Introduction
Lipopolysaccharides (LPSs, endotoxin) represent the
main surface antigens and virulence factors of gram-negative
bacteria. LPSs when isolated from smooth strains of bacteria are
composed of three distinct regions: lipid A, a core
oligosaccharide, and the O-specific polysaccharide (O-antigen). The
lipid A of E. coli type is built of a GlcN-containing
disaccharide substituted with six acyl residues. This hexaacylated
form critically affects the biological activity of endotoxins by
mediating the interaction of LPS with pattern recognition receptors
presented on a wide range of cells, mainly on
monocytes/macrophages, neutrophils and endothelial cells (1). LPSs elicit both systemic and local
reactions by induction of a massive proinflammatory response which
involves the release of cytokines participating also in anticancer
activity (2). Therefore, for several
decades, it has been postulated that LPSs or synthetic lipid A
molecules can be used as anticancer agents. Unfortunately, both of
them demonstrated only a slight therapeutic effect and overall
toxicity (3). The relationship
between chronic inflammation and carcinogenesis can therefore be
considered in the context of the delivery of various LPS doses into
the host environment (4,5) which can affect tumor growth or delay
tumor progression. A better understanding of the mode of
multifaceted action generated by LPSs or their derivatives in
induction of antitumor immune activity is still of interest.
In contrast to the smooth type of LPSs, the rough
LPS synthesized by strains such as E. coli B are devoid of
the O-specific chain (lipooligosaccharide, LOS). It may be related
to a partial reduction in virulence and increase in susceptibility
to phagocytosis and serum bactericidal reactions (6,7), but it is
still able to be an immune stimulator (8).
It has been reported that changes in the
proinflammatory activity of LPS derivatives could be induced by the
modified structure of lipid A associated with a different number of
fatty acid residues (9). Recently, we
identified de-O-acylated LOS E. coli B (LOS-OH) as a
major constituent of bacteriophage T4 lysate with reduced
biological activity.
In the present study, an LOS derivative with four
ester-bound fatty acid residues removed from the lipid A was
prepared by de-O-acylation using anhydrous hydrazine. There
are a few reports concerning such derivatives differing in the
presence of fatty acid residues which may modulate LOS activity.
However, a variety of de-O-acylated analogues characterized
by lower toxicity have been proposed as anticancer agents or
adjuvants for anticancer vaccines (3).
To investigate the effect of structural
modifications of E. coli B LOS-OH (LOS-OH) on its activity,
in vitro experiments using myeloid and lymphoid cells were
performed. Moreover, to examine this compound's effect on lung
colonization by B16 cells, a B16 melanoma metastasis in vivo
model was employed.
Our findings revealed that LOS-OH in in vitro
experiments induced lower stimulation of myeloid cells, resulting
in low cell surface expression, low cytokine production and limited
capacity of activation of the BM-DCs to trigger splenocyte priming.
Moreover, multiple administration of this compound led to a
statistically significant (P<0.05; P<0.01) reduction in lung
metastatic foci formation. It was accompanied by substantial (but
not statistically significant) changes in the ratio of granulocytic
to monocytic cells in blood and prolonged, low-level activation of
myeloid but not lymphoid spleen cells. Thus, LOS-OH demonstrated
weaker ability than LOS to stimulate and polarize an innate immune
response both in vitro and in vivo.
Materials and methods
LOS and LOS-OH preparation
Bacteria were grown for 24 h and harvested as
previously reported (9). E.
coli B LOS was extracted from bacterial cells by the PCP method
(10) and purified by
ultracentrifugation (105,000 × g, 4 h, 4°C). The yield of LOS was
4% of the bacterial cells.
LOS (50 mg) was de-O-acylated by treatment
with anhydrous hydrazine as previously described (11) (3 ml, 30 min, 37°C), followed by
precipitation with cooled acetone (−20°C) and centrifugation
(27,000 × g, 30 min, −10°C). The sediment was washed with acetone,
then dissolved in water, and freeze-dried (yield of
de-O-acylated LOS E. coli B: 34 mg, 68% of LOS)
(12). Both LOS and LOS-OH compounds
were diluted in PBS for in vitro and in vivo tests.
Applied doses did not affect cell viability in in vitro and
ex vivo cultures.
Mice and ethics statement
Female 6–9 week-old C57BL/6 (B6) mice were
maintained in the Animal Facility of the Ludwik Hirszfeld Institute
of Immunology and Experimental Therapy (Wroclaw, Poland) under
specific pathogen-free (SPF) conditions. Mice were supplied from
the Center for Experimental Medicine of the Medical University of
Bialystok (Bialystok, Poland). All experiments were performed
according to the EU Directive 2010/63/EU for animal experiments and
were approved by the 1st Local Ethics Committee for Experiments
with the Use of Laboratory Animals, Wroclaw, Poland (number
48/2008).
Cell lines and in vitro
stimulation
All cells were cultured in standard conditions at
37°C in a humid atmosphere (5% CO2). All culture media
were supplemented with 100 mg/ml streptomycin, 100 U/ml penicillin
and 0.5% sodium pyruvate (all from Sigma-Aldrich Chemie GmbH; Merck
KGaA).
B16 cells of a mouse melanoma line (ATCC; Rockville,
Maryland, USA) were maintained in vitro in RPMI-1640
GlutaMAX and Opti MEM GlutaMAX (1:1) (both from Gibco; Thermo
Fisher Scientific, Inc.,) additionally supplemented with 10% fetal
bovine serum (FBS, Sigma-Aldrich Chemie GmbH; Merck KGaA).
RAW264.7 cells of a monocyte line (ATCC) were
maintained in DMEM culture medium (ATCC) supplemented with 10% FBS.
For tests, cells were stimulated with LOS or LOS-OH (both E.
coli B) at concentrations of 50, 10, 1 or 0.1 µg/ml for 24 h in
24-well plates (5×105 /ml/well).
JAWS II cells of a dendritic cell line (ATCC) were
cultured in RPMI-1640 GlutaMAX and αMEM (both from Gibco; Thermo
Fisher Scientific, Inc.) in a ratio of 1:1 supplemented with 10%
FBS and 5 ng/ml rmGM-CSF (Immunotools, Germany). Cells were
stimulated with LOS or LOS-OH at concentrations of 10, 1, 0.1 µg/ml
for 24 h in 12-well plates (5×105 /ml/well).
The supernatants from these myeloid cultures were
collected and analyzed using commercially available ELISA sets.
Cell culture and ex vivo
stimulation
Bone marrow-derived dendritic cells (BM-DCs)
obtained from six-day culture in medium RPMI-1640 GlutaMAX
supplemented with 10% FBS, GM-CSF (40 ng/ml) and IL-4 (10 ng/ml)
(both from Immunotools, Germany) [as described in (13,14)] were
applied into 12-well plates (1×106 /ml/well). The next
day (7th day), cells were stimulated with LOS or LOS-OH at
concentrations of 10, 1, 0.1 µg/ml for 24 h in 12-well plates.
Concentrations of cytokines in the collected supernatants were
measured using ELISA. Phenotypic characteristics of stimulated
BM-DCs were analyzed using flow cytometry.
Co-culture of spleen cells with
preincubated BM-DCs
Spleen cells were obtained from healthy C57BL/6
mice, wiped through a sterile nylon filter into a RPMI-1640
GlutaMAX culture medium with 3% FBS, washed, resuspended and stored
in liquid nitrogen. After thawing, splenocytes were primed with
BM-DCs which were preincubated with LOS and LOS-OH at
concentrations of 10, 1, 0.1 µg/ml for 24 h in 12-well plates
(5×105 /ml/well) prior to mixed culture. The co-culture
was carried out for 5 days (10:1 ratio) in RPMI-1640 GlutaMAX
culture medium with 10% FBS and 200 U/ml IL-2. Afterwards the cells
and supernatants were harvested. The supernatants were analyzed
using commercially available ELISA sets. Phenotypic characteristics
of spleen cells were analyzed using flow cytometry. Splenocytes
were separated in FACS analysis based on SSC-FSC parameters.
Phenotypic characterization of the
BM-DCs
For phenotypic characterization of eight-day BM-DCs
the following fluorophore-labeled anti-mouse monoclonal antibodies
(mAbs) were used: mouse anti-mouse FITC I-Ab (BD
Pharmingen; clone 25-9-17), rat anti-mouse RPE-CD40 (BD Pharmingen;
clone 3/23), hamster anti-mouse APC-CD80 (BD Pharmingen; clone
16-10A1) and rat anti-mouse PE-Cy7-CD86 (BD Pharmingen; clone GL1),
and the appropriate isotype controls: FITC-labeled mouse IgG2a (BD
Pharmingen; clone G155-178), R-phycoerythrin (RPE)-labeled IgG2a
(BD Pharmingen; clone R35-95), APC-labeled Hamster IgG2k (BD
Pharmingen; clone B83-3), PE-Cy7-labeled Rat IgG2a (BD Pharmingen;
clone R35-95). The cells were stained for 45 min at 4°C. Expression
of the cell-surface molecules was analyzed by a FACSCalibur flow
cytometer with CellQuest software (Becton Dickinson; BD
Biosciences).
Evaluation of cytokine production
Supernatants from all cultures were analyzed using
commercially available ELISA kits (IL-6, IL-10 from Becton
Dickinson (BD Biosciences) and TNF-α, IL-12, IL-17A, IFN-γ from
eBioscience) in accordance with the manufacturer's
instructions.
In vivo experiments and determination
of antimetastatic activity of LOS-OH
B16 cells of a melanoma cell line were harvested
from in vitro cultures. A single-cell suspension in Hank's
buffered salt solution (2×105 cells/200 ml/mouse) with
cell viability over 90% was inoculated intravenously (i.v.) into
the lateral tail vein. LOS and LOS-OH were administered
intraperitoneally (i.p.) at doses of 250 and/or 2,500 µg/kg body
weight (b.w.)/dose, 4 times (1 h before the B16 cell inoculation
and on the 1st, 7th and 14th day following it). During the
experiments, mouse body weight and temperature were monitored, but
no changes were observed. Experiments were terminated on the 14th
and/or 21st day. Mice were sacrificed by cervical dislocation. For
visualization of lung metastases, organs (from 8–10 mice per group)
were fixed in formalin overnight, which allowed metastatic foci to
be distinguished from lung tissue (15). Lung colonies were counted under a
dissecting stereomicroscope (×10 magnification). The blood
morphology was evaluated in each blood sample using the Mythic 18
analyzers (C2 Diagnostics, Montpellier).
Analysis of spleen cells
Spleens obtained from tumor-bearing mice were
prepared as described above. Then, parts of the cells
(2×106 cells/ml) were stimulated with ConA (0.5 µg/ml)
or LOS (1 µg/ml) for 48 h and afterwards the supernatants were
collected. Concentration of cytokines in collected supernatants
were measured using ELISA sets. For lymphoid cell analysis,
splenocytes were stained with the following fluorophore-labeled
anti-mouse monoclonal antibodies (mAb): anti-CD4-APC (BD
Pharmingen; clone RM4-5), anti-CD8-PE-Cy7 (BD Pharmingen; clone
53-6.7), anti-CD49b-PE (BD Pharmingen; clone DX5) and
anti-CD19-FITC (BD Pharmingen; clone 1D3). For determination of
myeloid splenocytes, anti-B220-APC (BD Pharmingen; clone RA3-6B2),
anti-CD11b-PerCP-Cy5.5 (BD Pharmingen; M1/70), anti-Ly6C-PE (BD
Pharmingen; clone AL-21), anti-Ly6G-APC-Cy7 (BD Pharmingen; clone
1A8), anti-MHC class II-FITC (BD Pharmingen; clone 25-9-17) and
anti-CD86-PE-Cy7 (BD Pharmingen; clone GL1) antibodies were used.
To determine their viability DAPI was used. Phenotypic analysis was
carried out using an LSRFortessa cell analyzer with Diva software
(Becton Dickinson; BD Biosciences).
Statistical analysis
In all remaining analyses, the statistical
differences were calculated using the nonparametric Kruskal-Wallis
test for multiple independent groups followed by Dunn's multiple
comparison post hoc test or two-way analysis of variance (ANOVA)
followed by Sidak's multiple comparison test. Analyses were
performed using the GraphPad Prism 7.02 software (GraphPad, San
Diego, CA, USA). A P-value <0.05 was considered to be indicative
of statistical significance. Asterik(s) at the top of a histogram
bar describe statistically significant differences of the
experimental group when compared to the control group (*P<0.05,
**P<0.01, ***P<0.001, ****P<0.0001), while hash symbol(s)
placed above brackets indicate statistically significant
differences between the two indicated groups
(#P<0.05, ##P<0.01,
###P<0.001).
Results
LOS-OH demonstrates weaker ability
than LOS to stimulate myeloid cells for production of IL-6 and
TNF-α and phenotypic maturity
We evaluated the biological effect of LOS-OH on the
RAW 264.7 macrophage-like cell line (Fig.
1A) and JAWS II dendritic cell line (Fig. 1B) in comparison to LOS activity. For
this purpose, RAW 264.7 cells were exposed to both compounds at
concentrations ranging from 50 to 0.1 µg/ml while JAWS II cells
were exposed to concentrations ranging from 10 to 0.1 µg/ml. The
unstimulated cells were treated as a control. Observed differences
in the cytokine production depended on the concentration and nature
of the stimulators. RAW264.7 cells treated with LOS-OH produced
IL-6 (P<0.05) and TNF-α (P<0.01, P<0.0001) at extremely
low levels, showing a statistically significant decrease in
relation to the effect of LOS (Fig.
1A). JAWS II cells were also able to respond to compounds in a
concentration-dependent manner. However, in contrast to former
cells, differences in the level of cytokine production between LOS
and LOS-OH were less expressed. Compounds induced a statistically
significant difference in TNF-α production only at a concentration
of 0.1 µg/ml (P<0.05) (Fig. 1B)
Hence, the concentration range within which LOS-OH appeared to be
able to stimulate cytokine production is extremely narrow.
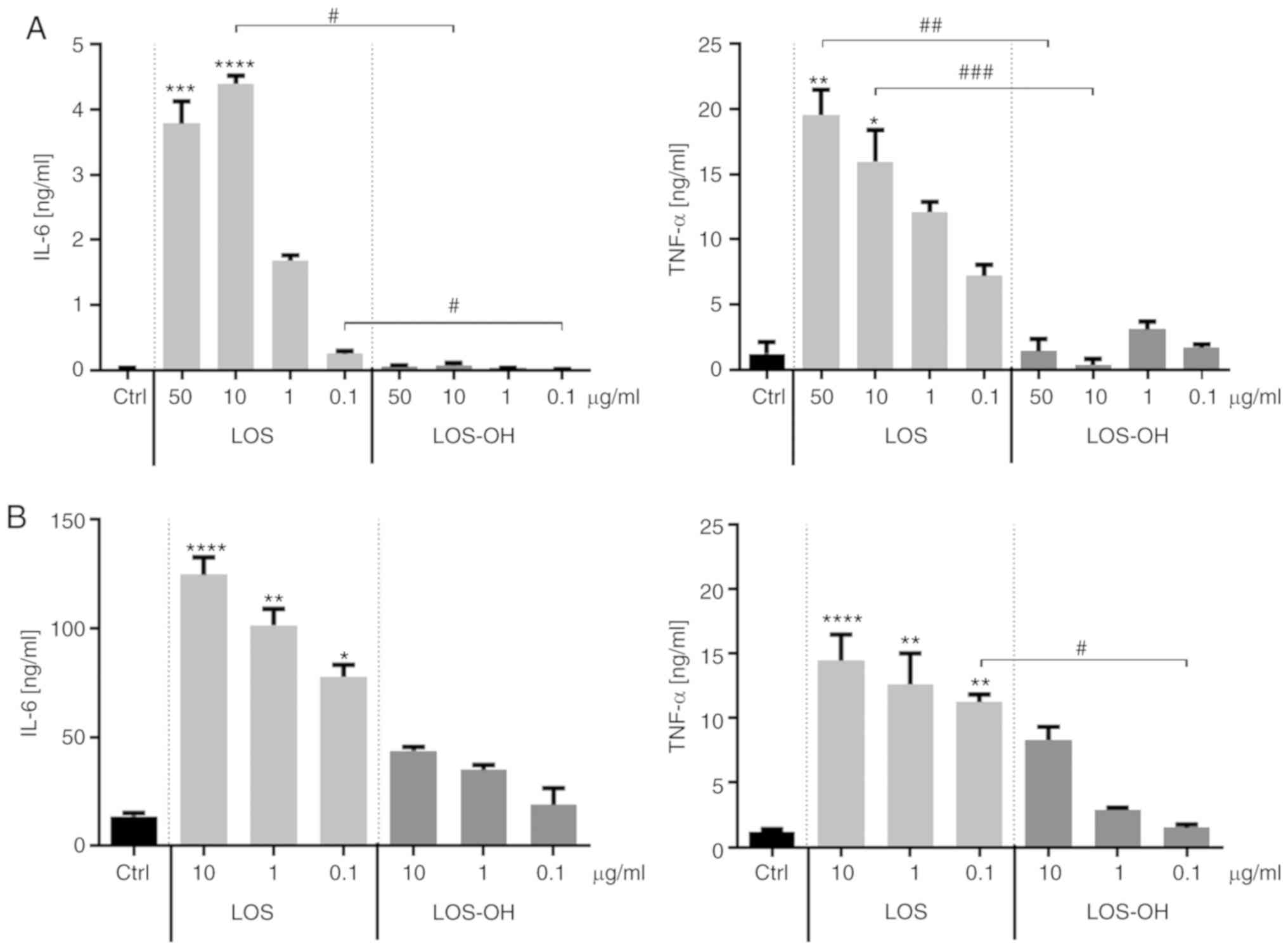 | Figure 1.Effects of LOS and LOS-OH on the
capacity of RAW 264.7 and JAWS II cells for cytokine production.
Concentrations of IL-6 and TNF-α in supernatants collected after 24
h of culture in (A) RAW264.7 and (B) JAWS II cells with LOS or
LOS-OH as measured using ELISA. Unstimulated cells were used as a
control (Ctrl). The results are shown as the mean ± SD (n=6). The
differences between groups were estimated using the nonparametric
Kruskal-Wallis test followed by Dunn's multiple comparison test
(*P<0.05, **P<0.01, ***P<0.001, ***P<0.0001 vs. Ctrl;
#P<0.05, ##P<0.01,
###P<0.001 between the two indicated groups). LOS,
Escherichia coli lipooligosaccharide; LOS-OH,
de-O-acylated derivative of LOS; Ctrl, control; IL-6,
interleukin 6; TNF-α, tumor necrosis factor-α. |
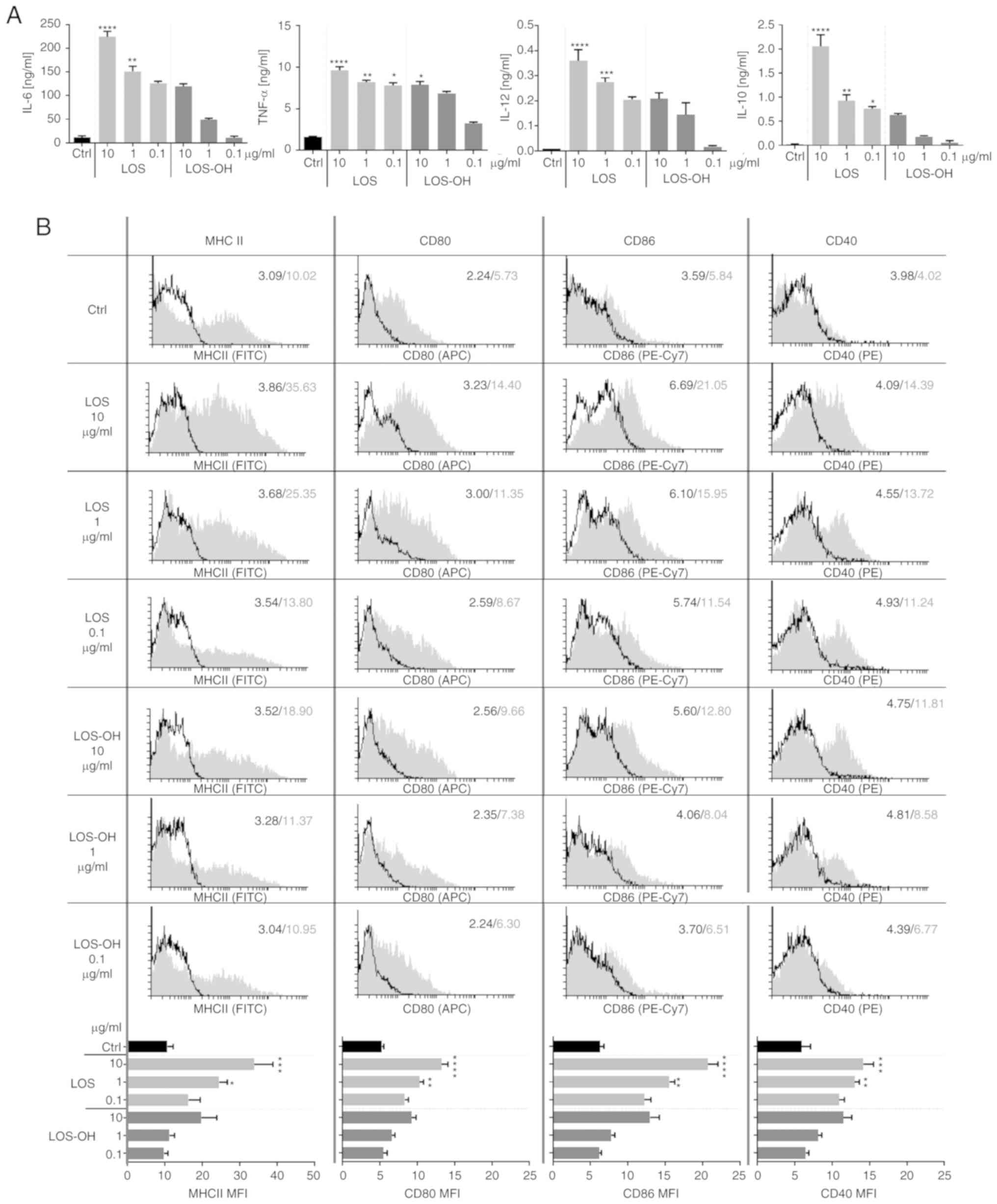
BM-DCs were exposed to LOS-OH for 24 h. We found
that their response was very similar to that observed for JAWS II
cells. Initially high IL-6 production diminished along with the
decrease in concentrations of both compounds. However, cytokine
production induced by LOS-OH at 10 µg/ml corresponded to that
obtained for LOS at 0.1 µg/ml and was subsequently drastically
decreased. A similar tendency of TNF-α and IL-12 production was
observed (Fig. 2A). BM-DCs responded
to a high LOS concentration with strong IL-10 production but
reduction of the compound's concentration, and the use of
corresponding LOS-OH concentrations markedly reduced cytokine
production. Thus, LOS-OH was less potent in the stimulation of
BM-DCs compared to LOS, regardless of the type of cytokine
produced.
The effect of both stimulators on the changes in
expression of BM-DC surface markers (MHC class II, CD40, CD80,
CD86) was evaluated (Fig. 2B). Cell
stimulation with LOS caused statistically significant increases in
surface marker expression over the control at the concentration of
10 µg/ml [MHCII, CD40 (P<0.001); CD80, CD86 (P<0.0001)] and 1
µg/ml [MHCII (P<0.05); CD80, CD86, CD40 (P<0.01)]. After
LOS-OH stimulation, we observed a slight increase in the expression
of surface markers compared to untreated cells; however, these
changes were not statistically significant. The obtained data
confirmed that LOS-OH exhibited substantially lower efficacy in the
activation of BM-DCs than LOS but still in a
concentration-dependent manner.
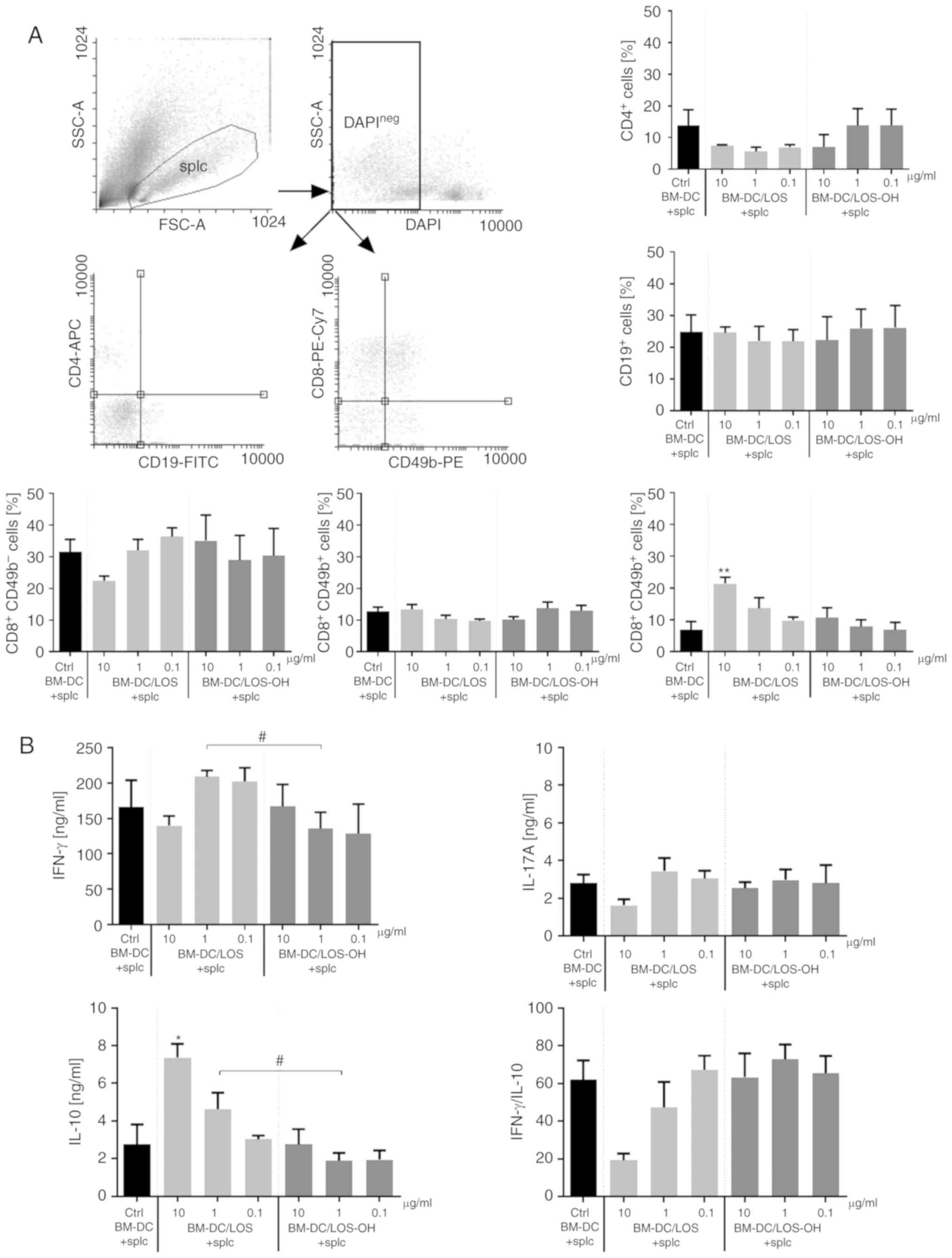
In order to analyze the indirect effect of both
compounds on lymphoid cells, we used a mixed cell culture of
splenocytes and BM-DCs preincubated for 24 h with LOS (BM-DC/LOS)
or LOS-OH (BM-DC/LOS-OH) prior to the test and compared it to
untreated cells (Fig. 3A).
Preincubated BM-DCs were used in a role of antigenic stimulators.
No statistically significant changes in the percentage of
CD4+ T helper cells and CD19+ B cells were
observed. Although there were some statistically significant
differences between BM-DC/LOS-OH groups, changes in the percentage
of CD8+CD49b− T cytotoxic (P<0.05) or
CD8−CD49b+ natural killer (NK) cells were
slight compared to the BM-DC/LOS groups. We found only an increase
in the CD8+49b+ natural killer T (NKT) cell
percentage. Thus, the treatment with BM-DC/LOS-OH did not cause any
substantial changes in the percentages of spleen-derived lymphoid
cell subpopulations. Even if changes were observed, they were
comparable with those induced by BM-DC/LOS at 0.1 µg/ml.
We also evaluated the ability of splenocytes
co-cultured with BM-DC/LOS or BM-DC/LOS-OH to produce cytokines
(Fig. 3B). It was observed that
stimulation with BM-DC/LOS-OH resulted in IFN-γ production equal
(at 10 µg/ml of LOS-OH) or lower (at subsequent doses of LOS-OH)
compared to splenocytes stimulated with untreated BM-DCs. Moreover,
we demonstrated a statistically significant reduction in the IFN-γ
production by splenocytes co-cultured with BM-DC/LOS-OH at 1 µg/ml
in relation to the production of this cytokine by splenocytes
stimulated with BM-DC/LOS at 1 µg/ml (P <0.05). We did not
observe significant differences in the production of IL-17A by
splenocytes stimulated with BM-DC/LOS, BM-DC/LOS-OH or untreated
BM-DCs. Splenocyte activation for IL-10 production by BM-DCs
preincubated with both compounds was altered in a
concentration-dependent manner and the difference between LOS-OH
and LOS at 1 µg/ml was statistically significant (P<0.05).
Regardless of this fact, considerable differences between the
levels of IFN-γ and IL-10 production were found, and the
IFN-γ/IL-10 ratio showed that the effect of activated BM-DCs on
splenocyte priming was found only when LOS was used at 10 µg/ml
(Fig. 3B). Also, BM-DC/LOS-OH were
not able to activate a lymphocyte-dependent immune response. In our
previous study, spleen cells obtained from healthy mice were able
to produce IL-10 after stimulation with LOS and LOS-OH (10, 1, 0.1
µg/ml). The concentration of IL-10 in the supernatants was in the
range 0.5–2.0 ng/ml after LOS use and after LOS-OH stimulation was
in the range 0–0.5 ng/ml and was dependent on the compound dose
(data not shown). In contrast to the effects obtained from mixed
cultures, splenocytes directly exposed to compounds did not reveal
capacity for IL-10 production. Moreover, they did not produce IFN-γ
or IL-17A.
LOS-OH exhibits an anti-metastatic
effect on pulmonary metastasis of B16 cells but did not affect
lymphoid cell activity
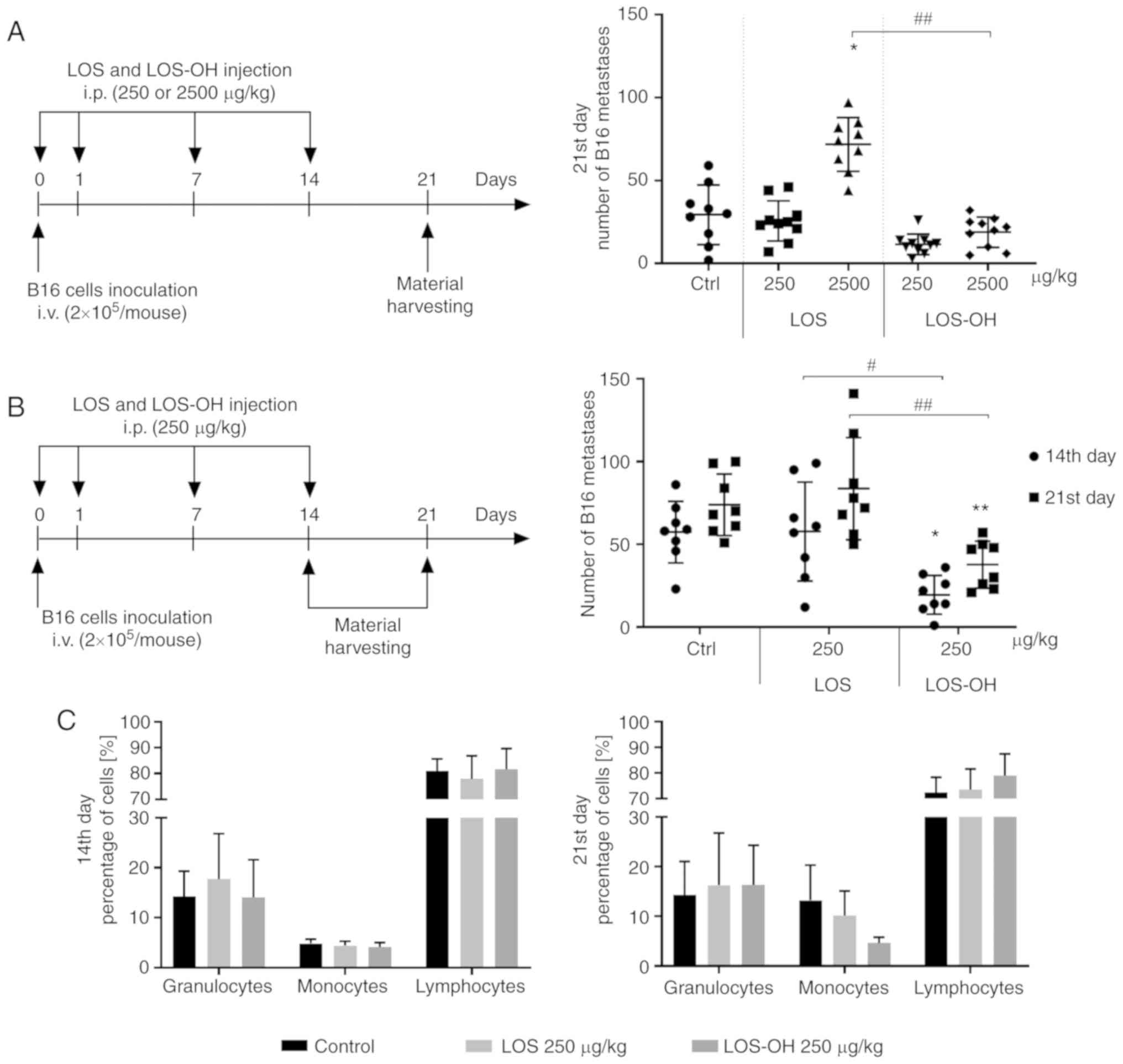
Since LOS-OH appeared to be non-toxic at a wide
range of doses (data not shown), for evaluation of its
anti-metastatic activity (vs. LOS) two widely different doses of
the compounds (250 or 2,500 µg/kg b.w.) were used (Fig. 4A). The lowest numbers of B16 pulmonary
metastatic foci were observed in the LOS-OH treated groups,
regardless of the dose administered. The administration of LOS at
2,500 µg/kg/dose caused a statistically significant (P<0.05)
increase in the number of pulmonary metastases compared to
untreated mice, whereas the use of LOS-OH at the same dose resulted
in a significant reduction in metastatic foci compared to the
LOS-treated mice (P<0.01).
In the next experiment we analyzed the time relation
of the anti-metastatic effect of LOS-OH applied at 250 µg/kg/dose
(Fig. 4B). The average number of
pulmonary metastases in the LOS-OH-treated mice varied between 20
(the 14th day) and 38 (the 21st day), while in mice treated with
LOS the number of metastases did not decrease and was estimated as
58 (the 14th day) and 84 (the 21st day). We found a time-dependent,
statistically significant reduction in pulmonary metastasis number
when mice were treated with LOS-OH compared to the control group
(P<0.05, P<0.01 at the 14th and 21st day, respectively) or
mice treated with LOS (P<0.05, P<0.01 at the 14th and 21st
day, respectively).
In the course of the second experiment, blood
morphological analysis was performed. The changes in cell number
were related to the type of the compound and time point of
observation (Fig. 4C). On the 14th
day of the experiment, lymphocytes obtained from metastasis-bearing
mice revealed a similar percentage regardless of the nature of
stimulators. There were no significant differences in percentages
of either monocytes or granulocytes at this time point. Meanwhile,
inconsiderable changes in the percentage of lymphocyte and monocyte
subpopulation in the blood obtained from mice on the 21st day of
the experiment were observed. However, no changes in the
granulocyte subpopulation were observed.
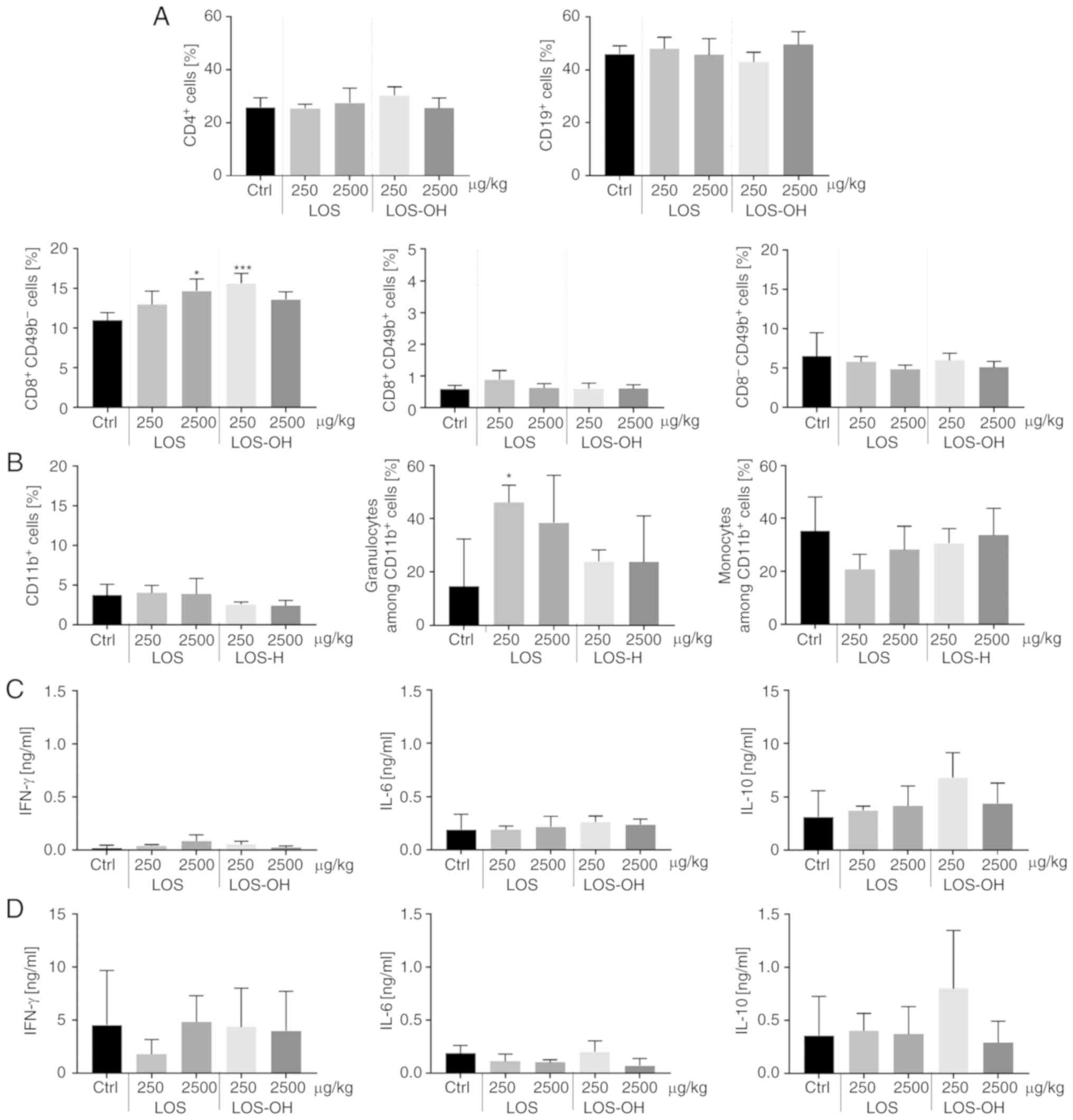
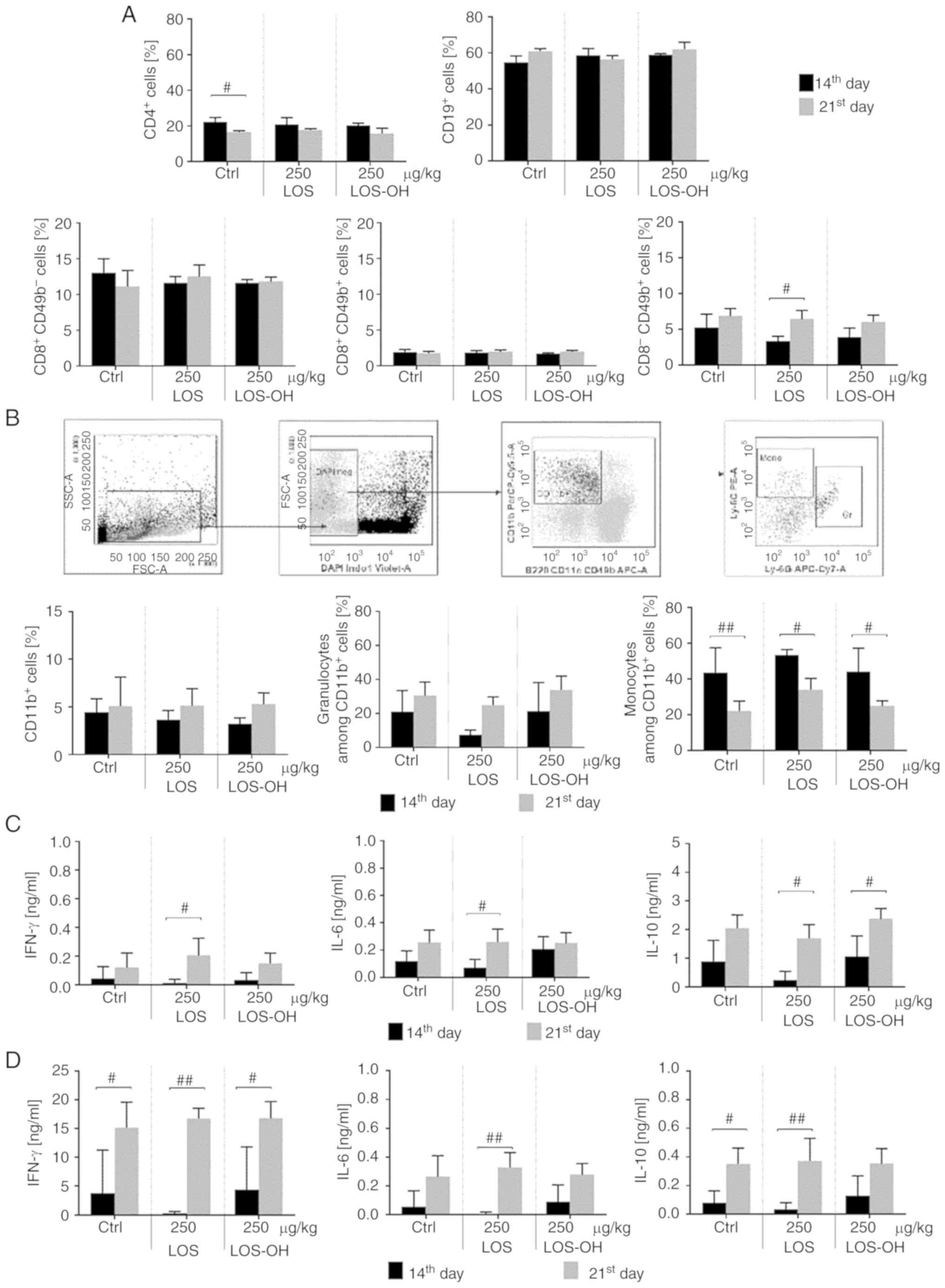
We also analyzed the influence of the administration
of two doses of compounds on activation of the systemic response of
treated mice, revealing that LOS-OH caused changes in the number of
lymphoid and myeloid cells in the spleen. The percentage of
CD4+ T helper, CD19+ B and
CD8−CD49b+ NK or
CD8+CD49b+ NKT cells did not show differences
between groups. However, after administration of LOS-OH at 250
µg/kg/dose, a statistically significant (P<0.001) increase in
the percentage of CD8+CD49b− T cytotoxic
cells was noted compared to the control group (Fig. 5A). On the other hand, treatment with
the compounds did not increase the number of CD11b+
cells but induced significant (P<0.05) changes within this
population after LOS (250 µg/kg) stimulation, particularly in the
granulocytic subpopulation (Fig.
5B).
We also evaluated the influence of both compounds on
specific cytokine production. For this purpose, splenocytes from
treated mice were ex vivo re-stimulated with LOS or
stimulated with concanavalin A (Con A). Splenocytes which responded
to LOS (Fig. 5C) produced a low
amount of IL-6, a minor amount of IFN-γ but a high amount of IL-10
compared to the control, especially when spleen cells originated
from mice treated with 250 µg/kg of LOS-OH. Splenocytes which
responded to ConA (Fig. 5D) produced
a similar amount of IL-6 but lower amount of IL-10 with the same
tendency corresponding with mouse treatment.
Subsequently, we analyzed the kinetics of activation
of the systemic response after treatment with 250 µg/kg of LOS-OH
or LOS (Fig. 6). No significant
changes in the percentage of CD4+ T helper,
CD8+CD49b+/− cells, or CD19+ B
splenocyte subpopulations were observed. An increase in the
percentage of CD8−CD49b+ NK cells during the
experiment (Fig. 6A) as well as a
time-related increase in the myeloid cell number was also found
(Fig. 6B). However, all observed
changes were only time-dependent, and they were not associated with
the applied treatment.
To conclude, the administration of LOS-OH was not
able to cause differences in the size of spleen cell subpopulations
compared to LOS or control groups even when some time-dependent
changes could be observed (NK and myeloid splenocytes).
We also evaluated the changes in the level of
cytokine production by splenocytes obtained on the 14th or the 21st
day of the experiment (Fig. 6C). The
data showed a time-dependent increase in cytokine production.
However, differences between types of cytokines were related to the
type of ex vivo stimulation rather than to the type of the
administered compound; higher production of IL-10 was induced ex
vivo by LOS restimulation, and a high amount of IFN-γ was
produced by ConA-stimulated splenocytes.
Discussion
The variability of lipid A structures depends on the
degree of acylation and the nature of the fatty acids. Removal of
four ester-bound fatty acid residues from hexaacyl lipid A of
Escherichia coli lipooligosaccharide (LOS) resulted in the
de-O-acylated derivative (LOS-OH). Since some aspects of
structure-function relationships for various lipopolysaccharides
(LPS) are not fully understood (16),
we investigated the influence of the structural modification of one
of these on the biological activity. Toward this aim, macrophages
or dendritic cells of the myeloid lines were exposed to LOS-OH for
24 h and revealed lower induction of IL-6 and TNF-α production
compared to LOS possessing hexaacyl lipid A activity. Hence, a
question has been raised whether LOS-OH would affect bone
marrow-derived dendritic cells (BM-DCs) in a similar manner. Based
on cytokine production, regardless of the type of secreted
cytokines (proinflammatory IL-6, TNF-α, IL-12 or anti-inflammatory
IL-10) we confirmed that LOS-OH demonstrated lower ability to
stimulate BM-DCs than LOS. BM-DCs are believed to be programmed for
a strong response to LPS stimulation, which resulted in
upregulation of MHC class II and CD80 and CD86 expression (17–19). A
similar effect was exhibited by BM-DCs stimulated with LOS
(8). In contrast, LOS-OH caused only
a slight increase in expression of these antigens. Similar
downregulation of the immune reaction has been described both in
human and mouse macrophages (20) and
could be associated with low TNF-α production leading to induction
of immune tolerance (3,21,22). The
relationship between chronic inflammation and carcinogenesis can be
considered in the context of the delivery of various doses of LPS
and its derivatives into the host environment (4,5), provoking
not only tumor growth. The effect associated with low LPS
concentration probably results from endotoxin-induced immune
tolerance (21,23,24).
LOS-OH appeared to be unable to directly trigger
splenocyte reactivity in vitro. When these cells were
co-cultured with BM-DC/LOS-OH, the cell interaction did not cause
changes in surface antigen expression on splenocytes. In these
mixed cultures, IFN-γ production did not exceed the control level
(untreated BM-DCs). In turn, BM-DCs preincubated with LOS-OH or LOS
provoked splenocytes to produce IL-10 at a higher level with a
statistically significant concentration of 1 µg/ml (P<0.05), but
significant changes in IL-10 production were observed only after
the use of BM-DC/LOS at 10 µg/ml (P<0.05). Moreover, the
calculation of IFN-γ to IL-10 ratio revealed that BM-DC/LOS-OH were
not able to modulate priming of splenocytes.
The above findings suggest that LOS-OH is a weaker
stimulator for in vivo activation and polarization of the
immune response, consistent with its non-toxicity over a wide dose
range in comparison to LOS. Therefore, we conducted in vivo
experiments with a melanoma B16 metastasis model in which B16
melanoma cells were inoculated intravenously in mice and treated
with LOS-OH.
LOS-OH given i.p. at a dose of 250 µg/kg b.w.,
caused a time-dependent, statistically significant reduction in the
number of experimental metastases compared to the control group or
mice treated with LOS. However, changes in the percentage of
monocyte and lymphocyte cells in the blood, examined 7 days after
the last compound administration in the second experiment, appeared
to be inconsiderable. Admittedly, the time interval in which the
immune cells retain increased activity after treatment with LPS
does not exceed 3 to 4 days (25),
but we observed in our previous report (8) the prolonged presence of low
concentrations of IL-1β and TNF-α in blood, even 7 days after the
application of LOS. However, this could be the result of a
secondary action of myeloid cells rather than a direct effect of
LOS administration (8). Thus, E.
coli B LOS-OH-mediated activation of myeloid cells in blood,
appeared to have low efficacy. The other issue can be the influence
of multiple LPS administration on reduction of immune cell
response, perhaps associated with induction of immune tolerance.
However, it is unlikely that a weak stimulator such as the LOS-OH
compound could cause tolerance. Alternatively, LPSs playing a role
in interaction between bacteria and the cell surface especially of
myeloid cells (26) trigger innate
inflammatory reaction. The structure of the LOS derivative with
four ester-bound fatty acid residues removed from the lipid A, such
as E. coli B LOS-OH, led to low-level activation of myeloid
cells which rather prevented proinflammatory activity. Due to
increasing evidence confirming the macrophage interactions with
development of metastases, we anticipated such a relationship in
the case of our experiments (27). It
cannot be excluded that a decrease in myeloid cell reactivity
hindered B16 cell migration and anchoring in the lung. These
findings suggest that a temporary anti-metastatic effect of LOS-OH
might be elicited via downregulation of myeloid cell activity.
Thus, it may be responsible for reduction in the local immune cell
response, but not the systemic response.
We also considered the interaction of blood
leukocytes and auxiliary cells of vascular endothelium, which are
well known to be sensitive to LPS action. The almost unaltered
percentage of granulocytes suggested that these cells may also be
responsible for inhibition of B16 cell migration to the lung, when
they are considered in the context of inflammatory reactions.
Granulocytes may be of great importance in the formation of a
metastatic niche, but they (at least neutrophils in humans) can
also reduce LPS toxicity by enzymatic activity (26).
Administration of LOS-OH to mice did not affect the
diversity of the spleen cell populations, and even if the changes
were visible, they were only time-dependent, and the multiple use
of LOS-OH led to low-level activation of myeloid but not lymphoid
spleen cells. The multiple administration of LOS as well as LOS-OH
provoked splenocytes to respond to secondary stimulation
(re-stimulated with LOS or stimulated with ConA), which resulted in
a time- and stimuli-dependent increase in IL-10 and IFN-γ
production. However, there were no differences between groups of
treated mice and control suggesting that no systemic response was
activated as a result of compound administration.
Altogether, multiple applications of E. coli
B LOS-OH derivative of E. coli B LOS were able to elicit a
statistically significant decrease in the number of metastatic
foci, presumably via silencing of myeloid cell reactivity as well
as the inability to stimulate lymphoid cells both directly and
indirectly.
These findings suggest that LOS-OH maintained in the
body of the metastasis-bearing mice seems to modulate or even
downregulate the innate response, leading to the inability of blood
myeloid cells to support the migration of melanoma cells to lung
tissue.
Acknowledgements
Not applicable.
Funding
This study was supported by the Polish Ministry of
Science and Higher Education-The National Centre for Research and
Development grant no. 13-00-89-06/2010 and Wroclaw Centre of
Biotechnology.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JM, AS, JW, JB and EPP conceived and designed the
experiments. JM, AS, NAG, JR, JJ, EPP and JW performed the
experiments. JM, AS, JR, JJ, JW and EPP collected and analyzed the
data. MK, AM, WJ, TN, JL and CL prepared and analyzed LOS and
LOS-OH of E. coli B and in this manner facilitated
conduction of the experiments. JM, AS, EPP, JW, JR and JL wrote the
manuscript. All the authors read and approved the manuscript and
provided critical feedback and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
All experiments were performed according to EU
Directive 2010/63/EU for animal experiments and were approved by
the 1st Local Ethics Committee for Experiments with the Use of
Laboratory Animals, Wroclaw, Poland (number 48/2008).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lien E, Heine H and Golenbock DT: LPS
receptorsImmune Response in the Critically Ill. Marshall JC and
Cohen J: Springer Berlin Heidelberg; Berlin, Heidelberg: pp.
164–172. 2002, https://doi.org/10.1007/978-3-642-57210-4_11
View Article : Google Scholar
|
|
2
|
Raetz CR and Whitfield C:
Lipopolysaccharide endotoxins. Annu Rev Biochem. 71:635–700. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lundin JI and Checkoway H: Endotoxin and
cancer. Environ Health Perspect. 117:1344–1350. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Puntoni M, Marra D, Zanardi S and Decensi
A: Inflammation and cancer prevention. Ann Oncol. 19 (Suppl
7):vii225–vii229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schottenfeld D and Beebe-Dimmer J: Chronic
inflammation: A common and important factor in the pathogenesis of
neoplasia. CA Cancer J Clin. 56:69–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Caroff M and Karibian D: Structure of
bacterial lipopolysaccharides. Carbohydr Res. 338:2431–2447. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Caroff M, Karibian D, Cavaillon JM and
Haeffner-Cavaillon N: Structural and functional analyses of
bacterial lipopolysaccharides. Microbes Infect. 4:915–926. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kicielińska J, Szczygieł A, Rossowska J,
Anger N, Kempińska K, Świtalska M, Kaszowska M, Wietrzyk J,
Boratyński J and Pajtasz-Piasecka E: Oral administration of
Polymyxin B modulates the activity of lipooligosaccharide E.
coli B against lung metastases in murine tumor models. PLoS
One. 11:e01481562016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kaszowska M, Niedziela T, Maciejewska A,
Lukasiewicz J, Jachymek W and Lugowski C: Core oligosaccharide of
Escherichia coli B-the structure required for bacteriophage
T4 recognition. Carbohydr Res. 413:51–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lukasiewicz J, Jachymek W, Niedziela T,
Malik-Gebicka M, Dzieciatkowska M and Lugowski C: Comparison of
serological specificity of anti-endotoxin sera directed against
whole bacterial cells and core oligosaccharide of Escherichia
coli J5-tetanus toxoid conjugate. Acta Biochim Pol. 49:721–734.
2002.PubMed/NCBI
|
|
11
|
Holst O: Deacylation of
lipopolysaccharides and isolation of oligosaccharide phosphates.
Methods Mol Biol. 145:345–353. 2000.PubMed/NCBI
|
|
12
|
Sturiale L, Palmigiano A, Silipo A, Knirel
YA, Anisimov AP, Lanzetta R, Parrilli M, Molinaro A and Garozzo D:
Reflectron MALDI TOF and MALDI TOF/TOF mass spectrometry reveal
novel structural details of native lipooligosaccharides. J Mass
Spectrom. 46:1135–1142. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rossowska J, Pajtasz-Piasecka E, Szyda A,
Krawczenko A, Zietara N and Dus D: Tumour antigen-loaded mouse
dendritic cells maturing in the presence of inflammatory cytokines
are potent activators of immune response in vitro but not
in vivo. Oncol Rep. 21:1539–1549. 2009.PubMed/NCBI
|
|
14
|
Rossowska J, Pajtasz-Piasecka E, Anger N,
Wojas-Turek J, Kicielińska J, Piasecki E and Duś D:
Cyclophosphamide and IL-12-transduced DCs enhance the antitumor
activity of tumor antigen-stimulated DCs and reduce Tregs and MDSCs
number. J Immunother. 37:427–439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mohanty S and Xu L: Experimental
metastasis assay. J Vis Exp:. (pii): 19422010.doi: 10.3791/1942.
PubMed/NCBI
|
|
16
|
Korneev KV, Kondakova AN, Arbatsky NP,
Novototskaya-Vlasova KA, Rivkina EM, Anisimov AP, Kruglov AA,
Kuprash DV, Nedospasov SA, Knirel YA and Drutskaya MS: Distinct
biological activity of lipopolysaccharides with different lipid A
acylation status from mutant strains of Yersinia pestis and some
members of genus Psychrobacter. Biochemistry (Mosc).
79:1333–1338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Górska S, Sandstrőm C, Wojas-Turek J,
Rossowska J, Pajtasz-Piasecka E, Brzozowska E and Gamian A:
Structural and immunomodulatory differences among lactobacilli
exopolysaccharides isolated from intestines of mice with
experimentally induced inflammatory bowel disease. Sci Rep.
6:376132016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Miernikiewicz P, Dąbrowska K, Piotrowicz
A, Owczarek B, Wojas-Turek J, Kicielińska J, Rossowska J,
Pajtasz-Piasecka E, Hodyra K, Macegoniuk K, et al: T4 phage and its
head surface proteins do not stimulate inflammatory mediator
production. PLoS One. 8:e710362013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Szczygieł A and Pajtasz-Piasecka E:
Between biology and medicine: Perspectives on the use of dendritic
cells in anticancer therapy. Postepy Hig Med Dosw (Online).
71:921–941. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maeshima N and Fernandez RC: Recognition
of lipid A variants by the TLR4-MD-2 receptor complex. Front Cell
Infect Microbiol. 3:32013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cross AS: Endotoxin tolerance-current
concepts in historical perspective. J Endotoxin Res. 8:83–98. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gioannini TL, Teghanemt A, Zarember KA and
Weiss JP: Regulation of interactions of endotoxin with host cells.
J Endotoxin Res. 9:401–408. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mackensen A, Galanos C, Wehr U and
Engelhardt R: Endotoxin tolerance: Regulation of cytokine
production and cellular changes in response to endotoxin
application in cancer patients. Eur Cytokine Netw. 3:571–579.
1992.PubMed/NCBI
|
|
24
|
Rockwell CE, Morrison DC and Qureshi N:
Lipid A-mediated tolerance and cancer therapy. Adv Exp Med Biol.
667:81–99. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ruggiero V, D'Urso CM, Albertoni C, Campo
S, Foresta P and Martelli EA: LPS-induced serum TNF production and
lethality in mice: Effect of L-carnitine and some acyl-derivatives.
Mediators Inflamm. 2 (Suppl):S43–S50. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Munford RS and Hall CL: Detoxification of
bacterial lipopolysaccharides (endotoxins) by a human neutrophil
enzyme. Science. 234:203–205. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nielsen SR and Schmid MC: Macrophages as
key drivers of cancer progression and metastasis. Mediators
Inflamm. 2017:96247602017. View Article : Google Scholar : PubMed/NCBI
|