Introduction
Colorectal cancer (CRC) is a serious health concern
worldwide although technologies for early detection and early
treatment are progressing. Globally, CRC is the third most commonly
diagnosed cancer and the fourth leading cause of cancer-related
deaths in males, the second and the third, respectively, in females
(1). Recent evidence has indicated
that obesity and its associated metabolic abnormalities, such as
diabetes and dyslipidemia are related to an increased risk of CRC
(2,3).
Type 2 diabetes mellitus is a major complication of obesity and its
prevalence is high and increasing. In addition to cardiovascular
and cerebrovascular events, cancer is also a major cause of
diabetes- and obesity-related deaths (4–6).
The patients with diabetes have increased risk of
many types of cancer, especially cancer of the colon, liver, and
pancreas (7). The mechanisms by which
diabetes promotes the development and progression of cancer
including CRC have been only partially elucidated. These include
chronic inflammation, oxidative stress, hyperglycemia, and insulin
resistance (8–11), which are thought to be caused by
metabolic abnormalities. Previously, it was reported that diet- or
drug-induced diabetic and obese mice were significantly susceptible
to colon tumorigenesis in carcinogen-induced colon cancer models
(12,13). In addition, recent studies have
revealed that several types of natural compounds and medicines,
including catechins obtained from green tea, curcumin, and
pentoxifylline suppressed the development of diabetes- and
obesity-related colorectal tumorigenesis partially by attenuating
chronic inflammation (14–16). These findings indicated that diabetes-
and obesity-associated metabolic abnormalities may be important
targets for preventing colon cancer development in diabetic and/or
obese individuals (10). Several
clinical studies have actually demonstrated that certain types of
anti-diabetic drugs could reduce the risk of CRC (17,18).
Sodium-glucose cotransporter 2 (SGLT2) inhibitors
were developed for the treatment of diabetes by preventing
reabsorption of glucose filtered through the glomeruli and
increasing its urinary excretion (19,20). This
phenomenon reduces blood glucose levels and improves insulin
resistance in patients with diabetes as well as in several rodent
models of diabetes (21–23). Cancer cells demonstrate uptake of
glucose, a major metabolic substrate required for cancer growth via
glucose transporters including SGLT. Therefore, the anticancer
effects of SGLT inhibitors have gained much attention. Recent
publications have indicated that SGLT2 inhibitors prevented
carcinogenesis and inhibited cancer growth in several types of
cancer (24–26), however, little is known on whether the
SGLT2 inhibitors have efficacy against CRC.
In the present study, in order to examine the
effects of an SGLT2 inhibitor on CRC, it was investigated whether
the development of azoxymethane (AOM)-induced colorectal
pre-neoplastic lesions in diabetic and obese db/db mice was
suppressed by a highly selective SGLT2 inhibitor tofogliflozin
(27). In addition, the direct
effects of tofogliflozin on CRC cell proliferation were also
examined.
Materials and methods
Animals and housing conditions
Twenty-four male db/db mice (5 weeks old)
were obtained from Japan SLC Inc. They were acclimatized for a week
before initiation of the experiment (16). They were carefully handled and
humanely maintained at the Gifu University Life Science Research
Center in accordance with the Institutional Animal Care Guidelines.
The mice were housed in cages and were provided basic diet CE-2
obtained from Oriental Yeast and sterilized tap water ad
libitum. The environmental conditions inside the room were as
follows: Temperature, 23±1°C; humidity, 50±20%; and 12-h light/dark
cycle.
Chemicals and methods of drug
administration
AOM was obtained from Wako Pure Chemical Co. and was
intraperitoneally injected once weekly from weeks 1 to 4 after
initiation of the experiment. AOM was diluted with ultra-pure water
to administer a dose of 15 mg/kg body weight. Tofogliflozin was
kindly provided by Kowa Co., Ltd. Tofogliflozin (1 mg/kg body
weight and 10 mg/kg body weight) was dissolved in ultra-pure water.
The solution was poured into the bottles and exchanged twice a
week.
Procedure of the animal
experiment
At five weeks of age, the mice were randomly divided
into four experimental groups and treated as follows: No treatment
(Group 1, n=5), AOM alone (Group 2, n=9), AOM followed by
tofogliflozin (1 mg/kg body weight) in drinking water (Group 3,
n=5), and tofogliflozin alone (Group 4, n=5). Group 2 and 3 mice
were injected with AOM (15 mg/kg body weight) intraperitoneally
once weekly from weeks 1 to 4 after initiation of the experiment.
Mice were not anesthetized prior to AOM injection. At 9 weeks of
age, the Group 3 and 4 mice received drinking water containing
tofogliflozin. At 24 weeks of age, counting from 14 weeks of
tofogliflozin treatment, all the mice were euthanized after 12 h of
fasting. Then, blood samples were collected from the inferior vena
cava for clinical chemistry and organs and tissues were removed for
histopathological examination. For euthanasia, after inhalation of
CO2 the chest cavity was opened, and the heart was
exposed and viewed directly to confirm death. The experimental
protocol was approved by the Committee of Institutional Animal
Experiments of Gifu University (authorization code: 30-7, dated 13
April 2018).
Histopathological examination
The livers, kidneys, white adipose tissues, and
colorectal tissues were excised. The colon and rectum were opened
longitudinally and fixed on a filter paper in 10% buffered formalin
for more than 24 h. It was divided into a rectal portion
(approximately 1 cm in length on the oral side from the dentate
line) and colonic portion. Each segment and the other tissues were
paraffin-embedded. From the rectal tissue blocks, two serial
sections were subjected to hematoxylin and eosin (H&E) staining
for histopathology and immunohistochemistry for β-catenin to
evaluate the development of β-catenin accumulated crypts (BCACs)
(16). Immunohistochemical analyses
for β-catenin (1:1,000 dilution, BD Transduction Laboratories) was
performed using a labeled streptavidin-biotin method (LSAB kit;
DAKO; Agilent Technologies, Inc.). Immunohistochemical staining for
F4/80 was also performed to examine macrophage infiltration in the
adipose tissues, according to a previous study (28) with primary antibody (1:100 dilution;
product code ab111101; Abcam). Immunoreactivity was considered as
positive when apparent staining was detected in the cytoplasm
and/or nuclei (28,29).
Clinical chemistry
Serum was centrifuged (1,600 × g for 15 min) from
the whole blood and was used for chemical analyses. Serum glucose,
insulin, total cholesterol, free fatty acid (FFA), and triglyceride
(TG) levels were determined by a commercial laboratory (SRL, Inc.).
Serum TNF-α levels were estimated by mouse TNF-α ELISA kit
(AKMTM-011; Shibayagi Co. Ltd.) in accordance with the
manufacturer's protocol.
mRNA extraction and qRT-PCR
analysis
The mRNA expression in the colonic mucosa of the
experimental mice was examined using quantitative real-time reverse
transcription-polymerase chain reaction (qRT-PCR) analysis. Total
RNA was isolated from the scraped colonic mucosa of all the
experimental mice using PureLinkTM RNA Mini Kit (Invitrogen; Thermo
Fisher Scientific, Inc.), according to the manufacturer's
instructions. The complementary DNA (cDNA) was amplified from 1.5
µg of total-RNA of each sample using High Capacity cDNA Reverse
Transcription Kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The primers used for the amplification of C-C motif
chemokine-2 (CCL-2), tumor necrosis factor-α (TNF-α),
F4/80, insulin-like growth factor-1 (IGF-1), insulin-like
growth factor-2 (IGF-2), insulin-like growth factor binding
protein-3 (IGFBP-3), and 18S specific genes were
previously reported (30,31) or as follows: IGF-1 forward,
5′-TCGGCCTCATAGTACCCACT-3′ and reverse,
5′-ACGACATGATGTGTATCTTTATTGC-3′; IGF-2 forward,
5′-ACCTTCGGCCTTTGTCTGGTA-3′ and reverse,
5′-CGAAGGCCAAAGAGATGAGA-3′; and IGFBP-3 forward,
5′-GACGACGTACATTGCCTCAG-3′, and reverse,
5′-GTCTTTTGTGCAAAATAAGGCATA-3′. Real-time PCR was performed using a
Light Cycler with FastStart Essential DNA Green Master (both from
Roche Diagnostics) (Table III). The
expression of these genes was normalized to 18S.
 | Table III.Steps and conditions of thermocycling
for qRT-PCR. |
Table III.
Steps and conditions of thermocycling
for qRT-PCR.
| Step | Target
temperature | Hold time | Cycles |
|---|
| Initial
denaturation | 95°C | 10
min | 1 |
|
Amplification-denaturation | 95°C | 10 sec | 45 |
|
Amplification-annealing | 60°C | 10 sec |
|
|
Amplification-elongation | 72°C | 15 sec |
|
| Melting-initial
stage | 60°C (5°C/sec) | 20 sec | 1 |
| Melting-final
stage | 95°C
(0.1°C/sec) | 20 sec |
|
| Cooling | 40°C (2°C/sec) | 10 sec | 1 |
Cell lines and culture conditions
Expression of SGLT2 was confirmed by western blot
for the HT29, HCT116, SW837, and SW480 human CRC cell lines, and
Huh7 human hepatoma cell line. Huh7 was used as a positive control
(25). These cell lines were obtained
from the JCRB Cell Bank (Osaka, Japan). All cell lines were
authenticated via short tandem repeat analysis by PCR and were
certified to be free of bacteria, fungi and mycoplasmas by the cell
bank. These cell lines were grown in appropriate media according to
the instructions of cell bank. Cells were subjected to experiments
within 6 months after receipt. All the cells were maintained in
Dulbecco's modified Eagle's medium supplemented with 10% fetal
bovine serum and 1% penicillin/streptomycin (all from
Sigma-Aldrich; Merck KGaA) in an incubator at 37°C under a
humidified atmosphere containing 5% CO2.
Protein extraction and western blot
analysis
Total protein was extracted from the cultured cells
and equivalent amounts of protein (20 µg/lane) were examined by
western blot as previously reported (32). The primary antibodies against SGLT2
(cat. no. 24654-1-AP) were obtained from ProteinTech Group, Inc.
and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; product no.
2118) was obtained from Cell Signaling Technology, Inc. GAPDH
served as a loading control.
Cell proliferation assays
SW480 and HT29 cells were seeded in 96-well plates.
On the following day, the cells were treated with indicated
concentrations (0, 0.05, 0.5, 5, and 50 µM) of tofogliflozin for 48
h. The cell proliferation assays were performed by
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) assay (Promega Corporation) in accordance with the
manufacturer's protocol.
Statistical analyses
The data are represented as the mean ± standard
error (SE). Differences between groups were analyzed using the
Kruskal-Wallis test, then the Steel-Dwass test was performed
between the groups on data to confirm statistical significance. A
P-value of <0.05 was considered to indicate a statistically
significant difference.
Results
General observations
There was no significant difference in relative
kidney weight, and white adipose tissue weight among all the groups
(Table I). The body weight of
tofogliflozin-treated mice was relatively increased compared to the
control, which is consistent with a previous study demonstrating
the body weight gain in SGLT2 inhibitor-treated mice (33). During the experiment, there were no
clinical symptoms in all the groups. Histopathological examination
did not reveal toxicity of tofogliflozin in the livers and the
kidneys (data not shown).
 | Table I.General observations of the
experimental mice. |
Table I.
General observations of the
experimental mice.
|
|
|
|
| Relative weight
(g/100 g body weight) of: |
|---|
|
|
|
|
|
|
|---|
| Group | Treatment | No. of mice | Body weight
(g) | Liver | Kidneys | White adipose
tissue |
|---|
| 1 | No treatment | 5 |
40.4±4.5a | 6.4±0.3 | 1.6±0.3 | 5.8±0.5 |
| 2 | AOM | 9 | 40.9±2.1 | 4.7±0.3 | 1.1±0.1 | 5.5±0.2 |
| 3 |
AOM/tofogliflozin | 5 | 39.0±2.0 | 5.1±0.1 | 1.2±0.1 | 5.1±0.2 |
| 4 | Tofogliflozin | 5 | 52.0±2.9 | 4.4±0.2 | 1.0±0.1 | 5.3±0.3 |
Serum parameters
The serum concentrations of glucose, insulin, total
cholesterol, FFA, and TG in each group are listed in Table II. There were no significant
differences in the results between the groups of mice treated with
AOM alone and AOM followed by tofogliflozin. In this study, no
adverse effect on serum parameters was observed after tofogliflozin
administration in the experimental mice.
 | Table II.Serum parameters of the experimental
mice. |
Table II.
Serum parameters of the experimental
mice.
| Group | Treatment | Glucose
(mg/dl) | Insulin
(ng/ml) | Total cholesterol
(mg/dl) | FFA(µEQ/l) | TG (mg/dl) |
|---|
| 1 | No treatment |
581.0±19.0a | 4.5±1.2 | 203.6±39.9 | 1542.8±350.0 | 194.4±52.5 |
| 2 | AOM | 304.9±50.8 | 2.6±0.4 | 155.9±33.6 | 1614.7±187.5 |
40.8±5.4b |
| 3 |
AOM/tofogliflozin |
223.4±71.8b | 2.7±0.6 | 122.4±9.3 |
956.8±54.5c |
28.4±7.7b,c |
| 4 | Tofogliflozin | 329.6±69.8 | 3.5±0.3 | 167.2±12.3 | 1446.8±135.9 |
214.4±45.8d |
AOM-induced colorectal pre-neoplastic
lesions in the experimental mice
Colorectal pre-neoplastic lesions BCAC (34,35) were
developed in the colons of AOM-injected mice with and without
administration of tofogliflozin. Optical microscopic representative
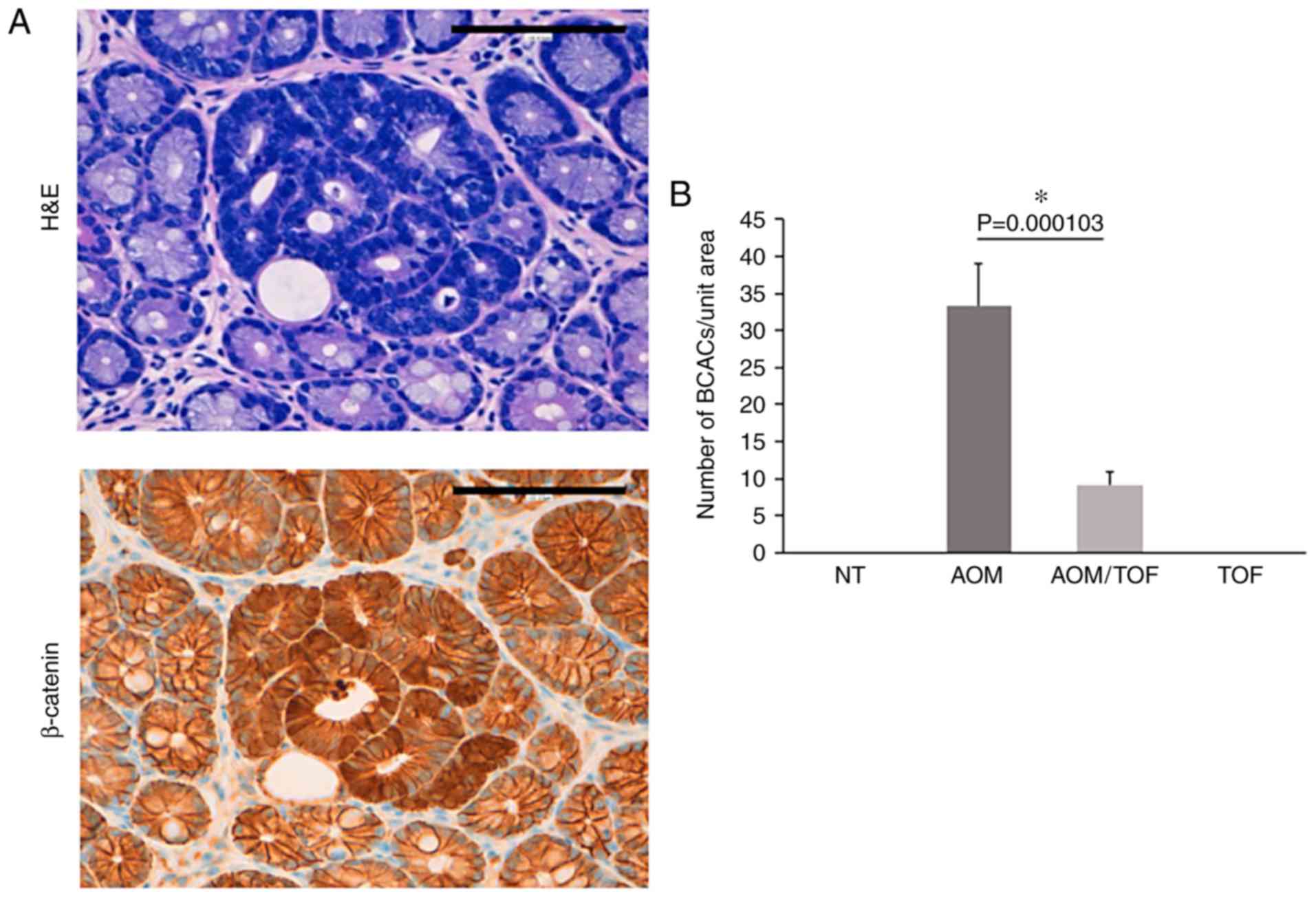
images of AOM-induced BCACs are presented in Fig. 1A. BCACs developed in the colon and
rectum of all the mice injected with AOM (Groups 2 and 3), but not
in the Group 1 and 4 mice that did not receive AOM. As revealed in
Fig. 1B, the number of BCACs was
significantly less in the mice treated with AOM plus tofogliflozin
as compared to the ones treated with AOM alone (72% reduction,
P<0.05).
Effects of tofogliflozin on serum
levels of TNF-α, chronic inflammation, and insulin-like growth
factor signals in colorectal mucosa of the experimental mice
TNF-α is an essential tumor promoter involved in
obesity, inflammation, and carcinogenesis (36,37).
Previous studies have indicated that chronic inflammation and
activation of the IGF/IGF-IR axis are involved in colorectal
carcinogenesis (16,38,39).
Therefore, the serum levels of TNF-α were estimated by
enzyme-linked immunosorbent assay (ELISA). The mRNA expression of
the specific molecules associated with chronic inflammation and IGF
signals, such as TNF-α, F4/80, CCL2, IGF1, IGF2, and IGFBP3, in
colonic mucosa, was evaluated by qRT-PCR. Serum TNF-α levels tended
to be lower in the AOM plus tofogliflozin-treated group as compared
to the AOM-treated group, however the change was not statistically
significant (Fig. 2A). There were
also no statistically significant differences in the expression of
the analyzed genes between the AOM plus tofogliflozin-treated group
and the AOM-treated group, however, IGF1 was decreased by treatment
with tofogliflozin. In addition, although there were no significant
differences, the expression levels of TNF-α and F4/80 in the colon
mucosa were also decreased in the tofogliflozin-treated group
compared to the no treatment group (Fig.
2B and C).
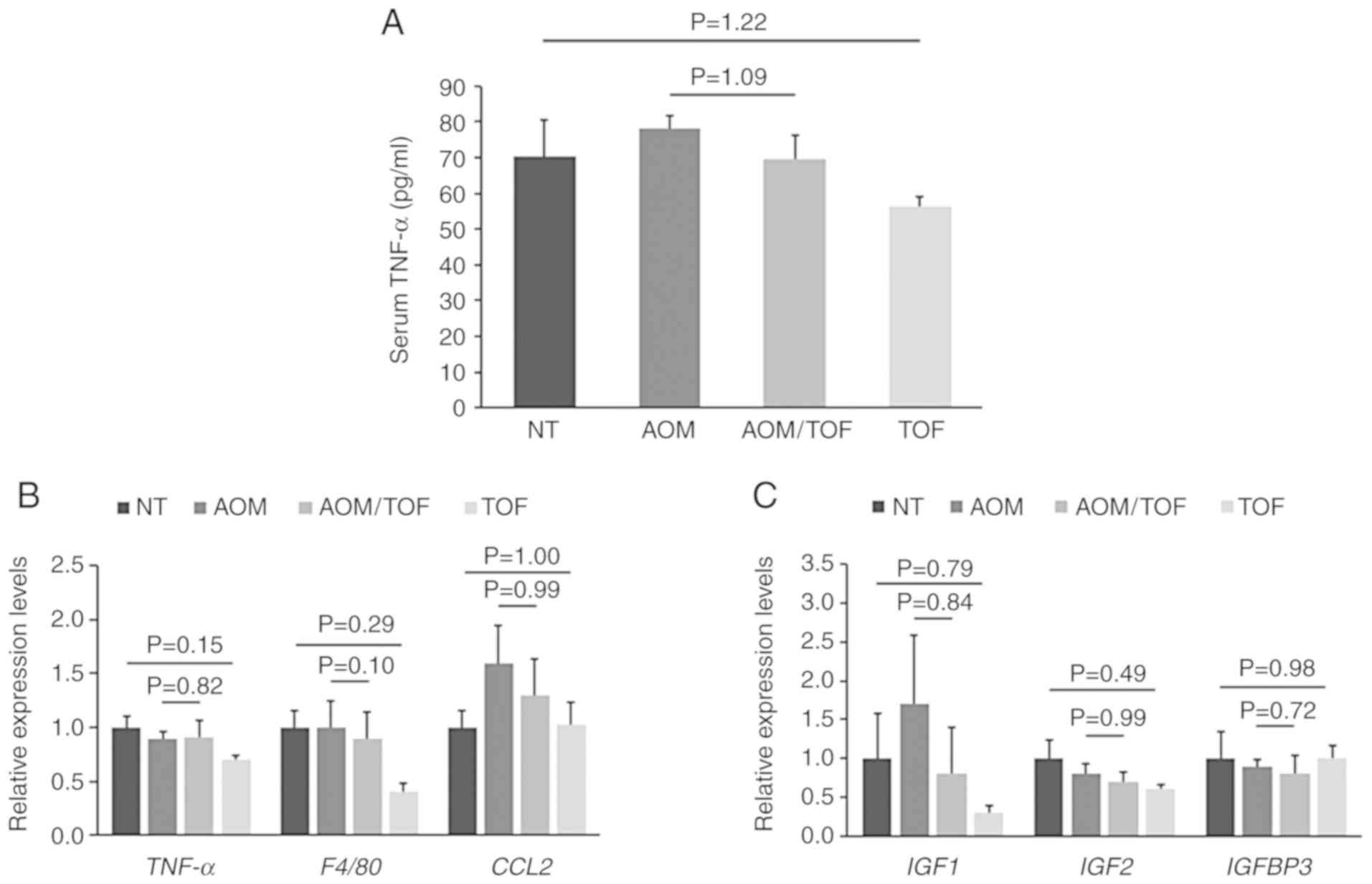 | Figure 2.Effects of tofogliflozin on the serum
levels of TNF-α and the expression levels of mRNA associated with
inflammation and IGF signaling in the colonic mucosa of the
experimental mice. (A) The serum concentrations of TNF-α were
assessed by an enzyme immunoassay. Total RNA was isolated from
scraped colonic mucosa of mice of all groups, and the expression
levels of mRNA involved in (B) inflammation (TNF-α, F4/80, and
CCL2) and (C) IGF signaling (IGF1, IGF2, and IGFBP3) were examined
by qRT-PCR with specific primers. Values are expressed as the mean
± SE. NT, no treatment. AOM, azoxymethane. TOF, tofogliflozin.
TNF-α, tumor necrosis factor-α; CCL-2, C-C motif chemokine-2;
IGF-1, insulin-like growth factor-1; IGF-2, insulin-like growth
factor-2; IGFBP-3, insulin-like growth factor binding
protein-3. |
Effects of tofogliflozin on chronic
inflammation and macrophage infiltration in white adipose tissue of
experimental mice
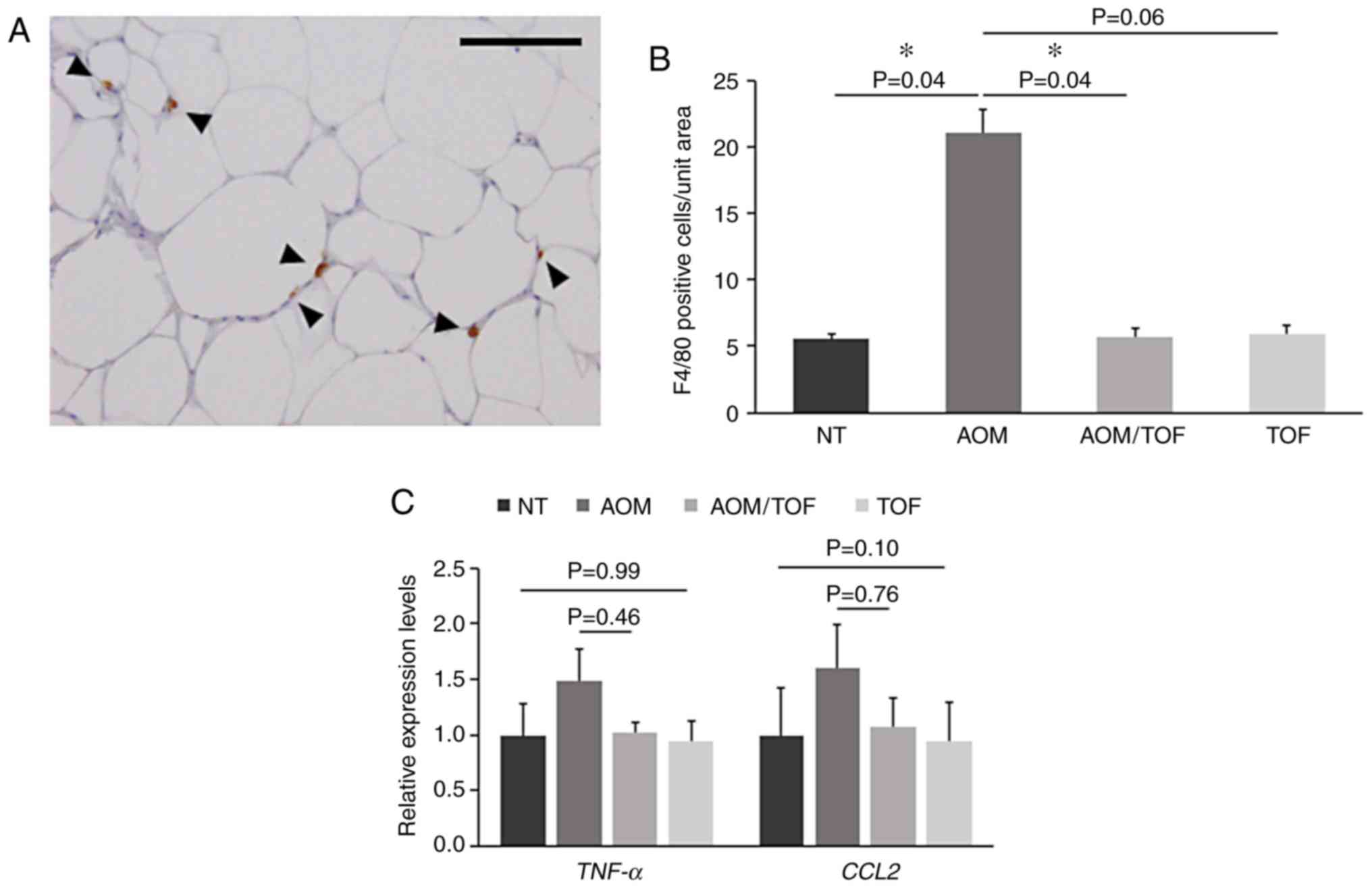
Macrophages play significant roles in
pro-inflammation in obese adipose tissues (40,41). In
the present study, immunohistochemical staining for F4/80 was
performed, which revealed significant higher macrophage
infiltrations in the white adipose tissues of the AOM-treated mice
as compared to the mice without AOM treatment (Fig. 3A). However, the number of F4/80
positive cells were decreased 0.25-fold in the mice treated with
AOM/tofogliflozin as compared to those treated with AOM alone
(Fig. 3B). In addition, it was
investigated whether tofogliflozin administration attenuated
chronic inflammation and decreased macrophage infiltration in the
white adipose tissues. The expression of the genes associated with
chronic inflammation, such as TNF-a and CCL2,
analyzed by qRT-PCR in the white adipose tissues tended to be lower
in the AOM plus tofogliflozin-treated group as compared to the
AOM-treated group, although the differences were not statistically
significant (Fig. 3C). These results
indicated that administration of tofogliflozin attenuated the
inflammatory response in the white adipose tissues of the diabetic
mice.
Effects of tofogliflozin on cellular
proliferation in the colorectal cancer cells
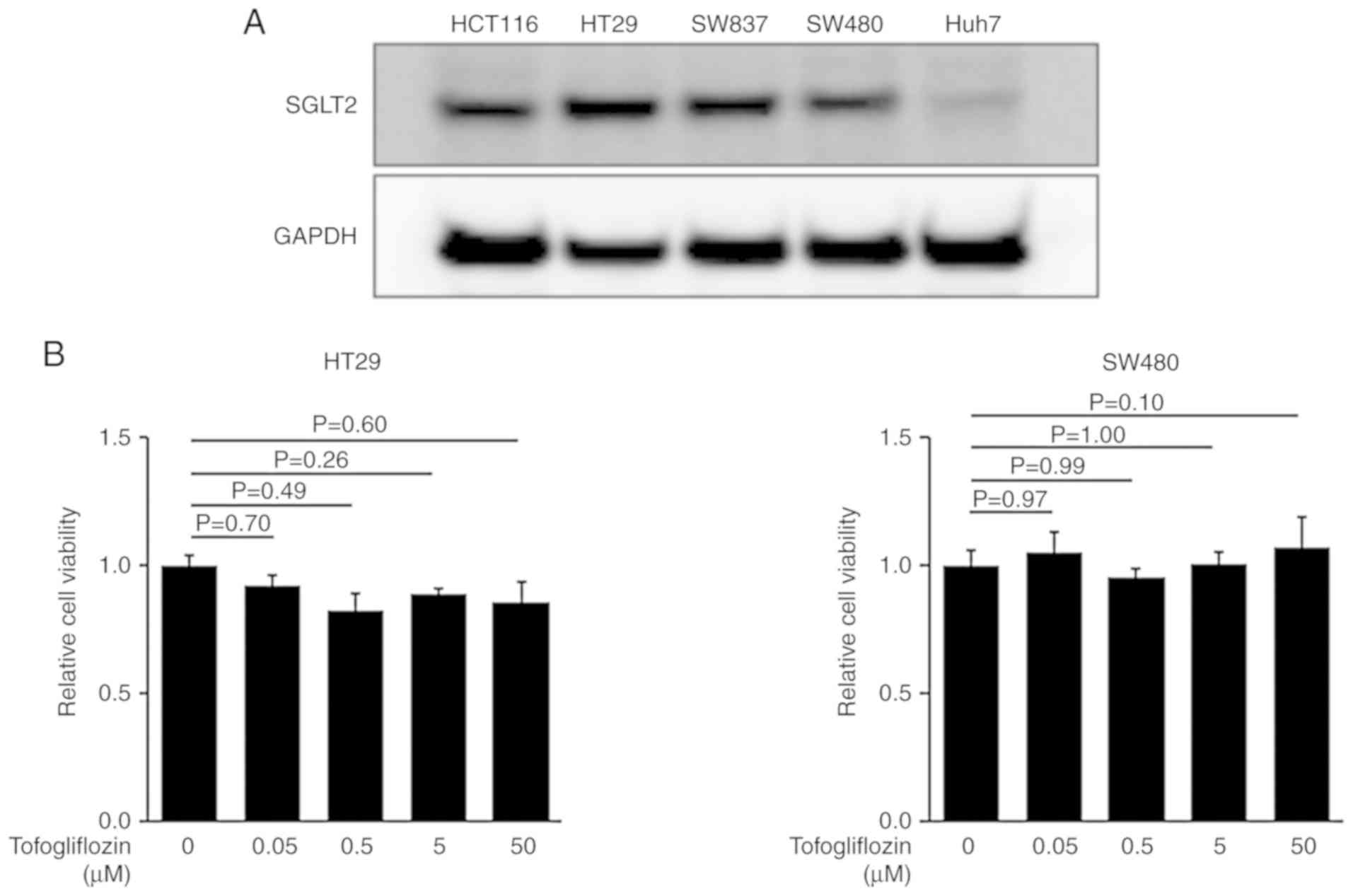
Recent studies have demonstrated that several types
of cancer cells exhibit SGLT2 expression (24–26).
Therefore, the expression of SGLT2 in four human CRC cell lines,
HT29, HCT116, SW837, and SW480 cells were examined. As revealed in
Fig. 4A, western blot analysis
revealed that SGLT2 was detected in these cells as well as the
hepatoma cell line Huh7 (positive control) (25). To evaluate whether tofogliflozin could
directly inhibit the growth of CRC cells, a cell proliferation
assay was performed using SGLT2-expressing HT29 and SW480 cells.
The MTS assay indicated that treatment with tofogliflozin exhibited
no effects on the proliferation of these cells (Fig. 4B).
Discussion
Diabetes mellitus is associated with a greater risk
of CRC. According to a meta-analysis, the overall hazard ratio for
CRC-specific incidence was 1.26, and CRC-specific mortality was
1.38 in patients with diabetes as compared to those without
diabetes (42). CRC and diabetes
share several cellular and molecular pathways, including epithelial
cell injury, activation of inflammation, and Wnt/β-catenin pathways
(43). Recently, researches on
inhibition of colorectal carcinogenesis by agents for diabetes have
been actively conducted. Among them, metformin was revealed to
reduce the risk of CRC-specific death by 34% in the CRC patients as
compared to those in the non-users (17). Intake of α-glucosidase inhibitors is
an independent factor associated with a decreased risk of
colorectal neoplasia (18). In the
present study, we have provided, to the best of our knowledge for
the first time, the evidence to demonstrate that the anti-diabetic
SGLT2 inhibitor tofogliflozin suppressed AOM-induced colorectal
carcinogenesis in diabetic and obese db/db mice. SGLT2
inhibitors are oral anti-diabetic medicines that promote urinary
glucose excretion leading to a reduction of hyperglycemia (20).
Chronic inflammation is one of the key mechanisms in
diabetes- and obesity-related CRC development. Notably, enhanced
inflammation in the adipose tissue is known as a key feature
linking obesity with the development and progression of CRC
(3). Infiltration of the macrophages
into white adipose tissues is considered an important phenomenon
for the development of chronic systemic inflammation at an early
phase, which usually coexists with the production of TNF-α
(40). In this study, treatment with
tofogliflozin reduced the expression of TNF-α and
CCL2 in the white adipose tissues of the AOM-administered
mice. In addition, macrophage accumulation in adipose tissue,
analyzed by immunohistochemistry for F4/80, was significantly
inhibited by tofogliflozin. These findings indicated that
attenuation of adipose tissue inflammation through inhibition of
macrophage infiltration is important to prevent the development of
colorectal carcinogenesis in db/db mice. The decreasing
tendency of the level of TNF-α in serum in the
tofogliflozin-treated mice was due to the reduction of chronic
inflammation in adipose tissues. Consistent with these findings,
recent studies have also indicated that SGLT2 inhibitors could
attenuate chronic inflammation in the adipose tissues as well as in
the liver (25,44), suggesting that the anti-inflammatory
effects of SGLT2 inhibitors may be a novel action of these agents
apart from lowering blood glucose.
The IGF/IGF-IR system is generally considered to
play a significant role in colorectal carcinogenesis and is one of
the molecular targets for the prevention and treatment of CRC
(16,45). An increased level of IGF-1 is related
to a higher risk of CRC, while genetic reduction of IGF-1 could
suppress colorectal carcinogenesis (46,47). The
results of the present study demonstrated that the expression of
IGF-1 in the colonic mucosa of mice was decreased by tofogliflozin.
In a similar experimental model, a type of green tea-containing
catechins was revealed to suppress CRC tumorigenesis by blocking
IGF signals in the colorectal mucosa (16). Collectively, these results indicated
that tofogliflozin may prevent the development of diabetes- and
obesity-related colorectal tumorigenesis, at least in part, through
amelioration of the hyperglycemic condition and inhibition of IGF
signaling.
Tofogliflozin has been reported to be a potent and
highly specific SGLT2 inhibitor which improves glycemic control in
diabetic rodents and human patients (48,49).
Consistent with previous studies, the present study demonstrated
that administration of tofogliflozin lowered blood glucose levels
in experimental mice, although no statistical significance was
reached. Recently, it was reported by Wu et al that one of
the factors contributing as a link between diabetes and cancer may
be a high glucose environment (11).
They indicated that a hyperglycemic state could drive genetic and
epigenetic alterations, in which high glucose may lead to
destabilization of the tumor suppressor ten-eleven translocation
(TET)-2 through dysregulation of 5-hydroxymethylcytosine.
Originally, only proximal renal tubules were
reported to contain SGLT2 (50).
However, recent studies have demonstrated that cancer cells also
express SGLT2 (24,25) and inhibition of this glucose
transporter leads to the reduction in the growth of several types
of cancer, including that of the pancreas, prostate, and liver
(24–26). In the present study, it was
demonstrated for the first time that SGLT2 is also expressed in CRC
cell lines. A cell proliferation assay revealed that tofogliflozin
displayed no significant alteration of CRC cell growth, suggesting
that this agent has no direct effects on CRC and possibly on
colonic tumorigenesis. These findings are consistent with a
previous study in which tofogliflozin was not found to exhibit
suppressive effects on the growth of hepatoma cell lines (25). Other SGLT2 inhibitors, however, have
been reported to show direct effects on cancer proliferation
(24). The difference among several
SGLT2 inhibitors may be due to SGLT2 selectivity. Most of the SGLT2
inhibitors have the inhibitory effects on SGLT1 as well as SGLT2.
SGLT1 is reported to display antitumor effects, especially when
co-expressed with receptor tyrosine kinase EGFR, in many types of
cancers including CRC (51–55), indicating that SGLT1 may have an
important efficacy on cancer proliferation. The selectivity of
tofogliflozin toward SGLT2 vs. SGLT1 was revealed to be the highest
among SGLT2 inhibitors under clinical development (49), suggesting that inhibition of SGLT1 by
‘SGLT2’ inhibitors played a role when using the inhibitors other
than tofogliflozin. Further studies to investigate the roles of
SGLTs and other glucose transporters in cancer cells are
required.
In summary, the present study demonstrated that
administration of the SGLT2 inhibitor tofogliflozin markedly
suppressed colorectal carcinogenesis in AOM-injected db/db
mice, presumably through attenuation of chronic inflammation
induced by obesity and diabetes. The risk of CRC is increased by
diabetes and obesity and their related metabolic abnormalities.
Therefore, the abnormalities, including chronic inflammation and
hyperglycemic state, may be effective therapeutic targets for
preventing CRC in patients having these co-existing conditions.
Since SGLT2 inhibitors are used in clinical practice without
causing severe adverse reactions (56), further studies should investigate
whether this class of drug can be used for CRC chemoprevention in
diabetic individuals.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Akihiro Abe and
Toshiki Ohta for technical assistance with the experiments. The
authors are also grateful to Miho Yagi, Chiyoko Sano, Hitomi
Fujisawa, and Eriko Kunishima for secretarial assistance.
Funding
The present study was supported in part by
Grants-in-Aid from the Ministry of Education, Science, Sports, and
Culture of Japan (nos. 25860529, 16K09352, and 17K15936).
Availability of data and materials
The datasets used and analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JK, YS, MS and MO conceived and designed the
experiments. JK, YS, MO, TM, MK, HS and TT performed the
experiments. JK, YS, MO, TI and TT analyzed the data. JK, YS and MS
wrote the paper. All authors read and approved the manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The experimental protocol was approved by the
Committee of Institutional Animal Experiments of Gifu University
(authorization code: 30-7, dated 13 April 2018).
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Giovannucci E and Michaud D: The role of
obesity and related metabolic disturbances in cancers of the colon,
prostate, and pancreas. Gastroenterology. 132:2208–2225. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gunter MJ and Leitzmann MF: Obesity and
colorectal cancer: Epidemiology, mechanisms and candidate genes. J
Nutr Biochem. 17:145–156. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cade WT: Diabetes-related microvascular
and macrovascular diseases in the physical therapy setting. Phys
Ther. 88:1322–1335. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Calle EE, Rodriguez C, Walker-Thurmond K
and Thun MJ: Overweight, obesity, and mortality from cancer in a
prospectively studied cohort of U.S. adults. N Engl J Med.
348:1625–1638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giovannucci E, Harlan DM, Archer MC,
Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG and
Yee D: Diabetes and cancer: A consensus report. Diabetes Care.
33:1674–1685. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kasuga M, Ueki K, Tajima N, Noda M, Ohashi
K, Noto H, Goto A, Ogawa W, Sakai R, Tsugane S, et al: Report of
the Japan diabetes Society/Japanese cancer association joint
committee on diabetes and cancer. Cancer Sci. 104:965–976. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ishino K, Mutoh M, Totsuka Y and Nakagama
H: Metabolic syndrome: A novel high-risk state for colorectal
cancer. Cancer Lett. 334:56–61. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pais R, Silaghi H, Silaghi AC, Rusu ML and
Dumitrascu DL: Metabolic syndrome and risk of subsequent colorectal
cancer. World J Gastroenterol. 15:5141–5148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shimizu M, Kubota M, Tanaka T and Moriwaki
H: Nutraceutical approach for preventing obesity-related colorectal
and liver carcinogenesis. Int J Mol Sci. 13:579–595. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu D, Hu D, Chen H, Shi G, Fetahu IS, Wu
F, Rabidou K, Fang R, Tan L, Xu S, et al: Glucose-regulated
phosphorylation of TET2 by AMPK reveals a pathway linking diabetes
to cancer. Nature. 559:637–641. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hata K, Kubota M, Shimizu M, Moriwaki H,
Kuno T, Tanaka T, Hara A and Hirose Y: Monosodium glutamate-induced
diabetic mice are susceptible to azoxymethane-induced colon
tumorigenesis. Carcinogenesis. 33:702–707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tuominen I, Al-Rabadi L, Stavrakis D,
Karagiannides I, Pothoulakis C and Bugni JM: Diet-induced obesity
promotes colon tumor development in azoxymethane-treated mice. PLoS
One. 8:e609392013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fukuta K, Shirakami Y, Maruta A, Obara K,
Iritani S, Nakamura N, Kochi T, Kubota M, Sakai H, Tanaka T and
Shimizu M: Preventive effects of pentoxifylline on the development
of colonic premalignant lesions in obese and diabetic mice. Int J
Mol Sci. 18(pii): E4132017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kubota M, Shimizu M, Sakai H, Yasuda Y,
Terakura D, Baba A, Ohno T, Tsurumi H, Tanaka T and Moriwaki H:
Preventive effects of curcumin on the development of
azoxymethane-induced colonic preneoplastic lesions in male
C57BL/KsJ-db/db obese mice. Nutr Cancer. 64:72–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shimizu M, Shirakami Y, Sakai H, Adachi S,
Hata K, Hirose Y, Tsurumi H, Tanaka T and Moriwaki H:
(−)-Epigallocatechin gallate suppresses azoxymethane-induced
colonic premalignant lesions in male C57BL/KsJ-db/db mice. Cancer
Prev Res (Phila). 1:298–304. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang YT, Tsai HL, Kung YT, Yeh YS, Huang
CW, Ma CJ, Chiu HC and Wang JY: Dose-dependent relationship between
metformin and colorectal cancer occurrence among patients with type
2 Diabetes-A nationwide cohort study. Transl Oncol. 11:535–541.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Horibe Y, Adachi S, Ohno T, Goto N, Okuno
M, Iwama M, Yamauchi O, Kojima T, Saito K, Ibuka T, et al:
Alpha-glucosidase inhibitor use is associated with decreased
colorectal neoplasia risk in patients with type 2 diabetes mellitus
receiving colonoscopy: A retrospective study. Oncotarget.
8:97862–97870. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Imamura M, Nakanishi K, Suzuki T, Ikegai
K, Shiraki R, Ogiyama T, Murakami T, Kurosaki E, Noda A, Kobayashi
Y, et al: Discovery of ipragliflozin (ASP1941): A novel C-glucoside
with benzothiophene structure as a potent and selective sodium
glucose co-transporter 2 (SGLT2) inhibitor for the treatment of
type 2 diabetes mellitus. Bioorg Med Chem. 20:3263–3279. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tahrani AA, Barnett AH and Bailey CJ: SGLT
inhibitors in management of diabetes. Lancet Diabetes Endocrinol.
1:140–151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fonseca VA, Ferrannini E, Wilding JP,
Wilpshaar W, Dhanjal P, Ball G and Klasen S: Active- and
placebo-controlled dose-finding study to assess the efficacy,
safety, and tolerability of multiple doses of ipragliflozin in
patients with type 2 diabetes mellitus. J Diabetes Complications.
27:268–273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tahara A, Kurosaki E, Yokono M, Yamajuku
D, Kihara R, Hayashizaki Y, Takasu T, Imamura M, Li Q, Tomiyama H,
et al: Effects of sodium-glucose cotransporter 2 selective
inhibitor ipragliflozin on hyperglycaemia, oxidative stress,
inflammation and liver injury in streptozotocin-induced type 1
diabetic rats. J Pharm Pharmacol. 66:975–987. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tahara A, Kurosaki E, Yokono M, Yamajuku
D, Kihara R, Hayashizaki Y, Takasu T, Imamura M, Qun L, Tomiyama H,
et al: Pharmacological profile of ipragliflozin (ASP1941), a novel
selective SGLT2 inhibitor, in vitro and in vivo. Naunyn
Schmiedebergs Arch Pharmacol. 385:423–436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaji K, Nishimura N, Seki K, Sato S,
Saikawa S, Nakanishi K, Furukawa M, Kawaratani H, Kitade M, Moriya
K, et al: Sodium glucose cotransporter 2 inhibitor canagliflozin
attenuates liver cancer cell growth and angiogenic activity by
inhibiting glucose uptake. Int J Cancer. 142:1712–1722. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Obara K, Shirakami Y, Maruta A, Ideta T,
Miyazaki T, Kochi T, Sakai H, Tanaka T, Seishima M and Shimizu M:
Preventive effects of the sodium glucose cotransporter 2 inhibitor
tofogliflozin on diethylnitrosamine-induced liver tumorigenesis in
obese and diabetic mice. Oncotarget. 8:58353–58363. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Scafoglio C, Hirayama BA, Kepe V, Liu J,
Ghezzi C, Satyamurthy N, Moatamed NA, Huang J, Koepsell H, Barrio
JR and Wright EM: Functional expression of sodium-glucose
transporters in cancer. Proc Natl Acad Sci USA. 112:E4111–E4119.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Utsunomiya K, Shimmoto N, Senda M,
Kurihara Y, Gunji R, Kameda H, Tamura M, Mihara H and Kaku K:
Japanese study of tofogliflozin with type 2 diabetes mellitus
patients in an observational study of the elderly (J-STEP/EL): A
12-week interim analysis. J Diabetes Investig. 7:755–763. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Terakura D, Shimizu M, Iwasa J, Baba A,
Kochi T, Ohno T, Kubota M, Shirakami Y, Shiraki M, Takai K, et al:
Preventive effects of branched-chain amino acid supplementation on
the spontaneous development of hepatic preneoplastic lesions in
C57BL/KsJ-db/db obese mice. Carcinogenesis. 33:2499–2506. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hata K, Tanaka T, Kohno H, Suzuki R, Qiang
SH, Yamada Y, Oyama T, Kuno T, Hirose Y, Hara A and Mori H:
Beta-catenin-accumulated crypts in the colonic mucosa of juvenile
ApcMin/+ mice. Cancer Lett. 239:123–128. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miyazaki T, Shirakami Y, Kubota M, Ideta
T, Kochi T, Sakai H, Tanaka T, Moriwaki H and Shimizu M: Sodium
alginate prevents progression of non-alcoholic steatohepatitis and
liver carcinogenesis in obese and diabetic mice. Oncotarget.
7:10448–10458. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shirakami Y, Shimizu M, Kubota M, Ohno T,
Kochi T, Nakamura N, Sumi T, Tanaka T, Moriwaki H and Seishima M:
Pentoxifylline prevents nonalcoholic steatohepatitis-related liver
pre-neoplasms by inhibiting hepatic inflammation and lipogenesis.
Eur J Cancer Prev. 25:206–215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shirakami Y, Gottesman ME and Blaner WS:
Diethylnitrosamine-induced hepatocarcinogenesis is suppressed in
lecithin:retinol acyltransferase-deficient mice primarily through
retinoid actions immediately after carcinogen administration.
Carcinogenesis. 33:268–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nagata T, Fukuzawa T, Takeda M, Fukazawa
M, Mori T, Nihei T, Honda K, Suzuki Y and Kawabe Y: Tofogliflozin,
a novel sodium-glucose co-transporter 2 inhibitor, improves renal
and pancreatic function in db/db mice. Br J Pharmacol. 170:519–531.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bird RP and Good CK: The significance of
aberrant crypt foci in understanding the pathogenesis of colon
cancer. Toxicol Lett. 112-113:395–402. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamada Y and Mori H: Pre-cancerous lesions
for colorectal cancers in rodents: A new concept. Carcinogenesis.
24:1015–1019. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kubota M, Shimizu M, Sakai H, Yasuda Y,
Ohno T, Kochi T, Tsurumi H, Tanaka T and Moriwaki H:
Renin-angiotensin system inhibitors suppress azoxymethane-induced
colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice.
Biochem Biophys Res Commun. 410:108–113. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Szlosarek P, Charles KA and Balkwill FR:
Tumour necrosis factor-alpha as a tumour promoter. Eur J Cancer.
42:745–750. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen J, Wu A, Sun H, Drakas R, Garofalo C,
Cascio S, Surmacz E and Baserga R: Functional significance of type
1 insulin-like growth factor-mediated nuclear translocation of the
insulin receptor substrate-1 and beta-catenin. J Biol Chem.
280:29912–29920. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Itzkowitz SH and Yio X: Inflammation and
cancer IV. Colorectal cancer in inflammatory bowel disease: The
role of inflammation. Am J Physiol Gastrointest Liver Physiol.
287:G7–G17. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Weisberg SP, McCann D, Desai M, Rosenbaum
M, Leibel RL and Ferrante AW Jr: Obesity is associated with
macrophage accumulation in adipose tissue. J Clin Invest.
112:1796–1808. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xu H, Barnes GT, Yang Q, Tan G, Yang D,
Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA and Chen H:
Chronic inflammation in fat plays a crucial role in the development
of obesity-related insulin resistance. J Clin Invest.
112:1821–1830. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
De Bruijn KM, Arends LR, Hansen BE,
Leeflang S, Ruiter R and van Eijck CH: Systematic review and
meta-analysis of the association between diabetes mellitus and
incidence and mortality in breast and colorectal cancer. Br J Surg.
100:1421–1429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gonzalez N, Prieto I, Del Puerto-Nevado L,
Portal-Nuñez S, Ardura JA, Corton M, Fernández-Fernández B,
Aguilera O, Gomez-Guerrero C, Mas S, et al: 2017 update on the
relationship between diabetes and colorectal cancer: Epidemiology,
potential molecular mechanisms and therapeutic implications.
Oncotarget. 8:18456–18485. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu L, Nagata N, Nagashimada M, Zhuge F, Ni
Y, Chen G, Mayoux E, Kaneko S and Ota T: SGLT2 inhibition by
empagliflozin promotes fat utilization and browning and attenuates
inflammation and insulin resistance by polarizing M2 macrophages in
Diet-induced obese mice. EBioMedicine. 20:137–149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ealey KN, Xuan W, Lu S and Archer MC:
Colon carcinogenesis in liver-specific IGF-I-deficient (LID) mice.
Int J Cancer. 122:472–476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Giovannucci E, Pollak MN, Platz EA,
Willett WC, Stampfer MJ, Majeed N, Colditz GA, Speizer FE and
Hankinson SE: A prospective study of plasma insulin-like growth
factor-1 and binding protein-3 and risk of colorectal neoplasia in
women. Cancer Epidemiol Biomarkers Prev. 9:345–349. 2000.PubMed/NCBI
|
|
47
|
Olivo-Marston SE, Hursting SD, Lavigne J,
Perkins SN, Maarouf RS, Yakar S and Harris CC: Genetic reduction of
circulating insulin-like growth factor-1 inhibits
azoxymethane-induced colon tumorigenesis in mice. Mol Carcinog.
48:1071–1076. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kaku K, Watada H, Iwamoto Y, Utsunomiya K,
Terauchi Y, Tobe K, Tanizawa Y, Araki E, Ueda M, Suganami H, et al:
Efficacy and safety of monotherapy with the novel sodium/glucose
cotransporter-2 inhibitor tofogliflozin in Japanese patients with
type 2 diabetes mellitus: A combined Phase 2 and 3 randomized,
placebo-controlled, double-blind, parallel-group comparative study.
Cardiovasc Diabetol. 13:652014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Suzuki M, Honda K, Fukazawa M, Ozawa K,
Hagita H, Kawai T, Takeda M, Yata T, Kawai M, Fukuzawa T, et al:
Tofogliflozin, a potent and highly specific sodium/glucose
cotransporter 2 inhibitor, improves glycemic control in diabetic
rats and mice. J Pharmacol Exp Ther. 341:692–701. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chao EC and Henry RR: SGLT2 inhibition-a
novel strategy for diabetes treatment. Nat Rev Drug Discov.
9:551–559. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hanabata Y, Nakajima Y, Morita K, Kayamori
K and Omura K: Coexpression of SGLT1 and EGFR is associated with
tumor differentiation in oral squamous cell carcinoma. Odontology.
100:156–163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lai B, Xiao Y, Pu H, Cao Q, Jing H and Liu
X: Overexpression of SGLT1 is correlated with tumor development and
poor prognosis of ovarian carcinoma. Arch Gynecol Obstet.
285:1455–1461. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu H, Ertay A, Peng P, Li J, Liu D, Xiong
H, Zou Y, Qiu H, Hancock D, Yuan X, et al: SGLT1 is required for
the survival of triple-negative breast cancer cells via
potentiation of EGFR activity. Mol Oncol. 13:1874–1886. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Madunić IV, Madunić J, Breljak D, Karaica
D and Sabolić I: Sodium-glucose cotransporters: New targets of
cancer therapy? Arh Hig Rada Toksikol. 69:278–285. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ren J, Bollu LR, Su F, Gao G, Xu L, Huang
WC, Hung MC and Weihua Z: EGFR-SGLT1 interaction does not respond
to EGFR modulators, but inhibition of SGLT1 sensitizes prostate
cancer cells to EGFR tyrosine kinase inhibitors. Prostate.
73:1453–1461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Rosenwasser RF, Rosenwasser JN, Sutton D,
Choksi R and Epstein B: Tofogliflozin: A highly selective SGLT2
inhibitor for the treatment of type 2 diabetes. Drugs Today (Barc).
50:739–745. 2014. View Article : Google Scholar : PubMed/NCBI
|