Introduction
Head and neck squamous cell carcinoma (HNSCC) is a
common malignant tumor (1,2). Despite advances in the early detection,
diagnosis, and management of HNSCC, the long-term survival rate of
HNSCC patients has improved only marginally over the past decades,
and more effective strategies for the treatment of HNSCC are thus
required (3). Although immunotherapy
with anti-programmed cell death protein 1 (anti-PD-1) and therapy
with anti-epidermal growth factor receptor (anti-EGFR) were
recently approved in many countries for the treatment of HNSCC
patients, cisplatin is still the most widely used antitumor drug
for HNSCC (4–6).
First synthesized in 1844 (7), cisplatin, or
cis-diamminedichloroplatinum (II), is a metallic (platinum)
coordination compound with a square planar molecular geometry.
Cisplatin is activated via the replacement of a chloride ligand
with H2O in the cancer cell cytoplasm. Activated
cisplatin binds to the N7 atom of guanines in DNA, which in turn
generates intra-strand DNA crosslinks (ICLs) (8). ICLs are cytotoxic lesions with a
covalent linkage between opposite strands of double-stranded DNA.
ICLs lead to defects of vital DNA metabolic processes such as
transcription and DNA replication in cancer cells, resulting in
cancer cell death (9). HNSCC patients
usually exhibit a good response to cisplatin chemotherapy however
later relapse, since the development of cisplatin resistance
markedly reduces the clinical effectiveness of this agent (10). Some research indicates that cisplatin
resistance can result from variations of genetic and protein
expression at the cellular level (11,12).
Another mechanism of cisplatin resistance consists of reducing the
cellular accumulation of cisplatin by increasing its efflux and
suppressing its influx (13). It was
revealed that the copper transporter ATP7B (ATPase copper
transporting beta) is linked to cisplatin efflux from cancer cells
(14). Copper transporter expression
is closely linked to the serum copper concentration (15). The copper chelator ammonium
tetrathiomolybdate (TM) may be effective for the treatment of
copper metabolism disorder and Wilson's disease (16). We previously demonstrated that copper
chelators have a bone-protective effect against bone-invasive HNSCC
cells (17), however, the potential
influence of TM on the accumulation of cisplatin in cancer cells in
HNSCC is not clear. The present findings provide the first evidence
that the copper chelator TM enhances the antitumor effect of
cisplatin via ATP7B suppression in an HNSCC mouse model.
Materials and methods
Reagents
SLC31A1/CTR1 antibody (anti-rabbit, polyclonal; cat.
no. GTX48534) was purchased from GeneTex. ATP7A (anti-rabbit,
polyclonal; ID product code ab125137) was purchased from Abcam.
ATP7B (anti-rabbit, polyclonal; cat. no. NB100-360) was purchased
from Novus Biologicals. Cleaved caspase-3 (Asp175) (5A1E)
(anti-rabbit, monoclonal; product no. 9664), Ki-67 (D2H10)
(anti-rabbit, monoclonal; product no. 9027), and horseradish
peroxidase (HRP)-conjugated IgG antibody (goat anti-rabbit,
monoclonal; product no. 7074) were purchased from Cell Signaling
Technology, Inc.
Cell lines and culture conditions
The human oral squamous cell carcinoma lines HSC-2
(#JCRB0622), HSC-3 (#JCRB0623), and HSC-4 (#JCRB0624) and the human
skin squamous cell carcinoma line A431 (#JCRB004) were obtained
from the Human Science Research Resources Bank (Osaka, Japan). All
cell lines were cultured in Dulbecco's modified Eagle's medium
(DMEM) (Thermo Fisher Scientific, Inc.) supplemented with 10%
heat-inactivated fetal bovine serum (FBS) and 1%
penicillin-streptomycin. All cell lines were cultured in an
atmosphere of 10% CO2 at 37°C. The
cis-dichloro-diamine-platinum (CDDP)-resistant subline
A431/CDDP-R was derived from the previously established human
epidermoid carcinoma cell line A431 (18). The subline A431/CDDP-R, which was
established by mutagenic induction, was revealed to have 2.7 times
more resistance to CDDP than the parent cell line A431 based on the
half maximal inhibitory concentration (IC50) (19).
Tissue microarray analysis
The expression of ATP7B was analyzed in head and
neck cancer tissue and in a normal tissue microarray (#OR601c; US
Biomax). The antigen was activated by cooking in a citric acid
solution. For the immunohistochemical analysis, the specimens were
incubated with anti-ATP7B antibody (1:250) overnight at 4°C. The
slides were then treated with a streptavidin-biotin complex
(EnVision System Labeled Polymer, HRP; Dako; Agilent Technologies,
Inc.) for 60 min at a dilution of 1:100. The immunoreaction was
visualized with the use of a DAB substrate-chromogen solution (Dako
Cytomation Liquid DAB Substrate Chromogen System; Dako; Agilent
Technologies, Inc.). The cells were counted using a light
microscope and evaluated.
Western blot analysis
Protein determination performed by Bradford assay. A
total of 15 µg protein were mixed with 4X Laemmli sample buffer
(Bio-Rad Laboratories, Inc.) and boiled at 95°C for 5 min. The
samples were electrophoresed in 4–12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels, and the
proteins were transferred onto polyvinylidene difluoride (PVDF)
membranes (Bio-Rad Laboratories, Inc.) and blocking with 5% skim
milk for 1 h. The membranes were incubated with primary (4°C, for
24 h) and secondary (room temperature, for 1 h) antibodies
according to the ECL chemiluminescence protocol (product no.
RPN2109; Amersham Biosciences; GE Healthcare Life Sciences) to
detect secondary antibody binding. Antibodies against ATP7A
(1:1,000), ATP7B (1:1,000), CTR1 (1:1,000), caspase-3 (1:1,000),
cleaved caspase-3 (1:1,000), β-actin (1:10,000) and GAPDH (2,000)
were used as primary antibodies. HRP-conjugated anti-rabbit
antibody (1:2,000) was used as the secondary antibody. A ChemiDoc
MP system (Bio-Rad Laboratories, Inc.) was used for the analysis of
western blots.
Flow cytometric analysis
HSC-3 cells were treated with cisplatin with or
without TM for 24 h. Cells were washed and fixed, then incubated
with Annexin V-FITC and PE (cat. no. 88-8005-72, cat. no. 00−6990;
eBioscience; Thermo Fisher Scientific, Inc.). After staining, the
cells were washed, suspended in the FACS staining buffer, and
analyzed on a FACS Aria III flow cytometer using FlowJo. Ver.10
(both from BD Biosciences).
Real-time PCR analysis
Total RNA from cells was extracted using RNA easy
Mini kit (Qiagen Sciences, Inc.). cDNA synthesis was performed with
1 µg of total RNA using PrimeScript (Takara Bio, Inc.). Real time
PCR analysis was carried out with iQ SYBR Green Mix using the CFX
Connect Real-Time PCR Detection System (Bio-Rad Laboratories,
Inc.). Detection was carried under following cycle conditions.
Initial denaturation at 95°C for 30 sec, followed by 41 cycles of
95°C for 10 sec and 60°C for 35 sec.
The primer sequence for ATP7B was forward,
5-GCCAGCATTGCAGAAGGAAAG-3 and reverse, 5-TGATAAGTGATGACGGCCTCT-3;
and for β-actin, forward, 5-GAAAATCTGGCACCACACCTT-3 and reverse,
5-TTGAAGGTAGTTTCGTGGAT-3.
The relative fold change values were evaluated by
normalization to β-actin expression via the 2−ΔΔCq
method (20).
Cell proliferation assay
The HSC-2 and HSC-3 cells were each plated in
six-well plates at a density of 1×105 cells/well. Two
6-well plates, one for each cell line, were used for each of the
four groups: A control, cisplatin-treated, TM-treated, and
cisplatin+TM-treated group. After 72 h, the cells were counted
using a TC20 automated cell counter (Bio-Rad Laboratories,
Inc.).
Immunocytochemical analysis
HSC-3 cells were plated on culture slides (BD
Falcon; BD Biosciences) at a density of 1×103
cells/well. After cisplatin or TM treatment, the number of
apoptotic cells was assessed by the DeadEnd™ Fluorometric TUNEL
System (Promega, Corporation).
Cisplatin concentration assay
The cisplatin concentrations in the cell suspensions
were measured by a cisplatin assay kit (MicroMolar Cisplatin Assay
Kit; ProFoldin). The samples, buffer, and chelate color solution
were mixed and incubated for 60 min at 65°C. The absorbance was
then read at a wavelength of 535 nm using a microplate reader
(SH-1000; Hitachi).
Animal experiments
A mouse model of bone invasion by human oral
squamous cell carcinoma was established in 5-week-old male BALB/c
nude mice (n=6 per group; n=24 total; mean body weight, 19.5 g;
Charles River Laboratories) by i.p. inoculation of 1×105
HSC-3 cells into the bone marrow space of the right tibial
metaphysis under general anesthesia with 0.4 mg/kg of medetomidine,
4.0 mg/kg of midazolam and 5.0 mg/kg of butorphanol Mice were
maintained in SPF cages. Body condition scoring was applied and
body weight was monitored daily. At 7 days after the tumor cell
inoculation, the mice were divided into four groups (control,
cisplatin-treated, TM-treated, and cisplatin+TM-treated). The
cisplatin group was treated with a single intraperitoneal injection
of 100 µl of cisplatin (5 mg/kg). The TM group was orally
administered 200 µl of a solution containing TM (1 mg) in
phosphate-buffered saline (PBS) 5×/week for 2 weeks. The
cisplatin+TM-treated group was treated with both agents at the
doses used in the cisplatin group and TM group. At the end of the
experimental period (day 35), the mice were sacrificed with
cervical dislocation by formal trained researcher under anesthesia
with 0.4 mg/kg of medetomidine, 4.0 mg/kg of midazolam and 5.0
mg/kg of butorphanol (i.p) and the right tibias of the nude mice
that had been injected with the cancer cells were excised and then
fixed in 4% paraformaldehyde phosphate buffer solution. There were
no differences in body weight in the control (24.9 g), cisplatin
(24.1 g), TM (24.5 g) and dual-treated group (23.9 g) at the end of
the experiment. The criteria of humane endpoints for euthanasia was
loss of >20 percent of body weight compared to the age-matched
controls. Death of the animal was verified by cessation of
cardiovascular and respiratory movements. All of the animal
experimental protocols were approved by the Ethics Review Committee
for Animal Experimentation of the Okayama University Graduate
School of Medicine and Dentistry (approval no. OKU-2018663).
In vivo radiography and assessment of
osteolytic lesion areas
Osteolytic bone destruction in the mice was assessed
on radiographs. The bones were placed against films (22×27 cm; Fuji
Industrial Film FR; Fuji Photo Film) and exposed to soft X-rays at
35 kV for 15 sec with the use of a Sofron apparatus (Sofron). The
radiolucent bone lesions were observed microscopically (IX81;
Olympus Corporation), and the areas were quantified with Lumina
Vision/OL image software (Mitani Corporation). A micro-CT image was
obtained with a SKYSCAN scanner (Bruker Japan).
Immunohistochemical analysis
Each tibial bone was fixed in 10% formalin at room
temperature for 48 h, decalcified, and then embedded in paraffin.
Serial sections were then prepared (5 µm-thick). The specimens were
incubated with ATP7B (1:250), Ki-67 (1:250) or IL-6 (1:100)
antibodies overnight at 4°C, followed by Alexa Fluor 488
anti-rabbit IgG (1:1,000) as a secondary antibody. Nuclei were
counterstained with Fluoroshield mounting medium with DAPI (product
no. ab104139; Abcam).
Statistical analysis
The data were analyzed using an unpaired Student's
t-test for comparisons of two groups and by performing a one-way
analysis of variance (ANOVA) and a post hoc Bonferroni or Dunnett's
test for multiple group comparisons. Graph Pad Prism, ver. 7.0
(GraphPad Software, Inc., La Jolla, CA, USA). was used for all
analyses. The results are expressed as the mean ± standard
deviation (SD). Probability (P)-values <0.05 were considered to
indicate a statistically significant difference.
Results
ATP7B expression in the human HNSCC
tissue
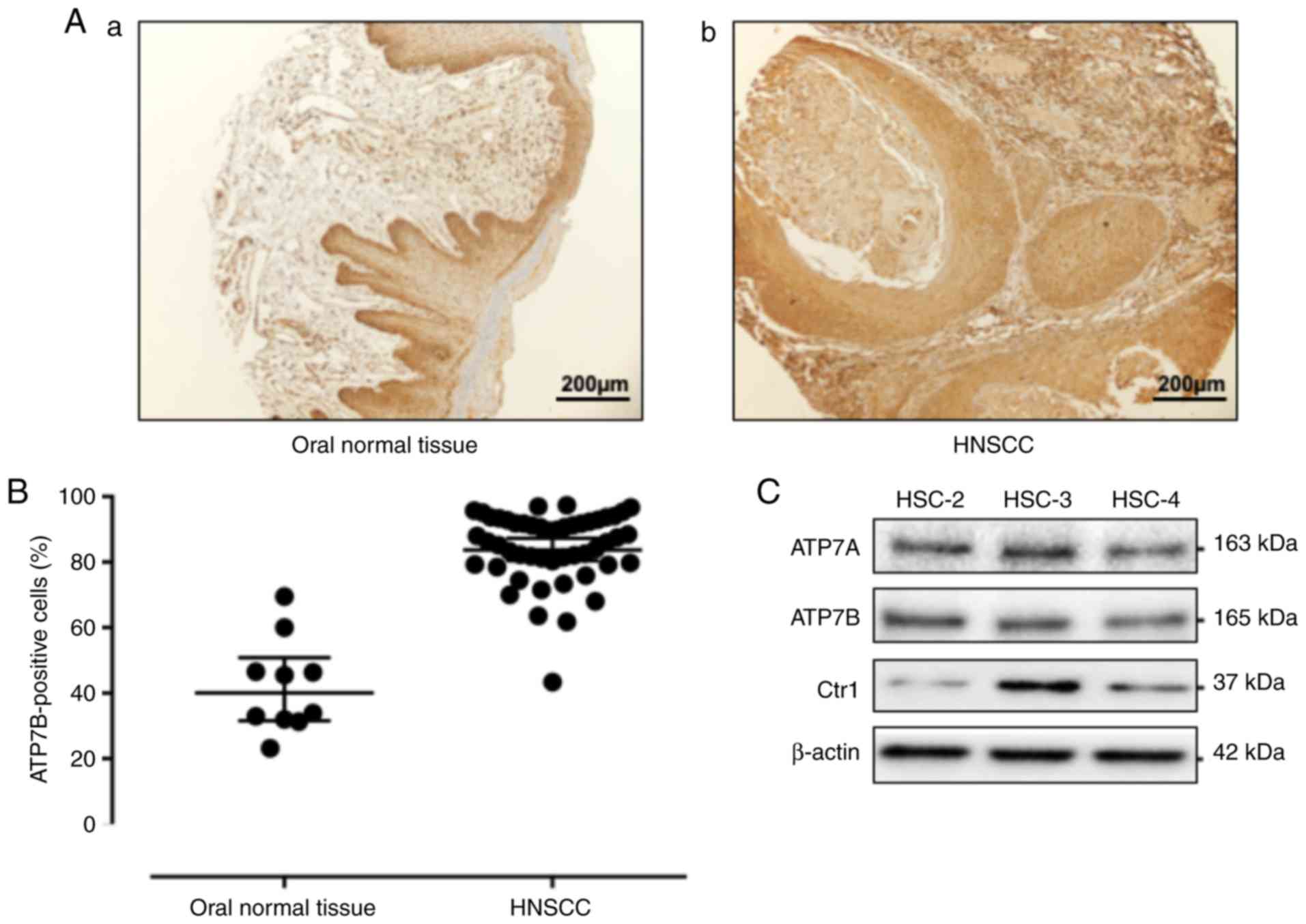
Fig. 1A provides a
representative histological pattern of normal oral tissue and HNSCC
tissue. ATP7B was expressed significantly higher in the HNSCC
samples compared to the normal epithelium samples (P<0.0001)
(Fig. 1B). To determine whether HNSCC
cells expressed ATP7B in vitro, western blot analysis was
performed in HSC-2, HSC-3 and HSC-4 cells. As revealed in Fig. 1C, the results of the western blot
analysis revealed a high expression of ATP7B in the HNSCC cells.
CTR1 is a cisplatin influx transporter. The human head and neck
carcinoma cell line HSC-3 markedly expressed CTR1. Thus, HSC-3
cells were used for the subsequent experiment.
TM enhances the antitumor effect of
cisplatin on oral squamous cell carcinoma cells
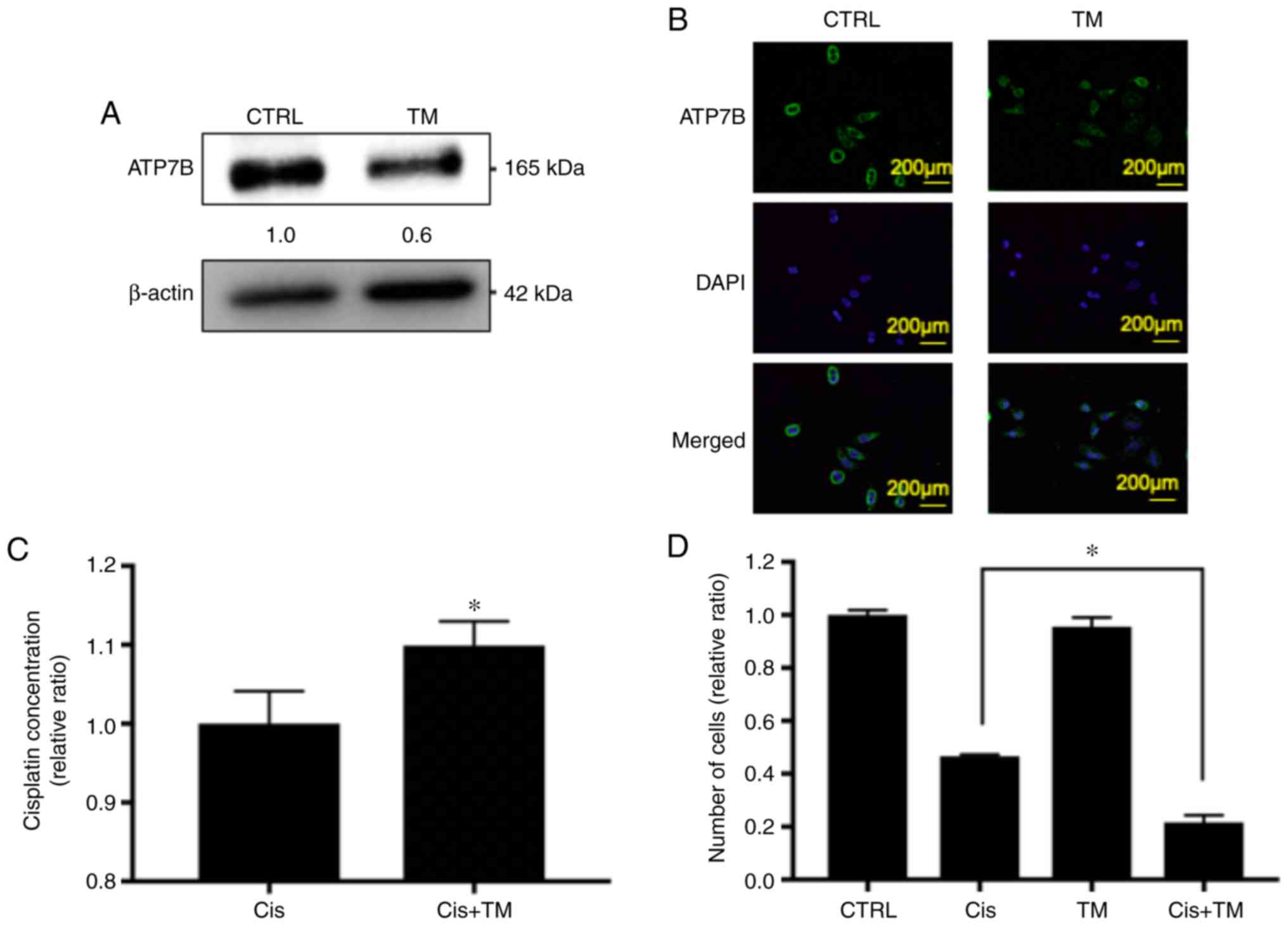
The effect of TM on the expression of the cisplatin
efflux transporter ATP7B was then assessed in HNSCC cells by
western blotting, immunocytochemical analysis and Real-time PCR.
First, TM was added to an HSC-3 culture medium for 24 h, and as
revealed in Figs. 2A and S1, the protein expression of ATP7B in the
TM-treated HSC-3 cells was decreased by 40%, while the mRNA
expression of ATP7B in the TM-treated HSC-3 cells was not altered.
Next, immunocytochemical analysis was performed, and the results
indicated that ATP7B expression in the cell membrane was decreased
by TM treatment, confirming the results of the western blotting
(Fig. 2B).
Based on these data, it was hypothesized that TM may
enhance the antitumor effect of cisplatin in HNSCC cells via an
accumulation of cisplatin. It was therefore evaluated whether TM
increased the cisplatin accumulation in the HNSCC cell lines, by
performing a platinum assay. As revealed in Fig. 2C, the cisplatin concentration in the
HSC-3 cells was increased by pretreatment with TM. Next, to analyze
the additive antitumor effect of TM and cisplatin against HNSCC
cells in vitro, a trypan blue staining assay was performed.
As revealed in Fig. 2D, TM did not
affect the number of viable HSC-3 cells up to 48 h after treatment
compared with the control. Cisplatin decreased the proliferation of
HSC-3 cells compared with the control group. Notably, the
TM+cisplatin dual treatment significant decreased the cell
proliferation compared to the single treatment of cisplatin. The
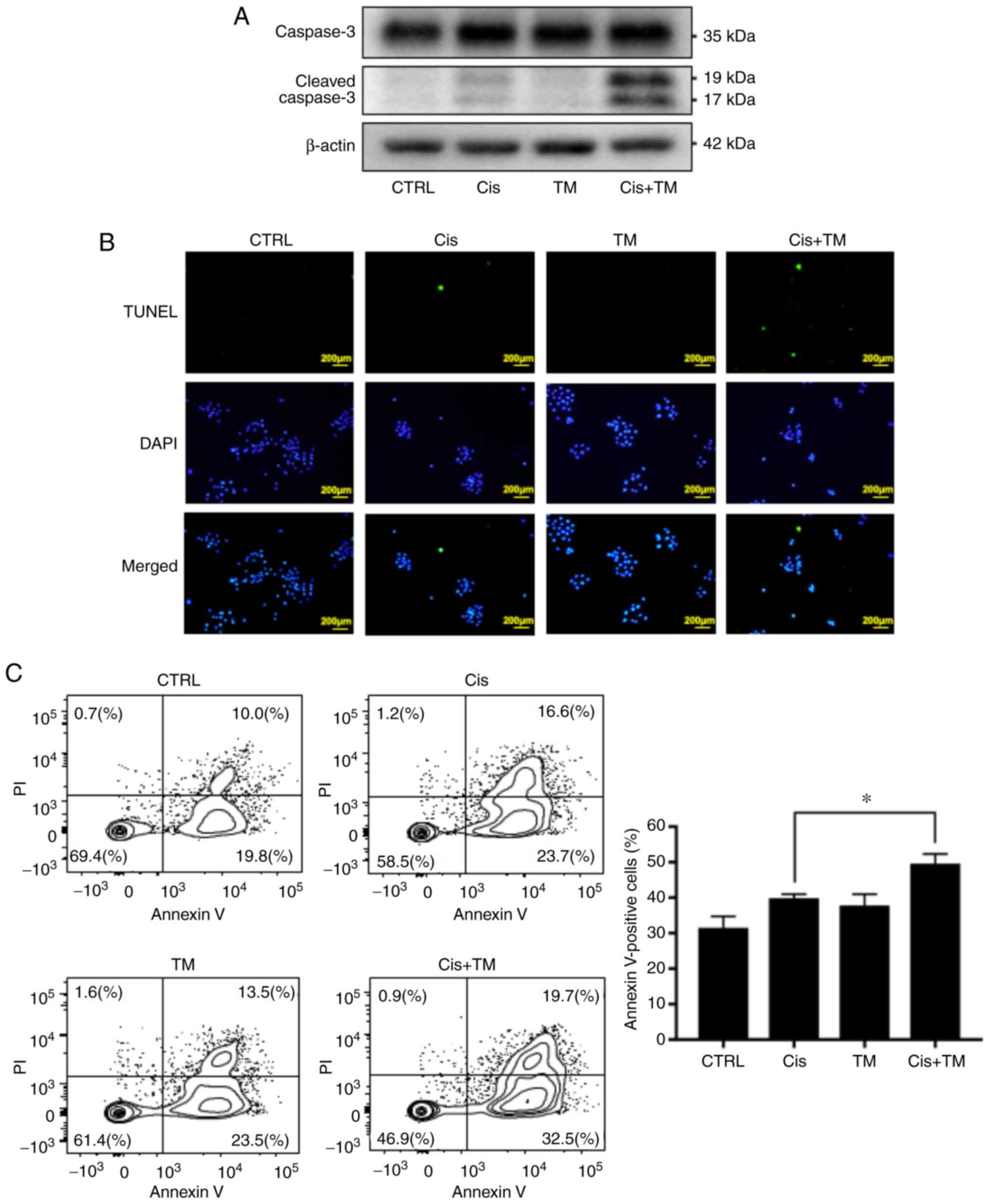
antitumor effect of cisplatin consists of inducing apoptosis by DNA
cross-linking in cancer cells. The cleavage of caspase-3 is well
known as an indicator of apoptosis. The effect of TM on
cisplatin-induced cleavage of caspase-3 was therefore assessed by
performing a western blot analysis. As revealed in Fig. 3A, TM and cisplatin did not affect the
expression of total caspase-3. Furthermore, TM did not directly
induce the cleavage of caspase-3. However, TM enhanced the
cisplatin-induced cleavage of caspase-3. Moreover, TM enhanced the
cisplatin-induced DNA fragmentation as evaluated by fluorescence
tunnel staining assay (Fig. 3B). Flow
cytometric analysis was also performed to evaluate the effect of TM
on cisplatin-induced HNSCC apoptosis. The plots of Annexin V/FITC-A
vs. propidium iodide-A from the gated cells revealed the
populations corresponding to viable and non-apoptotic (Annexin
V−PI−) and (Annexin
V+PI+, Annexin V+PI−)
apoptotic cells. TM did not directly induce apoptosis of HSC-3
cells (control 29.8%, TM 37.0%). However, TM significantly enhanced
the effect of cisplatin-induced apoptosis (cisplatin 40.3%,
cisplatin with TM 52.2%) (Fig.
3C).
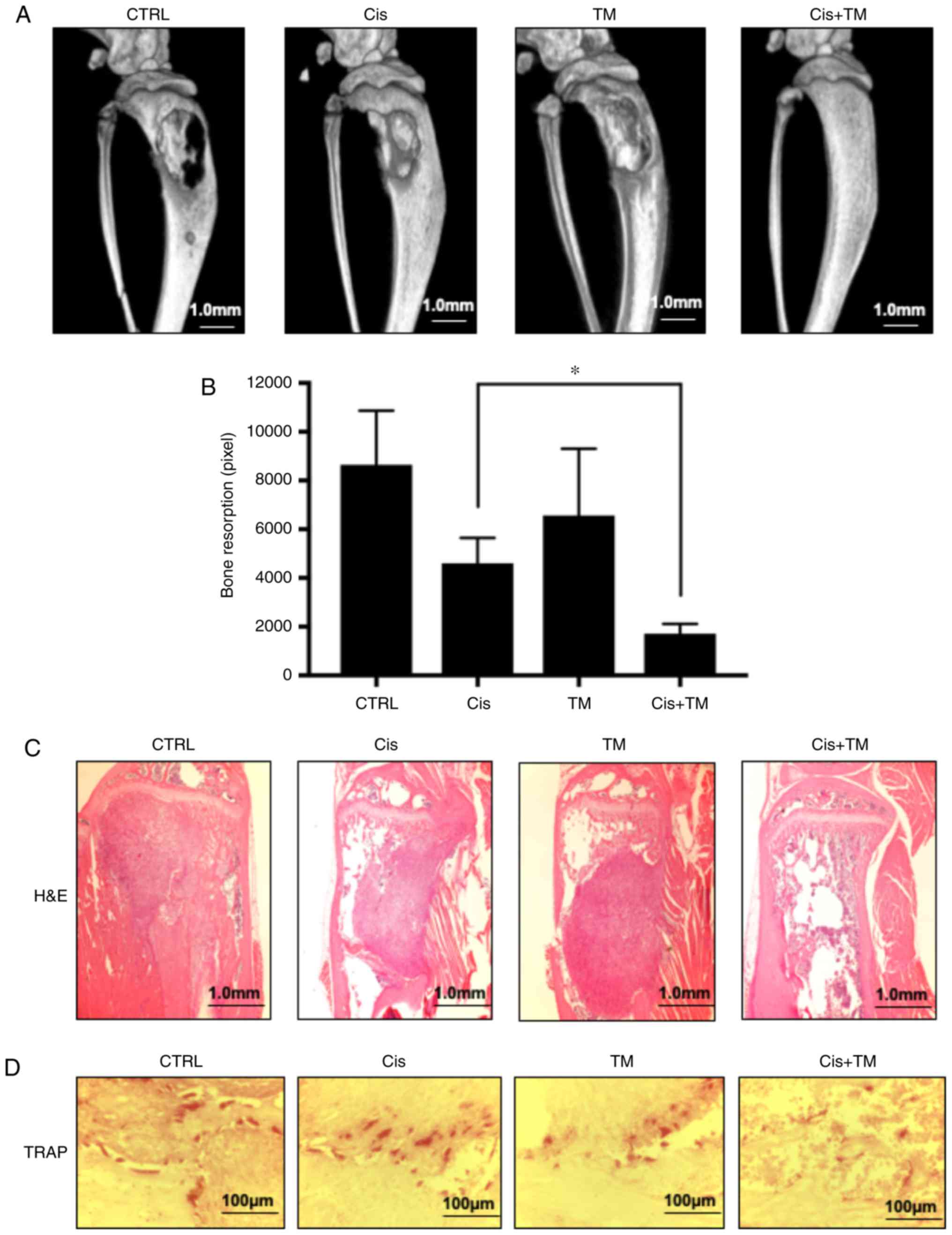
TM enhances the anticancer effect of
cisplatin and bone resorption in vivo
To analyze the dual treatment effect of TM+cisplatin
in vivo, an HNSCC bone invasion mouse model was established
using HSC-3 cells. The mice were administered TM (1 mg/kg) and/or a
low dose cisplatin (5 mg/kg) 5×/week beginning 7 days after the
tumor inoculation, and the tumor volume was measured on day 35. The
dual treatment effect of TM+low-dose cisplatin on osteolytic bone
destruction induced by oral squamous carcinoma was determined by
conducting soft X-ray and micro-CT examinations. As revealed in
Fig. 4A and B, the osteolytic lesions
were clearly visible in the tibiae of the mice with bone invasion
induced by HSC-3 cells treated with the vehicle (control) only. The
use of TM or low-dose cisplatin alone tended to suppress the
osteolytic bone destruction and tumor burden in the bone marrow.
Notably, few destructive lesions were detected in the tibiae of the
mice treated with TM + low-dose cisplatin. The total area of
radiographic osteolytic lesions from all tibiae was significantly
suppressed by the TM+cisplatin treatment compared to treatment with
cisplatin treatment (P<0.05). Hematoxylin and eosin (H&E)
staining revealed tumor growth in bone marrow. The dual treatment
with TM+cisplatin markedly decreased the tumor burden (Fig. 4C). Morisawa et al demonstrated
that TM has an anti-bone resorption effect by suppressing
osteoclastogenesis via suppression of RANKL in osteoblasts
(17). As revealed in Fig. 4D, treatment with TM decreased the
number of tartrate-resistant acid phosphatase (TRAP)-positive
osteoclasts in the bone marrow tumor invasion front. The present
findings are consistent with those of Morisawa et al
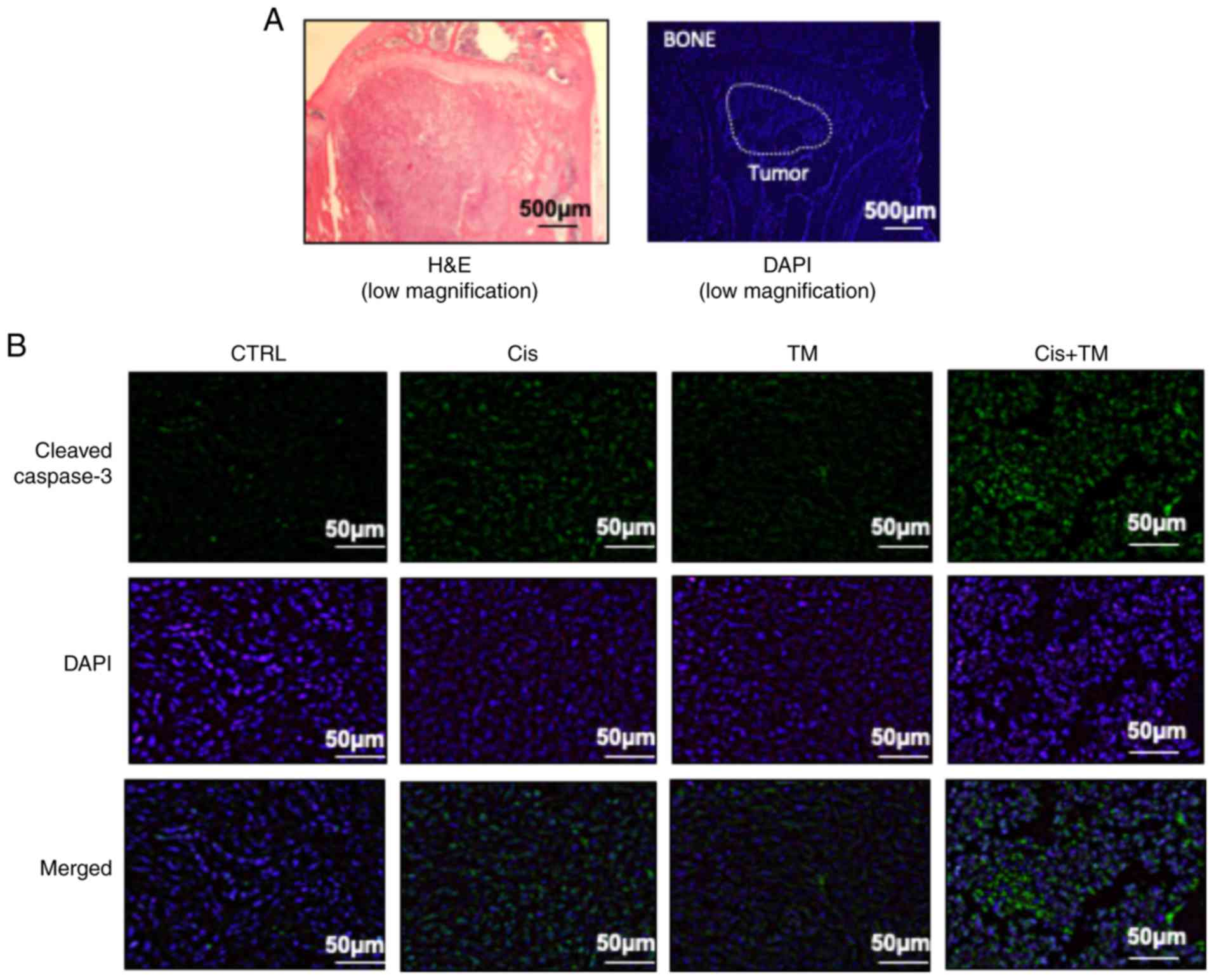
Imunohistochemical analysis of tumor-inoculated mouse tibia bone
marrow was performed. Fig. 5A reveals
a representative low magnification image of an HSC-3-inoculated
mouse tibial bone marrow section stained with H&E and DAPI. The
immunohistochemical analysis revealed a marked increase in the
number of cleaved caspase-3-positive tumor cells in HSC-3 tumor
sections from the cisplatin-treated mice. Whereas TM alone did not
increase the expression of cleaved caspase-3, the dual treatment of
TM+cisplatin enhanced the expression of cleaved caspase-3 compared
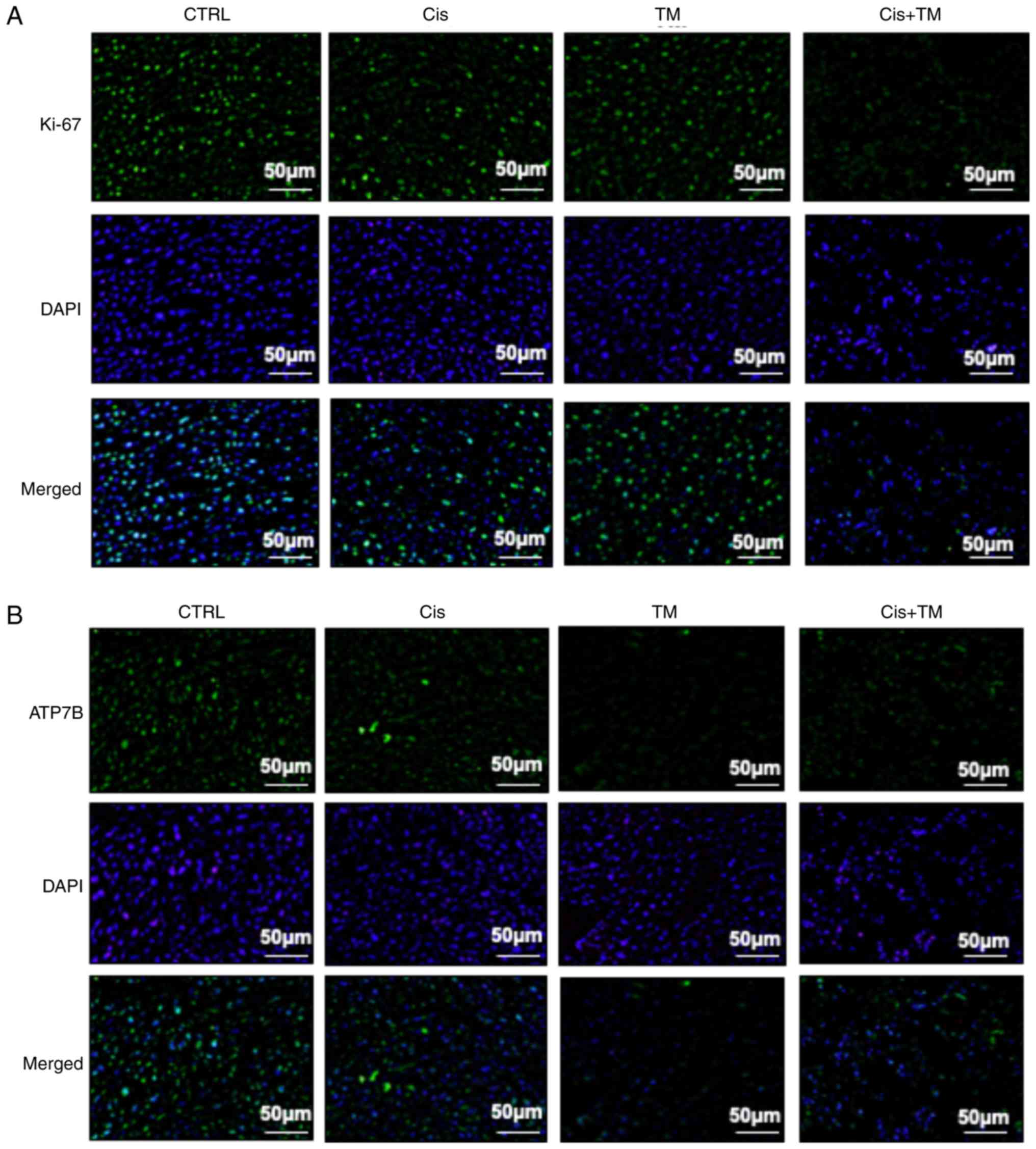
to the treatment with either TM or cisplatin alone (Fig. 5B). The expression of the cell growth
indicator Ki-67 was also evaluated in low-dose cisplatin-treated
mice. Although TM did not affect Ki-67 expression, the TM+cisplatin
dual treatment significantly decreased the expression of Ki-67
(Fig. 6A). TM treatment did not
affect the expression of IL-6 which is an important bone remodeling
factor (Fig. S2). Collectively these
results indicated that TM enhanced the antitumor effect of
cisplatin. Finally, immunohistochemical evaluation was conducted to
determine the effect of TM on the in vivo expression of the
copper transporter ATP7B. Notably, TM decreased the expression of
ATP7B compared with the control group. These data indicated that TM
enhanced the antitumor effect of cisplatin via an accumulation of
cisplatin in the cancer cells (Fig.
6B).
TM overcomes cisplatin resistance
The accumulation of cisplatin is decreased in most
of the available cisplatin-resistant cell lines, and an active
efflux system for cisplatin exists in some of these cell lines
(4–12). The results of the present
investigations indicated that TM treatment may overcome cisplatin
resistance. Mese et al established the cisplatin-resistant
human skin squamous cell carcinoma cell line A431 (18). This cell line (A431-CDDP-R) was used
to evaluate the ability of TM treatment to overcome cisplatin
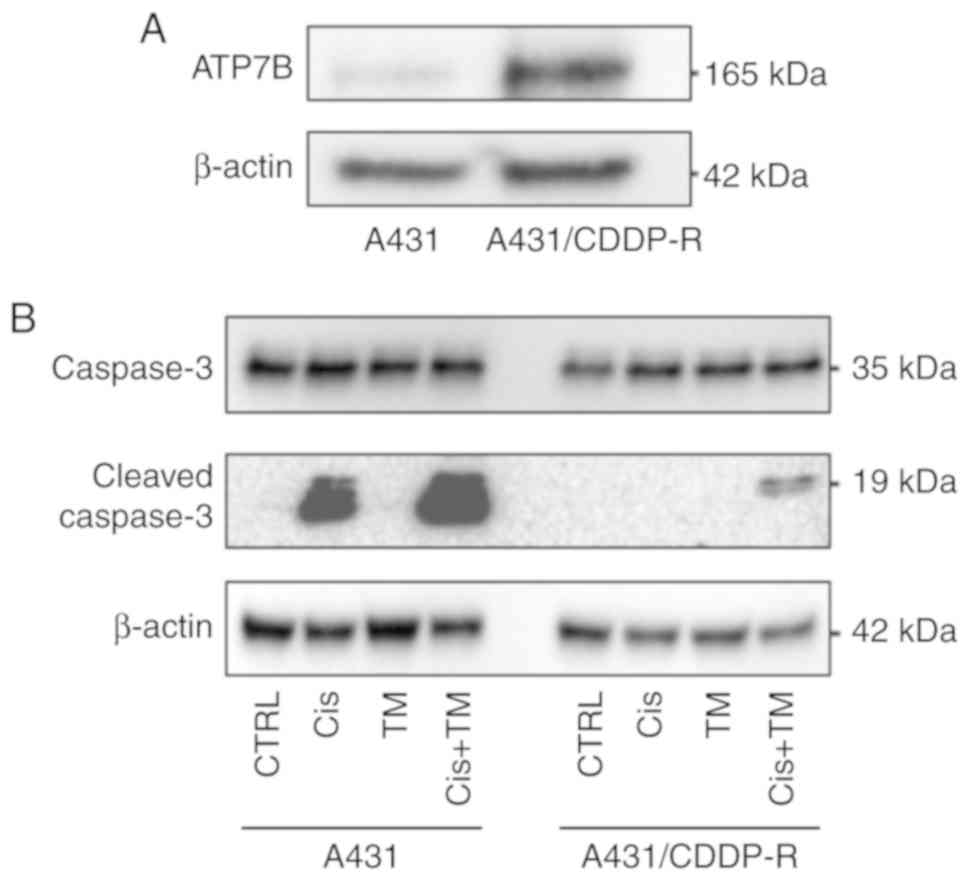
resistance. Notably, the parental A431 cells only slightly
expressed ATP7B. In contrast, the A431/CDDP-R cells strongly
expressed ATP7B (Fig. 7A). To assess
the ability of TM treatment to overcome cisplatin resistance in
A431/CDDP-R cells, the cells were pretreated with TM for 24 h. The
cells were then treated with cisplatin for 24 h. A western blot
analysis revealed that the TM treatment slightly enhanced the
cisplatin-induced cleaved caspase-3 expression in parental A431
cells. Treatment with cisplatin alone did not induce cell apoptosis
in A431/CDDP-R cells. Notably, pretreatment with TM strongly
enhanced the apoptosis effect of cisplatin in A431/CDDP-R cells
(Fig. 7B).
Discussion
Copper chelators have been reported to inhibit
cancer cell growth in vitro and in vivo (21–23).
Copper metabolism is critical for cell proliferation, and it is
strictly regulated by the copper transporters Crt1, ATP7A, and
ATP7B. ATP7B is expressed in mitochondria and excretes copper from
the cytoplasm to the extracellular space (15). Cisplatin is the most frequently used
platinum-based alkylating agent for several cancers. Cisplatin
binds to DNA and causes intra-strand crosslinking, leading to
apoptosis (9). Cisplatin is
transported into cancer cells via the copper transporter Ctr1
(24). Some clinical studies have
indicated that the CTR1 expression in tumors was correlated with
the therapeutic efficacy of platinum drugs (25,26). It
was also indicated that high extracellular copper levels suppressed
CTR1 expression, thereby preventing excess copper influx (27). However, the role of ATP7B in cancer
cells remains unknown. The present findings are the first, to the
best of our knowledge, to demonstrate that the copper chelator TM
enhanced the efficacy of cisplatin in HNSCC via a decrease in the
expression of ATP7B. However, the detailed mechanism underlying the
decrease in ATP7B expression remains unclear. A recent study
indicated that TM induces dimerization of ATP7B, leading to loss of
the copper efflux transporter function (28). Furthermore, the copper chelator
decreased the expression of ATP7B in liver cancer cell lines
(14). This mechanism may be
associated with our results. The present experiments revealed that
tissues from patients with HNSCC expressed high levels of ATP7B
compared to normal oral tissue (Fig.
1). Τhe expression of ATP7B in several HNSCC cell lines was
thus evaluated. In addition, since it has been hypothesized that
the copper concentration in the extracellular space mediates the
expression of copper transporter proteins (26), the effect of the copper chelator TM
was investigated on the expression of the copper efflux transporter
ATP7B in HNSCC cell lines. The present findings are the first to
demonstrate that the chelation of copper ions by TM inhibited the
ATP7B expression in HSC-3 HNSCC cells. Thus, it was surmised that
this effect was due to the maintenance of the copper metabolism of
cancer cells.
Based on our aforementioned results, it was
hypothesized that TM inhibited the cisplatin efflux from cancer
cells. Then, it was evaluated whether TM accelerates the apoptotic
effect of cisplatin, and it was observed that TM increased the
intracellular cisplatin concentration in HNSCC, resulting in an
inhibition of the cell proliferation in vitro. These results
indicate that the copper efflux transporter was a critical mediator
of cisplatin efficacy against cancer cells. The present experiments
also revealed that TM did not affect the proliferation of HSC-3
HNSCC cells. We reported previously that TM did not inhibit the
growth of fibroblasts, osteocytes, osteoblasts, or T cells, which
is consistent with other studies (17,29). We
also reported the molecular mechanism of TM in osteoclastogenesis
in bone marrow (17). TM decreased
the activation of LOX and the RANKL expression in osteoblasts,
resulting in a decrease in osteoclast formation in the
HNSCC-induced bone resorption area.
HNSCC frequently invades facial bone, which is a
source of growth factors for cancer cells (30). To assess the clinical synergistic
effects of copper-lowering agents and platinum agents, TM and
low-dose cisplatin was administered in a mouse model of
bone-destructive HNSCC, and the results indicated that treatment
with either low-dose cisplatin or TM alone partially reduced the
tumor growth in bone (Fig. 4A-C).
Notably, the combination treatment of cisplatin+TM significantly
decreased the tumor growth and bone resorption, Ki-67 expression
and cleaved caspase-3 expression compared to the single treatment
with either agent (Figs. 5 and
6). This additive effect was due to
the suppression of HNSCC cell proliferation via an accumulation of
cisplatin in HNSCC cells and to osteoclast formation by TM
(Fig. 4D).
In a clinical setting, the efficacy of
cisplatin-based chemotherapy against cancers is limited by the
occurrence of innate and acquired drug resistance, and a recent
study revealed that the mechanism of cisplatin resistance is based
on the copper transporter regulation in cancer cells (31). In the present study, the
administration of cisplatin increased the level of the cisplatin
efflux transporter ATP7B. We hypothesized that cancer cells
increase the expression of ATP7B to escape from cisplatin
accumulation and cell death. To evaluate the mechanism of the
function of ATP7B in cisplatin resistance, (epidermoid carcinoma)
A431 cells were used. Mese et al established
cisplatin-resistant A431 cells (18,19). The
expression of ATP7B in parental A431 and cisplatin-resistant A431
(A431 CDDP-R) cells was evaluated, and notably, the ATP7B
expression in the A431CDDP-R cells was markedly increased compared
to the expression in the parental A431 cells. It was speculated
that the cisplatin-resistant A431 cells discharged cisplatin via an
increase in ATP7B expression. The present experiments demonstrated
that TM enhanced the caspase-3 cleavage by cisplatin. These results
indicated that the mechanism of cisplatin resistance in A431 CDDP-R
cells is attributable to an acceleration of the efflux of cisplatin
from cells. However, the present data revealed one possibility that
ATP7B expression is associated to cisplatin resistance. ATP7B
knockdown or a knock-out HNSCC cell line must be established to
demonstrate this hypothesis in a future experiment.
In summary, to the best of our knowledge, the
present study is the first to reveal that a copper-lowering agent
could be an adjuvant to therapy with platinum agents against HNSCC,
and the present findings strongly indicate that TM with cisplatin
may be an effective approach for treating advanced HNSCC. A
clinical study conducted at the MD Anderson Cancer Center revealed
that copper-lowering agents have the potential to overcome
cisplatin resistance in ovarian cancer patients by regulating the
copper transporter hCtr1 (32). The
present in vitro and in vivo results strongly support
the notion that copper-lowering agents including TM could be a
clinically effective breakthrough for overcoming cisplatin
resistance. A copper-lowering agent could be an adjuvant to therapy
with platinum agents against HNSCC, and the present findings
strongly suggest that TM with cisplatin may be an effective
approach to treat advanced HNSCC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present research was funded by a Grant-in-Aid
for Young Scientists (JSPS KAKENHI grant no. 18K17225) to TO and
the Grant-in-Aid for Scientific Research (B) (JSPS KAKENHI grant
no. 17H04405) to AS from the Ministry of Education, Culture,
Sports, Science, and Technology of Japan.
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
TO conceived and designed the experiments. SR, TO,
TS and KH performed the experiments. TO, SR, KK, SI, NMMH, AS
analyzed and interpreted the data. SR, TO, KH, SI, YK, KA, NTTH
performed the data acquisition. TO wrote the paper. KK, TS, SI,
NMMH and AS revised/reviewed the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All of the animal experimental protocols were
approved by the Ethics Review Committee for Animal Experimentation
of the Okayama University Graduate School of Medicine and Dentistry
(approval no. OKU-2018663).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Global Burden of Disease Cancer
Collaboration, ; Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T,
Alizadeh-Navaei R, Allen C, Alsharif U, Alvis-Guzman N, Amini E, et
al: Global, Regional, and national cancer incidence, mortality,
years of life lost, years lived with disability, and
disability-adjusted life-years for 29 cancer groups, 1990 to 2016:
A Systematic analysis for the global burden of disease study. JAMA
Oncol. 4:1553–1568. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Argiris A, Karamouzis MV, Raben D and
Ferris RL: Head and neck cancer. Lancet. 371:1695–1709. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carvalho AL, Nishimoto IN, Califano JA and
Kowalski LP: Trends in incidence and prognosis for head and neck
cancer in the United States: A site-specific analysis of the SEER
database. Int J Cancer. 114:806–816. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guan J, Li Q, Zhang Y, Xiao N, Chen M,
Zhang Y, Li L and Chen L: A meta-analysis comparing cisplatin-based
to carboplatin-based chemotherapy in moderate to advanced squamous
cell carcinoma of head and neck (SCCHN). Oncotarget. 7:7110–7119.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nagasaka M, Zaki M, Issa M, Kim H, Abrams
J and Sukari A: Definitive chemoradiotherapy with carboplatin for
squamous cell carcinoma of the head and neck. Laryngoscope.
127:2260–2264. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao Y and Liu D: The roles of excision
repair cross-complementation group 1 in objective response after
cisplatin-based concurrent chemoradiotherapy and survival in head
and neck cancers: A systematic review and meta-analysis. Oral
Oncol. 51:570–577. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mehmood RK: Review of Cisplatin and
oxaliplatin in current immunogenic and monoclonal antibody
treatments. Oncol Rev. 8:2562014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chvalova K, Brabec V and Kasparkova J:
Mechanism of the formation of DNA-protein cross-links by antitumor
cisplatin. Nucleic Acids Res. 35:1812–1821. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chung CH, Lee JW, Slebos RJ, Howard JD,
Perez J, Kang H, Fertig EJ, Considine M, Gilbert J, Murphy BA, et
al: A 3′-UTR KRAS-variant is associated with cisplatin resistance
in patients with recurrent and/or metastatic head and neck squamous
cell carcinoma. Ann Oncol. 25:2230–2236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Damia G and Broggini M: Platinum
resistance in ovarian cancer: Role of DNA repair. Cancers (Basel).
11:E1192019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fadejeva I, Olschewski H and Hrzenjak A:
MicroRNAs as regulators of cisplatin-resistance in non-small cell
lung carcinomas. Oncotarget. 8:115754–115773. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lambert IH and Sorensen BH: Facilitating
the cellular accumulation of Pt-based chemotherapeutic drugs. Int J
Mol Sci. 19(pii): E22492018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leonhardt K, Gebhardt R, Mossner J,
Lutsenko S and Huster D: Functional interactions of Cu-ATPase ATP7B
with cisplatin and the role of ATP7B in the resistance of cells to
the drug. J Biol Chem. 284:7793–7802. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Inesi G: Molecular features of copper
binding proteins involved in copper homeostasis. IUBMB Life.
69:211–217. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roberts EA: Update on the diagnosis and
management of wilson disease. Curr Gastroenterol Rep. 20:562018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morisawa A, Okui T, Shimo T, Ibaragi S,
Okusha Y, Ono M, Nguyen TTH, Hassan NMM and Sasaki A: Ammonium
tetrathiomolybdate enhances the antitumor effects of cetuximab via
the suppression of osteoclastogenesis in head and neck squamous
carcinoma. Int J Oncol. 52:989–999. 2018.PubMed/NCBI
|
|
18
|
Mese H, Sasaki A, Alcalde RE, Nakayama S
and Matsumura T: Establishment and characterization of
cisplatin-resistant human epidermoid carcinoma cell line, A431
cell. Chemotherapy. 44:414–420. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mese H, Sasaki A, Nakayama S, Alcalde RE
and Matsumura T: The role of caspase family protease, caspase-3 on
cisplatin-induced apoptosis in cisplatin-resistant A431 cell line.
Cancer Chemother Pharmacol. 46:241–245. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chan N, Willis A, Kornhauser N, Ward MM,
Lee SB, Nackos E, Seo BR, Chuang E, Cigler T, Moore A, et al:
Influencing the tumor microenvironment: A phase II study of copper
depletion using tetrathiomolybdate in patients with breast cancer
at high risk for recurrence and in preclinical models of lung
metastases. Clin Cancer Res. 23:666–676. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chisholm CL, Wang H, Wong AH,
Vazquez-Ortiz G, Chen W, Xu X and Deng CX: Ammonium
tetrathiomolybdate treatment targets the copper transporter ATP7A
and enhances sensitivity of breast cancer to cisplatin. Oncotarget.
7:84439–84452. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park SJ, Kim MJ, Kim YK, Kim SM, Park JY
and Myoung H: Combined cetuximab and genistein treatment shows
additive anti-cancer effect on oral squamous cell carcinoma. Cancer
Lett. 292:54–63. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ishida S, Lee J, Thiele DJ and Herskowitz
I: Uptake of the anticancer drug cisplatin mediated by the copper
transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci USA.
99:14298–14302. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu X, Duan L, Zhou B, Ma R, Zhou H and Liu
Z: Genetic polymorphism of copper transporter protein 1 is related
to platinum resistance in Chinese non-small cell lung carcinoma
patients. Clin Exp Pharmacol Physiol. 39:786–792. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim ES, Tang X, Peterson DR, Kilari D,
Chow CW, Fujimoto J, Kalhor N, Swisher SG, Stewart DJ, Wistuba II
and Siddik ZH: Copper transporter CTR1 expression and tissue
platinum concentration in non-small cell lung cancer. Lung Cancer.
85:88–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Molloy SA and Kaplan JH: Copper-dependent
recycling of hCTR1, the human high affinity copper transporter. J
Biol Chem. 284:29704–29713. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fang T, Chen W, Sheng Y, Yuan S, Tang Q,
Li G, Huang G, Su J, Zhang X, Zang J and Liu Y: Tetrathiomolybdate
induces dimerization of the metal-binding domain of ATPase and
inhibits platination of the protein. Nat Commun. 10:1862019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim KK, Han A, Yano N, Ribeiro JR, Lokich
E, Singh RK and Moore RG: Tetrathiomolybdate mediates
cisplatin-induced p38 signaling and EGFR degradation and enhances
response to cisplatin therapy in gynecologic cancers. Sci Rep.
5:159112015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Okui T, Shimo T, Fukazawa T, Kurio N,
Hassan NM, Honami T, Takaoka M, Naomoto Y and Sasaki A: Antitumor
effect of temsirolimus against oral squamous cell carcinoma
associated with bone destruction. Mol Cancer Ther. 9:2960–2969.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kilari D, Guancial E and Kim ES: Role of
copper transporters in platinum resistance. World J Clin Oncol.
7:106–113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fu S, Naing A, Fu C, Kuo MT and Kurzrock
R: Overcoming platinum resistance through the use of a
copper-lowering agent. Mol Cancer Ther. 11:1221–1225. 2012.
View Article : Google Scholar : PubMed/NCBI
|