Introduction
Gastric cancer is an aggressive disease and a common
cause of cancer-associated mortality worldwide. The highest
mortality rates for this disease have been noted in East Asian
countries, including Japan, South Korea and China (1–3). Although
a marked decline in the incidence of gastric cancer has occurred in
some regions, such as in Western countries, gastric cancer remains
a major clinical challenge due to limited treatment methods, poor
prognosis and poor early diagnosis (4,5). Despite
improvements being made in oncological treatment, such as in
surgery, which is the most common therapeutic strategy, the
prognosis of patients with gastric cancer remains poor, with a
5-year survival rate of <25% (6).
Numerous molecular marker alterations are associated
with gastric cancer, including the inactivation of tumor suppressor
genes, such as APC, WNT signaling pathway regulator, cadherin 1,
retinoblastoma, p53 and DCC netrin 1 receptor, and the activation
of oncogenes, such as KRAS proto-oncogene, GTPase, erb-b2 receptor
tyrosine kinase 2, hepatocyte growth factor receptor, β-catenin 1,
cyclin E1 and fibroblast growth factor receptor 2 (1,7,8). Kim et al observed that
follistatin-like protein 1 (FSTL-1) is highly expressed in patients
with gastric cancer; however, the function of FSTL-1 in cancer
biology remains unknown (1). FSTL-1,
also known as follistatin-related protein or transforming growth
factor (TGF)-β stimulating clone-36, is a 308-amino acid soluble
extracellular glycoprotein, which is a member of the secreted
protein acidic and rich in cysteine and follistatin families
(9–12).
As a TGF-β stimulating clone-36 protein, FSTL-1 is
able to inhibit TGF-β superfamily proteins and is secreted under
inflammatory conditions. In addition, as a proinflammatory protein
it is highly expressed and serves a role in inflammatory diseases,
such as rheumatoid arthritis (13–15).
Alongside its proinflammatory role, FSTL-1 serves roles in numerous
pathological processes, such as fibrogenesis (16), vascularization (17), embryonic development (18–20),
immunomodulation (21) and
tumorigenesis (22). Furthermore,
FSTL1 is associated with cell biology processes, including cell
differentiation, migration, proliferation and apoptosis (23). Accumulating evidence has indicated
that FSTL1 is widely expressed in cancer cells, including human
lung cancer cells, clear-cell renal cell carcinoma cells, glioma
cells and gastric cancer cells (1,15,24,25).
Signal transducer and activator of transcription 6
(STAT6) belongs to the STAT family, which contains seven members,
including STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b and STAT6
(26). The activation of STAT6 is
tightly associated with inflammatory cytokines, such as interleukin
(IL)-4 and IL-13 signaling (27).
Jayakumar and Bothwell confirmed that STAT6 promotes intestinal
tumorigenesis in a mouse model of adenomatous polyposis (28). Furthermore, Wang et al
suggested that STAT6 is involved in the function of FSTL1 in
dendritic cell-mediated immunity (29). Therefore, it was hypothesized that the
proinflammatory protein FSTL-1 may mediate the expression of STAT6
in gastric cancer cells.
The present study aimed to determine the role and
the mechanism of FSTL-1 in gastric cancer progression. The results
revealed that FSTL-1 was highly expressed in gastric cancer cells,
and knockdown of FSTL-1 resulted in gastric cancer cell growth
inhibition and apoptosis promotion. In addition, the effects of
FSTL-1 on gastric cancer cell biology were blocked by STAT6
overexpression.
Materials and methods
Cell culture
The human gastric cancer cell lines (AGS, MGC-803,
SGC-7901, BGC-823 and MKN-45) and the human gastric mucosal
epithelial cell line (GES-1) were obtained from American Type
Culture Collection (Manassas, VA, USA). Cells were cultured in
Dulbecco's modified Eagle's medium supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) in a humidified incubator containing 5% CO2 at
37°C.
Cell transfection
For the construction of FSTL-1 knockdown cells, 25
pmol FSTL-1-specific small interfering RNA (siRNA; Shanghai
GenePharma Co., Ltd., Shanghai, China) and a negative control siRNA
(si-NC; Shanghai GenePharma Co., Ltd.) were transfected into
MGC-803 and MKN45 cells using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Briefly, cells were seeded in 6-well
plates at a density of 1×105 cells/well and transfection
was performed once cells reached 60% confluence. Cells were
transfected with the siRNAs at 37°C for 48 h. The siRNA sequences
were as follows: FSTL1 siRNA, sense 5′-GAAACUGCCAUCAAUAUUATT-3′,
anti-sense 5′-UAAUAUUGAUGGCAGUUUCTT-3′; and si-NC, sense
5′-UUCUCCGAACGUGUCACGUTT-3′ and anti-sense
5′-ACGUGACACGUUCGGAGAATT-3′. For transient expression of STAT6, 60
nM pcDNA3.1-STAT6 or an empty vector (pcDNA3.1) were co-transfected
with FSTL-1 siRNA or si-NC into MGC-803 and MKN45 cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Briefly, cells were seeded in 6-well plates at a
density of 1×105 cells/well and transfection was
performed once reached at 60% confluence. The cells were
transfected at 37°C for 48 h.
Cell survival assay
MGC-803 and MKN45 cells were cultured and
transfected as aforementioned. Cell viability was determined using
the CellTiter-Glo® Luminescent Cell Viability Assay
(Promega Corporation, Madison, WI, USA), according to the
manufacturer's protocol.
Cell cytotoxicity assay
MGC-803 and MKN45 cell cytotoxicity was measured
using an lactate dehydrogenase (LDH) assay, as previously reported
(30). LDH release from cells
transfected with FSTL-1 siRNA for 48 h was measured using a
cytotoxicity detection kit (Roche Applied Science, Penzberg,
Germany), according to the manufacturer's protocol.
Cell apoptosis
MGC-803 and MKN45 cells were cultured and
transfected as aforementioned. Cell apoptosis was ascertained using
the Cell Death Detection ELISA assay (cat. no. 11544675001; Roche
Diagnostics GmbH, Mannheim, Germany), according to the
manufacturer's protocol. The amount of histone-complexed DNA
fragments in MGC-803 and MKN45 cells was quantified, in order to
analyze apoptosis. The absorbance of MGC-803 and MKN45 cells was
measured using an ELISA reader at 405 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from MGC-803 and MKN45 cells
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol, and
the concentration of RNA was determined using a spectrophotometer.
cDNA was generated using a High-Capacity cDNA RT kit (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. The
relative mRNA expression levels of FSTL-1 were determined using an
Applied Biosystems 7500 Real Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.) with SYBR Green PCR Master Mix
(Thermo Fisher Scientific, Inc); results were quantified using the
(2−ΔΔCq) method (31).
β-actin was used as a control gene. The following primers were used
in the present study: FSTL-1, forward 5′-CCTGTGTGTGGCAGTAATGG-3′
and reverse 5′-TCAGGAGGGTTGAAAGATGG-3′; and β-actin, forward
5′-GCACCACACCTTCTACAATG-3′ and reverse 5′-TGCTTGCTGATCCACATCTG-3′.
Cycling conditions were as follows: Initial denaturation at 95°C
for 5 min, followed by 38 cycles of denaturation at 95°C for 15
sec, annealing at 58°C for 30 sec and extension at 55°C for 30 sec,
and a final extension step at 72°C for 1 min.
Western blot analysis
MGC-803 and MKN45 cells were transfected with FSTL-1
siRNA, or co-transfected with FSTL-1 siRNA and pcDNA3.1-STAT6 and
their corresponding controls for 48 h. Total proteins were
extracted using radioimmunoprecipitation assay (RIPA) buffer
(Thermo Fisher Scientific, Inc.) and the concentration was
determined by using the Bio-Rad Protein Assay kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Proteins (50 µg/lane) were
separated by 12% SDS-PAGE and were electrophoretically transferred
to nitrocellulose membranes. The membranes were blocked with 5%
nonfat dry milk for 1 h at room temperature. Subsequently, the
membranes were incubated with specific antibodies, including FSTL-1
(cat. no. ab71548; 1:1,000; Abcam, Cambridge, MA, USA),
anti-β-actin (cat. no. ab8226; 1:1,000; Abcam),
phosphorylated-STAT6 (cat. no. ab28829; 1:500; Abcam) and STAT6
(cat. no. ab227497; 1:1,000; Abcam) overnight at 4°C. Subsequently,
the membrane was incubated with a horseradish peroxidase-conjugated
goat anti-rabbit immunoglobulin G secondary antibody (cat. no.
ab6721; 1:5,000; Abcam) for 1 h at room temperature. An enhanced
chemiluminescence detection kit (Amersham; GE Healthcare, Chicago,
IL, USA) was used to detect immunoreactive proteins. β-actin was
used as control.
Caspase-3/9 activity
Caspase-3/9 activity was assessed using colorimetric
substrates, according to the methods described previously (32). MGC-803 and MKN45 cells were lysed with
RIPA lysis buffer, collected and incubated with colorimetric
caspase-3 substrate (Ac-DEVD-Pna; Promega Corporation) and
caspase-9 substrate (Ac-LEHD-Pna; Promega Corporation) at 37°C for
3 h in dark. The activity of caspase-3/9 in MGC-803 and MKN45 cells
was then measured using a spectrophotometer at a wavelength of 405
nm.
Statistical analysis
All experiments were conducted at least three times
in triplicate. SPSS 19.0 (SPSS, Inc., Chicago, IL, USA) was used
for data analyses. Data are presented as the means ± standard
deviation. Statistical analyses were performed by one-way analysis
of variance followed by Fisher's least significant difference.
P<0.05 was considered to indicate a statistically significant
difference.
Results
FSTL-1 is highly expressed in gastric
cancer cells
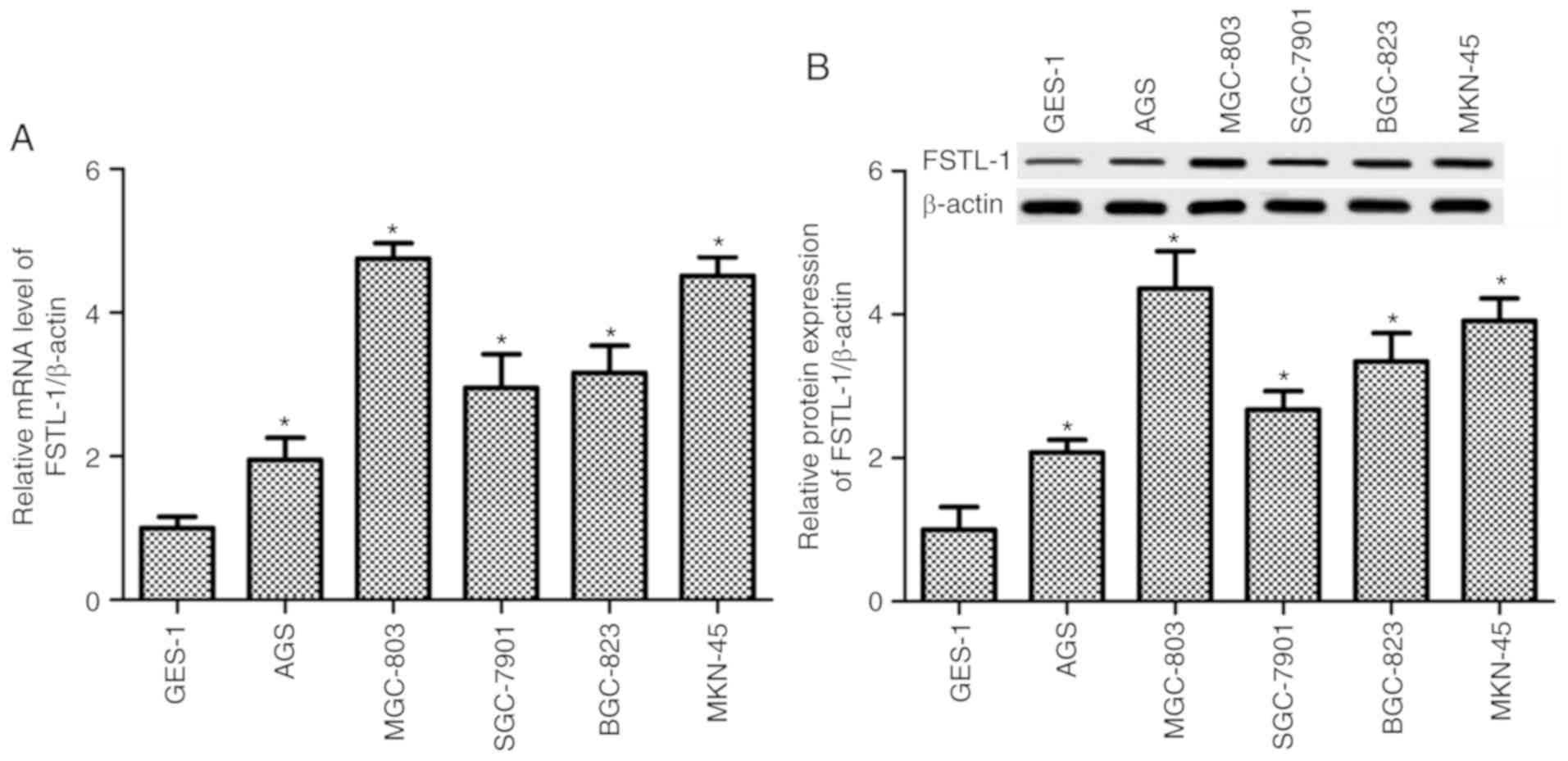
The present study demonstrated that compared with in
the control GES-1 gastric mucosal epithelial cell line, the mRNA
expression levels of FSTL-1 in the gastric cancer cell lines, AGS,
MGC-803, SGC-7901, BGC-823 and MKN-45, were significantly enhanced
(P<0.05, Fig. 1A). The protein
expression levels of FSTL-1 in the cells were detected by western
blotting, which was normalized to β-actin. The results revealed
that FSTL-1 protein expression was increased in all gastric cancer
cell lines compared with in the GES-1 cells (P<0.05, Fig. 1B). Expression was highest in MGC-803
and MKN45 cells; therefore, these two cell lines were used in the
subsequent experiments (Fig. 1).
FSTL-1 inhibition reduces MGC-803 and
MKN45 cell survival and induces cytotoxic effects
To determine the function of FSTL-1 in gastric
cancer cell biology, FSTL-1 was inhibited in MGC-803 and MKN45
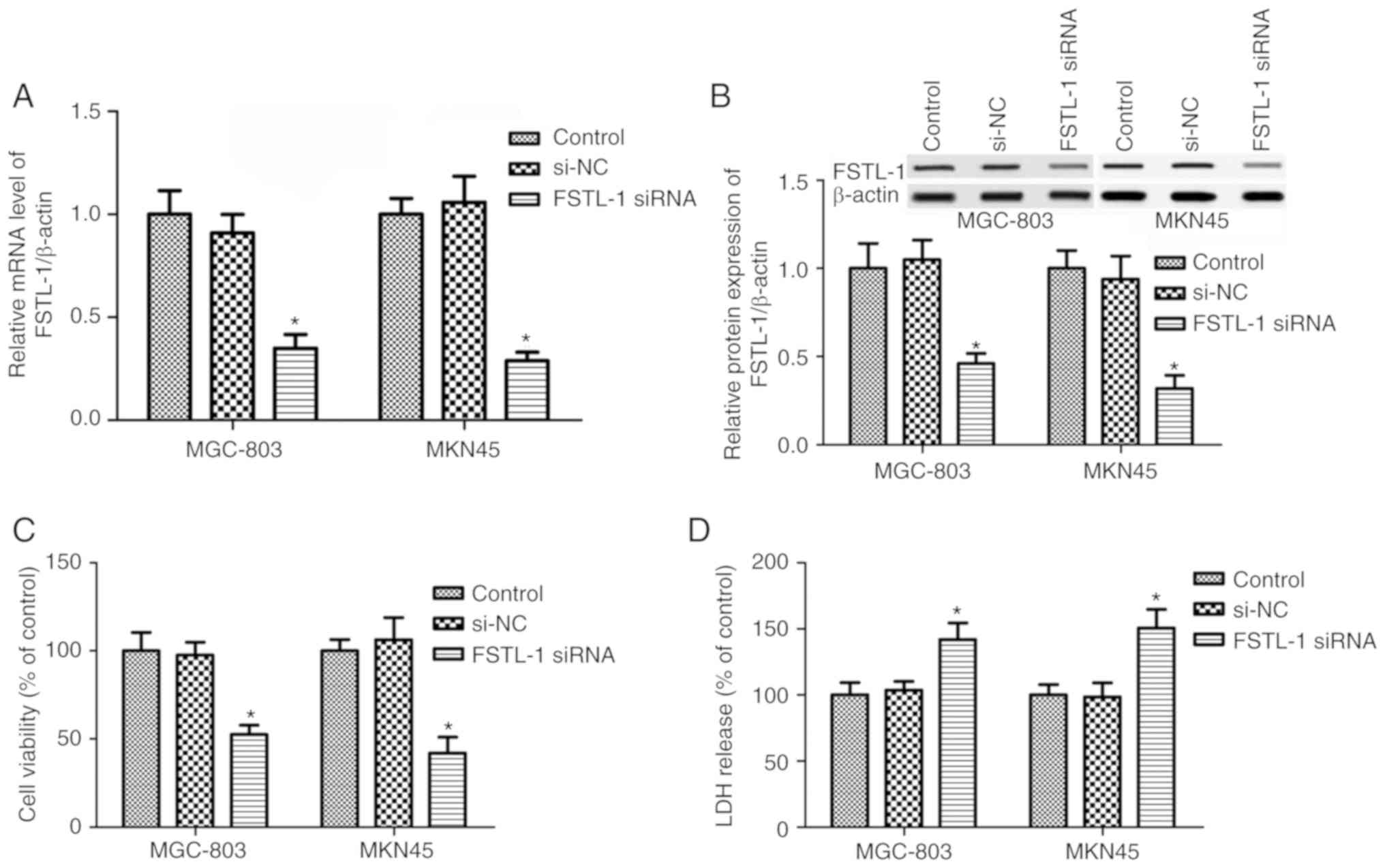
cells by transfection with FSTL-1 siRNA. The results suggested that
FSTL-1 mRNA and protein expression levels were decreased in MGC-803
and MKN45 cells post-transfection with FSTL-1 siRNA (P<0.05,
Fig. 2A and B). Subsequently, the
CellTiter-Glo® Luminescent Cell Viability assay was
performed to determine the effects of FSTL-1 depletion on MGC-803
and MKN45 cells. As shown in Fig. 2C,
compared with in the control and si-NC groups, FSTL-1 silencing
markedly reduced the viability of MGC-803 and MKN45 cells
(P<0.05). Further studies confirmed that FSTL-1 siRNA
transfection exhibited significant cytotoxic effects on MGC-803 and
MKN45 cells compared with the control group; there were no
significant differences between the si-NC group and the control
group (P>0.05, Fig. 2D).
FSTL-1 depletion promotes cell
apoptosis and mediates apoptosis-associated gene activity
Since FSTL-1 silencing inhibited MGC-803 and MKN45
cell viability and induced cytotoxic effects, the Cell Death
Detection ELISA assay and caspase-3/9 activity assay were performed
to determine the effects of FSTL-1 depletion on MGC-803 and MKN45
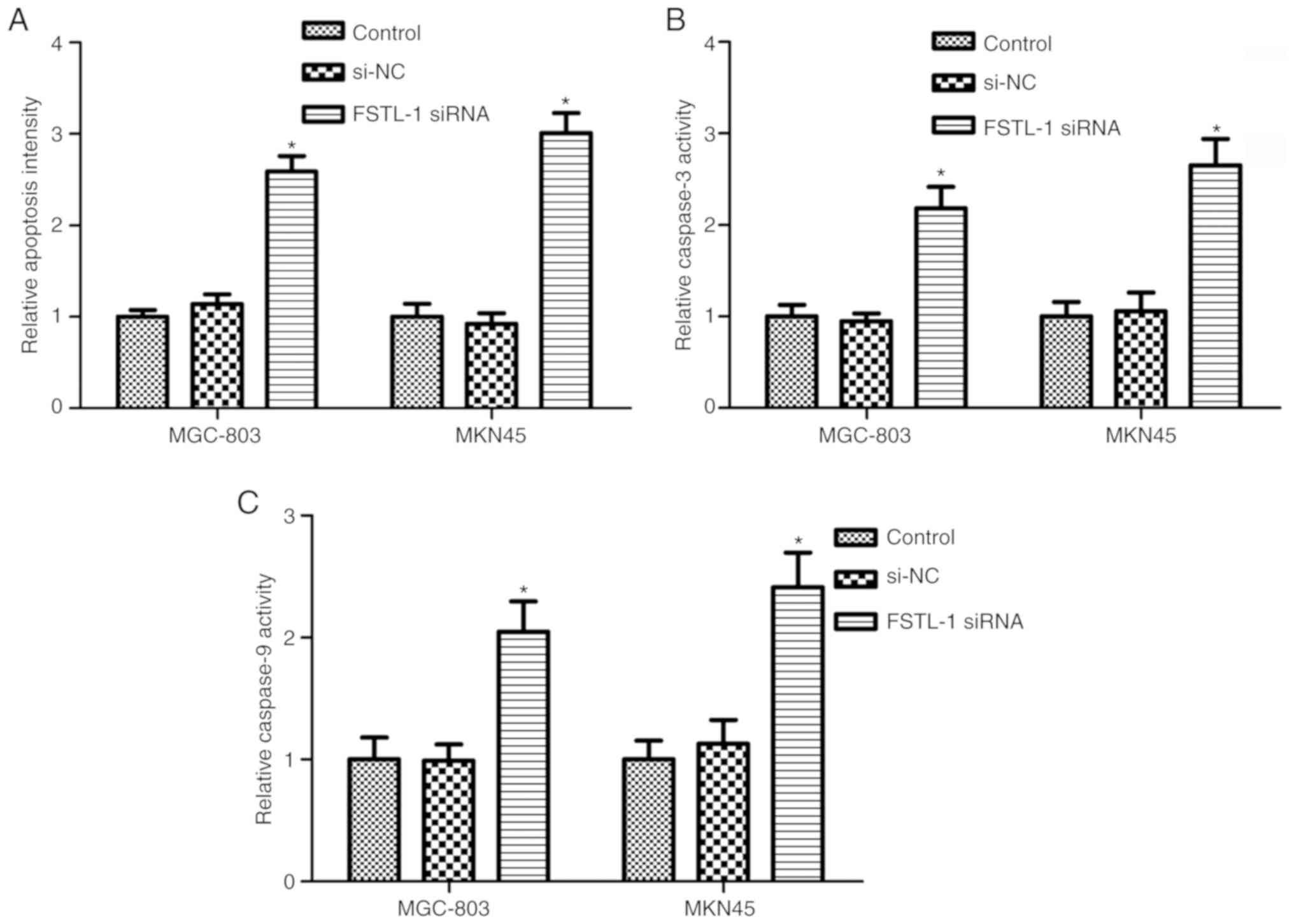
cell apoptosis. As shown in Fig. 3A,
FSTL-1 knockdown in MGC-803 and MKN45 cells for 48 h resulted in an
increase in the cell apoptotic ratio compared with in the control
group (P<0.05). To validate the results, caspase-3 and caspase-9
activity was determined. The results indicated that inhibiting the
expression of FSTL-1 markedly increased caspase-3 and caspase-9
activity in MGC-803 and MKN45 cells (P<0.05, Fig. 3B and C). These results suggested that
FSTL-1 may serve fundamental roles in gastric cancer cell survival
and apoptosis.
FSTL-1 silencing inhibits STAT6
phosphorylation in gastric cancer cells
FSTL-1 is an inflammatory cytokine regulator; in
addition, inflammatory cytokines can activate STAT6 (14,33).
Therefore, it was hypothesized that FSTL-1 may mediate STAT6
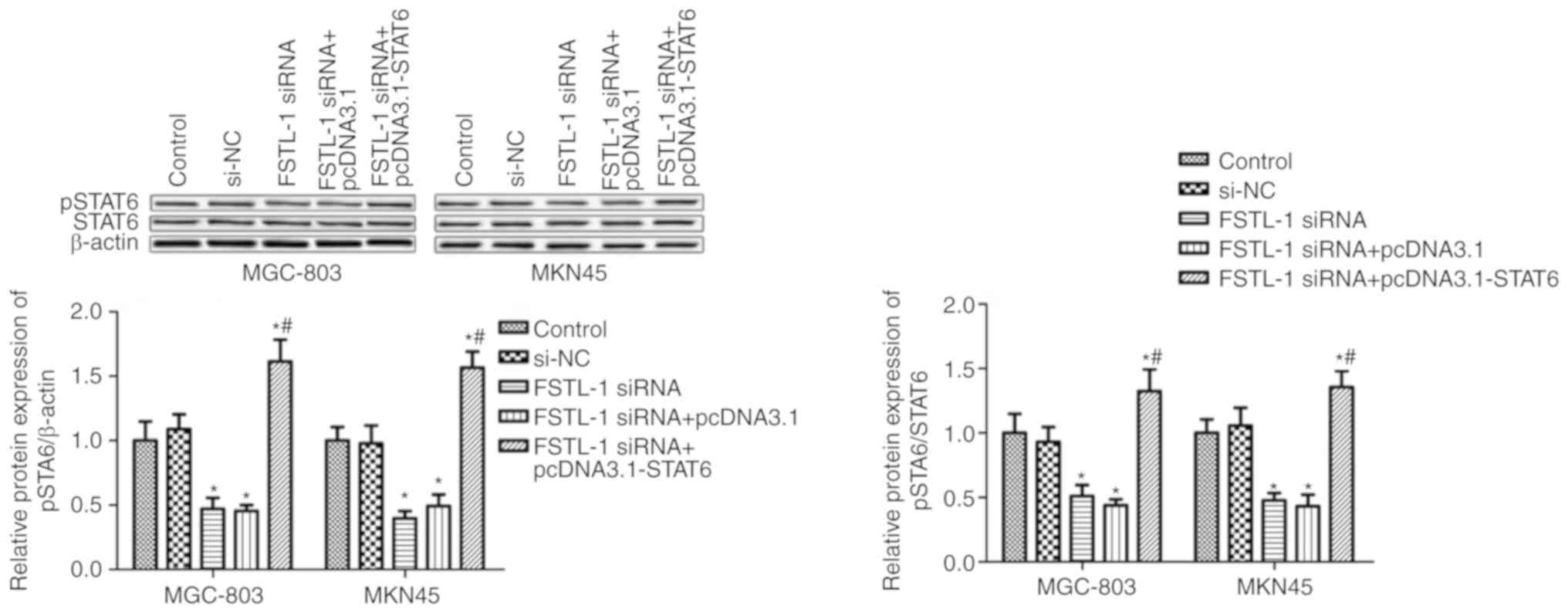
phosphorylation. The results of a western blot analysis indicated
that, compared with in the control group, FSTL-1 knockdown reduced
STAT6 phosphorylation in MGC-803 and MKN45 cells (P<0.05)
normalized to total STAT6 and the loading control β-actin, whereas
transfection with si-NC had no obvious effect on STAT6
phosphorylation (P>0.05, Fig. 4).
Furthermore, to confirm FSTL-1 knockdown decreased phosphorylation
of STAT6, total STAT6 levels were determined; the results revealed
that FSTL-1 depletion had no obvious effect on total STAT6 levels
(Fig. 4).
FSTL1 knockdown is implicated in
gastric cancer cell apoptosis via the STAT6 pathway
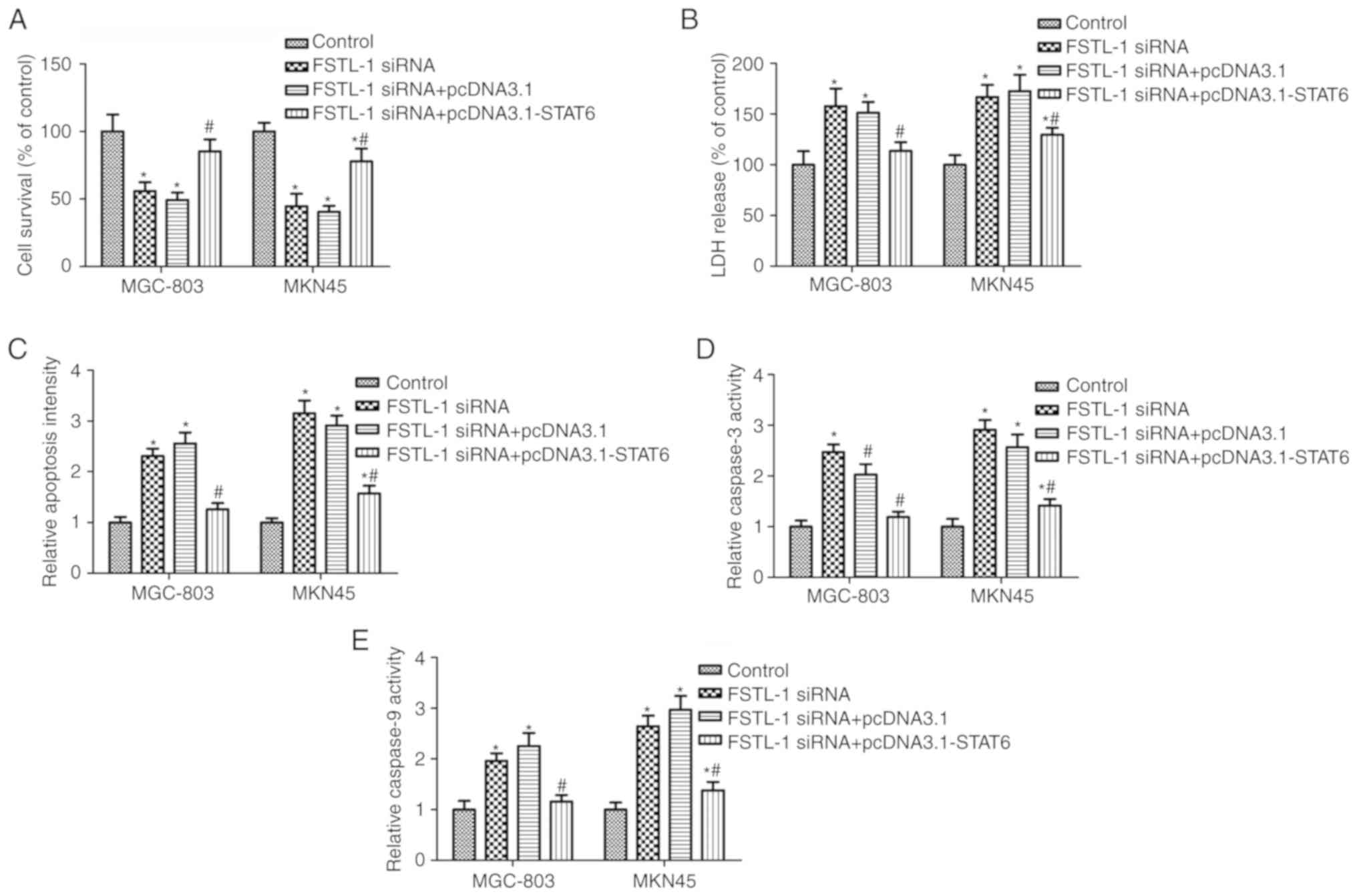
To determine whether STAT6 is involved in FSTL-1
knockdown-induced cell apoptosis, MGC-803 and MKN45 cells were
co-transfected with FSTL-1 siRNA and pcDNA3.1-STAT6. Western
blotting suggested that pcDNA3.1-STAT6 transfection increased STAT6
phosphorylation compared with in the FSTL-1 siRNA group (Fig. 4A). Transfection with FSTL1 siRNA
significantly inhibited MGC-803 and MKN45 cell survival and induced
cytotoxicity, both of which were rescued by pcDNA3.1-STAT6
transfection (Fig. 5A and B).
Furthermore, pcDNA3.1-STAT6 transfection reversed the effects of
FSTL-1 siRNA on MGC-803 and MKN45 cell apoptosis and caspase-3/9
activity (Fig. 5C-E). These results
indicated that FSTL1 knockdown induced cell apoptosis, which may be
dependent on inhibition of STAT6 phosphorylation.
Discussion
Gastric cancer is a common cause of
cancer-associated mortality worldwide (34). Early diagnosis is a main challenge for
cancer treatment, and molecular markers serve important roles in
cancer diagnosis and treatment. In the present study, FSTL1 was
revealed as a potential useful biomarker for gastric cancer
diagnosis and therapy.
The secreted glycoprotein FSTL-1 is associated with
several physiological and pathological processes, including
tumorigenesis (22,35). However, the function of FSTL-1 in
cancer progression remains controversial. A recent study
demonstrated that FSTL-1 knockdown with short hairpin RNA (shRNA)
sequences increases lung cancer cell growth, migration and
invasion, and decreases apoptosis (36). In addition, increasing FSTL-1 levels
via the melanoma antigen-A11 and androgen receptor increases growth
and progression of castration-resistant/recurrent prostate cancer
(37). Jin et al suggested
that FSTL-1 overexpression promotes glioma cell proliferation, cell
cycle progression and colony formation, whereas FSTL-1 depletion
inhibits these processes (25).
Furthermore, FSTL-1 has been revealed to be highly expressed in
patients with gastric cancer (1). The
present results were in accordance with some of these previous
reports; the findings confirmed that FSTL-1 expression was
increased in gastric cancer cells compared with in control cells,
and knockdown of FSTL-1 inhibited gastric cancer cell growth and
promoted apoptosis. It has been suggested that FSTL-1 antibodies
may be useful in FSTL-high cells to inhibit FSTL expression, and
previous evidence has suggested that FSTL plasmid-induced
overexpression or shRNA/siRNA knockdown studies could significantly
promote or suppress FSTL expression (25,38). We
aim to use FSTL-1 commercial recombinant proteins and antibodies to
study its function in future studies.
FSTL-1 has been reported to mediate inflammatory
cytokine expression and evidence has suggested that inflammatory
cytokines, such as IL-4, IL-5 and IL-13, may activate STAT6
(14,33). Furthermore, it has been demonstrated
that FSTL-1 is involved in dendritic cell-based immunity in
patients with nasopharyngeal carcinoma, and is associated with the
activation of STAT6 (29). Consistent
with these previous reports, the present study revealed that FSTL-1
knockdown in gastric cancer cells markedly suppressed the
activation of STAT6. STAT6 is a member of the STAT family, which
has fundamental roles in cancer, such as breast cancer (39), non-small-cell lung cancer (40), melanoma (27), hepatocellular carcinoma (41) and colon cancer (42). A recent study indicated that
inhibition of STAT6 activation serves inhibitory roles in gastric
cancer cell proliferation and invasion (43). In agreement with these earlier
reports, the present study demonstrated that STAT6 was involved in
the functions of FSTL-1 in gastric cancer cell growth and
apoptosis, and the effects of FSTL-1 knockdown were reversed with
STAT6 overexpression.
To the best of our knowledge, the present study is
the first to report on the function and mechanism of FSTL-1 in
gastric cancer development. In the present study, the effects of
FSTL-1 knockdown on cancer inhibition were only investigated in
vitro, and the lack of in vivo animal experiments is a
main limitation to the study. We aim to study the expression and
function of FSTL-1 in gastric cancer tissues and in a mouse model
in future studies. In conclusion, the present study revealed that
FSTL-1 was highly expressed in gastric cancer cells, and knockdown
of its expression induced tumor inhibitory effects via the
promotion of cell apoptosis and the suppression of cell survival.
STAT6 was also confirmed to be involved in the effects of FSTL-1 on
cancer progression.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XP designed the study and obtained data. PW, SL, YJ
and CW obtained and analyzed data. XP, SL and YJ wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim H, Eun JW, Lee H, Nam SW, Rhee H, Koh
KH and Kim H: Gene expression changes in patient-matched gastric
normal mucosa, adenomas, and carcinomas. Exp Mol Pathol.
90:201–209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Togo R, Ishihara K, Mabe K, Oizumi H,
Ogawa T, Kato M, Sakamoto N, Nakajima S, Asaka M and Haseyama M:
Preliminary study of automatic gastric cancer risk classification
from photofluorography. World J Gastrointest Oncol. 10:62–70. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Manzoni G, Marrelli D, Baiocchi GL,
Morgagni P, Saragoni L, Degiuli M, Donini A, Fumagalli U, Mazzei
MA, Pacelli F, et al: The Italian research group for gastric cancer
(GIRCG) guidelines for gastric cancer staging and treatment: 2015.
Gastric Cancer. 20:20–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brenner H: Long-term survival rates of
cancer patients achieved by the end of the 20th century: A period
analysis. Lancet. 360:1131–1135. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Werner M, Becker KF, Keller G and Höfler
H: Gastric adenocarcinoma: Pathomorphology and molecular pathology.
J Cancer Res Clin Oncol. 127:207–216. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tahara E: Genetic pathways of two types of
gastric cancer. IARC Sci Publ. 157:327–349. 2004.
|
|
9
|
Wei Q, Wang YN, Liu HY, Yang J, Yang CY,
Liu M, Liu YF, Yang P and Liu ZH: The expression and role of
activin A and follistatin in heart failure rats after myocardial
infarction. Int J Cardiol. 168:2994–2997. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shibanuma M, Mashimo J, Mita A, Kuroki T
and Nose K: Cloning from a mouse osteoblastic cell line of a set of
transforming-growth-factor-beta 1-regulated genes, one of which
seems to encode a follistatin-related polypeptide. Eur J Biochem.
217:13–19. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sylva M, Moorman AF and van den Hoff MJ:
Follistatin-like 1 in vertebrate development. Birth Defects Res C
Embryo Today. 99:61–69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Prakash S, Borreguero LJJ, Sylva M, Flores
Ruiz L, Rezai F, Gunst QD, de la Pompa JL, Ruijter JM and van den
Hoff MJB: Deletion of Fstl1 (follistatin-like 1) from the
endocardial/endothelial lineage causes mitral valve disease.
Arterioscler Thromb Vasc Biol. 37:e116–e130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kawabata D, Tanaka M, Fujii T, Umehara H,
Fujita Y, Yoshifuji H, Mimori T and Ozaki S: Ameliorative effects
of follistatin-related protein/TSC-36/FSTL1 on joint inflammation
in a mouse model of arthritis. Arthritis Rheum. 50:660–668. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miyamae T, Marinov AD, Sowders D, Wilson
DC, Devlin J, Boudreau R, Robbins P and Hirsch R: Follistatin-like
protein-1 is a novel proinflammatory molecule. J Immunol.
177:4758–4762. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bae K, Park KE, Han J, Kim J, Kim K and
Yoon KA: Mitotic cell death caused by follistatin-like 1 inhibition
is associated with up-regulated Bim by inactivated Erk1/2 in human
lung cancer cells. Oncotarget. 7:18076–18084. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong Y, Geng Y, Li L, Li X, Yan X, Fang Y,
Li X, Dong S, Liu X, Li X, et al: Blocking follistatin-like 1
attenuates bleomycin-induced pulmonary fibrosis in mice. J Exp Med.
212:235–252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ouchi N, Oshima Y, Ohashi K, Higuchi A,
Ikegami C, Izumiya Y and Walsh K: Follistatin-like 1, a secreted
muscle protein, promotes endothelial cell function and
revascularization in ischemic tissue through a nitric-oxide
synthase-dependent mechanism. J Biol Chem. 283:32802–32811. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Geng Y, Dong Y, Yu M, Zhang L, Yan X, Sun
J, Qiao L, Geng H, Nakajima M, Furuichi T, et al: Follistatin-like
1 (Fstl1) is a bone morphogenetic protein (BMP) 4 signaling
antagonist in controlling mouse lung development. Proc Natl Acad
Sci USA. 108:7058–7063. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sylva M, Li VS, Buffing AA, van Es JH, van
den Born M, van der Velden S, Gunst Q, Koolstra JH, Moorman AF,
Clevers H and van den Hoff MJ: The BMP antagonist follistatin-like
1 is required for skeletal and lung organogenesis. PLoS One.
6:e226162011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu J, Qi X, Gong J, Yu M, Zhang F, Sha H
and Gao X: Fstl1 antagonizes BMP signaling and regulates ureter
development. PLoS One. 7:e325542012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murakami K, Tanaka M, Usui T, Kawabata D,
Shiomi A, Iguchi-Hashimoto M, Shimizu M, Yukawa N, Yoshifuji H,
Nojima T, et al: Follistatin-related protein/follistatin-like 1
evokes an innate immune response via CD14 and toll-like receptor 4.
FEBS Lett. 586:319–324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou X, Xiao X, Huang T, Du C, Wang S, Mo
Y, Ma N, Murata M, Li B, Wen W, et al: Epigenetic inactivation of
follistatin-like 1 mediates tumor immune evasion in nasopharyngeal
carcinoma. Oncotarget. 7:16433–16444. 2016.PubMed/NCBI
|
|
23
|
Liu X, Liu Y, Li X, Zhao J, Geng Y and
Ning W: Follistatin like-1 (Fstl1) is required for the normal
formation of lung airway and vascular smooth muscle at birth. PLoS
One. 12:e01778992017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu Y, Tan X, Liu W, Chen X, Hou X, Shen
D, Ding Y, Yin J, Wang L, Zhang H, et al: Follistatin-like protein
1 plays a tumor suppressor role in clear-cell renal cell carcinoma.
Chin J Cancer. 37:22018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin X, Nie E, Zhou X, Zeng A, Yu T, Zhi T,
Jiang K, Wang Y, Zhang J and You Y: Fstl1 promotes glioma growth
through the BMP4/Smad1/5/8 signaling pathway. Cell Physiol Biochem.
44:1616–1628. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ihle JN: The Stat family in cytokine
signaling. Curr Opin Cell Biol. 13:211–217. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Son DJ, Jung YY, Park MH, Lee HL, Song MJ,
Yoo HS, Hwang DY, Han SB and Hong JT: Activated natural killer
cells mediate the suppressive effect of interleukin-4 on tumor
development via STAT6 activation in an atopic condition melanoma
model. Neoplasia. 19:537–548. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jayakumar A and Bothwell ALM: Stat6
promotes intestinal tumorigenesis in a mouse model of adenomatous
polyposis by expansion of MDSCs and inhibition of cytotoxic CD8
response. Neoplasia. 19:595–605. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang H, Huang S, Wu S, Yin S, Tang A and
Wen W: Follistatin-like protein-1 upregulates dendritic cell-based
immunity in patients with nasopharyngeal carcinoma. J Interferon
Cytokine Res. 37:494–502. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hussain S, Kodavanti PP, Marshburg JD,
Janoshazi A, Marinakos SM, George M, Rice A, Wiesner MR and
Garantziotis S: Decreased uptake and enhanced mitochondrial
protection underlie reduced toxicity of nanoceria in human
monocyte-derived macrophages. J Biomed Nanotechnol. 12:2139–2150.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yadav V, Varshney P, Sultana S, Yadav J
and Saini N: Moxifloxacin and ciprofloxacin induces S-phase arrest
and augments apoptotic effects of cisplatin in human pancreatic
cancer cells via ERK activation. BMC Cancer. 15:5812015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Miyake T, Miyake T, Sakaguchi M, Nankai H,
Nakazawa T and Morishita R: Prevention of asthma exacerbation in a
mouse model by simultaneous inhibition of NF-κB and STAT6
activation using a chimeric decoy strategy. Mol Ther Nucleic Acids.
10:159–169. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mimura K, The JL, Okayama H, Shiraishi K,
Kua LF, Koh V, Smoot DT, Ashktorab H, Oike T, Suzuki Y, et al:
PD-L1 expression is mainly regulated by interferon gamma associated
with JAK-STAT pathway in gastric cancer. Cancer Sci. 109:43–53.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shimasaki S, Koga M, Esch F, Cooksey K,
Mercado M, Koba A, Ueno N, Ying SY, Ling N and Guillemin R: Primary
structure of the human follistatin precursor and its genomic
organization. Proc Natl Acad Sci USA. 85:4218–4222. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ni X, Cao X, Wu Y and Wu J: FSTL1
suppresses tumor cell proliferation, invasion and survival in
non-small cell lung cancer. Oncol Rep. 39:13–20. 2018.PubMed/NCBI
|
|
37
|
Su S, Parris AB, Grossman G, Mohler JL,
Wang Z and Wilson EM: Up-regulation of follistatin-like 1 by the
androgen receptor and melanoma antigen-A11 in prostate cancer.
Prostate. 77:505–516. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hirsch R and Wilson DC: Follistatin-like
protein-1 as a biomarker for inflammatory disorders. US Patent
8,741,584. Filed February 4 2013; issued June 2 2014.
|
|
39
|
Binnemars-Postma K, Bansal R, Storm G and
Prakash J: Targeting the Stat6 pathway in tumor-associated
macrophages reduces tumor growth and metastatic niche formation in
breast cancer. Faseb J. 32:969–978. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pastuszak-Lewandoska D,
Domańska-Senderowska D, Kordiak J, Antczak A, Czarnecka KH,
Migdalska-Sęk M, Nawrot E, Kiszałkiewicz JM and Brzeziańska-Lasota
E: Immunoexpression analysis of selected JAK/STAT pathway molecules
in patients with non- small-cell lung cancer. Pol Arch Intern Med.
127:758–764. 2017.PubMed/NCBI
|
|
41
|
Qing T, Yamin Z, Guijie W, Yan J and
Zhongyang S: STAT6 silencing induces hepatocellular
carcinoma-derived cell apoptosis and growth inhibition by
decreasing the RANKL expression. Biomed Pharmacother. 92:1–6. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Leon-Cabrera SA, Molina-Guzman E,
Delgado-Ramirez YG, Vázquez-Sandoval A, Ledesma-Soto Y,
Pérez-Plasencia CG, Chirino YI, Delgado-Buenrostro NL,
Rodriguez-Sosa M, Vaca-Paniagua F, et al: Lack of STAT6 attenuates
inflammation and drives protection against early steps of
colitis-associated colon cancer. Cancer Immunol Res. 5:385–396.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lu G, Shi W and Zheng H: Inhibition of
STAT6/anoctamin-1 activation suppresses proliferation and invasion
of gastric cancer cells. Cancer Biother Radiopharm. 33:3–7. 2018.
View Article : Google Scholar : PubMed/NCBI
|