau MO and Denizot Y: Hallmarks in
colorectal cancer: Angiogenesis and cancer stem-like cells. World J
Gastroenterol. 20:4189–4196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuipers EJ, Grady WM, Lieberman D,
Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ and Watanabe T:
Colorectal cancer. Nat Rev Dis Primers. 1:150652015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shepherd NA and Griggs RK: Bowel cancer
screening-generated diagnostic conundrum of the century:
Pseudoinvasion in sigmoid colonic polyps. Mod Pathol. 1 (Suppl
28):S88–S94. 2015. View Article : Google Scholar
|
|
5
|
Griggs RK, Novelli MR, Sanders DS, Warren
BF, Williams GT, Quirke P and Shepherd NA: Challenging diagnostic
issues in adenomatous polyps with epithelial misplacement in bowel
cancer screening: 5 years' experience of the bowel cancer screening
programme expert board. Histopathology. 70:466–472. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Panarelli NC, Somarathna T, Samowitz WS,
Kornacki S, Sanders SA, Novelli MR, Shepherd NA and Yantiss RK:
Diagnostic challenges caused by endoscopic biopsy of colonic
polyps: A systematic evaluation of epithelial misplacement with
review of problematic polyps from the bowel cancer screening
program, United Kingdom. Am J Surg Pathol. 40:1075–1083. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Loughrey MB and Shepherd NA: The pathology
of bowel cancer screening. Histopathology. 66:66–77. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aghagolzadeh P and Radpour R: New trends
in molecular and cellular biomarker discovery for colorectal
cancer. World J Gastroenterol. 22:5678–5693. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hauptman N, Boštjančič E, Žlajpah M,
Ranković B and Zidar N: Bioinformatics analysis reveals most
prominent gene candidates to distinguish colorectal adenoma from
adenocarcinoma. Biomed Res Int. 2018:94165152018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu P, Takai K, Weaver VM and Werb Z:
Extracellular matrix degradation and remodeling in development and
disease. Cold Spring Harb Perspect Biol. 3:a0050582011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu P, Weaver VM and Werb Z: The
extracellular matrix: A dynamic niche in cancer progression. J Cell
Biol. 196:395–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Venning FA, Wullkopf L and Erler JT:
Targeting ECM disrupts cancer progression. Front Oncol. 5:2242015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pickup MW, Mouw JK and Weaver VM: The
extracellular matrix modulates the hallmarks of cancer. EMBO Rep.
15:1243–1253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fang M, Yuan J, Peng C and Li Y: Collagen
as a double-edged sword in tumor progression. Tumour Biol.
35:2871–2882. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boudjadi S, Bernatchez G, Beaulieu JF and
Carrier JC: Control of the human osteopontin promoter by ERRalpha
in colorectal cancer. Am J Pathol. 183:266–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Imano M, Okuno K, Itoh T, Ishimaru E,
Satou T and Shiozaki H: Increased osteopontin-positive macrophage
expression in colorectal cancer stroma with synchronous liver
metastasis. World J Surg. 34:1930–1936. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rohde F, Rimkus C, Friederichs J,
Rosenberg R, Marthen C, Doll D, Holzmann B, Siewert JR and Janssen
KP: Expression of osteopontin, a target gene of de-regulated Wnt
signaling, predicts survival in colon cancer. Int J Cancer.
121:1717–1723. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Galamb O, Sipos F, Spisak S, Galamb B,
Krenacs T, Valcz G, Tulassay Z and Molnar B: Potential biomarkers
of colorectal adenoma-dysplasia-carcinoma progression: mRNA
expression profiling and in situ protein detection on TMAs reveal
15 sequentially upregulated and 2 downregulated genes. Cell Oncol.
31:19–29. 2009.PubMed/NCBI
|
|
19
|
Valcz G, Sipos F, Krenacs T, Molnar J,
Patai AV, Leiszter K, Toth K, Solymosi N, Galamb O, Molnar B and
Tulassay Z: Elevated osteopontin expression and
proliferative/apoptotic ratio in the colorectal
adenoma-dysplasia-carcinoma sequence. Pathol Oncol Res. 16:541–545.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Augoff K, Rabczynski J, Tabola R, Czapla
L, Ratajczak K and Grabowski K: Immunohistochemical study of
decorin expression in polyps and carcinomas of the colon. Med Sci
Monit. 14:CR530–CR535. 2008.PubMed/NCBI
|
|
21
|
Liu QZ, Gao XH, Chang WJ, Wang HT, Wang H,
Cao GW and Fu CG: Secreted protein acidic and rich in cysteine
expression in human colorectal cancer predicts postoperative
prognosis. Eur Rev Med Pharmacol Sci. 19:1803–1811. 2015.PubMed/NCBI
|
|
22
|
Nyman MC, Sainio AO, Pennanen MM, Lund RJ,
Vuorikoski S, Sundström JT and Järveläinen HT: Decorin in human
colon cancer: Localization in vivo and effect on cancer cell
behavior in vitro. J Histochem Cytochem. 63:710–720. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cai X, Liu C, Zhang TN, Zhu YW, Dong X and
Xue P: Down-regulation of FN1 inhibits colorectal carcinogenesis by
suppressing proliferation, migration, and invasion. J Cell Biochem.
119:4717–4728. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thorsen SB, Lundberg M, Villablanca A,
Christensen SL, Belling KC, Nielsen BS, Knowles M, Gee N, Nielsen
HJ, Brunner N, et al: Detection of serological biomarkers by
proximity extension assay for detection of colorectal neoplasias in
symptomatic individuals. J Transl Med. 11:2532013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang S, Zhang C, Zhang Z, Qian W and Sun
Y, Ji B, Zhang Y, Zhu C, Ji D, Wang Q and Sun Y: Transcriptome
analysis in primary colorectal cancer tissues from patients with
and without liver metastases using next-generation sequencing.
Cancer Med. 6:1976–1987. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Q, Wang XQ, Wang J, Cui SJ, Lou XM,
Yan B, Qiao J, Jiang YH, Zhang LJ, Yang PY and Liu F: Upregulation
of spondin-2 predicts poor survival of colorectal carcinoma
patients. Oncotarget. 6:15095–15110. 2015.PubMed/NCBI
|
|
27
|
Zhao M, Liang F, Zhang B, Yan W and Zhang
J: The impact of osteopontin on prognosis and clinicopathology of
colorectal cancer patients: A systematic meta-analysis. Sci Rep.
5:127132015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sethi MK, Thaysen-Andersen M, Kim H, Park
CK, Baker MS, Packer NH, Paik YK, Hancock WS and Fanayan S:
Quantitative proteomic analysis of paired colorectal cancer and
non-tumorigenic tissues reveals signature proteins and perturbed
pathways involved in CRC progression and metastasis. J Proteomics.
126:54–67. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Oshima T, Akaike M, Yoshihara K, Shiozawa
M, Yamamoto N, Sato T, Akihito N, Nagano Y, Fujii S, Kunisaki C, et
al: Overexpression of EphA4 gene and reduced expression of EphB2
gene correlates with liver metastasis in colorectal cancer. Int J
Oncol. 33:573–577. 2008.PubMed/NCBI
|
|
30
|
Adany R and Iozzo RV: Hypomethylation of
the decorin proteoglycan gene in human colon cancer. Biochem J.
276:301–306. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang RY, Wang Z, Chen HQ and Zhu SB:
Negative correlation between miR-200c and decorin plays an
important role in the pathogenesis of colorectal carcinoma. Biomed
Res Int. 2017:10389842017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liang JF, Wang HK, Xiao H, Li N, Cheng CX,
Zhao YZ, Ma YB, Gao JZ, Bai RB and Zheng HX: Relationship and
prognostic significance of SPARC and VEGF protein expression in
colon cancer. J Exp Clin Cancer Res. 29:712010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Heitzer E, Artl M, Filipits M, Resel M,
Graf R, Weissenbacher B, Lax S, Gnant M, Wrba F, Greil R, et al:
Differential survival trends of stage II colorectal cancer patients
relate to promoter methylation status of PCDH10, SPARC, and UCHL1.
Mod Pathol. 27:906–915. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang S, Jin J, Tian X and Wu L:
hsa-miR-29c-3p regulates biological function of colorectal cancer
by targeting SPARC. Oncotarget. 8:104508–104524. 2017.PubMed/NCBI
|
|
35
|
Viana Lde S, Affonso RJ Jr, Silva SR,
Denadai MV, Matos D, Salinas de Souza C and Waisberg J:
Relationship between the expression of the extracellular matrix
genes SPARC, SPP1, FN1, ITGA5 and ITGAV and clinicopathological
parameters of tumor progression and colorectal cancer
dissemination. Oncology. 84:81–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mlakar V, Berginc G, Volavsek M, Stor Z,
Rems M and Glavac D: Presence of activating KRAS mutations
correlates significantly with expression of tumour suppressor genes
DCN and TPM1 in colorectal cancer. BMC Cancer. 9:2822009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chew A, Salama P, Robbshaw A, Klopcic B,
Zeps N, Platell C and Lawrance IC: SPARC, FOXP3, CD8 and CD45
correlation with disease recurrence and long-term disease-free
survival in colorectal cancer. PLoS One. 6:e220472011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Choe EK, Yi JW, Chai YJ and Park KJ:
Upregulation of the adipokine genes ADIPOR1 and SPP1 is related to
poor survival outcomes in colorectal cancer. J Surg Oncol.
117:1833–1840. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Archer. PreSeq™ RNA QC Assay. Tecnical
note. https://archerdx.com/support/preseq-dnaJune
17–2019
|
|
40
|
Latham GJ: Normalization of microRNA
quantitative RT-PCR data in reduced scale experimental designs.
Methods Mol Biol. 667:19–31. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Goni R, Garcia P and Foissac S: The qPCR
data statistical analysis. Integromics White Paper 2009. https://gene-quantification.de/integromics-qpcr-statistics-white-paper.pdfJune
17–2019
|
|
42
|
Wei R, Wong JPC and Kwok HF: Osteopontin-a
promising biomarker for cancer therapy. J Cancer. 8:2173–2183.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shevde LA and Samant RS: Role of
osteopontin in the pathophysiology of cancer. Matrix Biol.
37:131–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang E, Kang HJ, Koh KH, Rhee H, Kim NK
and Kim H: Frequent inactivation of SPARC by promoter
hypermethylation in colon cancers. Int J Cancer. 121:567–575. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cheetham S, Tang MJ, Mesak F, Kennecke H,
Owen D and Tai IT: SPARC promoter hypermethylation in colorectal
cancers can be reversed by 5-Aza-2′deoxycytidine to increase SPARC
expression and improve therapy response. Br J Cancer. 98:1810–1819.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tai IT and Tang MJ: SPARC in cancer
biology: Its role in cancer progression and potential for therapy.
Drug Resist Updat. 11:231–246. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Vaz J, Ansari D, Sasor A and Andersson R:
SPARC: A potential prognostic and therapeutic target in pancreatic
cancer. Pancreas. 44:1024–1035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yuan X, Bian T, Liu J, Ke H, Feng J, Zhang
Q, Qian L, Li X, Liu Y and Zhang J: Spondin2 is a new prognostic
biomarker for lung adenocarcinoma. Oncotarget. 8:59324–59332. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Feng Y, Hu Y, Mao Q, Guo Y, Liu Y, Xue W
and Cheng S: Upregulation of Spondin-2 protein expression
correlates with poor prognosis in hepatocellular carcinoma. J Int
Med Res. 47:569–579. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jin C, Lin JR, Ma L, Song Y, Shi YX, Jiang
P, Dong Y and Li XS: Elevated spondin-2 expression correlates with
progression and prognosis in gastric cancer. Oncotarget.
8:10416–10424. 2017.PubMed/NCBI
|
|
51
|
Jarvinen TA and Prince S: Decorin: A
growth factor antagonist for tumor growth inhibition. Biomed Res
Int. 2015:6547652015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu Z, Yang Y, Zhang X, Wang H, Xu W, Wang
H, Xiao F, Bai Z, Yao H, Ma X, et al: An oncolytic adenovirus
encoding decorin and granulocyte macrophage colony stimulating
factor inhibits tumor growth in a colorectal tumor model by
targeting pro-tumorigenic dignals and via immune activation. Hum
Gene Ther. 28:667–680. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yi W, Xiao E, Ding R, Luo P and Yang Y:
High expression of fibronectin is associated with poor prognosis,
cell proliferation and malignancy via the NF-κB/p53-apoptosis
signaling pathway in colorectal cancer. Oncol Rep. 36:3145–3153.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kullander K and Klein R: Mechanisms and
functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol.
3:475–486. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
55
|
de Marcondes PG and Morgado-Diaz JA: The
role of EphA4 signaling in radiation-induced EMT-like phenotype in
colorectal cancer cells. J Cell Biochem. 118:442–445. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Patel PG, Selvarajah S, Guerard KP,
Bartlett JMS, Lapointe J, Berman DM, Okello JBA and Park PC:
Reliability and performance of commercial RNA and DNA extraction
kits for FFPE tissue cores. PLoS One. 12:e01797322017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
von Ahlfen S, Missel A, Bendrat K and
Schlumpberger M: Determinants of RNA quality from FFPE samples.
PLoS One. 2:e12612007. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Guerra JM, Monteiro RL, Gonzalez L, Kimura
LM, Cirqueira CDS and de Araujo LJT: Against all odds: RNA
extraction from different protocols adapted to formalin-fixed
paraffin-embedded tissue. Appl Immunohistochem Mol Morphol. May
23–2019.(Epub ahead of print). doi: 10.1097/PAI.0000000000000772.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bustin SA, Benes V, Garson JA, Hellemans
J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL,
et al: The MIQE guidelines: Minimum information for publication of
quantitative real-time PCR experiments. Clin Chem. 55:611–622.
2009. View Article : Google Scholar : PubMed/NCBI
|

















