Introduction
According to the National Cancer Institute of United
States, malignant gliomas (anaplastic astrocytoma and glioblastoma
multiforme) occur more frequently than other types of primary
central nervous system (CNS) tumors and account for over half of
all brain cancers (1). These tumors
are characterized by aggressive growth and are associated with a
mean patient survival time of 12–15 months (2,3). Although
the clinical application of temozolomide (TMZ) has been thoroughly
demonstrated to effectively prolong the survival time of patients
with brain tumors, unfortunately, glioma cells exhibit resistance
to TMZ under certain conditions (4–6). Surgical
resection is usually inadequate for local control, and residual
tumors often lead to recurrent disease (7). Although malignant gliomas are sensitive
to high doses of radiation, radiotherapeutic treatment is limited
by normal tissue toxicity (8).
Therefore, current therapies are unsatisfactory, indicating the
requirement for novel therapeutic agents and approaches to prolong
the survival time of patients with glioma.
Recently, an extensive study evaluated the safety
and therapeutic efficacy of natural compounds for treating cancer.
Tetrandrine (TET) is a bisbenzylisoquinoline alkaloid isolated from
the root of Han-Fang-Chi (Stephania tetrandra S. Moore),
which has been used in traditional Chinese medicine to treat
arthritis (9–11), silicosis (12–14) and
occlusive cardiovascular disorders (15–17) in
China for several decades. TET is also reported to exert
substantial inhibitory effects on various types of tumors (18–21), and
to reverse multidrug resistance (22–24).
Although several studies have addressed the effects of TET on
gliomas (25–28), its clinical use is inconvenient due to
its insolubility in water. Tetrandrine citrate (TetC), a novel TET
salt, was synthesized in-house. Compared with TET, TetC has higher
water solubility and can be administered by injection in an in
vivo study. The difference between TetC and TET is only the
difference in acid radicals. In the present study, the inhibitory
effect of TetC on human glioma U87 cell proliferation was
investigated in vitro and in vivo, and the potential
molecular mechanisms of its antitumor activity were explored.
Materials and methods
Reagents
A free base formulation of TET (purity, 98%) was
purchased from Nanjing Jingzhu Biotechnology Co., Ltd., and citrate
was purchased from Beijing Kehai Junzhou Biotechnology Center. TetC
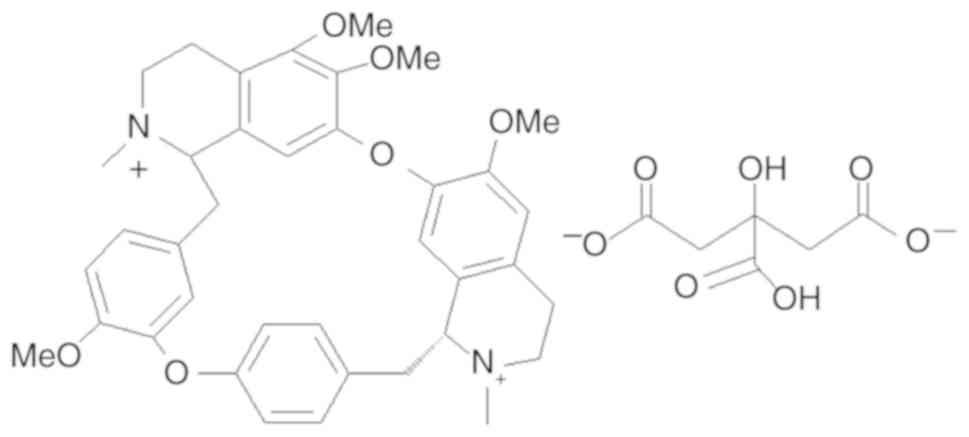
was synthesized in-house, and its structure is presented in
Fig. 1. MTT, DMSO, NP40 and
2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) were obtained
from Sigma-Aldrich; Merck KGaA. All other chemicals were of
standard analytical grade.
Cell culture
Human glioma U87 cells (ATCC version, glioblastoma
of unknown origin) and U251 cells were purchased from the Cell
Center of the Institute of Basic Medical Sciences, Chinese Academy
of Medical Sciences and Peking Union Medical College (Beijing,
China) and were validated by short tandem repeat DNA profiling. U87
and U251 cells were cultured in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% heat-inactivated FBS
(ScienCell Research Laboratories, Inc.), 100 U/ml penicillin and
100 µg/ml streptomycin at 37°C (5% CO2) in a humidified
atmosphere. Human umbilical vein endothelial cells (HUVECs) were
purchased from ScienCell Research Laboratories, Inc., and cultured
in endothelial cell medium containing 100 mg/ml streptomycin, 100
IU/ml penicillin, 40 µg/ml endothelial cell growth supplement and
5% heat-inactivated FBS (all from ScienCell Research Laboratories,
Inc.,) at 37°C in a humidified atmosphere containing 5%
CO2. Compared with other normal cells, such as primary
hepatocytes, HUVECs are easily obtained, cultured and have no
characteristics of tumor cells. Thus, HUVECs were selected as
normal control cells in the present study.
Cell viability assay
Cell viability assays were performed using the MTT
method according to the manufacturer's instructions (Sigma-Aldrich;
Merck KGaA). Cells were seeded into 96-well plates (Costar;
Corning, Inc.) at a density of 4×103 cells/well. After a
24-h incubation period, triplicate wells were treated with various
concentrations of TetC (0, 5, 10, 20 and 40 µmol/l) for 48 h. Next,
20 µl MTT solution (5 mg/ml in PBS) was added to each well and
incubated at 37°C for 4 h. The formazan crystals were dissolved by
adding 150 µl DMSO to each well, and the absorbance was measured
with a microplate reader (Multiskan™ MK3; Thermo Fisher Scientific,
Inc.) at a wavelength of 570 nm. IC50 values were
calculated from cytotoxicity curves using the Bliss independence
method (29).
Cell morphology and Hoechst
staining
U87 cells were seeded at a density of
2.5×105 cells/flask. Following a 24-h incubation period,
the cells were treated with 0, 5, 10, 20 and 40 µmol/l TetC. After
a further 48 h of treatment, images of the cells were captured
using an optical microscope (×20; Olympus Corporation). The cells
were then washed with pre-cooled PBS and incubated in fresh culture
medium containing 10 µg/ml Hoechst 33342 fluorescent dye (Beyotime
Institute of Biotechnology) at 37°C for 20 min. The cells were
washed twice with PBS to remove residual Hoechst, and photographed
using a fluorescence microscope (×20; Olympus Corporation). In
addition, U87 cells were treated with 20 µmol/l TetC for 0, 1.5, 3,
6, 12, 24 and 48 h, and photographed using an optical microscope
(×40; Olympus Corporation).
Detection of intracellular reactive
oxygen species (ROS)
Intracellular ROS measurements were performed by
detecting the fluorescence intensity of 2,7-dichlorofluorescein
(DCF). U87 cells in each treatment group (0, 5, 10, 20 and 40
µmol/l TetC) were incubated with the fluorescent probe DCFH-DA
(1:1,000) at 37°C for 10 min in the dark, and then washed three
times with ice-cold PBS. Images were acquired using an inverted
fluorescence microscope (×20; Olympus Corporation), and the
fluorescence intensity was detected using ImageJ software (version
1.0; National Institutes of Health). After imaging, the U87 cells
were centrifuged (250 × g at 4°C for 10 min) and washed with
ice-cold PBS prior to flow cytometric analysis with a FACSCalibur
flow cytometer and CellQuest software (version 5.1; BD
Biosciences).
FITC-Annexin V/propidium iodide (PI)
apoptosis analysis
Cells were seeded at a density of 2.5×105
cells/flask and incubated for 24 h at 37°C (5% CO2),
prior to treatment with 0, 5, 10, 20 or 40 µmol/l TetC. After 48 h,
the cells were collected and resuspended in 200 µl binding buffer;
10 µl FITC-labeled enhanced Annexin V and 10 µl PI were added and
the samples were gently mixed. After incubation in the dark for 15
min at room temperature, the samples were diluted with 300 µl
binding buffer and subjected to flow cytometric analysis using the
FACSCalibur flow cytometer with CellQuest software (version 5.1; BD
Biosciences).
Cell cycle assay
To determine the effect of TetC on cell cycle
progression, cells were cultured for 6 h (one cell cycle) in medium
containing 0, 5, 10, 20 or 40 µmol/l TetC. The cells were washed
with PBS, collected by trypsinization, fixed with 70% ethanol and
treated with 1% NP40 and 5 mg/ml RNase for 30 min. After staining
with 50 mmol/l PI, the cells were subjected to flow cytometric
analysis as aforementioned.
Western blotting
Cells were harvested and washed with PBS, and
whole-cell extracts were prepared by incubating the cells on ice in
lysis buffer containing phosphatase inhibitor cocktail (Roche
Diagnostics). The lysates were clarified by centrifugation (12,000
× g at 4°C for 20 min), and the total protein was quantified using
a BCA Protein Assay kit (Pierce; Thermo Fisher Scientific, Inc.).
Equal amounts of lysate (40 µg/lane) were resolved by 10% SDS-PAGE
and transferred to polyvinylidene difluoride membranes (EMD
Millipore). The membranes were blocked in Tris-buffered saline with
Tween-20 (TBST) containing 5% skim milk at room temperature for 2
h, and incubated with the corresponding primary antibodies at 4°C
overnight. The membranes were then incubated with horseradish
peroxidase-conjugated secondary antibodies for 1 h at room
temperature. All primary antibodies were purchased from Cell
Signaling Technology, Inc., and were targeted against cleaved (CL)
caspase-3 (cat. no. 9661; 1:1,000), caspase-3 (cat. no. 9662;
1:1,000), Fas (cat. no. 8023; 1:1,000), Bcl-2 (cat. no. 15071;
1:1,000), Bax (cat. no. 2772; 1:1,000), p-p38 (cat. no. 9216;
1:1,000), p38 (cat. no. 9212; 1:1,000), p-JNK (cat. no. 9255;
1:1,000), JNK (cat. no. 9252; 1:1,000) and β-actin (cat. no. 8457;
1:1,000). β-actin was used as the endogenous reference protein.
Secondary antibodies against rabbit (cat. no. 7074; 1:5,000) or
mouse (cat. no. 7076; 1:5,000 dilution) IgG were also purchased
from Cell Signaling Technology, Inc., and the pre-stained protein
marker p7708V was purchased from New England BioLabs, Inc. The
proteins were visualized using enhanced chemiluminescence western
blotting detection reagents (GE Healthcare). ImageJ software
(version 1.0; NIH) was used to quantify the optical density for
treated samples, which were normalized to the β-actin internal
controls.
In vivo assessment of the therapeutic
effects of TetC in BALB/c nude mice
A total of 14 female BALB/c nude mice (20±2 g) aged
4–6 weeks were obtained from Vital River Laboratories Co., Ltd.,
and used to create a human glioma U87 ×enograft model. The mice
were maintained in a temperature-controlled room (22±2°C) with a
12-h light/12-h dark cycle and a relative humidity of 40–60%, with
ad libitum access to food and water. All animal experiments
were approved by the Institutional Animal Care and Use Committee of
Beijing Hospital, and the U87 ×enograft mouse model was established
as previously described (30).
Briefly, U87 cells (5×106 cells per animal) were
injected into the armpit of each mouse. When the tumor volume had
reached a volume of 1 cm3, it was removed and cut into 2
mm3 pieces; these tissues were inoculated into the
armpits of another group of female nude mice. After 3 days of tumor
growth, the animals were randomly divided into the control or 200
mg/kg TetC (22 g/l)-treated groups (six mice per group). Each
animal received either 200 µl PBS (vehicle control) or TetC via
intraperitoneal injection, every other day for 14 days. Health
status and behavior of mice were monitored daily. At the end of the
experiment, the mice were anesthetized by intraperitoneal injection
with 10% chloral hydrate (300 mg/kg bodyweight) and were sacrificed
by cervical dislocation. When the mice did not move, death was
confirmed and then tumor tissues were removed and the body and
tumor weights were measured.
Statistical analysis
All experiments were repeated three times. SPSS 17.0
statistical software was used for statistical analysis, and the
results are presented as the means ± standard deviation. Treatment
effects were compared using one-way ANOVA and significance was
calculated using the LSD test. P<0.05 was considered to indicate
a statistically significant difference.
Results
Inhibition of human glioma U87, U251
and HUVEC growth by TetC
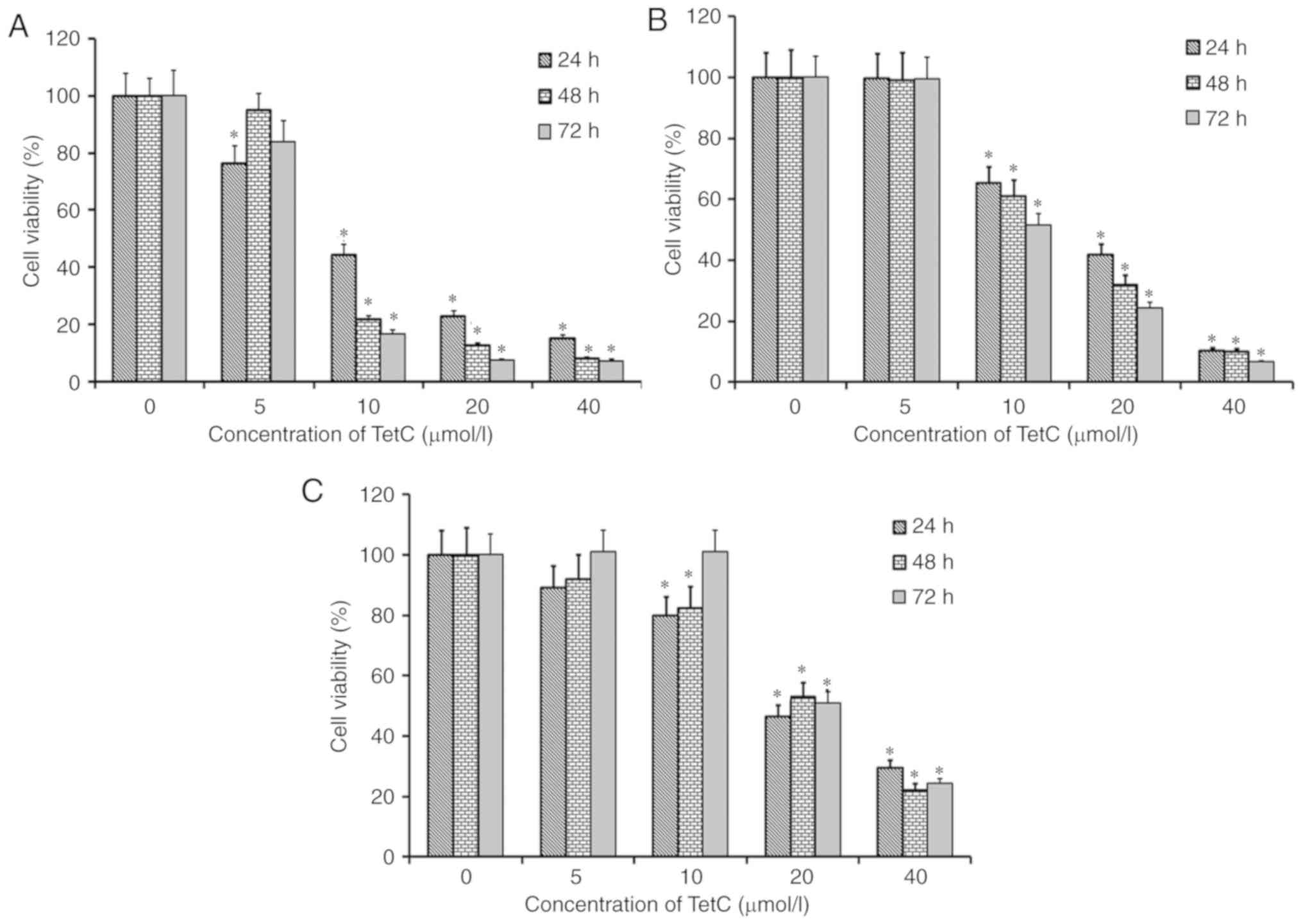
The growth-inhibitory effect of TetC on human glioma
U87 and U251 cells, in addition to HUVECs, was examined using an
MTT assay. The cells were cultured for 24, 48 and 72 h in the
presence of 0, 5, 10, 20 or 40 µmol/l TetC. Following treatment
with TetC, the proliferative rate of all three cell lines was
decreased in a dose-dependent manner (Fig. 2). The IC50 values of TetC
in U87 cells at 24, 48 and 72 h were 10.4±1.1, 9.1±0.7 and 7.3±0.6
µmol/l, respectively; in U251 cells, the IC50 values
were 16.6±1.6 (24 h), 12.5±1.2 (48 h) and 10.8±0.9 (72 h) µmol/l,
and 19.1±1.8 (24 h), 21.3±1.8 (48 h) and 20.3±2.1 (72 h) µmol/l in
HUVECs. The greatest growth-inhibitory effect was observed in U87
cells, and TetC was more cytotoxic to U87 cells than HUVECs.
Therefore, the U87 cell line was selected for use in subsequent
experimentation.
TetC induces the vacuolar degeneration
of human glioma U87 cells
Human glioma U87 cells were cultured for 48 h in the
presence of various concentrations of TetC (0, 5, 10, 20 and 40
µmol/l). Cell morphology was then observed by optical microscopy.
TetC (10 µmol/l) induced the intracellular vacuolization of U87
cells, and ≥20 µmol/l induced cell rounding (Fig. 3A). Fluorescence microscopy revealed
that markers of apoptosis, such as nuclear concentration and
apoptotic body formation, were induced by ≥20 µmol/l TetC (Fig. 3B). In addition, 20 µmol/l TetC induced
cell rounding with small vacuole formation in a time-dependent
manner (Fig. 3C).
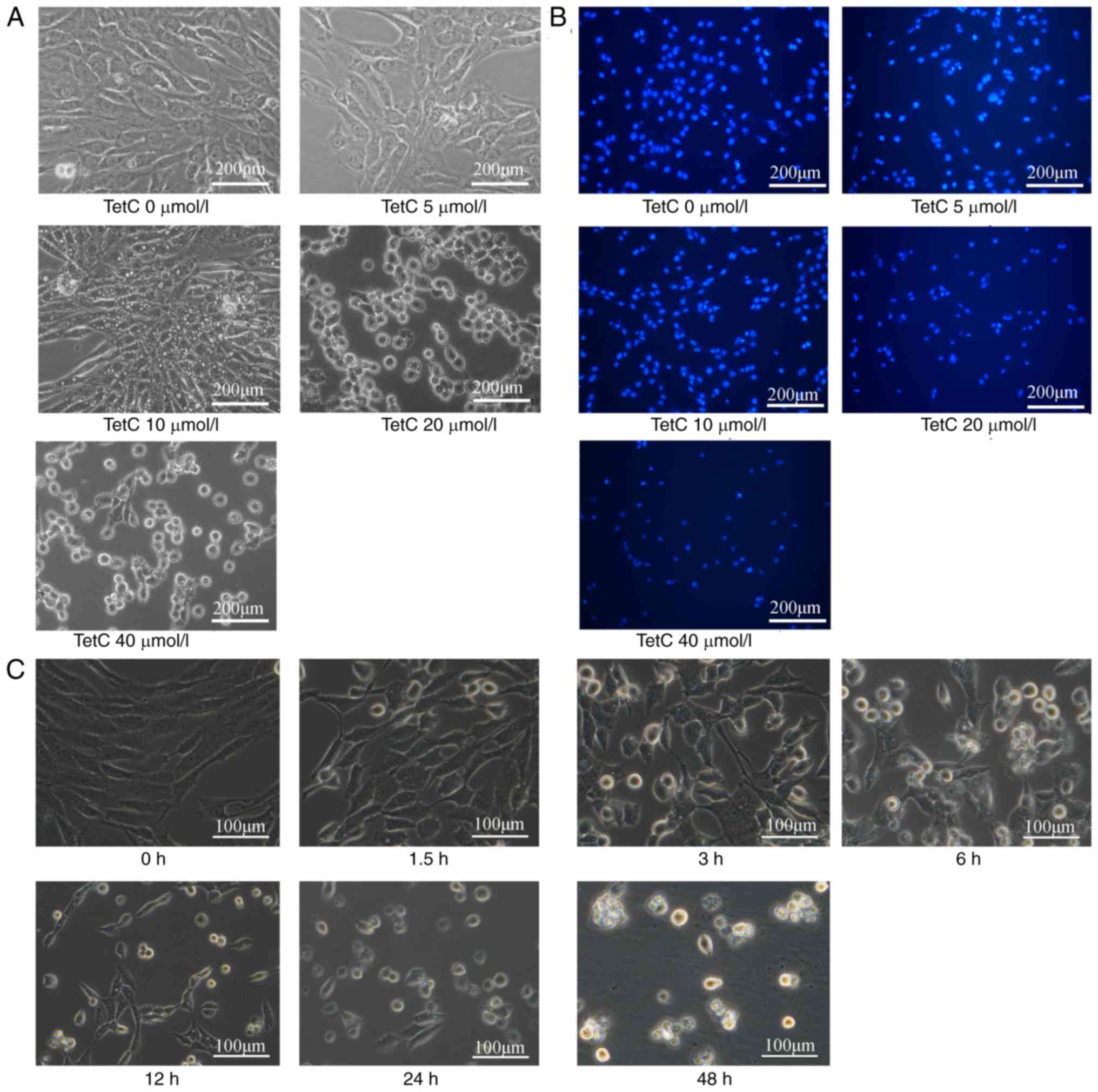 | Figure 3.Effect of TetC on intracellular
vacuolization and apoptosis in human glioma U87 cells. (A) Cells
were treated with 0, 5, 10, 20 and 40 µmol/l TetC for 48 h, and
observed under a microscope (×20). (B) Cells were then stained with
Hoechst 33342 for 20 min and photographed using a fluorescence
microscope (×20). (C) Cells were treated with 20 µmol/l TetC for 0,
1.5, 3, 6, 12, 24 and 48 h and observed under a microscope (×40).
TetC, tetrandrine citrate. |
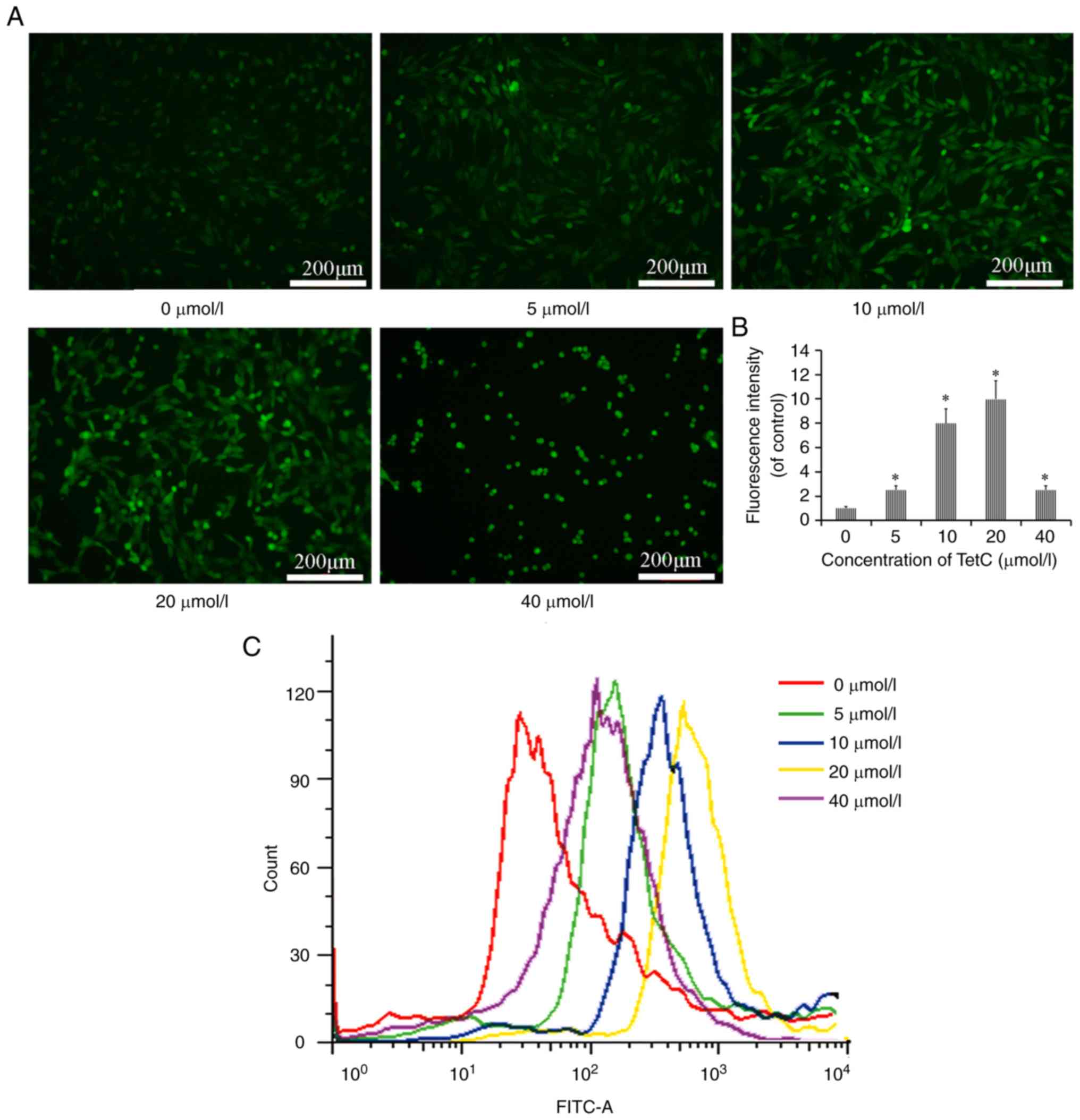
TetC increases ROS production in U87
cells
The effects of TetC on intracellular ROS production
in U87 cells were determined using a DCFH-DA fluorescence assay. As
revealed in Fig. 4A, the number of
fluorescent puncta, representing the concentration of ROS, was
higher in TetC-treated cells than in the control cells. In
addition, the flow cytometric results revealed that ROS levels were
increased by treatment with 0, 5, 10, 20 and 40 µmol/l TetC,
compared with those in the control cells. However, the levels of
ROS were increased to a lesser degree following treatment with 40
µmol/l, compared with the use of 10 or 20 µmol/l TetC (Fig. 4B and C). These results demonstrated
that ROS were produced in U87 cells in response to TetC.
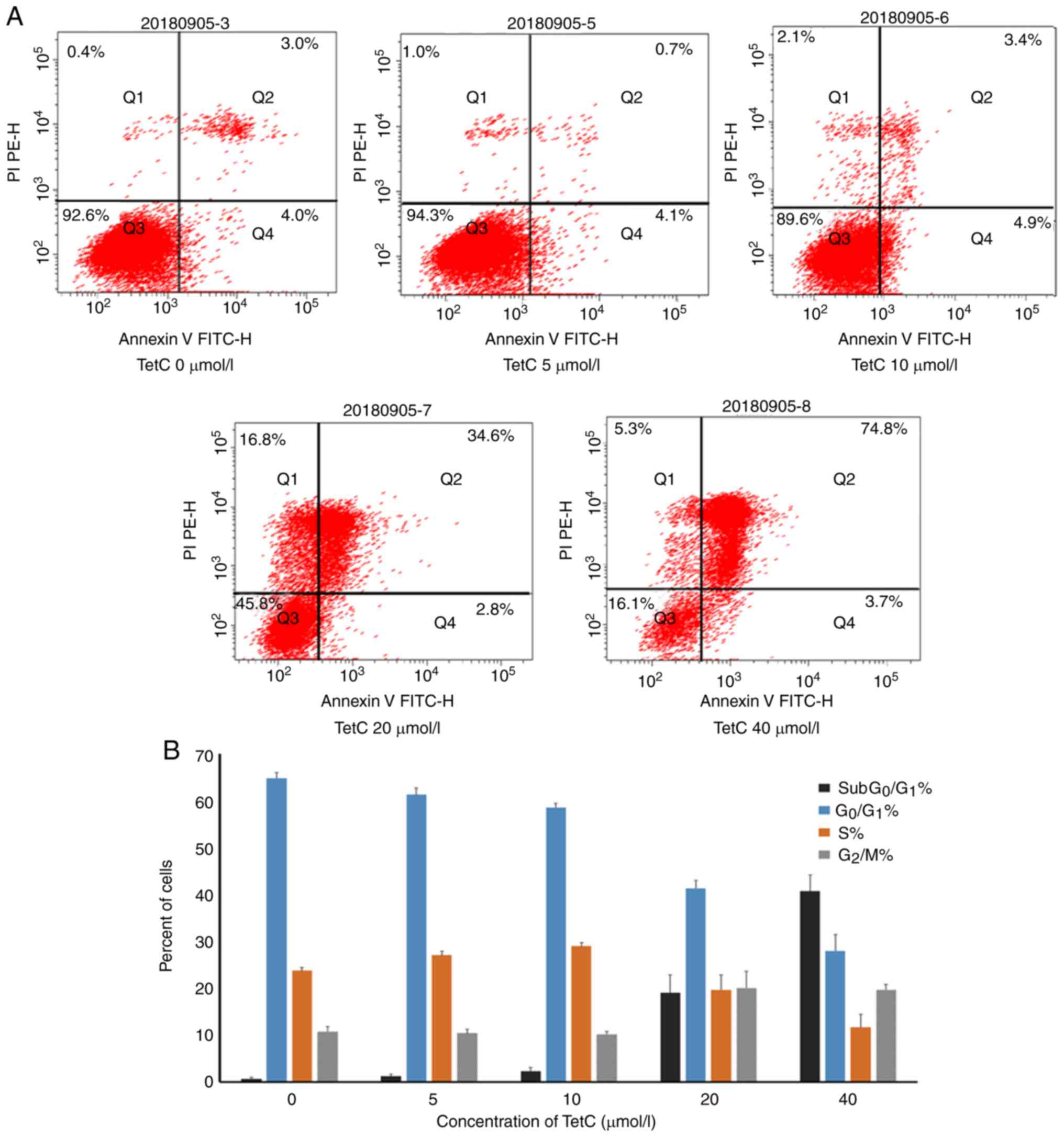
TetC induces U87 cell apoptosis and
decreases the percentage of cells in the
G0/G1 phase
To evaluate the effect of TetC on apoptosis, human
glioma U87 cells were treated with different concentrations of
TetC. The induction of apoptosis by TetC was confirmed by
FITC-Annexin V/PI staining. The apoptotic ratio was significantly
enhanced in cells incubated with 20 or 40 µmol/l TetC for 48 h,
compared with that of the control (Fig.
5A). To investigate the effect of TetC on the cell cycle, the
cells were treated with 0–40 µmol/l TetC, which significantly
altered the cell cycle distribution in a dose-dependent manner; the
percentage of the G0/G1 phase cells was
decreased, and the percentage of cells in the G2/M and
subG0/G1 phases was increased (Fig. 5B).
TetC regulates the expression of
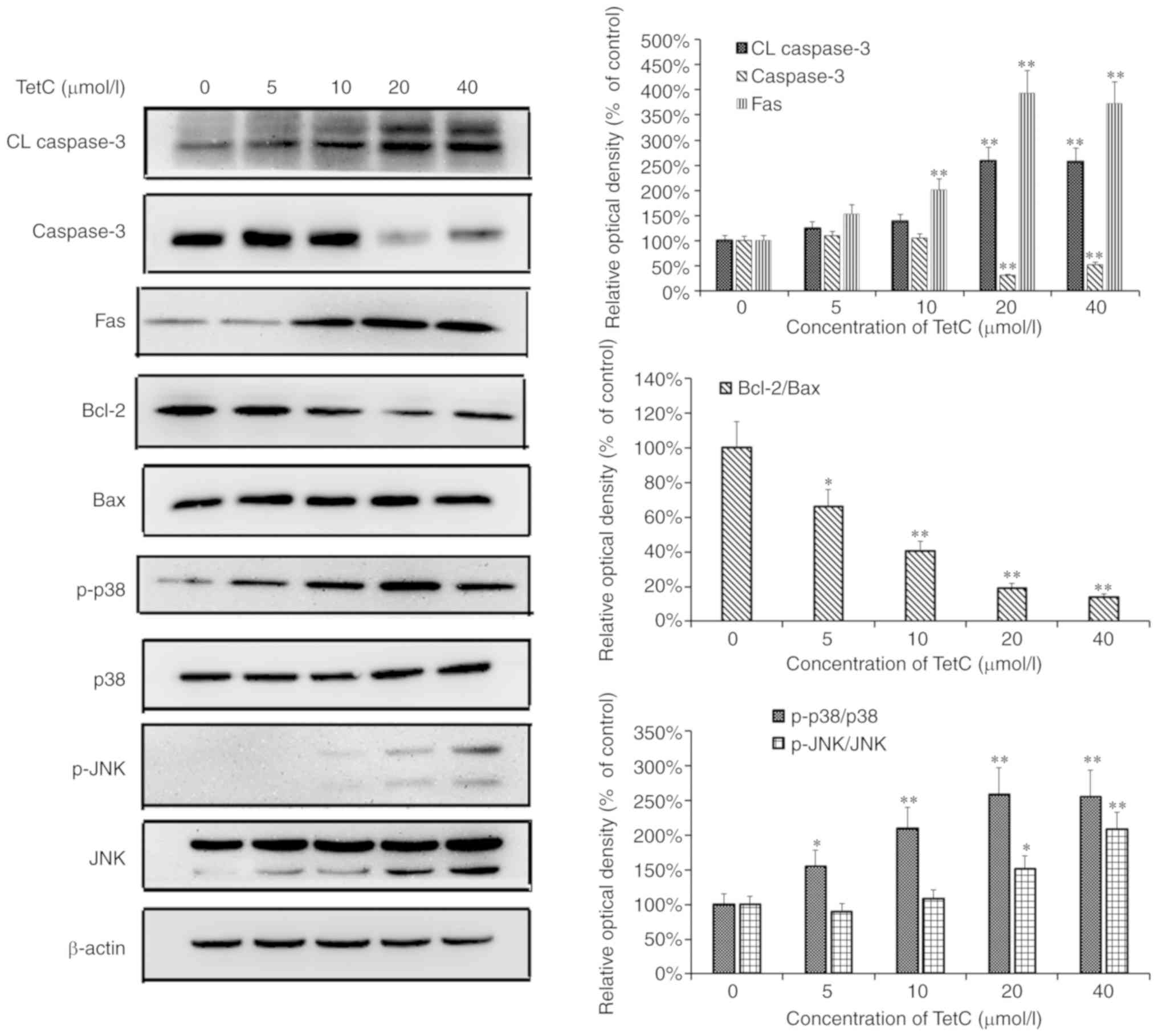
tumor-related genes in U87 cells
To investigate the mechanisms of TetC in human
glioma U87 cells, the effects of TetC treatment on the expression
levels of apoptosis-associated proteins were investigated. As
revealed in Fig. 6, the expression
levels of caspase-3 and Bcl-2 in U87 cells were markedly
downregulated following TetC treatment in dose-dependent manner,
whereas the levels of CL caspase-3, Fas, p-p38 and p-JNK were
increased.
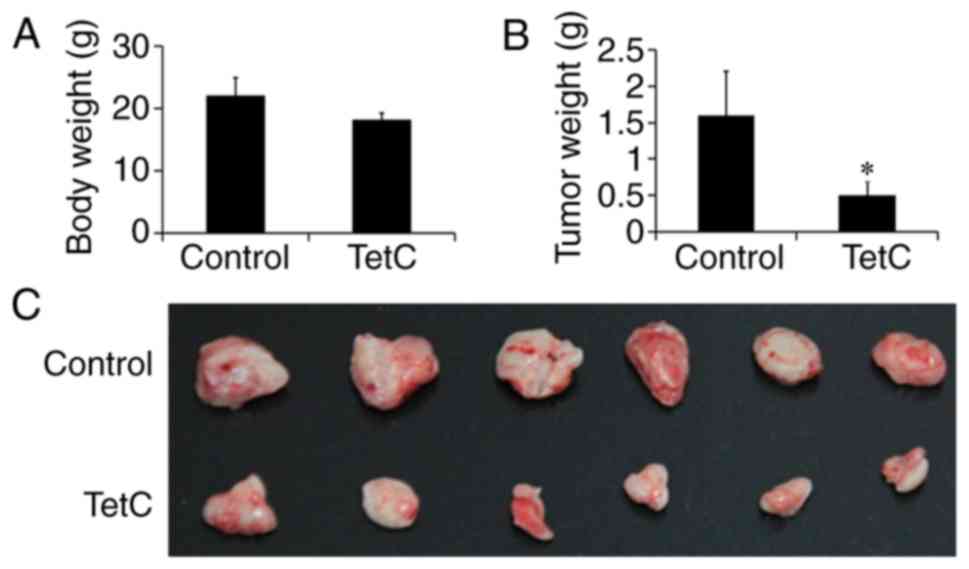
Inhibition of human glioma U87
×enograft growth in BALB/c nude mice
TetC treatment was initiated three days after tumor
implantation. TetC was intraperitoneally administered at a dose of
200 mg/kg, every other day for 14 days; the control mice were
administered PBS (vehicle only). The body weights of the animals in
the control and TetC-treated groups were not significantly
different (Fig. 7A), although tumor
growth in the TetC-treated BALB/c nude mice was significantly
suppressed compared with that in the control mice (Fig. 7B and C). Compared with the control
group (1.8±0.11 cm), the maximum diameter of tumors (0.8±0.06 cm)
in TetC-treated groups was shorter and there was only one tumor per
mouse. Treatment with TetC inhibited the growth of human glioma U87
×enografts by up to 68.7%, which suggests that TetC, at a
well-tolerated dose, markedly inhibits U87 ×enograft growth.
Discussion
Malignant glioma is among the neoplasms with the
highest mortality rates, and is associated with one of the worst
5-year overall survival rates among all human cancers (31). Although TMZ has consistently been
demonstrated to effectively prolong the survival time of patients
with brain tumors, under certain conditions, glioma cells exhibit
marked resistance to TMZ (4–6). Therefore, new therapeutic compounds and
treatment approaches are required to prolong the survival time of
glioma patients. In the present study, the effect of TetC on cell
proliferation was investigated using an MTT assay, which revealed
that TetC inhibited the proliferation of U87 and U251 cells, as
well as HUVECs, in a dose-dependent manner (Fig. 2). Furthermore, these growth-inhibitory
effects were most prominent in U87 cells, and a greater cytotoxic
effect was apparent in these cells, compared with that in HUVECs.
It can therefore be deduced that TetC has a more pronounced effect
on U87 cells; thus this cell line was utilized in subsequent
experimentation. In the cell viability assay, the vacuolization of
U87 cells was observed in the 10 µmol/l TetC-treated group, however
cell-rounding with reduced vacuolization was observed in the 20 and
40 µmol/l treatment groups. Furthermore, the change of vacuoles
over time at 20 µmol/l TetC was also observed (Fig. 3C). Numerous vacuoles of different
sizes appeared in each cell, so it was difficult to quantify the
number of vacuoles and size under the the current experimental
conditions. In addition, cell apoptosis would occur with the
prolongation of 20 µmol/l TetC treatment time. It was speculated
that different mechanisms of action were responsible for the
differences in response to 10 µmol/l and ≥20 µmol/l TetC treatment.
In the 10 µmol/l TetC-treated group, cell vacuolization was an
indication of mitochondrial or endoplasmic reticulum denaturation
(32), although some researchers
consider this to be the result of methuosis (33). In the 20 and 40 µmol/l TetC-treated
groups, cell rounding was more likely to be associated with
apoptosis. Subsequently, apoptosis was assessed using flow
cytometry and Hoechst staining, and the cell cycle status was
investigated by flow cytometry alone. The results of these assays
demonstrated that TetC induced apoptosis in a dose-dependent
manner. Hoechst staining revealed nuclear concentration and
apoptotic body formation in the 20 and 40 µmol/l TetC-treated
groups. This suggests that following treatment with TetC, apoptosis
contributes to the inhibition of U87 cell proliferation, which was
supported by the results of the cell cycle assay.
Mitochondria and the production of ROS serve
important roles in the induction of apoptosis under physiological
and pathological conditions. Notably, mitochondria are both a
source and a target of ROS (34). In
the present study, it was observed that compared with the vehicle
control, TetC treatment resulted in increased ROS production.
However, compared with the 10 and 20 µmol/l TetC-treated groups,
single-cell fluorescence intensity analysis revealed that 40 µmol/l
TetC decreased the levels of ROS (Fig.
4B), and it was hypothesized that this decrease in fluorescence
intensity was the result of apoptosis (Fig. 5). Cell apoptosis leads to the decrease
of intracellular ROS. The findings of the 40 µmol/l TetC group in
Figs. 4 and 5 were consistent.
Caspase-3 and members of the Bcl-2 protein family
act as key regulators of apoptosis, and are important determinants
of cellular sensitivity or resistance to chemotherapeutic drugs
(35). Bcl-2 and Bax belong to the
Bcl-2 protein family, and Bcl-2 inhibits, whilst Bax promotes
apoptosis (36). The present study
indicated that although the expression level of Bcl-2 was decreased
in a dose-dependent manner, that of Bax, a pro-apoptotic protein,
was not significantly altered, thus the Bcl-2/Bax ratio was
decreased. Bcl-2 and Bax may therefore be involved in TetC-induced
apoptosis. Moreover, an increase in the level of CL caspase-3, but
a decrease in the expression level of caspase-3 was observed
following TetC treatment; the CL caspase-3/caspase-3 ratio was thus
increased. Notably, caspase-3 activation was demonstrated to occur
after treatment with TetC. These events have been associated with a
decrease in the Bcl-2/Bax ratio (37). In addition, the reason why CL
caspase-3 was induced in the control group may be due to too many
cells in the control group, which causes apoptosis in few cells.
Further research will be performed on this phenomenon.
The JNK and p38 mitogen-activated protein kinase
(MAPK) pathways are critical to MAPK signaling. The activation of
JNK and p38 MAPK signaling is involved in the initiation of
apoptosis by different stimuli in a number of common malignant
tumors (38). Therefore, the effect
of TetC on the expression and phosphorylation of JNK and p38 MAPK
was also investigated. As revealed in Fig. 7, in U87 cells treated with TetC for 48
h, the protein levels of p-JNK and p-p38 MAPK were significantly
increased in a dose-dependent manner, compared with those in the
control cells. The increased activities of both of these pathways
may induce U87 cell apoptosis and thereby inhibit tumor cell
growth.
In vivo, it was determined that TetC
decreased tumor size and inhibited the growth of human glioma
U87-cell xenografts in BALB/c nude mice, without influencing body
weight. This result suggests that TetC not only inhibits tumor
growth, but is also well tolerated. The limitation of this study is
that it is not clear whether TetC can pass through the blood-brain
barrier. It is reported that tetrandrine liposomes could pass
through blood-brain barrier (39). If
TetC cannot pass the blood-brain barrier, Tet liposomes will be
used in a future study. In addition, only the effect of TetC on U87
cell apoptosis was investigated in the present study. It is
considered that TetC has a wide range of pharmacological effects
which will be addressed in a future study.
In conclusion, TetC induced apoptosis in human
glioma U87 cells by decreasing the Bcl-2/Bax ratio and increasing
the production of ROS, the CL caspase-3/caspase-3 ratio and JNK and
p38 phosphorylation. In vivo, TetC was highly effective at
inhibiting the growth of human glioma U87 ×enografts in BALB/c nude
mice, and is therefore a promising candidate for the treatment of
human gliomas.
Acknowledgements
We acknowledge the technical assistance and support
from Dr Jin Liu for flow cytometric analysis.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81671391),
the CAMS Innovation Fund for Medical Sciences (grant no.
2018-I2M-1-002) and the Beijing Hospital Nova Project (grant no.
BJ-2016-033 and BJ-2016-034).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL conceived and designed the experiments. JS and
YaZ performed the experiments. YoZ and JC analyzed the data. GH
assisted in the western blot analysis and manuscript preparation.
YL wrote the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All animal experimentation was approved by the
Institutional Animal Care and Use Committee of Beijing
Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Friedman HS, Kerby T and Calvert H:
Temozolomide and treatment of malignant glioma. Clin Cancer Res.
6:2585–2597. 2000.PubMed/NCBI
|
|
2
|
Gao J, Wang Z, Liu H, Wang L and Huang G:
Liposome encapsulated of temozolomide for the treatment of glioma
tumor: Preparation, characterization and evaluation. Drug Discov
Ther. 9:205–212. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kawaji H, Tokuyama T, Yamasaki T, Amano S,
Sakai N and Namba H: Interferon-beta and temozolomide combination
therapy for temozolomide monotherapy-refractory malignant gliomas.
Mol Clin Oncol. 3:909–913. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu Z, Xie G, Zhou G, Cheng Y, Zhang G, Yao
G, Chen Y, Li Y and Zhao G: NVP-BEZ235, a novel dual PI3K-mTOR
inhibitor displays anti-glioma activity and reduces chemoresistance
to temozolomide in human glioma cells. Cancer Lett. 367:58–68.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang X, Jia L, Jin X, Liu Q, Cao W, Gao X,
Yang M and Sun B: NF-κB inhibitor reverses temozolomide resistance
in human glioma TR/U251 cells. Oncol Lett. 9:2586–2590. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tian T, Li A, Lu H, Luo R, Zhang M and Li
Z: TAZ promotes temozolomide resistance by upregulating MCL-1 in
human glioma cells. Biochem Biophys Res Commun. 463:638–643. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dunn-Pirio AM and Vlahovic G:
Immunotherapy approaches in the treatment of malignant brain
tumors. Cancer. 123:734–750. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu Z, Wang G, Xu S, Li Y, Tian Y, Niu H,
Yuan F, Zhou F, Hao Z, Zheng Y, et al: Effects of tetrandrine on
glioma cell malignant phenotype via inhibition of ADAM17. Tumour
Biol. 35:2205–2210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li X, Wu Z, He B and Zhong W: Tetrandrine
alleviates symptoms of rheumatoid arthritis in rats by regulating
the expression of cyclooxygenase-2 and inflammatory factors. Exp
Ther Med. 16:2670–2676. 2018.PubMed/NCBI
|
|
10
|
Gao LN, Feng QS, Zhang XF, Wang QS and Cui
YL: Tetrandrine suppresses articular inflammatory response by
inhibiting pro-inflammatory factors via NF-κB inactivation. J
Orthop Res. 34:1557–1568. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lai JH: Immunomodulatory effects and
mechanisms of plant alkaloid tetrandrine in autoimmune diseases.
Acta Pharmacol Sin. 23:1093–1101. 2002.PubMed/NCBI
|
|
12
|
Miao RM, Fang ZH and Yao Y: Therapeutic
efficacy of tetrandrine tablets combined with matrine injection in
treatment of silicosis. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za
Zhi. 30:778–780. 2012.(In Chinese). PubMed/NCBI
|
|
13
|
Zhang HN, Xin HT, Zhang WD, Jin CJ, Huang
SY and Zhang Y: The anti-fibrotic effects of Qidan granule in
experimental silicosis. Zhonghua Yu Fang Yi Xue Za Zhi. 41:290–294.
2007.(In Chinese). PubMed/NCBI
|
|
14
|
Miao RM, Sun XF, Zhang YY, Wu W, Fang ZH,
Zhao R, Zhao DK, Qian GL and Ji J: Clinical efficacy of tetrandrine
combined with acetylcysteine effervescent tablets in treatment of
silicosis. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi.
31:857–858. 2013.(In Chinese). PubMed/NCBI
|
|
15
|
Zhang TJ, Guo RX, Li X, Wang YW and Li YJ:
Tetrandrine cardioprotection in ischemia-reperfusion (I/R) injury
via JAK3/STAT3/Hexokinase II. Eur J Pharmacol. 813:153–160. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang P, Xu Y, Wei R, Li H, Tang Y, Liu J,
Zhang SS and Zhang C: Efficacy of tetrandrine on lowering
intraocular pressure in animal model with ocular hypertension. J
Glaucoma. 20:183–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J, Yu B, Zhang XQ, Sheng ZF, Li SJ,
Wang ZJ, Cui XY, Cui SY and Zhang YH: Tetrandrine, an
antihypertensive alkaloid, improves the sleep state of
spontaneously hypertensive rats (SHRs). J Ethnopharmacol.
151:729–732. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yuan B, Yao M, Wang X, Sato A, Okazaki A,
Komuro H, Hayashi H, Toyoda H, Pei X, Hu X, et al: Antitumor
activity of arsenite in combination with tetrandrine against human
breast cancer cell line MDA-MB-231 in vitro and in vivo. Cancer
Cell Int. 18:1132018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
N B and K RC: Tetrandrine and cancer-an
overview on the molecular approach. Biomed Pharmacother.
97:624–632. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wong V, Zeng W, Chen J, Yao XJ, Leung ELH,
Wang QQ, Chiu P, Ko BCB and Law BYK: Tetrandrine, an activator of
autophagy, induces autophagic cell death via PKC-α inhibition and
mTOR-dependent mechanisms. Front Pharmacol. 8:3512017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu T, Liu X and Li W: Tetrandrine, a
Chinese plant-derived alkaloid, is a potential candidate for cancer
chemotherapy. Oncotarget. 7:40800–40815. 2016.PubMed/NCBI
|
|
22
|
Joshi P, Vishwakarma RA and Bharate SB:
Natural alkaloids as P-gp inhibitors for multidrug resistance
reversal in cancer. Eur J Med Chem. 138:273–292. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu L, Liang Y, Deng L, Ding Y, Chen L, Ye
Y, Yang X and Pan Q: Characterization of tetrandrine, a potent
inhibitor of P-glycoprotein-mediated multidrug resistance. Cancer
Chemother Pharmacol. 53:349–356. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin L, Xu M, Luo XH and Zhu XF: Stephania
tetrandra and ginseng-containing chinese herbal formulation NSENL
reverses cisplatin resistance in lung cancer xenografts. Am J Chin
Med. 45:385–401. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Y and Tseng SH: The potential of
tetrandrine against gliomas. Anticancer Agents Med Chem.
10:534–542. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen Y, Chen JC and Tseng SH: Tetrandrine
suppresses tumor growth and angiogenesis of gliomas in rats. Int J
Cancer. 124:2260–2269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang KH, Chen ML, Chen HC, Huang YW, Wu
TY and Chen YJ: Enhancement of radiosensitivity in human
glioblastoma U138MG cells by tetrandrine. Neoplasma. 46:196–200.
1999.PubMed/NCBI
|
|
28
|
Imoto K, Takemura H, Kwan CY, Sakano S,
Kaneko M and Ohshika H: Inhibitory effects of tetrandrine and
hernandezine on Ca2+ mobilization in rat glioma C6 cells. Res
Commun Mol Pathol Pharmacol. 95:129–146. 1997.PubMed/NCBI
|
|
29
|
Shi Z, Liang YJ, Chen ZS, Wang XW, Wang
XH, Ding Y, Chen LM, Yang XP and Fu LW: Reversal of
MDR1/P-glycoprotein-mediated multidrug resistance by vector-based
RNA interference in vitro and in vivo. Cancer Biol Ther. 5:39–47.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin YJ and Zhen YS: Rhein lysinate
suppresses the growth of breast cancer cells and potentiates the
inhibitory effect of Taxol in athymic mice. Anticancer Drugs.
20:65–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tykocki T and Eltayeb M: Ten-year survival
in glioblastoma. A systematic review. J Clin Neurosci. 54:7–13.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu J, Zhen YZ, Cui J, Hu G, Wei J, Xu R,
Tu P and Lin YJ: Dynamic influence of Rhein lysinate on HeLa cells.
Int J Oncol. 53:2047–2055. 2018.PubMed/NCBI
|
|
33
|
Li Z, Mbah NE, Overmeyer JH, Sarver JG,
George S, Trabbic CJ, Erhardt PW and Maltese WA: The JNK signaling
pathway plays a key role in methuosis (non-apoptotic cell death)
induced by MOMIPP in glioblastoma. BMC Cancer. 19:772019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gruia MI, Negoita V, Vasilescu M, Panait
M, Gruia I, Velescu BS and Uivarosi V: Biochemical action of new
complexes of ruthenium with quinolones as potential antitumor
agents. Anticancer Res. 35:3371–3378. 2015.PubMed/NCBI
|
|
35
|
Hu XH, Zhao ZX, Dai J, Geng DC and Xu YZ:
MicroRNA-221 regulates osteosarcoma cell proliferation, apoptosis,
migration, and invasion by targeting CDKN1B/p27. J Cell Biochem.
120:4665–4674. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Driak D, Dvorska M, Bolehovska P, Svandova
I, Novotny J and Halaska M: Bad and Bid-potential background
players in preneoplastic to neoplastic shift in human endometrium.
Neoplasma. 61:411–415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ye Y, Zhi F, Peng Y and Yang CC: MiR-128
promotes the apoptosis of glioma cells via binding to NEK2. Eur Rev
Med Pharmacol Sci. 22:8781–8788. 2018.PubMed/NCBI
|
|
38
|
Lee JY and Yune TY: Ghrelin inhibits
oligodendrocyte cell death by attenuating microglial activation.
Endocrinol Metab (Seoul). 29:371–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li XT, Tang W, Xie HJ, Liu S, Song XL,
Xiao Y, Wang X, Cheng L and Chen GR: The efficacy of RGD modified
liposomes loaded with vinorelbine plus tetrandrine in treating
resistant brain glioma. J Liposome Res. 29:21–34. 2019. View Article : Google Scholar : PubMed/NCBI
|