Introduction
As one of the most prevalent types of cancer,
colorectal cancer (CRC) is a considerable contributor to cancer
mortality worldwide (1). In spite of
recent improvements in surgical techniques, dosing and scheduling
of adjuvant therapy, the overall prognosis of CRC patients varies
substantially depending on tumour progression. Accordingly,
elucidating the molecular mechanisms underlying tumour development,
as well as identifying novel related factors, are crucial for the
development of new diagnostic and therapeutic methods against
CRC.
Kruppel-like factor 17 (KLF17) belongs to the KLF
family of transcription factors, which consists of 17 members. The
KLF family members play important roles in multifarious cellular
processes, including tumour development (2). Recent studies have investigated the
downregulation of KLF17 in multiple human malignancies, including
breast, liver, stomach and esophageal cancer, suggesting the
potential of KLF17 as a tumour suppressor in cancerous cells
(3–6).
Furthermore, KLF17 may be involved in the regulation of
epithelial-mesenchymal transition (EMT), which has been identified
as a critical process in the progression of cancer to the
metastatic state (7). Moreover, KLF17
has been revealed to be regulated by epigenetic mechanisms such as
DNA methylation, and identified as a possible prognostic biomarker
for CRC (6,8). However, the clinical significance and
regulatory mechanism of KLF17 remains unclear in CRC and needs to
be further explored.
The aim of the present study was to explore the
clinical significance of KLF17 expression in CRC and determine
whether KLF17 inhibited CRC cell growth and metastasis, as well as
the possible underlying molecular mechanism.
Materials and methods
Clinical samples and database
A total of 140 patients with CRC undergoing curative
surgeries between 2014 and 2016 at the Tenth People's Hospital
(Shanghai, China) were included in this study. No patients received
preoperative chemotherapy or radiotherapy. The paired adjacent
non-cancerous samples were obtained at a distance of at least 10 cm
from the tumour lesion. Demographic and clinicopathological
characteristics, including age, sex, tumour location, histologic
differentiation and TNM stage, were collected based on the UICC
criteria. Survival data were collected through follow-up visits and
telephone interviews. This study was approved by The Ethical
Committee of the Tenth People's Hospital.
Tissue microarray (TMA) construction
and immunohistochemistry (IHC)
The TMA slides included 140 CRC and matched adjacent
non-cancerous tissues. All tissues were formalin-fixed and paraffin
embedded, and diverted 2 mm cores from representative areas into
recipient block microarrays.
For IHC, the sections were mounted onto slides,
dewaxed in xylene, and then treated for 30 min with methanol
containing 1% hydrogen peroxide to block endogenous peroxidases. A
primary rabbit anti-human KLF17 polyclonal antibody (dilution,
1:100; cat. no. ab84196; Abcam) was used, according to the
manufacturer's instructions. For immunostaining, a
peroxidase-conjugated goat anti-rabbit secondary antibody (OriGene
Technologies, Inc.) was used, according the manufacturer's
instructions. IHC staining was assessed by two independent
investigators under light microscopy. KLF17 expression was
quantified by evaluating the percentage of positive tumour cells
(9). Positive expression was defined
as ≥5% stained cells in a sample (10).
Cell culture
The human colorectal cancer cell lines LoVo, SW620,
HT29 and Caco-2 cells were purchased from the American Type Culture
Collection. The HT29 cell line was recently authenticated in
Microread Gene Technology by performing a short tandem repeat (STR)
profiling analysis. All cells were cultured in recommended medium
supplemented with 10% fetal bovine serum. For the demethylation
experiments, 2 µM 5-Aza-dC (Merck KGaA) was added to the cell
medium and the exponentially growing cells were treated for 72
h.
Lentiviral infection and transient
transfection
The recombinant lentiviral vector pLV.0-KLF17
overexpressing KLF17 (lenti-KLF17) and control recombinant
lentivector carrying a scramble construct and green fluorescent
protein (lenti-GFP) were obtained from GeneCopoeia, Inc. RNA
interference recombinant lentiviral vector for UHRF1 (lenti-shF1)
was described in our previous study (11). Virus packaging was performed in 293T
cells, following standard procedures, and viral supernatant was
used to infect LoVo and SW620 cells. KLF17 small interfering RNA
(si-F17) and scramble oligonucleotide used as a negative control
(si-NC) were purchased from Thermo Fisher Scientific, Inc. and
transfected into the Lenti-shF1-infected LoVo cells using
Lipofectamine 2000 reagent (Thermo Fisher Scientific, Inc.) to
knockdown the expression of KLF17.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
The mRNA level was quantified by RT-qPCR using the
Quantitect SYBR Green PCR kit (Qiagen AB). β-Actin was used as the
internal control to normalize the results. Primers used in this
experiment were as follows: KLF17 forward,
5′-GCTGCCCAGGATAACGAGAAC-3′ and reverse,
5′-ATCTCTGCGCTGTGAGGAAAG-3′; UHRF1 forward,
5′-CAAGATTGAGCGGCCGGGTGAAGG-3′ and reverse,
5′-TGAGGGGCGGGTCCAGGCAGTAGA-3′; E-cadherin forward,
5′-CGAGAGCTACACGTTCACGG-3′ and reverse,
5′-GGGTGTCGAGGGAAAAATAGG-3′; vimentin forward,
5′-AGTCCACTGAGTACCGGAGAC-3′ and reverse,
5′-CATTTCACGCATCTGGCGTTC-3′; β-actin forward,
5′-CCTGTACGCCAACACAGTGC-3′ and reverse, 5′-ATACTCCTGCTTGCTGATCC-3′.
Amplification conditions were 94°C for 2 min followed by 40 cycles
of 94°C for 45 sec, 60°C for 40 sec and 72°C for 40 sec. The
2−ΔΔCq method was used to calculate the difference in
expression (12).
Western blotting
Total protein was extracted from cells and tissues
with the Total Protein Extraction kit (EMD Millipore), according to
the manufacturer's instructions. The protein content was determined
using a bicinchoninic acid (BCA) protein assay kit (Bio-Rad
Laboratories, Inc.) with bovine serum albumin as the standard.
Proteins (20 µg) were separated by 8% SDS-PAGE and transferred onto
polyvinylidene difluoride membranes (EMD Millipore). The membranes
were blocked in 5% non-fat milk in Tris-buffered saline with 0.05%
Tween-20 at room temperature for 2 h, then probed with antibodies
against KLF17 (cat. no. ab 84196; 1:1,000 dilution), UHRF1 (cat.
no. ab 57083; 1:2,000 dilution), E-cadherin (cat. no. ab 40772;
1:1,000 dilution; all from Abcam), vimentin (cat. no. 5741; 1:500
dilution; Cell Signalling Technology, Inc.) and β-actin (cat. no.
A2228, 1:5,000 dilution; Sigma-Aldrich; Merck KGaA) overnight at
4°C. After washing, membranes were incubated at 37°C for 1 h with
the horseradish peroxidase-conjugated secondary antibody (cat. no.
A0208, 1:1,000 dilution; Beyotime Institute of Biotechnology,
Haimen, China). The blotted proteins were visualized with an
enhanced chemiluminescence (ECLTM plus Western Blotting Detection
Kit; GE Healthcare) and images were obtained by using the
chemiluminescent image analyzer (LAS-4000; Fujifilm). The density
of protein band was quantified using Quantity One v4.4 software
(Bio-Rad Laboratories). Relevant protein expression levels were
defined as the ratio of the density of the specific band for a
target protein to that of the band for β-actin.
Cell proliferation assay
Cell proliferation rates were detected by MTT assay.
Cells (5×103) were seeded in 96-well plates and cultured
for 24, 48, 72 or 96 h. MTT (20 µl) was then added into the plates
and the cells were treated for another 2 h at 37°C. Following the
dissolution of the crystals using dimethyl sulphoxide (DMSO), the
optical density was determined using a microplate reader (BioTek
Instruments, Inc.).
Adhesion assay
CRC cells were placed on 24-well plates covered with
fibronectin (BD Biosciences) and cultured for 24 h. The medium was
then removed and the adherent cells were fixed and counted under a
fluorescence microscope (Olympus Corp.). Five random visual fields
were selected on each insert at a magnification of ×200.
Cell invasion assays
Next, 5×104 cells were seeded in a
Transwell chamber (Corning, Inc.) and covered with Matrigel (BD
Biosciences). The chambers were then filled with complete RPMI-1640
medium and incubated for 24 h. The cells on the top surface of the
membranes were removed, whereas those invading from the upper to
the lower surface were fixed and stained with 0.1% crystal violet
for 1 h at room temperature. Five random visual fields were
captured at a magnification of ×200.
Tumourigenicity and liver metastasis
assay in nude mice
Six-week-old female athymic BALB/c nude mice (n=60;
Chinese Academy of Sciences) were maintained under specific
pathogen-free conditions, using a 10-h light/14-h dark cycle at a
temperature of 25±1°C and relative humidity of 40–60% with free
access to food and water.
For the tumourigenicity assay, 30 mice were randomly
divided into three groups on average and subcutaneously injected in
the right flank with 1.5×106 LoVo (blank), lenti-KLF17-
or lenti-GFP-infected cells in 100 µl PBS. The length and width of
the tumours was measured daily and the volume was calculated using
the formula V=(LxW2)/2. Tumour volumes were observed for
28 days and the growth curves of the tumours for each group were
plotted. The mice were then euthanized by inhalation of a lethal
dose of isoflurane (concentration of 5%) in a closed chamber and
the resected tissues were collected for RNA detection and IHC.
A nude mouse model with CRC cells described
previously was used for the liver metastasis assay (13). First, a total of 30 nude mice were
randomly divided into three groups on average. After the mice were
anesthetised by inhalation of isoflurane, a small left abdominal
incision was performed to expose the spleen. Next, 1×106
LoVo (blank), lenti-KLF17- or lenti-GFP-infected cells in 100 µl
PBS were injected into the spleen of the mice from the three
groups. Splenectomy was performed 10 min later, and the abdomen was
closed. The mice were euthanized 6 weeks later, their livers were
examined for metastasis and the resected tissues were collected for
RNA detection and IHC.
All animal experiments were performed according to
protocols approved by the Ethics Committee of Animal Experiments of
the Tenth People's Hospital Affiliated to Tongji University.
DNA extraction and methylation
analysis
The CGI analysis software (www.urogene.org) was used to find typical CGIs in the
promoter region of KLF17. Two different methods were used to
determine the DNA methylation status of the KLF17 CGI in CRC. For
bisulfite sequencing PCR (BSP), the genomic DNA was
bisulfite-converted using the EZ DNA methylation kit (Zymo
Research, Corp.) and amplified using the following bisulfite PCR
primers: 5′-TTTGTTGTTTAGGTTGGAGTGTAAT-3′ and
5′-AATCACTTAAAATCAAAAATTTAAAACC-3′. The PCR products were cloned
into pMD-18T (Takara Bio, Inc.) and the sequencing was performed in
10 positive clones. QUMA analyser software was used to detect and
analyse the sequencing data. For methylation-specific PCR (MSP),
following treatment with the DNA methylation kit, the genomic DNA
was amplified using the following primers: Methylated,
5′-TTTAGGTTGGAGTGTAATGGC-3′ and 5′-ATTAACCAAACGTAATAACGCGTA-3′;
Unmethylated, 5′-GTTGTTTAGGTTGGAGTGTAATGGT-3′ and
5′-AATTAACCAAACATAATAACACATA-3′.
Statistical analysis
Data are presented as the mean ± SD of at least
three independent experiments. Comparisons between the two groups
were performed by Student's t-test, Dunnett's t-test, or the
Mann-Whitney U test, where appropriate, and comparisons among
multiple groups were performed by a one-way analysis of variance
(ANOVA) followed by Tukey's post hoc test. The relationship between
the expression of KLF17 and clinicopathological features was
calculated using χ2 and Fisher's exact tests.
Kaplan-Meier curves and log-rank analysis evaluated the effects of
KLF17 expression on overall survival. Multivariate cox regression
survival analysis with the stepwise backwards (Wald) method was
used to discover independent prognostic factors. P<0.05 was
considered to indicate a statistically significant difference. SPSS
version 13 (SPSS, Inc.) was used for statistical analysis.
Results
Downregulation of KLF17 in CRC tissue
samples and highly metastatic cells
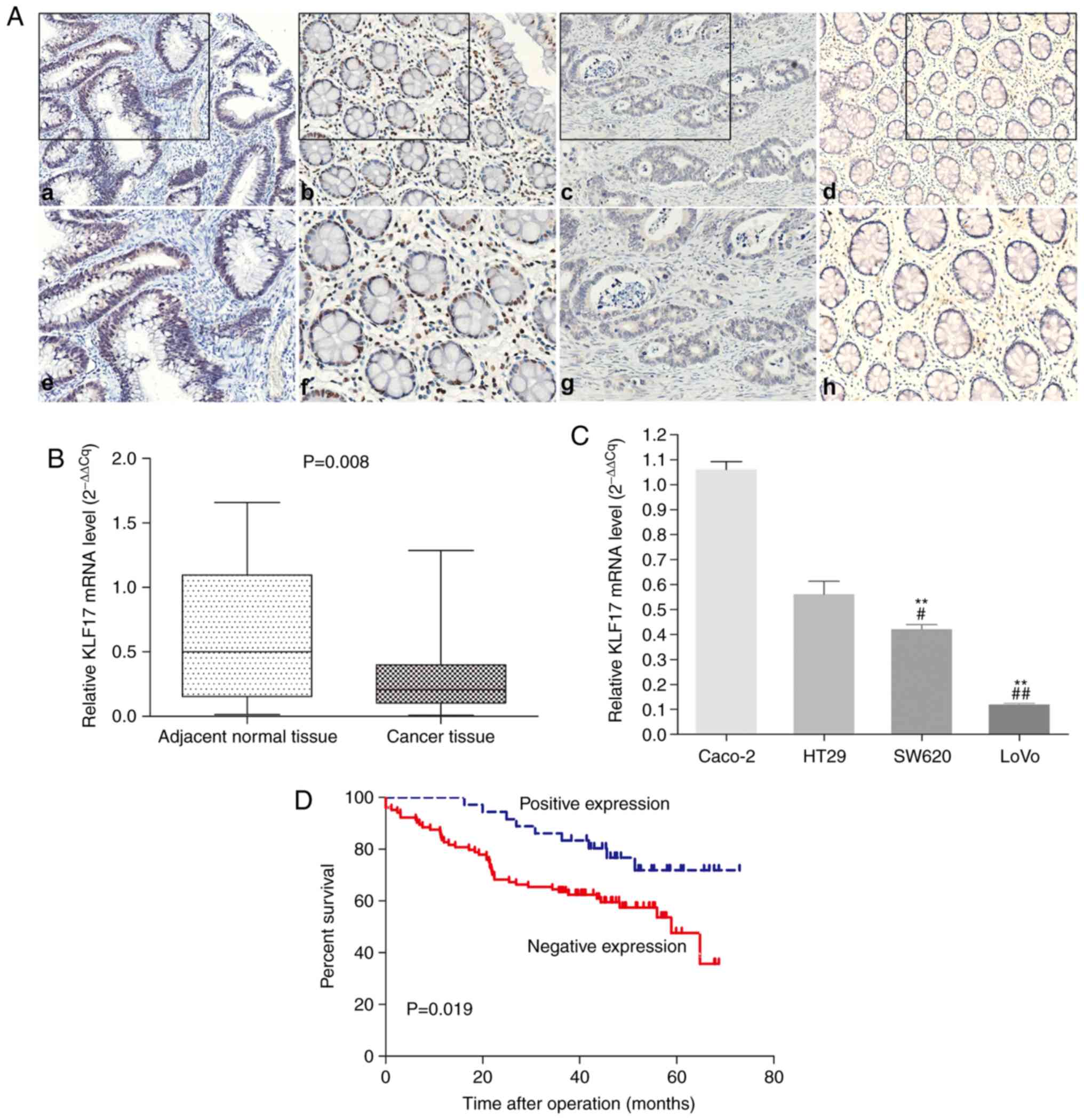
Positive KLF17 staining by IHC was observed in the
nucleus of the CRC (Fig. 1A-a and e)
and adjacent non-cancerous cells (Fig.
1A-b and -f). The IHC staining scores of 140 CRC and matched
adjacent non-cancerous tissues are included in Table SI. Among the 140 paired tissues, the
percentage of KLF17 positive cells in CRC (25.7%, 36/140) was
statistically lower than that in the adjacent non-cancerous samples
(72.9%, 102/140; P<0.001).
Next, RT-qPCR was performed to detect the KLF17 mRNA
expression in 20 paired CRC and adjacent non-tumour tissues; the
results revealed a significantly decreased KLF17 expression in CRC
tissues, as compared to the adjacent normal tissues (Fig. 1B). In addition, the highly metastatic
CRC LoVo and SW620 cells exhibited a lower KLF17 expression, as
compared to the lowly metastatic Caco- 2 and HT29 cells using
RT-qPCR detection (Fig. 1C).
KLF17 expression is inversely
correlated with lymph node metastasis and adverse prognosis in CRC
patients
The relationship between KLF17 expression and
patient characteristics is presented in Table I. The low expression of KLF17 was
correlated with lymph node metastasis (P=0.036; Table I) in CRC patients.
 | Table I.Association of KLF17 expression with
clinicopathological features in CRC patients. |
Table I.
Association of KLF17 expression with
clinicopathological features in CRC patients.
|
|
| KLF17
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
features | No. of patients
(%) | Positive
(n=36) | Negative
(n=104) | P-value |
|---|
| Age (years) |
|
|
|
|
|
<60 | 36 | 7 | 29 |
|
|
≥60 | 104 | 29 | 75 | 0.381 |
| Sex |
|
|
|
|
|
Female | 56 | 18 | 40 |
|
|
Male | 84 | 18 | 64 | 0.244 |
| Tumour
location |
|
|
|
|
| Right
colon | 49 | 14 | 35 |
|
| Left
colon | 48 | 10 | 38 |
|
|
Rectum | 43 | 12 | 31 | 0.632 |
|
Differentiation |
|
|
|
|
|
Well-Moderate | 104 | 25 | 79 |
|
|
Poor | 36 | 11 | 25 | 0.508 |
| Tumor size |
|
|
|
|
|
<5 | 82 | 17 | 65 |
|
| ≥5 | 58 | 19 | 39 | 0.12 |
| Depth of
invasion |
|
|
|
|
|
T0-T2 | 12 | 3 | 9 |
|
|
T3-T4 | 128 | 33 | 95 | 0.953 |
| Nodal status |
|
|
|
|
| N0 | 68 | 23 | 45 |
|
|
N1-N2 | 72 | 13 | 59 | 0.036 |
| Liver
metastasis |
|
|
|
|
|
Absent | 132 | 35 | 97 |
|
|
Present | 8 | 1 | 7 | 0.68 |
| TMN stage |
|
|
|
|
|
0-II | 65 | 18 | 48 |
|
|
III–IV | 75 | 18 | 56 | 0.703 |
Kaplan-Meier analysis revealed that the 5-year
overall survival rate was higher in patients with a high KLF17
expression, as compared to patients with a low KLF17 expression (75
vs. 56.7%; P=0.019; Fig. 1D).
Moreover, univariate and multivariate analysis revealed that KLF17
expression is an independent prognostic biomarker for CRC (RR:
0.444, 95% CI: 0.216–0.912, P=0.027; Table II).
 | Table II.Cox proportional hazard regression
model analysis (n=140) |
Table II.
Cox proportional hazard regression
model analysis (n=140)
| Variables | Categories | RR (95% CI) | Wald
χ2 | P-value |
|---|
|
|---|
| Univariate
analysis |
|---|
| Age (years) | ≥60 vs. <60 | 0.900
(0.496–1.633) | 0.121 | 0.728 |
| Sex | Male vs.
female | 0.840
(0.491–1.438) | 0.404 | 0.84 |
|
Differentiation | Poor vs.
well-moderate | 1.273
(0.709–2.289) | 0.653 | 0.419 |
| Tumor size | ≥5 vs. <5 | 1.056
(0.614–1.814) | 0.038 | 0.845 |
| Depth of
invasion | T3-T4 vs.
T0-T2 | 1.161
(0.419–3.217) | 0.082 | 0.775 |
| Nodal status | N1-N2 vs. N0 | 2.140
(1.212–3.779) | 6.872 | 0.009 |
| Metastasis to other
organs | Present vs.
absent | 5.191
(2.309–11.670) | 15.876 | <0.001 |
| TNM stage | III–IV vs.
0-II | 2.257
(1.267–4.019) | 7.639 | 0.006 |
| KLF17
expression | Positive vs.
negative | 0.432
(0.211–0.887) | 5.223 | 0.022 |
|
| Multivariate
analysisa |
|
| KLF17
expression | Positive vs.
negative | 0.444
(0.216–0.912) | 4.889 | 0.027 |
| Metastasis to other
organs | Present vs.
absent | 3.785
(1.634–8.771) | 9.64 | 0.002 |
| TNM stage | III–IV vs.
0-II | 1.925
(1.058–3.505) | 4.596 | 0.032 |
Ectopic KLF17 expression inhibits cell
growth, invasion and EMT in CRC cells
Considering that lower KLF17 levels were associated
with worse outcomes in CRC patients, the function of KLF17 in CRC
cells was subsequently evaluated. In accordance with the KLF17 mRNA
level in different CRC cell lines (Fig.
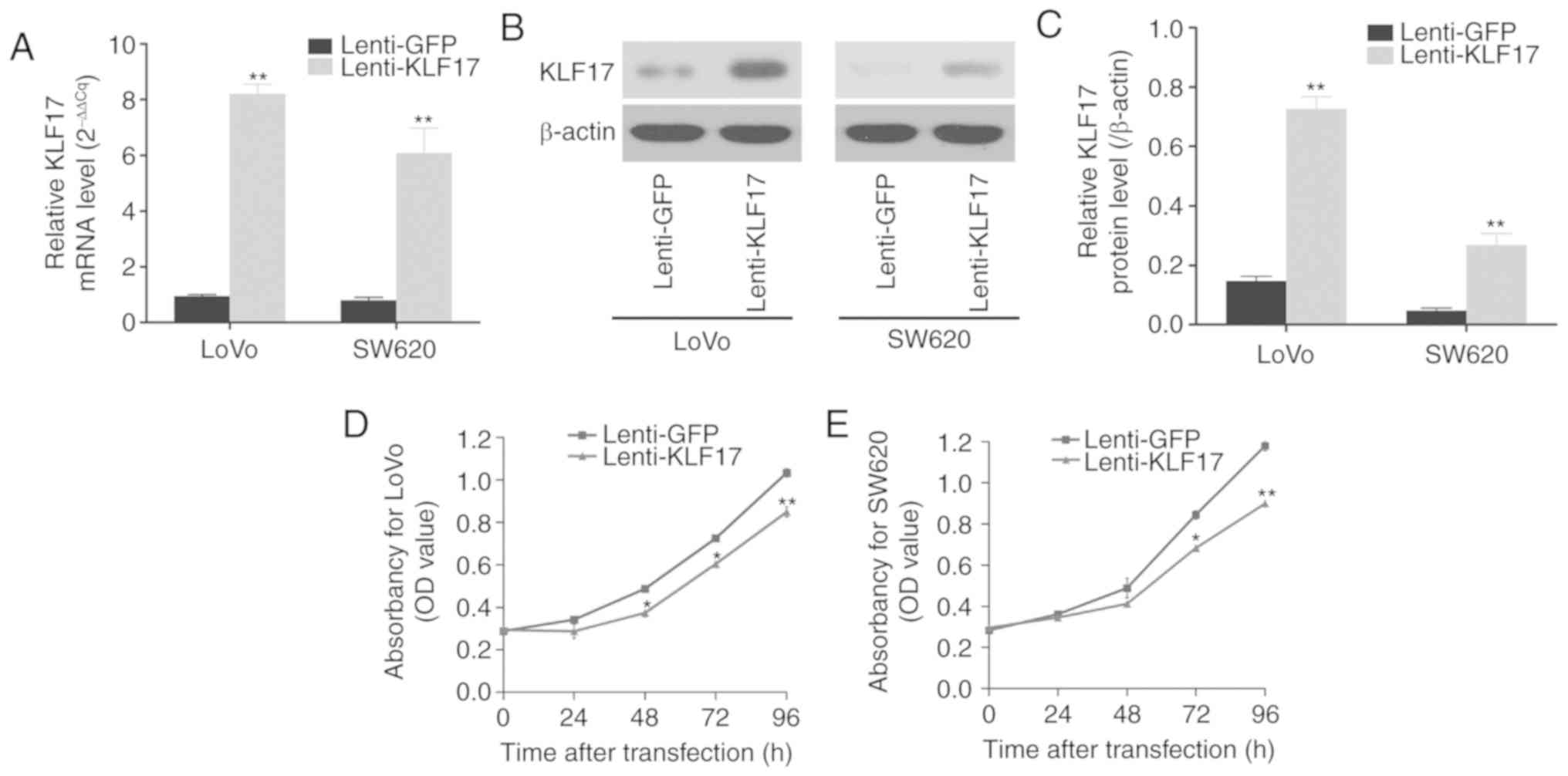
1C), stable LoVo and SW620 cell lines were established through
lentiviral infection that ectopically expressed KLF17.
As revealed in Fig.
2A-C, the expression levels of KLF17 were significantly
increased in LoVo and SW620 cells following lentivirus-mediated
KLF17 infection, as compared to cells infected with lenti-GFP
control.
The MTT assays then revealed that the upregulation
of KLF17 inhibited cell proliferation in LoVo and SW620 cells
(Fig. 2D and E). Similarly, the
potential of colony formation was substantially reduced in the
KLF17-upregulated CRC cells (Fig. 2F and
G).
To detect the effects of KLF17 expression on the
invasion and metastasis capacities of these cells, adhesion and
Transwell assays were performed and the results revealed that
increased KLF17 expression significantly reduced the ability of
adhesion and invasion in LoVo and SW620 cells (Fig. 2H-K).
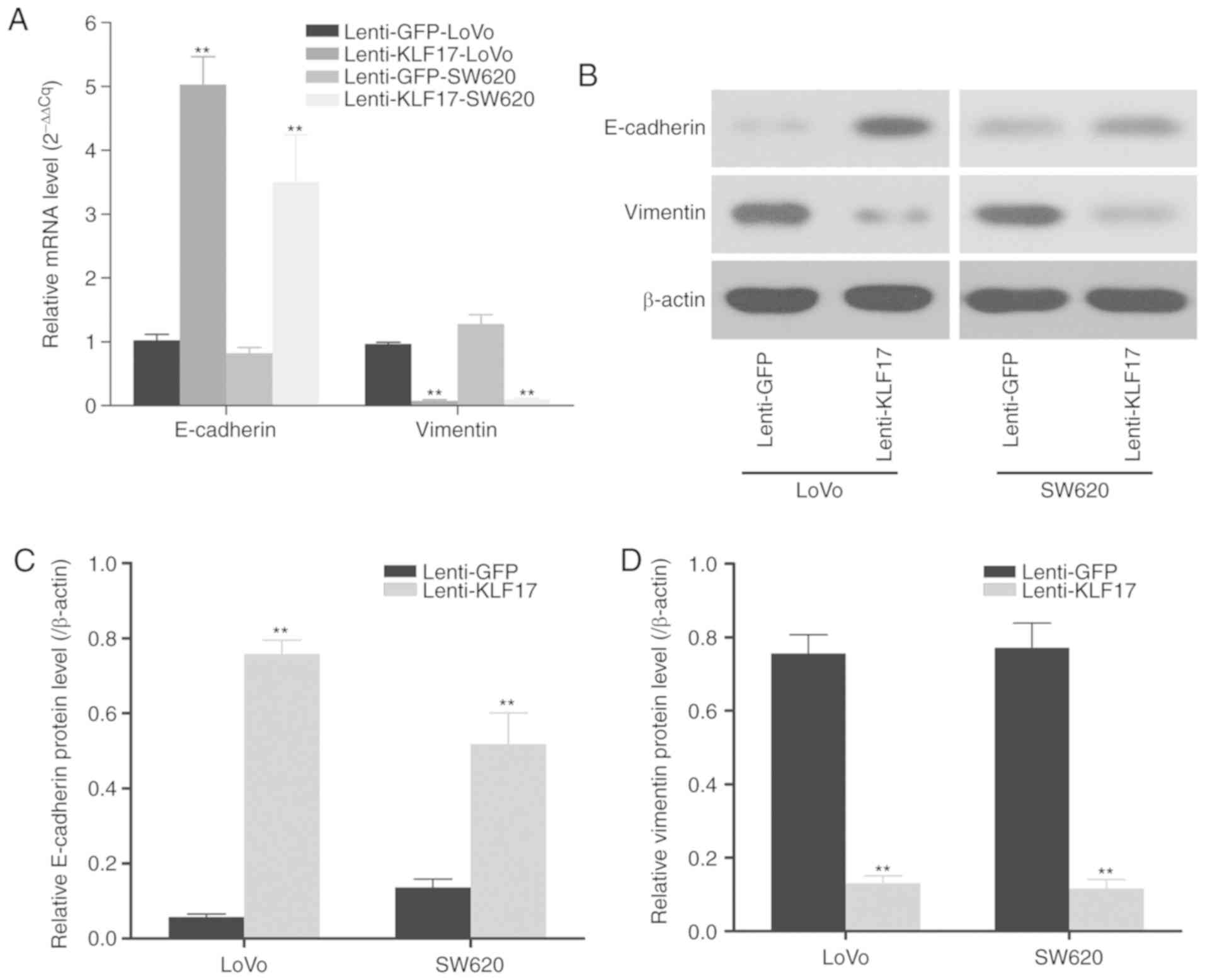
KLF17 has been demonstrated to be a negative
regulator of metastasis and EMT. It has been revealed to band
directly to the promoters of genes such as E-cadherin and vimentin,
which are involved in EMT, and inhibit their expression. Therefore,
the expression of certain EMT biomarkers was next investigated in
stable LoVo and SW620 cells with KLF17 overexpression to evaluate
the effects of KLF17 on EMT in CRC cells. As revealed in Fig. 3, ectopic KLF17 expression led to the
upregulation of epithelial biomarker E-cadherin and the
downregulation of mesenchymal biomarker vimentin, indicating that
KLF17 represses EMT in CRC cells.
Ectopic KLF17 expression suppressed
the tumour growth and metastasis of CRC in nude mice
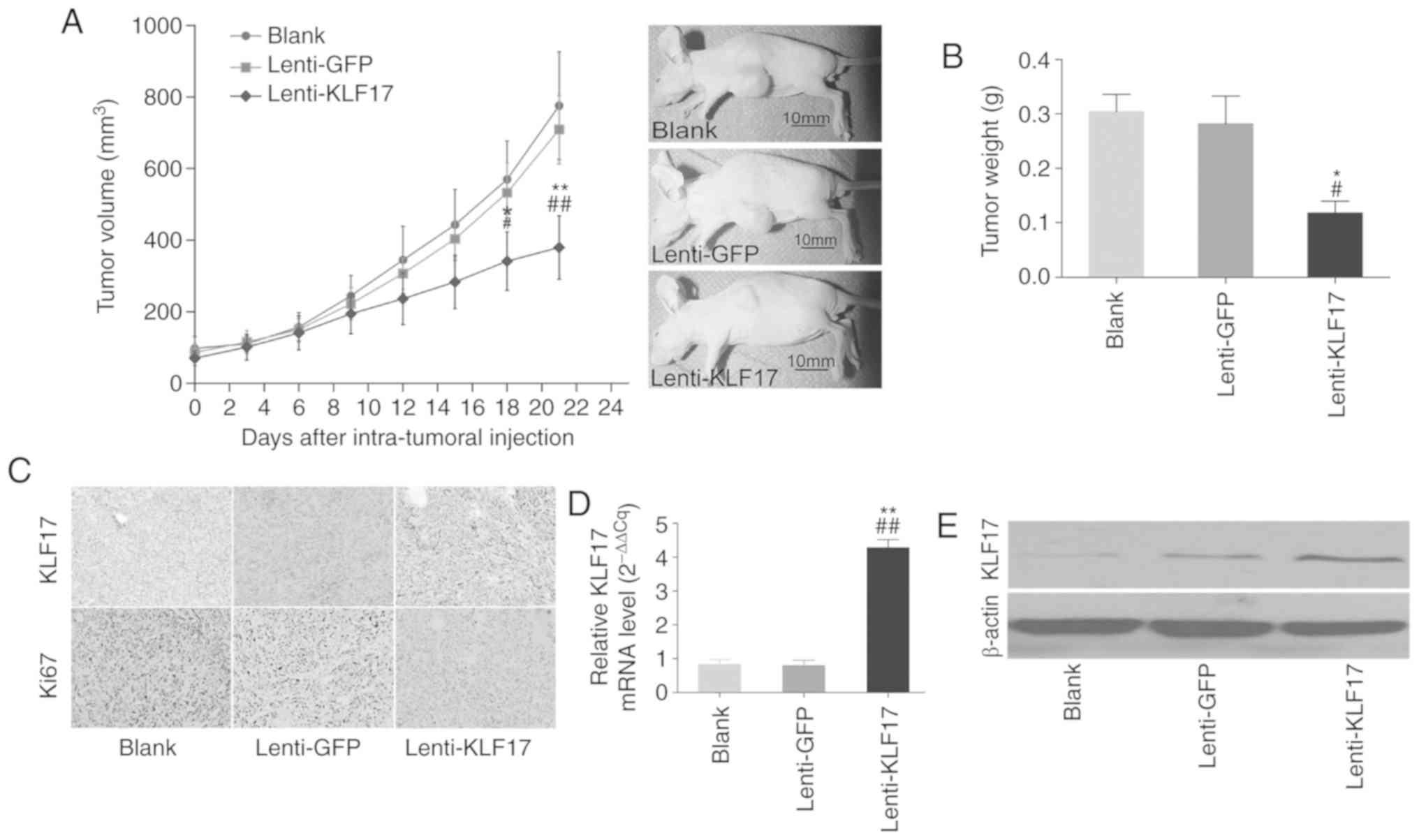
In the following experiment, a nude tumour model was
established to verify the in vitro findings as
aforementioned. As revealed in Fig.
4A, the mouse group subcutaneously injected with the
lenti-KLF17-infected LoVo cells exhibited a lower proliferation
capacity, as compared to the control groups. The tumour volume in
mice injected with lenti-KLF17-infected LoVo cells was considerably
smaller than that in mice injected with LoVo cells or
lenti-GFP-infected LoVo cells. At the end of the experiment, the
tumour weight was significant lower in the group with
lenti-KLF17-infected LoVo cells (0.118±0.021 g), as compared to the
groups with LoVo (0.304±0.032 g) and lenti-GFP-infected LoVo
(0.282±0.051 g) cells (Fig. 4B).
Using antibodies against Ki-67, a standard biomarker for cellular
proliferation, IHC was further performed, and the results revealed
that lenti-KLF17-transfected LoVo cells displayed increased levels
of KLF17 and decreased levels of Ki-67, as compared to the control
groups (Fig. 4C).
In addition, RT-qPCR and western blotting were
performed to detect the mRNA and protein levels in tumour specimens
when the mice were euthanized (Fig.
4D-F). The increased KLF17 expression in lenti-KLF17-infected
mice confirmed the successful transmission of KLF17 by
lentivirus-mediated infection.
The tumour metastasis potential of
lenti-KLF17-infected LoVo cells was analyzed using a nude mice
model of metastasis. As revealed in Fig.
4G and H, more hepatic metastatic nodules were found in the
lenti-GFP and blank control groups than in the lenti-KLF17 group.
In addition, the higher KLF17 expression level in the
lenti-KLF17-infected group confirmed that synthetic KLF17 was
successfully delivered into the LoVo cells (Fig. 4I).
UHRF1-regulated promoter methylation
suppresses KLF17 expression in CRC
It has been reported that KLF17 can be silenced by
the methylation of its promoter (6),
as the methylation of CpG islands (CGIs) in promoters suppresses
reciprocation with transcription factors and inhibits gene
expression (14). Therefore, it was
investigated whether promoter methylation in CRC is associated with
the downregulation of KLF17. Using CGI analysis software
(www.urogene.org), a typical CGI in the promoter
region of KLF17 was revealed (Fig.
5A).
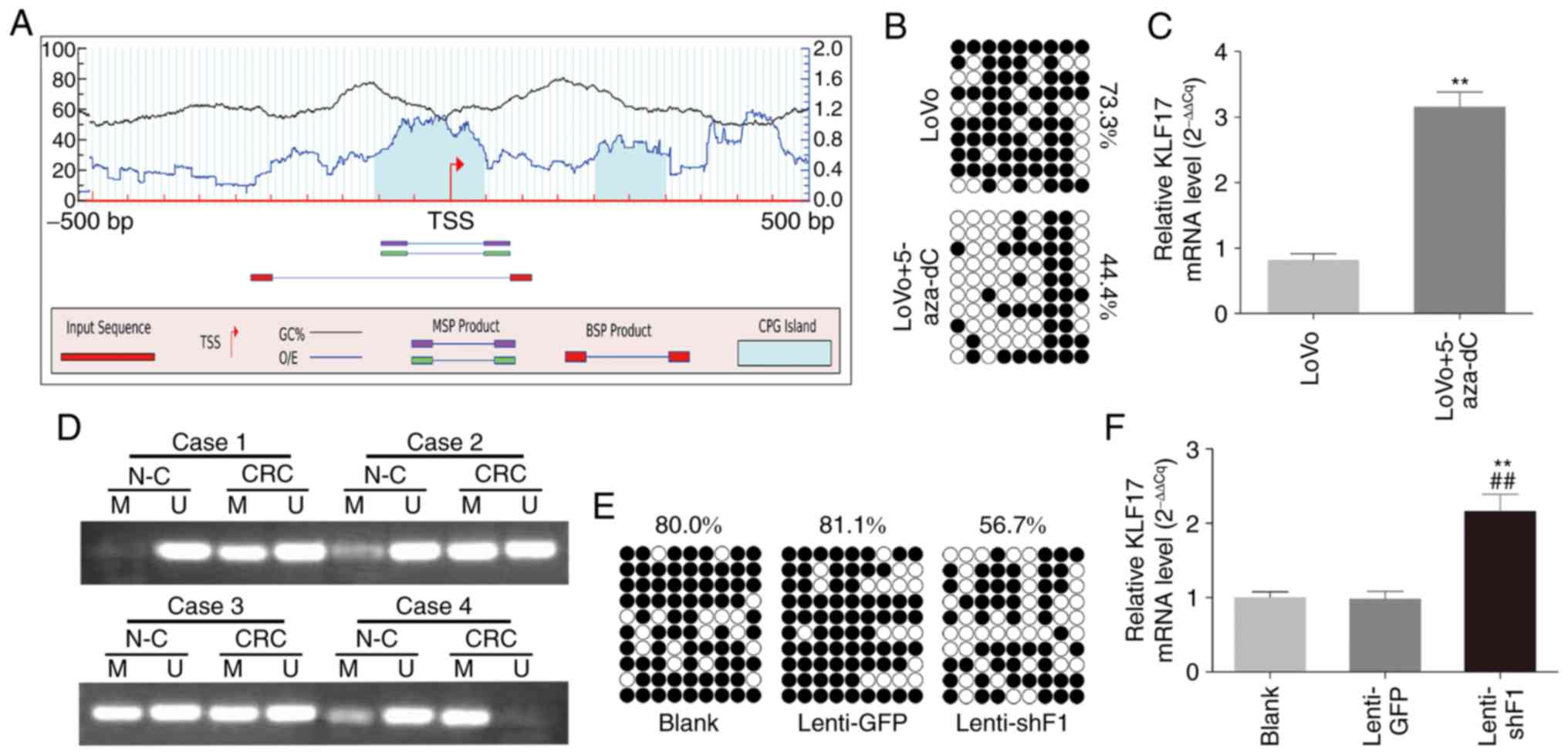 | Figure 5.Promoter methylation regulates by
UHRF1 suppresses KLF17 expression in CRC. (A) Predicted CGIs in the
KLF17 promoter, with the positions of TSS. (B) Methylation levels
of KLF17 CGIs detected by BSP in LoVo and 5-aza-dC-treated LoVo
cells. Black circle, methylated; white circle, unmethylated. (C)
Expression of KLF17 mRNA in LoVo and 5-aza-dC-treated LoVo cells.
**P<0.01. (D) Methylation status of KLF17 promoter measured by
MSP in CRC and corresponding normal mucosa. M, methylated; U,
unmethylated. (E) Methylation levels of KLF17 CGIs detected by BSP
in LoVo cells, lenti-GFP- or lenti-shF1-infected LoVo cells. Black
circle, methylated; white circle, unmethylated. (F) The expression
of KLF17 mRNA in LoVo cells, lenti-GFP- or lenti-shF1-infected LoVo
cells. **P<0.01 vs. the blank group; ##P<0.01 vs.
the lenti-GFP group. Results are presented as the mean ± SD from
three independent experiments. KLF17, kruppel-like factor 17; CRC,
colorectal cancer; CGIs, CpG islands; BSP, bisulfite sequencing
PCR; MSP, methylation-specific PCR; GFP, green fluorescent
protein. |
To determine whether promoter methylation directly
downregulated KLF17, LoVo cells were treated with methylation
inhibitor 5-aza-dc, and then BSP analysis was performed. Following
demethylation, it was observed that the methylation of KLF17
promoter decreased (Fig. 5B) and the
expression of KLF17 was restored (Fig.
5C). MSP was next used to analyze the CGI methylation level of
KLF17 in human CRC. As revealed in Fig.
5D, methylated PCR products were detected in 93.3% (28/30) and
53.3% (16/30) of CRC and paired normal mucosa specimens,
respectively. The methylation level of CRC tissue samples was
evidently higher than that of corresponding normal tissue samples
(P=0.001).
In our previous study, UHRF1 was highly expressed in
CRC and revealed to play an essential role in CRC carcinogenesis
(11). Given the potential of UHRF1
as a DNA methylation regulator and that CGI exists on the KLF17
promoter, we questioned whether the overexpression of UHRF1 is an
underlying mechanism of KLF17 DNA methylation in CRC.
Lentiviral-mediated RNAi of UHRF1 was then carried out to knock
down the UHRF1 expression of LoVo cells (lenti-shF1), and BSP
analysis was performed to detect the CGI methylation status. As
revealed in Fig. 5E and F, the
depletion of UHRF1 decreased CpG methylation and elevated
expression of KLF17 in CRC.
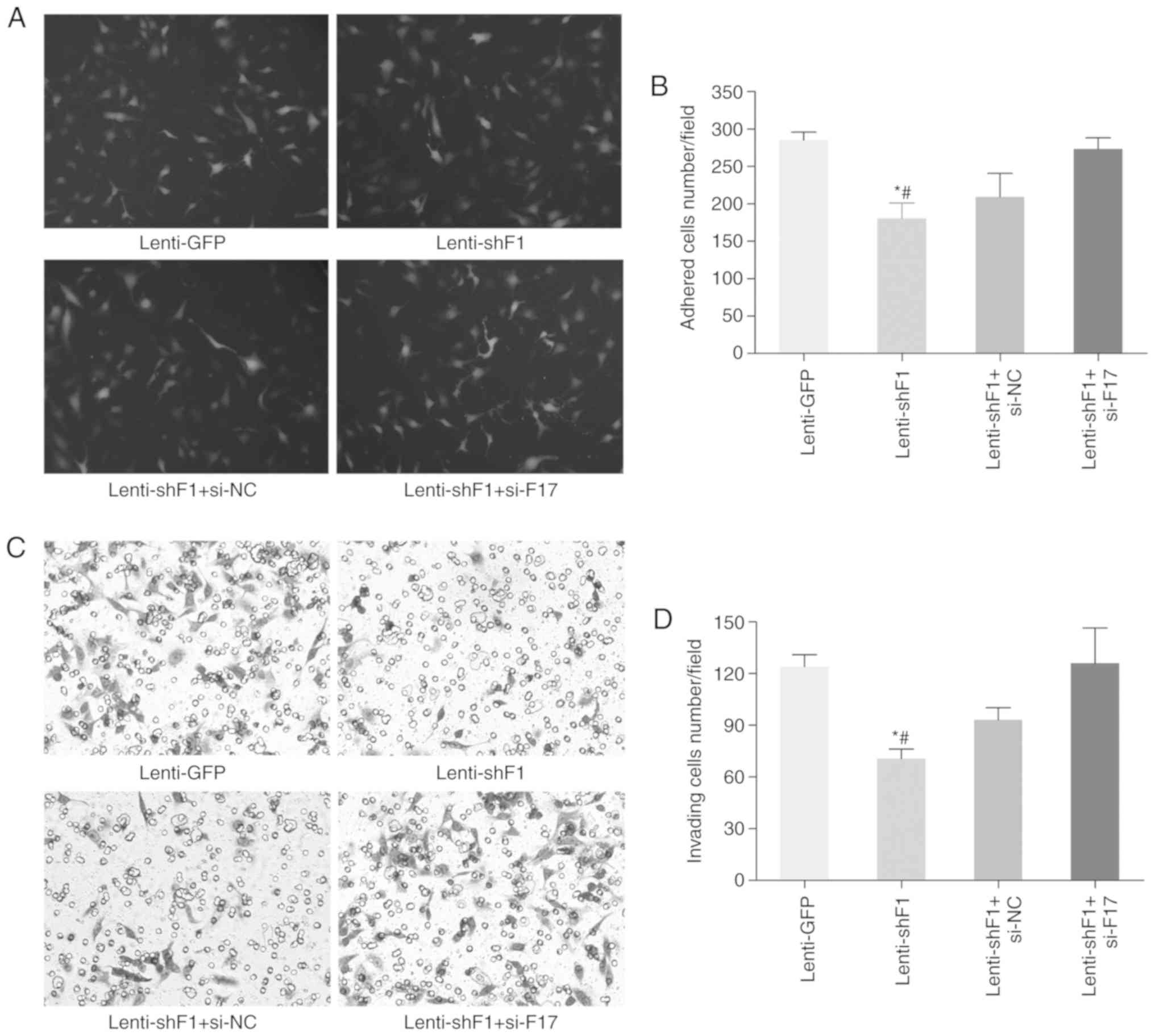
Rescue experiments were then performed to inspect
whether UHRF1 promotes CRC progression through the downstream KLF17
gene using lenti-shF1-infected LoVo cells co-transfected with KLF17
small interfering RNA. It was revealed that the decreased adhesion,
invasion and EMT of CRC cells induced by UHRF1 inhibition could be
partially rescued by KLF17 silencing (Fig. 6). Collectively, the present results
indicated that KLF17 may be a potent downstream gene of UHRF1 and
its downregulation by UHRF1 can be caused by DNA methylation.
Discussion
The KLF transcription factor family proteins have
vital functions in many physiological processes and cancer
development, including proliferation, invasion and metastasis.
Increasing evidence has revealed that KLF4, KLF6 and KLF9 were
downregulated (15–17), but KLF5 upregulated in CRC specimens,
as compared to normal epithelium specimens (18). Moreover, decreased KLF4 expression was
correlated with lymph node metastasis and poor survival in CRC
patients (19).
As an inhibitor of EMT and a potential tumour
suppressor gene in several types of cancer (6,20–22), KL17 has been reported to be negatively
correlated with a poor outcome in lung (23), liver (3), gastric (4)
and papillary thyroid cancer (24).
However, the impact of KLF17 on CRC development has not been fully
elucidated. In the present study, it was demonstrated that KLF17
downregulation was associated with lymph node metastasis and may be
an independent prognostic factor for CRC. In addition, a lower
KLF17 expression was observed in the highly metastatic LoVo and
SW620 CRC cell lines than that in the lowly metastatic CRC cell
lines, indicating that KLF17 could play a negative regulatory role
in CRC progression and metastasis.
Considering its higher transfection efficiency and
more sustained long-term gene expression, lentivirus-mediated
ectopic expression of KLF17 in 2 CRC cell lines was used in this
study to further investigate the biological potential of KLF17 in
the development of CRC (25). Our
in vitro functional experiments revealed that KLF17
overexpression inhibited the proliferation and colony formation
capacity of CRC cells. The in vivo tumourigenicity assay in
nude mice verified that KLF17 overexpression led to a significant
decrease in tumour growth in CRC. In an earlier study, Cai et
al revealed that forced KLF17 expression in lung cancer cells
inhibited the growth rate and colony formation in a time-dependent
manner (23). In a later study, Ye
et al reported that the loss of KLF17 enhances the
proliferation of papillary thyroid cancer cells (24). Furthermore, Ali et al observed
that ectopic KLF17 expression in breast cancer cells containing
mutant p53 reduced cell proliferation, while the depletion of KLF17
promoted cell growth and decreased the apoptotic level of
adriamycin-treated breast cancer cells (20).
A major cause of cancer mortality, metastasis, is a
complex process consisting of multiple steps (26). EMT is considered the key process for
cancer metastasis. During this cellular process, the epithelial
features are lost and mesenchymal features are acquired for the
epithelial cells, leading to the elimination of cell connection and
an increase in cell migration and invasion (27). Using both mouse and cell models,
Gumireddy et al (7) observed
that KLF17 knockdown resulted in EMT and spindle-like and
fibroblastic morphology of breast cancer cells. Furthermore, KLF17
has been revealed to inhibit EMT and cancer metastasis by
controlling related genes (28). The
lower KLF17 expression was associated with the alteration of
EMT-related gene expression in HCC patients. Specifically, the
depletion of KLF17 altered the expression of E-cadherin, vimentin
and ZO-1 in HepG2 cells (29). Sun
et al (3) revealed that KLF17
directly binds to the promoter of vimentin, ZO-1 and fibronectin,
suggesting that KLF17 is an upstream regulator of those EMT-related
genes.
Following lentivirus-mediated forced KLF17
expression, two CRC cell lines exhibited reduced adhesion and
invasion capacities. In addition, an in vivo liver
metastasis model further confirmed the in vitro results, in
which ectopic KLF17 expression significantly depressed LoVo cell
metastasis to the liver. Major EMT markers were then analysed in
CRC cells by RT-qPCR and western blotting. As anticipated, ectopic
KLF17 expression suppressed vimentin (the mesenchymal marker)
expression but promoted that of E-cadherin (the epithelial marker)
in CRC cell lines. Collectively, these results indicated that KLF17
may act as a negative tumour regulator by suppressing EMT
progression in CRC.
Given the potential diagnostic and prognostic value
of KLF17 and its function as a tumour suppressor during CRC
tumourigenesis, the mechanisms controlling KLF17 expression should
be fully elucidated. In a previous study, Sachdeva et al
observed the 5-Aza-dC-mediated induction of KLF3, a member of the
KLF family, in soft tissue sarcomas. As the DNA methylation
inhibitor, 5-Aza-dC induced KLF3 expression by three-fold.
Subsequently, experiments detected conserved CGI in both mouse and
human KLF3 promoters and discovered that the downregulation of KLF3
in sarcoma occurs due to promoter hypermethylation (30). Aberrant DNA methylation patterns are
commonly observed in many types of cancer, including CRC.
Cancer-specific CGI methylation blocks the initiation of gene
transcription and affects many genes in CRC; these modifications
were considered to be a key component of tumorigenesis (31). In the present study, a typical CGI was
revealed in the promoter region of KLF17, and its methylation led
to the suppression of KLF17 expression. In accordance with our
findings, another study demonstrated that the methylation of CGI in
the promoter of KLF17 by UHRF1 decreased KLF17 expression in breast
cancer (6). These results suggested
that the CGI in the promoter of KLF17 may be a cancer-related CGI,
and the regulation of UHRF1 to the expression of KLF17 by
methylation may be a crucial component in the mechanism underlying
tumourigenesis not limited to CRC.
As a vital regulator of DNA methylation, UHRF1 has
been revealed to be overexpressed and play an important role in the
carcinogenesis of several types of cancer (32–34). In
our previous study, UHRF1 expression was correlated with CRC
progression and promoted the growth and metastasis of CRC (11). In the present study, it was further
revealed that UHRF1 silences KLF17 expression through the
methylation of CGI in the promoter of KLF17, and mediates cellular
EMT and invasion in a KLF17-dependent manner in CRC cells. The
results additionally elucidated the underlying mechanism of KLF17
downregulation and the regulation of UHRF1 in CRC carcinogenesis,
invasion and metastasis.
Despite the considerable number of clinical
specimens used in the present study, larger-scale prospective
studies are required to further evaluate or confirm the potential
of KLF17 as a novel biomarker for CRC. Moreover, additional
extensive mechanistic studies are required to elucidate how KLF17
promoter methylation occurs and how KLF17 regulates downstream
targets in CRC carcinogenesis and progression.
In summary, these data indicated that KLF17 is
frequently silenced in CRC and may serve as a potential independent
prognostic CRC biomarker. KLF17 reduced CRC EMT and suppressed
tumour cell proliferation, adhesion, invasion and metastasis.
Moreover, the knockdown of UHRF1 decreased the methylation level of
the KLF17 promoter, triggered the expression of KLF17 and reduced
cell adhesion and invasion by inhibiting EMT in CRC. In
combination, the present study offered insights into KLF17
expression regulation in CRC progression and suggested that making
changes to this mechanism may represent a new therapeutic approach
to blocking CRC development.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (nos. 81301753, 81230057, 81730102 and
81472262).
Availability of data and materials
The data and materials used in the present study are
available for research purposes.
Authors' contributions
FW and HQ designed the experiments and executed
laboratory analysis. XJ, TYS, HL, CS and ZL conducted the
experiments and contributed to the data collection. XJ, FW and HQ
wrote the manuscript. All authors have approved the final version
of the publication and agree to be accountable for all aspects of
the research in ensuring that the accuracy or integrity of any part
of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The research protocol was assessed and permitted by
the Ethical Committee of the Tenth People's Hospital Affiliated to
Tongji University. Written informed consent was obtained from all
patients. All animal experiments were performed according to
protocols approved by the Ethics Committee of Animal Experiments of
the Tenth People's Hospital Affiliated to Tongji University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim C, He P, Bialkowska A and Yang V: SP
and KLF transcription factors in digestive physiology and diseases.
Gastroenterology. 152:1845–1875. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun Z, Han Q, Zhou N, Wang S, Lu S, Bai C
and Zhao R: MicroRNA-9 enhances migration and invasion through
KLF17 in hepatocellular carcinoma. Mol Oncol. 7:884–894. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peng JJ, Wu B, Xiao XB, Shao YS, Feng Y
and Yin MX: Reduced Krüppel-like factor 17 (KLF17) expression
correlates with poor survival in patients with gastric cancer. Arch
Med Res. 45:394–399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li S, Qin X, Cui A, Wu W, Ren L and Wang
X: Low expression of KLF17 is associated with tumor invasion in
esophageal carcinoma. Int J Clin Exp Pathol. 8:11157–11163.
2015.PubMed/NCBI
|
|
6
|
Gao SP, Sun HF, Li LD, Fu WY and Jin W:
UHRF1 promotes breast cancer progression by suppressing KLF17
expression by hypermethylating its promoter. Am J Cancer Res.
7:1554–1565. 2017.PubMed/NCBI
|
|
7
|
Gumireddy K, Li A, Gimotty PA,
Klein-Szanto AJ, Showe LC, Katsaros D, Coukos G, Zhang L and Huang
Q: KLF17 is a negative regulator of epithelial-mesenchymal
transition and metastasis in breast cancer. Nat Cell Biol.
11:1297–1304. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao HL, Zhou N, Sun Z, Dou XL, Guan M and
Bai CM: KLF17 expression in colorectal carcinoma and its clinical
significance. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 38:69–72.
2016.PubMed/NCBI
|
|
9
|
Zlobec I, Terracciano L, Jass J and Lugli
A: Value of staining intensity in the interpretation of
immunohistochemistry for tumor markers in colorectal cancer.
Virchows Arch. 451:763–769. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Crnogorac-Jurcevic T, Gangeswaran R,
Bhakta V, Capurso G, Lattimore S, Akada M, Sunamura M, Prime W,
Campbell F, Brentnall T, et al: Proteomic analysis of chronic
pancreatitis and pancreatic adenocarcinoma. Gastroenterology.
129:1454–1463. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang F, Yang YZ, Shi CZ, Zhang P, Moyer
MP, Zhang HZ, Zou Y and Qin HL: UHRF1 promotes cell growth and
metastasis through repression of p16(ink(4)a) in colorectal cancer.
Ann Surg Oncol. 19:2753–2762. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamamoto Y, Hirakawa E, Mori S, Hamada Y,
Kawaguchi N and Matsuura N: Cleavage of carcinoembryonic antigen
induces metastatic potential in colorectal carcinoma. Biochem
Biophys Res Commun. 333:223–229. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koch A, Joosten SC, Feng Z, de Ruijter TC,
Draht MX, Melotte V, Smits KM, Veeck J, Herman JG, Van Neste L, et
al: Analysis of DNA methylation in cancer: Location revisited. Nat
Rev Clin Oncol. 15:459–466. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao W, Hisamuddin IM, Nandan MO, Babbin
BA, Lamb NE and Yang VW: Identification of Kruppel-like factor 4 as
a potential tumor suppressor gene in colorectal cancer. Oncogene.
23:395–402. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reeves HL, Narla G, Ogunbiyi O, Haq AI,
Katz A, Benzeno S, Hod E, Harpaz N, Goldberg S, Tal-Kremer S, et
al: Kruppel-like factor 6 (KLF6) is a tumor-suppressor gene
frequently inactivated in colorectal cancer. Gastroenterology.
126:1090–1103. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kang L, Lu B, Xu J, Hu H and Lai M:
Downregulation of Kruppel-like factor 9 in human colorectal cancer.
Pathol Int. 58:334–338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakaya T, Ogawa S, Manabe I, Tanaka M,
Sanada M, Sato T, Taketo MM, Nakao K, Clevers H, Fukayama M, et al:
KLF5 regulates the integrity and oncogenicity of intestinal stem
cells. Cancer Res. 74:2882–2891. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu J, Lu B, Xu F, Gu H, Fang Y, Huang Q
and Lai M: Dynamic down-regulation of Kruppel-like factor 4 in
colorectal adenoma-carcinoma sequence. J Cancer Res Clin Oncol.
134:891–898. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ali A, Shah AS and Ahmad A:
Gain-of-function of mutant p53: Mutant p53 enhances cancer
progression by inhibiting KLF17 expression in invasive breast
carcinoma cells. Cancer Lett. 354:87–96. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ali A, Bhatti MZ, Shah AS, Duong HQ,
Alkreathy HM, Mohammad SF, Khan RA and Ahmad A: Tumor-suppressive
p53 signaling empowers metastatic inhibitor KLF17-dependent
transcription to overcome tumorigenesis in non-small cell lung
cancer. J Biol Chem. 290:21336–21351. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ali A, Zhang P, Liangfang Y, Wenshe S,
Wang H, Lin X, Dai Y, Feng XH, Moses R, Wang D, et al: KLF17
empowers TGF-β/Smad signaling by targeting Smad3-dependent pathway
to suppress tumor growth and metastasis during cancer progression.
Cell Death Dis. 6:e16812015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cai XD, Zhou YB, Huang LX, Zeng QL, Zhang
LJ, Wang QQ, Li SL, Feng JQ and Han AJ: Reduced expression of
Kruppel-like factor 17 is related to tumor growth and poor
prognosis in lung adenocarcinoma. Biochem Biophys Res Commun.
418:67–73. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ye WC, Gao L, Huang J, Fang XM and Xie G:
Suppressed Krüppel-like factor 17 expression induces tumor
proliferation, metastasis and a poor prognosis in papillary thyroid
carcinoma. Mol Med Rep. 10:2087–2092. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Milone MC and O'Doherty U: Clinical use of
lentiviral vectors. Leukemia. 32:1529–1541. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stuelten CH, Parent CA and Montell DJ:
Cell motility in cancer invasion and metastasis: Insights from
simple model organisms. Nat Rev Cancer. 18:296–312. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Campbell K: Contribution of
epithelial-mesenchymal transitions to organogenesis and cancer
metastasis. Curr Opin Cell Biol. 55:30–35. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou S, Tang X and Tang F: Krüppel-like
factor 17, a novel tumor suppressor: Its low expression is involved
in cancer metastasis. Tumour Biol. 37:1505–1513. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu FY, Deng YL, Li Y, Zeng D, Zhou ZZ,
Tian DA and Liu M: Down-regulated KLF17 expression is associated
with tumor invasion and poor prognosis in hepatocellular carcinoma.
Med Oncol. 30:4252013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sachdeva M, Dodd RD, Huang Z, Grenier C,
Ma Y, Lev DC, Cardona DM, Murphy SK and Kirsch DG: Epigenetic
silencing of Kruppel like factor-3 increases expression of
pro-metastatic miR-182. Cancer Lett. 369:202–211. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Feinberg AP: The key role of epigenetics
in human disease prevention and mitigation. N Engl J Med.
378:1323–1334. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim KB, Son HJ, Choi S, Hahm JY, Jung H,
Baek HJ, Kook H, Hahn Y, Kook H and Seo SB: H3K9 methyltransferase
G9a negatively regulates UHRF1 transcription during leukemia cell
differentiation. Nucleic Acids Res. 43:3509–3523. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Taniue K, Kurimoto A, Sugimasa H, Nasu E,
Takeda Y, Iwasaki K, Nagashima T, Okada-Hatakeyama M, Oyama M,
Kozuka-Hata H, et al: Long noncoding RNA UPAT promotes colon
tumorigenesis by inhibiting degradation of UHRF1. Proc Natl Acad
Sci U S A. 113:1273–1278. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mudbhary R, Hoshida Y, Chernyavskaya Y,
Jacob V, Villanueva A, Fiel M, Chen X, Kojima K, Thung S, Bronson
RT, et al: UHRF1 overexpression drives DNA hypomethylation and
hepatocellular carcinoma. Cancer Cell. 25:196–209. 2014. View Article : Google Scholar : PubMed/NCBI
|