Introduction
Human renal cell carcinoma (RCC) accounts for ~90%
of all cancers of the kidney, in which significantly advanced,
unresectable and metastatic RCC remains one of the most aggressive
and fatal subtypes (1). Furthermore,
the incidence of RCC is increasing at an annual rate of ~1.5–5.9%
worldwide (2). Generally, patients
with RCC respond poorly to conventional radiotherapy and standard
chemotherapy, which may be attributable to multidrug resistance
(3). Molecular targeting agents such
as immunotherapy, tyrosine kinase inhibitors and anti-angiogenic
agents have become additional options for the treatment of
metastatic RCC (4,5). Sunitinib, a tyrosine kinase inhibitor of
the vascular endothelial growth factor (VEGF) receptor, has been
used as a first line treatment in patients with metastatic RCC.
However, the initial treatment success of ~80% is overshadowed by
the occurrence of resistance after a drug-sensitive period
(6). Therefore, it is critical to
elucidate the mechanisms of RCC progression to identify novel
biomarkers and develop advanced management options for patients
with RCC.
MicroRNAs (miRNAs) are a class of endogenous short
18–25 nucleotide non-coding RNA molecules that bind to the
3′-untranslated region (UTR) of specific target mRNAs, thereby
triggering mRNA degradation and translational repression (7). Increasing evidence has suggested that
alterations in miRNA expression profiles contribute to cancer
pathogenesis through various biological processes (8,9). A recent
study demonstrated that the antitumor miRNA (miR)-101-mediated
ubiquitin-like with PHD and ring finger domains 1 pathway may be
suppressed by sunitinib treatment (10). miR-21 has also been indicated to
promote proliferation and differentiation, and decrease apoptosis
in human RCC cells by activating the mTOR-STAT3 signaling pathway
(11). Moreover, Machackova et
al (12) showed that miR-429
inhibits the loss of E-cadherin in RCC cells, and is associated
with poor prognosis in patients with RCC. Therefore, investigating
the function of aberrantly expressed miRNAs and the mechanisms
underlying miRNA regulation is important for elucidating the
molecular mechanisms of RCC tumorigenesis, metastasis and drug
resistance.
Prior et al (13) demonstrated that miR-942 was
upregulated in sunitinib-resistant Caki-2 cells, compared with the
sunitinib-sensitive counterparts. However, the exact role of
miR-942 in the sunitinib response is far from clear. Furthermore,
various long non-coding (lnc)RNAs have been proposed to function as
competing endogenous RNAs (ceRNAs) that modulate miRNA target gene
expression. In the present study, RNA sequencing was performed on
miR-942-transfected and untransfected control cells. In addition,
miRNA-seq and RNA-seq data were downloaded from The Cancer Genome
Atlas (TCGA) (14), and comprehensive
bioinformatics was used to analyze the significant functions
involved. Integrated analysis of the RNA sequencing and TCGA data
was performed to identify significant prognostic factors, and to
construct an miR-942-related ceRNA network. The present study aimed
to identify the target genes of miR-942 in promoting RCC cell
proliferation and sunitinib resistance, which may aid in the
development of novel therapies for RCC.
Materials and methods
Cell culture
Human renal cell carcinoma OS-RC-2 cells were
purchased from the Shanghai Institute of Biological Sciences
(Shanghai, China) and maintained in RPMI 1640 medium supplemented
with 10% fetal bovine serum (Sigma-Aldrich; Merck KGaA). The cells
were incubated at 37°C (5% CO2) in a humidified
incubator.
Establishment of sunitinib-resistant
cell lines
The sunitinib-resistant OS-RC-2 cell line was
established by continuous exposure to increasing concentrations of
sunitinib (Selleck Chemicals) for ~12 weeks. The initial
concentration of sunitinib was 1 µM, increased to 2 µM after 4
weeks, to 5 µM after 4 weeks, and maintained at 5 µM for the last 4
weeks.
Quantification of miRNA in
sunitinib-resistant cells
Reverse transcription-quantitative (RT-q)PCR was
used to quantify four miRNAs in the sunitinib-sensitive and
resistant cell lines. Total RNA was extracted from confluent cells
in 60 mm culture dishes using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol; 1 µg total RNA was reverse-transcribed
using HiScript Reverse Transcriptase (RNase H; Vazyme Biotech Co.,
Ltd.), and qPCR was performed using the SYBR®-Green
Master Mix with the ABI 7500 system (Applied Biosystems). The
thermocycling conditions were as follows: 50°C for 2 min, 95°C for
10 min, followed by 40 cycles of 95°C for 30 sec, and 60°C for 30
sec. All reactions were performed in triplicate and the expression
values were normalized to that of U6 (15). The PCR primers are displayed in
Table I.
 | Table I.Primers for microRNA reverse
transcription-quantitative PCR in sunitinib-resistant OS-RC-2
cells. |
Table I.
Primers for microRNA reverse
transcription-quantitative PCR in sunitinib-resistant OS-RC-2
cells.
| Name | Orientation | Sequence
(5′-3′) |
|---|
| U6 | Forward |
CGCTTCGGCAGCACATATAC |
|
| Reverse |
AAATATGGAACGCTTCACGA |
| hsa-miR-942-5p | Reverse |
CCAGTGCAGGGTCCGAGGTATT |
|
| Forward |
TGCGCTCTTCTCTGTTTTGGCC |
|
hsa-miR-133a-5p | Reverse |
CCAGTGCAGGGTCCGAGGTATT |
|
| Forward |
TGCGCAGCTGGTAAAATGGAAC |
| hsa-miR-628-5p | Reverse |
CCAGTGCAGGGTCCGAGGTATT |
|
| Forward |
TGCGCATGCTGACATATTTACT |
| hsa-miR-484 | Reverse |
CCAGTGCAGGGTCCGAGGTATT |
|
| Forward |
TGCGCTCAGGCTCAGTCCCCTC |
Overexpression of miR-942 in OS-RC-2
cells
The miR-942 mimic and mimic control were designed
and synthesized by Shanghai GenePharma Co., Ltd. The sequences are
as follows: miR-942 forward, 5′-UCUUCUCUGUUUUGGCCAUGUG-3′, and
reverse, 5′-CAUGGCCAAAACAGAGAAGAUU-3′; negative control forward,
5′-UUCUCCGAACGUGUCACGUTT-3′, and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′. Cells at 70–80% confluence were
transfected with the miR-942 mimic or mimic control (100 nmol/l)
using Lipofectamine 2000 transfection reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) following the manufacturer's protocol.
Each group contained three replicates, and the cells were harvested
48 h post-transfection.
RNA sequencing
RNA isolation and sequencing
Total RNA was extracted from confluent cells in 60
mm culture dishes using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. The purity, concentration and quality of the RNA was
assessed prior to sequencing using a NanoDrop Spectrophotometer
(Thermo Fisher Scientific, Inc.), the Qubit 2.0 Fluorometer
(Invitrogen; Thermo Fisher Scientific, Inc.) and the Agilent 2100
Bioanalyzer (Agilent Technologies, Inc.), respectively. Sequencing
libraries were generated using the NEBNext® Ultra™ RNA
Library Prep kit for Illumina (cat. no. NEB #E7530; New England
Biolabs Inc.). The Qubit 2.0 Fluorometer and Agilent 2100
Bioanalyzer were then used to verify the quality, concentration and
size of the cDNA libraries. The libraries were pooled and sequenced
on an Illumina HiSeq platform, and raw reads were generated. The
original sequencing data were uploaded to the NCBI SRA database
(https://www.ncbi.nlm.nih.gov/sra) with
the accession number SRP127372. Reads with adaptor sequences, those
containing >10% unknown nucleotide content, and those with low
quality bases accounting for >50% of the total nucleotides were
filtered out during data processing.
Sequence alignment and identification
of differentially expressed genes (DEGs)
Filtered reads were aligned to the reference genome
using Hierarchical Indexing For Spliced Alignment Of Transcripts
(16). Initially, the data were
normalized by counts per million (CPM), and low-level expressed
genes (expression values <0.1) were filtered out. The
quasi-likelihood F-tests method in the R package edge
(17) was used to determine DEGs
between the miR-942 mimic- and the mimic control-treated group. A
false discovery rate (FDR)-correction (18) was applied to account for multiple
testing and false-positives, where the threshold was set at
FDR<0.05. Furthermore, DEGs were classified as mRNAs and lncRNAs
using genomic annotation information in the GENCODE (v. 24)
database (19).
Prediction and screening of miR-942
target genes
Downregulated lncRNA and mRNA genes identified
during differential expression analysis were considered as
potential target genes for miR-942. miRanda software (v3.3a)
(20) was used to predict the binding
sites of miR-942 and these DEGs. The parameters were set as
follows: -sc 120, -en 0, -strict. If the genes possessed a
predicted miR-942 binding site, they were considered to be target
genes.
Protein-protein interaction (PPI)
analysis of predicted mRNAs
For the resulting miR-942 target mRNAs, PPI analysis
was performed using the Search Tool for the Retrieval of
Interacting Genes/Proteins (21). In
the present study, required confidence (combined score) >0.4 was
selected as the screening threshold. Next, Cytoscape (22) was used to construct a PPI network and
the topology of the network was analyzed. According to the ranking
of network connectivity, important nodes in the PPI network were
subsequently obtained.
Functional enrichment analysis of
putative miR-942 target mRNAs
For the resulting putative miR-942 target mRNAs,
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes
(KEGG) pathway enrichment analysis were performed on the DEGs using
the Database for Annotation, Visualization and Integrated Discovery
(v. 6.8) online tool (23). The
parameters were set as gene count ≥2, with a hypergeometric test
significance threshold of P<0.05. The results of enriched KEGG
pathways and GO terms in the biological process, molecular function
and cellular component categories (24) were obtained.
Analysis of TCGA data
Processing of TCGA data
Level 3 RNA-seq data containing the exon and
clinical data of 606 samples for kidney renal clear cell carcinoma
(KIRC) were downloaded from TCGA database (14), and the raw counts expression matrix
was extracted. The data were re-annotated by integrating the
genomic annotation information in the GENCODE database (V24lift)
(19), along with the chromosomal
location information of the exon. According to the re-annotation
data, the genes were classified into lncRNAs and mRNAs. The
expression values of multiple exons that corresponded to the same
gene were integrated to obtain corresponding gene expression
profiles. The gene expression matrix was subsequently normalized
using the CPM method.
Level 3 miRNA-seq data from KIRC 588 samples were
downloaded from the TCGA database (14). The data were also normalized by the
CPM method, and the miR-942 expression values were extracted.
Gene set enrichment analysis (GSEA) of
miR-942 and RNA-seq data from TCGA
For miRNA-seq and RNA-seq data, normal tissue
samples and samples with only one type of data (e.g. miRNA-seq or
RNA-seq) were excluded, leaving a total of 515 tumor tissue
samples. Subsequently, the GSEA tool (25) was used to analyze the KEGG pathways
influenced by miR-942. The coding gene expression profiles were set
as the expression dataset and the miR-942 expression value as
phenotype labels; GSEA analysis was performed using
c2.cp.kegg.v6.1.symbols, primarily focusing on the enriched
phenotype that was negatively correlated with miR-942.
Integrated analysis of RNA-sequencing and
TCGA data
Identification of prognostic
factors
To evaluate the prognostic capacity of each gene,
533 patient samples from TCGA database that had clinical survival
information and contained normalized target gene expression
profiles were included. The analysis was based on the median gene
expression values within these patient samples. The patients were
then divided into high- and low-expression groups using the median
gene expression values as the cutoff point. The difference in the
survival rates of patients between the high- and low-expression
groups was compared, and the genes potentially influencing the
prognosis of patients were predicted. The R survival package
(26) was used to construct
Kaplan-Meier curves, and the survival curves were compared using
the log-rank test. P<0.05 was considered to indicate a
statistically significant difference, and was used to assess the
contribution of lncRNA and/or coding genes to survival
prediction.
CeRNA network construction
The correlation coefficients for miRNA-lncRNA and
miRNA-mRNAs identified from TCGA data portal were calculated using
Pearson's correlation coefficient (27). The miRNA-lncRNA and miRNA-coding gene
pairs with correlation coefficients <0 (i.e., negative
correlation) and P<0.05 indicated that there was a negative
correlation between the expression of miRNA and lncRNA or coding
gene. Moreover, these results were compared with the miR-942 target
genes from the analysis of RNA-sequencing data. Overlapping target
genes were identified as the final potential miR-942 target genes.
The miR-942-related ceRNA network was constructed using Cytoscape
software (22).
RT-qPCR
Total RNA from OS-RC-2 cells in confluent 60-mm cell
culture dishes was extracted using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol; 1 µg total RNA was reverse transcribed
using the PrimeScript™ RT Master Mix (Takara Bio, Inc.).
Subsequently, qPCR was performed using SYBR® Premix EX
taq with the ABI 7500 system (Applied Biosystems; Thermo Fisher
Scientific, Inc., with GAPDH as the reference gene, according to
the manufacturer's protocol. The primers are listed in Table II. The thermocycling conditions were
as follows: 50°C for 3 min, 95°C for 3 min and then 40 cycles of
95°C for 10 sec, and 60°C for 30 sec. The relative gene expression
levels were calculated using the 2−ΔΔCq method (15).
 | Table II.Primers for mRNA reverse
transcription-quantitative PCR in OS-RC-2 cells. |
Table II.
Primers for mRNA reverse
transcription-quantitative PCR in OS-RC-2 cells.
| Gene | Orientation | Sequence
(5′-3′) |
|---|
| GAPDH |
|
TGACAACTTTGGTATCGTGGAAGG |
|
| Reverse |
AGGCAGGGATGATGTTCTGGAGAG |
| LINC00461 | Forward |
CAGCCTATGACAGACAGCCC |
|
| Reverse |
CCAGTTGGTGCTGCCATTTG |
| METAP1 | Forward |
CATCCAGGGCTCGTACTTCTG |
|
| Reverse |
TCTCGCTTCGCCTTTTCATCT |
| DCAF11 | Forward |
AGCTTGGGATGGTCGTCTTG |
|
| Reverse |
TCTCTGGTGCAACATTCGAGG |
| SALL1 | Forward |
GACGTGATGAACCAGATATTGCT |
|
| Reverse |
TTGACGAAAACGGCTTGTTAAAG |
| hsa-miR-942-5p | Reverse
transcription |
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCACATG |
|
| Stem-Loop |
GCGCTCTTCTCTGTTTTGGC |
lncRNA transfection
Small interfering (si)RNA LINC00641 and the negative
control (NC) siRNA were purchased from Guangzhou RiboBio Co., Ltd.,
and 200 nmol/l of each was transfected into cells using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.,) according to the manufacturer's instructions.
The mixture containing the synthesized nucleotide and the
transfection reagent was incubated at room temperature for 10 min
and subsequently added to the cultured cells. The cells were
harvested 48 h post-transfection.
MTT assay
Cell viability was assessed using an MTT assay.
Briefly, 100 µl transfected cells were seeded into 96-well plates
at a density of ~5×103 cells/well. After 24 h, 0, 2.5,
5, 10, 15 or 20 µM sunitinib was added and the cells were incubated
for 48 h. MTT (10 µl, 5 mg/ml) was added to each well prior to
culturing for a further 4 h at 37°C. To dissolve the formazan
crystals, 150 µl DMSO was added to each well and shaken gently for
5 min at room temperature. The absorbance was then measured at 550
nm using a microplate reader.
Statistical analysis
Statistical analyses were performed using SPSS v13.0
(SPSS, Inc.) software. The independent samples t-test and Pearson's
correlation analysis were used to compare the bioinformatics data.
All values are expressed as the mean ± standard deviation. The
experimental results were analyzed using the unpaired, two-tailed
Student's t-test (two groups) or ANOVA (≥3 groups) followed by
Bonferroni's correction. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression levels of miRNAs in
sunitinib-resistant cells
A total of four miRNAs (miR-942-5p, miR-133a-5p,
miR-484 and miR-628-5p) were quantified in sunitinib-sensitive or
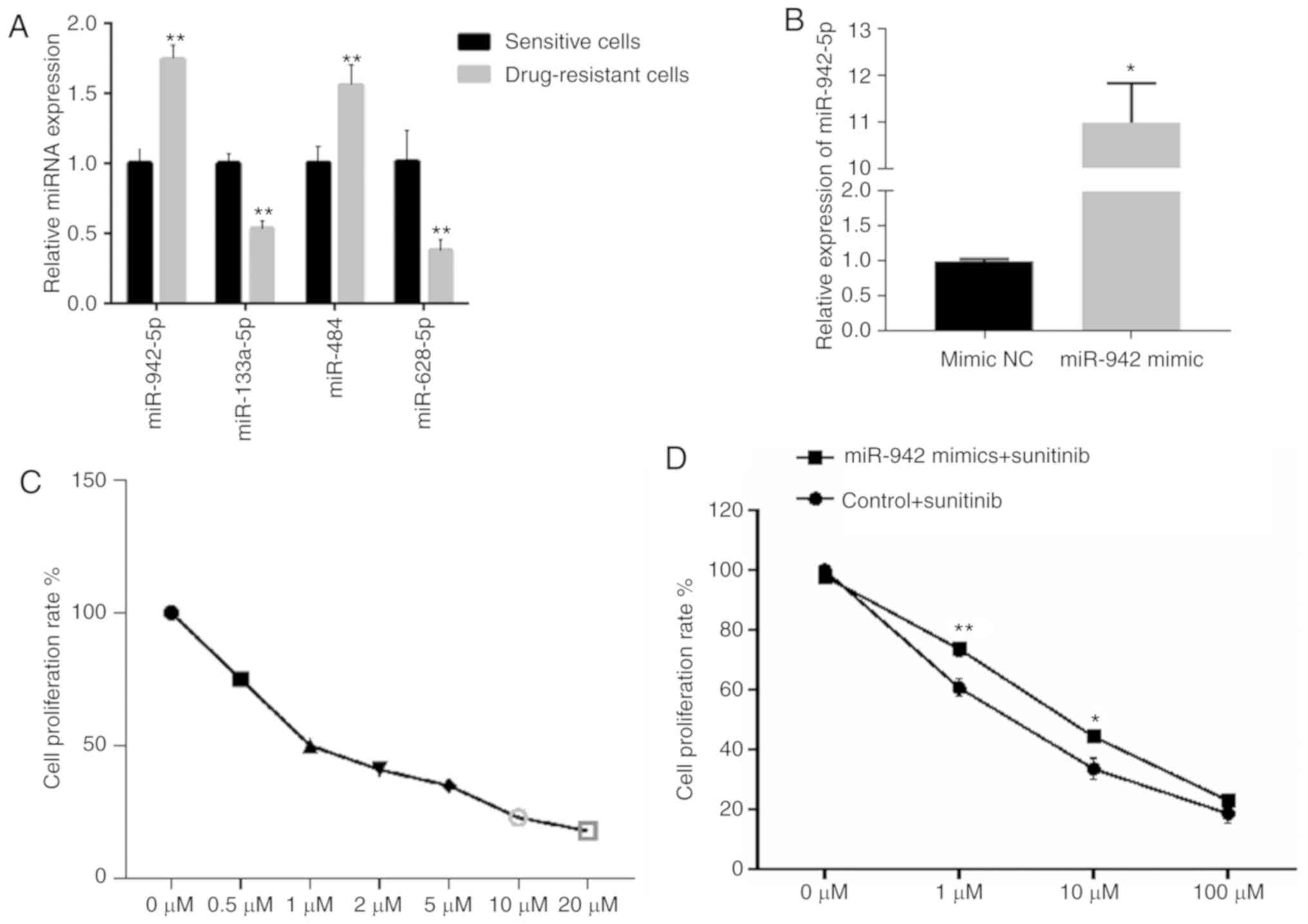
-resistant OS-RC-2 cells using RT-qPCR. As shown in Fig. 1A, the expression levels of miR-942-5p
and miR-484 were significantly increased, whilst those of
miR-133a-5p and miR-628-5p were significantly decreased in
sunitinib-resistant cells, compared with those in
sunitinib-sensitive cells (P<0.01). This result suggests that
miR-942-5p and miR-484 may be associated with sunitinib resistance.
As the fold-change of miR-942-5p was the largest between the
sunitinib-resistant and sensitive cell lines, this miRNA was
selected for further investigation.
Identification of differentially
expressed lncRNAs and mRNAs using RNA-sequencing data
miR-942 mimics were successfully transfected into
OS-RC-2 cells (Fig. 1B), and an MTT
assay was used to assess cell viability at different concentrations
of sunitinib (Fig. 1C). The MTT assay
demonstrated that cell viability was modestly, but significantly
increased following miR-942 transfection and treatment with 1 and
10 µM sunitinib (P<0.01 and P<0.05, respectively; Fig. 1D), suggesting that miR-942 may serve
an important role in RCC cell proliferation and sunitinib
resistance. RNA sequencing was conduced on the miR-942- and miR-942
NC transfected cells. With the threshold of FDR<0.05, a total of
95 differentially expressed lncRNAs, including 65 upregulated and
30 downregulated lncRNAs, were identified in the miR-942
mimic-treated group compared with the control-treated group.
Concurrently, 1,697 differentially expressed mRNAs were screened
out between the miR-942 mimic and the control-treated groups,
comprising 1,171 upregulated and 526 downregulated differentially
expressed mRNAs.
Prediction of miR-942 target
genes
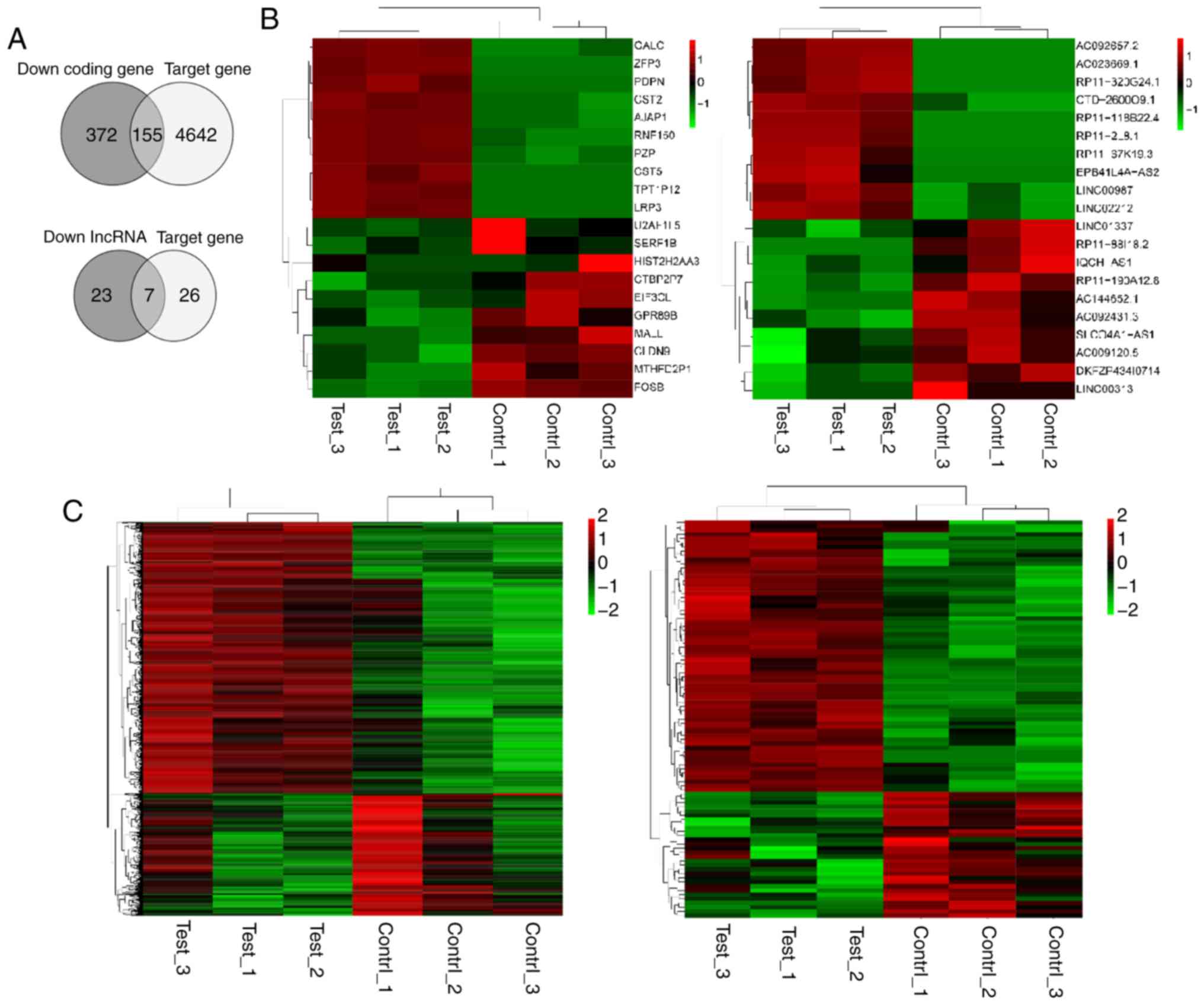
By combining the identified downregulated genes and
the binding sites between miR-942 and the predicted lncRNA/mRNAs,
seven lncRNAs and 155 mRNAs were predicted to be target genes of
miR-942, respectively (Fig. 2A). Heat
maps display the downregulation and upregulation of the top 10 DEGs
(Fig. 2B) and the total DEGs
(Fig. 2C).
Establishment of a PPI network based
on miR-942 target DEGs
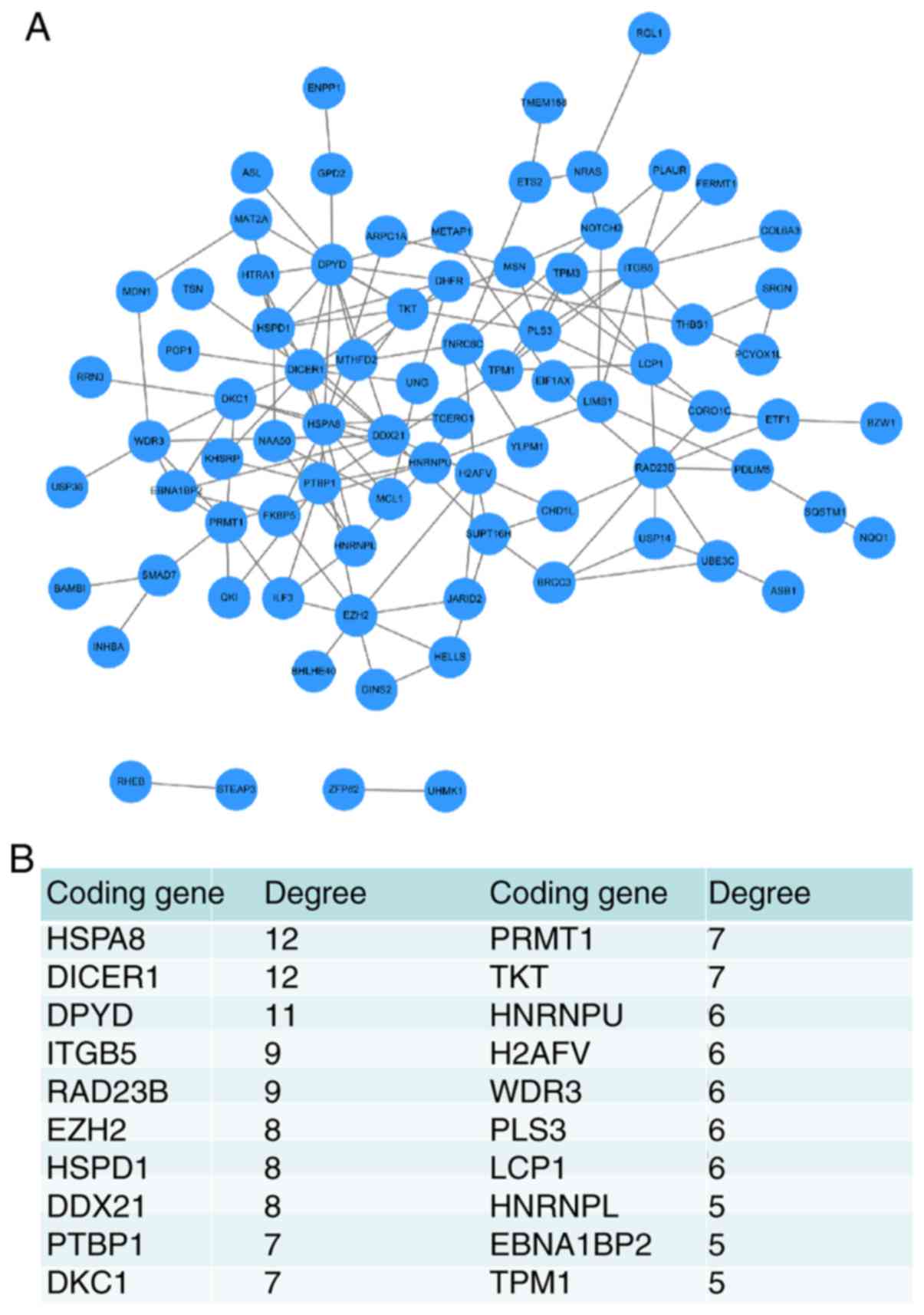
PPI network analysis was performed to evaluate which
of the miR-942 targets served a vital role in patient prognosis.
The PPI network contained 82 nodes and 148 edges (interactions),
and the hub nodes with a higher connectivity degree included the
heat shock protein family A (Hsp70) member 8 (HSPA8, degree=12),
Dicer 1, ribonuclease III (DICER1, degree=12), dihydropyrimidine
dehydrogenase (DPYD, degree=11), integrin subunit β5 (ITGB5,
degree=9), RAD23 Homolog B, nucleotide excision repair protein
(RAD23B, degree=8), enhancer Of Zeste 2 polycomb repressive complex
2 subunit (EZH2, degree=8), heat shock protein family D (Hsp60)
member 1 (HSPD1, degree=8), DExD-Box helicase 21 (DDX21, degree=8),
polypyrimidine tract binding protein 1 (PTBP1, degree=7), dyskerin
pseudouridine synthase 1 (DKC1, degree=7), protein arginine
methyltransferase 1 (PRMT1, degree=7) and transketolase (TKT,
degree=7) (Fig. 3).
GO and KEGG pathway enrichment
analysis of miR-942 target DEGs
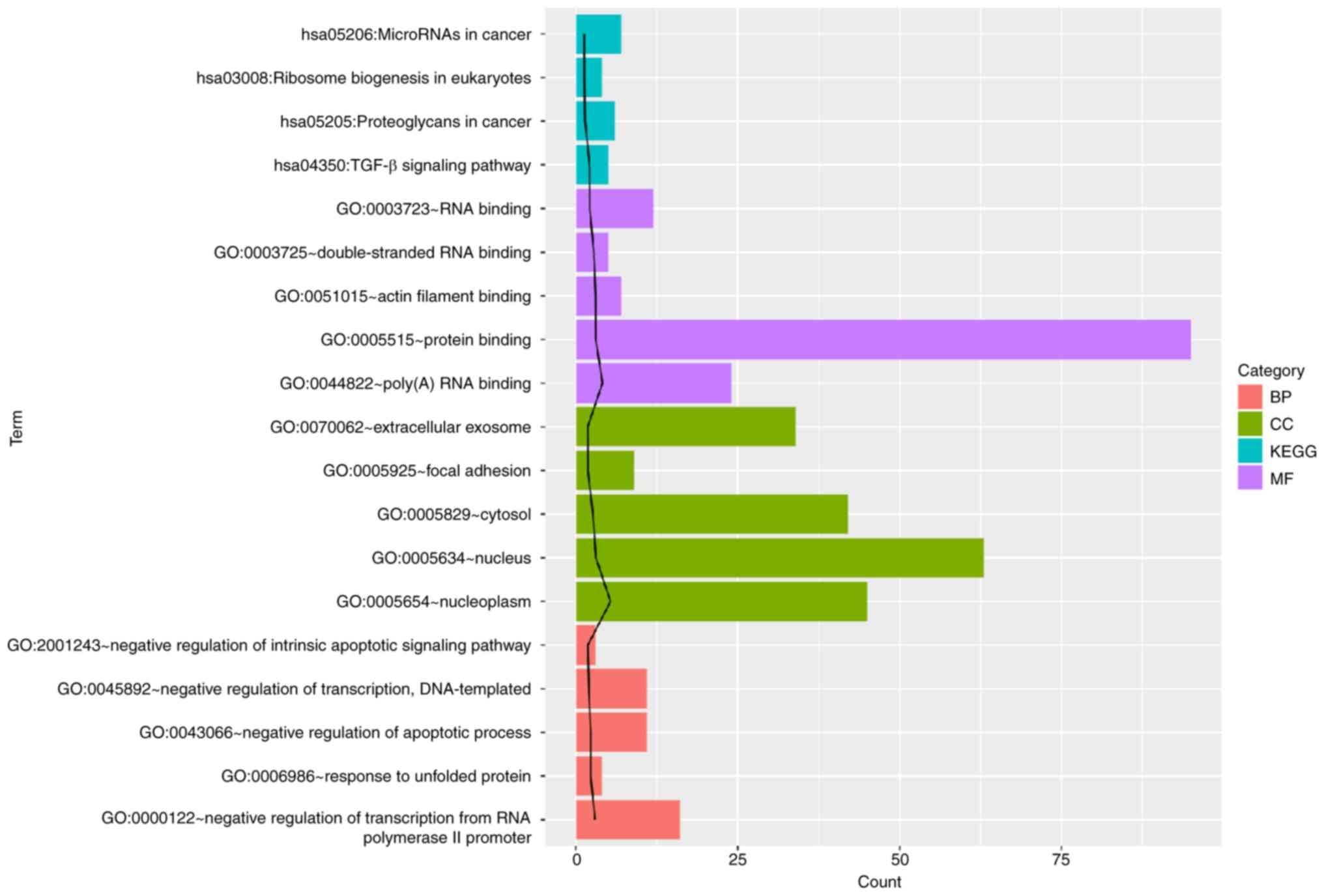
To further explore the function of miR-942 target
DEGs, GO and KEGG pathway analysis was performed (Fig. 4). The identified target genes were
found to be significantly associated with ‘protein binding’, ‘TGF-β
signaling pathway’, ‘negative transcriptional regulation’ and ‘RNA
binding’.
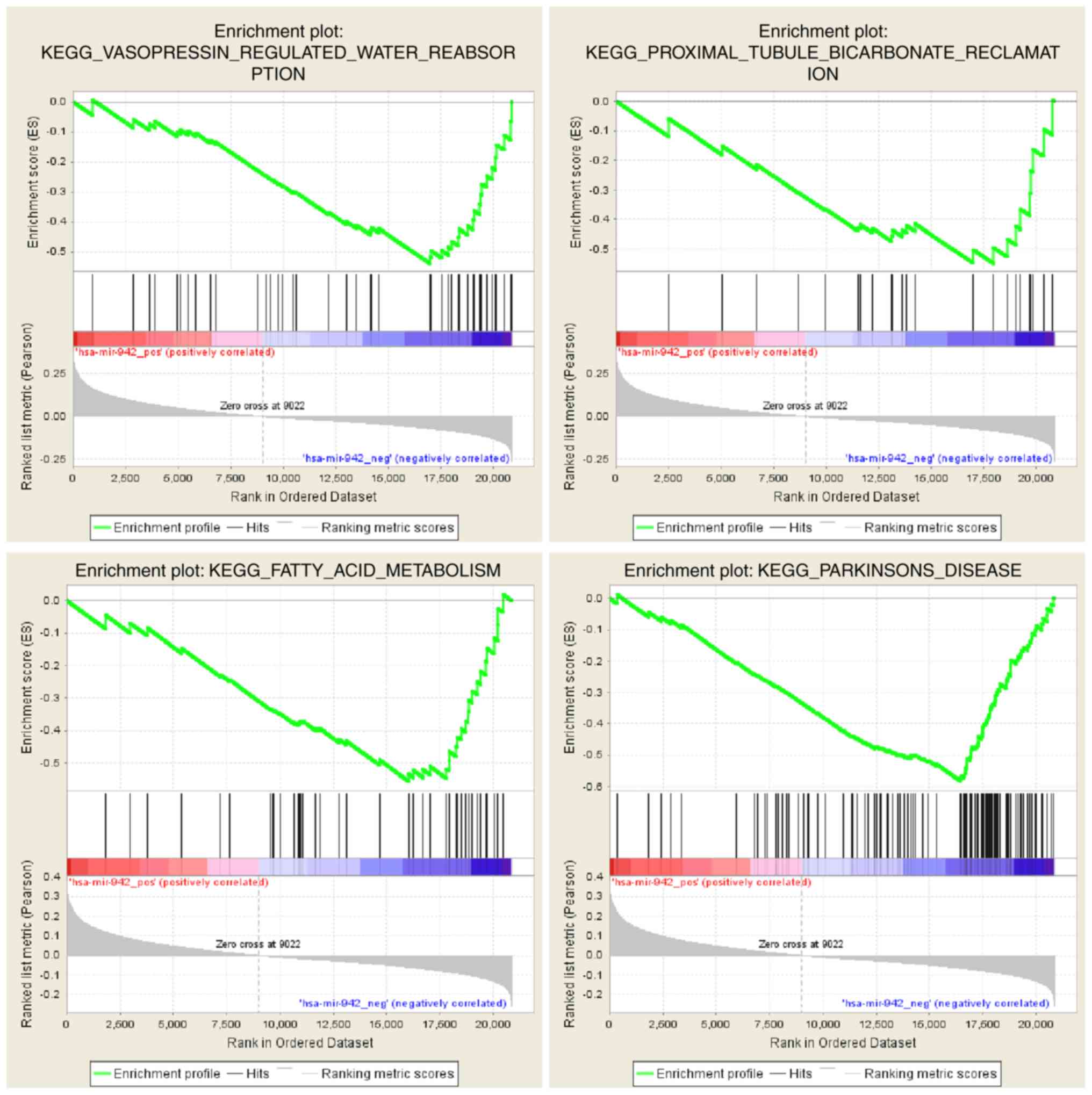
GSEA analysis of miR-942 and RNA-seq
data from TCGA
After preprocessing of the RNA-seq and miR-942 data
from TCGA, enrichment analysis was performed using the GSEA tool.
The results of negative regulation with miR-942 were selected using
a nominal P-value of <0.1 as a significant enrichment result.
GSEA analysis revealed significant enrichment for ‘vasopressin
regulated water reabsorption’, ‘proximal tubule bicarbonate
reclamation’, ‘fatty acid metabolism’ and ‘Parkinson's disease’
(Fig. 5 and Table III).
 | Table III.Gene set enrichment analysis of
microRNA-942 and RNA-seq data from The Cancer Genome Atlas. |
Table III.
Gene set enrichment analysis of
microRNA-942 and RNA-seq data from The Cancer Genome Atlas.
| Process | Size | ES | NES | NOM p-val |
|---|
| Vasopressin
regulated water reabsorption | 43 | −0.53886 | −1.72647 | 0.004504505 |
| Proximal tubule
bicarbonate reclamation | 23 | −0.54862 | −1.60212 | 0.040983606 |
| Fatty acid
metabolism | 42 | −0.55580 | −1.54426 | 0.092702170 |
| Parkinson's
disease | 112 | −0.58263 | −1.56579 | 0.098837210 |
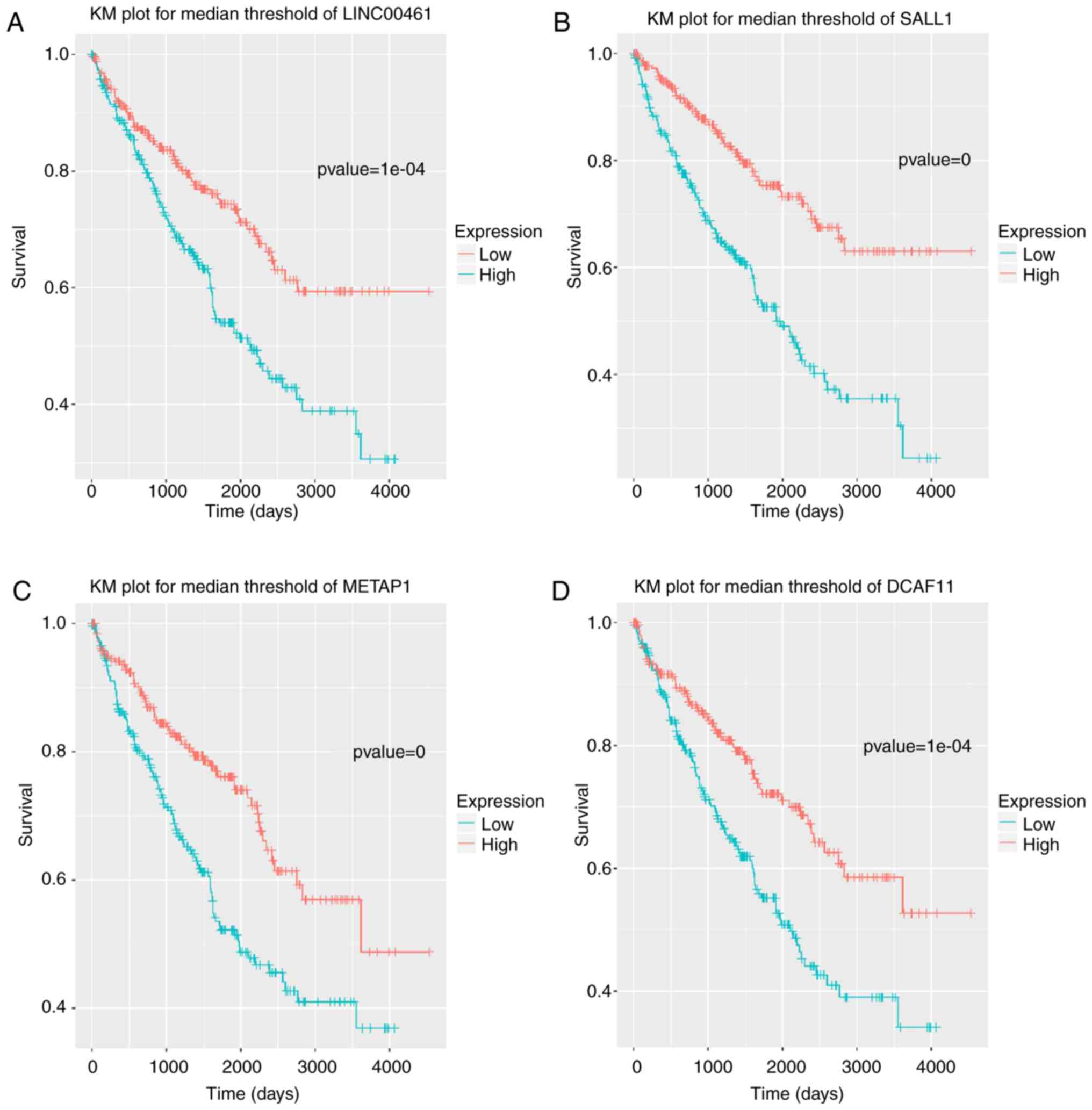
Identification of prognostic
factors
A total of 533 patient samples were categorized into
high- and low-expression groups based on the normalized expression
values of the identified lncRNAs/mRNAs. Using Kaplan-Meier
analysis, the high expression levels of 31 lncRNAs and/or mRNAs
were shown to result in a significant increase in patient survival
rate; the most significant 22 of the 31 lncRNAs and/or mRNAs are
shown in Table IV. These included
the lncRNA LINC00461 and the genes spalt-like transcription factor
1 (SALL1), methionyl aminopeptidase 1 (METAP1), and
DDB1 and CUL4 associated factor 11 (DCAF11) (Fig. 6).
 | Table IV.Long non-coding RNAs or
protein-coding genes that significantly effect patient
survival. |
Table IV.
Long non-coding RNAs or
protein-coding genes that significantly effect patient
survival.
| Gene | P-value |
|---|
| SSFA2 |
4.61×10−11 |
| SALL1 |
9.32×10−09 |
| BCAR3 |
1.38×10−05 |
| TBC1D14 |
3.54×10−05 |
| METAP1 |
3.80×10−05 |
| MBLAC2 |
5.96×10−05 |
| DCAF11 |
6.49×10−05 |
| LINC00461 |
1.02×10−04 |
| H2AFV |
5.81×10−04 |
| PDIK1L |
6.82×10−04 |
| LZTFL1 |
1.44×10−03 |
| PTMA |
2.07×10−03 |
| PLEKHB2 |
3.23×10−03 |
| HMGCR |
3.63×10−03 |
| TAF9B |
5.08×10−03 |
| ARSD |
6.67×10−03 |
| TMBIM6 |
9.49×10−03 |
| DPY19L3 |
1.58×10−02 |
| STARD7 |
2.11×10−02 |
| SEPT2 |
2.91×10−02 |
| DKFZP434I0714 |
3.00×10−02 |
| ZNF552 |
4.55×10−02 |
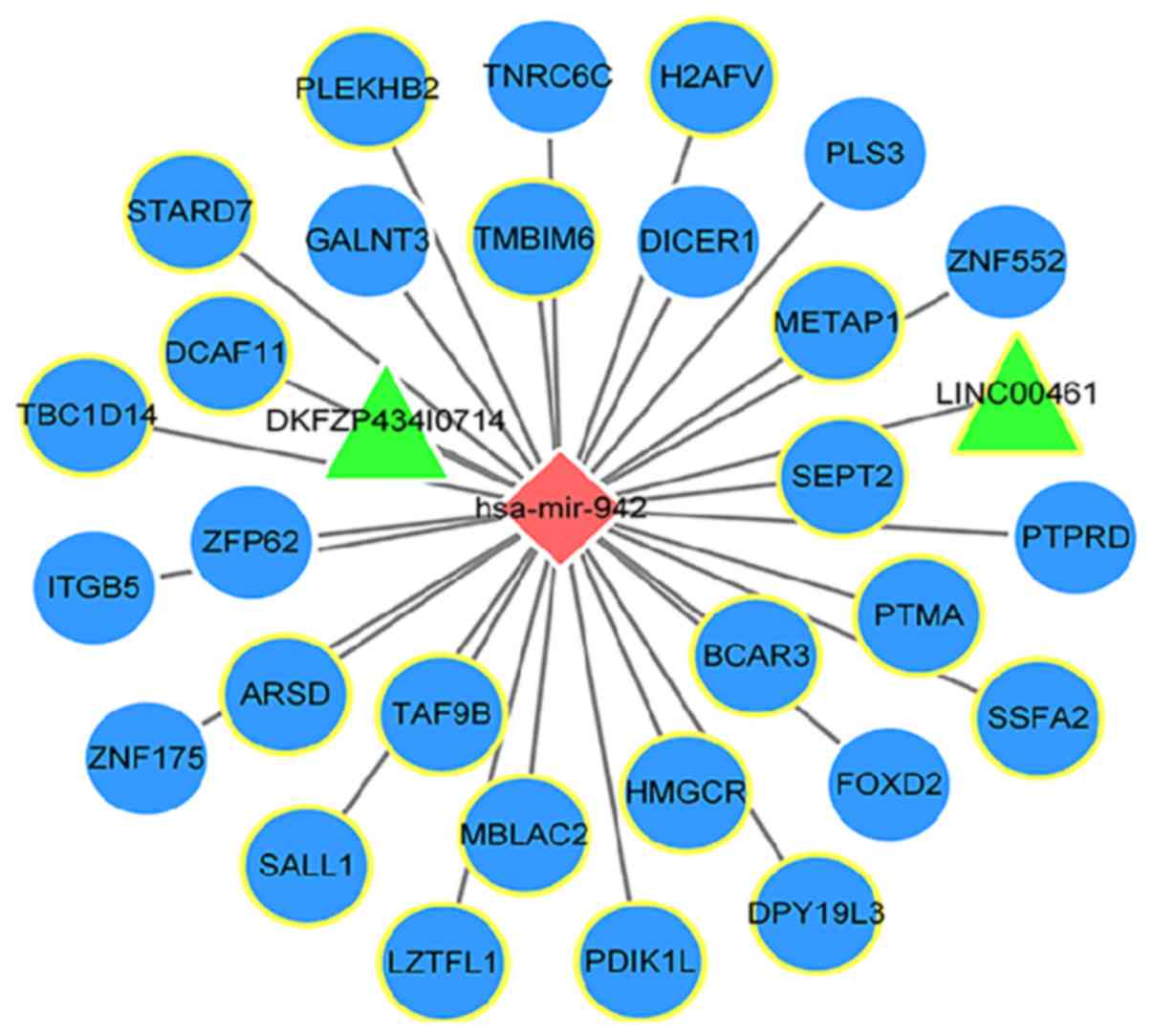
Construction of an miR-942-related
ceRNA network
Following integrated analysis of the miR-942 target
genes, and Pearson's correlation analysis between miR-942 and the
identified lncRNA/mRNAs, 31 potential target genes were identified
for miR-942 (29 mRNAs and 2 lncRNAs). A miR-942-related ceRNA
network was subsequently constructed (Fig. 7).
RT-qPCR analysis of miR-942 target
gene expression
The expression levels of LINC00461, miR-942,
SALL1, METAP1 and DCAF11 were further verified by
RT-qPCR. The expression levels of miR-942 in OS-RC-2 cells were
significantly upregulated compared with the NC mimic group
(Fig. 8A; P<0.01). The expression
level of LINC00461 was also significantly increased in the
miR-942-transfected group (Fig. 8B;
P<0.01). However, the expression levels of the miR-942 target
genes SALL1, METAP1 and DCAF11 were significantly
downregulated in the miR-942 mimic-transfected group compared with
the control-transfected group (Fig.
8C-E; P<0.01). Additionally, a miR-942 inhibitor was
transfected into sunitinib-resistant OS-RC-2 cells, and the
expression levels of LINC00461, miR-942, SALL1, METAP1 and DCAF11
were reversed compared with the miR-942 mimic-transfected group
(Fig. 8F-J; P<0.01).
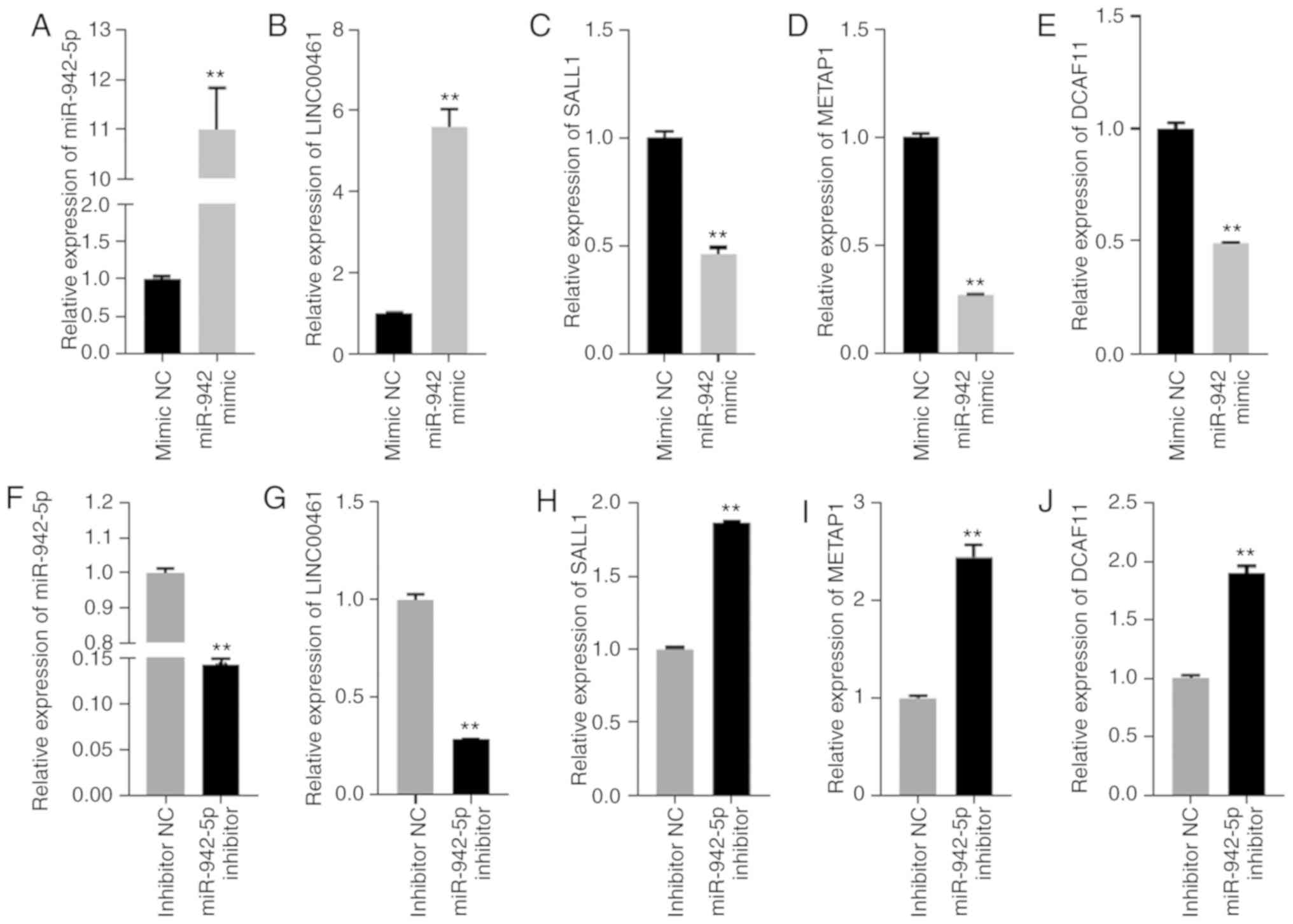 | Figure 8.(A-E) Relative expression levels of
miR-942, LINC00461, SALL1, METAP1 and DCAF11 in
OS-RC-2 cells transfected with miR-942 mimics or the NC. (F-J) The
relative expression levels of miR-942, LINC00461, SALL1,
METAP1 and DCAF11 in sunitinib-resistant OS-RC-2 cells
transfected with miR-942 inhibitor and the inhibitor NC.
**P<0.01. miR, microRNA; SALL1, spalt-like transcription factor
1; METAP1, methionyl aminopeptidase 1; DCAF11, DDB1 and CUL4
associated factor 1; NC, negative control. |
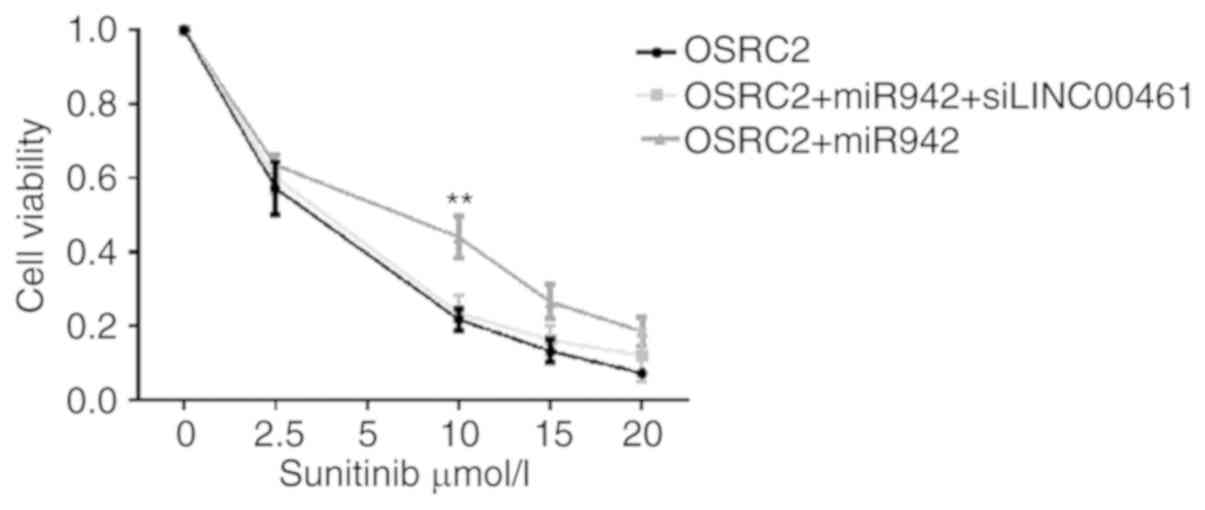
MTT assay
The viability of OS-RC-2 cells was measured using an
MTT assay following miR-942 transfection. As shown in Fig. 9, the viability of OS-RC-2 cells
transfected with miR-942 mimics was significantly higher at 10
µmol/l sunitinib compared with that in untreated cells (P<0.01).
However, the viability of OS-RC-2 cells was decreased following
co-transfection with miR-942 mimics and LINC00641 siRNA at all
concentrations of sunitinib, (compared with the cells transfected
with miR-942 mimics alone) and was comparable to that of the
untransfected cells. These results suggest that LINC00461 is
associated with sunitinib resistance.
Discussion
Sunitinib is one of the most widely used drugs in
the treatment of RCC. A Previous study has suggested that
miR-942-5p, miR-133a-5p, miR-484 and miR-628-5p are upregulated in
sunitinib-resistant RCC cells, and miR-942-5p was found to be the
most upregulated (13). However, the
exact mechanism was far from being elucidated. In the present
study, miR-942 mimics were transfected into RCC cells, and MTT
analysis demonstrated that cell viability was significantly
increased following treatment with sunitinib, compared with that in
negative control group. This result suggested that miR-942 may
serve important roles in RCC cell proliferation and sunitinib
resistance. In addition, RNA sequencing was performed to identify
the target genes of miR-942. When comparing miR-942 mimic-treated
with control-treated samples, a total of seven lncRNAs and 155
mRNAs were predicted to be target genes for miR-942. These genes
were significantly associated with ‘protein binding’, ‘TGF-β
signaling pathway’, ‘negative transcriptional regulation’ and ‘RNA
binding’. Using integrated analysis of the RNA-sequencing data and
that downloaded from TCGA, an miR-942-related ceRNA network was
constructed and included the lncRNA LINC00461, as well as the genes
SALL1, METAP1, and DCAF11, which were predicted to
have a significant effect on the survival of patients with RCC.
High miR-942 expression levels in metastatic RCC
cells have been demonstrated to upregulate the secretion of matrix
metalloproteinase-9 and VEGF, promoting endothelial cell migration
and sunitinib resistance (13). In
the present study, the overexpression of miR-942 in
sunitinib-sensitive OS-RC-2 cells was found to affect the
expression of a number of lncRNAs and mRNAs, including lncRNA
LINC00461. While few studies have reported a role for LINC00461, a
recent study revealed that LINC00461 was highly expressed in glioma
tissues, and is important for the proliferation and migration of
glioma cells (28). lncRNAs can act
as miRNA decoys, reducing their regulatory effect on target mRNAs.
In addition, studies have indicated a role for miRNA-lncRNA
interactions in cancer progression (29–31). In
the present study, it was speculated that LINC00461 may be a
prognostic factor for RCC that may regulate miR-942. Therefore, the
interaction between miR and 942-LINC00461 may serve a key role in
RCC tumorigenesis, metastasis and drug resistance, which should be
further verified by additional experiments.
Moreover, miR-942 was predicted to regulate the
expression of the prognosis-associated factors SALL1, METAP1
and DCAF11. The protein encoded by SALL1 is a zinc
finger transcriptional repressor that has been shown to regulate
the normal renal progenitor cell population. It is overexpressed in
embryonic kidney malignancy, Wilms' tumor (WT) and progressive
stem-like tumor xenografts derived from primary WT, which
contributes to nephron formation and regeneration, and is indicated
in WT oncogenesis (32), and METAP1
co-translationally removes the N-terminal methionine from nascent
proteins (33). In addition, human
METAP1 is reported to exert anti-proliferative activities in
different cancer cell lines, thus may be a potent anti-cancer agent
(33). A study also showed that
DCAF11 mediates the degradation of the stem-loop binding
protein at the end of the S phase, which is essential for cell
viability (34). Therefore, it was
speculated that miR-942 may interact with these potential target
genes in the progression of RCC. Further MTT experiments
demonstrated that the viability of OS-RC-2 cells was decreased
following co-transfection with miR-942 mimics and LINC00641 siRNA,
which was comparable with that of untransfected cells.
In conclusion, the present study indicated that
miR-942 may interact with lncRNA LINC00461 and the SALL1,
METAP1 and DCAF11 genes. Ongoing studies with these
molecular markers may result in the generation of novel
therapeutics for the prevention and treatment of RCC.
Acknowledgements
Not applicable.
Funding
The study was supported by the National Natural
Science Foundation of Zhejiang Province (grant no.
LY18H160002).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
GD and YiC conceived and designed the study and the
experiments. YiC acquired the funding. YiC, HW, WG, YL and YuC
performed the experiments. JH and CS analyzed the data and
conducted the statistical analysis. HW and YuC wrote the
manuscript. WG and YL critically revised the manuscript. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hsieh JJ, Purdue MP, Signoretti S, Swanton
C, Albiges L, Schmidinger M, Heng DY, Larkin J and Ficarra V: Renal
cell carcinoma. Nat Rev Dis Primers. 3:170092017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wong MCS, Goggins WB, Yip BHK, Fung FDH,
Leung C, Fang Y, Wong SYS and Ng CF: Incidence and mortality of
kidney cancer: Temporal patterns and global trends in 39 countries.
Sci Rep. 7:156982017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Walsh N, Larkin A, Kennedy S, Connolly L,
Ballot J, Ooi W, Gullo G, Crown J, Clynes M and O'Driscoll L:
Expression of multidrug resistance markers ABCB1 (MDR-1/P-gp) and
ABCC1 (MRP-1) in renal cell carcinoma. BMC Urol. 9:62009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choueiri TK, Escudier B, Powles T,
Mainwaring PN, Rini BI, Donskov F, Hammers H, Hutson TE, Lee JL,
Peltola K, et al: Cabozantinib versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1814–1823. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amin A, Dudek AZ, Logan TF, Lance RS,
Holzbeierlein JM, Knox JJ, Master VA, Pal SK, Miller WH Jr, Karsh
LI, et al: Survival with AGS-003, an autologous dendritic
cell-based immunotherapy, in combination with sunitinib in
unfavorable risk patients with advanced renal cell carcinoma (RCC):
Phase 2 study results. J Immunother Cancer. 3:142015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Joosten S, Hamming L, Soetekouw P, Aarts
M, Veeck J, van Engeland M and Tjan-Heijnen VC: Resistance to
sunitinib in renal cell carcinoma: From molecular mechanisms to
predictive markers and future perspectives. Biochim Biophys Acta.
1855:1–16. 2015.PubMed/NCBI
|
|
7
|
Ma R, Jiang T and Kang X: Circulating
microRNAs in cancer: Origin, function and application. J Exp Clin
Cancer Res. 31:382012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lim JH, Song MK, Cho Y, Kim W, Han SO and
Ryu JC: Comparative analysis of microRNA and mRNA expression
profiles in cells and exosomes under toluene exposure. Toxicol In
Vitro. 41:92–101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takahashi RU, Prieto-Vila M, Hironaka A
and Ochiya T: The role of extracellular vesicle microRNAs in cancer
biology. Clin Chem Lab Med. 55:648–656. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goto Y, Kurozumi A, Nohata N, Kojima S,
Matsushita R, Yoshino H, Ishida Y, Ichikawa T, Naya Y and Seki N:
The microRNA signature of patients with sunitinib failure:
Regulation of UHRF1 pathways by microRNA-101 in renal cell
carcinoma. Oncotarget. 7:59070–59086. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang T, Hu XY, Li YH, Tian BQ, Li ZW and
Fu Q: MicroRNA-21 regulates the proliferation, differentiation, and
apoptosis of human renal cell carcinoma cells by the mTOR-STAT3
signaling pathway. Oncol Res. 24:371–380. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Machackova T, Mlcochova H, Stanik M,
Dolezel J, Fedorko M, Pacik D, Poprach A, Svoboda M and Slaby O:
MiR-429 is linked to metastasis and poor prognosis in renal cell
carcinoma by affecting epithelial-mesenchymal transition. Tumor
Biol. 37:14653–14658. 2016. View Article : Google Scholar
|
|
13
|
Prior C, Perez-Gracia JL, Garcia-Donas J,
Rodriguez-Antona C, Guruceaga E, Esteban E, Suarez C, Castellano D,
del Alba AG, Lozano MD, et al: Identification of tissue microRNAs
predictive of sunitinib activity in patients with metastatic renal
cell carcinoma. PLoS One. 9:e862632014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tomczak K, Czerwińska P and Wiznerowicz M;
The Cancer Genome Atlas (TCGA), : An immeasurable source of
knowledge. Contemp Oncol (Pozn). 19:A68–A77. 2015.PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim D, Langmead B and Salzberg SL: HISAT:
A fast spliced aligner with low memory requirements. Nat Methods.
12:357–360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Y, McCarthy D, Robinson M and Smyth
GK: edgeR: Differential expression analysis of digital gene
expression data. User's Guide. 2014, https://www/genomatixde/online_help/help_regionminer/edgeR.pdf
|
|
18
|
Benjamini Y and Hochberg Y: Controlling
the false discovery rate: A practical and powerful approach to
multiple testing. J Royal Stat Soc Series B (Methodological).
57:289–300. 1995. View Article : Google Scholar
|
|
19
|
Harrow J, Frankish A, Gonzalez JM,
Tapanari E, Diekhans M, Kokocinski F, Aken BL, Barrell D, Zadissa
A, Searle S, et al: GENCODE: The reference human genome annotation
for The ENCODE Project. Genome Res. 22:1760–1774. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
John B, Sander C and Marks DS: Prediction
of human microRNA targets. Methods Mol Biol. 342:101–113.
2006.PubMed/NCBI
|
|
21
|
von Mering C, Huynen M, Jaeggi D, Schmidt
S, Bork P and Snel B: STRING: A database of predicted functional
associations between proteins. Nucleic Acids Res. 31:258–261. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kohl M, Wiese S and Warscheid B:
Cytoscape: Software for visualization and analysis of biological
networks. Methods Mol Biol. 696:291–303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gene Ontology Consortium, . Gene ontology
consortium: Going forward. Nucleic Acids Res. 43:D1049–D1056. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES and Mesirov JP: Gene set enrichment analysis: A
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Therneau T: A package for survival
analysis in S. R package version 2.37–7. 2014, http://cran/R-project
org/package=survival2015
|
|
27
|
Zhou M, Wang X, Shi H, Cheng L, Wang Z,
Zhao H, Yang L and Sun J: Characterization of long non-coding
RNA-associated ceRNA network to reveal potential prognostic lncRNA
biomarkers in human ovarian cancer. Oncotarget. 7:12598–12611.
2016.PubMed/NCBI
|
|
28
|
Yang Y, Ren M, Song C, Li D, Soomro SH,
Xiong Y, Zhang H and Fu H: LINC00461, a long non-coding RNA, is
important for the proliferation and migration of glioma cells.
Oncotarget. 8:84123–84139. 2017.PubMed/NCBI
|
|
29
|
Braconi C, Kogure T, Valeri N, Huang N,
Nuovo G, Costinean S, Negrini M, Miotto E, Croce CM and Patel T:
microRNA-29 can regulate expression of the long non-coding RNA gene
MEG3 in hepatocellular cancer. Oncogene. 30:4750–4756. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang WC, Fu WM, Wong CW, Wang Y, Wang WM,
Hu GX, Zhang L, Xiao LJ, Wan DC, Zhang JF and Waye MM: The lncRNA
H19 promotes epithelial to mesenchymal transition by functioning as
miRNA sponges in colorectal cancer. Oncotarget. 6:22513–22525.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma MZ, Chu BF, Zhang Y, Weng MZ, Qin YY,
Gong W and Quan ZW: Long non-coding RNA CCAT1 promotes gallbladder
cancer development via negative modulation of miRNA-218-5p. Cell
Death Dis. 6:e15832015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Metsuyanim S, Pode-Shakked N, Schmidt-Ott
KM, Keshet G, Rechavi G, Blumental D and Dekel B: Accumulation of
malignant renal stem cells is associated with epigenetic changes in
normal renal progenitor genes. Stem Cells. 26:1808–1817. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Frottin F, Bienvenut WV, Bignon J, Jacquet
E, Vaca Jacome AS, Van Dorsselaer A, Cianferani S, Carapito C,
Meinnel T and Giglione C: MetAP1 and MetAP2 drive cell selectivity
for a potent anti-cancer agent in synergy, by controlling
glutathione redox state. Oncotarget. 7:63306–63323. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Djakbarova U, Marzluff WF and Köseoğlu MM:
DDB1 and CUL4 associated factor 11 (DCAF11) mediates degradation of
stem-loop binding protein at the end of S phase. Cell Cycle.
15:1986–1996. 2016. View Article : Google Scholar : PubMed/NCBI
|