Introduction
Lung cancer is a leading cause of cancer-related
morbidity worldwide (1). As the
major histological subtype of lung cancer, non-small cell lung
cancer (NSCLC) has a prevalence of up to 80% in lung cancer
patients. Of these patients, 50% are diagnosed with lung
adenocarcinoma (LADC) (2). In
clinical settings, the treatment of lung cancer mainly consists of
surgery, chemotherapy and radiotherapy. However, the treatment
efficacy of these conventional therapies varies and is
unsatisfactory. Importantly, a group of drug candidates for
molecular-targeted therapy have been tested in trials with
promising beneficial effects (3–5). The
increased need for new diagnostic, prognostic and therapeutic tools
for NSCLC requires additional molecular and mechanistic studies
regarding the pathobiology of NSCLC.
Canopy fibroblast growth factor signaling regulator
2 (CNPY2), a member of the CNPY protein family, is an endoplasmic
reticulum (ER) luminal protein encoded by the CNPY2 gene
(6). CNYP2 contributes to
angiogenesis and the prevention of hypertrophic cardiomyopathy
(7,8). In addition, it modulates DNA
replication, cellular invasion, blood vessel development, and
metastasis of several malignancies including human prostate cancer,
colorectal cancer, and renal cell carcinoma (9–12). In
a recent study, an elevated CNPY2 expression level in NSCLC
patients was correlated with poor survival (13). However, the biological function and
regulation of CNPY2 in the pathogenesis of LADC are still
unknown.
miR-30a, a member of the human miRNA family, is
consistently downregulated in many malignancies including breast
cancer, renal cell carcinoma, hepatocellular carcinoma, colorectal
cancer and cervical cancer (14–18).
Interestingly, it was controversially considered to be
tumor-suppressive or oncogenic in distinct types of malignancies
(19–21). We believe that the differential
roles of miR-30 family members as tumor-suppressor genes or
oncogenes, are dependent on the target gene in the particular type
of cancer. There is a need to define the selective role of miR-30
family members in cancer regulation, in order to design more
specific and potent therapies for cancer subtypes. Here, we focus
on one member of the miR-30 family, miR-30a-3p, which is abundantly
expressed with an unclear role in LADC, and has been identified as
a CNPY2 regulator.
In the present study, for the first time, we
investigated both CNPY2 and miR-30a-3p expression in
LADC tissue samples and NSCLC cell lines. In addition, the
molecular function and interaction of CNPY2 and miR-30a-3p in LADC
cell lines were characterized. We identified CNPY2 as a
target gene of miR-30a-3p with a tumor-suppressive function. Thus,
miR-30a-3p activation or CNPY2 inhibition may be applicable for
treating LADC.
Materials and methods
Patients and tissue samples
Tissue samples were collected from 64 LADC patients
(mean age, 58 years; age range, 45–72 years; sex, 29 male and 35
female) undergoing surgery at the Department of Surgery, The First
Affiliated Hospital, Bengbu Medical College between January 2012
and December 2017. The diagnosis of LADC was based on pathological
findings. The samples were embedded in paraffin, and matched
healthy lung tissues (adjacent tumors) served as controls. Patients
with other types of tumors or chronic/acute lung diseases were
excluded from this study. Each patient signed an informed consent.
Sample handling was in line with the guidelines set out in the
Declaration of Helsinki. The study protocols were in line with the
guidelines of the Ethics Committee of Bengbu Medical College
(Anhui, China).
TCGA analysis
To analyze the clinicopathologic parameters and
overall survival curves of the LADC patients with high or low CNPY2
mRNA expression, data from The Cancer Genome Atlas (TCGA) dataset
were retrieved and analyzed.
Cell culture and transfection
A LADC-derived cell line (EKVX cells) was provided
by the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China). The control cells were normal human bronchial epithelial
(HBE) cells purchased from Gaining Biotech (Shanghai, China). Cells
were cultured in DMEM medium (Hyclone; GE Healthcare Life Sciences)
containing 10% fetal bovine serum (FBS; Sigma-Aldrich; Merck KGaA)
at 37°C in 5% CO2.
miR-30a-3p mimics and inhibitors were provided by
GeneCopoeia (Guangzhou, China), together with the corresponding
negative control vectors. The small interfering (si)RNA of
CNPY2 was obtained from EnoGene (Nanjing, China). To induce
overexpression and silencing of miR-30a-3p and CNPY2, EKVX cells
were transfected with the corresponding vectors using a commercial
Lipofectamine 2000 kit, according to standard protocols
(Invitrogen; Thermo Fisher Scientific, Inc.).
Immunohistochemical staining
evaluation
Following sample fixation using 10% formalin and
embedding in paraffin, the sections (3-µm) were subjected to
immunohistochemical staining using an established protocol
(22). In brief, the anti-human
CNPY2 antibody (dilution 1:500; cat. no. ab233136; Abcam) was
firstly used to stain the sections at 4°C overnight. Secondary
antibodies were then added to the mixture. For visualization of the
immunoreactions, a Vectastain® Elite
avidin-biotin-peroxidase (ABC) complex (Vector Laboratories) kit
was used. Non-specific binding was controlled by application of
secondary-only negative controls.
A visual grading system was used for quantification
of CNPY2 expression according to the extent and intensity of
staining. The immunoreactivity score was determined as described
previously (23). For
immunoreactivity scoring of each sample, 10 visual fields were
randomly selected from different areas. The average
immunoreactivity score was obtained based on these 10 visual
fields. High expression was defined as an immunoreactivity score of
4 or more. The slides were independently viewed by two experienced
physicians blinded to clinicopathological information and outcome
of the patients. In the cases of discrepancies, the data were
reviewed simultaneously until a consensus was reached.
Real-time PCR
Trizol reagent was used for total RNA extraction
from tissue samples and cultured cells. Synthesis of cDNA was
conducted using the First-Strand cDNA synthesis kit (Thermo Fisher
Scientific, Inc.). The miRNeasy Mini kit (Qiagen) was utilized to
extract miRNA from the tissue samples and cell lines. cDNA was
synthesized using the miScript II RT Kit (Qiagen). Quantitative
real-time PCR was conducted on an Applied Biosystems ABI
StepOnePlus Systems (Thermo Fisher Scientific, Inc.) using SYBR
Green (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
internal controls for CNPY2 and miR-30a-3p were GAPDH and U6 snRNA.
The quantification of miR-30a-3p and CNPY2 was performed based on
the 2−∆∆Cq method (24).
The specific primers for CNPY2, GAPDH, miR-30a-3p and U6 snRNA were
as follows: CNPY2, 5′-ATCCTTCCACCCATCGCAAG-3′ and
5′-AACATTGTCAGCCTCTCGGG-3′; GAPDH, 5′-GCACCGTCAAGGCTGAGAAC-3′ and
5′-TGGTGAAGACGCCAGTGGA-3′; miR-30a-3p, 5′-CGCTTTCAGTCGGATGTTTG-3′
and 5′-GTGCAGGGTCCGAGGT-3′; U6 snRNA, 5′-GCGCGTCGTGAAGCGTTC-3′ and
5′-GTGCAGGGTCCGAGGT-3′.
Western blot analysis
Protein was extracted from the tissue samples and
cells according to a previous report (21), followed by measurement with the BCA
Protein Assay kit. The protein samples then underwent SDS-PAGE
analysis, followed by transfer to polyvinylidene difluoride (PVDF)
membranes. Subsequently, 5% BSA was used to block the PVDF
membranes at room temperature for 1 h. The membranes were incubated
with antibodies against CNPY2 (dilution 1:1,000; cat. no. ab233136;
Abcam), N-cadherin (dilution 1:100; cat. no. ab18203; Abcam) or
E-cadherin (dilution 1:100; cat. no. ab15148; Abcam) for 2 h, and
then incubated with HRP-conjugated secondary antibodies (HRP-goat
anti-rabbit IgG, dilution 1:2,000; cat. no. ab205718; Abcam) for 1
h. Finally, the enhanced chemiluminescence method was used to
measure immunoreactivity using the ECL kit (Thermo Fisher
Scientific, Inc.). The same membrane probed with β-actin (dilution
1:2,000; cat. no. ab8227; Abcam) served as the loading control.
Luciferase reporter assay
Wild-type (WT) and mutant (Mut) CNPY2
3′-untranslated region (UTR) were amplified and cloned into the
pGL3-Basic vector purchased from Promega. Human 293T cells,
purchased from the Cell Bank at the Chinese Academy of Sciences
(Shanghai, China), were transfected with pGL3-Mut-3′-UTR-CNPY2 or
pGL3-WT-3′-UTR-CNPY2, as well as miR-30a-3p mimic, miR-30a-3p
inhibitor, control, or negative control vector. The pRL-TK vector
(Promega) was used as an internal control for normalization of
transfection efficacy. Approximately 48 h after transfection, the
relative luciferase activity was evaluated using the
dual-luciferase reporter assay system (Promega).
Invasion and migration assays
Quantitative assays of cellular migration and
invasion were carried out in a commercial chamber (Corning)
equipped with a polycarbonate filter (8.0 µm) inserted into 24-well
plates. A Transwell system purchased from Corning was utilized to
determine the invasion of EKVX cells. Matrigel (12.5 mg; BD
Biosciences) in 50 ml PBS was added to the filter. Cells suspended
in serum-free medium (100 µl) were transferred to the upper
chamber. Subsequently, the lower chamber was filled with medium
(500 µl) supplemented with 10% FBS. After incubation at 37°C for 48
h, the cells were removed from the upper surface of the filter
using a cotton swab, and then penetrated to the lower surface of
the filter followed by staining with crystal violet (0.1%, 15 min
at room temperature). The cells left on the lower side of each
membrane were counted under a light microscope for five fields of
view (magnification, ×200).
Determination of cellular
proliferation
Cell proliferation was measured with a commercial
CCK-8 kit (Beyotime Institute of Biotechnology). The harvested
cells (1.0×104/well) were then seeded into 96-well
plates. Subsequently, 10 µl of CCK-8 reagent was added to each well
at 0, 24, 48 and 72 h, respectively. The mixture was then incubated
for another 2 h at 37°C, followed by determination of the
absorbance at 450 nm using a microplate reader.
Colony formation assay
Approximately 48 h after cellular transfection, the
cells were suspended in culture medium supplemented with 0.3%
agarose. The cells were then plated on a layer containing 0.7%
agarose in growth medium in a 6-well plate and cultured for 14
days. The cells were then fixed using methanol (20 min) and stained
with crystal violet dye (0.1%, 15 min). Colonies consisting of more
than 50 cells were counted as a single colony under a light
inverted microscope (TS100; Nikon Corp.) and each assay was
performed in triplicate.
Statistical analysis
All experiments were repeated at least three times,
independently. Data are displayed as mean ± standard error of the
mean (SEM). SPSS 19.0 software (IBM Corp.) was used for data
analysis. We evaluated the statistical significance with the
performance of the one-way analysis of variance (ANOVA) test
followed by Dunnett's post hoc test for the comparison of >2
groups. A P-value of <0.05 was considered statistically
significant.
Results
CNPY2 is upregulated in LADC tissues
and the expression level of CNPY2 correlates with clinical outcomes
in LADC patients
CNPY2 expression in LADC tissues was first
investigated. The protein expression level of CNPY2 in LADC samples
was significantly increased compared to that of normal controls as
shown by immunohistochemical analysis (Fig. 1A and B). To confirm and validate the
relationship between clinicopathological parameters in LADC
patients and CYPY2 expression, we analyzed data from The Cancer
Genome Atlas (TCGA) database (https://cancergenome.nih.gov/). The
clinicopathological and demographic features are documented in
Table I. The TCGA cohort
demonstrated consistent CNPY2 upregulation in LADC samples
(Fig. 1C), regardless of clinical
stage (Fig. 1D) or gender (Fig. 1E). The expression of CNPY2 was also
correlated with LADC survival (Fig.
1F). These data confirmed that CNPY2 may serve as a marker of
LADC, with some diagnostic and prognostic power in LADC, similar to
that reported in other types of cancers (10,11).
However, it is not entirely clear how CNPY2 expression is modulated
in LADC.
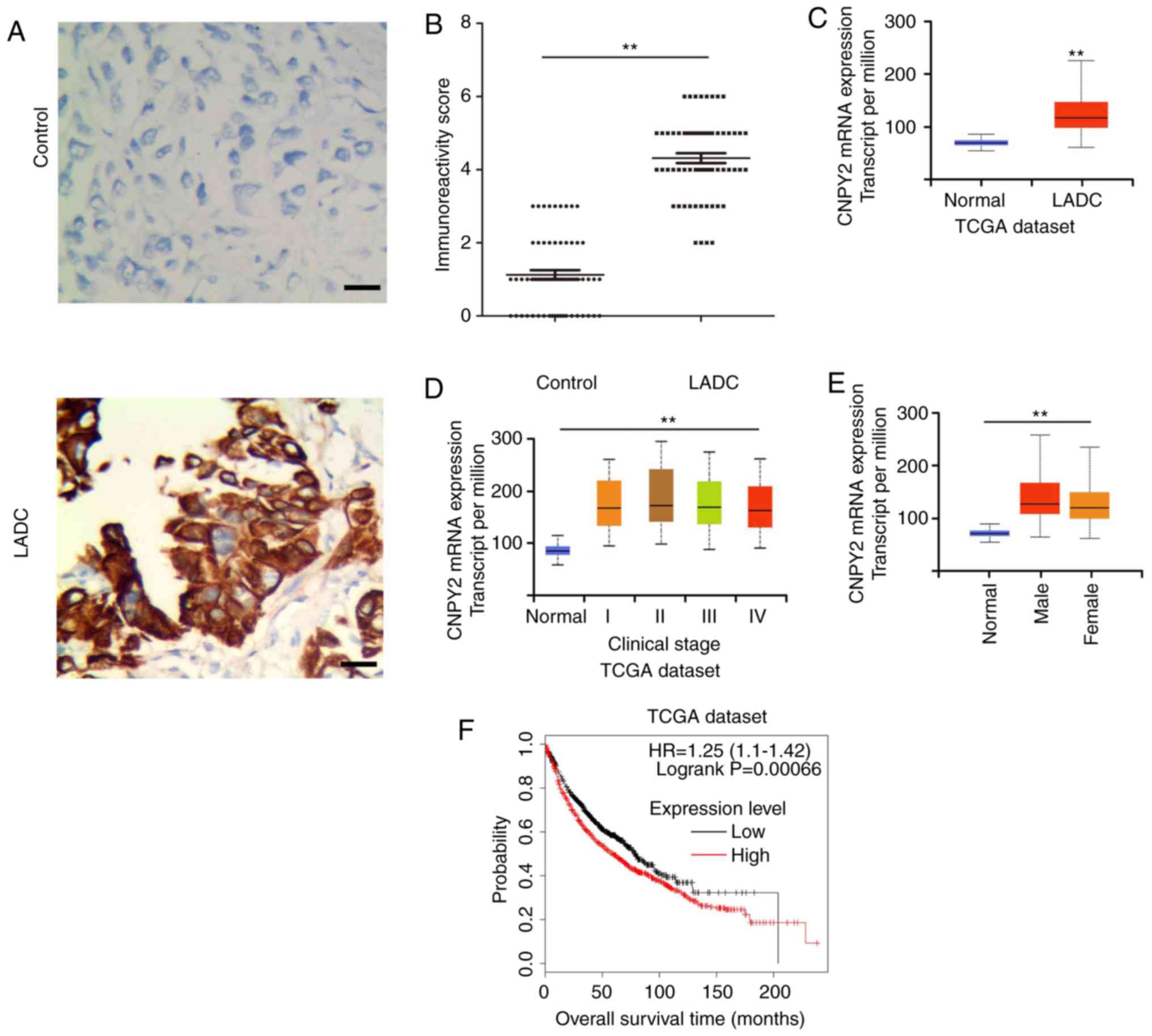 | Figure 1.CNPY2 is upregulated in LADC tissues
and the expression level of CNPY2 correlates with clinical outcome
in LADC patients. (A) Immunohistochemical analysis of CNPY2
expression in LADC tissues (n=64, bottom panel) and matched normal
lung tissues (n=64, top panel) (scale bar, 100 µm, magnification
×200). (B) CNPY2 immunoreactivity scores were determined by blinded
analysis. **P<0.01 vs. the control group. (C) Compared with
normal control tissues (n=59), (the controls were obtained from
paracancerous tissues in patients with LADC), CNPY2 was highly
expressed in 515 LADC samples (TCGA cohort). (D) CNPY2 expression
levels in patients with different clinical stages of LADC (TCGA
cohort): Normal (n=59), stage I (n=277), II (n=125), III (n=85) and
IV (n=28). (E) Expression of CNPY2 in LADC based on patient's sex
(TCGA cohort): Normal (n=59), male (n=238) and female (n=276). (F)
The overall survival curve for patients with low vs. high CNPY2
using the publicly available Kaplan-Meier plotter database
(www.kmplot.com): Low expression level (n=964),
high expression level (n=962). LADC, lung adenocarcinoma; CNPY2,
canopy fibroblast growth factor signaling regulator 2; TCGA, The
Cancer Genome Atlas. |
 | Table I.Correlation analysis between the
clinical features and expression of miR-30a-3p in the LADC
cases. |
Table I.
Correlation analysis between the
clinical features and expression of miR-30a-3p in the LADC
cases.
| Variables | Total no. of pts
(N=64) | miR-30a-3p low,
n | miR-30a-3p high,
n | χ2 |
P-valuea |
|---|
| Age (years) |
|
|
|
| 0.42 |
|
≥50 | 43 | 22 | 21 |
0.66 |
|
|
<50 | 21 | 13 | 8 |
|
|
| Sex |
|
|
|
| 0.66 |
|
Male | 29 | 15 | 14 |
0.19 |
|
|
Female | 35 | 20 | 15 |
|
|
| TNM stage |
|
|
|
| 0.0002b |
|
I+II | 24 | 6 | 18 | 13.66 |
|
|
III+IV | 40 | 29 | 11 |
|
|
| Metastasis |
|
|
|
| 0.0002b |
|
Yes | 42 | 30 | 12 | 13.81 |
|
| No | 22 | 5 | 17 |
|
|
Downregulated miR-30a-3p contributes
to CNPY2 dysregulation in LADC
Following confirmation of CNPY2 upregulation in
human LADC, we next characterized the mechanism of CNPY2
upregulation using molecular and cellular biology tools. The
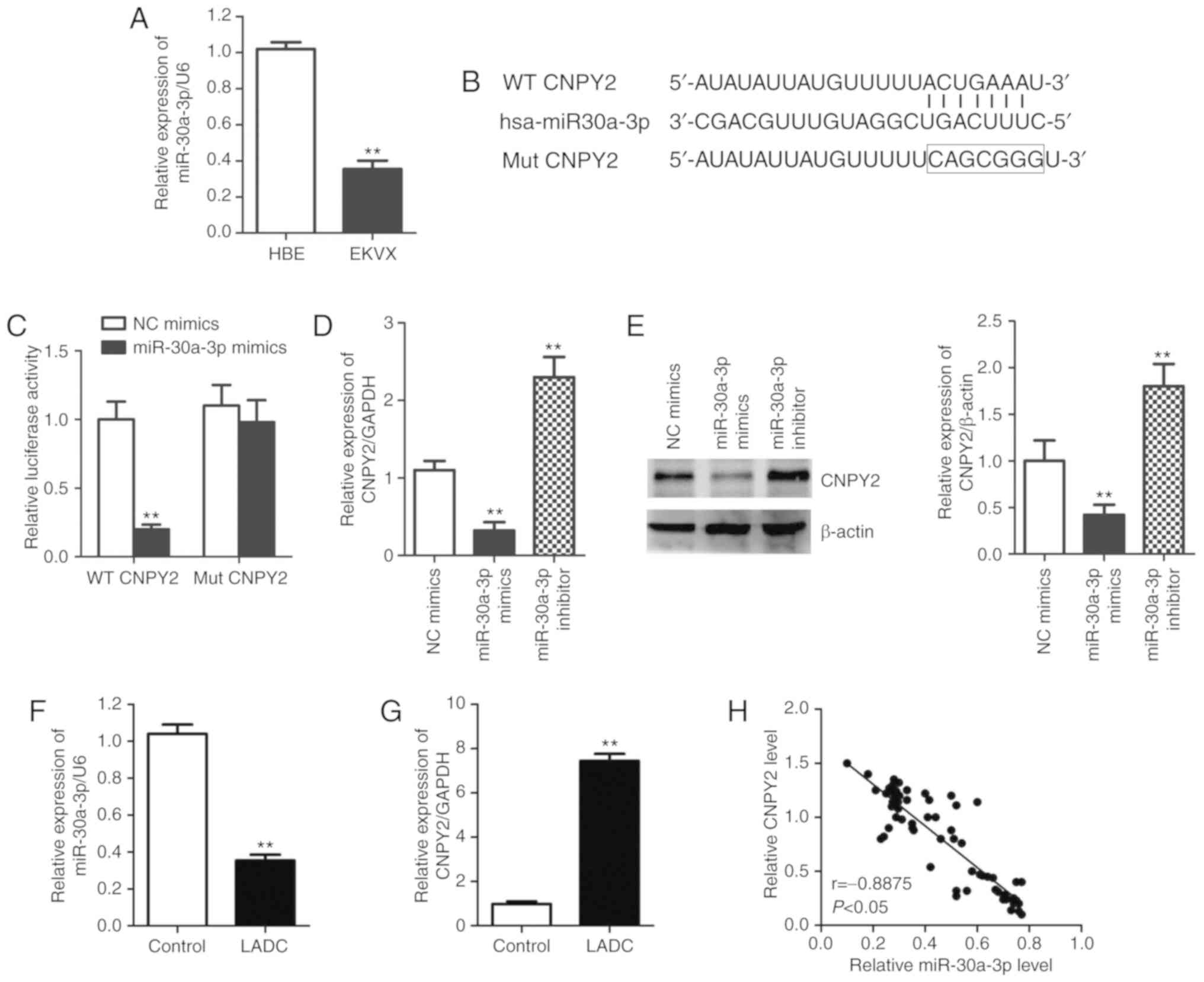
expression of miR-30a-3p was markedly reduced in LADC cells (EKVX)
compared with that noted in the control non-cancer (HBE) cells
(Fig. 2A), similar to previous
findings in other types of cancers (10,11).
TargetScan (http://www.targetscan.org/) and miRanda bioinformatics
databases (http://microrna.sanger.ac.uk/) identified the binding
sequence for miR-30a-3p in the 3′-UTR of the CNPY2 gene
(Fig. 2B). This binding and
consequent expression inhibition was confirmed by a CNPY2 3′-UTR
luciferase assay. The reporter luciferase activity with the
wild-type (WT) CNPY2 3′-UTR vector was significantly reduced after
treatment with miR-30a-3p mimics (Fig.
2C). However, the promoter luciferase activity with the mutant
(Mut) CNPY2 3′-UTR (Fig. 2B) showed
no significant changes (Fig. 2C)
following treatment with miR-30a-3p mimics. EKVX cells were
transfected with miR-30a-3p mimic or miR-30a-3p inhibitor to alter
the expression of miR-30a-3p, and the transfection efficiency was
identified by quantitative PCR analysis. The expression of
miR-30a-3p was significantly augmented in the miR-30a-3p
mimic-transfected cells but downregulated in miR-30a-3p
inhibitor-transfected cells relative to the NC miRNA-transfected
cells (Fig. S1). Then, the
downregulation of CNPY2 mRNA by miR-30a-3p mimics and significant
upregulation of CNPY2 mRNA mediated by miR-30a-3p inhibitors
(Fig. 2D) were further confirmed by
quantitative PCR. In addition, the regulation of CNPY2 expression
at the protein level was validated by western blot analysis
(Fig. 2E). These data directly
demonstrate that miR-30a-3p inhibited CNPY2 expression, via
specific miRNA binding at the post-transcriptional level, in LADC
cells. We next confirmed the inverse expression pattern of
miR-30a-3p and CNPY2 in LADC tissues. Compared to adjacent normal
tissues, LADC tissues exhibited a significantly lower level of
miR-30a-3p (Fig. 2F), and a higher
level of CNPY2 (Fig. 2G). A
significant negative correlation was noted between miR-30a-3p and
CNPY2 mRNA expression in tumor tissues following Spearman's rank
correlation analysis (r=−0.8875, P<0.05, Fig. 2H). Taken together, these findings
show that miR-30a-3p is a direct negative regulator of CNPY2 in
LADC under in vitro and in vivo conditions.
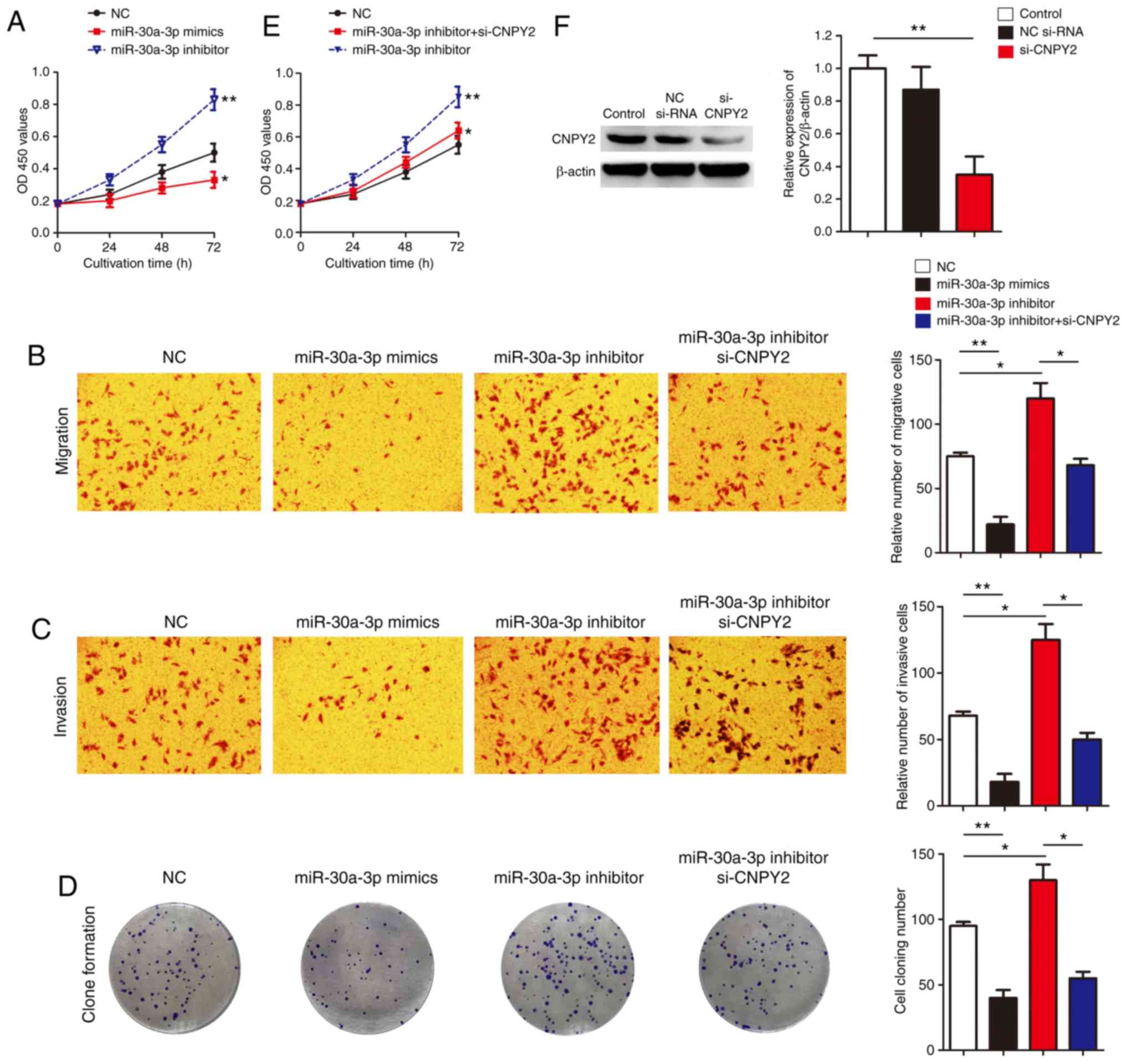
miR-30a-3p inhibits cellular
proliferation, invasion and migration by targeting CNPY2
Following confirmation of the negative regulation of
CNPY2 by miR-30a-3p in LADC both in vitro and in
vivo, we next evaluated the functional outcome of reduced
miR-30a-3p in LADC via CNPY2. miR-30a-3p overexpression (by
miR-30a-3p mimics) significantly reduced cell proliferation
(Fig. 3A), invasion (Fig. 3B), migration (Fig. 3C), and clone formation (Fig. 3D), whereas, miR-30a-3p inhibitors
significantly increased cell proliferation (Fig. 3A), invasion (Fig. 3B), migration (Fig. 3C), and clone formation (Fig. 3D). As CNPY2 is a direct target of
miR-30a-3p in LADC, we then determined where CNPY2 is responsible
for the oncogenic biological outcomes of miR-30a-3p. On this basis,
rescue experiments were performed by co-transfecting miR-30a-3p
inhibitor (to mimic the downregulation of miR-30a-3p in LADC cells)
and selective CNPY2 silencing (si-CNPY2) in EKVX cells. In this
study, the effectiveness of si-CNPY2 was confirmed by clearly
reduced CNPY2 protein expression post-transfection in EKVX cells
(Fig. 3F). In miR-30a-3p
inhibitor-treated cells, si-CNPY2 partially reversed the increased
cell proliferation rate, cell migration and invasion ability
(Fig. 3B-E). We also observed
similar results in A549 cells, another NSCLC cell line, where
miR-30a-3p was responsible for suppression of proliferation and
colony formation via CNPY2 (Fig.
S2). These findings indicate, for the first time, that
miR-30a-3p inhibits LADC cell proliferation, invasion and migration
by targeting CNPY2 in vitro.
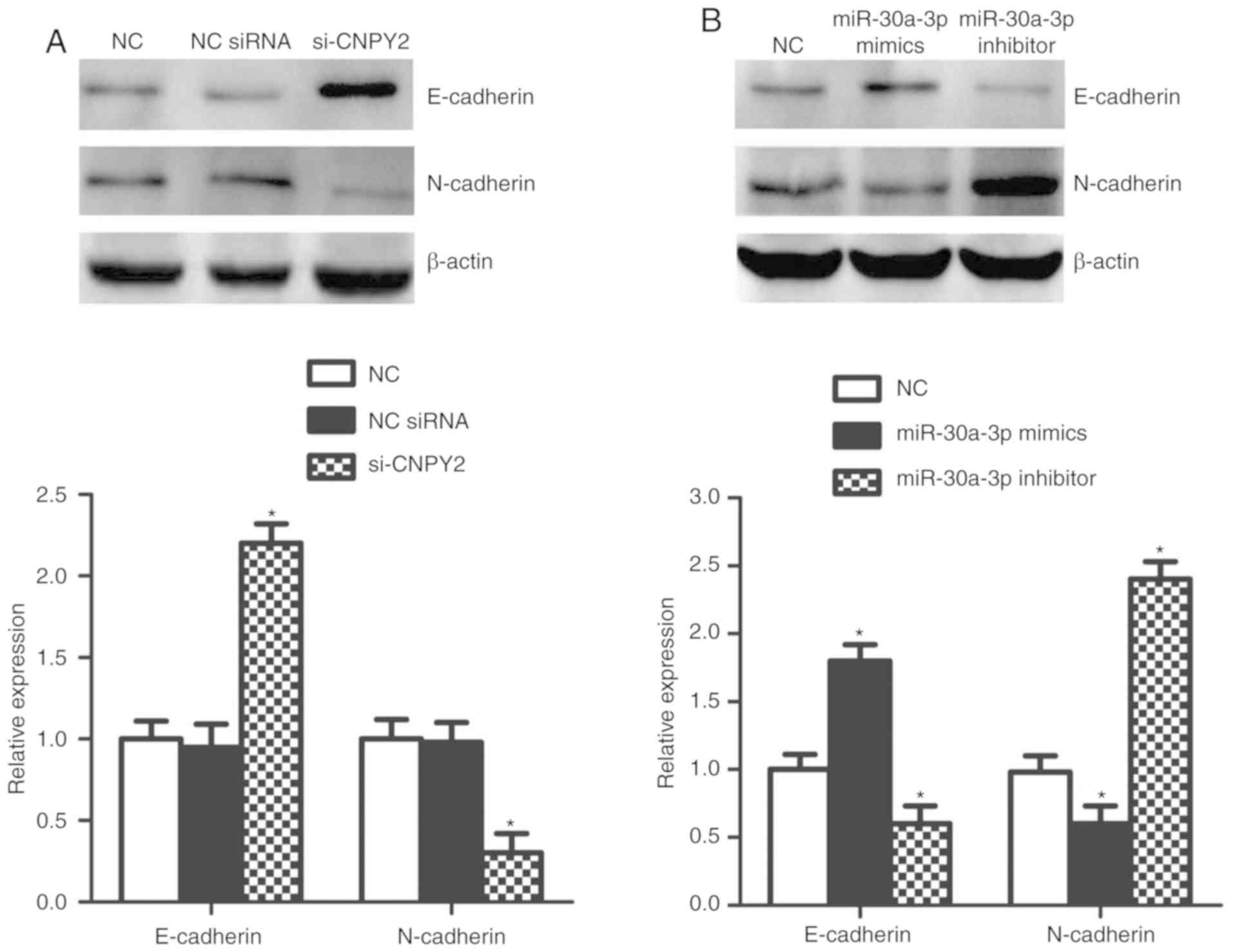
miR-30a-3p targets CNPY2 in regulating
EMT-associated proteins in vitro
CNPY2 was reported to enhance epithelial-mesenchymal
transition (EMT), an essential step of metastasis in NSCLC
(25). We next analyzed the roles
of miR-30a-3p and CNPY2 in regulating EMT in EKVX cells. Consistent
with a previous report (25), our
data showed that CNPY2 knockdown led to downregulation of
N-cadherin protein and upregulation of E-cadherin expression
(Fig. 4A), suggesting EMT
suppression. Notably, overexpression of miR-30a-3p exhibited
effects similar to those for CNPY2 silencing, while miR-30a-3p
inhibitor induced EMT, with increased N-cadherin and reduced
E-cadherin (Fig. 4B). Our results
further illustrate that CNPY2 serves as a target of miR-30a-3p in
regulating EMT-associated proteins in LADC cells.
Discussion
microRNAs are a class of post-transcriptional
regulatory molecules, some of which have been reported to be
closely related to tumorigenesis by targeting a variety of
tumor-associated genes (17–19,26).
The miR-30a family, including miR-30a-3p and miR-30a-5p, is
strongly related to the progression of different malignant tumors,
including hepatocellular carcinoma, breast cancer (14,15),
colon cancer (16), cervical cancer
(17), and osteosarcoma (26). To note, miR-30a abnormally mediated
tumor suppression or oncogenesis in various cancers (17,19).
However, only a few studies have investigated the roles of
miR-30a-3p in lung adenocarcinoma (LADC). Our data demonstrated
significant downregulation of miR-30a-3p in LADC tissues and EKVX
cells. In addition, miR-30a-3p was found to play a suppressive role
in LADC as its overexpression inhibited cellular proliferation,
invasion and migration of LADC cells. Importantly, we predicted and
validated binding and negative regulation of the 3′UTR of canopy
fibroblast growth factor signaling regulator 2 (CNPY2) by
miR-30a-3p with various computational biology and molecular biology
tools. We investigated the relationship between CYPY2 and the
clinicopathological parameters of LADC by analyzing the TCGA
database, which indicated that high expression of CNPY2 in LADC
samples was significantly correlated with staging and metastasis.
In recent studies, CNPY2 was found to promote tumor growth and
angiogenesis by activating p53 in human colorectal cancer and renal
cell carcinoma (10,11). For example, CNPY2, overexpressed in
non-small cell lung cancer (NSCLC) tissues, was negatively
correlated with the prognosis of NSCLC patients, while its
overexpression inhibited cisplatin-induced apoptosis of a NSCLC
cell line (13). We then
investigated CNPY2 expression in EKVX cells following artificial
alteration of miR-30a-3p levels. Western blotting showed that
miR-30a-3p was involved in the negative regulation of CNPY2.
Furthermore, miR-30a-3p regulated cellular viability and invasion
of LADC cells, which indicated that miR-30a-3p may be associated
with the pathogenesis of LADC by modulating the expression of
CNPY2.
NSCLC mainly includes squamous cell carcinoma and
adenocarcinoma, and is considered one of the major causes of
cancer-related death. Metastasis is involved and responsible for
the high mortality rate of LADC patients. Therefore, it is
necessary to understand the mechanism of invasion and metastasis of
LADC, which may be beneficial for molecular therapy with the aim of
inhibiting cancer progression and metastasis. Several mechanisms
have been reported to be related to the local progression and
metastasis of NSCLC, particularly EMT (27,28).
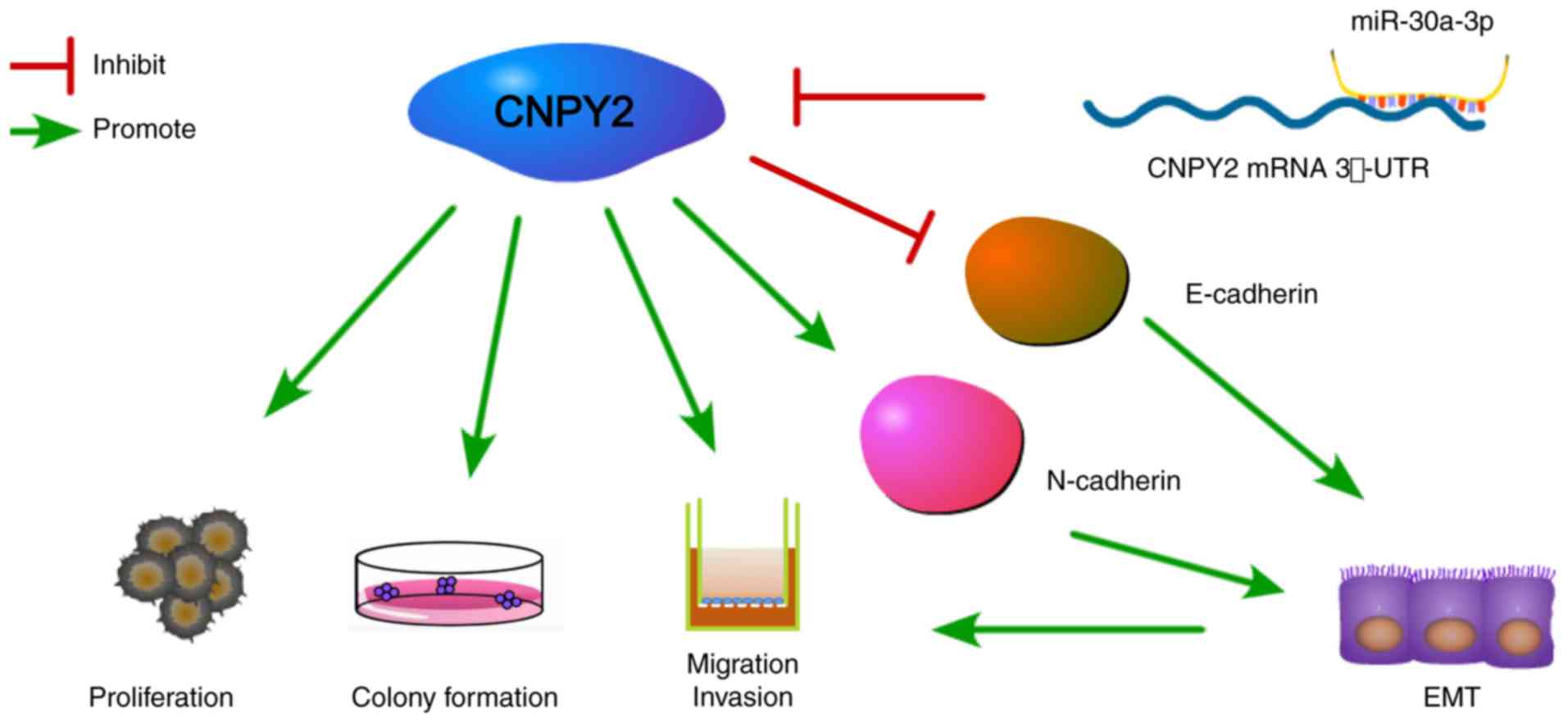
In this study, overexpression of miR-30a-3p or knockdown of CNPY2
in EKVX cells resulted in downregulation of N-cadherin and
upregulation of E-cadherin (Fig.
5). Therefore, we confirmed that in EKVX cells, CNPY2 is a
target of miR-30a-3p in the regulation of EMT-associated proteins.
Our results are consistent with those of Dou et al, who
found that CNPY2 contributes to EMT through activation of the
AKT/GSK3β pathway in NSCLC (25).
The changes in protein expression led to a decrease in cell-cell
adhesion, resulting in cancer cell proliferation and EMT.
Therefore, miR-30a-3p activation or CNPY2 inhibition may be a novel
strategy for the treatment of LADC.
The present study provides clear evidence that i)
miR-30a-3p is reduced in LADC, ii) suppression of miR-30a-3p leads
to upregulation of CNPY2, a known oncogene, iii) miR-30a-3p
modulation regulates cancer-related cellular function in LADC
cells, and iv) the biological functions of miR-30a-3p, at least in
part, are dependent on CNPY2. However, due to the nonselective
nature of the epigenetic regulatory role of miRNA, the provided
data do not distinguish the effects of miR-30a-3p on CNPY2 from
those of other targets. miR-30a-3p is reported to bind and
downregulate the expression of WNT2, a central regulator of the WNT
pathway (29). In order to better
define the mechanistic role of miR-30a-3p in different cancers, a
more selective approach is required, such as ‘Target Protectors’
(30), to define the selective role
of an miRNA-targeted mRNA in a specific phenotypic readout. This
will be a future research direction based on the results obtained
in the current study.
The human miR30a gene encodes two miRNA types
including miR-30a-3p and miR-30a-5p (GENECARDS: http://www.genecards.org/), with different sequences
and targets. miR-30a-5p has been extensively studied, with more
than twice the number of peer-reviewed research articles published
up to 2019 compared to miR-30a-3p. In NSCLC, miR-30a-5p is known as
a potential biomarker (31) with
significant downregulation, similar to miR-30a-3p. This is the
first study on the association between miR-30a-3p and CNPY2 in
LADC. We confirmed that CNPY2 mRNA selectively binds to miR-30a-3p,
compared to miR-30a-5p, further confirming the selective regulation
of CNPY2 via miR-30a-3p in LADC. A consistent downregulation of
miR-30a in cancer tissues has been observed (32), although the mechanism leading to
this downregulation has not been defined. It has been suggested
that dysregulated MIR30A gene promoter methylation is
involved (33). Further studies are
needed to define the mechanism of miR-30a-3p suppression and
selectivity of the miRNA target in various types of cancer.
In conclusion, the present study demonstrated that
miR-30a-3p acts as a tumor inhibitor in LADC via CNPY2
downregulation. In the future, potential molecular therapies
targeting the miRNA/CNPY2 axis should be explored as novel
strategies with which to treat LADC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81772493 and
81570011), Science and Technology Program of Anhui Province (Key
Laboratories project: 1606c08225, 2016080503B035, 2017070503B037)
and Bengbu Medical College Science and Technology Development Fund
(BYKY1644).
Availability of data and materials
The TCGA data which was used to support this study
is available at TCGA website (https://cancergenome.nih.gov/).
Authors' contributions
HW and TW participated in the design and
coordination of the study. DK, RL and XX performed the experiments.
XX and RL performed the statistical analysis. HW and ZQ wrote the
paper. XW, HW and TW reviewed and edited the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Sample handling was in line with the guidelines set
out in the Declaration of Helsinki. The study protocols were in
line with the guidelines of the Ethics Committee of Bengbu Medical
College (Anhui, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: North-East japan study group: Gefitinib or chemotherapy for
non-small-cell lung cancer with mutated EGFR. N Engl J Med.
362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fukuoka M, Wu YL, Thongprasert S,
Sunpaweravong P, Leong SS, Sriuranpong V, Chao TY, Nakagawa K, Chu
DT, Saijo N, et al: Biomarker analyses and final overall survival
results from a phase III, randomized, open-label, first-line study
of gefitinib versus carboplatin/paclitaxel in clinically selected
patients with advanced non-small-cell lung cancer in asia (IPASS).
J Clin Oncol. 29:2866–2874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jänne PA, Yang JC, Kim DW, Planchard D,
Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, et al: AZD9291
in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J
Med. 372:1689–1699. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bruhn H: A short guided tour through
functional and structural features of saposin-like proteins.
Biochem J. 389:249–257. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo J, Zhang Y, Mihic A, Li SH, Sun Z,
Shao Z, Wu J, Weisel RD and Li RK: A secreted protein (Canopy 2,
CNPY2) enhances angiogenesis and promotes smooth muscle cell
migration and proliferation. Cardiovasc Res. 105:383–393. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo J, Mihic A, Wu J, Zhang Y, Singh K,
Dhingra S, Weisel RD and Li RK: Canopy 2 attenuates the transition
from compensatory hypertrophy to dilated heart failure in
hypertrophic cardiomyopathy. Eur Heart J. 36:2530–2540. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ito S, Ueda T, Ueno A, Nakagawa H,
Taniguchi H, Kayukawa N and Miki T: A genetic screen in drosophila
for regulators of human prostate cancer progression. Biochem
Biophys Res Commun. 451:548–555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan P, Gong H, Zhai X, Feng Y, Wu J, He S,
Guo J, Wang X, Guo R, Xie J and Li RK: Decreasing CNPY2 expression
diminishes colorectal tumor growth and development through
activation of p53 pathway. Am J Pathol. 186:1015–1024. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taniguchi H, Ito S, Ueda T, Morioka Y,
Kayukawa N, Ueno A, Nakagawa H, Fujihara A, Ushijima S, Kanazawa M,
et al: CNPY2 promoted the proliferation of renal cell carcinoma
cells and increased the expression of TP53. Biochem Biophys Res
Commun. 485:267–271. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shimura M, Mizuma M, Takadate T, Katoh Y,
Suzuki T, Iseki M, Hata T, Aoki S, Suzuki Y, Sakata N, et al: A
novel liver metastasis-correlated protein of pancreatic
neuroendocrine neoplasm (PanNEN) discovered by proteomic analysis.
Oncotarget. 9:24291–24303. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu D, Qin Y, Jun-Qiang L and Shun-Lin G:
CNPY2 enhances resistance to apoptosis induced by cisplatin via
activation of NF-kB pathway in human non-small cell lung cancer.
Biomed Pharmacother. 103:1658–1663. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fu J, Xu X, Kang L, Zhou L, Wang S, Lu J,
Cheng L, Fan Z, Yuan B, Tian P, et al: MiR-30a suppresses breast
cancer cell proliferation and migration by targeting eya2. Biochem
Biophys Res Commun. 445:314–319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang W, Lin H, Zhou L, Zhu Q, Gao S, Xie
H, Liu Z, Xu Z, Wei J, Huang X and Zheng S: MicroRNA-30a-3p
inhibits tumor proliferation, invasiveness and metastasis and is
downregulated in hepatocellular carcinoma. Eur J Surg Oncol.
40:1586–1594. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu M, Huang F, Zhang D, Ju J, Wu XB, Wang
Y, Wang Y, Wu Y, Nie M, Li Z, et al: Heterochromatin protein HP1γ
promotes colorectal cancer progression and is regulated by miR-30a.
Cancer Res. 75:4593–4604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Z, Chen L, Zhang X, Xu X, Xing H,
Zhang Y, Li W, Yu H, Zeng J and Jia J: RUNX3 regulates vimentin
expression via miR-30a during epithelial-mesenchymal transition in
gastric cancer cells. J Cell Mol Med. 18:610–623. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao J, Li B, Shu C, Ma Y and Gong Y:
Downregulation of miR-30a is associated with proliferation and
invasion via targeting MEF2D in cervical cancer. Oncol Lett.
14:7437–7442. 2017.PubMed/NCBI
|
|
19
|
Wang Z, Dai X, Chen Y, Sun C, Zhu Q, Zhao
H, Liu G, Huang Q and Lan Q: MiR-30a-5p is induced by Wnt/β-catenin
pathway and promotes glioma cell invasion by repressing NCAM.
Biochem Biophys Res Commun. 465:374–380. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang HY, Li YY, Fu S, Wang XP, Huang MY,
Zhang X, Shao Q, Deng L, Zeng MS, Zeng YX and Shao JY: MicroRNA-30a
promotes invasiveness and metastasis in vitro and in vivo through
epithelial-mesenchymal transition and results in poor survival of
nasopharyngeal carcinoma patients. Exp Biol Med (Maywood).
239:891–898. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park D, Kim H, Kim Y and Jeoung D: MiR-30a
regulates the expression of CAGE and p53 and regulates the response
to anti-cancer drugs. Mol Cells. 39:299–309. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng Z, Gan H, Cai Z, Li N, Yang Z, Lu G
and Chen J: Aberrant expression of hypoxia-inducible factor 1α,
TWIST and E-cadherin is associated with aggressive tumor phenotypes
in endometrioid endometrial carcinoma. Jpn J Clin Oncol.
43:396–403. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang H, Liu H, Min S, Shen Y, Li W and
Wang X: CDK16 overexpressed in non-small cell lung cancer and
regulates cancer cell growth and apoptosis via a p27-dependent
mechanism. Biomed Pharmacother. 103:399–405. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dou Y, Lei JQ, Guo SL, Zhao D, Yue HM and
Yu Q: The CNPY2 enhances epithelial-mesenchymal transition via
activating the AKT/GSK3β pathway in non-small cell lung cancer.
Cell Biol Int. 42:959–964. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhong B, Guo S, Zhang W and Zhang C, Wang
Y and Zhang C: Bioinformatics prediction of miR-30a targets and its
inhibition of cell proliferation of osteosarcoma by up-regulating
the expression of PTEN. BMC Med Genomics. 10:642017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chaffer CL, San Juan BP, Lim E and
Weinberg RA: EMT, cell plasticity and metastasis. Cancer Metastasis
Rev. 35:645–654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Santamaria PG, Moreno-Bueno G, Portillo F
and Cano A: EMT: Present and future in clinical oncology. Mol
Oncol. 11:718–738. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qi B, Wang Y, Chen ZJ, Li XN, Qi Y, Yang
Y, Cui GH, Guo HZ, Li WH and Zhao S: Down-regulation of
miR-30a-3p/5p promotes esophageal squamous cell carcinoma cell
proliferation by activating the Wnt signaling pathway. World J
Gastroenterol. 23:7965–7977. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Staton AA and Giraldez AJ: Use of target
protector morpholinos to analyze the physiological roles of
specific miRNA-mRNA pairs in vivo. Nat Protoc. 6:2035–2049. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Świtlik W, Karbownik MS, Suwalski M, Kozak
J and Szemraj J: MiR-30a-5p together with miR-210-3p as a promising
biomarker for non-small cell lung cancer: A preliminary study.
Cancer Biomark. 21:479–488. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu Q, Li H, Li Y and Jiang L:
MicroRNA-30a functions as tumor suppressor and inhibits the
proliferation and invasion of prostate cancer cells by
down-regulation of SIX1. Hum Cell. 30:290–299. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Han X, Zhen S, Ye Z, Lu J, Wang L, Li P,
Li J, Zheng X, Li H, Chen W and Zhao L: A feedback loop between
miR-30a/c-5p and DNMT1 mediates cisplatin resistance in ovarian
cancer cells. Cell Physiol Biochem. 41:973–986. 2017. View Article : Google Scholar : PubMed/NCBI
|