Introduction
Breast cancer is the most common cancer among
females in China, and is the second leading cause of
cancer-associated mortality within this population. Recurrence and
metastasis are the most important factors affecting the survival of
patients (1). Tumor cells depend on
the tumor microenvironment to complete the process of invasion and
metastasis. Macrophages are the main component of the tumor
microenvironment (1). In particular,
M2-type macrophages (also known as TAMs) account for ~30–50% of
inflammatory cells in the tumor stroma, and contribute to the
occurrence and development of breast cancer (2,3). Clinical
studies have linked the quantity and density of TAM to poor
prognosis in most tumors, including malignant melanomas and breast,
prostate, ovarian, cervical, and lung cancer (3). Clinically, TAMs mainly originate from
monocytes in the peripheral blood, and are recruited by chemokines
in the vicinity of the tumor, which influence the microenvironment
leading to tumor metastasis (4,5). For
example, TAMs secrete various extracellular matrix degradation
enzymes [such as tissue proteases, matrix metalloproteinases
(MMPs), and serine proteases] that degrade the extracellular matrix
and promote tumor metastasis (6).
C-C motif chemokine ligand 5 (CCL5) is a chemotactic
cytokine or chemokine that is widely secreted from natural killer
cells, T cells, fibroblasts, epithelial cells, and platelets
(7). In parallel, CCL5 contributes to
the recruitment and stimulation of these cells; however, CCL5 is
also secreted by certain tumor cells, such as malignant melanoma
cells (8) and ovarian (9), prostate (10), and breast cancer cells (11). The expression of CCL5 is lower in
normal breast epithelium cells, but higher in the primary breast
tumor site, local lymph nodes, and metastasis sites (11). Therefore, CCL5 must be expressed
during the process of malignant transformation in breast cancer
(11). Chemokine levels are also
positively correlated with the density of TAM and negatively
correlated with the prognosis of patients (11). Thus, blocking chemokine receptors or
inhibiting their production could inhibit the recruitment of
macrophages by tumor cells.
Nuclear factor (NF)-κB regulates the expression of
many chemokines. Out of these chemokines, CCL5 serves as the target
gene of NF-κB, promoting tumor development (12). NF-κB and hypoxia inducible factors-1α
(HIF-1α) may regulate the function of TAMs, being important for the
progression and metastasis of tumors (13). Therefore, the current study aimed to
detect the expression of CCL5 and its receptor in breast cancer
tissues and different breast cancer cell lines. The effects of CCL5
on the proliferation, apoptosis, migration, and invasion of breast
cancer cells was investigated by activating or silencing the
expression of CCL5. In addition, the chemotactic effect of CCL5 to
TAM was studied. Through these analyses, we explored molecular
mechanism of CCL5 in the interaction between breast cancer cells
and TAM. Our findings nay serve as a theoretical basis for
targeting TAMs during the treatment of breast cancer.
Materials and methods
Human tissue specimens
A total of 65 patients with complete clinical data
who underwent breast cancer surgery in The First Affiliated
Hospital of Xi'an Jiaotong University and The First Affiliated
Hospital of China Medical University from 2010 to 2012 were
evaluated. Samples were excluded if the patients received any
preoperatively adjuvant chemotherapy, radiotherapy or hormone
therapy. All patients were female, aged 29–73 years old. The
tumor-node-metastasis (TNM) stage of the surgical tissue samples
was determined according to the American Join Committee on Cancer
(6th version, 2002) (14). The
survival status of all patients was determined from the clinical
medical records and by telephone follow-up.
Cell lines and culture
The human breast cancer cell lines MCF-7,
MDA-MB-231, SK-BR-3, and T47D were derived from Shanghai Cell Bank,
Chinese Academy of Science (Shanghai, China). All cells were
cultured in RPMI-1640 medium that was supplemented with 10% (v/v)
fetal bovine serum (FBS; Biological Industries) and 1% (v/v)
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.) at
37°C in a humidified incubator with 5% CO2.
Cell co-culture
Two ml cell suspension (density 5×104
cells/ml) of MCF-7/small interfering RNA (siRNA)-negative control
(siNC) or MCF-7/siCCL5 cells were inoculated into 6-well plates and
incubated at 37°C for 24 h. Then, 1 ml cell suspension of TAMs
(density 1×106/ml) induced from THP-1 cells (derived
from Kunming Cell Bank, Chinese Academy of Sciences) were
inoculated in the upper chamber of the co-culture chamber. To
further verify that the migration and invasion of tumor cells
induced by co-culture of MCF-7 with TAM were caused by the
activation of NF-κB signaling, the co-culture was pre-treated with
PDTC (cat. no. S1808; Beyotime Institute of Biotechnology), a
specific blocking reagent of NF-κB. The optical density (OD) value,
as determined by an MTT assay, indicated the proliferation index of
cells in different groups: MCF-7/siNC group, MCF-7/siNC + TAM
group, MCF-7/siCCL5 group, and MCF-7/siCCL5 + TAM group.
TAM in vitro model
In this study, human monocyte leukemia cell line
(THP-1) was selected to obtain a TAM cell model in vitro.
THP-1 cells were seeded at 1×106 cells/well into 6-well
plates, and phorbol-12-myristate-13-acetate (PMA) was added into
the well to make its final concentration of 320 nmol/l and
incubated at 37°C for 6 h. Then, 20 ng/ml IL-4 and IL-13 was added
at 37°C for 18 h, to obtain M2 macrophages (also known as TAM)s.
This group was hence defined as PMA+IL4/IL-13 group. Cells treated
with PMA only for 24 h were defined as the PMA group.
Flow cytometry
Cells of THP-1 group, PMA group and PMA + IL-
4/IL-13 group were trypsinized and centrifuged at 4°C at 300 × g
for 5 min, then washed with PBS twice and centrifuged at 4°C at 300
× g for another 5 min. A total of 1×106 cells were
suspended with 100 µl PBS before 10 µl CD204 antibody or 5 µl CD206
antibody was added, and incubated in the dark at 37°C for 60 min.
Finally, cells were suspended in 500 µl PBS and detected by flow
cytometry.
CCL5 RNA interference (RNAi)
CCL5 specific siRNA was purchased from Qiagen, Inc.
The CCL5 siRNA sequence was: Sense 5′-GAAGAAGUGGGUUCAAGAATT-3′ and
antisense: 5′-UUCUUGAACCCACUUCUUCTT-3′. The NC sequence was: Sense
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense:
5′-ACGUGACACGUUCGGAGAATT-3′. Transfection was performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Cells (5×104 per well) were seeded in
twelve-well plates 24 h prior to transfection and transfected with
80 pM siRNA. The effect of siRNA treatment on secretion and
expression of CCL5 was determined by ELISA and quantitative
real-time PCR 24 or 48 h post-transfection.
MTT assay
Cells in the logarithmic growth stage were digested
by 0.25% trypsin and were then resuspended in complete medium. Cell
number was counted and adjusted, and 100 µl of MDA-MB-231
(5×104 cells/ml) or MCF-7 (1×105 cells/ml)
cell suspension was inoculated in 96-well plates. Then, the old
culture medium was discarded and the cells were gently washed with
PBS. Then, 100 µl 5% FBS (Biological Industries) containing medium
was added with different concentrations of exogenous CCL5 (5, 10,
20, 30, 40 and 50 ng/ml, PeproTech, Inc.) to each group. After
incubation at 37°C for 24 or 48 h, 20 µl of MTT reagent (5 mg/ml)
was added to each well. Incubation of the cells was continued at
37°C for 4 h. Next, 100 µl dimethyl sulfoxide was added to each
well after the supernatant was discarded. The wells were oscillated
for 5–10 min to dissolve the crystals fully. The OD value of each
well was measured at 492 nm with a multi-function microplate
reader.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from breast cancer cells or
specimens using TRIzol reagent (Thermo Fisher Scientific, Inc.).
cDNA was synthesized using a PrimeScript RT reagent kit (Fermentas;
Thermo Fisher Scientific, Inc.). RT was performed on the PCR
amplification instrument at 37°C for 15 min, 85°C for 5 sec, 4°C
maintained to terminal. The synthesized cDNA was stored at −20°C.
qPCR was carried out according to the manufacturer's instructions
for the SYBR® Green PCT kit (Takara Bio, Inc.). The
primers used in the present study were: CCL5 sense,
5′-ACACCCTGCTGCTTTGCCTACA-3′, antisense,
5′-TCCCGAACCCATTTCTTCTCTG-3′, GAPDH sense,
5′-ACCACAGTCCATGCCATCAC-3′, antisense, 5′-TCCACCACCCTGTTGCTGTA-3′;
C-C chemokine receptor type 5 (CCR5) sense,
5′-ATCACTTGGGTGGTGGCTGTGTTTG-3′, antisense:
5′-CCCTGTGCCTCTTCTTCTCATTTCG-3′; CCR1 sense,
5′-ACCACAGAGTTTGACTATGGGGATG-3′, antisense,
5′-AGGGAAGCGTGAACAGGAAGAGCAG-3′; CCR3 sense,
5′-GAGACTGAAGAGTTGTTTGAAGAGA-3′, antisense,
5′-GATTGATAGGAAGAGAGAAGGATAG-3′ Blank controls with no cDNA
templates were used to rule out contamination. The thermocycling
conditions were as follows: 95°C for 30 sec, followed by 40 cycles
of 95°C for 5 sec, 60°C for 30 sec, one cycle of 95°C for 15 sec,
60°C for 30 sec and 95°C for 15 sec. The specificity of the PCR
product was confirmed by melting curve analysis. At the extension
stage of each cycle, the value of the quantification threshold
cycle (Ct) value was recorded. GAPDH was used as an internal
control. The relative expression level of the target genes was
assessed using the ΔΔCq method.
Enzyme-linked immunosorbent assay
(ELISA)
To quantify the secretion of CCL5, cells were
cultured in normal medium. The supernatant was collected after 24,
48, and 72 h incubation. The secretion of CCL5 was quantified using
commercial Human RANTES and CCL5 ELISA kits (R&D Systems, Inc.)
following the manufacturer's instructions.
Cell migration and invasion assay
A cell migration assay was carried out using a
24-well chamber. In total, 5×103 cells/well of
MDA-MB-231 and 1×104 cells/well of MCF-7 were suspended
in the upper chamber of the well in 200 µl RPMI-1640 with no serum.
Then, 600 µl RPMI-1640 containing 10% FBS with rhCCL5 (R&D
Systems, Inc.) at different concentrations (0, 10, 20 ng/ml) was
loaded in the bottom chamber. After incubation at 37°C for 18 h
(MDA-MB-231 cells) or 24 h (MCF-7 cells), the Transwell chambers
were fixed in 4% formaldehyde for 30 min and stained with 0.01%
crystal violet at room temperature for 20 min. Non-migrating cells
were carefully removed from the upper surface of the inside well.
Cells that had migrated to the bottom surface of the filter were
counted. Five random high-power fields were visualized using an
inverted phase-contrast microscope at ×200 magnification. The means
were obtained for the statistical analysis. Invasion assays were
performed in a similar manner to migration assays, except that the
cells were placed in the upper chamber with a Matrigel-coated
membrane. After incubation at 37°C for 36 h (MDA-MB-231 cells) or
48 h (MCF-7 cells), fixation and staining were performed.
Western blot analysis
Cells were harvested, and total protein was
extracted from the cell lines. Nuclear protein was isolated by
using a Nuclear Extraction kit, according to the manufacturer's
instructions (Pioneer Biotechnology, Inc.), and protein
concentrations were determined using the BCA Protein Assay kit
(Pierce; Thermo Fisher Scientific, Inc.). Equal amounts of protein
(150 µg) were separated by 10% SDS-PAGE and transferred to a
polyvinylidene difluoride (PVDF) membrane (Roche Diagnostics,
Inc.). After blocking with 5% (w/v) skim milk powder dissolved in
TBST at 37°C for 1 h, the PVDF membrane was incubated with the
primary antibody at 4°C overnight. The following primary antibodies
(1:1,000) were used: Anti-β-actin antibody (sc-47778, Santa Cruz,
Biotechnology, Inc.), anti-CCR5 antibody (PA1-41303, Invitrogen;
Thermo Fisher Scientific, Inc.), anti-E-cadherin antibody (cat. no.
4065, Cell Signaling Technology, Inc.), anti-N-cadherin antibody
(ab76057, Abcam), anti-Vimentin antibody(cat. no. 5741, Cell
Signaling Technology, Inc.), anti-Occludin antibody (71–1500,
Invitrogen; Thermo Fisher Scientific, Inc.), anti-NF-κB p65
antibody(3034, Cell Signaling Technology, Inc.), and anti-MMP9
antibody (ab73734, Abcam). After extensive washing using TBST, the
membrane was incubated with the HRP-conjugated secondary antibodies
(1:8,000; goat anti-rabbit, sc-2004; goat anti-mouse, sc-2005) at
room temperature for 1 h. Protein bands were visualized with an
enhanced chemiluminescence reagent (EMD Millipore), and were
analyzed with Quantity One 6.0 software (Bio-Rad Laboratories,
Inc). The relative expression of the target protein was expressed
with the grayscale value of the target protein/β-actin.
Xenograft tumor model
Eight female athymic nude mice (4 weeks old; 18–20
g) were purchased from Shanghai Silaike Laboratory Animal Co., Ltd.
Mice were raised in the specific-pathogen free grade experimental
animal center of the Medical School of Xi'an Jiaotong University.
The experimental process was approved by the ethics committee of
Medical and Biological Research of Xi'an Jiaotong University. MCF-7
cells were orthotopically injected into the mammary fat pads of 5
week-old female nude mice. Tumor growth was observed every 2 to 3
days. When the tumors reached 100 mm3 in size, the mice
were randomly separated into two groups. The experimental group was
intraperitoneally injected with 0.1 ml anti-CCL5 neutralizing
antibody (1 µg/ml, ProteinTech Group, Inc.) every 3 days, while the
control group was administered the same volume of RPMI-1640 medium.
After 30 days, the animals were sacrificed, and the tumors were
isolated for immunohistochemical staining. Tumor volume=(a ×
b2)/2, where a denotes the longest diameter and b
denotes the shortest diameter of the tumor.
Immunohistochemistry
Paraffin-embedded tissues were retrieved from the
Department of Pathology of The First Affiliated Hospital of Xi'an
Jiaotong University and The First Affiliated Hospital of China
Medical University, and 4 µm tissue sections were prepared.
Paraffin sections were deparaffinized in xylene and were rehydrated
through graded alcohols. Antigen retrieval was performed on
sections heated in a citrate buffer (pH=6.0) for 5 min at 92°C, and
were then cooled naturally at room temperature. The sections were
rinsed with TBS after endogenous peroxidase was inactivated with 3%
hydrogen peroxide. After protein blocking with 10% goat serum
(Abcam) at room temperature for 30 min, sections were incubated
with primary antibodies (all 1:100) against human CCL5 (cat. no.
P230E; Invitrogen; Thermo Fisher Scientific, Inc.), F4/80, CD16,
and CD206 (cat. nos. bs-11182R, bs-6028R and bs-21473R,
respectively; Beijing Bioss Biotechnology, Ltd.) overnight at 4°C
and rinsed with TBS. Next, the sections were incubated with
secondary antibody for 15 min at 37°C, followed by incubation with
streptavidin-HRP (Dako; Agilent Technologies, Inc.) for 15 min at
37°C, and rinsing with TBS. Peroxidase reactivity was visualized
using diaminobenzidine (Dako; Agilent Technologies, Inc.) at room
temperature for 3–5 min. Finally, the sections were counterstained
with hematoxylin at room temperature for 30 sec and mounted. The
number of macrophages in tumor stroma treated with anti-CCL5
neutral antibodies were counted. The positive cell count method was
adopted to observe the densest expressed areas of positive cells in
tumor stroma under a low power light microscope. Five
representative areas were selected from each section, and the
number of macrophages was counted and averaged under a 400-power
microscope.
Statistical analysis
All data were expressed as mean ± standard
deviation. Data were analyzed by a Student's t-test or One-way
analysis of variance (ANOVA) as appropriate. The Least Significant
Difference post-hoc test was used for the post-hoc test following
the ANOVA. The relationship between CCL5 protein expression and
patient age, lymph node status, and TNM stage was evaluated using a
χ2 test. P<0.05 was considered to indicate a
statistically significant difference. SPSS v 17.0 software (SPSS
Inc.) was used. All experiments were repeated independently at
least three times.
Results
Expression of CCL5 in breast cancer
tissues, and its relationship with clinical pathology and
prognosis
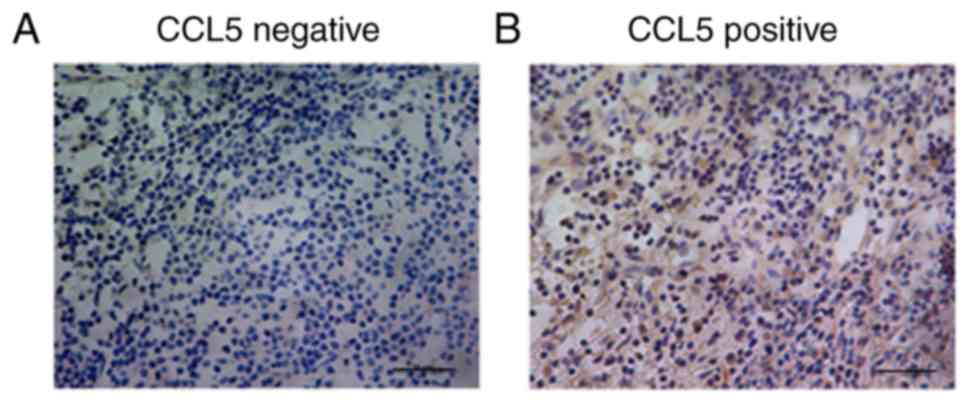
Different expression levels of CCL5 were detected in
the cytoplasm of breast cancer cells (Fig. 1). The association between the
expression level of CCL5 and the clinicopathological features of
breast cancer was analyzed using the immunohistochemical results
(Table SI). Stratification analysis
showed that there was no significant association between CCL5
expression and patient age, hormone receptor status, or human
epidermal growth factor-2 status. However, CCL5 expression was
significantly associated with lymph node status and TNM stage
(P<0.05; Table SI). Thus, CCL5
may be involved in the invasion and metastasis of breast
cancer.
Expression of CCL5 and its receptor in
different breast cancer cell lines, and the secretion of CCL5
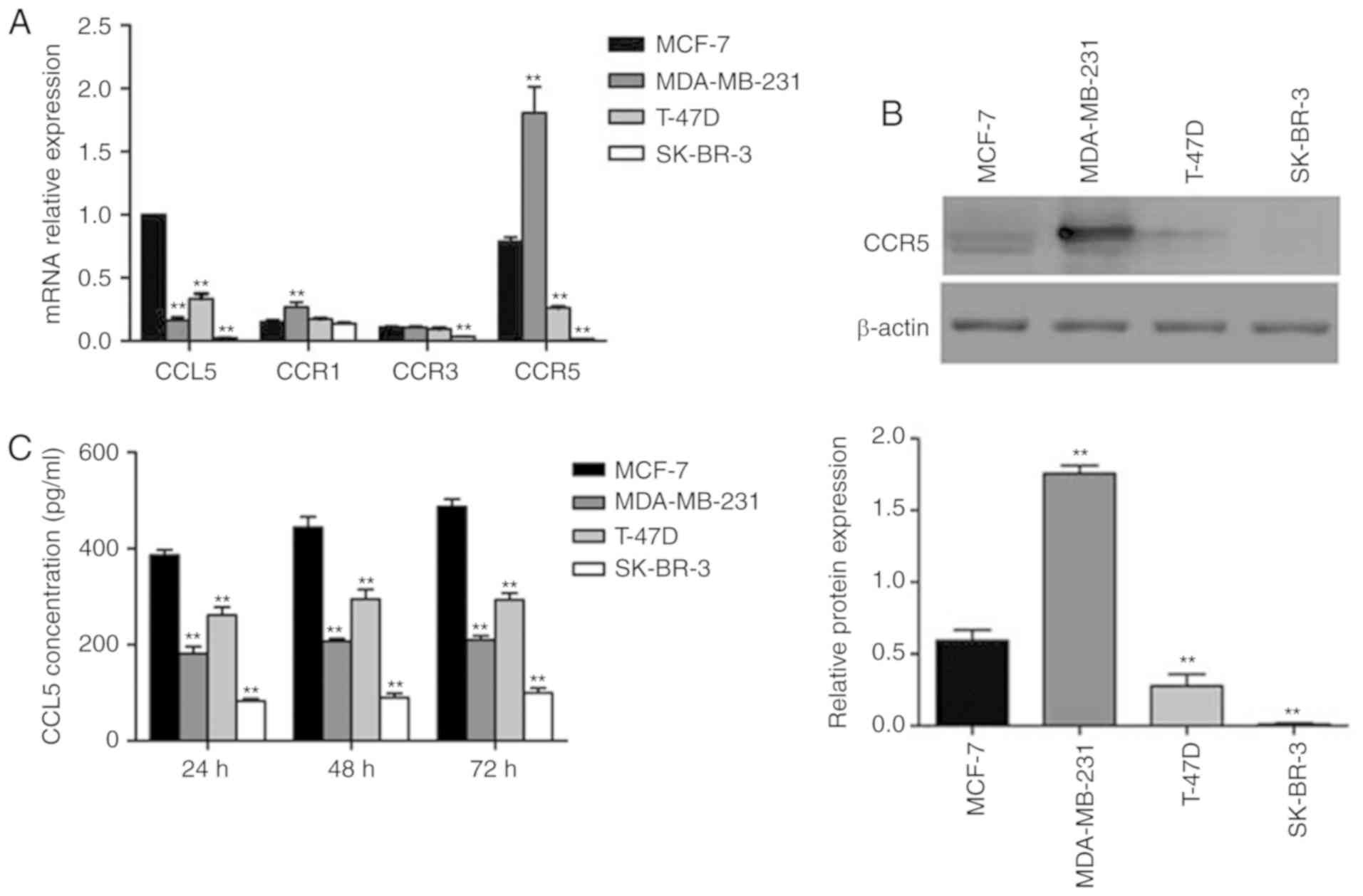
RT-qPCR was used to determine the levels of CCL5
expression and its receptor in four types of human breast cancer
cell lines (MCF-7, MDA-MB-231, SK-BR-3, T-47D). CCL5 expression was
the highest in MCF-7, and lowest in SK-BR-3 cells (Fig. 2A). CCR1 and CCR3 expression was
expressed at relatively low levels in all four cell lines, while
high CCR5 expression was reported in MCF-7 and MDA-MB-231 cell
lines. As a chemokine ligand, CCL5 must combine with the
corresponding receptor to function; thus, the relative levels of
CCR5 was characterized by Western blot assay. Significantly
increased expression of CCR5 was detected in MDA-MB-231 cells
compared with MCF-7 cells (P<0.05; Fig. 2B). This result was consistent with the
results of mRNA analysis. CCL5 secretion was detected by ELISA,
which revealed that MCF-7 cells secreted the most CCL5, while the
other three cell lines secreted less (Fig. 2C). CCL5 secretion increased over time
(Fig. 2C). In the present study, the
association between the constitutive expression of CCL5 in human
breast cancer cell lines was investigated. However, the expression
levels of CCL5 in breast cancer cells were not consistent with the
invasion ability of cell lines. The possible reason is that the
expression of CCL5 is affected by certain factors existing around
these cells, since the baseline constitutive expression of CCL5
mRNA in these four breast cancer cells was detected without any
induction in our study. Therefore, this may not reflect the real
ability of different breast cancer cells to express CCL5. We aim to
conduct a series of experiments in the future to investigate the
migration and invasion of SK-BR-3 cells, which have lower
expression levels of CCL5 and CCR5, to serve as a negative
control.
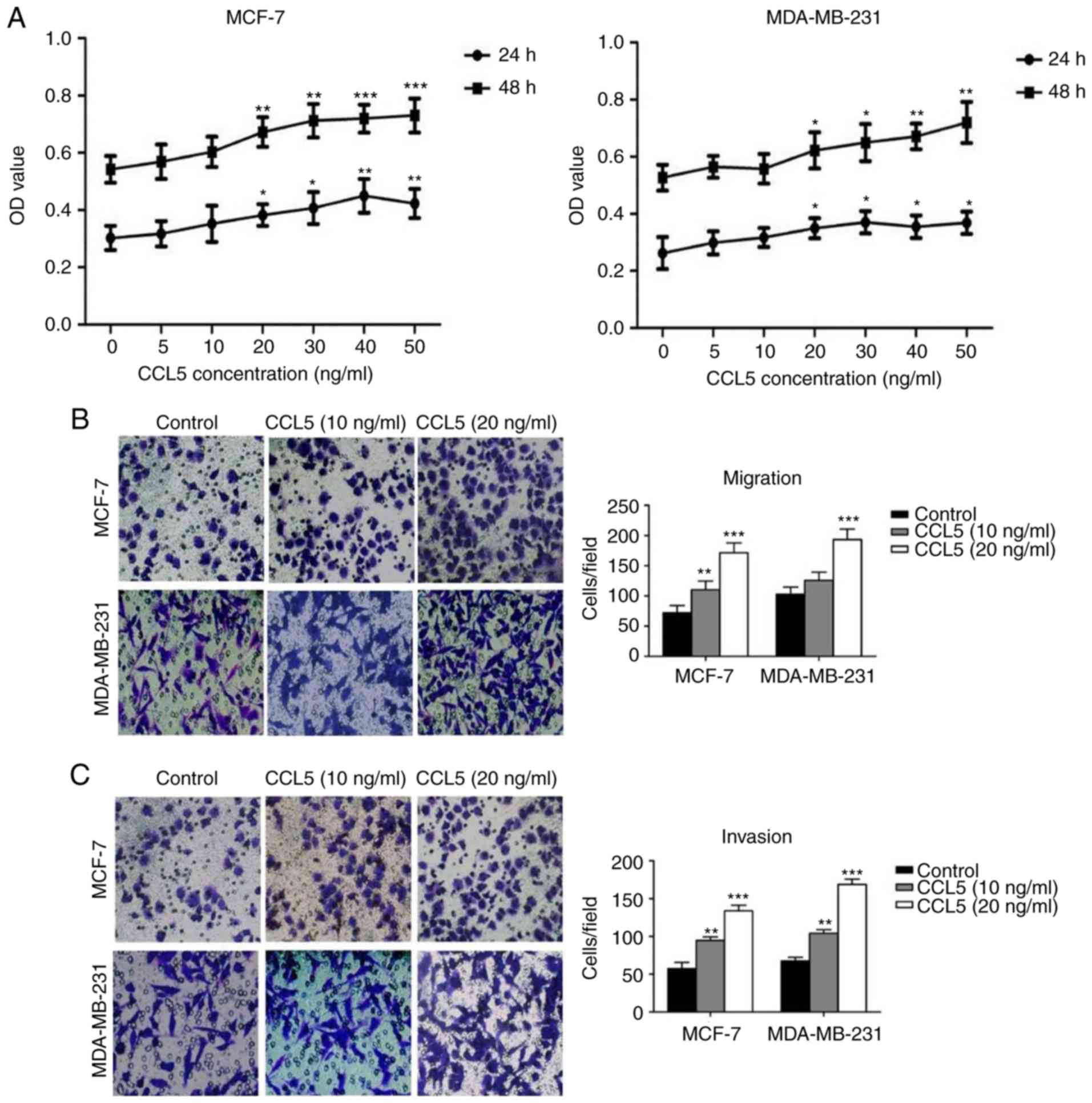
CCL5 promotes the proliferation,
migration, and invasion of breast cancer cells
An MTT assay was used to detect the effect of
different concentrations of CCL5 on cell proliferation. The
proliferation of MCF-7 and MDA-MB-231 cells increased significantly
following treatment with ≥20 ng/ml CCL5 compared with the blank
control (P<0.05; Fig. 3A). Cell
migration and invasion assays showed that the migration and
invasion capacity of MCF-7 and MDA-MB-231 cells were enhanced when
exogenous CCL5 was added (Fig. 3B and
C). When 10 ng/ml CCL5 was added, both the migration and
invasion capacity of MCF-7 cells were significantly enhanced
(P<0.05), while the migration of MDA-MB-231 cells was not;
invasion was significantly enhanced (P<0.01; Fig. 3C). When 20 ng/ml CCL5 was added, the
migration and invasion capacity of the two cell lines were
significantly increased compared with the control (P<0.001;
Fig. 3B and C). Thus, CCL5 was
proposed to promote the proliferation, migration and invasion
capabilities of MCF-7 and MDA-MB-231 cells, especially at 20 ng/ml
or greater.
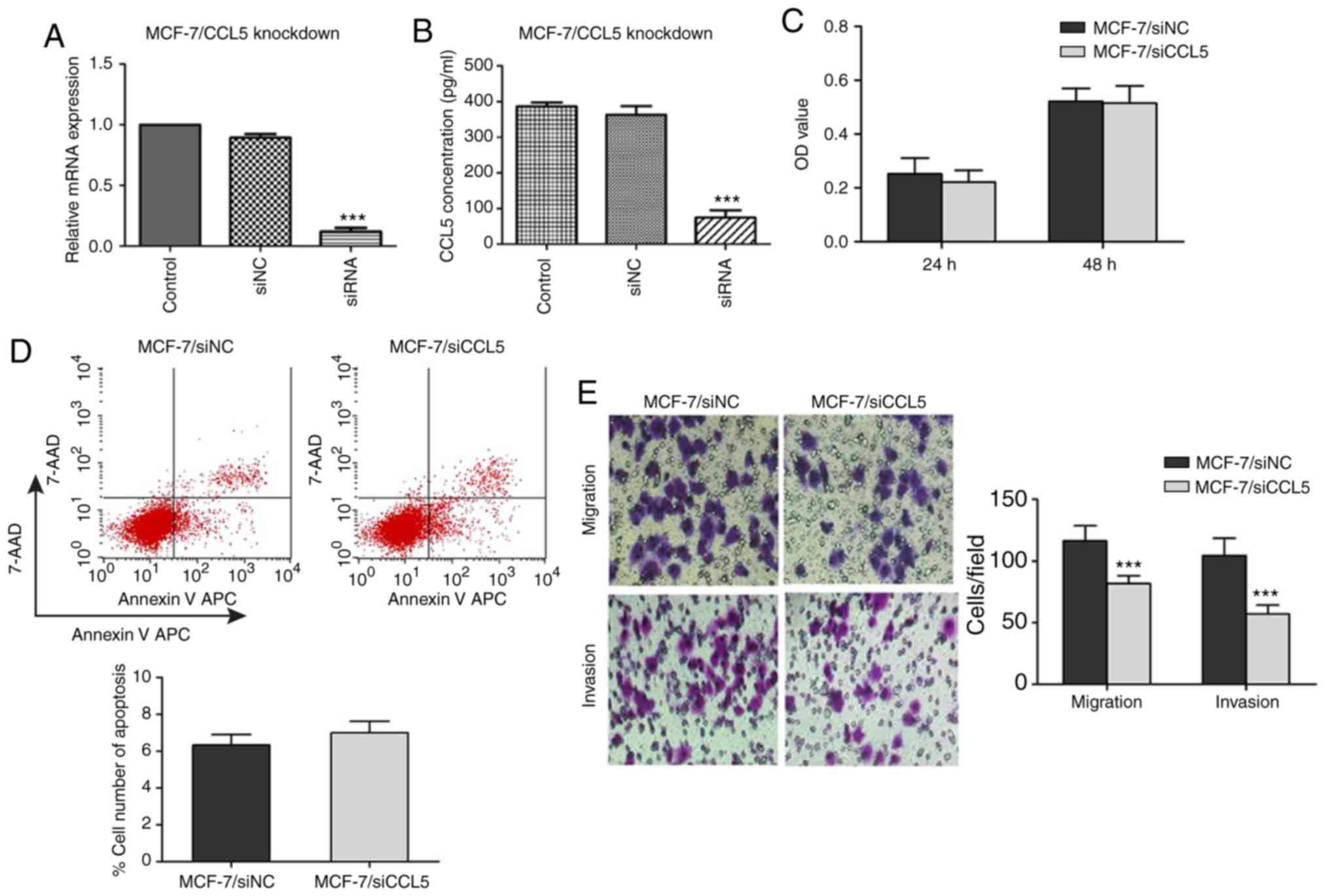
Inhibition of CCL5 affects different
biological behaviors in MCF-7 cells
To verify the inhibitory effect of siRNA on CCL5
expression in MCF-7 cells, RT-qPCR and ELISA were used to detect
the mRNA expression of CCL5 and CCL5 secretion in the supernatant.
Compared with the control and siNC groups, CCL5 mRNA expression and
CCL5 secretion in supernatant significantly decreased in the siCCL5
group (P<0.001; Fig. 4A and
B).
An MTT assay was used to detect the proliferation of
MCF-7 cells after CCL5 interference. When the CCL5 gene was
silenced, there was no statistical difference in the OD values at
24 or 48 h compared with the NC group (P>0.05; Fig. 4C). Flow cytometry showed that, after
inhibiting CCL5 expression, the early apoptosis of MCF-7/siCCL5
group cells was not significantly different to that of the
MCF-7/siNC group (6.99±0.62 vs. 6.33±0.58%, P>0.05; Fig. 4D). The Transwell assay demonstrated
that, when inhibiting CCL5 expression, the migration and invasion
capacities of MCF-7/siCCL5 cells were significantly reduced
compared with the control (P<0.001; Fig. 4E). CCL5 expression in MCF-7 was
significantly inhibited by RNA interference; the proliferation and
apoptosis of MCF-7 was not markedly affected when CCL5 was
downregulated, whereas the migration and invasion capacity of MCF-7
were significantly inhibited.
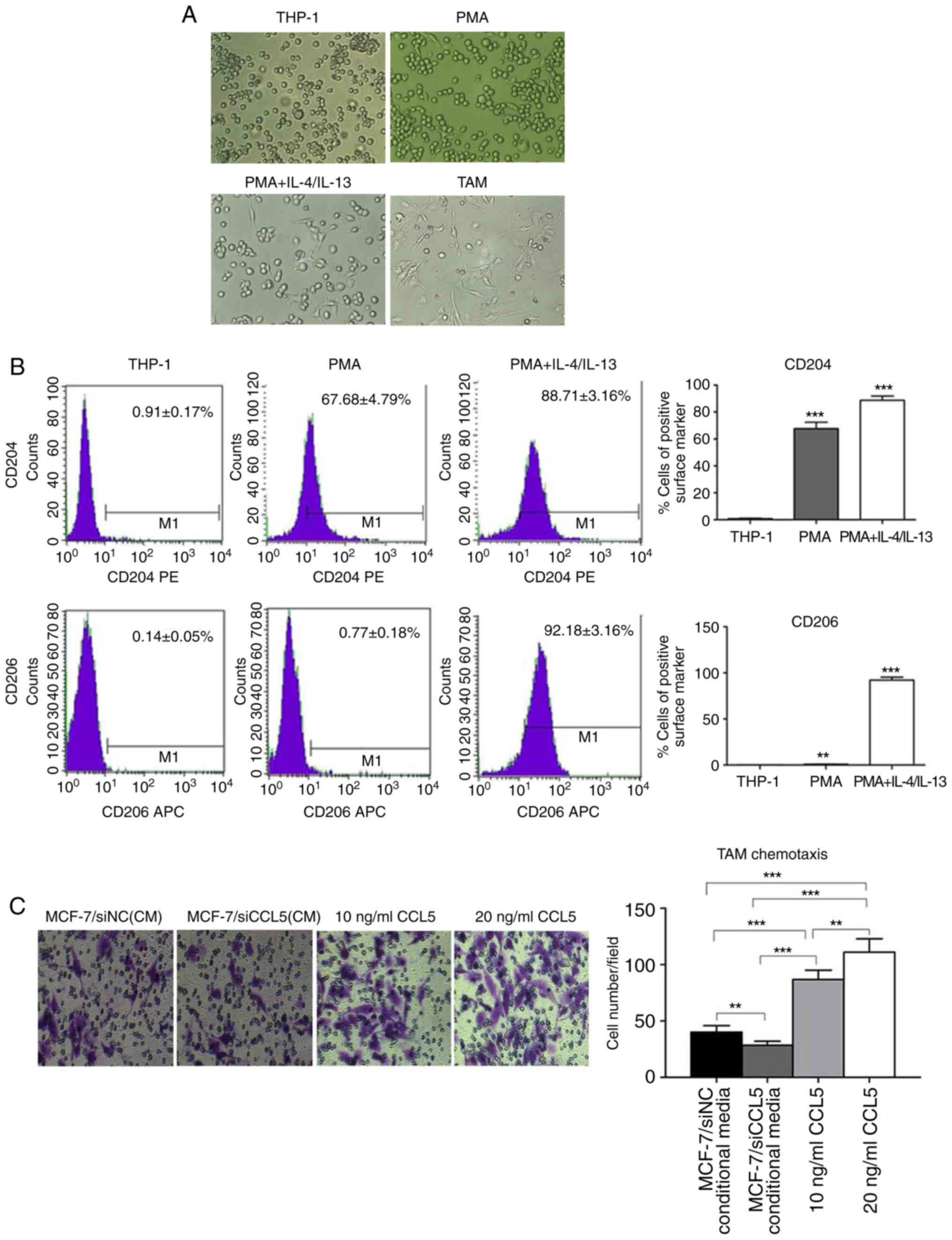
Generation of TAMs in vitro
Currently, methods for obtaining TAMs include the
isolation macrophages from the bone marrow of tumor-bearing mice,
or human monocyte THP-1 and mouse monocytes U937 induced by Th2
factor (15). THP-1 is a stable cell
line, which can exhibit M2 phenotypic characteristics after
induction with PMA and IL-4/IL-13 in vitro, and is
recognized as a good model cell to simulate TAMs. In the present
study, the human monocyte leukemia cell line THP-1 was selected to
obtain TAMs in vitro. CD16 was employed as the molecular
marker of M1-type macrophages, and CD206 as the molecular marker of
M2-type macrophages. Morphological observations revealed that THP-1
cells grew in suspension with a round shape, while some cells grew
in clusters and forming grape-like clusters under normal conditions
(Fig. 5A). After adding 320 nmol/l
PMA for 24 h, the morphology of THP-1 cells changed; some were
larger with a long fusiform shape and stretched out pseudopods,
presenting macrophage morphology (Fig.
5A).
After 320 nmol/l PMA was added for 6 h, THP-1 cells
adhered to the cell wall. When 20 ng/ml IL-4 and IL-13 were added
for 18 h, the shape of THP-1 cells became irregular, with most
extending their pseudopods, namely TAMs (Fig. 5A). After induction, TAMs were cultured
for 24 h, with most cells being irregular in shape with multiple
pseudopod protrusions (Fig. 5A). Flow
cytometry showed that, in the untreated THP-1 cells, the cell
surface expression was 0.91±0.17% for CD204 and 0.14±0.05% for
CD206. However, the cell surface of the PMA group was 67.68±4.79%
for CD204 and 0.77±0.18% for CD206. In the PMA + IL-4/IL-13 group,
CD204 expression was 88.71±3.16%, while that of CD206 was
92.18±3.16% (Fig. 5B). In comparison,
CD204 and CD206 expression on the cell surface of the PMA +
IL-4/IL-13 group was significantly higher than THP-1 (P<0.0001;
Fig. 5B), suggesting the successful
induction of TAMs.
A Transwell assay was used to detect CCL5-induced
chemotaxis of TAM (Fig. 5C). The
chemotaxis potential of MCF-7/siCCL5-conditioned medium to TAMs was
significantly weak compared with that of MCF-7/siNC. The complete
medium containing CCL5 exerted significant chemotactic ability to
TAMs compared with the control (P<0.01; Fig. 5C). Thus, CCL5 was proposed to recruit
TAMs in vitro, and that the chemotaxis of TAMs increased
with the increasing CCL5 concentration.
MCF-7 cells co-cultured with TAMs
promotes cell proliferation, migration and invasion
An MTT assay was used to detect changes to cell
proliferation before and after TAMs were co-cultured with
MCF-7/siNC or MCF-7/siCCL5. The OD value of MCF-7/siNC co-cultured
with TAMs significantly increased compared with MCF-7/siNC
(P<0.05; Fig. 6A). However, after
interference of endogenous CCL5 in MCF-7 cells, no significant
changes in the OD before and after co-culturing with TAMs were
reported. The OD was statistically significantly different for
MCF-7/siNC + TAMs compared with MCF-7/siCCL5+TAM (P<0.05,
Fig. 6A). Therefore, the
proliferation capacity of tumor cells had increased after
co-culturing, with CCL5 contributing to this process.
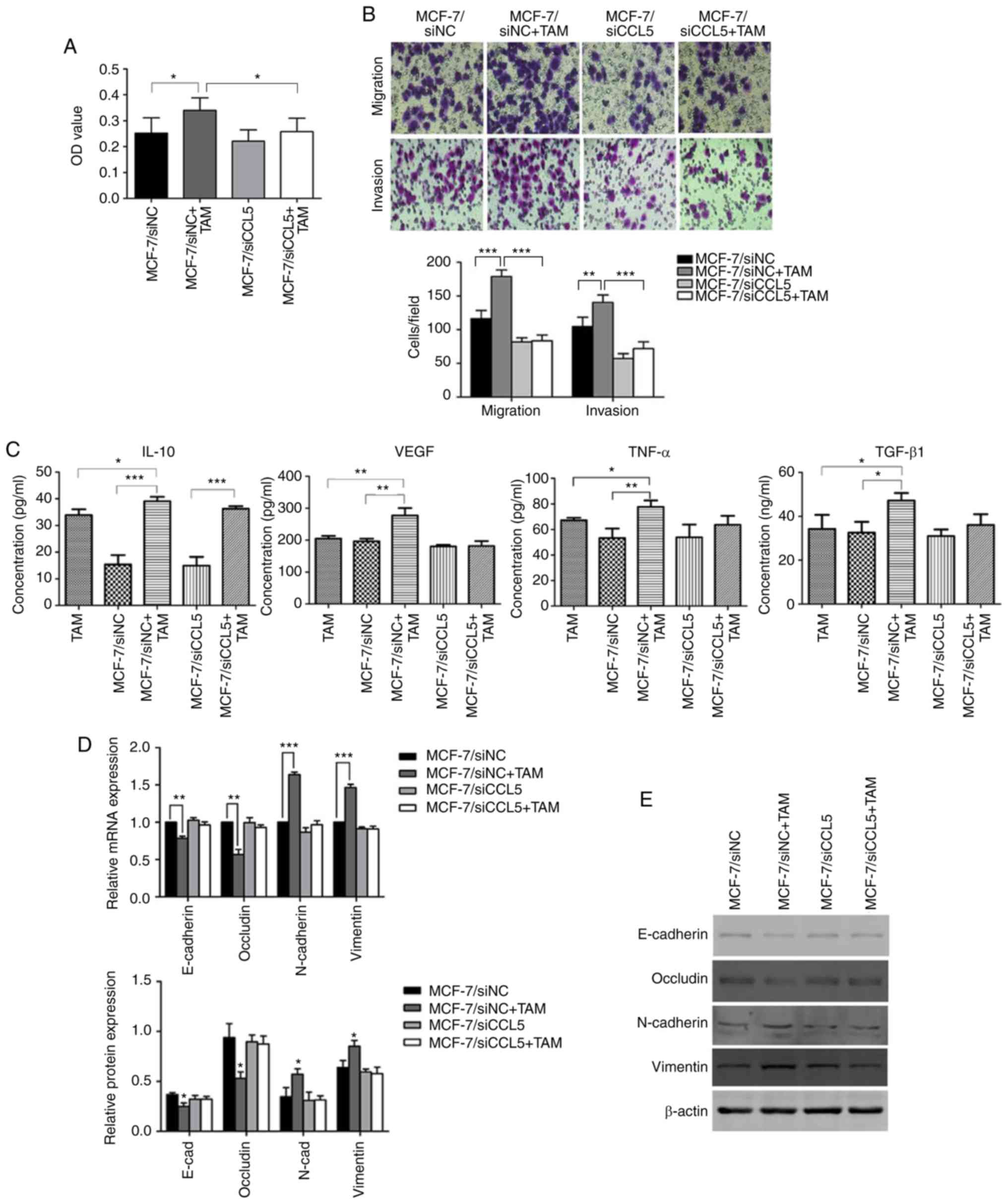 | Figure 6.Changes in cell proliferation,
migration and invasion before and after MCF-7 cell co-culture with
TAMs. (A) The OD value before and after co-culture of MCF-7 cells
and TAMs. *P<0.05. (B) Changes in cell migration and invasion
before and after co-culture of MCF-7 cells with TAMs, as determined
by a Transwell assay (magnification ×200), **P<0.01,
***P<0.001. (C) ELISA detection of IL-10, VEGF, TNF-α and TGF-β1
in the supernatant before and after co-culture of MCF-7 cells with
TAMs. *P<0.05, **P 0.01, ***P<0.001. (D) The mRNA expression
of EMT markers before and after MCF-7 cells were co-cultured with
TAMs. **P<0.01, ***P<0.001. (E) Expression of EMT-related
proteins before and after co-culture of MCF-7 with TAMs. *P<0.05
vs. MCF-7/siNC group. E-cad, E-cadherin; EMT,
epithelial-mesenchymal transition; OD, optical density; IL,
interleukin; N-cad, N-cadherin; siNC, small interfering RNA
negative control; siCCL5, small interfering RNA C-C motif chemokine
ligand 5; TAMs, associated macrophages; TGF-β1, transforming growth
factor-β1; TNF-α, tumor necrosis factor-α; VEGF, vascular
endothelial growth factor. |
The Transwell assay showed that the migration
(P<0.01) and invasion capability (P<0.001) of MCF-7/siNC
cells were significantly enhanced after co-culturing with TAM
compared with MCF-7/siNC alone (Fig.
6B). However, endogenous CCL5 interference induced significant
differences between MCF-7/siNC + TAM and MCF-7/siCCL5 + TAM
(P<0.05; Fig. 6B). Thus, cell
migration and invasion abilities of cells were enhanced by CCL5
after MCF-7 cells were co-cultured with TAMs. The ELISA assay
showed that, after co-culturing, the secretion of IL-10, vascular
endothelial growth factor (VEGF), tumor necrosis factor (TNF)-α and
transforming growth factor (TGF)-β1 in the supernatant increased to
different extent, and was statistically significant compared with
MCF-7/siNC (P<0.05; Fig. 6C).
However, when MCF-7/siCCL5 cells were co-cultured with TAMs, a
significant increase was only observed for the secretion of IL-10
compared with MCF-7/siCCL5 cells (P<0.001; Fig. 6C). Thus, the secretion of cytokines by
TAMs may depends on CCL5.
RT-qPCR showed that the expression of epithelial
markers (such as E-cadherin and Occludin) was significantly reduced
after co-culturing MCF-7/siNC cells with TAM compared with
MCF-7/siNC alone (P<0.01; Fig.
6D). The mRNA expression levels of mesenchymal markers (such as
N-cadherin and Vimentin) were significantly increased compared with
MCF-7/siNC alone (P<0.001; Fig.
6D). In comparison, the mRNA expression of EMT markers did not
change significantly after MCF-7/siCCL5 cells were co-cultured with
TAM (Fig. 6D).
Western blot analysis was conducted to detect the
expression of these cell markers (Fig.
6E). After MCF-7/siNC cells were co-cultured with TAMs, the
expression of E-cadherin and Occludin was significantly
downregulated (P<0.05, Fig. 6E),
whereas the expression of N-cadherin and Vimentin was significantly
upregulated (P<0.05; Fig. 6E). The
expression of these proteins did not significantly change after
MCF-7/siCCL5 cells were co-cultured with TAMs, supporting the
results of RT-qPCR results (Fig. 6E).
Thus, CCL5 may mediate affect the EMT of MCF-7 cells co-cultured
with TAMs.
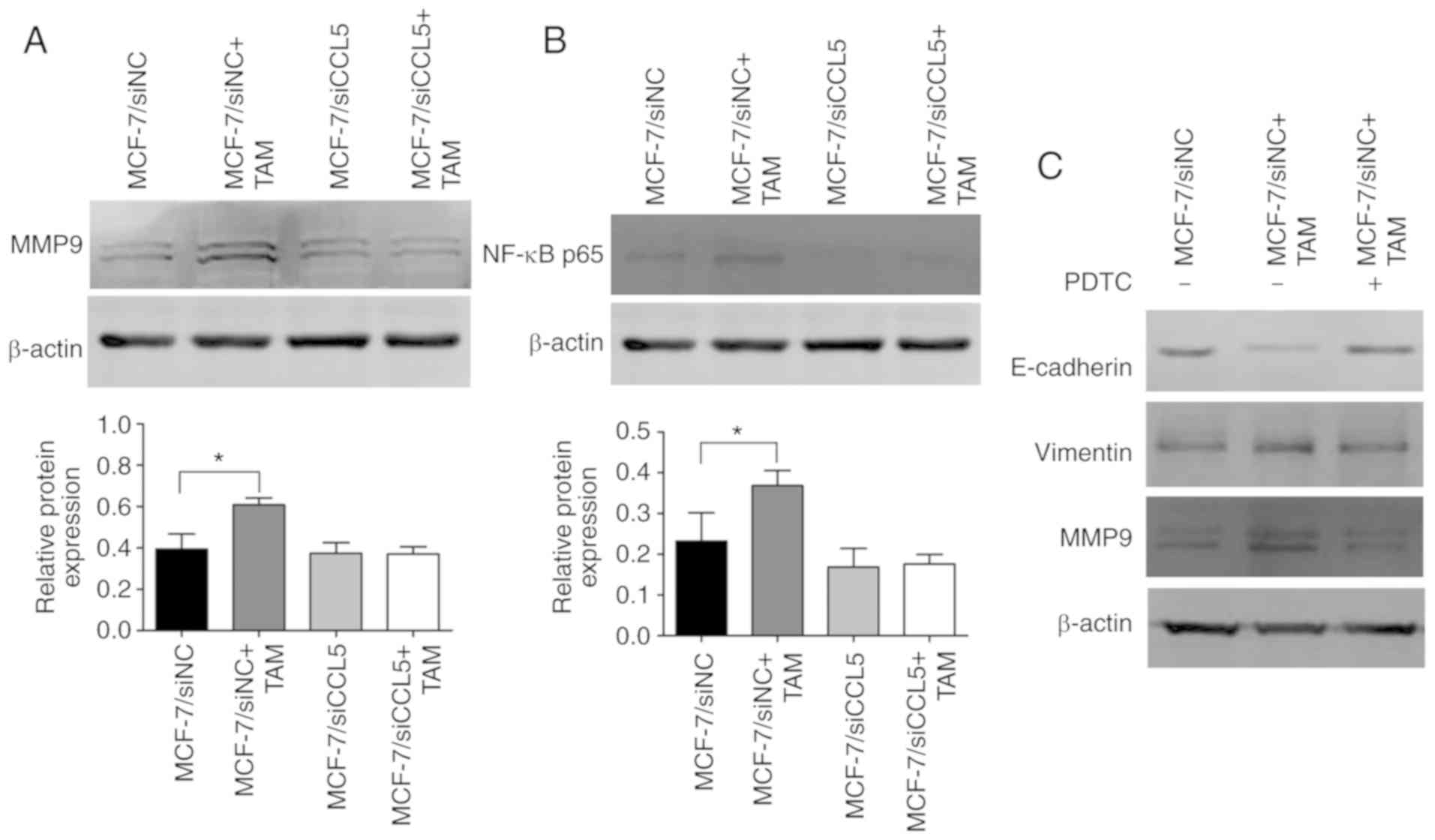
Furthermore, MMP9 protein expression was
significantly upregulated after MCF-7/siNC cells were co-cultured
with TAMs compared with MCF-7/siNC cells alone (P<0.05; Fig. 7A). However, there were no significant
changes in MMP9 protein expression before and after MCF-7/siCCL5
cells were co-cultured with TAMs (Fig.
7A). Thus, MCF-7 cells co-cultured with TAM were proposed to
effectively degrade the extracellular matrix in a manner dependent
on CCL5.
CCL5 activated the NF-κB signaling
pathway by recruiting TAMs to promote tumor migration and
invasion
Western blotting revealed that NF-κB p65 expression
was significantly upregulated after MCF-7/siNC cells were
co-cultured with TAMs compared with MCF-7/siNC cells alone
(P<0.05; Fig. 7B). However, this
difference was not statistically significant in MCF-7/siCCL5 cells
co-cultured with TAMs or alone. NF-κB p65 expression was markedly
decreased in MCF-7/siCCL5 cells compared with the MCF-7/siNC group
(Fig. 7B). Thus, MCF-7 cells
co-cultured with TAM may activate the NF-κB signaling pathway. On
the contrary, the signaling pathway was inhibited after CCL5
interference. After blocking NF-κB activation with its specific
blocker (PDTC), the EMT process was suggested to be reversed and
MMP9 expression was downregulated (Fig.
7C). MCF-7 cells co-cultured with TAMs were suggested to
promote the EMT process of breast cancer cells and increased MMP9
expression by activating the NF-κB pathway, which enhanced the
migration and invasion of cells. However, silencing the expression
of CCL5 did not activate the NF-κB signaling pathway. Thus, CCL5
could contribute to the effects of co-culture by activating the
NF-κB signaling pathway to promote the migration and invasion of
tumors.
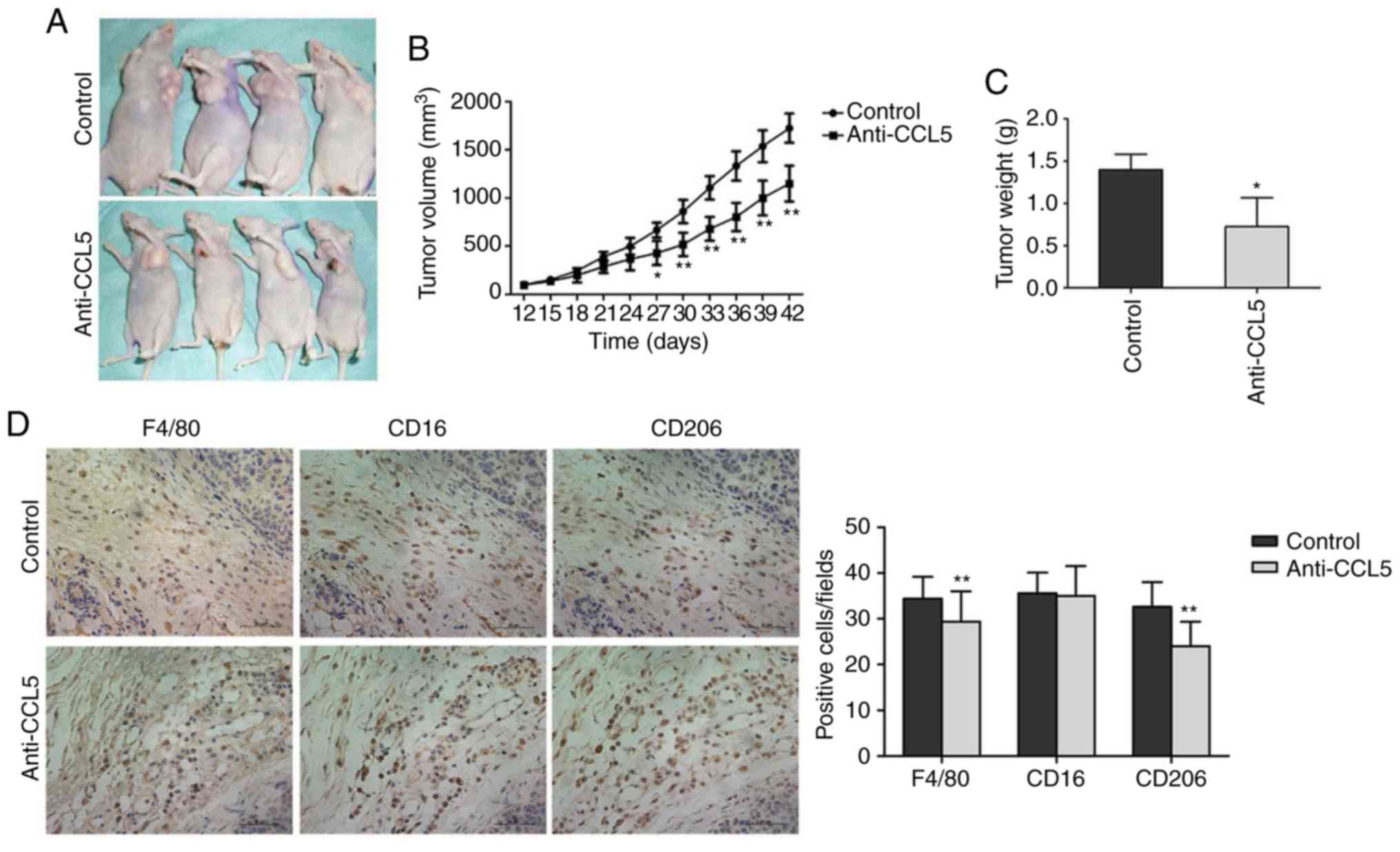
CCL5 promotes the growth of human
breast cancer xenograft tumor in nude mice
The size of the transplanted tumor significantly
differed between the experimental and control groups on day 27 (15
days after CCL5 injection, P<0.05; Fig. 8A and B). Tumor volume was
significantly lower in the experimental group compared with the
control group on day 42 (CCL5 injection after 30 days, P<0.05;
Fig. 8B). After collecting the tumor
tissue, the tumor weight in the experimental group was
significantly reduced compared with the control group (P<0.05,
Fig. 8C). Thus, CCL5 was suggested to
promote the growth of the breast cancer xenograft tumors.
Immunohistochemistry was applied to detect the
infiltration of macrophages in tumor tissues after blocking CCL5
secretion (Fig. 8D). Compared with
the control group, the number of macrophages (Table SII) in tumor stroma treated with
anti-CCL5 neutral antibodies was significantly lower compared with
the control (34.93±4.213 vs. 30.59±5.705, P<0.01; Fig. 8D). The number of M1 type cells did not
significantly change (36.06±3.930 vs. 35.97±6.058, P=0.876;
Fig. 8D). The number of M2 type cells
was significantly lowered following anti-CCL5 treatment
(33.32±4.857 vs. 24.95±4.752, P<0.01; Fig. 8D). Thus, CCL5 in the tumor
microenvironment may facilitate TAM recruitment, while blocking
CCL5 secretion inhibits TAM recruitment.
Discussion
Studies have shown that tumor invasion and
metastasis are closely related to cells in the tumor
microenvironment, with TAMs receiving considerable attention in
recent years (16,17). TAMs enhance the migration and invasion
abilities of tumor cells by secreting a variety of growth factors
and cytokines, promoting the generation of blood vessels and
lymphatic vessels, and inducing immune suppression within the
microenvironment (16). Angiogenesis,
hypoxia and TAM infiltration are associated with the poor prognosis
of breast cancer. Correspondingly, decreasing the number of TAMs
could reduce tumor progression (18).
Lewis et al (18) reported
that TAMs secrete VEGF to promote tumor angiogenesis, while hypoxia
upregulates the expression of VEGF in TAMs. Nagakawa et al
(19) confirmed that TAMs produce
various enzymes (such as MMP2 and MMP9) that degrade the
extracellular matrix (ECM) and enhance the activity of tumor cells.
Soria et al (20) determined
that the expression of IL-1, TNF-α, CCL5 and CCL2 in breast cancer
tissues is significantly higher than that in normal breast tissue.
Thus, these four factors may synergistically promote the
progression of breast cancer.
The current study established an indirect co-culture
system by co-culturing MCF-7 cells with TAMs in a simulated tumor
microenvironment in vitro to observe the morphological
changes to tumor cells. We reported that MCF-7 cells exhibited a an
epithelial-like phenotype, which changed to an interstitial
phenotype, with greater intercellular space and reduced adhesion
upon induction. An ELISA assay was used to detect the secretion of
IL-10, VEGF, TNF-α and TGF-β in the supernatant of TAMs only or
co-cultured with MCF-7 cells. TAMs and MCF-7 cells secreted all
four factors. After co-culture, the secretion of the four factors
in supernatant increased. Thus, this co-culture system induces the
secretion of a variety of materials that promotes the
proliferation, migration and invasion of tumor cells. For instance,
Hagemann et al (21) showed
that TAMs co-cultured with tumor cells promotes the expression of
MMPs, particularly MMP2 and MMP9; this process was conducted in a
manner dependent on TNF. In this study, western blotting
demonstrated that MMP9 was upregulated after co-culturing MCF-7
cells with TAMs; thus, the co-culture of tumor cells and
macrophages increased the secretion of chemical factors and the
expression of MMP9.
EMT is an important physiological and pathological
process. During the ‘lifetime’ of tumor cells, the EMT process is
continuously activated, causing epithelial cells to lose polarity
and gain the properties of mesenchymal cells (22). Consequently, the migration and
invasion ability of cancer cells is enhanced, as well as resistance
to apoptosis, leading to the secretion of various components that
degrade ECM (22). We reported that,
after co-culture with TAMs, the migration and invasion abilities of
MCF-7 cells were enhanced. Analysis of cell morphology also
revealed changes from an epithelial phenotype to a mesenchymal
phenotype. RT-qPCR and western blot analyses were used to test the
mRNA and protein expression levels of EMT markers in MCF-7 cells.
These approaches confirmed that tumor cells underwen EMT changes
after co-culture with TAMs. Thus, the migration and invasion of
cells was enhanced by EMT.
The high expression of chemokines and their
receptors in various tumors activates abnormal signaling pathways,
leading to the inactivation of the tumor suppressor gene or the
abnormal activation of proto-oncogenes (23,24). As a
result, these genes might contribute to the occurrence, metastasis,
angiogenesis, EMT, and immune suppression of tumors. The current
study demonstrated that the co-culture of MCF-7 cells and TAMs
promoted the secretion of various chemical factors, induces the
occurrence of EMT, and up-regulates the expression of MMP9;
however, the inhibition of CCL5 expression did not cause these
changes. We speculated that CCL5 contributes to signal transmission
in tumor cells and their microenvironment.
Tumor invasion and metastasis require the occurrence
of EMT, as well as tumor angiogenesis, the regulation of various
transcriptions and chemical factors, and the secretion of MMPs
(25,26). The signaling pathways are involved in
ERK/MAPK, PI3K/Akt, Notch, Wnt, Hedgehog, and NF-κB pathways
(25,26). Activated NF-κB promotes the occurrence
and development of many tumors, regulating the expression of
chemokines, which are mainly homodimers and heterodimers (13). For instance, the heterodimer formed by
P65/P50 exists in almost all cells in the body. Heterodimers are
present in the cytoplasm in an inactive form in dormant cells.
NF-κB is activated when cells are stimulated by various factors
(13). P65 and P50 are released into
the nucleus, causing transcriptional activation or inhibition of
corresponding target genes. It has been suggested that
transcription factors NF-κB and HIF-1α regulate the function of
macrophages (13).
CCL5 is a target gene of NF-κB. Huang et al
(27) showed that CCL5 activates αvβ3
integrin through the PI3K/Akt pathway to promote cell migration. In
turn, αvβ3 integrin activates the IKKα/β and NF-κB pathways
(27). The activation of NF-κB also
promotes the secretion of MMP9. Grivennikov et al (28) had reported that TNF-α secreted by
cells causes the secretion of chemical factors, while TAMs are
activated by NF-κB, which activates the pre-feedback loop. The
activation of NF-κB also increases the production of MMPs, further
promoting the degradation of ECM and the release of growth factors
(29).
Applying NF-κB inhibitors or anti-inflammatory
drugs, like aspirin, reduces the concentration of NF-κB downstream
protein IL-8 and slows down the progression of colon cancer and
breast cancer (30). In the preset
study, western blotting was performed to detect the protein
expression of NF-κB p65 before and after MCF-7 cells were
co-cultured with TAMs. We found that NF-κB p65 expression was
upregulated after co-culture; thus, it activates the NF-κB
signaling pathway. When blocking NF-κB activation using the
inhibitor PDTC, the EMT process was reversed, and the expression of
MMP9 was downregulated. Thus, MCF-7 cells co-cultured with TAMs
activate the NF-κB signaling pathway. Finally, through EMT
occurrence and MMP9 upregulation, NF-κB pathway activation promotes
the migration and invasion of tumor cells. However, CCL5
intervention prevented the co-culture of MCF-7 cells and TAMs
showing these changes. Also, the migration and invasion ability of
the cells did not significantly change. Thus, CCL5 plays an
important role in the interaction between tumor cells and TAM.
The current study revealed that CCL5 promotes tumor
migration and invasion. For instance, breast cancer cells and
different cells in the tumor microenvironment (such as MSCs)
secrete CCL5. The activation of CCL5 recruits TAM into the tumor
microenvironment. TAMs interact with tumor cells to secrete a
variety of factors (such as VEGF and TNF-β) and promotes the
release of MMPs, which activate the NF-κB signaling pathway to
induce EMT occurrence (15). All of
these factors promote the proliferation of tumor cells and
angiogenesis, as well as the degradation of ECM; consequently, the
invasion and metastasis of the tumor are promoted. However, the
invasion and metastasis of the tumor are complex processes
involving in the interaction of various factors and multiple
signaling pathways. In conclusion, it is important to determine
whether chemokines, other than CCL5, activate this pathway, and
whether other signaling pathways are involved.
Supplementary Material
Supporting Data
Acknowledgements
We sincerely thank Professor Wei Duan (Deakin
University, Australia) for insightful discussions.
Funding
This study was supported by grants from Basic
Research Project of Natural Science in Shaanxi Province: Multi-mode
nano-targeted probe for molecular imaging of HER-2 positive breast
cancer (grant no. 2017JM8106).
Availability of data and materials
The datasets supporting the conclusions of this
article are included within this article and its additional images.
Raw data are available from the corresponding author on reasonable
request.
Authors' contributions
XZ designed the study. GA, FW, SH, JB, LF and SG
performed the experiments and analyzed the data. GA and FW wrote
the manuscript. XZ and SG helped to revise the manuscript. All
authors read and approved the final manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Ethics approval for the use of human samples was
obtained from the Ethical Committee of The First Affiliated
Hospital of Xi'an Jiaotong University (approval no.
XJTU1AF2018LSK-247). Written informed consent was obtained from
patients. The animal studies were approved by the ethics committee
of Medical and Biological Research of Xi'an Jiaotong
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TAM
|
tumor-associated macrophage
|
|
MMP
|
matrix metalloprotease
|
|
ECM
|
extracellular matrix
|
|
FBS
|
fetal bovine serum
|
|
NC
|
negative control
|
|
CCL5
|
C-C motif chemokine ligand 5
|
|
PMA
|
phorbol-12-myristate-13-acetate
|
|
NF-κB
|
nuclear factor-κB
|
|
HIF-1α
|
hypoxia inducible factors-1α
|
References
|
1
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sica A, Allavena P and Mantovani A: Cancer
related inflammation: The macrophage connection. Cancer Lett.
267:204–215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin EY, Nguyen AV, Russell RG and Pollard
JW: Colony-stimulating factor 1 promotes progression of mammary
tumors to malignancy. J Exp Med. 193:727–740. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin EY and Pollard JW: Macrophages:
Modulators of breast cancer progression. Novartis Found Symp.
256:158–168. 2004.PubMed/NCBI
|
|
6
|
Yang J, Liao D, Chen C, Liu Y, Chuang TH,
Xiang R, Markowitz D, Reisfeld RA and Luo Y: Tumor-associated
macrophages regulate murine breast cancer stem cells through a
novel paracrine EGFR/Stat3/Sox-2 signaling pathway. Stem Cells.
31:248–258. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Donlon TA, Krensky AM, Wallace MR, Collins
FS, Lovett M and Clayberger C: Localization of a human
T-cell-specific gene, RANTES (D17S136E), to chromosome 17q11.2-q12.
Genomics. 6:548–553. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mrowietz U, Schwenk U, Maune S, Bartels J,
Küpper M, Fichtner I, Schröder JM and Schadendorf D: The chemokine
RANTES is secreted by human melanoma cells and is associated with
enhanced tumour formation in nude mice. Br J Cancer. 79:1025–1031.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wertel I, Tarkowski R, Bednarek W and
Kotarski J: Relationship between RANTES and dendritic cells in
ovarian cancer patients. Front Biosci (Elite Ed). 3:227–232. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vaday GG, Peehl DM, Kadam PA and Lawrence
DM: Expression of CCL5 (RANTES) and CCR5 in prostate cancer.
Prostate. 66:124–134. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Soria G and Ben-Baruch A: The inflammatory
chemokines CCL2 and CCL5 in breast cancer. Cancer Lett.
267:271–285. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Milliken D, Scotton C, Raju S, Balkwill F
and Wilson J: Analysis of chemokines and chemokine receptor
expression in ovarian cancer ascites. Clin Cancer Res. 8:1108–1114.
2002.PubMed/NCBI
|
|
13
|
Tang X: Tumor-associated macrophages as
potential diagnostic and prognostic biomarkers in breast cancer.
Cancer Lett. 332:3–10. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Greene FL, Page DL, Fleming ID, et al:
AJCC cancer staging manual6th. Springer-Verlag; New York, NY:
2002
|
|
15
|
Tjiu JW, Chen JS, Shun CT, Lin SJ, Liao
YH, Chu CY, Tsai TF, Chiu HC, Dai YS, Inoue H, et al:
Tumor-associated macrophage-induced invasion and angiogenesis of
human basal cell carcinoma cells by cyclooxygenase-2 induction. J
Invest Dermatol. 129:1016–1025. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Siveen KS and Kuttan G: Role of
macrophages in tumour progression. Immunol Lett. 123:97–102. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu H, Xu JB, He YL, Peng JJ, Zhang XH,
Chen CQ, Li W and Cai SR: Tumor-associated macrophages promote
angiogenesis and lymphangiogenesis of gastric cancer. J Surg Oncol.
106:462–468. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lewis JS, Landers RJ, Underwood JC, Harris
AL and Lewis CE: Expression of vascular endothelial growth factor
by macrophages is up-regulated in poorly vascularized areas of
breast carcinomas. J Pathol. 192:150–158. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nagakawa Y, Aoki T, Kasuya K, Tsuchida A
and Koyanagi Y: Histologic features of venous invasion, expression
of vascular endothelial growth factor and matrix
metalloproteinase-2 and matrix metalloproteinase-9, and the
relation with liver metastasis in pancreatic cancer. Pancreas.
24:169–178. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Soria G, Ofri-Shahak M, Haas I,
Yaal-Hahoshen N, Leider-Trejo L, Leibovich-Rivkin T, Weitzenfeld P,
Meshel T, Shabtai E, Gutman M and Ben-Baruch A: Inflammatory
mediators in breast cancer: Coordinated expression of TNFα &
IL-1β with CCL2 & CCL5 and effects on epithelial-to-mesenchymal
transition. BMC Cancer. 11:1302011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hagemann T, Robinson SC, Schulz M, Trümper
L, Balkwill FR and Binder C: Enhanced invasiveness of breast cancer
cell lines upon co-cultivation with macrophages is due to TNF-alpha
dependent up-regulation of matrix metalloproteases. Carcinogenesis.
25:1543–1549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lim J and Thiery JP:
Epithelial-mesenchymal transitions: Insights from development.
Development. 139:3471–3486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aldinucci D and Colombatti A: The
inflammatory chemokine CCL5 and cancer progression. Mediators
Inflamm. 2014:2923762014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cambien B, Richard-Fiardo P, Karimdjee BF,
Martini V, Ferrua B, Pitard B, Schmid-Antomarchi H and
Schmid-Alliana A: CCL5 neutralization restricts cancer growth and
potentiates the targeting of PDGFRβ in colorectal carcinoma. PLoS
One. 6:e288422011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huber MA, Kraut N and Beug H: Molecular
requirements for epithelial-mesenchymal transition during tumor
progression. Curr Opin Cell Biol. 17:548–558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huber MA, Azoitei N, Baumann B, Grünert S,
Sommer A, Pehamberger H, Kraut N, Beug H and Wirth T: NF-kappaB is
essential for epithelial-mesenchymal transition and metastasis in a
model of breast cancer progression. J Clin Invest. 114:569–581.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang CY, Fong YC, Lee CY, Chen MY, Tsai
HC, Hsu HC and Tang CH: CCL5 increases lung cancer migration via
PI3K, Akt and NF-kappaB pathways. Biochem Pharmacol. 77:794–803.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bond M, Fabunmi RP, Baker AH and Newby AC:
Synergistic upregulation of metalloproteinase-9 by growth factors
and inflammatory cytokines: An absolute requirement for
transcription factor NF-kappa B. FEBS Lett. 435:29–34. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen JJ, Lin YC, Yao PL, Yuan A, Chen HY,
Shun CT, Tsai MF, Chen CH and Yang PC: Tumor-associated
macrophages: The double-edged sword in cancer progression. J Clin
Oncol. 23:953–964. 2005. View Article : Google Scholar : PubMed/NCBI
|