Introduction
An endometrial polyp is defined as a localized,
disorganized proliferation of benign glandular and stromal elements
protruding from the surface of the endometrium. Polyps are common
and can occur at any age but are mostly observed in the
perimenopausal period (1).
Endometrial polyps are usually asymptomatic and found incidentally;
however, they sometimes cause abnormal uterine bleeding and
infertility. They arise as monoclonal overgrowths of genetically
altered endometrial stromal cells with secondary induction of
polyclonal benign glands (2).
Tamoxifen and estrogen exposure are known to increase the frequency
and number of endometrial polyps, and several studies have shown
that body mass index, waist circumference, and insulin resistance
are related to the presence of these polyps (3,4). The
relationship of tamoxifen with endometrial polyps has been widely
investigated, and it has been suggested that tamoxifen may induce
KRAS mutations in endometrial cells and cause the recurrence
of endometrial polyps and cancer (5,6). Several
studies have also investigated the relationships of endometrial
polyps with gene expression, mutation, and the immune system
(7–10). However, the pathogenesis of
endometrial polyps is still debated.
Next-generation sequencing has produced a
breakthrough in genomic research and has facilitated mutational
analysis by whole exome sequencing (11). To explore cancer genomic alterations,
a large number of cancer genomes have been sequenced worldwide,
resulting in the implementation of projects such as The Cancer
Genome Atlas (TCGA) and The International Cancer Genome Consortium
(ICGC). Whole exome sequencing has been the main platform for these
sequencing initiatives, and data on the mutation of protein-coding
regions have been accumulated in relation to all types of cancers
(12,13).
In this study, we performed whole exome sequencing
and targeted mutation analysis to verify the presence of somatic
mutations in benign endometrial polyps and identify the relevant
driver mutations. Our results indicated the importance of oncogenic
mutation in benign endometrial polyps and may lead to a new
discovery in the tumorigenesis of endometrial tumors.
Materials and methods
Patient selection and tissue samples
for whole exome sequencing
After approval of the Ethics Committee of Keio
University School of Medicine (No. 2015-0032) and provision of
written informed consent, four patients were recruited for whole
exome analysis. These patients were scheduled for transcervical
resection of endometrial polyps due to their symptoms (infertility,
abnormal uterine bleeding). They were premenopausal and not taking
any drugs that might affect the endometrium (e.g. oral
contraceptives, tamoxifen). Endometrial polyps were removed by
transcervical resection, and peripheral blood lymphocytes were
collected as control samples. One polyp was randomly selected for
analysis in patients with multiple polyps. These polyps were
dissected for analysis and pathological assessment. Samples were
stained with hematoxylin and eosin, and those containing about
20–50% of stromal components were selected. Two independent
pathologists confirmed the diagnosis of endometrial polyp. Clinical
data (age, body mass index, medical history) were obtained from the
clinical records. All patients received an outpatient hysteroscopy
prior to transcervical resection. The endometrial polyps were
counted by two independent physicians who were blinded to
information on gene analysis. Specifically, one physician counted
the polyps at the time of outpatient hysteroscopy and another
physician counted them by reviewing the recorded videos and photos
of the hysteroscopy.
Whole exome sequencing
DNA was extracted from endometrial polyps and
peripheral blood lymphocytes, and whole exome sequencing was
performed using a next-generation sequencer Hiseq 2500 (Illumina,
San Diego, CA, USA) according to the manufacturer's protocol and
previous reports (14–16). In short, a sequencing library was
build using a SureSelect XT Human All Exon kit and sequencing
analysis was performed using a HiSeq 2500 system with HiSeq Rapid
SBS Kit v2-HS (Illumina) according to the manufacturer's
instructions. The sequencing quality was evaluated by a
bio-analysis company (Takara Bio Inc., Shiga, Japan).
Polyp-specific somatic mutations were derived by subtracting
lymphocyte sequencing data from those of the polyps.
Confirmation of observed RAS
mutation
We focused on one somatic mutation that was found in
endometrial polyps. Validation was performed by targeted mutation
analysis via the polymerase chain reaction-reverse
sequence-specific oligonucleotide (PCR-rSSO) method. Specifically,
34 new patients were recruited by the same selection criteria that
had been used for whole exome sequencing (endometrial polyp,
premenopausal, no medication). Patient recruitment was approved by
the Ethics Committee of Keio University School of Medicine (No.
2007-0081) and written informed consent was obtained from all
participating patients. Tissue samples from endometrial polyps were
formalin-fixed and paraffin-embedded (FFPE), and were diagnosed by
two independent pathologists. A total of 35 samples were collected
from the 34 patients, including one recurrence of endometrial
polyps. DNA extraction and mutation analysis for RAS genes
were conducted at LSI Medience Corp. (Tokyo, Japan). Genomic DNA
was extracted from tissue and serial slices of 7 µm were prepared.
After deparaffinization with xylene, the tissue sections were
stained with hematoxylin and eosin, and the target lesions were
dissected for analysis. PCR-rSSO with a Mebgen™ Rasket Kit (Medical
and Biological Laboratories Co., Nagoya, Japan) was performed for
RAS mutation analysis. This kit can detect 48 mutation
hotspots of RAS (KRAS and NRAS) and is used
clinically to detect RAS mutations in colorectal cancer as
biomarkers of unfavorable response to the anti-EGFR antibody
(17).
Laser-capture microdissection and
RAS-targeted sequencing
We analyzed the RAS mutations in stromal and
glandular components of endometrial polyps using laser-capture
microdissection. An NRAS-mutated case was selected based on
the presence of an adequate DNA quantity from each component. The
RAS mutation was analyzed in separate glandular and stromal
components. Serial slices of 7 µm were prepared from a block. After
deparaffinization with xylene, the tissue sections were stained
with hematoxylin and eosin, and the glandular and stromal lesions
were dissected by a PALM-IV system (Carl Zeiss Microscopy,
Oberkochen, Germany). All glandular lesions were microdissected and
collected, and the remaining regions on the slide were regarded as
stromal lesions. Each sample was placed in a 200-µl microtube and
DNA was extracted using a NucleoSpin DNA FFPE XS kit (Takara Bio,
Inc.). The NRAS exon 2 was amplified from the extracted DNA
by Applied Biosystems AmpliTaq Gold DNA polymerase (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with the NRAS exon2
primer (Table SI). PCR products were
purified using a NucleoSpin Gel and PCR Clean-Up Kit (Takara Bio)
and then ligated to a pGEM-T easy vector (Promega, Fitchburg, WI,
USA), followed by transformation of Sure2 competent cells (Agilent,
Santa Clara, CA, USA). Competent cells were cultured on LB plates
with ampicillin, and 30 colonies were chosen for each sample by
color selection. The inserted DNA was amplified from each colony
using SP6 and T7 primers (Table SI)
and PCR products were analyzed by Sanger sequencing using the same
NRAS exon2 primer pair.
Additional RAS mutation analysis for
atypical polypoid adenomyoma
The RAS mutation was also investigated in
atypical polypoid adenomyoma (APAM) by the PCR-rSSO method used in
the analysis of endometrial polyps. Ten patients with APAM without
premalignant or malignant components were recruited and provided
written informed consent. In total, 12 samples from 10 patients
were analyzed. APAM samples were obtained from two patients by
diagnostic biopsy or resection and therapeutic hysterectomy. The
pathological diagnosis was confirmed by two independent
pathologists.
Statistical analysis
The clinical findings were compared by t-test and
Pearson's Chi-squared test using Graph Pad Prism 7 (GraphPad
Software, La Jolla, CA, USA), with the significance threshold set
at P<0.05.
Results
Whole exome analysis of endometrial
polyps
Whole exome sequencing was performed on samples from
4 patients using DNA isolated from endometrial polyps and
peripheral blood lymphocytes. We identified 22 nonsynonymous
somatic mutations, including 21 missense and 1 nonsense mutations
in endometrial polyps. No indel, frameshift or synonymous mutations
were detected. The number of mutations varied from 2 to 10 per
patient. Two of 4 endometrial polyps harbored KRAS
mutations: c.37G>T (p.G13C) in patient 1 and c.35G>T (p.G12V)
in patient 4. Additional mutations, including PPP2R1A (protein
phosphatase 2 regulatory subunit A α) and ARHGAP35 (Rho GTPase
activating protein 35), were found (Tables I and SII).
 | Table I.Results of the whole exome sequencing
for 4 cases of endometrial polyps. |
Table I.
Results of the whole exome sequencing
for 4 cases of endometrial polyps.
| Case | Age (years) | BMI
(kg/m2) | Parity | Genes derived from
NGS |
|---|
| 1 | 35 | 21.3 | G0P0 | STIL
(c.3425C>G, p.P1142R) ICA1L (c.6T>G, p.D2E)
NT5E (c.1060C>T, p.R354C) KRAS (c.37G>T, p.G13C)
SRSF9 (c.293G>T, p.R98L) CNTNAP4 (c.2225C>T,
p.A742V) PPP2R1A (c.767C>A, p.S256Y) |
| 2 | 36 | 21.9 | G0P0 | PPP1R12B
(c.2444A>T, p.K815M) RAD21 (c.428T>C, p.I143T) |
| 3 | 36 | 24.4 | G1P1 | HECTD3
(c.1711C>T, p.R571C) RAP1GAP2 (c.1243C>T, p.P415S)
ARHGAP35 (c.1190T>C, p.M397T) MAGEA4 (c.616G>A,
p.V206I) |
| 4 | 35 | 21.0 | G0P0 | MORN1
(c.254C>T, p.T85I) DZIP3 (c.2587G>A, p.E863K)
SCUBE2 (c.1724G>C, p.R575P) KRAS (c.35G>T, p.G12V)
DHRS12 (c.488C>A, p.T163K) YLPM1 (c.5183A>G,
p.Y1728C) TIMM21 (c.629C>T, p.A210V) MAGEB2
(c.350C>A, p.S117*) MED12 (c.3412C>G, p.R1138G) |
RAS mutation analysis by polymerase
chain reaction-reverse sequence-specific oligonucleotide
method
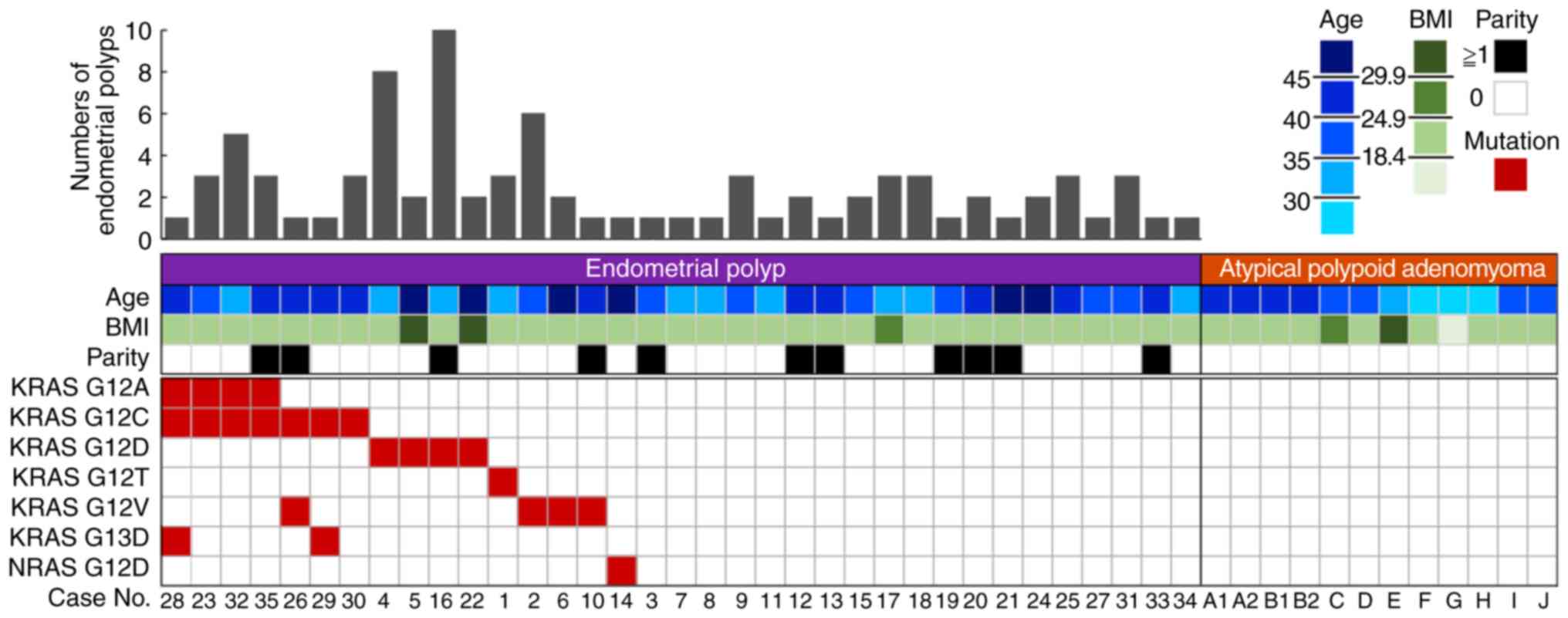
RAS mutations were analyzed in 35 endometrial
polyp samples from 34 patients (including one recurrent case) and
12 APAM samples from 10 patients by PCR-rSSO for validation. This
analysis revealed that 16 of 35 cases (45.7%) of endometrial polyps
harbored RAS mutations (15 KRAS, one NRAS); 10
cases had a single RAS mutation, and 6 had multiple
RAS mutations, varying from 2 to 3 per endometrial polyp.
The RAS mutations were all found in exon 2 of KRAS
and NRAS (KRAS G12V, G12D, G12C, G12A, G13D;
NRAS G12D). Two metachronous endometrial polyp samples
(cases 5 and 22) from a patient with recurrence of endometrial
polyps harbored the same mutation (KRAS G12D). There were no
mutations in the 12 atypical polypoid adenomyoma samples (Fig. 1). The comparison between 16 cases with
RAS mutations and 19 cases without RAS mutations
showed that the number of endometrial polyps was significantly
higher in RAS-mutated cases, as assessed by outpatient
hysteroscopy (3.25±2.70 vs. 1.74±0.87, P=0.045). There were no
differences in age, body mass index, and parity between these two
groups (Table II).
 | Table II.Clinical characteristics of the cases
with endometrial polyps with or without RAS mutation. |
Table II.
Clinical characteristics of the cases
with endometrial polyps with or without RAS mutation.
| Clinical
characteristics | RAS
mutation | Others | P-value |
|---|
| Age (years) | 40.9 | ±5.6 | 38.5 | ±4.7 | 0.165a |
| Body mass index
(kg/m2) | 21.9 | ±4.6 | 20.5 | ±2.8 | 0.258a |
| Multigravida | 4 | (25.0%) | 7 | (36.8%) | 0.493b |
| Numbers of
polyps | 3.25 | ±2.70 | 1.74 | ±0.87 | 0.045b |
RAS mutations are present in both
stromal and glandular components of endometrial polyps
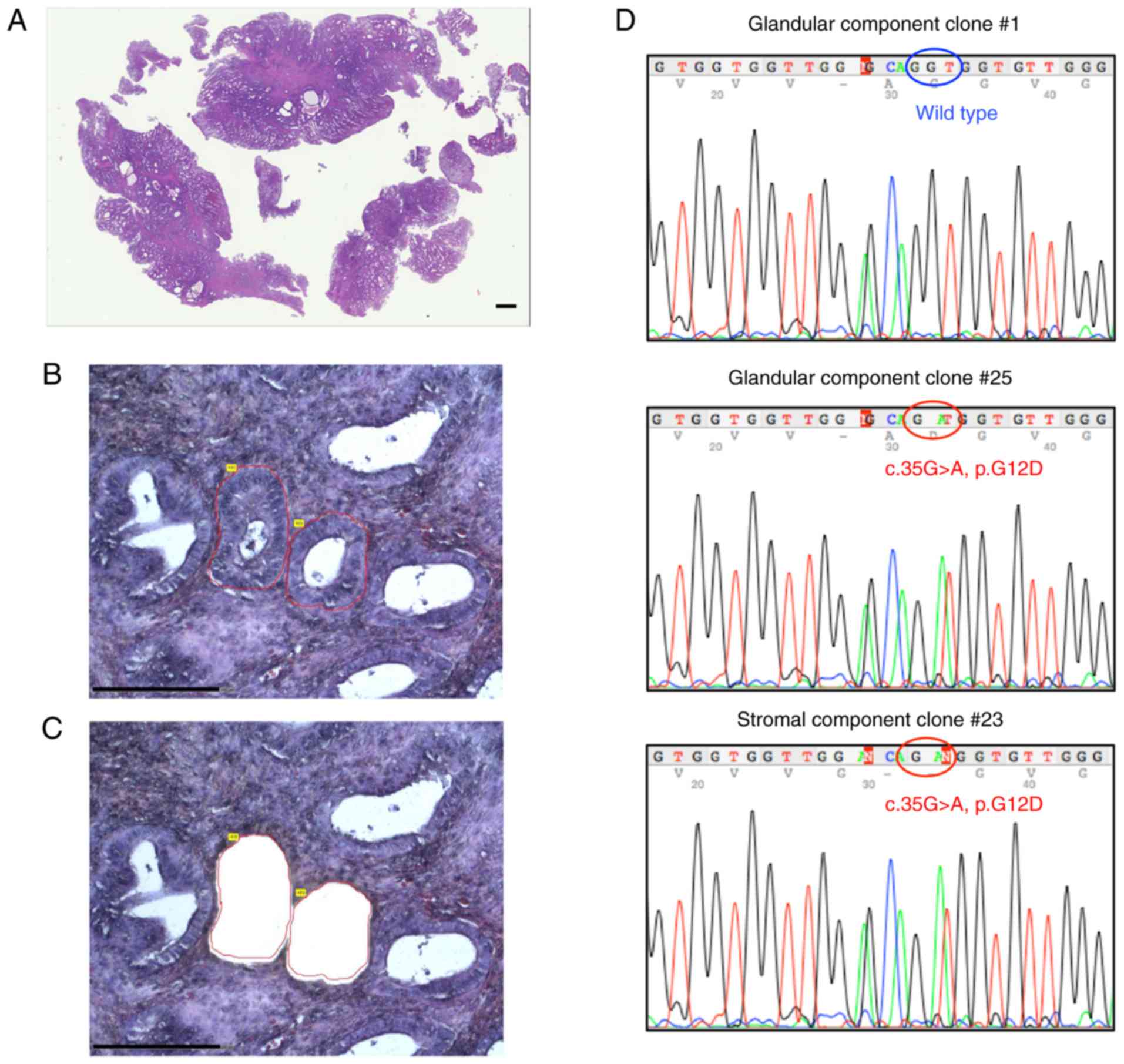
We next used laser-capture microdissection to
investigate whether the RAS mutations were harbored by
glandular or stromal components of endometrial polyps. Case 14,
carrying the NRAS mutation, was selected for this analysis
because it contained an adequate quantity of DNA from each cellular
component. The single endometrial polyp was 25 mm in size and had
developed from the fundus of the endometrial cavity. Sanger
sequencing of the glandular and stromal components showed that both
harbored an NRAS mutation (c.35C>A, p.G12D; Fig. 2).
Discussion
In the present study, 45.7% of the examined
endometrial polyps harbored RAS mutations. It is well known
that endometrial cancers may contain KRAS mutations, with a
reported rate of 10–30% (18–20). Furthermore, KRAS mutations were
detected in 30–50% of POLE mutated (ultramutated) or
microsatellite instability (hypermutated) endometrial cancers
(21,22). We found a surprisingly high frequency
of RAS mutations in endometrial polyps, a type of lesion
generally regarded as benign.
Endometrial polyps are monoclonal overgrowths of
endometrial stromal cells with secondary induction of polyclonal
benign glands (2). These non-atypical
polyps usually do not develop into carcinoma, while colon polyps
can sequentially advance to colon carcinoma. KRAS mutations
in colon polyps are correlated with the development of advanced
polyps and adenomas. Moreover, KRAS mutations in colon
polyps correlate with a larger lesion size and a higher number of
polyps, and may be a useful marker for predicting the development
of metachronous advanced neoplasia (23–25). In
contrast, the pathogenesis of endometrial polyps is not well known,
except for the fact that tamoxifen and estrogen exposure are risk
factors. KRAS mutations in endometrial polyps have been
found in elderly, tamoxifen-treated patients (5,6). We
investigated endometrial polyps from premenopausal, drug-free
patients to exclude age and artificial hormonal factors. In this
study, several cases carried multiple RAS mutations,
indicating that these events may favor the development of
endometrial polyps.
The association between oncogenic mutations and
benign tumors has been extensively investigated, and several
oncogenes have been reported in benign conditions (26). Recent reports revealed that 26% of
cases of deep infiltrating endometriosis harbor somatic cancer
driver mutations (KRAS, PIK3CA, ARID1A, PPP2R1A) in
glandular, but not stromal, compartments of deep endometriotic
lesions (27,28). Furthermore, KRAS, ARHGAP35, and
PPP2R1A mutations, which were detected by whole exome
sequencing in this study, were recently found in uterine
endometrial epithelium (29). Our
result added a new information that RAS mutation in
endometrial polyps has an important role in their multiple
development.
Atypical polypoid adenomyoma (APAM) is defined as a
mixture of polypoid lesion consisting of glands with cytological
atypia and fibromuscular stroma (2).
APAM is generally regarded as a benign lesion, however it
frequently recurs and often coexists with atypical endometrial
hyperplasia and endometrial adenocarcinoma (30). Therefore, we expected that APAM
harbors RAS mutation more frequently than endometrial
polyps. Previous small-scale studies have shown that APAM is
associated with MLH1 hypermethylation, and microdissected
glandular components were shown to contain CTNNB1 mutations
(31,32). In addition, KRAS mutation was
found in 4/16 cases (25%) without BRAF mutation (33), in contrast with the absence of
RAS mutations in our APAM samples (0/12). Larger studies
employing laser-microdissection followed by RAS mutation
analysis in individual APAM components, will be necessary to draw
final conclusions on this issue.
In conclusion, we found that RAS genes were
frequently mutated in endometrial polyps, as assessed by whole
exome sequencing and targeted mutation analysis. This is the first
report showing a high frequency of pathogenic RAS mutations
in non-treated endometrial polyps. RAS mutations may have an
important role in tumorigenesis and in the formation of multiple
endometrial polyps.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the Japan Society for the
Promotion of Science (JSPS) through a Grant-in-Aid for Scientific
Research (C) (16K11154), and by the Keio Gijuku Academic
Development Fund. The funders had no role in the study design, in
the collection, analysis and interpretation of data, in the writing
of the report, and in the decision to submit the manuscript for
publication.
Availability of data and materials
The data analyzed during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
The concept was designed by TT, KB and KK. draft
preparation, writing, and editing of the manuscript were undertaken
by TT and KB. Molecular analyses were performed by TT, KB, MA and
MY. Clinical procedures and sample collection were carried out by
TT, KB, YK, MA, ET and DA. TT, KB and DA prepared the revised
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The Ethics Committees of Keio University School of
Medicine approved the study. All the participants signed informed
consent prior to participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
ORCID ID for authors: TT, 0000-0001-6686-5742; KB,
0000-0003-2610-2833; YK, 0000-0002-4503-2845; MA,
0000-0003-2974-8795; MY, 0000-0002-1505-3194; ET,
0000-0001-9243-0267; KK, 0000-0002-6798-8151; DA,
0000-0002-9596-8326.
References
|
1
|
Dreisler E, Stampe Sorensen S, Ibsen PH
and Lose G: Prevalence of endometrial polyps and abnormal uterine
bleeding in a Danish population aged 20–74 years. Ultrasound Obstet
Gynecol. 33:102–108. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kurman RJ, Carcangiu ML, Herrington CS and
Young RH: WHO Classification of Tumours of Female Reproductive
Organs4th. WHO Classification of Tumours. 6. World Health
Organization; Lyon: 2014
|
|
3
|
Özkan NT, Tokmak A, Güzel Aİ, Özkan S and
çİçek MN: The association between endometrial polyps and metabolic
syndrome: A case-control study. Aust N Z J Obstet Gynaecol.
55:274–278. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Serhat E, Cogendez E, Selcuk S, Asoglu MR,
Arioglu PF and Eren S: Is there a relationship between endometrial
polyps and obesity, diabetes mellitus, hypertension? Arch Gynecol
Obstet. 290:937–941. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsujioka H, Hachisuga T, Fukuoka M, Ueda
T, Miyahara D, Horiuchi S, Shirota K, Yoshizato T, Emoto M,
Miyamoto S and Kawarabayashi T: Monitoring of endometrial K-ras
mutation in tamoxifen-treated patients with breast cancer. Int J
Gynecol Cancer. 19:1052–1056. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsujioka H, Hachisuga T, Hikita S, Ueda T,
Yotsumoto F, Shirota K, Yoshizato T, Kawarabayashi T, Kuroki M and
Miyamoto S: Apoptosis as a possible candidate mechanism for removal
of tamoxifen-related endometrial cells with KRAS mutations.
Anticancer Res. 30:3119–3123. 2010.PubMed/NCBI
|
|
7
|
Dal Cin P, Wanschura S, Kazmierczak B,
Tallini G, Dei Tos A, Bullerdiek J, Van den Berghe I, Moerman P and
Van den Berghe H: Amplification and expression of the HMGIC gene in
a benign endometrial polyp. Genes Chromosomes Cancer. 22:95–99.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Indraccolo U, Di Iorio R, Matteo M, Corona
G, Greco P and Indraccolo SR: The pathogenesis of endometrial
polyps: A systematic semi-quantitative review. Eur J Gynaecol
Oncol. 34:5–22. 2013.PubMed/NCBI
|
|
9
|
Zhu Y, Du M, Yi L, Liu Z, Gong G and Tang
X: CD4+ T cell imbalance is associated with recurrent
endometrial polyps. Clin Exp Pharmacol Physiol. 45:507–513. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rios SS, Andrade RV, Pereira RW, Wall NR,
Bahjri K, Caldas É, Cavalcante L and Figueiredo F: Microsatellite
instability in endometrial polyps. Eur J Obstet Gynecol Reprod
Biol. 153:193–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sikkema-Raddatz B, Johansson LF, de Boer
EN, Almomani R, Boven LG, van den Berg MP, van Spaendonck-Zwarts
KY, van Tintelen JP, Sijmons RH, Jongbloed JD and Sinke RJ:
Targeted next-generation sequencing can replace Sanger sequencing
in clinical diagnostics. Hum Mutat. 34:1035–1042. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Forbes SA, Beare D, Gunasekaran P, Leung
K, Bindal N, Boutselakis H, Ding M, Bamford S, Cole C, Ward S, et
al: COSMIC: Exploring the world's knowledge of somatic mutations in
human cancer. Nucleic Acids Res 43 (Database Issue). D805–D811.
2015. View Article : Google Scholar
|
|
13
|
Nakagawa H and Fujita M: Whole genome
sequencing analysis for cancer genomics and precision medicine.
Cancer Sci. 109:513–522. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ng SB, Buckingham KJ, Lee C, Bigham AW,
Tabor HK, Dent KM, Huff CD, Shannon PT, Jabs EW, Nickerson DA, et
al: Exome sequencing identifies the cause of a mendelian disorder.
Nat Genet. 42:30–35. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Margulies M, Egholm M, Altman WE, Attiya
S, Bader JS, Bemben LA, Berka J, Braverman MS, Chen YJ, Chen Z, et
al: Genome sequencing in microfabricated high-density picolitre
reactors. Nature. 437:376–380. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bentley DR, Balasubramanian S, Swerdlow
HP, Smith GP, Milton J, Brown CG, Hall KP, Evers DJ, Barnes CL,
Bignell HR, et al: Accurate whole human genome sequencing using
reversible terminator chemistry. Nature. 456:53–59. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoshino T, Muro K, Yamaguchi K, Nishina T,
Denda T, Kudo T, Okamoto W, Taniguchi H, Akagi K, Kajiwara T, et
al: Clinical validation of a multiplex kit for RAS mutations in
colorectal cancer: Results of the RASKET (RAS KEy Testing)
prospective, multicenter study. EBioMedicine. 2:317–323. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pappa KI, Choleza M, Markaki S, Giannikaki
E, Kyroudi A, Vlachos G, Voulgaris Z and Anagnou NP: Consistent
absence of BRAF mutations in cervical and endometrial cancer
despite KRAS mutation status. Gynecol Oncol. 100:596–600. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Garcia-Dios DA, Lambrechts D, Coenegrachts
L, Vandenput I, Capoen A, Webb PM, Ferguson K; ANECS, ; Akslen LA,
Claes B, et al: High-throughput interrogation of PIK3CA, PTEN,
KRAS, FBXW7 and TP53 mutations in primary endometrial carcinoma.
Gynecol Oncol. 128:327–334. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van der Putten LJM, van Hoof R, Tops BBJ,
Snijders MPLM, van den Berg-van Erp SH, van der Wurff AAM, Bulten
J, Pijnenborg JMA and Massuger LFAG: Molecular profiles of benign
and (pre)malignant endometrial lesions. Carcinogenesis. 38:329–335.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cancer Genome Atlas Research Network, ;
Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H,
Robertson AG, Pashtan I, Shen R, et al: Integrated genomic
characterization of endometrial carcinoma. Nature. 497:67–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yeramian A, Moreno-Bueno G, Dolcet X,
Catasus L, Abal M, Colas E, Reventos J, Palacios J, Prat J and
Matias-Guiu X: Endometrial carcinoma: Molecular alterations
involved in tumor development and progression. Oncogene.
32:403–413. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lorentzen JA, Grzyb K, De Angelis PM, Hoff
G, Eide TJ and Andresen PA: Oncogene mutations in colorectal polyps
identified in the norwegian colorectal cancer prevention (NORCCAP)
screening study. Clin Med Insights Pathol. 9:19–28. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Juárez M, Egoavil C, Rodriguez-Soler M,
Hernández-Illán E, Guarinos C, García-Martínez A, Alenda C,
Giner-Calabuig M, Murcia O, Mangas C, et al: KRAS and BRAF somatic
mutations in colonic polyps and the risk of metachronous neoplasia.
PLoS One. 12:e01849372017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamane LS, Scapulatempo-Neto C, Alvarenga
L, Oliveira CZ, Berardinelli GN, Almodova E, Cunha TR, Fava G,
Colaiacovo W, Melani A, et al: KRAS and BRAF mutations and MSI
status in precursor lesions of colorectal cancer detected by
colonoscopy. Oncol Rep. 32:1419–1426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kato S, Lippman SM, Flaherty KT and
Kurzrock R: The conundrum of genetic ‘Drivers’ in benign
conditions. J Natl Cancer Inst. 108(pii): djw0362016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Anglesio MS, Papadopoulos N, Ayhan A,
Nazeran TM, Noë M, Horlings HM, Lum A, Jones S, Senz J, Seckin T,
et al: Cancer-associated mutations in endometriosis without cancer.
N Engl J Med. 376:1835–1848. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Noe M, Ayhan A, Wang TL and Shih IM:
Independent development of endometrial epithelium and stroma within
the same endometriosis. J Pathol. 245:265–269. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Suda K, Nakaoka H, Yoshihara K, Ishiguro
T, Tamura R, Mori Y, Yamawaki K, Adachi S, Takahashi T, Kase H, et
al: Clonal expansion and diversification of cancer-associated
mutations in endometriosis and normal endometrium. Cell Rep.
24:1777–1789. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Heatley MK: Atypical polypoid adenomyoma:
A systematic review of the English literature. Histopathology.
48:609–610. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ota S, Catasus L, Matias-Guiu X, Bussaglia
E, Lagarda H, Pons C, Muñoz J, Kamura T and Prat J: Molecular
pathology of atypical polypoid adenomyoma of the uterus. Hum
Pathol. 34:784–788. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takahashi H, Yoshida T, Matsumoto T,
Kameda Y, Takano Y, Tazo Y, Inoue H and Saegusa M: Frequent
β-catenin gene mutations in atypical polypoid adenomyoma of the
uterus. Hum Pathol. 45:33–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Němejcová K, Kenny SL, Laco J, Škapa P,
Staněk L, Zikán M, Kleiblová P, McCluggage WG and Dundr P: Atypical
polypoid adenomyoma of the uterus: An immunohistochemical and
molecular study of 21 Cases. Am J Surg Pathol. 39:1148–1155. 2015.
View Article : Google Scholar : PubMed/NCBI
|