Introduction
Cervical cancer ranks as the fourth most common type
of cancer and the fourth leading cause of cancer-related mortality
in women worldwide (1). Although the
persistent infection of high-risk human papillomaviruses (HPV) has
been demonstrated to be associated with the development of cervical
cancer by epidemiological, molecular and functional studies
(2), the molecular mechanisms
underlying the transition from HPV infection to cervical
carcinogenesis and progression are not yet fully understood.
Inhibitor of differentiation 1 (Id-1) is a member of
the helix-loop-helix (HLH) transcription factor family (3). As the protein lacks the basic domain for
DNA binding, Id-1 acts as the dominant inhibitor of the basic HLH
transcription proteins by forming heterodimers. It has been
indicated that Id-1 is involved in cell differentiation,
proliferation, invasion, apoptosis and chemoresistance (4). Recently, Id-1 has been suggested as a
possible oncogene. Overexpression of Id-1 has been found in
numerous types of cancer, including gastric carcinoma (5), hepatocellular carcinoma (6), breast cancer (7) and prostate cancer (8). Our previous study also found that
increased Id-1 expression was associated with aggressive behavior
of cervical cancer and poor overall survival (9). Although Id-1 has been extensively
investigated in recent years, the molecular mechanisms of Id-1 in
cervical cancer development have not yet been addressed.
NF-κB is a prominent survival-regulatory
transcription factor that can be activated by various stimuli
(10). The predominant form of active
NF-κB in cells consists of a p65/p50 heterodimer, which is
sequestered as an inactive state in the cytoplasm by the inhibitor
of κB (IκB) protein. The phosphorylation and degeneration of IκB
releases NF-κB and promotes its translocation into the nucleus,
where it binds to target genes (11,12). It
has been previously demonstrated that NF-κB p65 is significantly
activated in cervical cancer and is associated with cancer
progression (13). However, the
mechanisms responsible for NF-κB activation in cervical cancer have
not yet been well determined.
Although Id-1 and NF-κB are known to be associated
with tumor development and progression, limited studies are
available regarding the association between Id-1 and NF-κB in the
uterine cervix. In this study, clinical and laboratorial evidence
was used to assess the co-expression status of Id-1 and NF-κB, and
their prognostic significance in cervical cancer. Furthermore, the
effects of silencing or promoting Id-1 gene expression on the NF-κB
signaling pathway, properties and chemoresistance in vitro
were investigated.
Materials and methods
Human tissues
Paraffin-embedded cervical tissue samples (normal,
n=20; squamous cervical cancer, n=65) were collected from the
Pathology Department of West China Second Hospital, Sichuan
University during the year 2006. The details of these samples had
been previously described (14). The
human investigations were approved by and carried out in accordance
with an assurance filed with the Ethics Committee of the West China
Second University Hospital, Sichuan University. Moreover, informed
consents were obtained from all the patients.
Immunohistochemistry (IHC)
The procedure of IHC has been previously described
(14). This analysis was performed to
detect the specificity of mouse anti-human Id-1 (sc-133104) and
NF-κB p65 (sc-135769) antibodies (diluted 1:100; Santa Cruz
Biotechnology, Inc.) in cervical tissue samples. Horse anti-mouse
IgG (PK-4002, 1:200 dilution in PBS; Zhongshan Jinqiao
Biotechnology Co., Ltd., Beijing, China) was used as secondary
antibody. Cells with brown staining in the cytoplasm or nucleus
were considered positive. Five fields of view (magnification, ×40)
were selected for each slice and the percentage of positive cells
was calculated. The immunoreactivities were assessed as a continuum
from the undetected level (0%) to diffused staining (100%), and the
positive immunoreactivity was defined as >10%.
Cells and cell culture
The human cervical cancer cell lines, HeLa and SiHa,
and the E6/E7 immortalized human cervical epithelial cell line
(H8), were all obtained from the China Center for Type Culture
Collection [obtained in October, 2016; cell line characterizations
were performed by DNA (STR) profiling]. Four stable reconstructive
cell lines were established as follows: SiHa-knock down (KD) cells
(infected with pGIPZ-hId-1-shRNAmir lentivirus, Sequences (target
sequence was underlined): Sense 5′-AAAAGGAGCTGAACTCTGAGTCTGACTCGAGTCAGACTCAGAGTTCAGCTCC−3′,
antisense 5′-GGAGCTGAACTATGAGTCTGACTCGAGTCAGACTCAGAGTTCAGCTCCTTTT-3′),
SiHa-KD-control cells (infected with pGIPZ-shRNAmir-control
lentivirus, Sequences (target sequence was underlined): Sense
5′-AAAATTCTCACGAACGTAGTCACGTCTCGAGACGTGACTACGTTCGTGAGAA−3′,
antisense 5′-TTTTTTCTCACGAACGTAGTCACGTCTCGAGACGTGACTACGTTCGTGAGAA−3′),
SiHa-Id-1 cells (infected with pWPI-hId-1 lentivirus) and
SiHa-Id-1-control cells (infected by empty-pWPI-vector lentivirus),
respectively. The Id-1 vectors and viral packaging system were
kindly provided by Professor Wenming Xu, Sichuan University. The
SiHa cells were inoculated in 6-well plates at 5×105
cells/well. When the cells grew to 80% confluence, the medium was
replaced with medium/Polybrene (5 µg/ml; Santa Cruz Biotechnology,
Inc.) mixture, and lentiviral particles (1×105 IFU) were
added to the culture. All cells were maintained in RPMI-1640 or
DMEM medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented
with 10–15% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.) and antibiotics (100 µg/ml streptomycin and 100 U/ml
penicillin; Gibco; Thermo Fisher Scientific, Inc.) at 37°C in 5%
CO2.
Transient transfections
The H8 cells were transfected with pcDNA expression
plasmid Id-1 which contains the full-length human Id-1 gene or
pcDNA3.1(−) as the control (kindly provided by Professor Xia Wang,
Sichuan University). Transient transfections were performed using
the FuGENE 6 transfection reagent (Promega Corporation) according
to the manufacturers' recommendations. The siRNA oligonucleotides
for Id-1 (sequences: Sense, 5′-CGACAUGAACGGCUGUUACTT-3′, antisense,
5′-GUAACAGCCGUUCAUGUCGTT-3′) and non-targeting siRNA as the control
(sequences: Sense, 5′-UUCUCCGAACGUGUCACGUTT-3′, antisense,
5′-ACGUGACACGUUCGGAGAATT-3′) (Santa Cruz Biotechnology, Inc.) were
transiently transfected into SiHa cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. After
incubation for 4 h, the medium was removed and replaced with fresh
medium. Cells were collected at the indicated time points (0, 15,
30, 60 and 120 min). The expression of nuclear NF-κB p65 and Id-1
was examined by western blot analysis.
NF-κB reporter gene assay
In order to detect NF-κB transcriptional activity, a
dual-luciferase reporter gene assay (Promega Corporation) was used
according to the manufacturer's instructions. The cells were
co-transfected with the pGL4.32 (luc2P/NF-κB-RE/Hygro) plasmid and
pRL-TK-Luc reference plasmid (Lipofectamine® 2000).
After 48 h, cell lysates were analyzed for luciferase assay by
Thermo Scientific Varioskan® Flash (Thermo Fisher
Scientific, Inc.). NF-κB transcriptional activity was calculated as
relative luciferase units ratio of pNF-κB-Luc/pRL-TK-Luc.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from the cultured cells and
reverse transcribed into cDNA using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) and the PrimeScript RT reagent kit
(Takara Biotechnology Co., Ltd.) with following protocol: 15 min at
37°C and 5 sec at 85°C. β-2-microglobulin (B2M) was detected as an
internal reference. The sequences of the primers were as follows:
Human Id-1, forward 5′-TCGGGCTTCCACCTCATTT-3′ and reverse
5′-CTCCACCTTGCTCACCTTGC-3′; human B2M, forward
5′-ATGAGTATGCCTGCCGTGTGA-3′ and reverse 5′-GGCATCTTCAAACCTCCATG-3′.
PCR was performed using the CFX96 real-time system using SsoFast
EvaGreen® Supermix kit (Bio-Rad Laboratories, Inc.) with
the following protocol: 20 sec at 95°C, 2 sec at 98°C and 5 sec at
65°C (40 cycles). The relative gene expression (folds) was
calculated using the 2−ΔΔCq method (15).
Western blot analysis
Whole-cell proteins and nuclear extracts from the
cultured cells were prepared using the BCA Protein Extraction kit
(Beyotime Institue of Biotechnology). Protein concentration was
quantified by BCA Protein Assay kit (Beyotime Institue of
Biotechnology). Cell proteins (100 µg) were loaded onto a 10–15%
gel for SDS-PAGE, and then transferred onto a PVDF membrane (EMD
Millipore). The membranes were blocked with 5% skim milk for 1 h at
room temperature and then incubated with primary antibodies against
Id-1 (sc-133104), NF-κB p65 (sc-135769), IκBα (sc-1643), Bcl-2
(sc-7382), Bcl-xl (sc-8392), Survivin (sc-17779), X-linked
inhibitor of apoptosis (XIAP, sc-55550), inhibitor of apoptosis-1
(IAP-1, sc-271419), inhibitor of apoptosis-2 (IAP-2, sc-517317),
Bax (sc-7480), Bak (sc-517390), histone H1 (sc-393358) and β-actin
(sc-47778) (Santa Cruz Biotechnology, Inc.) overnight at 4°C. After
washing, the membrane was incubated with HRP labelled goat
anti-mouse IgG (ZB-2305; Zhongshan Jinqiao Biotechnology Co., Ltd.,
Beijing, China) for 1 h at room temperature. Finally, protein
signals were visualized using the eECL Western Blot Kit (CoWin
Biosciences, Inc.) using the ChemiDoc XRS system (Bio-Rad
Laboratories, Inc.).
Co-immunoprecipitation analysis
Whole-cell proteins from the SiHa cells were
extracted using cell lysis buffer. This extraction was then divided
into four equal volume samples (1–4), and the
volumes of samples 1–3 were made up to 500 µl with ice-cold PBS.
Antibody-IgG was added to sample 2 (diluted 1:500), and antibody
Id-1/NF-κB p65 was added to sample 3 (diluted 1:500), while sample
1 was supplemented only with ice-cold PBS as the blank control.
Samples 1–3 were rocked at 4°C for 5 h, and subsequently 10 µl
Protein A/G PLUS-Agarose beads (Santa Cruz Biotechnology, Inc.) was
added and rocked overnight at 4°C. Subsequently, the beads were
pelleted at ~1,000 × g for 5 min and washed three times in ice-cold
PBS. Samples 1–3 were resuspended in 10 µl ice-cold PBS and mixed
with 10 µl 2X SDS loading buffer, together with sample 4, then
subjected to SDS-PAGE on a 12% gel. The subsequent procedure was
essentially the same as that of the western blot analysis described
above.
Proliferative and cytotoxicity
assay
For the cell proliferation assay, 5×103
cells/well were plated into a 96-well plate and incubated for 7
days at 37°C in 5% CO2. For this assay, every 24 h 10
µl/well WST-1 (Roche Diagnostics) was added to the cells and
incubated for 4 h at 37°C. Subsequently, the absorbance of the
samples against a background control was measured at 440 nm by an
enzymatic labeling instrument (Thermo Fisher Scientific, Inc.),
which was regarded as the indicator of viable cells. For cell
cytotoxicity assay, 5×104 cells/well were plated into a
96-well plate and incubated at 37°C for 24 h. Various different
concentrations of cisplatin (0, 5 and 10 µg/ml) were then added to
the medium for 24 h of co-incubation. A WST assay was then
performed out to detect cell viability as described above.
Cell migration assay
The wound healing assay was used to examine
directional cell migration in vitro. The cells were seeded
into a 6-well plate and incubated for 24 h until a confluent
monolayer was formed. Cells were subsequently serum-starved, and
linear wounds of similar width were created by manually scraping
the cell monolayer with sterile plastic tips. Images were acquired
at 0 and 48 h under a microscope (Nikon Corporation), and the width
of the wounds were evaluated by using the reference mark (the cell
boundary marked with a white line). The percentage of cell
migration was calculated using the following formula: (Width of the
wound at 0 h-width of the wound at 48 h)/width of the wound at 0
h.
Matrigel invasion assay
The cell invasion assay was performed using
Transwell Chambers (Corning, Inc.) with an 8-µm pore membrane
inserted into a 24-well plate. The upper membranes of the Transwell
chambers were coated with 40 µl Matrigel (BD Biosciences) for 4 h
at 37°C. 1×104 cells in serum-free medium were added
into the upper chamber, while the lower chamber was supplemented
with 600 µl serum-containing medium (15%). After 48 h, non-invaded
cells were scraped off with a cotton swab from the upper side of
the chambers. Cells on the lower side of the chambers were fixed
and stained with 0.1% crystal violet for 20 min at room
temperature. The number of invaded cells was counted under a
microscope (Nikon Corporation).
Detection of apoptotic cells by
5,5′,6,6′-tetrachloro-1,1′,3,3′tetraethylbenzimidazolylcarbocyanine
iodide (JC-1) staining
JC-1 (Beyotime Institute of Biotechnology) is a
fluorescent cationic dye widely used for measuring mitochondrial
membrane potential (ΨΔm), which is a hallmark of apoptosis.
5×104 cells/well were cultured in a 24-well plate.
Various concentrations of cisplatin were then added to the medium
for 24 h. After washing the cells, 500 µl 1X JC-1 reagent solution
was added followed by incubation at 37°C for 20 min. Finally, the
cells were observed under a fluorescence microscope (Nikon Eclipse
Ti; Nikon Corporation). The percentage of survival cells was
calculated using the following formula: The number of viable
cells/the total number of cells.
Statistical analysis
The patient characteristics were compared using
Fisher's exact test. One-way ANOVA or multivariate analysis of
variance (post hoc test: Least significant difference) were used
where appropriate. The Kaplan-Meier method and the log-rank test
were performed to assess the univariate cumulative survival.
Pearson's correlation test was used for correlation analysis.
Multivariate analysis was performed using a Cox regression model
analysis. For all tests, P<0.05 (two-sided) was considered to
indicate a statistically significant difference.
Results
Co-expression of Id-1 and nuclear
NF-κB p65 in cervical cancer tissues
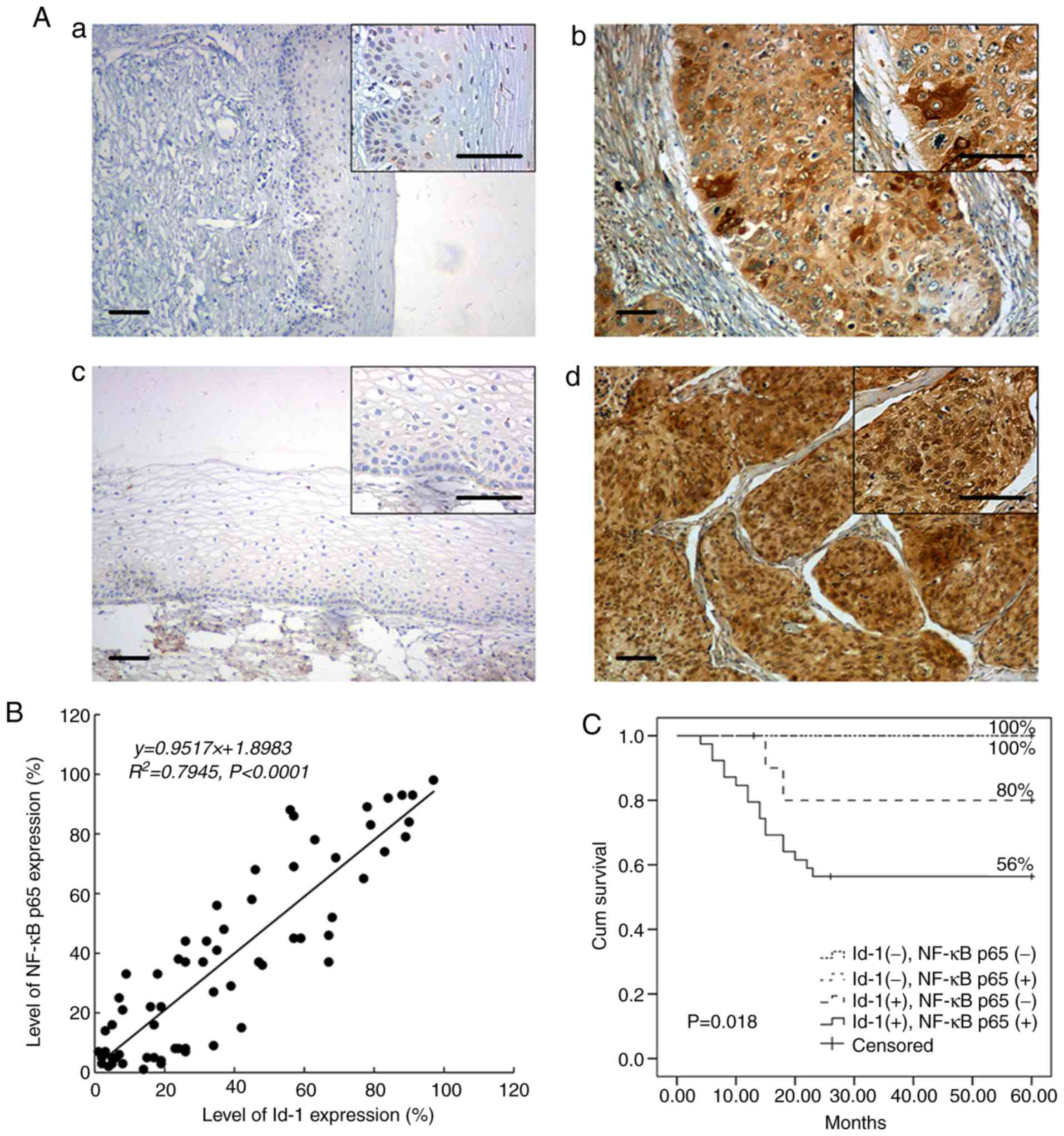
Id-1 immunopositivity was most abundant in the
cytoplasm of the tumor cells, whereas NF-κB p65 was predominantly
detected within the nucleus of the tumor cells (Fig. 1A). Essentially, no immunoreactivity
for Id-1 or nuclear NF-κB p65 was observed in the 20 normal samples
(5 and 11%, respectively). However, in the 65 cancer tissues,
diffused Id-1 or focal NF-κB p65 staining was frequently detected
(75.4 and 67.7%, respectively). Moreover, the expression status of
Id-1 and nuclear NF-κB p65 exhibited a significant positive
association (Table I). Of the 65
cancer samples, 39 (60%) exhibited the co-expression of Id-1 and
nuclear NF-κB p65. There was a significant difference in the
positive rates of nuclear NF-κB p65 in the Id-1 negative and Id-1
positive group [Table I, 5/16 and
39/49 (31.3 vs. 79.6%); P=0.001]. Additionally, there was a strong
linear correlation between the NF-κB p65 and Id-1 expression
(Pearson's correlation; R2=0.7945; P<0.001; Fig. 1B).
 | Table I.Association of Id-1 and nuclear NF-κB
p65 expression with patient characteristics. |
Table I.
Association of Id-1 and nuclear NF-κB
p65 expression with patient characteristics.
|
| Id-1 (−)
(n=16) | Id-1 (+)
(n=49) |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | NF-κB p65 (−)
(a) | NF-κB p65 (+)
(b) | NF-κB p65 (−)
(c) | NF-κB p65 (+)
(d) |
P-valuea |
|---|
| No. of
patients | 11 | 5 | 10 | 39 | 0.001 |
| FIGO stage |
|
|
|
| 0.108 |
|
I–II | 8 | 2 | 4 | 29 |
|
|
III–IV | 3 | 3 | 6 | 10 |
|
| Histological
grades |
|
|
|
| 0.002 |
|
WDSCC | 9 | 1 | 2 | 8 |
|
| MDSCC +
PDSCC | 2 | 4 | 8 | 31 |
|
| Lymph node
metastasis |
|
|
|
| 0.026 |
| No | 10 | 2 | 4 | 17 |
|
|
Yes | 1 | 3 | 6 | 22 |
|
| Vascular
invasion |
|
|
|
| 0.001 |
| No | 11 | 4 | 5 | 14 |
|
|
Yes | 0 | 1 | 5 | 25 |
|
| Invasive
interstitial depth |
|
|
|
| 0.009 |
|
≤1/2 | 9 | 1 | 4 | 11 |
|
|
>1/2 | 2 | 4 | 6 | 28 |
|
| Tumor size |
|
|
|
| 0.912 |
|
Diameter ≤4 cm | 5 | 2 | 6 | 19 |
|
|
Diameter >4 cm | 6 | 3 | 4 | 20 |
|
Association of Id-1 and NF-κB p65
expression with patient characteristics and prognosis
All patients were stratified into the four groups
according to the immunopositivity of Id-1 and nuclear NF-κB p65
(Table I): ‘a’ Id-1 negative/nuclear
NF-κB p65-negative group; ‘b’ Id-1 negative/nuclear NF-κB
p65-positive group; ‘c’ Id-1 positive/nuclear NF-κB p65-negative
group; ‘d’ Id-1-positive/nuclear NF-κB p65-positive group. In
addition to the clinical stages and tumor size, significant
differences were found in terms of histological grade, lymph node
metastasis, vascular invasion or invasive interstitial depth in the
four groups. The ‘d’ group exhibited a tendency to be more
frequently associated with cervical cancer progression and
malignancy, involving poor histological grades, lymph node
metastasis, vascular invasion and invasive interstitial depth
(P<0.05).
The 5-year cumulative survival rate for the 65
patients was 70%. In total, 19 patients died due to tumor
recurrence within 2 years (29.2%). As shown in Fig. 1C, subjects in the ‘d’ group had a
poorer prognosis compared with the controls (log rank P=0.018). As
expected, apart from the co-expression of Id-1 and nuclear NF-κB
p65, either Id-1 or nuclear NF-κB p65 expression was also a
statistically significant prognostic indicator (Table II). However, only the co-expression
of Id-1 and nuclear NF-κB p65 and the expression of nuclear NF-κB
p65 were independent prognostic factors, as shown by the
multivariate analysis (P=0.031 and P=0.01, respectively; Table II).
 | Table II.Relationship between Id-1 and nuclear
NF-κB p65 expression and survival. |
Table II.
Relationship between Id-1 and nuclear
NF-κB p65 expression and survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Prognostic
variants | P-value | HR | 95% CI | P-value |
|---|
| Id-1 (negative vs.
positive) | 0.006 | 33.731 |
0.481–2,365.901 | 0.105 |
| NF-κB p65 (negative
vs. positive) | 0.016 | 5.005 | 1.155–21.684 | 0.031 |
| Id-1 & NF-κB
p65 (negative/negative or positive/positive) | 0.002 | 6.953 | 1.605–30.123 | 0.01 |
Co-expression of Id-1 and nuclear
NF-κB p65 in cervical cancer cell lines
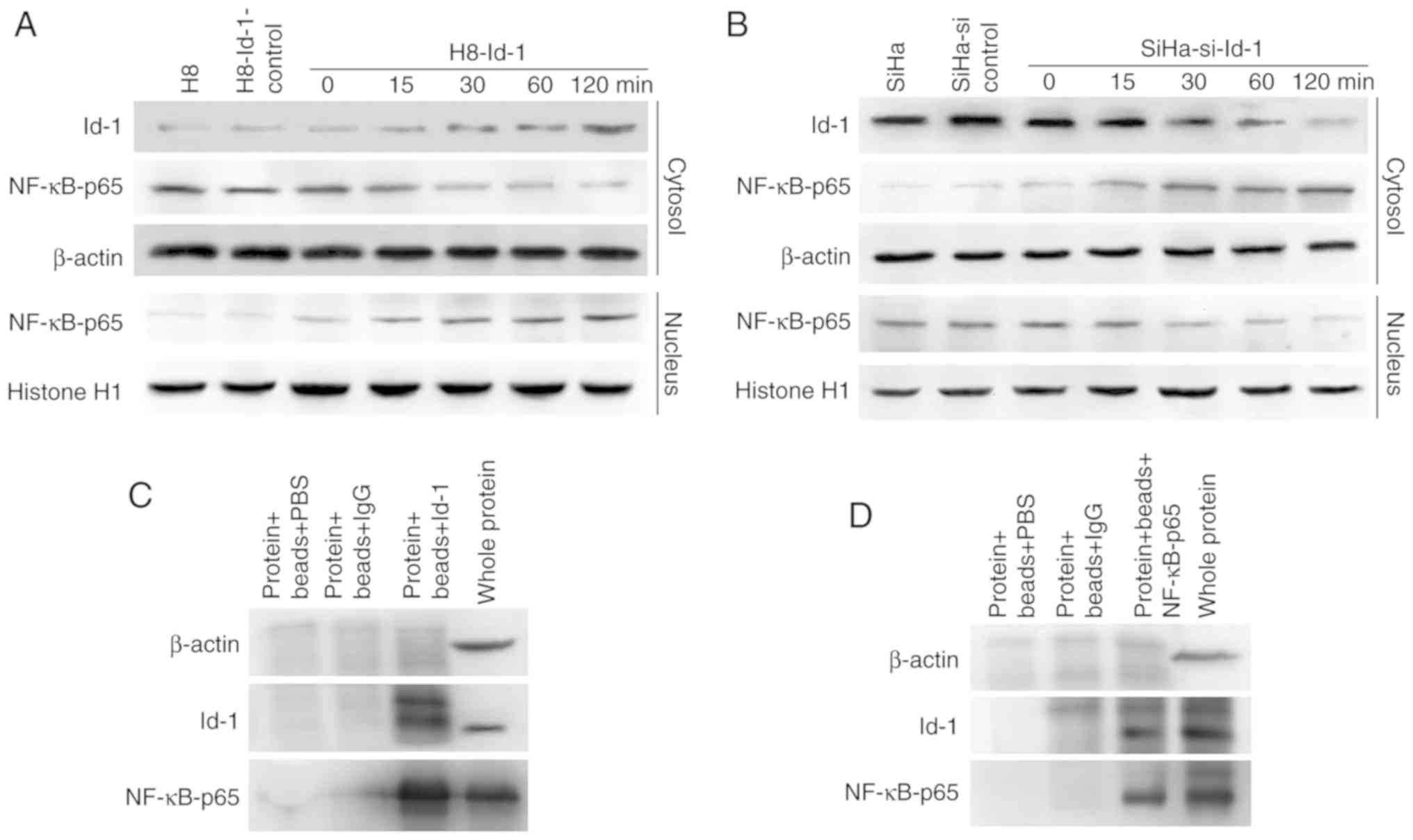
To determine the protein expression levels of Id-1,
nuclear NF-κB p65 and IκB-α in vitro, western blot analysis
was performed. As shown in Fig. 2A,
Id-1 and nuclear NF-κB p65 were expressed at significantly higher
levels in SiHa or HeLa cells than in H8, whereas the lower
expression of IκB-α was detected in the cancer cell lines. RT-qPCR
was then performed to detect the relative mRNA level of Id-1
(Fig. 2B). Significant differences
were found when comparing the SiHa (36.15±2.63) and HeLa
(13.29±0.83) with H8 (1.01±0.15; P<0.001). These results in
vitro were in agreement with the findings in human tissues.
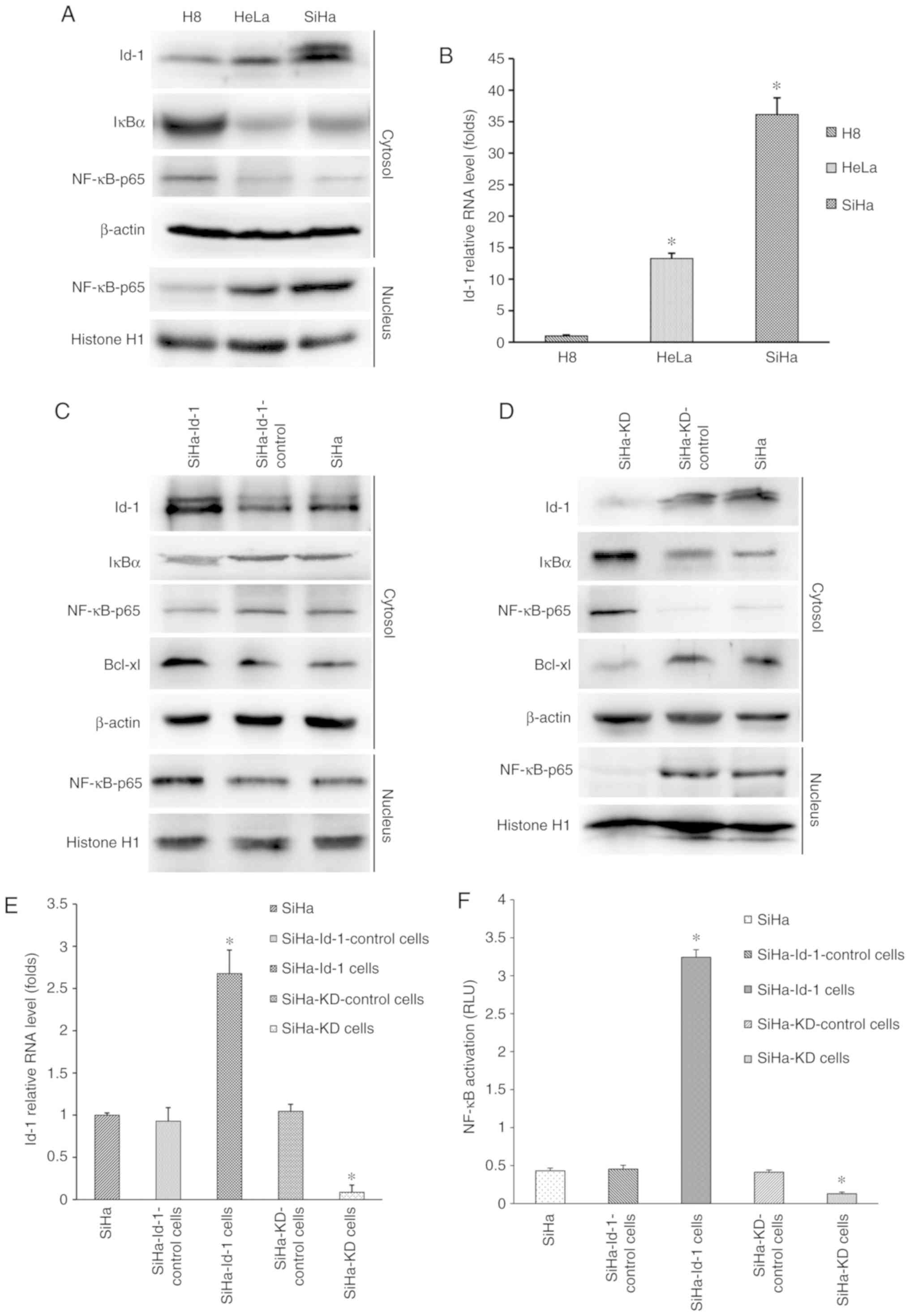 | Figure 2.Synchronous co-expression of Id-1 and
nuclear NF-κB p65 in cervical cell lines and the effects of Id-1
expression on NF-κB signaling pathway. (A) Western blot analysis
was performed to determine the expression levels of Id-1, IκB-α and
nuclear NF-κB p65. β-actin or histone H1 was used as an internal
control for whole or nuclear protein, respectively. Representative
images are shown. (B) Id-1 mRNA was detected by reverse
transcription-quantitative PCR. B2M was used as an internal
control. The data are expressed as folds of increase compared to H8
(*P<0.001 vs. H8). Western blot analysis was performed to
determine the expression levels of Id-1, IκBα, Bcl-xl and nuclear
NF-κB p65 in the (C) SiHa, SiHa-Id-1 and SiHa-Id-1-control cells
and (D) SiHa, SiHa-KD, SiHa-KD-control. β-actin or histone H1 was
used as an internal control. Representative images are shown. (E)
Id-1 mRNA was detected by quantitative RT-PCR in the corresponding
cells. B2M was used as an internal control. The data are expressed
as folds of increase compared to the controls (*P<0.001 vs.
respective control). (F) NF-κB transcription activity was detected
by a dual-luciferase reporter gene assay in the corresponding
cells. The data are presented as relative luciferase units
(*P<0.001 vs. respective control). Id-1, Id-1, inhibitor of
differentiation 1; IκBα, inhibitor of κB. |
Id-1 expression is closely associated
with the activity of the NF-κB signaling pathway
Initially, the four stable reconstructive cells were
examined for the expression status of Id-1 transcripts by RT-qPCR
and western blot analysis (Fig.
2C-E). Id-1 expression at the mRNA and protein level was
markedly downregulated in the SiHa-KD cells compared with the
controls (P<0.001; Fig. 2E),
indicating that the Id-1 gene was effectively silenced. Conversely,
the SiHa-Id-1 cells exhibited significantly elevated levels of Id-1
transcripts in comparison to the controls (P<0.001; Fig. 2E), demonstrating that the activity of
the Id-1 gene was effectively intensified. However, there were no
significant differences between the SiHa, SiHa-KD-control and
SiHa-Id-1-control cells (P>0.05; Fig.
2E).
Subsequently, the expression levels of nuclear NF-κB
p65 and IκB-α were determined by western blot analysis (Fig. 2C and D). The up or downregulation of
nuclear NF-κB p65 was observed in the SiHa-Id-1 cells or SiHa-KD
cells, respectively compared with the controls, whereas IκB-α was
expressed at a lower or higher level in the corresponding cells.
Moreover, NF-κB transcriptional activity was also markedly
increased and decreased in the SiHa-Id-1 cells or SiHa-KD cells,
respectively (P<0.001; Fig. 2F).
These results suggested that the expression status or activity of
nuclear NF-κB p65 was closely associated with Id-1, while IκB-α
essentially displayed an opposite change in expression. Moreover,
the expression of the immediate downstream effector of NF-κB,
Bcl-xl, was also found to be increased or decreased with Id-1 up-
or downregulation, respectively (Fig. 2C
and D).
Time-dependent activation of NF-κB by
Id-1, and co-immunoprecipitation
Id-1 expression exhibited a trend towards a positive
association with nuclear NF-κB p65 activity; however, the mechanism
involved is not clear. Since NF-κB translocates to the nucleus upon
activation and is time-dependent, the expression of NF-κB p65 in
the nuclear extracts and Id-1 in whole lysates was examined by
western blot analysis of H8-Id-1 cells and SiHa-si-Id-1 cells. As
shown in Fig. 3A and B, the transient
transfection of H8 cells with Id-1 overexpression plasmids and SiHa
cells with Id-1 siRNA plasmids indicated that the expression of
Id-1 resulted in a time-dependent synchronized alteration in the
nuclear expression of NF-κB p65. Furthermore, it was sought to
determine whether an Id-1-NF-κB p65 complex or interaction existed
in the SiHa cells. As shown in Fig. 3C
and D, immunoprecipitation with Id-1 protein from the SiHa
cells and hybridization to NF-κB p65 antibody indicated the binding
of NF-κB p65 protein. This interaction was further confirmed by the
hybridization of Id-1 antibody to immunoprecipitates of NF-κB p65
protein from SiHa cells.
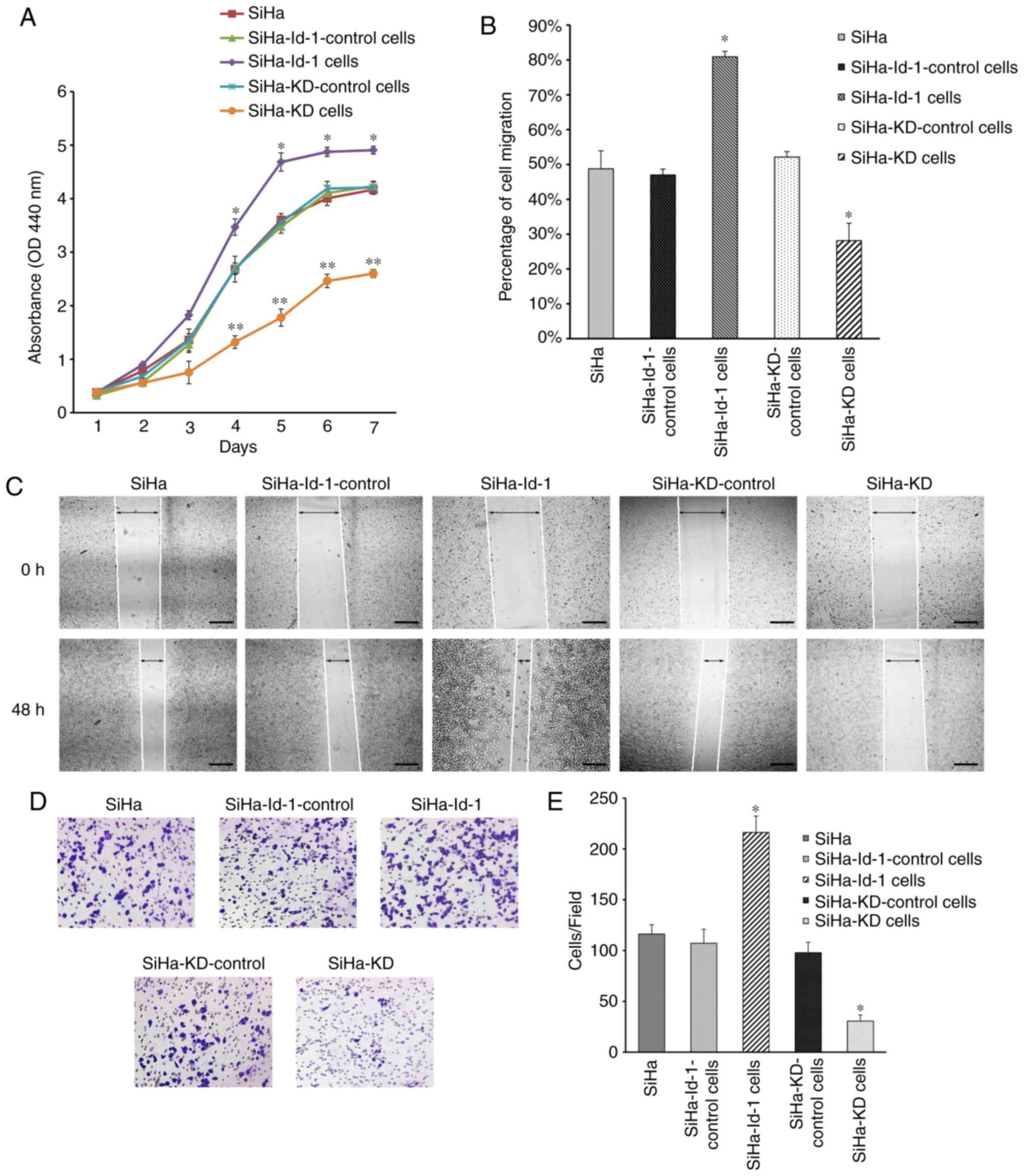
Effects of Id-1 on tumor cell
properties in vitro
WST assay was performed to detect tumor cell
proliferative potential (Fig. 4A).
The SiHa-KD cells grew at a significantly slower rate than the
controls (P<0.001). Conversely, the overexpression of Id-1
transcripts in the SiHa-Id-1 cells significantly increased cell
proliferative activity (P<0.01).
The cell migratory potential was measured by a wound
healing assay (Fig. 4B and C). The
migratory activity of the SiHa-KD cells was significantly decreased
following Id-1 gene knockdown (P<0.001), whereas the SiHa-Id-1
cells exhibited a significantly increased migratory ability in
comparison to the controls (P<0.001).
Tumor cell invasive potential was evaluated by
Matrigel invasion assay. As shown in Fig.
4D and E, a lower Id-1 expression in SiHa-KD cells was found to
effectively inhibit invasion (P<0.001), while the invasive
potential of the SiHa-Id-1 cells was increased significantly
following Id-1 gene overexpression (P<0.001).
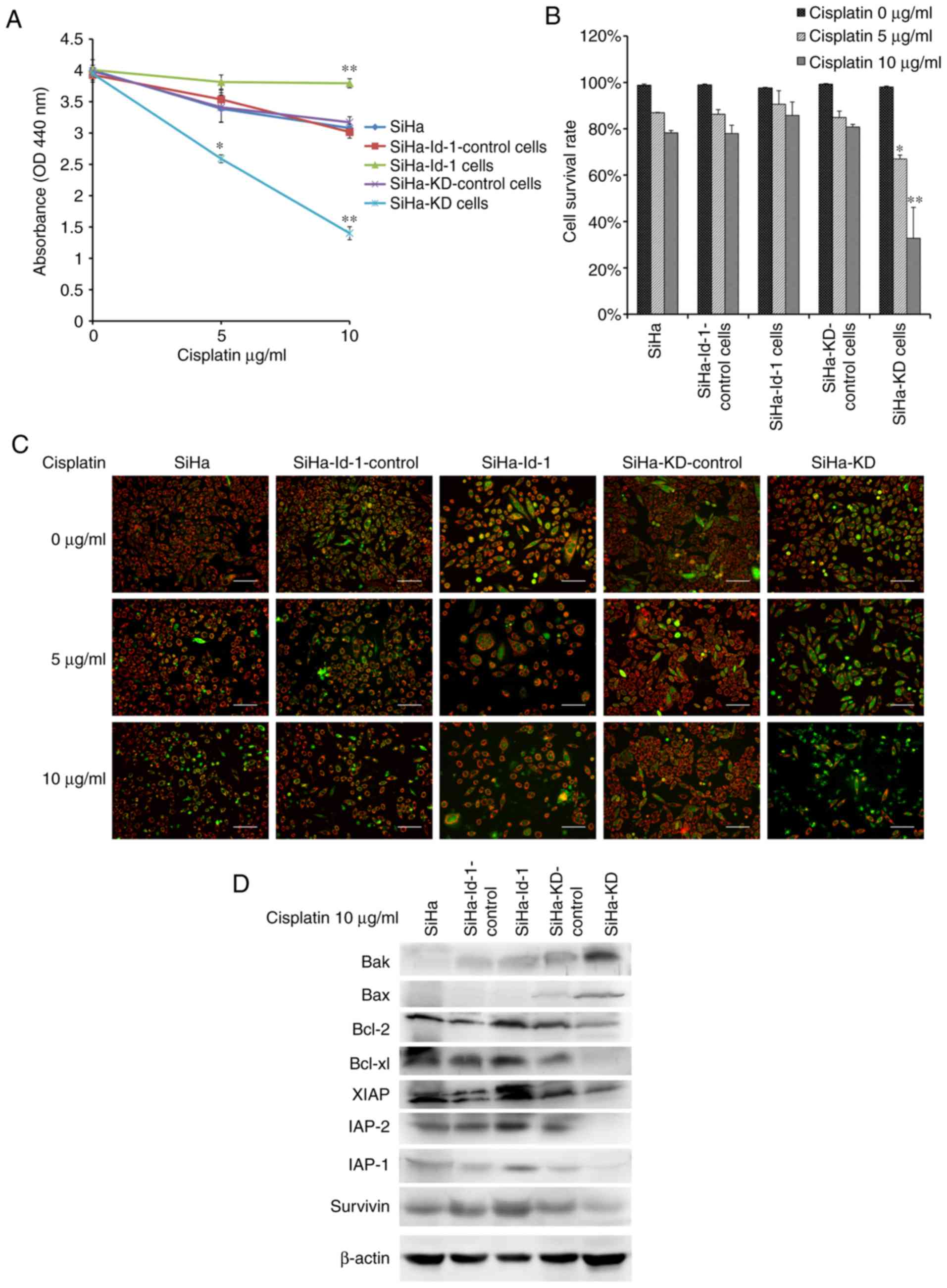
Id-1 is associated with cell
chemosensitivity to cisplatin by regulating the expression of
survival genes related to NF-κB
At first, a WST assay was performed to detect the
cytotoxic effects of cisplatin and cell viability. As shown in
Fig. 5A, the viability of the SiHa-KD
cells treated with cisplatin was significantly decreased compared
with the SiHa-KD control (5 µg/ml, P<0.01; 10 µg/ml,
P<0.001). By contrast, the upregulation of Id-1 in the SiHa-Id-1
cells led to an enhanced chemoresistance compared to SiHa-Id-1
control (10 µg/ml, P<0.001).
The detection of apoptotic cells using JC-1 was then
performed. As shown in Fig. 5B and C,
the apoptotic cells, exhibiting primarily green fluorescence and a
shrinkage in morphology, were easily differentiated from the viable
cells emitting red and green fluorescence. The effect of
cisplatin-induced apoptosis was concentration-dependent. Notably,
the percentage of surviving cells was significantly reduced in the
SiHa-KD cells compared with the SiHa-KD control cells (5 µg/ml
P<0.01, 10 µg/ml P=0.001). However, only a few apoptotic cells
were observed in the SiHa-Id-1 cells following exposure to
cisplatin (P>0.05).
The immediate downstream effectors of NF-κB,
including Bcl-2, Bcl-xl, Survivin, XIAP, IAP-1, IAP-2, Bax and Bak,
were then detected by western blot analysis (Fig. 5D). Following exposure to 10 µg/ml
cisplatin, expression of anti-apoptotic proteins, including Bcl-2,
Bcl-xl, Survivin, XIAP, IAP-1 and IAP-2, was significantly
decreased in the SiHa-KD cells, while the expression levels of the
pro-apoptotic proteins, Bax and Bak, were higher than the controls.
However, the proteins associated with apoptosis exhibited the
opposite change in expression in the SiHa-Id-1 cells compared with
the controls.
Discussion
The present study demonstrated that the elevated
co-expression of Id-1 and nuclear NF-κB p65 was more frequently
associated with cervical cancer progression, aggressive behaviors
and poorer clinical outcomes, further suggesting an increased
resistance to chemotherapy. Simultaneously, targeting Id-1 with
shRNA or Id-1 overexpression lentivirus in SiHa cells had a trend
towards a synchronous association with nuclear NF-κB p65 activity
and cell survival capacity. More importantly, the current study
demonstrated physical interaction of Id-1 and NF-κB p65 in SiHa
cells. This study also indicated that the survival-promoting effect
of Id-1 may be attributed to the subsequent activation of the NF-κB
signaling pathway, suggesting the possible mechanisms for tumor
cell survival and chemoresistance.
Although Id-1 is known to have a critical role in
tumorigenesis, metastasis and progression in a variety of cancers
(16–18), the exact role of Id-1 in cervical
cancer remains unclear. In this study, it was demonstrated that
Id-1 expression was significantly elevated in cervical cancer
tissues and in cultured cancer cell lines. Recently, a few studies
have suggested the potential association between Id-1 and NF-κB in
human cancers, indicating that NF-κB, an important downstream
signaling pathway of Id-1, may be responsible for some effects of
Id-1 (19–21). However, the association of Id-1 with
NF-κB has not yet been addressed yet in cervical cancer to date, to
the best of our knowledge. This study provided the evidence of the
synchronous elevated co-expression of Id-1 and nuclear NF-κB p65
both in vivo and in vitro; moreover, the data
suggested a strong linear correlation between them. The association
of these oncoproteins with the patient characteristics and
prognosis were also evaluated. The findings demonstrated that
cervical cancers with the co-expression of Id-1 and nuclear NF-κB
p65 were more frequently linked to a variety of clinical parameters
associated with cancer aggressiveness and metastasis. Perhaps the
most novel points arising from the current study is that compared
with the separate expression of Id-1 or nuclear NF-κB p65,
co-expression of the both conferred significantly poorer prognosis
and was an independent, strong indicator of survival in cervical
cancer. However, previous studies have only indicated that either
of the two oncoproteins was closely associated with cervical cancer
malignancy and a poor prognosis (9,13).
Nevertheless, it remains to be determined whether
the coordinated regulation of Id-1 and nuclear NF-κB p65
contributes to cervical cancer progression and metastasis. In this
study, shRNA and the Id-1 overexpression lentivirus were used to
modulate expression of Id-1 transcripts in SiHa cells. Since the
nuclear translocation and activation of NF-κB is essentially
dependent on IκB-α degradation in the cytoplasm (22), IκB-α was detected in the corresponding
cells. IκB-α essentially displayed an opposite change in expression
to Id-1 and NF-κB p65. After promoting Id-1 gene expression in the
SiHa cells, expression of IκB-α was significantly decreased and
nuclear translocation of NF-κB p65 was significantly increased,
indicating that Id-1 is a positive regulator of the NF-κB signaling
pathway. These findings are in agreement with earlier observations
in lung cancer, colon cancer and esophageal cancer (23–25).
Moreover, the data indicated that silencing the Id-1 gene markedly
inhibited cell proliferation, migration and invasion, whereas the
ectopic expression of Id-1 resulted in the stimulation of cell
malignant properties. These observations are consistent with
previous findings (26). Given these
observations, it is apparent that Id-1 is crucial gene in tumors.
The mechanisms may involve several molecules associated with NF-κB,
which controls aggressive cellular phenotypes (27–29).
However, the mechanisms responsible for Id-1
activation of NF-κB have not yet been fully elucidated. Since this
study provides the first evidence, to the best of our knowledge,
that an Id-1-NF-κB p65 complex may exist in the SiHa cells, the two
oncoproteins may cooperate to promote cervical carcinogenesis.
However, the details underlying their physical combination are far
from being understood. Li et al (30) indicates that the PI3K/Akt signaling
pathway serves as a link between Id-1 and NF-κB in esophageal
cancer cells. However, Yang et al (31) demonstrated that Id-1 potentiates NF-κB
activation upon T cell receptor signaling in T cells. It is
speculated that there may be more potential mechanisms responsible
for Id-1 and NF-κB interaction, which warrants further
investigations.
The present study further suggested that Id-1
expression was associated with cell chemosensitivity to cisplatin,
and the activation of survival genes via NF-κB. The results
revealed that reduced activity of the NF-κB signaling pathway
mediated by the silencing of the Id-1 gene in SiHa cells led to
significantly increased anti-tumor responses to cisplatin compared
with the controls, suggesting that Id-1 was able to protect tumor
cells from apoptosis through the activation of the NF-κB signaling
pathway. The result is supported by the studies of Li et al
(23) and Lin et al (29) in other types of cancer. As the
Id-1/NF-κB signaling pathway is widely involved in cervical cancer
progression, invasion and chemoresistance, this may suggest a
prospective therapeutic strategy for cervical cancer. Some new
agents targeting NF-κB have been developed (32), while Id-1, as the upstream regulator
of NF-κB, should also be considered as an alternative target for
cancer therapy.
In conclusion, the synchronous co-expression of Id-1
and nuclear NF-κB p65 has a prominent role in many aspects of
cervical cancer, suggesting that the combined analysis of Id-1 and
NF-κB may help to predict malignant properties and a poor
prognosis. Apart from NF-κB, Id-1 should also be developed as a
promising therapeutic strategy for cervical cancer. Reagents
targeting Id-1/NF-κB may have important clinical implications.
Acknowledgements
Not applicable.
Funding
This study was supported by Sichuan Science and
Technology Program of China (grant nos. 2018SZ0248 and
2019YFS0404)
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZW, YZ, and XP performed the experiments, acquired
the data and drafted the manuscript. JL, LH and XP collected the
tissue samples. YZ and JL analyzed and interpreted the data. All
authors have read and approved the final manuscript and agreed to
be accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All procedures performed in experiments involving
human participants were in accordance with the ethical standards of
the institutional and/or national research committee and with the
1964 Helsinki declaration and its later amendments or comparable
ethical standards. The present study was approved by the
Institutional Ethical Board of the West China Second University
Hospital, Sichuan University. Informed consent was obtained from
all individual participants included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fernandes JV, DE Medeiros Fernandes TA, DE
Azevedo JC, Cobucci RN, DE Carvalho MG, Andrade VS and DE Araújo
JM: Link between chronic inflammation and human
papillomavirus-induced carcinogenesis (Review). Oncol Lett.
9:1015–1026. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perk J, Iavarone A and Benezra R: Id
family of helix-loop-helix proteins in cancer. Nat Rev Cancer.
5:603–614. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wong YC, Wang X and Ling MT: Id-1
expression and cell survival. Apoptosis. 9:279–289. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han S, Gou C, Hong L, Liu J, ZheyiHa n,
Liu C, Wang J, Wu K, Ding J and Fan D: Expression and significances
of Id1 helix-loop-helix protein overexpression in gastric cancer.
Cancer Lett. 216:63–71. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho Y, Cho EJ, Lee JH, Yu SJ, Kim YJ, Kim
CY and Yoon JH: Fucoidan-induced ID-1 suppression inhibits the in
vitro and in vivo invasion of hepatocellular carcinoma cells.
Biomed Pharmacother. 83:607–616. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jang KS, Han HX, Paik SS, Brown PH and
Kong G: Id-1 overexpression in invasive ductal carcinoma cells is
significantly associated with intratumoral microvessel density in
ER-negative/node-positive breast cancer. Cancer Lett. 244:203–210.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zielinski AJ, Fong S, Allison J, Kawahara
M, Coppe JP, Feiler H, Lee NM and Desprez PY: The helix-loop-helix
Id-1 inhibits PSA expression in prostate cancer cells. Int J
Cancer. 126:2490–2496. 2010.PubMed/NCBI
|
|
9
|
Li J, Jia H, Xie L, Wang X, He H, Lin Y
and Hu L: Correlation of inhibitor of differentiation 1 expression
to tumor progression, poor differentiation and aggressive behaviors
in cervical carcinoma. Gynecol Oncol. 114:89–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pikarsky E, Porat RM, Stein I, Abramovitch
R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E and
Ben-Neriah Y: NF-kappaB functions as a tumour promoter in
inflammation-associated cancer. Nature. 431:461–466. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chandrika G, Natesh K, Ranade D, Chugh A
and Shastry P: Suppression of the invasive potential of
Glioblastoma cells by mTOR inhibitors involves modulation of NFKB
and PKC-α signaling. Sci Rep. 6:224552016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Jia H, Xie L, Wang X, Wang X, He H,
Lin Y and Hu L: Association of constitutive nuclear factor-kappaB
activation with aggressive aspects and poor prognosis in cervical
cancer. Int J Gynecol Cancer. 19:1421–1426. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peng X, Wu Z, Yu L, Li J, Xu W, Chan HC,
Zhang Y and Hu L: Overexpression of cystic fibrosis transmembrane
conductance regulator (CFTR) is associated with human cervical
cancer malignancy, progression and prognosis. Gynecol Oncol.
125:470–476. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hao L, Liao Q, Tang Q, Deng H and Chen L:
Id-1 promotes osteosarcoma cell growth and inhibits cell apoptosis
via PI3K/AKT signaling pathway. Biochem Biophys Res Commun.
470:643–649. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shuno Y, Tsuno NH, Okaji Y, Tsuchiya T,
Sakurai D, Nishikawa T, Yoshikawa N, Sasaki K, Hongo K, Tsurita G,
et al: Id1/Id3 knockdown inhibits metastatic potential of
pancreatic cancer. J Surg Res. 161:76–82. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Forootan SS, Wong YC, Dodson A, Wang X,
Lin K, Smith PH, Foster CS and Ke Y: Increased Id-1 expression is
significantly associated with poor survival of patients with
prostate cancer. Hum Pathol. 38:1321–1329. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun W, Guo MM, Han P, Lin JZ, Liang FY,
Tan GM, Li HB, Zeng M and Huang XM: Id-1 and the p65 subunit of
NF-KB promote migration of nasopharyngeal carcinoma cells and are
correlated with poor prognosis. Carcinogenesis. 33:810–817. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Su Y, Gao L, Teng L, Wang Y, Cui J, Peng S
and Fu S: Id1 enhances human ovarian cancer endothelial progenitor
cell angiogenesis via PI3K/Akt and NF-KB/MMP-2 signaling pathways.
J Transl Med. 11:1322013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsai CH, Yang MH, Hung AC, Wu SC, Chiu WC,
Hou MF, Tyan YC, Wang YM and Yuan SF: Identification of Id1 as a
downstream effector for arsenic-promoted angiogenesis via PI3K/Akt,
NF-KB and NOS signaling. Toxicol Res (Camb). 5:151–159. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shin CH and Choi DS: Essential roles for
the Non-canonical IKB kinases in linking inflammation to cancer,
obesity, and diabetes. Cells. 8(pii): E1782019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li J, Li Y, Wang B, Ma Y and Chen P: Id-1
promotes migration and invasion of non-small cell lung cancer cells
through activating NF-KB signaling pathway. J Biomed Sci.
24:952017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li B, Cheung PY, Wang X, Tsao SW, Ling MT,
Wong YC and Cheung AL: Id-1 activation of PI3K/Akt/NFkappaB
signaling pathway and its significance in promoting survival of
esophageal cancer cells. Carcinogenesis. 28:2313–2320. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Porquet N, Poirier A, Houle F, Pin AL,
Gout S, Tremblay PL, Paquet ER, Klinck R, Auger FA and Huot J:
Survival advantages conferred to colon cancer cells by
E-selectin-induced activation of the PI3K-NFKB survival axis
downstream of Death receptor-3. BMC Cancer. 11:2852011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma H, Wei Y, Leng Y, Li S, Gao L, Hu H,
Chen L, Wang F, Xiao H, Zhu C and Liang C: TGF-β1-induced
expression of Id-1 is associated with tumor progression in gastric
cancer. Med Oncol. 31:192014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roschger C and Cabrele C: The Id-protein
family in developmental and cancer-associated pathways. Cell Commun
Signal. 15:72017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma J, Shi M, Li G, Wang N, Wei J, Wang T,
Ma J and Wang Y: Regulation of Id1 expression by
epigallocatechin-3-gallate and its effect on the proliferation and
apoptosis of poorly differentiated AGS gastric cancer cells. Int J
Oncol. 43:1052–1058. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin J, Guan Z, Wang C, Feng L, Zheng Y,
Caicedo E, Bearth E, Peng JR, Gaffney P and Ondrey FG: Inhibitor of
differentiation 1 contributes to head and neck squamous cell
carcinoma survival via the NF-kappaB/survivin and phosphoinositide
3-kinase/Akt signaling pathways. Clin Cancer Res. 16:77–87. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li B, Tsao SW, Li YY, Wang X, Ling MT,
Wong YC, He QY and Cheung AL: Id-1 promotes tumorigenicity and
metastasis of human esophageal cancer cells through activation of
PI3K/AKT signaling pathway. Int J Cancer. 125:2576–2585. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang Y, Liou HC and Sun XH: Id1
potentiates NF-kappaB activation upon T cell receptor signaling. J
Biol Chem. 281:34989–34996. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thulasidasan AKT, Retnakumari AP, Shankar
M, Vijayakurup V, Anwar S, Thankachan S, Pillai KS, Pillai JJ,
Nandan CD, Alex VV, et al: Folic acid conjugation improves the
bioavailability and chemosensitizing efficacy of
curcumin-encapsulated PLGA-PEG nanoparticles towards paclitaxel
chemotherapy. Oncotarget. 8:107374–107389. 2017. View Article : Google Scholar : PubMed/NCBI
|