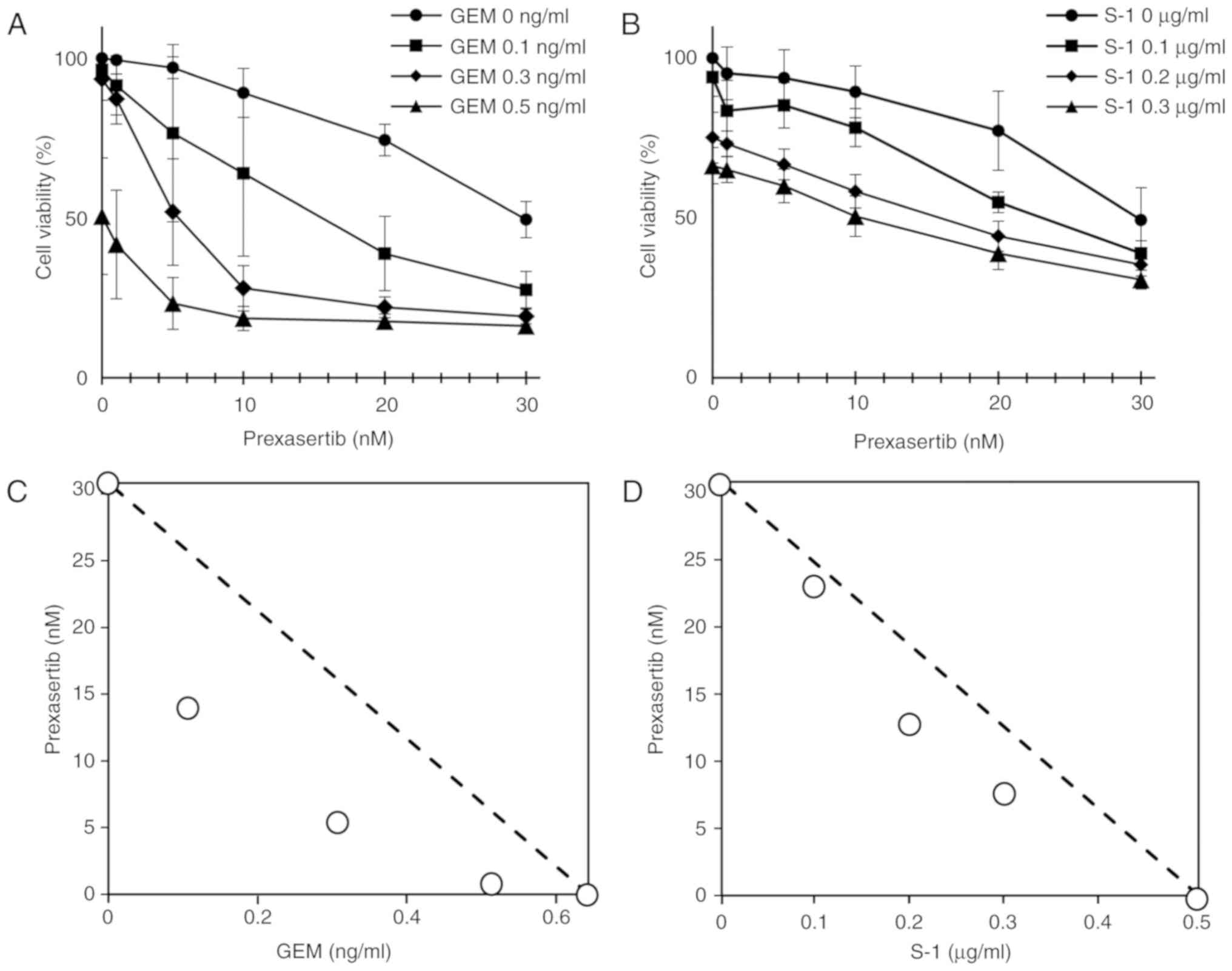
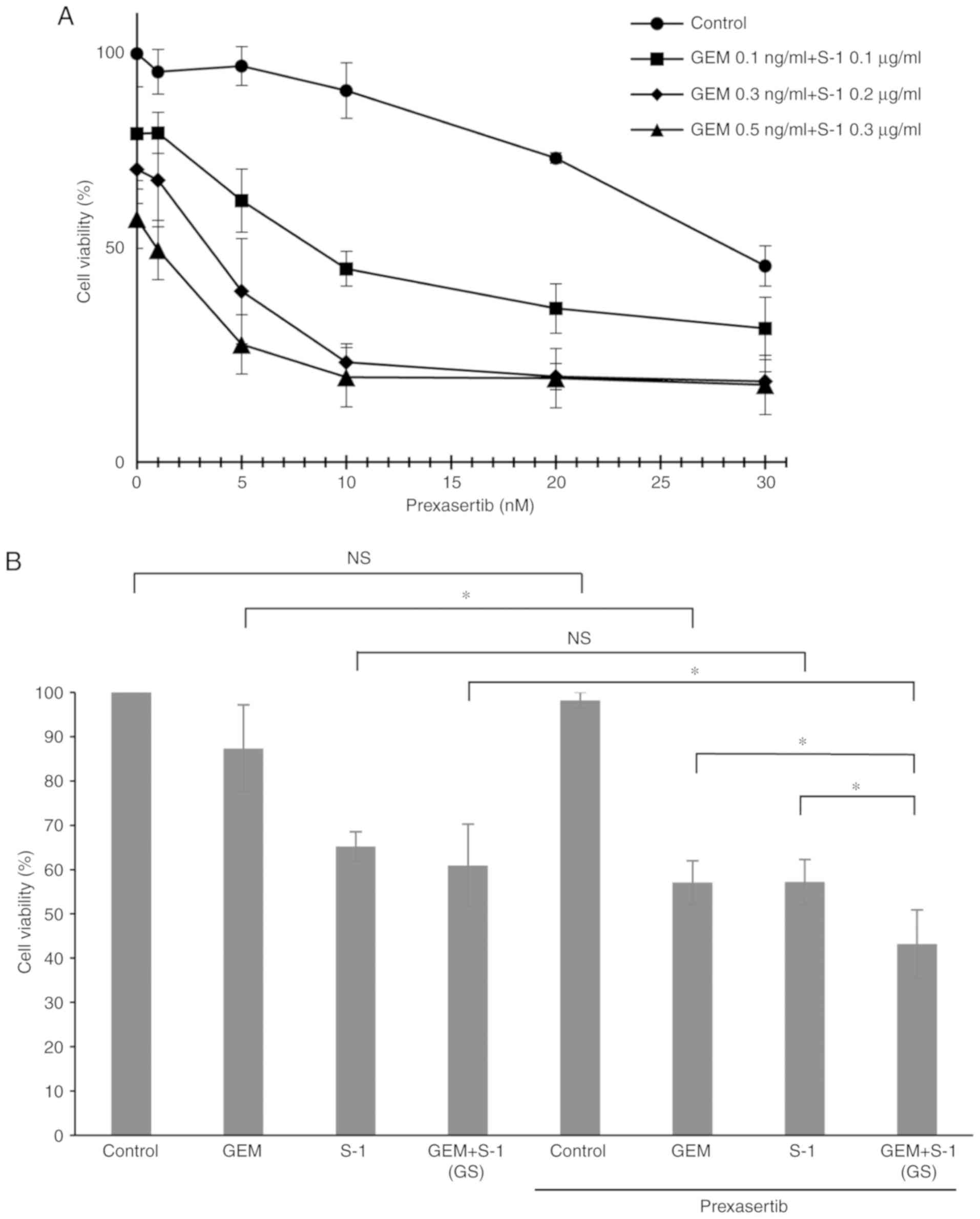
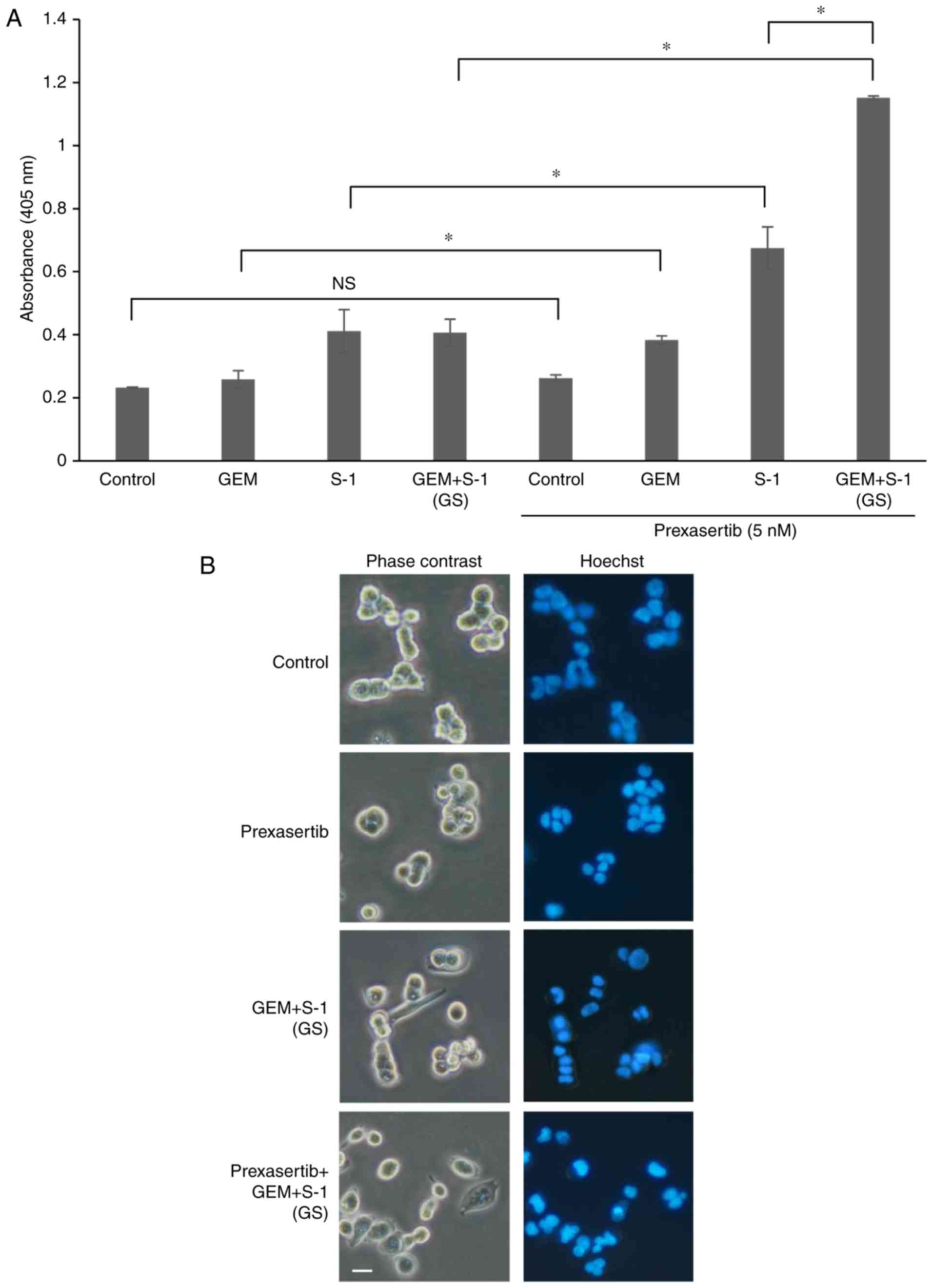
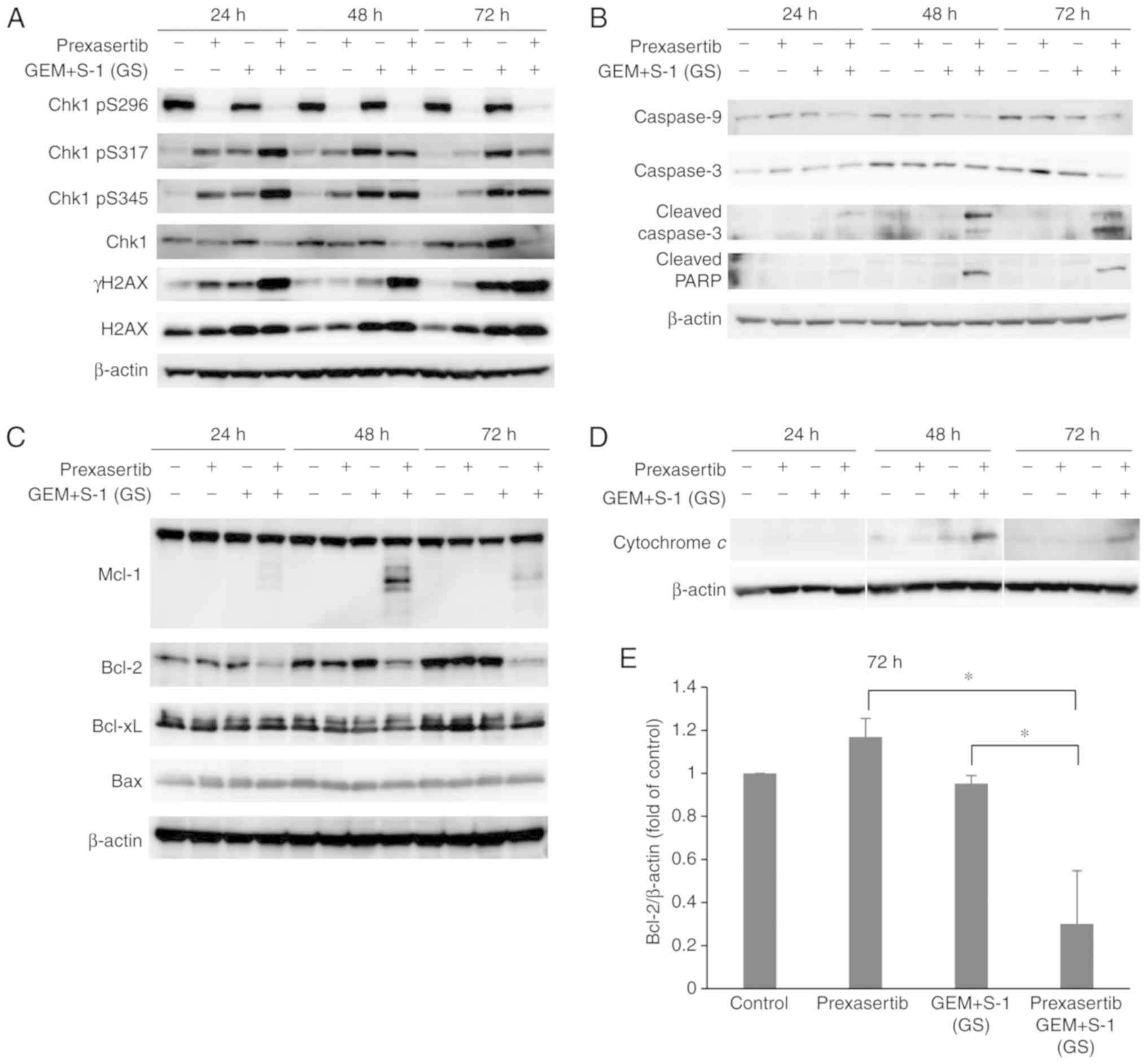
|
1
|
Burris HA III, Moore MJ, Andersen J, Green
MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo
AM, Tarassoff P, et al: Improvements in survival and clinical
benefit with gemcitabine as first-line therapy for patients with
advanced pancreas cancer: A randomized trial. J Clin Oncol.
15:2403–2413. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shirasaka T, Shimamato Y, Ohshimo H,
Yamaguchi M, Kato T, Yonekura K and Fukushima M: Development of a
novel form of an oral 5-fluorouracil derivative (S-1) directed to
the potentiation of the tumor selective cytotoxicity of
5-fluorouracil by two biochemical modulators. Anticancer Drugs.
7:548–557. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tatsumi K, Fukushima M, Shirasaka T and
Fujii S: Inhibitory effects of pyrimidine, barbituric acid and
pyridine derivatives on 5-fluorouracil degradation in rat liver
extracts. Jpn J Cancer Res. 78:748–755. 1987.PubMed/NCBI
|
|
4
|
Fukui Y, Oka T, Nagayama S, Danenberg PV,
Danenberg KD and Fukushima M: Thymidylate synthase,
dihydropyrimidine dehydrogenase, orotate phosphoribosyltransferase
mRNA and protein expression levels in solid tumors in large scale
population analysis. Int J Mol Med. 22:709–716. 2008.PubMed/NCBI
|
|
5
|
Shirasaka T, Nakano K, Takechi T, Satake
H, Uchida J, Fujioka A, Saito H, Okabe H, Oyama K, Takeda S, et al:
Antitumor activity of 1 M tegafur-0.4 M
5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against
human colon carcinoma orthotopically implanted into nude rats.
Cancer Res. 56:2602–2606. 1996.PubMed/NCBI
|
|
6
|
Shirasaka T, Shimamoto Y and Fukushima M:
Inhibition by oxonic acid of gastrointestinal toxicity of
5-fluorouracil without loss of its antitumor activity in rats.
Cancer Res. 53:4004–4009. 1993.PubMed/NCBI
|
|
7
|
Sasako M, Sakuramoto S, Katai H, Kinoshita
T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T and
Ohashi Y: Five-year outcomes of a randomized phase III trial
comparing adjuvant chemotherapy with S-1 versus surgery alone in
stage II or III gastric cancer. J Clin Oncol. 29:4387–4393. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, et al: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): A phase III
trial. Lancet Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ohtsu A, Baba H, Sakata Y, Mitachi Y,
Horikoshi N, Sugimachi K and Taguchi T: Phase II study of S-1, a
novel oral fluorophyrimidine derivative, in patients with
metastatic colorectal carcinoma. S-1 cooperative colorectal
carcinoma study group. Br J Cancer. 83:141–145. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okamoto I, Yoshioka H, Morita S, Ando M,
Takeda K, Seto T, Yamamoto N, Saka H, Asami K, Hirashima T, et al:
Phase III trial comparing oral S-1 plus carboplatin with paclitaxel
plus carboplatin in chemotherapy-naïve patients with advanced
non-small-cell lung cancer: Results of a west Japan oncology group
study. J Clin Oncol. 28:5240–5246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kubota K, Sakai H, Katakami N, Nishio M,
Inoue A, Okamoto H, Isobe H, Kunitoh H, Takiguchi Y, Kobayashi K,
et al: A randomized phase III trial of oral S-1 plus cisplatin
versus docetaxel plus cisplatin in Japanese patients with advanced
non-small-cell lung cancer: TCOG0701 CATS trial. Ann Oncol.
26:1401–1408. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ueno H, Ioka T, Ikeda M, Ohkawa S,
Yanagimoto H, Boku N, Fukutomi A, Sugimori K, Baba H, Yamao K, et
al: Randomized phase III study of gemcitabine plus S-1, S-1 alone,
or gemcitabine alone in patients with locally advanced and
metastatic pancreatic cancer in Japan and Taiwan: GEST study. J
Clin Oncol. 31:1640–1648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Uesaka K, Boku N, Fukutomi A, Okamura Y,
Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto
H, et al: Adjuvant chemotherapy of S-1 versus gemcitabine for
resected pancreatic cancer: A phase 3, open-label, randomised,
non-inferiority trial (JASPAC 01). Lancet. 388:248–257. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshizawa J, Takizawa A, Takeuchi O,
Hiraku O, Sasaki K, Morimoto Y, Atsuda K, Inoue G, Suzuki Y,
Asanuma F and Yamada Y: Experimental study of combination therapy
with S-1 against pancreatic cancer. Cancer Chemother Pharmacol.
64:1211–1219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morimoto Y, Takeuchi O, Takizawa A,
Yoneyama H, Asanuma F, Suzuki Y, Atsuda K and Yamada Y: Effect of a
combination of S-1 and gemcitabine on cell cycle regulation in
pancreatic cancer cell lines. Anticancer Drugs. 23:505–514. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jackson SP and Bartek J: The DNA-damage
response in human biology and disease. Nature. 461:1071–1078. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dent P, Tang Y, Yacoub A, Dai Y, Fisher PB
and Grant S: CHK1 inhibitors in combination chemotherapy: Thinking
beyond the cell cycle. Mol Interv. 11:133–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li T, Christensen SD, Frankel PH, Margolin
KA, Agarwala SS, Luu T, Mack PC, Lara PN Jr and Gandara DR: A phase
II study of cell cycle inhibitor UCN-01 in patients with metastatic
melanoma: A California cancer consortium trial. Invest New Drugs.
30:741–748. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Webster JA, Tibes R, Morris L, Blackford
AL, Litzow M, Patnaik M, Rosner GL, Gojo I, Kinders R, Wang L, et
al: Randomized phase II trial of cytosine arabinoside with and
without the CHK1 inhibitor MK-8776 in relapsed and refractory acute
myeloid leukemia. Leuk Res. 61:108–116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sausville E, Lorusso P, Carducci M, Carter
J, Quinn MF, Malburg L, Azad N, Cosgrove D, Knight R, Barker P, et
al: Phase I dose-escalation study of AZD7762, a checkpoint kinase
inhibitor, in combination with gemcitabine in US patients with
advanced solid tumors. Cancer Chemother Pharmacol. 73:539–549.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wehler T, Thomas M, Schumann C,
Bosch-Barrera J, Viñolas Segarra N, Dickgreber NJ, Dalhoff K,
Sebastian M, Corral Jaime J, Alonso M, et al: A randomized, phase 2
evaluation of the CHK1 inhibitor, LY2603618, administered in
combination with pemetrexed and cisplatin in patients with advanced
nonsquamous non-small cell lung cancer. Lung Cancer. 108:212–216.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Karp JE, Thomas BM, Greer JM, Sorge C,
Gore SD, Pratz KW, Smith BD, Flatten KS, Peterson K, Schneider P,
et al: Phase I and pharmacologic trial of cytosine arabinoside with
the selective checkpoint 1 inhibitor Sch 900776 in refractory acute
leukemias. Clin Cancer Res. 18:6723–6731. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
King C, Diaz HB, McNeely S, Barnard D,
Dempsey J, Blosser W, Beckmann R, Barda D and Marshall MS:
LY2606368 causes replication catastrophe and antitumor effects
through CHK1-dependent mechanisms. Mol Cancer Ther. 14:2004–2013.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wagenpfeil S, Treiber U and Lehmer A:
Statistical analysis of combined dose effects for experiments with
two agents. Artif Intell Med. 37:65–71. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hirata K, Horikoshi N, Aiba K, Okazaki M,
Denno R, Sasaki K, Nakano Y, Ishizuka H, Yamada Y, Uno S, et al:
Pharmacokinetic study of S-1, a novel oral fluorouracil antitumor
drug. Clin Cancer Res. 5:2000–2005. 1999.PubMed/NCBI
|
|
26
|
Goto H, Izawa I, Li P and Inagaki M: Novel
regulation of checkpoint kinase 1: Is checkpoint kinase 1 a good
candidate for anti-cancer therapy? Cancer Sci. 103:1195–1200. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ghelli Luserna Di Rorà A, Iacobucci I,
Imbrogno E, Papayannidis C, Derenzini E, Ferrari A, Guadagnuolo V,
Robustelli V, Parisi S, Sartor C, et al: Prexasertib, a Chk1/Chk2
inhibitor, increases the effectiveness of conventional therapy in
B-/T- cell progenitor acute lymphoblastic leukemia. Oncotarget.
7:53377–53391. 2016.PubMed/NCBI
|
|
28
|
Zeng L, Beggs RR, Cooper TS, Weaver AN and
Yang ES: Combining Chk1/2 inhibition with Cetuximab and radiation
enhances in vitro and in vivo cytotoxicity in head and neck
squamous cell carcinoma. Mol Cancer Ther. 16:591–600. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lowery CD, VanWye AB, Dowless M, Blosser
W, Falcon BL, Stewart J, Stephens J, Beckmann RP, Bence Lin A and
Stancato LF: The checkpoint kinase 1 inhibitor prexasertib induces
regression of preclinical models of human neuroblastoma. Clin
Cancer Res. 23:4354–4363. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brill E, Yokoyama T, Nair J, Yu M, Ahn YR
and Lee JM: Prexasertib, a cell cycle checkpoint kinases 1 and 2
inhibitor, increases in vitro toxicity of PARP inhibition by
preventing Rad51 foci formation in BRCA wild type high-grade serous
ovarian cancer. Oncotarget. 8:111026–111040. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Barnard D, Diaz HB, Burke T, Donoho G,
Beckmann R, Jones B, Barda D, King C and Marshall M: LY2603618, a
selective CHK1 inhibitor, enhances the anti-tumor effect of
gemcitabine in xenograft tumor models. Invest New Drugs. 34:49–60.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Montano R, Thompson R, Chung I, Hou H,
Khan N and Eastman A: Sensitization of human cancer cells to
gemcitabine by the Chk1 inhibitor MK-8776: Cell cycle perturbation
and impact of administration schedule in vitro and in vivo. BMC
Cancer. 13:6042013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fujinaka Y, Matsuoka K, Iimori M, Tuul M,
Sakasai R, Yoshinaga K, Saeki H, Morita M, Kakeji Y, Gillespie DA,
et al: ATR-Chk1 signaling pathway and homologous recombinational
repair protect cells from 5-fluorouracil cytotoxicity. DNA Repair
(Amst). 11:247–258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang J, Liu X, Bhalla K, Kim CN, Ibrado
AM, Cai J, Peng TI, Jones DP and Wang X: Prevention of apoptosis by
Bcl-2: Release of cytochrome c from mitochondria blocked. Science.
275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Seymour JF, Kipps TJ, Eichhorst B, Hillmen
P, D'Rozario J, Assouline S, Owen C, Gerecitano J, Robak T, De la
Serna J, et al: Venetoclax-Rituximab in relapsed or refractory
chronic lymphocytic leukemia. N Engl J Med. 378:1107–1120. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dong J, Zhao YP, Zhou L, Zhang TP and Chen
G: Bcl-2 upregulation induced by miR-21 via a direct interaction is
associated with apoptosis and chemoresistance in MIA PaCa-2
pancreatic cancer cells. Arch Med Res. 42:8–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hakata S, Terashima J, Shimoyama Y, Okada
K, Fujioka S, Ito E, Habano W and Ozawa S: Differential
sensitization of two human colon cancer cell lines to the antitumor
effects of irinotecan combined with 5-aza-2′-deoxycytidine. Oncol
Lett. 15:4641–4648. 2018.PubMed/NCBI
|
|
38
|
Wang T, Yang Z, Zhang Y, Zhang X, Wang L,
Zhang S and Jia L: Caspase cleavage of Mcl-1 impairs its
anti-apoptotic activity and proteasomal degradation in non-small
lung cancer cells. Apoptosis. 23:54–64. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: FOLFIRINOX versus gemcitabine for
metastatic pancreatic cancer. N Engl J Med. 364:1817–1825. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Motoi F, Ishida K, Fujishima F, Ottomo S,
Oikawa M, Okada T, Shimamura H, Takemura S, Ono F, Akada M, et al:
Neoadjuvant chemotherapy with gemcitabine and S-1 for resectable
and borderline pancreatic ductal adenocarcinoma: Results from a
prospective multi-institutional phase 2 trial. Ann Surg Oncol.
20:3794–3801. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ishii H, Yamashita N, Ueno M, Ohkawa S,
Saito AM and Sekimoto M: A randomised controlled trial of
gemcitabine hydrochloride plus S-1 combination therapy versus
gemcitabine hydrochloride therapy alone in pancreatic cancer
patients aged ≥75 years: A study protocol for an open-label
randomised feasibility study. BMJ Open Gastroenterol.
5:e0001872018.PubMed/NCBI
|
|
43
|
Mizusawa J, Morizane C, Okusaka T,
Katayama H, Ishii H, Fukuda H and Furuse J; Hepatobiliary and
Pancreatic Oncology Group of the Japan Clinical Oncology Group, :
Randomized phase III study of gemcitabine plus S-1 versus
gemcitabine plus cisplatin in advanced biliary tract cancer: Japan
clinical oncology group study (JCOG1113, FUGA-BT). J Clin Oncol.
46:385–388. 2016.
|
|
44
|
Scagliotti G, Kang JH, Smith D, Rosenberg
R, Park K, Kim SW, Su WC, Boyd TE, Richards DA, Novello S, et al:
Phase II evaluation of LY2603618, a first-generation CHK1
inhibitor, in combination with pemetrexed in patients with advanced
or metastatic non-small cell lung cancer. Invest New Drugs.
34:625–635. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Laquente B, Lopez-Martin J, Richards D,
Illerhaus G, Chang DZ, Kim G, Stella P, Richel D, Szcylik C,
Cascinu S, et al: A phase II study to evaluate LY2603618 in
combination with gemcitabine in pancreatic cancer patients. BMC
Cancer. 17:1372017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee JM, Nair J, Zimmer A, Lipkowitz S,
Annunziata CM, Merino MJ, Swisher EM, Harrell MI, Trepel JB, Lee
MJ, et al: Prexasertib, a cell cycle checkpoint kinase 1 and 2
inhibitor, in BRCA wild-type recurrent high-grade serous ovarian
cancer: A first-in-class proof-of-concept phase 2 study. Lancet
Oncol. 19:207–215. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hong DS, Moore K, Patel M, Grant SC,
Burris HA III, William WN Jr, Jones S, Meric-Bernstam F, Infante J,
Golden L, et al: Evaluation of prexasertib, a checkpoint kinase 1
inhibitor, in a phase Ib study of patients with squamous cell
carcinoma. Clin Cancer Res. 24:3263–3272. 2018. View Article : Google Scholar : PubMed/NCBI
|