Introduction
The rate of lung cancer in tuberculosis (TB)
patients is 7–30% higher than that in healthy individuals. TB also
predicts an increased long-term risk of cancer (1). Non-small cell lung cancer (NSCLC), the
leading cause of cancer-related death, is responsible for millions
of new cases and deaths every year worldwide (2). The most commonly used therapeutic
methods for patients with NSCLC include surgery, radiation and
systemic therapy. Systemic therapy is recommended since the
consistent late diagnosis of NSCLC is a major obstacle to using
surgical procedures (3–5). Cisplatin (CDDP) and doxorubicin (DOX)
are commonly used as lung cancer treatment regimes in the clinic
(6,7).
However, the toxicity and drug resistance associated with CDDP and
DOX result in a high level of mortality in NSCLC (8). Thus, the delivery of anticancer agents
at higher concentrations, direct targeting to the tumor site and
reduced accumulation into non-tumor organs are urgently sought
(8).
Epidermal growth factor receptor (EGFR) is a
promising target, which is overexpressed in lung carcinomas
(9,10). EGFR plays an important role in
regulating cell proliferation, survival and growth (11,12). Many
researchers have demonstrated that EGFR-targeted therapy achieves
higher precision and has fewer side effects (13,14). In
the present study, EGFR-targeted nanoparticles were constructed and
co-delivered cisplatin (CDDP) and doxorubicin (DOX) for lung cancer
therapy.
Combinatorial strategies have emerged as promising
therapeutic regimens to improve the anticancer efficacy,
simultaneously reducing side effects (15,16). Drug
combinations could inhibit tumor growth through different and
synergistic effect mechanisms. CDDP, a front-line DNA alkylating
agent, has widely been used in solid tumors due to its various
mechanisms such as DNA damage, cellular damage, mitochondria damage
and dysfunction, and other deleterious effects (17–19). DOX,
an anthracycline antibiotic, has antitumor cytotoxicity by
interfering with DNA synthesis (20).
However, the clinical application of CDDP and DOX has been severely
impeded for severe toxicities, drug resistance and low aqueous
solubility (21,22). Current strategies to co-deliver drugs
have focused on fabricating polymeric nanoparticles and lipid
nanoparticles.
Lipid polymeric nanoparticles (LPNs) combine the
merits of both polymeric nanoparticles and lipid nanoparticles.
Therefore, LPNs have been demonstrated to display distinctive
features in combinational therapy such as high biocompatibility,
high drug loading and in vivo stability, low cytotoxicity,
controlled release and capability for modifications and
conjugations (23). In the present
study, EGF-PEG-DSPE was synthesized. Then, EGFR-targeted LPNs were
fabricated, which consisted of a CDDP-loaded hybrophobic polymeric
core, a DOX-loaded phospholipid layer, and an outer layer of
EGF-PEG-DSPE ligand. The particle size, ζ potential, stability, and
release behavior of the LPNs were characterized. The antitumor
ability of LPNs was assessed in vitro and in
vivo.
Materials and methods
Materials
Murine EGF was purchased from PeproTech (Rocky Hill,
NJ, USA). CDDP injection was provided by Hanson Pharma Co., Ltd.
(Lianyungang, China). CDDP, DOX, PLA, fetal bovine serum (FBS),
Roswell Park Memorial Institute (RPMI)-1640 medium and
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
(MTT) were purchased from Sigma-Aldrich; Merck KGaA.
Miglyol® 812 was provided by Beijing Fengli Jingqiu
Pharmaceutical Co., Ltd. (Beijing, China).
DSPE-PEG2000-maleimide was purchased from Peng Sheng
Biological (Shanghai, China).
Cell line and culture
A549 cells were obtained from the American Type
Culture Collection (ATCC; Manassas, VA, USA). Cells were maintained
in RPMI-1640 medium supplemented with 10% (v/v) FBS, 50 µM
2-mercaptoethanol, and 100 µg/ml kanamycin at 37°C in a humidified
atmosphere of 5% CO2.
Animal model
Male C57BL/6 mice (6 weeks of age, 20–25 g) were
purchased from Beijing Vital River Laboratory Animal Technology
Co., Ltd. (Beijing, China) and raised under conventional conditions
with a 12-h light/dark cycle, constant temperature (25°C) and
humidity (60%), and free access to standard food and water
(24). To produce the animal model,
the mice were injected with A549 cells (1×106 cells in
100 µl PBS/mouse) into the right flank, followed by assays for
tumor growth. Tumor volume (TV) was determined by the formula:
(Largest superficial diameter) × (Smallest superficial
diameter)2/2. When the tumor diameter reached 12 mm, the
body weight lost was over 25%, or the mice were too weak to take
food and water, they were sacrificed. Eighty mice were used and
euthanized; no mouse was found dead. Animal health and behavior
were monitored every 12 h along with the tumor growth. When any
animal experienced mild pain (shortly arched back, especially after
administration; partly hair rising; occasional salivation; and
transient tremor) during the experiments, oxymorphone (0.05 mg/kg,
s.c.) was administrated. Barbital sodium was administrated (100
mg/kg, i.p. injection) to the mice and then mice were sacrificed by
cervical dislocation. All animal experiments were approved by the
Ethics Committee of the Affiliated Hospital of Hebei University and
followed the SUNY Upstate Medical University, Department of
Laboratory Animal Resources Guidelines for Anesthesia and Analgesia
in Laboratory Animals and Guidelines for the Care and Use of
Laboratory Animals and the National Animal Laboratory Center of
China (http://www.cmu.edu.cn/sydwb/info/1841/1211.htm).
Synthesis and characterization of
EGF-PEG-DSPE
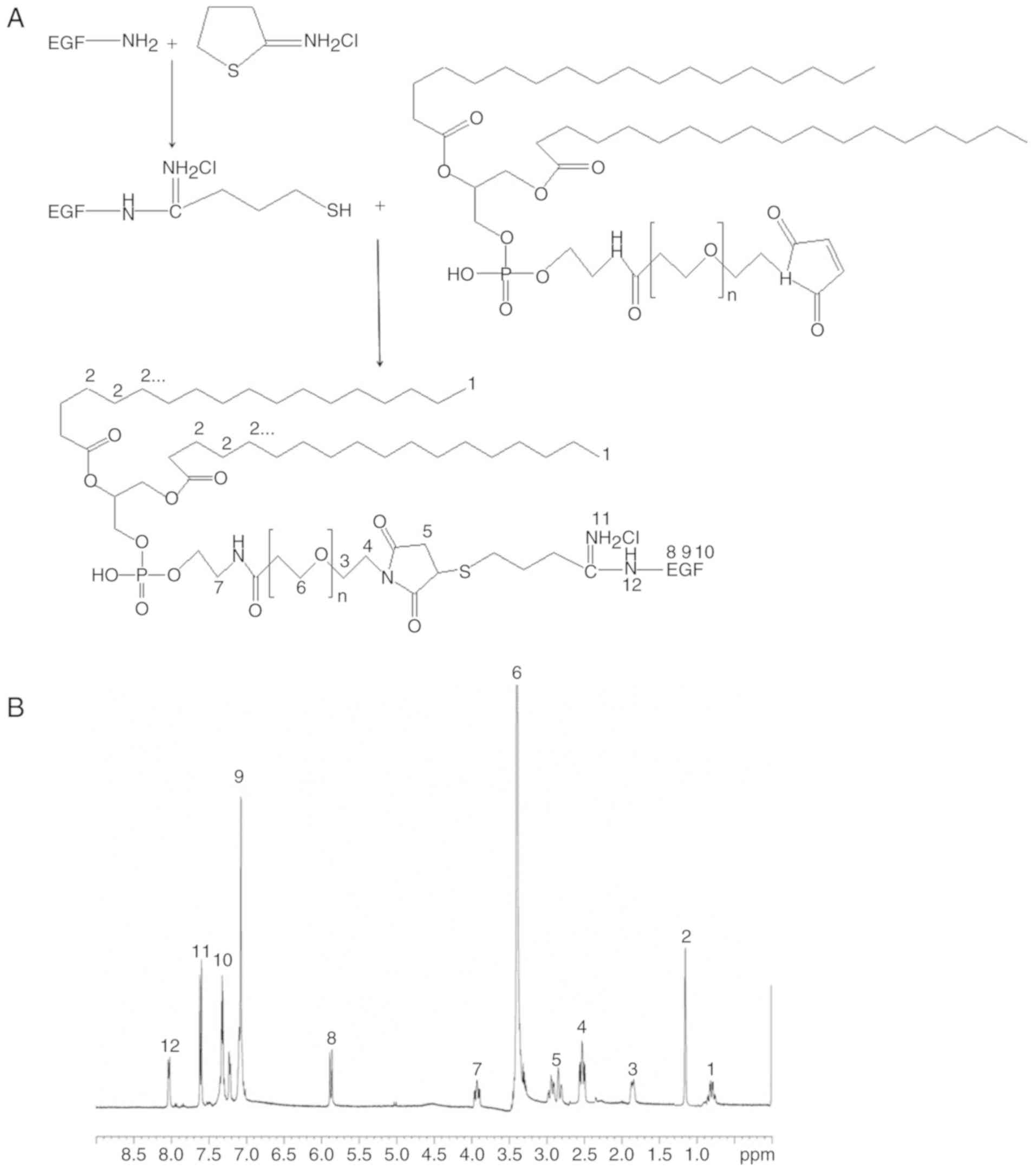
EGF was modified with an excess of Traut's reagent
for 1 h at room temperature under nitrogen to obtain thiolated EGF
(Fig. 1A) (25). Excess thiolated EGF was mixed with
DSPE-PEG2000-maleimide and stirred at 400 rpm at room
temperature overnight. To remove unbound EGF, the sample was
separated by gel filtration with Sephadex G-150 gel and eluted with
HEPES buffer in 600 µl fractions. 1H-NMR (DMSO-d6, 300
MHz) spectroscopy was used for the confirmation of the chemical
structure (26).
Preparation of EGFR-targeted LPNs
EGFR-targeted LPNs loaded with CDDP and DOX (EGF C/D
LPNs, Fig. 2) were prepared by a
solvent extraction/evaporation method (27). Organic phase was formed by dissolving
CDDP (100 mg) and PLA (200 mg) in dichloromethane (5 ml). An
aqueous phase was obtained by dispersing DOX (50 mg),
Miglyol® 812 (100 mg) and EGF-PEG-DSPE (100 mg) in water
(20 ml) by ultrasonication. The organic solution phase was then
added into the aqueous phase and ultrasonicated for 5 min in an ice
bath. The organic solvent was then removed by stirring at the speed
of 300 rpm with a magnetic stirrer.
EGFR-targeted LPNs loaded with CDDP (EGF C LPNs)
were prepared using the same method without adding DOX.
EGFR-targeted LPNs loaded with DOX (EGF D LPNs) were
constructed in a similar manner without adding CDDP.
Drug-free EGFR-targeted LPNs (EGF LPNs) were
constructed in a similar manner without adding any drug.
EGFR-free LPNs loaded with CDDP and DOX (C/D LPNs)
were constructed in a similar manner using PEG-DSPE instead of
EGF-PEG-DSPE.
Free-CDDP and DOX drug combination (Free C/D) was
constructed by dissolving DOX (25 mg) in CDDP injection (10 ml, 5
mg/ml).
Characterization of LPNs
The size, polydispersity index (PDI), and ζ
potential of the LPNs were measured by a Malvern Zetasizer Nano
ZS90 (Malvern Instruments, Malvern, UK) (28). A UV-vis spectrophotometric method was
used to measure the drug encapsulation efficiency (EE) and drug
loading (DL) capacity of CDDP or DOX in LPNs (29). LPNs were dissolved in
o-phenylenediamine and dimethylformamide (DMF) and heated at 90°C
for 30 min. DMF-water mixture (7:3, v/v, pH 6.2) was used to dilute
the product and estimated at 478 nm (for DOX) and 705 nm (for CDDP)
on the UV-vis spectrophotometer (Simadzu, Japan) (30). The EE and DL were determined according
to the formulas: EE (%) = (weightentapped
drug/weighttotal drug) ×100; DL (%) =
(weightentapped drug/weightLPNs) ×100.
Stability of LPNs
LPNs were stored at 2–8°C. The size and EE of the
LPNs were measured for 3 month to determine the storage stability
(8). At 0, 5, 10, 20, 30, 60, and 90
days, LPNs were taken out and assessed by the methods mentioned in
‘Characterization of LPNs’ section.
Drug release from LPNs
Drug release from LPNs was evaluated by a dialysis
method (31). Various types of LPNs
(2 ml) were sealed in dialysis bags (molecular cut-off: 1 kDa)
separately, which were incubated in PBS (pH 7.4, 37°C, 20 ml) and
shaken at a speed of 100 rpm. Incubation medium was collected and
replaced by the same volume of fresh pre-heated medium at specific
time points. The medium containing the released drugs was analyzed
by the methods described in the above ‘Characterization of LPNs’
section.
Cytotoxicity of LPNs
Cytotoxicity of the LPNs was evaluated on A549 cells
by MTT assay (32). Cells
(2×105 per well) were seeded into 6-well microplates and
allowed to grow for 24 h to a subconfluent state. The various types
of LPNs and Free C/D were added along with fresh medium (contained
10% of FBS) and incubated for 72 h. MTT solution (50 µl in culture
medium) was then added after suspensions were removed. Thereafter,
the cells were incubated at 37°C in 5% CO2 for another 4
h and the medium was removed. DMSO (100 µl) was then added to
dissolve the crystals and the absorbance was measured using a
microplate reader at 570 nm. The formula: (Absorbancetest
cells)/(Absorbancecontrol) ×100 was used to
calculate the viability of the treated cells.
Synergistic effects of LPNs
Combination index (CI) analysis was undertaken to
study the synergistic effect of the CDDP and DOX combination
formulation (8). A CI value>1
indicates an antagonistic effect, <1 indicates a synergistic
effect. To evaluate the CI value, IC50 values of the
various types of LPNs described in the above section were
calculated. CI values were calculated according to the following
formula: CI50 =
DCDDP/(D50)CDDP
+DDOX/(D50)DOX. DCDDP
and DDOX mean the IC50 value of CDDP and DOX,
separately. (D50)CDDP and
(D50)DOX referred to the concentrations of
CDDP and DOX in the LPN formulations at the IC50
value.
In vivo tissue distribution of
LPNs
The mice bearing the lung carcinoma model were
randomly divided into several groups and intravenously administered
(through the tail vein) 200 µl of the various types of LPNs and
Free C/D (each contained 5 mg CDDP per kg and/or 2.5 mg DOX per kg
of the mice), separately (33). At 1
and 24 h after injection, the mice were sacrificed and the normal
and tumor tissues of the mice were collected, using 0.9% saline
solution to homogenize. The tissue content of CDDP and DOX was
determined as described in the above ‘Characterization of LPNs’
section.
In vivo antitumor efficiency of
LPNs
The same amount of drugs were administered to lung
carcinoma-bearing mice using the same method every three days,
respectively (32). Mice injected
with 0.9% saline were used as control. The tumor growth and body
weight changes of each mice were measured every three days. At day
18 after first drug administration, mice were sacrificed by
cervical dislocation and the tumors were excised and weighed. The
formula: (Tumor weightcontrol-tumor
weighttreated)/(tumor weightcontrol) ×100 was
applied to calculate the tumor inhibition ratio (%).
Statistical analysis
The study data are expressed as the mean ± standard
deviation. Statistical analysis was performed using ANOVA followed
by a post hoc test (S-N-K method). A P-value <0.05 (*P<0.05)
was considered to indicate a statistically significant result.
Results
Characterization of EGF-PEG-DSPE
1H-NMR spectroscopy was utilized to
determine the chemical structure of EGF-PEG-DSPE. Fig. 1B presents the chemical structure
shifts and are marked with numbers according to the structure, δ:
(1) 0.79 (CH3, DSPE);
(2) 1.18 (CH2, DSPE);
(3) 1.89 (CH2, beside the
O side of PEG); (4) 2.47
(CH2, beside the N side of thiolated EGF); (5) 2.89 (CH2, thiolated EGF);
(6) 3.37 (CH2, PEG);
(7) 3.88 (CH2, beside the
NH side of amide linkage); (8–10)
5.86–7.37 (CH2, EGF); (11) 7.57 (=NH2Cl); (12) 8.03 (-NH-EGF).
Characterization of LPNs
Table I shows the
physicochemical property of LPNs. When EGF was added to the
formulation, particle size was increased from 118.7 nm (C/D LPNs)
to 141.6 nm (EGF C/D LPNs). However, the size did not increase with
the feeding of drugs; EGF LPNs and EGF C/D LPNs had similar sizes
(~140 nm). The PDIs of LPNs were between 0.10 and 0.20. Negative ζ
potential was achieved by the LPNs and LPNs containing EGF were
more negatively charged. More than 80% (EE) of the drugs were
loaded into the LPNs, with various DL capacities from 2.2 to 4.7%.
The stability of LPNs was then evaluated over the period of 90
days. Fig. 3 shows that the size and
EE of LPNs did not change significantly.
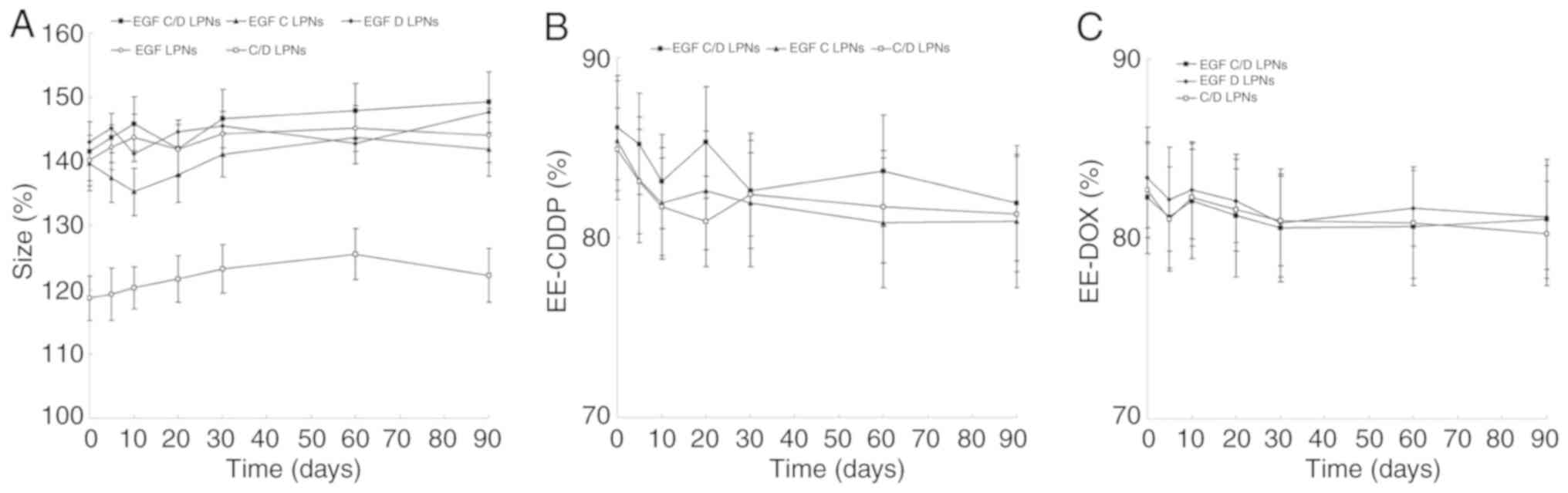 | Figure 3.Stability of the LPNs was evaluated
by the changes in size (A) and EE of CDDP (B) and DOX (C) over a
period of 90 days. LPNs were stored at 2–8°C. The size and EE LPNs
were measured at 0, 5, 10, 20, 30, 60, and 90 days. Data are
expressed as mean ± standard deviation (n=6). EGF C/D LPNs,
EGFR-targeted LPNs loaded with CDDP and DOX; EGF C LPNs,
EGFR-targeted LPNs loaded with CDDP; EGF D LPNs, EGFR-targeted LPNs
loaded with DOX; EGF LPNs, Drug-free EGFR-targeted LPNs; C/D LPNs,
EGFR-free LPNs loaded with CDDP and DOX. CDDP, cisplatin; DOX,
doxorubicin; EGFR, epidermal growth factor receptor; LPNs, lipid
polymeric nanoparticles; EE, encapsulation efficiency. |
 | Table I.Physicochemical properties of the
LPNs. |
Table I.
Physicochemical properties of the
LPNs.
|
Characteristics | EGF C/D LPNs | EGF C LPNs | EGF D LPNs | EGF LPNs | C/D LPNs |
|---|
| Size (nm) | 141.6±4.6 | 139.8±4.3 | 143.1±4.9 | 140.2±3.9 | 118.7±3.5 |
| PDI | 0.19±0.02 | 0.17±0.02 | 0.18±0.03 | 0.15±0.01 | 0.13±0.01 |
| ζ potential
(mV) | −39.2±3.7 | −36.7±3.5 | −38.1±3.1 | −37.3±2.9 | −28.4±2.6 |
| EE-CDDP (%) | 86.1±2.9 | 85.4±3.3 | NA | NA | 84.9±2.3 |
| EE-DOX (%) | 82.3±3.1 | NA | 83.4±2.8 | NA | 82.7±2.6 |
| DL-CDDP (%) | 3.8±0.5 | 3.6±0.4 | NA | NA | 4.7±0.5 |
| DL-DOX (%) | 2.2±0.3 | NA | 2.3±0.3 | NA | 3.2±0.3 |
Drug release from LPNs
In vitro CDDP and DOX release profile of
various types of LPNs all showed sustained behaviors (Fig. 4). However, the release rates of DOX
were faster than CDDP, which may be explained by the drugs loaded
in the different parts of LPNs. In addition, LPNs containing EGFR
released drugs more slowly than the EGFR-free LPNs.
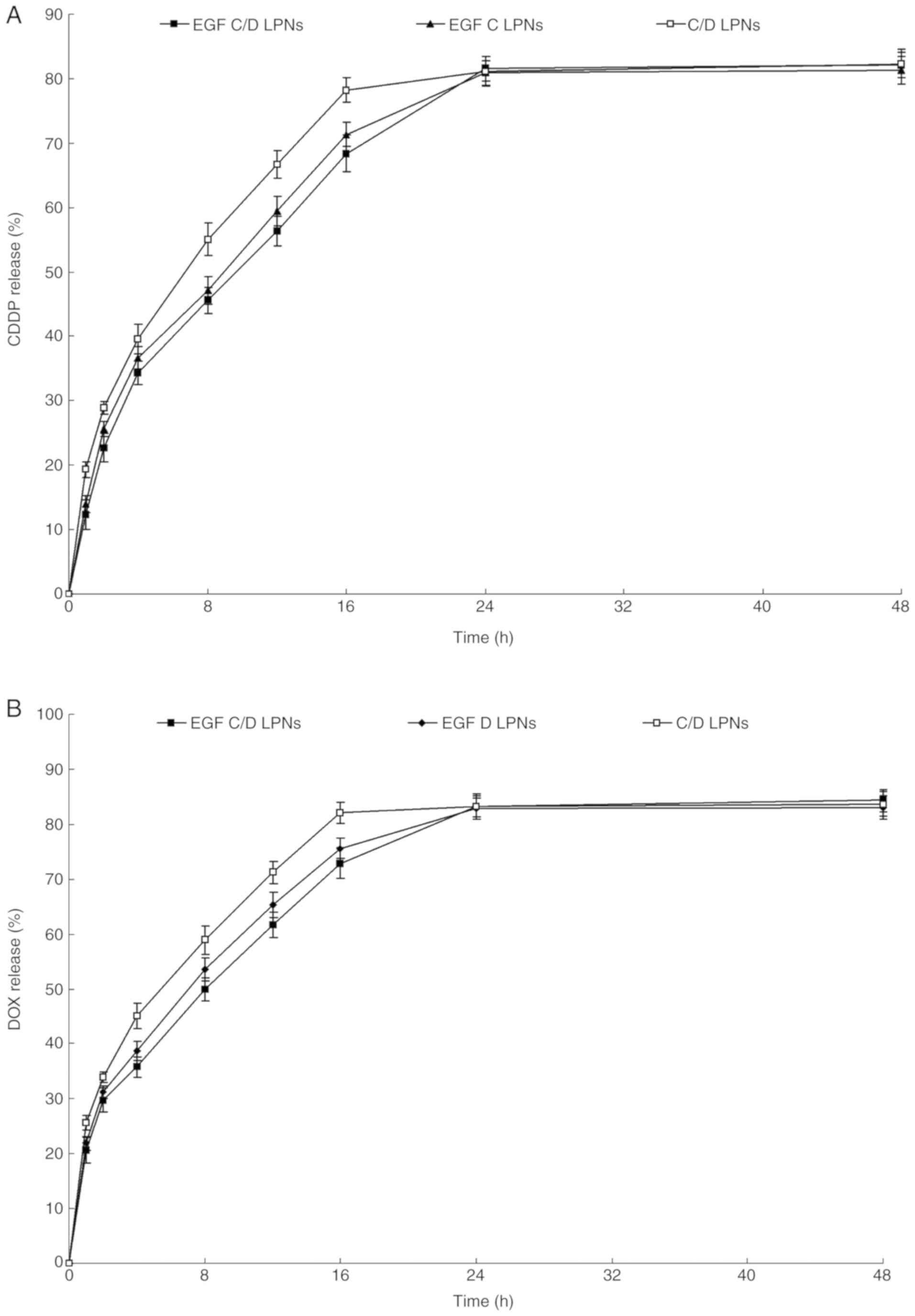 | Figure 4.In vitro CDDP (A) and DOX (B)
release profile of various types of LPNs. Drug release from LPNs
was evaluated by dialysis method. Various types of LPNs (2 ml) were
sealed in dialysis bags (molecular cut-off: 1 kDa) separately,
which were incubated in PBS (pH 7.4, 37°C, 20 ml) and shaken at the
speed of 100 rpm. Data are expressed as mean ± standard deviation
(n=6). EGF C/D LPNs, EGFR-targeted LPNs loaded with CDDP and DOX;
EGF C LPNs, EGFR-targeted LPNs loaded with CDDP; EGF D LPNs,
EGFR-targeted LPNs loaded with DOX; C/D LPNs, EGFR-free LPNs loaded
with CDDP and DOX. CDDP, cisplatin; DOX, doxorubicin; EGFR,
epidermal growth factor receptor; LPNs, lipid polymeric
nanoparticles. |
Synergistic effects of LPNs
Table II summarized
the CI50 of EGF C/D LPNs on A549 cells when different
CDDP/DOX ratios were applied. Synergistic effect
(CI50=0.57) was observed at the ratio of 2:1 (CDDP/DOX,
w/w), suggesting the suitable drug ratios for the LPN preparation.
Other weight ratios tested all showed CI50 values >1,
which indicated antagonistic or no obvious synergistic effect.
 | Table II.CI50 values of EGF C/D
LPNs. |
Table II.
CI50 values of EGF C/D
LPNs.
| LPNs | CDDP/DOX (w/w) | CI50 of
CDDP (mg/ml) | CI50 of
DOX (mg/ml) |
CI50 |
|---|
| EGF C LPNs | NA | 9.26 | NA | NA |
| EGF D LPNs | NA | NA | 11.37 | NA |
| EGF C/D LPNs | 10:1 | 8.76 | 0.88 | 1.05 |
| EGF C/D LPNs | 5:1 | 8.13 | 1.63 | 1.02 |
| EGF C/D LPNs | 2:1 | 3.76 | 1.88 | 0.57 |
| EGF C/D LPNs | 1:1 | 5.97 | 5.97 | 1.17 |
| EGF C/D LPNs | 1:2 | 3.91 | 7.82 | 1.11 |
| EGF C/D LPNs | 1:5 | 1.09 | 10.91 | 1.08 |
Cytotoxicity of LPNs
Fig. 5 shows that
there was no significant cytotoxicity of the drug-free EGF LPNs.
This indicates the safety of the materials used in the studied
concentrations. Drug-containing formulations showed cytotoxicity in
dose-dependent manners. EGF C/D LPNs showed markedly higher
inhibition efficiency in lung carcinoma cells compared with C/D
LPNs, EGF C LPNs and EGF D LPNs (P<0.05). C/D LPNs showed more
efficiency than Free C/D (P<0.05).
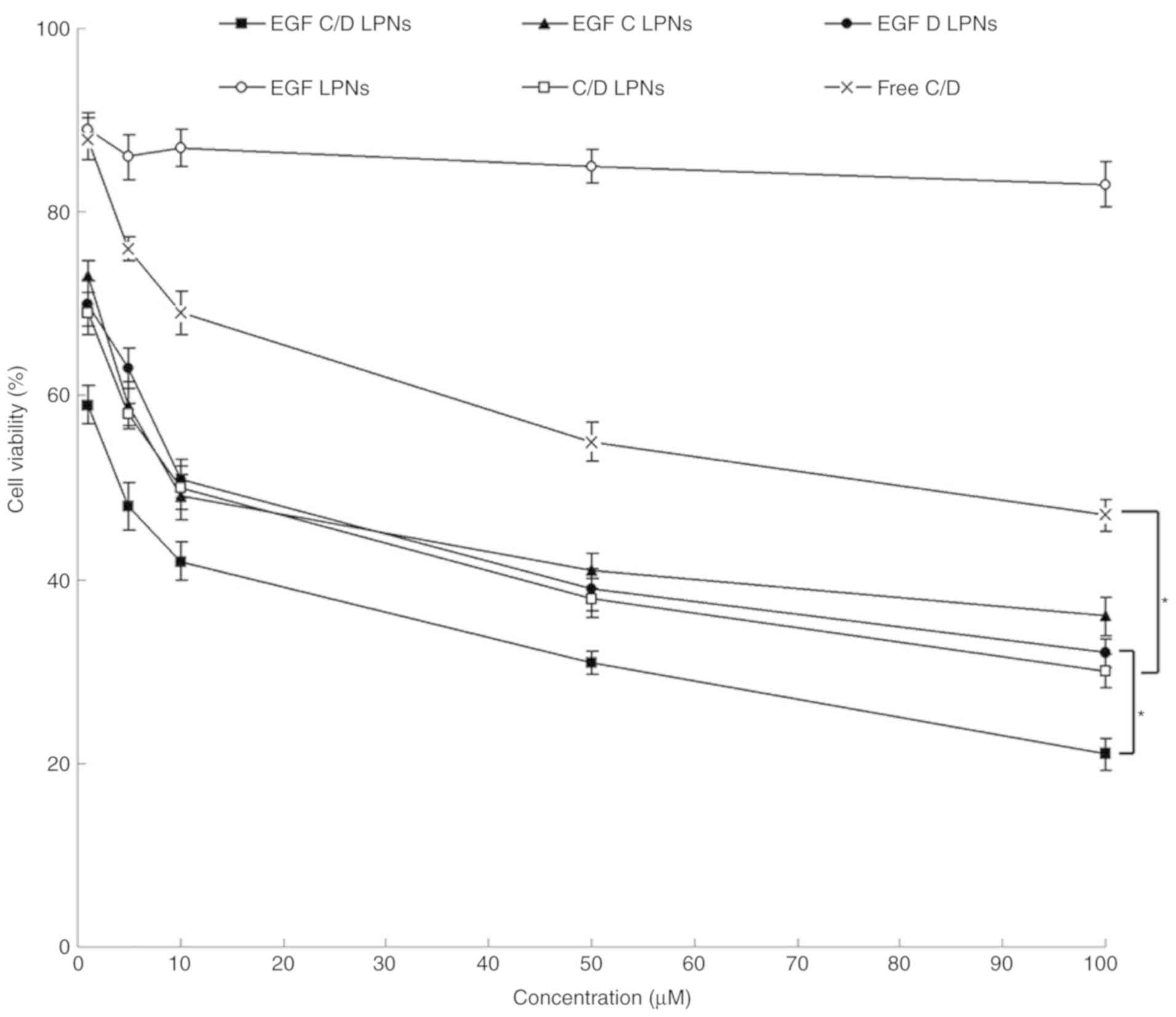 | Figure 5.Cytotoxicity of LPNs evaluated using
A549 cells by MTT assay. Cytotoxicity of LPNs was evaluated on A549
cells by MTT assay. Cells (2×105 per well) were seeded
into 6-well microplates and allowed to grow for 24 h to a
subconfluent state. Various types of LPNs and Free C/D were added
along with fresh medium (contained 10% of FBS) and incubated for 72
h. Data are expressed as mean ± standard deviation (n=8).
*P<0.05. EGF C/D LPNs, EGFR-targeted LPNs loaded with CDDP and
DOX; EGF C LPNs, EGFR-targeted LPNs loaded with CDDP; EGF D LPNs,
EGFR-targeted LPNs loaded with DOX; EGF LPNs, Drug-free
EGFR-targeted LPNs; C/D LPNs, EGFR-free LPNs loaded with CDDP and
DOX; Free C/D, Free-CDDP and DOX drug combination. CDDP, cisplatin;
DOX, doxorubicin; EGFR, epidermal growth factor receptor; LPNs,
lipid polymeric nanoparticles. |
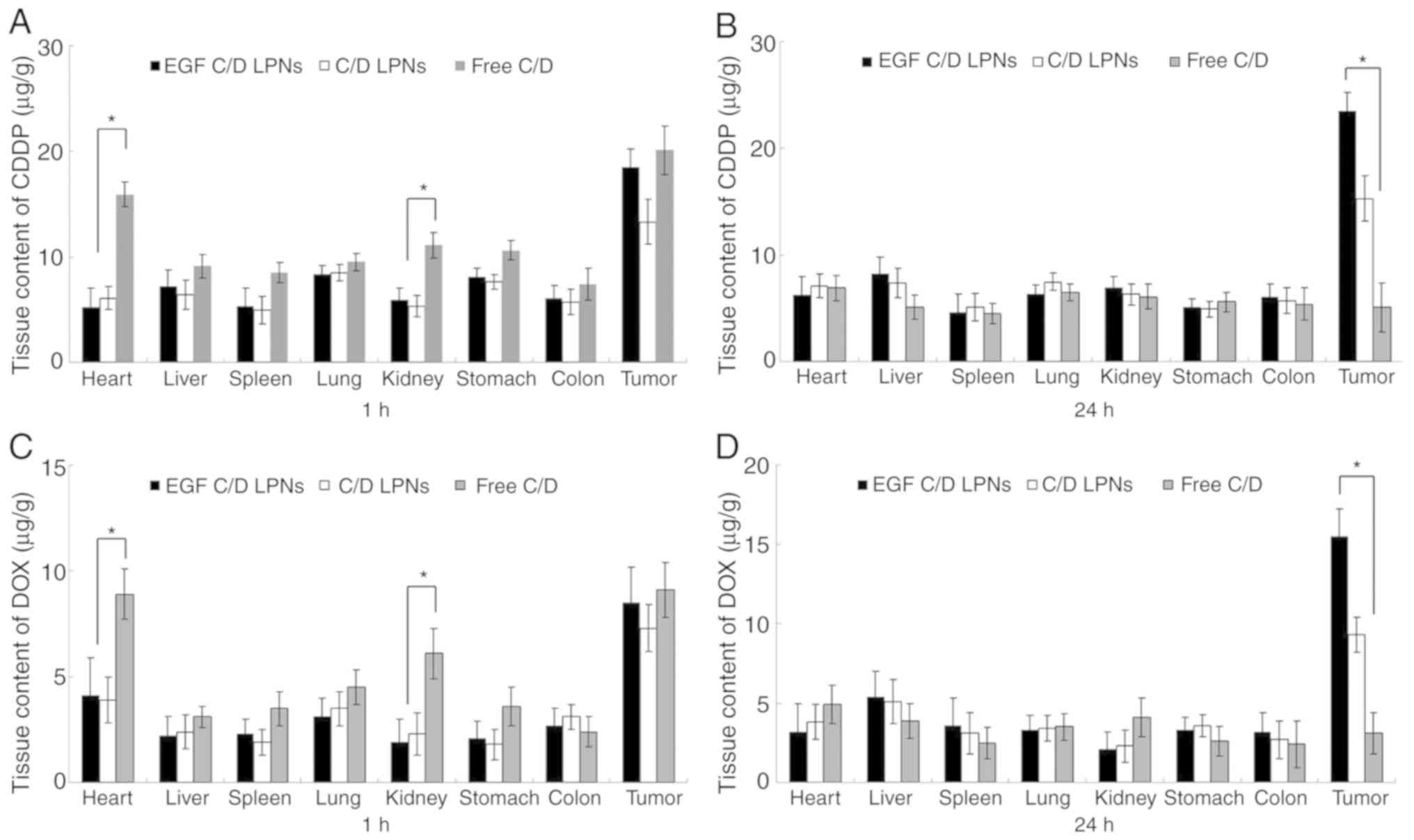
In vivo tissue distribution of
LPNs
Fig. 6 shows the in
vivo drug tissue distribution of EGF C/D LPNs, C/D LPNs and
Free C/D in the lung carcinoma mouse model. At 24 h after
injection, the CDDP and DOX tumor distribution of EGF C/D LPNs was
higher in the tumor tissue than that of the C/D LPNs and Free C/D
(P<0.05). CDDP and DOX containing LPNs accumulated less in the
heart and kidney than Free C/D at 1 h after injection
(P<0.05).
In vivo antitumor efficiency of
LPNs
The tumor volume curves illustrated that treatment
with drug-free EGF LPNs did not show any effect on suppressing
tumor growth when compared with the 0.9% saline control (Fig. 7A). However, when loaded with drugs,
LPNs showed profound tumor growth inhibition ability, compared with
Free C/D group (P<0.05). The tumor volume following treatment
with EGF C/D LPNs at day 18 was 253 mm3, which was the
smallest among all the tested groups. The larger tumor size
following treatment with C/D LPNs (512 mm3) compared
with EGF C/D LPNs at the end of the study could prove the
efficiency of EGF modification. Tumor inhibition ratios of EGF C/D
LPNs, EGF C LPNs and EGF D LPNs were 74.5, 45.6, and 49.7%,
respectively (Fig. 7B). This revealed
the superior antitumor ability of the dual drug LPNs than the
single drug LPNs. The drug-loaded LPN groups caused a slightly
increase in the weights of mice, while the body weights of the Free
C/D, blank LPNs and saline control groups were significantly
decreased (Fig. 7C).
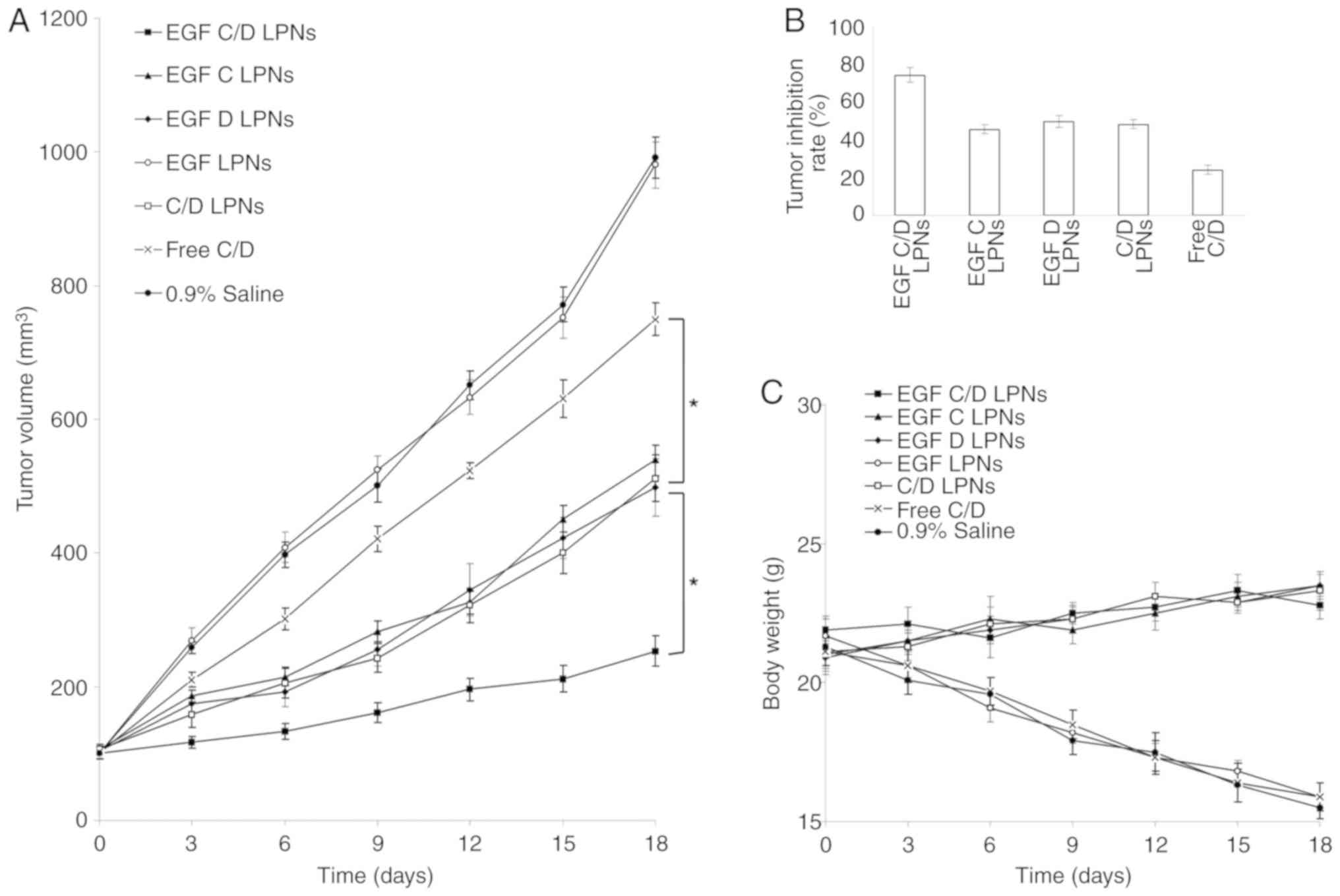 | Figure 7.In vivo tumor inhibitory
effect evaluated using a mouse-bearing lung carcinoma model: (A)
Tumor volume, (B) tumor inhibition rate and (C) body weight. Data
are expressed as mean ± standard deviation (n=8). *P<0.05. EGF
C/D LPNs, EGFR-targeted LPNs loaded with CDDP and DOX; EGF C LPNs,
EGFR-targeted LPNs loaded with CDDP; EGF D LPNs, EGFR-targeted LPNs
loaded with DOX; EGF LPNs, Drug-free EGFR-targeted LPNs; C/D LPNs,
EGFR-free LPNs loaded with CDDP and DOX; Free C/D, Free-CDDP and
DOX drug combination. CDDP, cisplatin; DOX, doxorubicin; EGFR,
epidermal growth factor receptor; LPNs, lipid polymeric
nanoparticles. |
Discussion
Epidermal growth factor receptor (EGFR) is
overexpressed in many carcinomas and has been used as a promising
target for drug delivery. For example, Singh et al developed
EGFR-targeted gelatin nanoparticles for systemic administration of
gemcitabine in an orthotopic pancreatic cancer model. They
synthesized a thiolated gelatin conjugate which they used for the
loading of gemcitabine (34). Gill
and colleagues reported the preparation of poly(lactic-co-glycolic
acid) (PLGA) nanoparticles surface conjugated to
diethylenetriaminepentaacetic acid-human EGF encapsulating the
ruthenium-based DNA replication inhibitor and radiosensitizer for
EGFR-targeted combination therapy in esophageal cancer cells
(35). Kuai et al designed
EGFR-targeted immune magnetic liposomes which could capture
circulating colorectal tumor cells efficiently (36). In the present study, EGF-PEG-DSPE was
synthesized and applied as materials for lipid polymeric
nanoparticle (LPN) preparation. The core-shell structure of the
LPNs allows them to achieve high drug encapsulation yield,
excellent stability, tunable and sustained drug release profile,
and potential for differential targeting of cells or tissues
(37). The stability of the LPNs was
essential to be evaluated since disruption of the particles could
affect the therapeutic potential of the drug delivery systems
(38). The sizes and encapsulation
efficiency (EE) of all LPNs tested showed no obvious change during
90 days, indicating that the LPNs were stable during 3 months of
storage without the incidence of disassembly or aggregation.
For the preparation of EGF C/D LPNs, cisplatin
(CDDP) was encapsulated in the polymeric PLA core and doxorubicin
(DOX) was beneath the lipid shell. The release of DOX from LPNs was
faster than CDDP. This may be explained by the fact that the lipid
shell on the surface of LPNs enabled DOX to be released faster than
CDDP. Compared with C/D LPNs, drugs release from EGF C/D LPNs were
in a more sustained behavior, which may be due to the modification
of EGF that hindered the drug release from the systems. The
prolongation of release time may be attributed to the slow
degradation of the nanomaterials, which let the drugs slowly
diffuse from the matrix (39).
Cytotoxicity of LPNs was tested in A549 cells. The
retained cytotoxicity of the LPNs in a long therapeutic period is
important for treatment (40). The
results showed that there was no significant cytotoxicity of
drug-free EGF LPNs, which may be proof of the low toxicity of the
materials used in the preparation. Significant improvement in
cytotoxicity was achieved by the drug-loaded LPNs when compared to
the free drugs. EGF C/D LPNs inhibited the growth of lung carcinoma
cells more significantly compared with that noted in the C/D LPNs,
which indicated that EGF modification enhanced the cytotoxicity of
the system (41). Combination index
(CI) was calculated to validate the synergistic effect of the drug
co-loaded LPNs using the isobologram equation of Chou and Talalay
(42). EGF C/D LPNs displayed a CI
value <1 (0.57) when the CDDP/DOX ratio was 2:1, suggesting the
suitable CDDP to DOX ratio in the LPNs system. However, other
ratios showed no synergistic effect than the single drug-loaded
LPNs. The reason might be attributed to differences in the
controlled release manner and also different dosages of drugs have
different effects on cancer cells (24,43). The
drug-loaded particles were mainly taken up by cells via the
endocytosis pathway and then exerted antitumor activity after the
drug molecules were released from the NPs, thus different dosages
and ratios of drugs may have different effects, some synergistic
and others antagonistic.
In vivo tissue biodistribution behavior of
EGF C/D LPNs, C/D LPNs and Free C/D were evaluated in a lung
carcinoma mouse model. At 24 h after injection, tumor tissue showed
a significantly higher accumulation of EGF C/D LPNs than those in
other normal tissues, which supported the preferential accumulation
of EGF C/D LPNs in the tumor based on the enhanced permeability and
retention (EPR) effect (44). The
long circulating effect of LPNs was attributed to the presence of a
PEG chain on the surface of the particles, which provided stealth
effect to the NLCs (45). Less CDDP
and DOX containing LPNs accumulated in the heart and kidney than
Free C/D at 1 h after injection; this may lead to lower toxicity in
the heart and kidney.
In vivo antitumor efficiency of EGF C/D LPNs
was more prominently than C/D LPNs and the single drug-loaded LPNs.
This may be attributed to the synergistic anti-lung carcinoma
effects of the dual drug-loaded LPNs. C/D LPNs exhibited more
profound efficiency than the Free C/D, which could be explained by
the high structural integrity, high biocompatibility and
bioavailability and controlled release capability attributed to the
polymer core of the lipid layers of the LPNs (46). The lipid shell enveloping the core is
biocompatible and exhibits behavior similar to that of cell
membranes. Thus, LPNs have good affinity to the cell membranes,
allow fusion of the particles to the cell surface and drugs are
able to be delivered more efficiently into tumor cells (47). Considering the lower toxicity of LPNs
due to the body weight loss of the animals, EGF C/D LPNs exhibited
improved anticancer activity along with lower toxicity than the
free drugs. The tumor inhibition ratio of the EGF C/D LPNs was
significantly higher than that of the EGF C LPNs, EGF D LPNs and
C/D LPNs. These results are in accordance with the above results,
indicating a superior efficiency of EGF C/D LPNs for lung carcinoma
therapy.
In conclusion, EGF-PEG-DSPE was synthesized and
EGFR-targeted LPNs were constructed, which consisted of a
CDDP-loaded hydrophobic polymeric core, DOX-loaded phospholipid
layer, and an outer layer of EGF-PEG-DSPE ligand. EGF C/D LPNs were
stable and could release drugs in a sustained manner. In
vitro and in vivo studies revealed that the EGF C/D LPNs
exhibited improved anticancer activity along with lower toxicity.
These results indicated superior efficiency of the EGF C/D LPNs for
lung carcinoma therapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YN designed the research, performed the experiments
and analyzed the data. YN wrote the paper and approved the
manuscript and agrees to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All animal experiments were approved by the Ethics
Committee of the Affiliated Hospital of Hebei University and
followed the SUNY Upstate Medical University, Department of
Laboratory Animal Resources Guidelines for Anesthesia and Analgesia
in Laboratory Animals and Guidelines for the Care and Use of
Laboratory Animals and the National Animal Laboratory Center of
China.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Simonsen DF, Farkas DK, Søgaard M,
Horsburgh CR, Sørensen HT and Thomsen RW: Tuberculosis and risk of
cancer: A Danish nationwide cohort study. Int J Tuberc Lung Dis.
18:1211–1219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Song Z, Shi Y, Han Q and Dai G:
Endothelial growth factor receptor-targeted and reactive oxygen
species-responsive lung cancer therapy by docetaxel and resveratrol
encapsulated lipid-polymer hybrid nanoparticles. Biomed
Pharmacother. 105:18–26. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carney DN: Lung cancer-time to move on
from chemotherapy. N Engl J Med. 346:126–128. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reck M, Heigener DF, Mok T, Soria JC and
Rabe KF: Management of non-small-cell lung cancer: Recent
developments. Lancet. 382:709–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murren JR, Durivage HJ, Rosenberg AH, Chen
Y, Del Prete SA, Murphy GJ, Buzaid AC and Hait WN: Cisplatin,
doxorubicin, mitomycin C, and 5-fluorouracil for the treatment of
metastatic non-small cell lung cancer. Limited activity of an
aggressive chemotherapy regimen. Am J Clin Oncol. 17:239–241. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Z, Song M, He Z, Zong L, Jiang B, Zhang
T and Hu Z: Comparison of quick recovery outcome of inhalable
doxorubicin and cisplatin in lung cancer patients: A randomized,
double-blind, single-center trial. Drug Deliv Transl Res.
8:985–993. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu J, Cheng H, Han L, Qiang Z, Zhang X,
Gao W, Zhao K and Song Y: Synergistic combination therapy of lung
cancer using paclitaxel- and triptolide-coloaded lipid-polymer
hybrid nanoparticles. Drug Des Devel Ther. 12:3199–3209. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Prabhu VV and Devaraj N: Epidermal growth
factor receptor tyrosine kinase: A potential target in treatment of
non-small-cell lung carcinoma. J Environ Pathol Toxicol Oncol.
36:151–158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pancewicz-Wojtkiewicz J: Epidermal growth
factor receptor and notch signaling in non-small-cell lung cancer.
Cancer Med. 5:3572–3578. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schrank Z, Chhabra G, Lin L, Iderzorig T,
Osude C, Khan N, Kuckovic A, Singh S, Miller RJ and Puri N: Current
molecular-targeted therapies in NSCLC and Their mechanism of
resistance. Cancers (Basel). 10(pii): E2242018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mendelsohn J and Baselga J: Epidermal
growth factor receptor targeting in cancer. Semin Oncol.
33:369–385. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gerber DE: Targeted therapies: A new
generation of cancer treatments. Am Fam Physician. 77:311–319.
2008.PubMed/NCBI
|
|
14
|
Tsai WH, Yu KH, Huang YC and Lee CI:
EGFR-targeted photodynamic therapy by curcumin-encapsulated
chitosan/TPP nanoparticles. Int J Nanomedicine. 13:903–916. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iram S, Zahera M, Khan S, Khan I, Syed A,
Ansary AA, Ameen F, Shair OHM and Khan MS: Gold nanoconjugates
reinforce the potency of conjugated cisplatin and doxorubicin.
Colloids Surf B Biointerfaces. 160:254–264. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun TM, Du JZ, Yao YD, Mao CQ, Dou S,
Huang SY, Zhang PZ, Leong KW, Song EW and Wang J: Simultaneous
delivery of siRNA and paclitaxel via a ‘two-in-one’ micelleplex
promotes synergistic tumor suppression. ACS Nano. 5:1483–1494.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Browning RJ, Reardon PJT, Parhizkar M,
Pedley RB, Edirisinghe M, Knowles JC and Stride E: Drug delivery
strategies for platinum-based chemotherapy. ACS Nano. 11:8560–8578.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oberoi HS, Nukolova NV, Kabanov AV and
Bronich TK: Nanocarriers for delivery of platinum anticancer drugs.
Adv Drug Deliv Rev. 65:1667–1685. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marullo R, Werner E, Degtyareva N, Moore
B, Altavilla G, Ramalingam SS and Doetsch PW: Cisplatin induces a
mitochondrial-ROS response that contributes to cytotoxicity
depending on mitochondrial redox status and bioenergetic functions.
PLoS One. 8:e811622013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ramasamy T, Tran TH, Choi JY, Cho HJ, Kim
JH, Yong CS, Choi HG and Kim JO: Layer-by-layer coated
lipid-polymer hybrid nanoparticles designed for use in anticancer
drug delivery. Carbohydr Polym. 102:653–661. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ruttala HB, Ramasamy T, Gupta B, Choi HG,
Yong CS and Kim JO: Multiple polysaccharide-drug complex-loaded
liposomes: A unique strategy in drug loading and cancer targeting.
Carbohydr Polym. 173:57–66. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo S, Wang Y, Miao L, Xu Z, Lin CM, Zhang
Y and Huang L: Lipid-coated Cisplatin nanoparticles induce
neighboring effect and exhibit enhanced anticancer efficacy. ACS
Nano. 7:9896–9904. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu B, Han L, Liu J, Han S, Chen Z and
Jiang L: Co-delivery of paclitaxel and TOS-cisplatin via
TAT-targeted solid lipid nanoparticles with synergistic antitumor
activity against cervical cancer. Int J Nanomedicine. 12:955–968.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kou CH, Han J, Han XL, Zhuang HJ and Zhao
ZM: Preparation and characterization of the Adriamycin-loaded
amphiphilic chitosan nanoparticles and their application in the
treatment of liver cancer. Oncol Lett. 14:7833–7841.
2017.PubMed/NCBI
|
|
25
|
Bohl Kullberg E, Bergstrand N, Carlsson J,
Edwards K, Johnsson M, Sjöberg S and Gedda L: Development of
EGF-conjugated liposomes for targeted delivery of boronated
DNA-binding agents. Bioconjug Chem. 13:737–743. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qiu J, Cai G, Liu X and Ma D:
αvβ3 integrin receptor specific peptide
modified, salvianolic acid B and panax notoginsenoside loaded
nanomedicine for the combination therapy of acute myocardial
ischemia. Biomed Pharmacother. 96:1418–1426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Zhang P and Zhu T: Ovarian
carcinoma biological nanotherapy: Comparison of the advantages and
drawbacks of lipid, polymeric, and hybrid nanoparticles for
cisplatin delivery. Biomed Pharmacother. 109:475–483. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tan S and Wang G: Redox-responsive and
pH-sensitive nanoparticles enhanced stability and anticancer
ability of erlotinib to treat lung cancer in vivo. Drug Des Devel
Ther. 11:3519–3529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tan S and Wang G: Lung cancer targeted
therapy: Folate and transferrin dual targeted, glutathione
responsive nanocarriers for the delivery of cisplatin. Biomed
Pharmacother. 102:55–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Duan W and Liu Y: Targeted and synergistic
therapy for hepatocellular carcinoma: Monosaccharide modified lipid
nanoparticles for the co-delivery of doxorubicin and sorafenib.
Drug Des Devel Ther. 12:2149–2161. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang F, Li A, Liu H and Zhang H: Gastric
cancer combination therapy: Synthesis of a hyaluronic acid and
cisplatin containing lipid prodrug coloaded with sorafenib in a
nanoparticulate system to exhibit enhanced anticancer efficacy and
reduced toxicity. Drug Des Devel Ther. 12:3321–3333. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Z, Wei Y, Fang G, Hong D, An L, Jiao
T, Shi Y and Zang A: Colorectal cancer combination therapy using
drug and gene co-delivered, targeted poly(ethylene
glycol)-ε-poly(caprolactone) nanocarriers. Drug Des Devel Ther.
12:3171–3180. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gao Z, Li Z, Yan J and Wang P: Irinotecan
and 5-fluorouracil-co-loaded, hyaluronic acid-modified
layer-by-layer nanoparticles for targeted gastric carcinoma
therapy. Drug Des Devel Ther. 11:2595–2604. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Singh A, Xu J, Mattheolabakis G and Amiji
M: EGFR-targeted gelatin nanoparticles for systemic administration
of gemcitabine in an orthotopic pancreatic cancer model.
Nanomedicine. 12:589–600. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gill MR, Menon JU, Jarman PJ, Owen J,
Skaripa-Koukelli I, Able S, Thomas JA, Carlisle R and Vallis KA:
111In-labelled polymeric nanoparticles incorporating a
ruthenium-based radiosensitizer for EGFR-targeted combination
therapy in oesophageal cancer cells. Nanoscale. 10:10596–10608.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kuai JH, Wang Q, Zhang AJ, Zhang JY, Chen
ZF, Wu KK and Hu XZ: Epidermal growth factor receptor-targeted
immune magnetic liposomes capture circulating colorectal tumor
cells efficiently. World J Gastroenterol. 24:351–359. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang L, Chan JM, Gu FX, Rhee JW, Wang AZ,
Radovic-Moreno AF, Alexis F, Langer R and Farokhzad OC:
Self-assembled lipid-polymer hybrid nanoparticles: A robust drug
delivery platform. ACS Nano. 2:1696–1702. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
You P, Yuan R and Chen C: Design and
evaluation of lidocaine- and prilocaine-coloaded nanoparticulate
drug delivery systems for topical anesthetic analgesic therapy: A
comparison between solid lipid nanoparticles and nanostructured
lipid carriers. Drug Des Devel Ther. 11:2743–2752. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhang S, Li J, Hu S, Wu F and Zhang X:
Triphenylphosphonium and D-α-tocopheryl polyethylene glycol 1000
succinate-modified, tanshinone IIA-loaded lipid-polymeric
nanocarriers for the targeted therapy of myocardial infarction. Int
J Nanomedicine. 13:4045–4057. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Miao JF, Peng YF, Chen S, Gao WJ, Yang QX,
Zhu P, Guo J, Tao J, Luo L, Zhang Y and Ling Y: A novel harmine
derivative,
N-(4-(hydroxycarbamoyl)benzyl)-1-(4-methoxyphenyl)-9H-pyrido[3,4-b]indole-3-carboxamide
(HBC), as histone deacetylase inhibitor: In vitro
antiproliferation, apoptosis induction, cell cycle arrest, and
antimetastatic effects. Eur J Pharmacol. 824:78–88. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nishikawa K, Asai T, Shigematsu H, Shimizu
K, Kato H, Asano Y, Takashima S, Mekada E, Oku N and Minamino T:
Development of anti-HB-EGF immunoliposomes for the treatment of
breast cancer. J Control Release. 160:274–280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: The combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gawde KA, Sau S, Tatiparti K, Kashaw SK,
Mehrmohammadi M, Azmi AS and Iyer AK: Paclitaxel and di-fluorinated
curcumin loaded in albumin nanoparticles for targeted synergistic
combination therapy of ovarian and cervical cancers. Colloids Surf
B Biointerfaces. 167:8–19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang H, Sun G, Zhang Z and Ou Y:
Transcription activator, hyaluronic acid and tocopheryl succinate
multi-functionalized novel lipid carriers encapsulating etoposide
for lymphoma therapy. Biomed Pharmacother. 91:241–250. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cui T, Zhang S and Sun H: Co-delivery of
doxorubicin and pH-sensitive curcumin prodrug by
transferrin-targeted nanoparticles for breast cancer treatment.
Oncol Rep. 37:1253–1260. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhu B, Yu L and Yue Q: Co-delivery of
vincristine and quercetin by nanocarriers for lymphoma combination
chemotherapy. Biomed Pharmacother. 91:287–294. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mandal B, Bhattacharjee H, Mittal N, Sah
H, Balabathula P, Thoma LA and Wood GC: Core-shell-type
lipid-polymer hybrid nanoparticles as a drug delivery platform.
Nanomedicine. 9:474–491. 2013. View Article : Google Scholar : PubMed/NCBI
|