Introduction
Osteosarcoma is the most common malignant bone
cancer in children and young adults occurring during growth spurts
(1,2).
At diagnosis 20% of patients present with metastatic osteosarcoma
and 30–40% patients diagnosed with a local tumor develop metastasis
later (3,4). The 5 year survival rate in cases with
early diagnosis is 60–75%, however, in cases with metastatic
disease it is approximately 30% (5,6).
Unfortunately, osteosarcoma treatment outcomes for metastatic
disease or recurrence have not improved in over two decades
(7–9).
Cancer cells gain growth advantages over normal
cells by exploiting various growth signaling pathways (10). Many cancer types also express
glutamate receptors, suggesting that glutamate may play a
significant role in these types of cancers (11,12).
Importantly, glutamate signaling is exploited by cancers of the
breast, prostate and skin to enhance their growth (13–17). More
recently, a genome wide association study (GWAS) found a single
nucleotide alteration in the metabotropic glutamate receptor 4
(MGluR4) gene in osteosarcoma patients (17,18).
Spontaneous secretion of glutamate and expression of glutamate
receptors were demonstrated in osteosarcoma MG63 and Saos-2 cell
lines (19). Since glutamate
signaling plays a crucial role in tumor growth, it is crucial to
investigate the mechanisms underlying glutamate signaling and to
discover strategies to interrupt this signaling to prevent tumor
growth.
Glutamate secretion was first shown to be prevented
by a drug, riluzole, in brain slices (20). Although the mechanism of action of
riluzole is not clear, it was shown to block sodium channels as
well as glutamate signaling (21,22).
Through an unknown mechanism, riluzole was found to increase
cytosolic Ca2+ levels in MG63 cells (23). Clinically used as a neuroprotectant
drug in several neurological diseases such as amyotrophic lateral
sclerosis (ALS) and Parkinson's disease, riluzole is currently
being tested on several cancers for therapeutic purposes (24). For instance, it was found that
treatment of triple-negative breast cancer cells with riluzole
inhibited cell proliferation (25).
In addition, riluzole was observed to reduce the growth of cancer
cells in culture or in xenograft models for brain, skin, breast and
prostate cancers (25–31). In a clinical trial for melanoma
patients, riluzole decreased tumor size in a number of patients
(32). Furthermore, in a phase II
trial in patients with advanced GRM1-positive melanoma, riluzole
showed some clinical benefits (33).
Previously, we successfully used human metastatic osteosarcoma LM7
cells derived from Saos-LM6 cells to study the effect of riluzole
(34). We demonstrated that LM7 cells
secrete glutamate and riluzole blocks secretion of glutamate,
thereby inhibiting the autocrine effect on LM7 cells (28). Moreover, we demonstrated that riluzole
inhibited proliferation and migration and induced apoptosis in LM7
cells. We further demonstrated that LM7 cells express metabotropic
glutamate receptor, mGluR5, and knockdown of mGluR5 prevented the
colony forming ability of LM7 cells (28). Thus, from many studies it is apparent
that riluzole is an effective drug that inhibits cell proliferation
in several types of cancers. However, the methods of delivery of
riluzole which may impact the effectiveness and the outcome of
riluzole therapy have not been investigated.
Nanoparticles of various natures, both organic and
inorganic matter such as liposomes, peptides, cyclodextrin, viral
particles, carbon nanotubes (CNTs), nano-diamonds, graphene,
quantum dots, and metal-based nanoparticles, are used as
theranostic agents with which to deliver cancer drugs (35,36).
Nanoparticle size ranges from 3 to 200 nm; however, for escape from
mononuclear phagocytosis, the size of the nanoparticle needs to be
<100 nm. As is already known, nanoparticles with sizes of <50
nm achieve better biodistribution, escape the immune system, and
have improved clearance (35,37,38). Small
nanoparticles penetrate tumor tissue more effectively through the
enhanced permeability and retention effect (EPR). Nanoparticle
surface characteristics also play an important role in nanoparticle
lifespan and escape from the immune system. Therefore, the surface
needs to be hydrophilic, which is achieved by coating the
nanoparticle surface with a hydrophilic polymer (39). Nanoparticle-mediated drug delivery
improves bioavailability, enhances drug delivery, and serves as
diagnostic agents (36,40,41). The
shape of the nanoparticles is critical to their effectiveness as a
drug carrier (42–44). The shape of the nanoparticles
determines the surface area to volume properties so that
nanoparticles of the same size but different shapes may show
different drug-loading capacity and release. Therefore, we used two
iron oxide nanoparticles of the same size (15±2.5 nm); one a solid
spherical structure (IO-sphere), and the other a cage with a hollow
interior (IO-cage) offering a larger surface area and increased
surface to volume ratio with higher loading and release compared to
a solid IO-sphere.
We previously compared the effectiveness of free
riluzole and riluzole released from nanocages or nanospheres on the
apoptosis of LM7 cells in culture. We showed that riluzole released
from nanoparticles is more effective in inducing apoptosis in
cultured LM7 cells when compared to free riluzole (45). To determine whether this effect occurs
in vivo, we tested the effect of riluzole delivery via
nanospheres (IO-sphere) and nanocages (IO-cage) on osteosarcoma
xenografts implanted in nude mice. Our results demonstrated that
groups of nude mice injected with riluzole showed a decreased
bioluminescence signal at the tumor site when compared to the
control groups (PBS, nanocage, nanosphere). Moreover, the group
with nanocage-delivered-riluzole showed the least intense signal.
Similarly, tumor volume calculated from manual measurements
demonstrated that riluzole released from the nanocages was most
effective in reducing the tumor size when compared to the free
riluzole or riluzole released from the nanospheres. Furthermore,
tumors from the groups injected with nanocage+riluzole showed the
highest percentage of apoptosis followed by the nanosphere+riluzole
groups and free riluzole group. Riluzole-treated groups displayed
significantly higher apoptosis compared to the control groups (PBS,
nanocage or nanosphere). Thus, we showed that delivery of the drug
via nanocage enhanced tumor control.
Materials and methods
Materials
3,4-Dihydroxyhydrocinnamic acid (DHCA) (Alfa Aesar)
and manganese (II) acetate, oleylamine, oleic acid, iron (II)
perchlorate, and 2-(N-morpholino)ethane sulfonic acid (MES) were
purchased from Sigma-Aldrich;Merck KGaA. p-Xylene,
1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide (EDC) and
N-hydroxysuccinimide (NHS) were purchased from Thermo Fisher
Scientific, Inc. Tetrahydrofuran (THF), hexane, ferric chloride
hexahydrate, phosphate-buffered saline (PBS), sodium carbonate and
sodium bicarbonate were purchased from Thermo Fisher Scientific,
Inc. Optimum cutting temperature compound (O.C.T. Compound) was
purchased from Sakura Tech. D-luciferin was purchased from Xenogen.
Riluzole was purchased from R&D Systems (Tocris).
Methods
Iron oxide nanocage (IO-cage)
synthesis
First, IO-cages were synthesized with oleic acid by
a modified version of a previously published method (46). Manganese (II) acetate (0.17 g),
oleylamine (0.82 ml) and oleic acid (0.16 ml) were added to
p-xylene (15 ml) in a three-necked 50 ml flask with a reflux
condenser. The flask was heated to 90°C in air under magnetic
stirring, and then 1 ml of deionized water was rapidly injected
into the flask. The reaction mixture was heated at 90°C for 1.5 h,
producing Mn3O4 nanoparticles. One milliliter
of 2.0 M aqueous iron (II) perchlorate solution was added and the
mixture was maintained at 90°C for an additional 1.5 h to produce
IO-cages by galvanic replacement. After cooling, IO-cages were
collected by centrifugation, rinsed with ethanol, and dispersed in
an organic solvent such as hexane or THF. Then, these hydrophobic
IO-cages/IO-sphere were coated with DHCA and transferred to the
aqueous phase using a modified version of a previously published
method (47). First, 300 mg of DHCA
was dissolved in 6 ml of THF in a three-neck flask (25 ml). The
resulting solution was heated to 50°C after bubbling for 30 sec
with flowing nitrogen gas. Then, 100 mg of hydrophobic IO-cage or
IO-sphere capped by oleic acid were dispersed in 1 ml of THF which
was added dropwise to the solution. The solution was heated to 50°C
for 3 h, and then cooled to room temperature, and 500 µl NaOH (0.5
M) was introduced to precipitate the magnetic nanoparticles. The
precipitate was collected by centrifugation and resuspended in 2 ml
water, and then dialyzed overnight.
Capping of the IO-cage/IO-sphere
First, MES buffer (pH 6.0) was prepared from 0.1 M
MES and 0.1 M NaCl. Next, 9.6 mg (50 µmol) EDC was dissolved in 200
µl of MES buffer (pH 6.0) and 10.9 mg (50 µmol) NHS
(N-hydroxysuccinimide) was also dissolved in 200 µl of MES buffer
(pH 6.0). Then, to a working solution of IO-cage or IO-sphere in
MES buffer (pH 6.0) was added 100 nmol of EDC and 125 nmol of NHS
per mg of IO-cage/IO-sphere. After reacting this mixture for 15
min, 1 mg PEG-10k-diamine per mg IO-cage/IO-sphere was dissolved
and reacted in the MES buffer solution for 6 h while on a rocker.
Then the resulting solution was dialyzed overnight with a 3,500 Da
molecular weight membrane.
IO-cage/IO-sphere drug loading
Riluzole hydrochloride (25 mg) was dissolved in DI
H2O using serial dilutions down to 2.5 mg/ml with 40°C
DI H2O and light rocking. Alternatively, riluzole
hydrochloride could also be dissolved in DMSO, however, it is then
necessary to dialyze overnight with a 3,500 Da weight membrane to
remove excess DMSO from the nanoparticle solutions. Riluzole (2.5
mg/ml) was incubated in aliquots with IO-cage/sphere concentrations
of 5 mg/ml, and was left to shake on a rocker at 4°C for 6 h. The
magnetic nanoparticles containing riluzole were washed using a 1.5
T bar magnet to separate free riluzole hydrochloride and then
subsequently resuspended in DI water. Iron contents in each aliquot
of nanoparticles were quantified by UV/Vis spectroscopy (a ferric
chloride peak at 351 nm) after an acid digestion of iron oxide
nanoparticles in 5 N HCl.
Cell culture
LM7 and LM7.eGFP.ffLuc cells (34) were obtained from Eugenie E. Kleinerman
and were maintained in DMEM without glutamine supplemented with
4.5% glucose (Gibco; Thermo Fisher Scientific, Inc.) 1 mM pyruvate
(Gibco; Thermo Fisher Scientific, Inc.), 10% fetal bovine serum
(FBS) (Gibco; Thermo Fisher Scientific, Inc.), 2 mmol/l GlutaMAX–I
(Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and
100 µg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.) as
previously described (34). Cells
were passaged every 4 days. Cells were maintained at 37°C in 95%
air and 5% CO2. LM7 and LM7.eGFP.ffLuc cells were tested
for and were free of mycoplasma contamination.
In vivo drug delivery
Animal experiments were performed following animal
protocols approved by the Institutional Animal Care and Use
Committee (IACUC, protocol #2015-0038 to Olorunsuen Ogunwobi from
Hunter College) at Weill Cornell Medical College and Hunter
College. Thirty-six 5 week-old NOD.Cg-Prkdcscid
Il2rgtm1Wjl/SzJ (NSG) male mice weighing 25 g
were implanted subcutaneously with 1 million LM7.eGFP.FFLuc cells
in 100 µl of PBS in the right flank. After tumors were detectable
at day 5, the animals were randomly grouped into 6 groups with 6
animals in each group. The mice were treated once every day when
tumors reached 200 mm3 in size, receiving daily
injections of treatments via intraperitoneum (i.p.) injection for 9
days with i) PBS, or ii) riluzole or iii) neat IO-sphere or iv)
IO-sphere loaded with riluzole or v) neat IO-cage, and vi) IO-cage
loaded with riluzole. An injected concentration of IO-cages and
IO-spheres was 75 µg/kg, corresponding to a riluzole concentration
of 2.5 mg/kg.
Bioluminescence imaging
First, the animals were injected i.p. with 150 mg/kg
body weight luciferin (Xenogen), and after 10 min they were
anesthetized using 2% isofluorane and were imaged using the IVIS
in vivo imaging system (Xenogen). Photons emitted from the
luciferase-expressing LM7.eGFP.FFLuc cells in the area of the tumor
in the mouse body were quantified using ‘Living Image,’ a software
program, version 4.3.1(https://ctac.mbi.ufl.edu/files/2017/02/@-IVIS-Spectrum-User-Manual-4.3.1.
Grayscale reference images were superimposed over the pseudocolor
images, representing the emitted light intensity around the tumor
site (blue least intense and red most intense). Bioluminescence
imaging results were confirmed by macroscopic examination of the
tumor by measurement and resection of the tumor from the euthanized
animals. Animals were imaged once 2 days before they were
euthanized to excise tissues (N=2, total experimental duration=14
days).
Histological sections for TUNEL assay
and hematoxylin and eosin (H&E) staining
A mixture of 10% formalin and 4% paraformaldehyde
was used to fix the tumor tissues. The following day tumor tissue
was incubated in a series of ethanol concentration (70, 85, 95, 95,
100, 100%, respectively) and Histo-Clear (National Diagnostics),
followed by three exchanges of paraffin at 60°C for 1 h. Tumor
tissue was sectioned and sections were deparaffinized in xylene and
a series of ethanol (with high to low concentrations), followed by
rehydration in deionized water prior to the TUNEL staining. The
sections were permeabilized with 0.5% Triton X-100 in PBS for 5 min
followed by washing with PBS and TUNEL staining. TUNEL staining was
performed as per the TUNEL staining kit instructions (Roche). Then,
the sections were rinsed in three exchanges of deionized water
after staining and were mounted with DAPI mounting medium. The
mounted histological tumor sections were imaged multiple times
using a Zeiss fluorescence microscope at ×20 magnification. Ten
images were obtained per tumor sample. The images were quantified
by counting the number of DAPI-positive nuclei and TUNEL-positive
nuclei. Tumor sections from all samples were stained using H&E
and imaged at ×20 using bright fields using a Zeiss microscope.
Statistical analysis
Tumor apoptosis analysis was conducted by one way
ANOVA and two different post-hoc analyses were performed by Tukey's
and Bonferroni's tests. One way ANOVA was performed for the tumor
volume and two different post-hoc analyses were carried out by
Tukey's and Bonferroni's tests. Significance was defined at P≤0.05
for the analyses.
Results
Scanning electron microscope images
show comparable size of the iron oxide nanospheres and iron oxide
nanocages
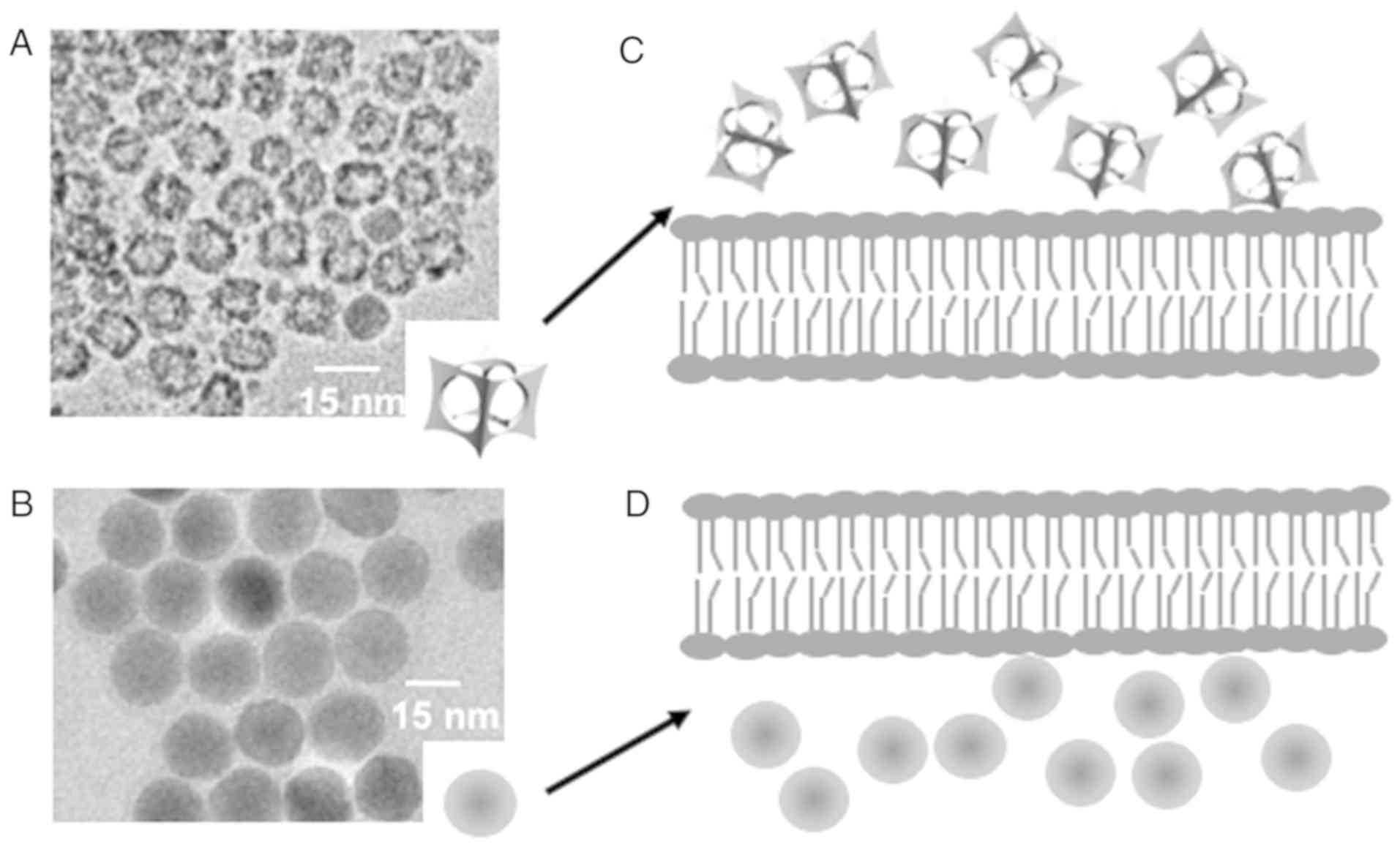
We performed transmission electron microscopy (TEM)
of the nanoparticles (Fig. 1A and B),
which showed that the IO-cages and IO-spheres were the same size
(Fig. 1A and B), using a JEOL JEM
2100). The size range of both IO-nanoparticles, IO-cage and
IO-sphere was 15±2.5 nm and these nanoparticles were capped by
polyethylene glycol (PEG) after completion of drug incorporation to
yield a hydrodynamic size of 25±2.5 nm, measured by Dynamic Light
Scattering (DLS) using a Malvern Zetasizer Nano S system. Both the
IO-cage and IO-sphere contained ~30 molecules of riluzole each,
which was measured by assaying the riluzole concentration remaining
in the supernatant. We had previously demonstrated that cellular
internalization of the cage was much slower when compared to
IO-spheres in LM7 cells in vitro (45). Slower cellular internalization of the
IO-cages compared to the IO-spheres is depicted in a diagram
(Fig. 1C and D).
Nanocage-delivered riluzole is most
effective in tumor control
We previously demonstrated that IO-cage-delivered
riluzole is more effective in inducing apoptosis in LM7 cells in
vitro (45). We aimed to test the
efficacy of riluzole delivery via IO-cages in reducing tumor size
in a xenograft nude mouse model (protocol no. 2015-0038). For the
in vivo study, we implanted one million LM7-eGFP-ff-Luc
cells in 5 week-old NOD.Cg-Prkdcscid
Il2rgtm1Wjl/SzJ (NSG) mice in the right flank
region via a subcutaneous injection. Once tumor size reached ~200
mm3 on day 5, the animals were randomly placed in 6
groups of 6 animals each and drugs were i.p. injected daily. The
animals received either PBS, IO-sphere, IO-cage for controls and
free riluzole, IO-sphere+riluzole or IO-cage+riluzole. We measured
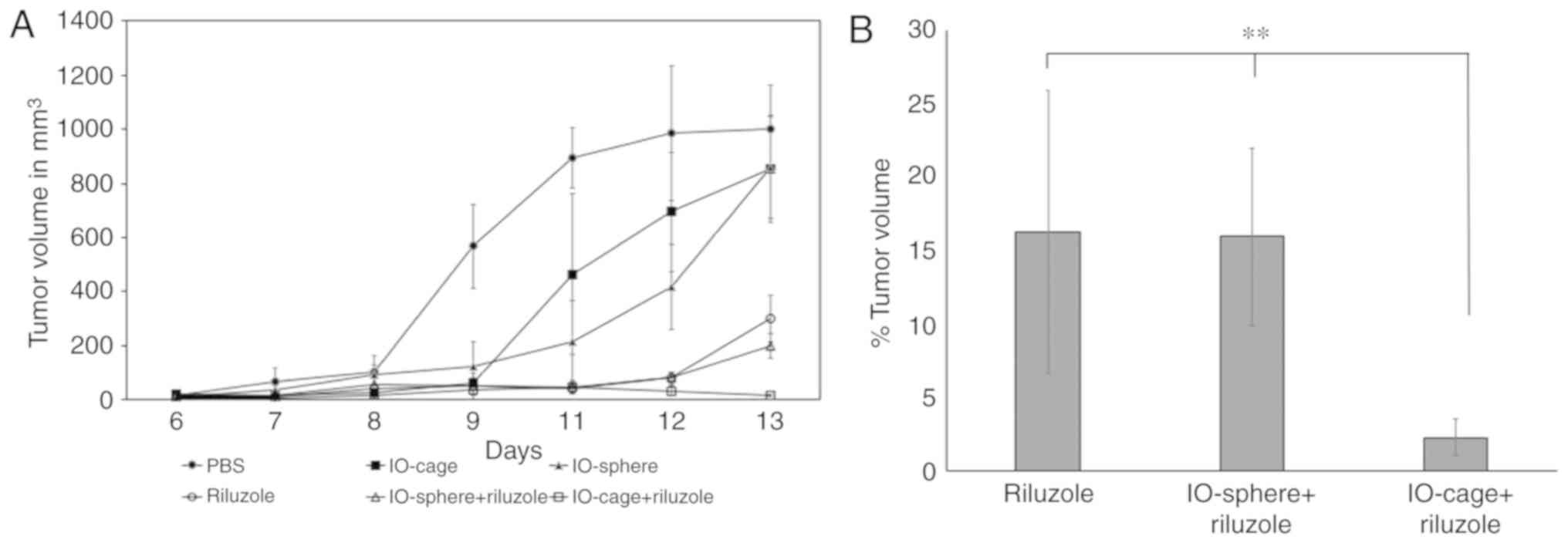
the tumor volume using Vernier calipers every day for 2 weeks. We
calculated the tumor volume by using the formula (π × length ×
width2)/6. The PBS group showed the largest tumors and
rapid growth followed by IO-cage and IO-sphere in the control
groups. Riluzole and IO-sphere+riluzole groups had significantly
decreased tumor size until day 12. However, there was a slight
increase in the tumor size from these groups on day 13. Samples in
the control groups were not significantly different from each
other. Importantly, the IO-cage-delivered riluzole group had the
smallest tumor size throughout the study compared to the control
groups (PBS or IO-cages alone) (Fig.
2A). We then calculated the remaining tumor volume in the
groups that received riluzole either free or via IO-cage or
IO-sphere. The data showed the highest tumor shrinkage in the group
of mice that received riluzole via IO-cage (Fig. 2B) and was significantly different
(P≤0.05) when compared to the riluzole group and IO-sphere+riluzole
group.
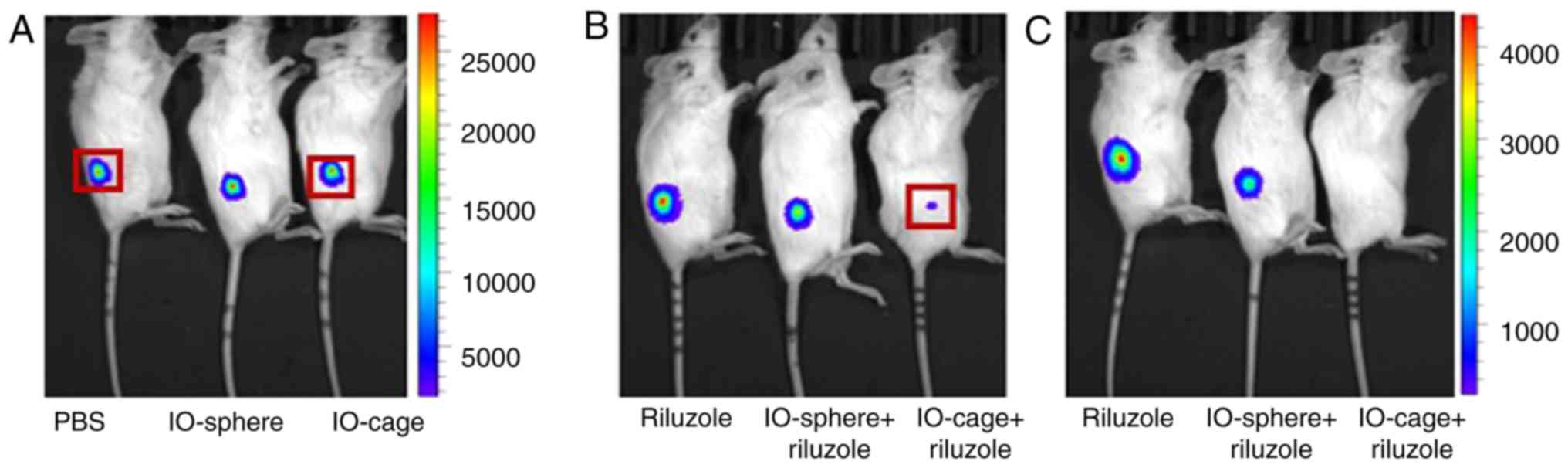
The regression of the tumors was analyzed by
bioluminescence of luciferase-expressing osteosarcoma after 12 days
of the tumor implantation. We anesthetized the mice with isoflurane
and i.p. injected the mice with luciferin D at 150 mg/kg body
weight and imaged the mice using an IVIS machine. As expected,
similar to the in vitro data, riluzole released from the
nanocage was found to be most effective and the mice showed the
least intense signal for luciferase activity while the luciferase
activity was prominent in the control groups (PBS, IO-cage alone,
IO-sphere alone) (Fig. 3A). Although
the riluzole and the IO-sphere+riluzole group showed a luciferase
signal, the signal intensity was significantly reduced as indicated
by the luminescence intensity bar (Fig.
3B and C). The control groups displayed a maximum luciferase
bioluminescence at 25,000 photons per sec while the
riluzole-treated groups (free riluzole, IO-sphere+riluzole or
IO-cage+riluzole) showed a maximum of 4,000 photons per sec. In
vivo luciferase data was corroborated by the measurement of the
remaining tumor volume (Fig. 2B) in
the groups of mice with riluzole treatment. These data support the
outcome that riluzole delivery through the IO-cages was most
effective compared to free riluzole or IO-sphere-delivered riluzole
in reducing tumor size in the xenograft mouse model. Therefore, we
conclude that riluzole was most effective in reducing tumor size
when delivered via IO-cage.
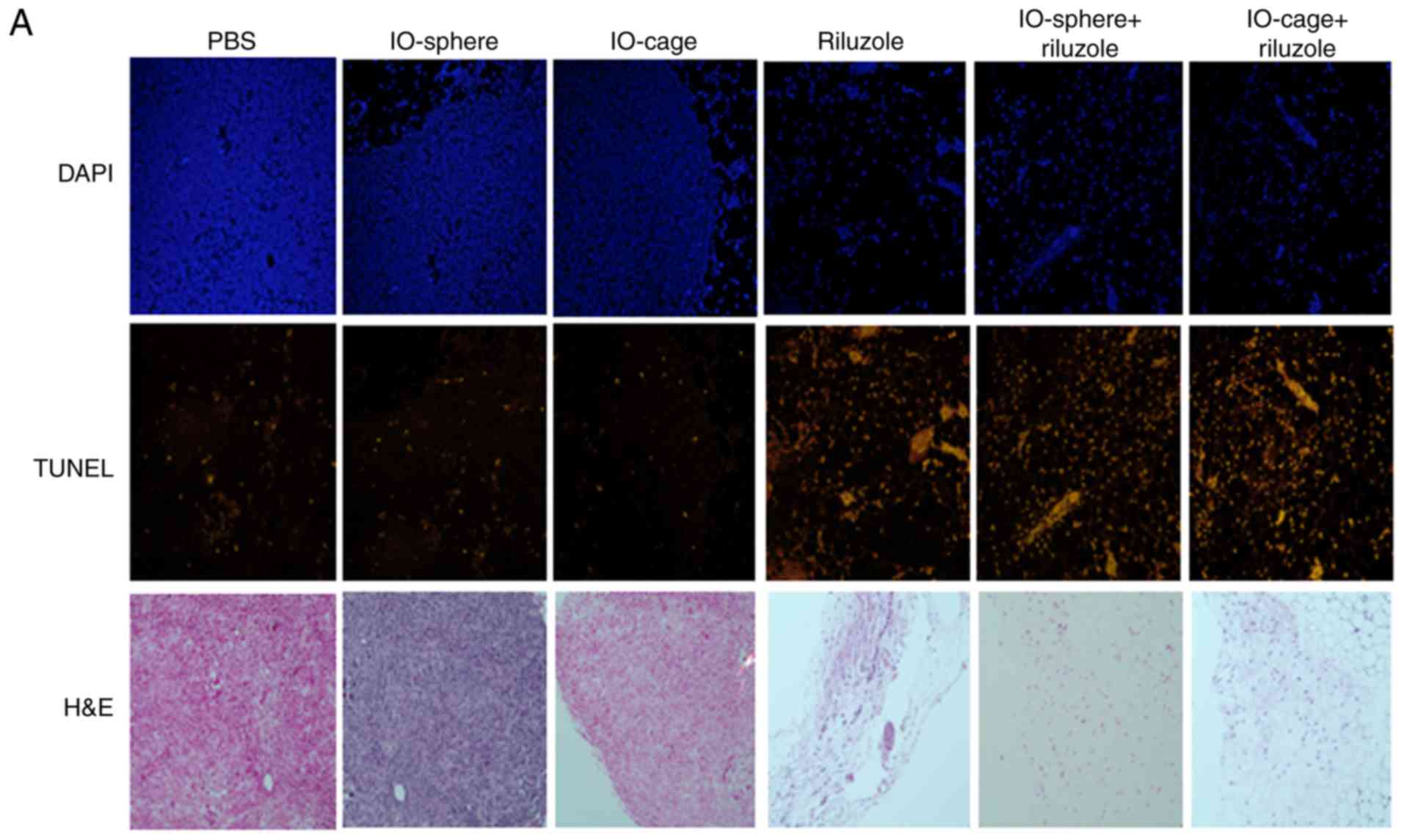
Tumors from mice treated with
nanocage-delivered riluzole exhibit increased apoptosis
Since riluzole delivery via IO-cage showed reduced
tumor size, we aimed to determine the extent of apoptosis as a
reason for tumor shrinkage. We performed TUNEL staining to assess
the apoptosis in the tumor tissues. Tumor tissue sections were
deparaffinized, fixed, permeabilized, and TUNEL assay was
performed. The sections were subsequently mounted in DAPI
containing mounting media and imaged using Zeiss fluorescence
microscope at ×20. Riluzole induced apoptosis in the free riluzole,
IO-sphere+riluzole and IO-cage+riluzole groups (Fig. 4A). The tumor sections stained with
hematoxylin and eosin (H&E) showed more cells in the control
samples compared to fewer cells in the riluzole, IO-sphere+riluzole
and IO-cage+riluzole treated sections. Analysis of DAPI-positive
and TUNEL-positive cells revealed that riluzole treatment induced a
higher percentage of apoptotic cells in tumor tissues of all
riluzole-treated animals when compared to the control groups (PBS,
IO-sphere or IO-cage) (Fig. 4B and C)
with a statistically significant difference (P≤0.05). Moreover, the
highest percentage of apoptosis was noted in the
IO-cage+riluzole-treated group as indicated by percentage of
apoptosis in the tumor tissues (Fig.
4C) and was significantly higher than that of the riluzole or
IO-sphere+riluzole-treated samples (P≤0.05). Based on the apoptosis
data, we found that riluzole induced apoptosis in tumors of the
mice treated with riluzole (riluzole, IO-sphere+riluzole and
IO-cage+riluzole groups) and the highest percentage of apoptotic
cells was observed in tumors of mice when riluzole was delivered
through the IO-cage.
Discussion
In the present study, we demonstrated that IO-cages
and IO-spheres were of the same size and were loaded with the same
number of Rilzuole molecules. We then demonstrated that LM7 cells
expressing luciferase and GFP, when injected in 5 week-old nude
mice formed tumors that were reduced in size by treatment with
riluzole. However, the delivery of riluzole from IO-cages was most
effective in shrinking tumors compared to free riluzole or riluzole
released from IO-spheres, which was evident both by the in
vivo bioluminescent assay and by manual measurements using
Vernier calipers. Furthermore, apoptosis, measured by TUNEL assay,
showed that riluzole released from IO-cages was the most effective
inducer of apoptosis in tumor sections. Overall, our study
demonstrated that riluzole delivery via IO-cage was more effective
than free riluzole for the potential therapy for osteosarcoma.
The slower internalization of IO-cages in turn
influenced drug efficacy by releasing riluzole near ion channels on
membrane surfaces that alter membrane potential which subsequently
inhibits glutamate release and thus prevents autocrine signaling by
glutamate (28,45). As described previously, the IO-cages
can conceal the charges on drugs due to drug loading in the cavity,
thereby highly charged drugs can be delivered using IO-cages
(45).
In vivo tumor measurement using
bioluminescence in living animals is an important tool for
detecting and following the growth/reduction in tumor burden over
time (48). We used both
bioluminescence in vivo assay as well as manual measurement
to assess tumor growth/shrinkage. Although our results from both
methods agreed, we believe that bioluminescence assay could be used
periodically at regular intervals to monitor tumor growth shrinkage
and may provide accurate data. Additionally, we observed that the
tumor growth shrinkage was most significant on the last day, day
13, when compared to free riluzole or riluzole released from the
IO-sphere, thus we believe that a relatively longer duration of the
experiment may further discriminate the effectiveness of the cage
vs. sphere as suggested by luciferase assay and apoptosis in the
tumor tissue. Recent studies with gold particles with an average
size of 200 nm have demonstrated that star-shaped and rod-shaped
gold nanoparticles showed the highest cytotoxicity in osteosarcoma
and pancreatic duct cell lines compared to spherical gold
nanoparticles of the same size in vitro (49). Furthermore, star-shaped and rod-shaped
gold nanoparticles stimulated expression of Bax protein and caused
increased cytotoxicity. Therefore, the shape of the nanoparticle is
crucial in determining delivery of the drug as well as inducing
cytotoxicity.
The effective serum concentration of riluzole in
humans determined from previous clinical trials is ~50 mg oral
daily dose; the area under the curve (AUC) in serum at 24 h is
approximately 2,000 ng/ml (50,51). We
injected 50 µM riluzole intraperitoneally (i.p.) in the mice daily
for the in vivo experiments, which is approximately 400
ng/ml blood in mice. The serum concentration with the i.p. injected
riluzole, which does not undergo hepatic clearance, was 5 times
less compared to the oral dose in humans and was effective and well
within the tolerated dose in humans. Riluzole released from
IO-cages or IO-spheres may show differences in biodistribution and
pharmacokinetics and pharmacodynamics when compared to free
riluzole. This issue warrants further investigation. Interestingly,
riluzole delivered via 88 nm liposomes for targeted delivery to the
brain in rats showed lower biodistribution in other organs
(52). In our study, the half-life of
riluzole may have been prolonged due to delivery via the IO-cage
where riluzole is incorporated in the cavity of the IO-cage
preventing it from the hydrophilic environment until release thus
prolonging the half-life of riluzole. This needs to be further
investigated. Furthermore, in the present study, the tumor burden
in the PBS group was too large and prevented further investigation
on the effect of riluzole delivery on metastasis. Therefore, future
investigations need to focus on riluzole delivery using IO-spheres
or IO-cages and the efficacy on metastasis in a metastasis
model.
We conclude that the delivery of riluzole was most
effective in reducing osteosarcoma tumor size in nude mice via the
IO-cage when compared to free riluzole and IO-sphere-delivered
riluzole. The effectiveness of riluzole may be due to the slower
internalization of IO-cages and riluzole loading at a high
concentration in the hollow core of the IO-cage. The small size of
IO-cages may serve several advantages including evasion from the
immune system, better biodistribution and delivery of a high dose
of riluzole. The IO-cage-mediated delivery of riluzole may be
applied to other cancer models that depend on glutamate for growth
signaling or for the delivery of drugs that carry charge.
Acknowledgements
We thank Eugenie E. Kleinerman from the MD Anderson
Cancer Center for the generous gift of LM7 and LM7-eGFP-ff-Luc
cells. We thank Dr Olorunseun Ogunwobi for the animal protocol. We
thank Dr Upal Basu-Roy for the statistical analysis of the data. We
thank Dr Alka Mansukhani and Dr Muktar Mahajan for critical
comments on the manuscript.
Funding
The research study was funded by PSC CUNY #47 to
Shahana S. Mahajan and the material parts were supported by the
National Institute on Minority Health and Health Disparities
(NIMHD) of NIH (MD007599).
Availability of data and materials
Data and material will be made available upon
request.
Authors' contributions
MR carried out the in vivo experiment and
analyzed the data. SSM conceived and supervised the study. JF and
HM organized the nanoparticle synthesis, drug loading, capping
modification, and TEM imaging. CNR performed the apoptosis assay.
All authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Animal study was preapproved by the IACUC Committee
at Weill Cornell Medical College, and the studies were carried out
in accordance with the approved protocol. The approved protocol
number is #2015-0038.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interest.
References
|
1
|
Dorfman HD and Czerniak B: Bone cancers.
Cancer. 75:203–210. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Whelan JS: Osteosarcoma. Eur J Cancer.
33:1611–1618. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaste SC, Pratt CB, Cain AM, Jones-Wallace
DJ and Rao BN: Metastases detected at the time of diagnosis of
primary pediatric extremity osteosarcoma at diagnosis: Imaging
features. Cancer. 86:1602–1608. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mialou V, Philip T, Kalifa C, Perol D,
Gentet JC, Marec-Berard P, Pacquement H, Chastagner P, Defaschelles
AS and Hartmann O: Metastatic osteosarcoma at diagnosis: Prognostic
factors and long-term outcome-the french pediatric experience.
Cancer. 104:1100–1109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Berner K, Johannesen TB, Berner A,
Haugland HK, Bjerkehagen B, Bohler PJ and Bruland OS: Time-trends
on incidence and survival in a nationwide and unselected cohort of
patients with skeletal osteosarcoma. Acta Oncol. 54:25–33. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology, and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Geller DS and Gorlick R: Osteosarcoma: A
review of diagnosis, management, and treatment strategies. Clin Adv
Hematol Oncol. 8:705–718. 2010.PubMed/NCBI
|
|
8
|
Lindsey BA, Markel JE and Kleinerman ES:
Osteosarcoma overview. Rheumatol Ther. 4:25–43. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morrow JJ and Khanna C: Osteosarcoma
genetics and epigenetics: Emerging biology and candidate therapies.
Crit Rev Oncog. 20:173–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martin GS: Cell signaling and cancer.
Cancer Cell. 4:167–174. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stepulak A, Luksch H, Gebhardt C,
Uckermann O, Marzahn J, Sifringer M, Rzeski W, Staufner C, Brocke
KS, Turski L and Ikonomidou C: Expression of glutamate receptor
subunits in human cancers. Histochem Cell Biol. 132:435–445. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stepulak A, Rola R, Polberg K and
Ikonomidou C: Glutamate and its receptors in cancer. J Neural
Transm (Vienna). 121:933–944. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Koochekpour S: Glutamate, a metabolic
biomarker of aggressiveness and a potential therapeutic target for
prostate cancer. Asian J Androl. 15:212–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pollock PM, Cohen-Solal K, Sood R,
Namkoong J, Martino JJ, Koganti A, Zhu H, Robbins C, Makalowska I,
Shin SS, et al: Melanoma mouse model implicates metabotropic
glutamate signaling in melanocytic neoplasia. Nat Genet.
34:108–112. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Willard SS and Koochekpour S: Glutamate
signaling in benign and malignant disorders: Current status, future
perspectives, and therapeutic implications. Int J Biol Sci.
9:728–742. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Willard SS and Koochekpour S: Glutamate,
glutamate receptors, and downstream signaling pathways. Int J Biol
Sci. 9:948–959. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu LJ, Wall BA, Wangari-Talbot J and Chen
S: Metabotropic glutamate receptors in cancer. Neuropharmacology.
15:193–202. 2016.
|
|
18
|
Savage SA, Mirabello L, Wang Z,
Gastier-Foster JM, Gorlick R, Khanna C, Flanagan AM, Tirabosco R,
Andrulis IL, Wunder JS, et al: Genome-Wide association study
identifies two susceptibility loci for osteosarcoma. Nat Genet.
45:799–803. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kalariti NP, Lembessis P and Koutsilieris
M: Characterization of the glutametergic system in MG-63
osteoblast-like osteosarcoma cells. Anticancer Res. 24:3923–3929.
2004.PubMed/NCBI
|
|
20
|
Martin D, Thompson MA and Nadler JV: The
neuroprotective agent riluzole inhibits release of glutamate and
aspartate from slices of hippocampal area CA1. Eur J Pharmacol.
250:473–476. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Doble A: The pharmacology and mechanism of
action of riluzole. Neurology. 47 (Suppl 4):S233–S241. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hubert JP, Delumeau JC, Glowinski J,
Premont J and Doble A: Antagonism by riluzole of entry of calcium
evoked by NMDA and veratridine in rat cultured granule cells:
Evidence for a dual mechanism of action. Br J Pharmacol.
113:261–267. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jan CR, Lu YC, Jiann BP, Chang HT and
Huang JK: Effect of riluzole on cytosolic Ca2+ increase
in human osteosarcoma cells. Pharmacology. 66:120–127. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu J and Wang LN: The efficacy and safety
of riluzole for neurodegenerative movement disorders: A systematic
review with meta-analysis. Drug Deliv. 25:43–48. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Speyer CL, Smith JS, Banda M, DeVries JA,
Mekani T and Gorski DH: Metabotropic glutamate receptor-1: A
potential therapeutic target for the treatment of breast cancer.
Breast Cancer Res Treat. 132:565–573. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Akamatsu K, Shibata MA, Ito Y, Sohma Y,
Azuma H and Otsuki Y: Riluzole induces apoptotic cell death in
human prostate cancer cells via endoplasmic reticulum stress.
Anticancer Res. 29:2195–2204. 2009.PubMed/NCBI
|
|
27
|
Le MN, Chan JL, Rosenberg SA, Nabatian AS,
Merrigan KT, Cohen-Solal KA and Goydos JS: The glutamate release
inhibitor Riluzole decreases migration, invasion, and proliferation
of melanoma cells. J Invest Dermatol. 130:2240–2249. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liao S, Ruiz Y, Gulzar H, Yelskaya Z, Ait
Taouit L, Houssou M, Jaikaran T, Schvarts Y, Kozlitina K, Basu-Roy
K, et al: Osteosarcoma cell proliferation and survival requires
mGluR5 receptor activity and is blocked by riluzole. PLoS One.
12:e01712562017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sperling ST, Aung S, Martin V, Rohde V and
Ninkovic M: Riluzole: A potential therapeutic intervention in human
brain tumor stem-like cells. Oncotarget. 8:96697–96709. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yelskaya Z, Carrillo E, Dubisz E, Gulzar
H, Morgan D and Mahajan SS: Synergistic inhibition of survival,
proliferation, and migration of U87 cells with a combination of
LY341495 and iressa. PLoS One. 8:e645882013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang C, Yuan XR, Li HY, Zhao ZJ, Liao YW,
Wang XY, Su J, Sang SS and Liu Q: Anti-cancer effect of
metabotropic glutamate receptor 1 inhibition in human glioma U87
cells: Involvement of PI3K/Akt/mTOR pathway. Cell Physiol Biochem.
35:419–432. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yip D, Le MN, Chan JL, Lee JH, Mehnert JA,
Yudd A, Kempf J, Shih WJ, Chen S and Goydos JS: A phase 0 trial of
riluzole in patients with resectable stage III and IV melanoma.
Clin Cancer Res. 15:3896–3902. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mehnert JM, Silk AW, Wen Y, Lee JH, Dudek
L, Jeong BS, Li J, Schenkel JM, Sadimin E, Kane M, et al: A phase
II trial of riluzole, an antagonist of metabotropic glutamate
receptor 1 (GRM1) signaling, in patients with advanced melanoma.
Pigment Cell Melanoma Res. 31:534–540. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jia SF, Worth LL and Kleinerman ES: A nude
mouse model of human osteosarcoma lung metastases for evaluating
new therapeutic strategies. Clin Exp Metastasis. 17:501–506. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Caldorera-Moore M, Guimard N, Shi L and
Roy K: Designer nanoparticles: Incorporating size, shape and
triggered release into nanoscale drug carriers. Expert Opin Drug
Deliv. 7:479–495. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tomuleasa C, Braicu C, Irimie A, Craciun L
and Berindan- Neagoe I: Nanopharmacology in translational
hematology and oncology. Int J Nanomedicine. 9:3465–3479.
2014.PubMed/NCBI
|
|
37
|
Dadwal A, Baldi A and Kumar Narang R:
Nanoparticles as carriers for drug delivery in cancer. Artif Cells
Nanomed Biotechnol. 46:295–305. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sykes EA, Chen J, Zheng G and Chan WC:
Investigating the impact of nanoparticle size on active and passive
tumor targeting efficiency. ACS Nano. 8:5696–5706. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Moghimi SM and Szebeni J: Stealth
liposomes and long circulating nanoparticles: Critical issues in
pharmacokinetics, opsonization and protein-binding properties. Prog
Lipid Res. 42:463–478. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gao Y, Xie J, Chen H, Gu S, Zhao R, Shao J
and Jia L: Nanotechnology-Based intelligent drug design for cancer
metastasis treatment. Biotechnol Adv. 32:761–777. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim PS, Djazayeri S and Zeineldin R: Novel
nanotechnology approaches to diagnosis and therapy of ovarian
cancer. Gynecol Oncol. 120:393–403. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Champion JA, Katare YK and Mitragotri S:
Particle shape: A new design parameter for micro- and nanoscale
drug delivery carriers. J Control Release. 121:3–9. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Toy R, Peiris PM, Ghaghada KB and
Karathanasis E: Shaping cancer nanomedicine: The effect of particle
shape on the in vivo journey of nanoparticles. Nanomedicine (Lond).
9:121–134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Truong NP, Whittaker MR, Mak CW and Davis
TP: The importance of nanoparticle shape in cancer drug delivery.
Expert Opin Drug Deliv. 12:129–142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rampersaud S, Fang J, Wei Z, Fabijanic K,
Silver S, Jaikaran T, Ruiz Y, Houssou M, Yin Z, Zheng S, et al: The
effect of cage shape on nanoparticle-based drug carriers:
Anticancer drug release and efficacy via receptor blockade using
dextran-coated iron oxide nanocages. Nano Lett. 16:7357–7363. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Oh MH, Yu T, Yu SH, Lim B, Ko KT,
Willinger MG, Seo DH, Kim BH, Cho MG, Park JH, et al: Galvanic
replacement reactions in metal oxide nanocrystals. Science.
340:964–968. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu Y, Chen T, Wu C, Qiu L, Hu R, Li J,
Cansiz S, Zhang L, Cui C, Zhu G, et al: Facile surface
functionalization of hydrophobic magnetic nanoparticles. J Am Chem
Soc. 136:12552–12555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ray P, Wu AM and Gambhir SS: Optical
bioluminescence and positron emission tomography imaging of a novel
fusion reporter gene in tumor xenografts of living mice. Cancer
Res. 63:1160–1165. 2003.PubMed/NCBI
|
|
49
|
Steckiewicz KP, Barcinska E, Malankowska
A, Zauszkiewicz- Pawlak A, Nowaczyk G, Zaleska-Medynska A and
Inkielewicz-Stepniak I: Impact of gold nanoparticles shape on their
cytotoxicity against human osteoblast and osteosarcoma in in vitro
model. Evaluation of the safety of use and anti-cancer potential. J
Mater Sci Mater Med. 30:222019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Groeneveld GJ, Van Kan HJ, Kalmijn S,
Veldink JH, Guchelaar HJ, Wokke JH and Van den Berg LH: Riluzole
serum concentrations in patients with ALS: Associations with side
effects and symptoms. Neurology. 61:1141–1143. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Groeneveld GJ, van Kan HJ, Lie AHL,
Guchelaar HJ and van den Berg LH: An association study of riluzole
serum concentration and survival and disease progression in
patients with ALS. Clin Pharmacol Ther. 83:718–722. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bondi ML, Craparo EF, Giammona G and Drago
F: Brain-targeted solid lipid nanoparticles containing riluzole:
Preparation, characterization and biodistribution. Nanomedicine
(Lond). 5:25–32. 2010. View Article : Google Scholar : PubMed/NCBI
|